Abstract
The present study aimed to investigate the effect of α7 nicotinic acetylcholine receptor (α7nAChR) agonist on the damage of hippocampal neurons and the expression of toll like receptor 4 (TLR4)/myeloid differentiation primary response 88 (Myd88)/nuclear factor (NF)-κB signal pathway-associated factors in cardiopulmonary bypass (CPB). Sprague Dawley rats were randomly divided into five groups: Sham operation (Sham); CPB; CPB + α7nAChR agonist PHA568487 (PHA); CPB + α7nAChR inhibitor MLA (MLA); and CPB + PHA568487 + TLR4 antagonist (CPT). Blood and brain tissue samples were harvested at 12 h following the withdrawal of CPB. Levels of serum inflammatory factors [interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α] and brain injury markers [S-100β and neuron-specific enolase (NSE)] were measured using ELISA. In addition, pathological histology and apoptosis changes were observed using hematoxylin and eosin staining, and Tunnel assays. Quantitative polymerase chain reaction and western blot assays were used to determine the expression of TLR4, Myd88 and NF-κB mRNA, and protein in the hippocampus. The morphology of hippocampal pyramidal cells in the Sham group was observed to be normal. Pyramidal cells in the CPB, MLA and CPT groups were loosely arranged, and the baselines had disappeared, with clear nucleus pyknosis and neuronal apoptosis. Furthermore, the cells in the PHA group were slightly damaged. IL-1β, IL-6, TNF-α, S-100β and NSE expression levels in the CPB, MLA, and CPT groups were significantly higher compared with that in the Sham group (P<0.05). Compared with CPB group, the expression of inflammatory cytokines in the PHA group was significantly lower (P<0.05). The expression of TLR4, Myd88 and NF-κB mRNA, and protein in the hippocampus of CPB, MLA and CPT groups were significantly higher compared with that in the Sham group, and the PHA group expression was significantly lower compared with the CPB group (P<0.05). α7nAChRs agonist can inhibit the apoptosis of rat brain neurons induced by CPB, and may protect against brain injury through the TLR4/Myd88/NF-κB signaling pathway.
Keywords: α7 nicotinic acetylcholine receptor, cardiopulmonary bypass, neuronal cell, inflammation, TLR4 signaling pathway
Introduction
Since cardiopulmonary bypass (CPB) technology is used in open heart surgery, the morality caused by postoperative myocardial infarction, heart failure, fatal arrhythmia and other cardiac causes has been significantly reduced (1). However, recent studies have found that patients often appear cognitive dysfunction, represented by neuropsychiatric disorders and mental disorders. The mortality rate due to neurological disorders and neuropsychiatric disorders following CPB is increasing from 7.2 to 19.6% (2,3). Temperature changes, hypotension, and other operations on the heart during CPB can cause low perfusion and hypoxia of brain tissue, ultimately leading to the occurrence of oxygen debt. Brain oxygen exposure triggers the post-trauma inflammatory response, and is a decisive factor in the cascade of inflammatory responses mediated by autoinflammatory mediators. On the other hand, full contact between blood and non-biological materials during CPB, focal cerebral ischemia and reperfusion injury due to cerebral embolism, and endotoxemia can all induce systemic inflammatory response syndrome (SIRS) (4–6). Therefore, inflammatory response plays an important role in CPB-induced brain injury.
Excessive activation-caused inflammatory response is a crucial reason for the occurrence of brain nerve functional damage after CPB. Cholinergic anti-inflammatory pathway (CAP) is an endogenous neurofeedback mechanism that regulates inflammatory response of the body. α7 nicotinic acetylcholine receptor (α7nAchR) is necessary for mediating CAP, agonist CAP pathway regulates the over-activated inflammatory response by inhibiting the activation of downstream inflammatory signaling pathways (7). Τoll like receptor 4 (TLR4)/myeloid differentiation primary response 88 (Myd88)/nuclear factor (NF)-κB pathway plays a vital role in inflammatory signaling, activation and regulation. Studies have shown that activation of α7nAchR can inhibit the binding between microglia and lipopolysaccharide, significantly reduce TNF-α release, and exert anti-inflammatory effect in neonatal brain injury (8,9). Activation of α7nAchR can antagonize the toxicity of β-amyloid and serves as a treatment approach of Alzheimer's disease. Therefore, activation of α7nAChRs can inhibit the inflammation via CAP and inhibit brain tissue injury.
Previous clinical and basic researches have demonstrated that CPB can cause systemic inflammatory response syndrome and brain tissue injury, induce postoperative cognitive dysfunction. CAP plays an important role in these studies. Whether α7nAChRs can inhibit the CPB-induced neuronal damage and the regulation of TLR4/Myd88/NF-κB pathway remains unclear. In the present study, we established CPB model in non-blood pre-filling rats and treated the models with α7nAChR agonist PHA568487 and specific α7nAChR blocker MLA, aiming to investigate the protective effects and underlying mechanism of α7nAChR agonist, provide evidence for formulating treatment approach of brain injury and reducing cardiovascular complications of CPB cardiovascular surgery.
Materials and methods
Animals and ethics approval
SD rats, weighing 350–400 g, were provided by Experimental Animal Center of China Medical University (production license No. SCXK (Liao)-2013-0001, application license No. SYXK (Liao)-2013-0007). The present study was approved by the China Medical University Laboratory Animal Welfare and Ethics Committee (IACUC). Experimental animals were feed in the barrier system and managed by the experimental animal professionals.
Establishment of CPB rat model
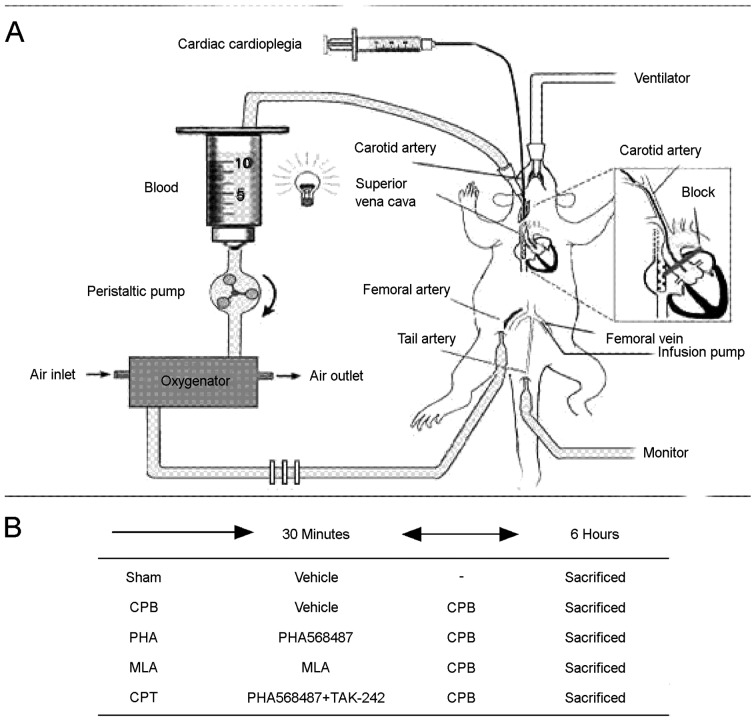
CPB was performed as previously reported with minor modifications (10). Briefly, rats were anesthetized with isoflurane; a 24G trocar was inserted into the right femoral vein, a 22G trocar was inserted into the left femoral artery, another 22G trocar puncture catheter was used as a perfusion artery for extracorporeal circulation. Self-made multi-hole 18G puncture needle arrived the right atrium through puncture catheter via the right internal jugular vein, serving as the extracorporeal venous outflow end. For the establishment of CPB model, we applied the following equipment connected with PVC tube (internal diameter 1.6 mm), including venous drainage tube, blood reservoir, arterial infusion tube, filter and connecting pipe. A total of 15 ml of circulating prefilled solution was consisted of 6 ml hydroxyethyl starch, 6 ml lactate Ringer's solution, 1 ml heparin (250 IU kg−1), 5% sodium bicarbonate, and 1 ml of 20% mannitol (Fig. 1-A). When CPB began, mechanical ventilation was terminated, CPB flow rate was more than 80 ml/kg/min, circulation volume was timely added to ensure the flow rate, flow time was 60 min. During CPB, the oxygen-air mixture (at a ratio of 1:4) flew through the membrane lung at a rate of 800 ml/min; mechanical ventilation was restarted before the flow was reduced, circulation volume was gradually decreased by adjusting the diameter of venous outflow end; the right internal jugular vein and caudal artery were ligated, followed by the removal of the catheter and suture of incision; after rats resumed spontaneous breathing, tracheal intubation was also removed and rats were fed in laboratory for observation.
Figure 1.
Schematic diagram of experimental design. (A) CPB rat model. (B) Experimental protocol in CPB rat model. CPB, cardiopulmonary bypass.
Groups and treatments
Rats were randomly divided into five groups: The sham operation group (Sham group; n=10), the CPB group (CPB group; n=10), the CPB + α-7nAchR agonist (PHA568487) group (PHA group; n=10), the CPB + α-7nAchR antagonist (methyllycaconitine, MLA) group (MLA group; n=10), the CPB + PHA568487 + TLR4 shRNA (CPT group; n=10). In the Sham group, the intubation and mechanical ventilation were performed in the right femoral artery only and the right internal jugular vein was catheterized without bypass;in the CPB group, the CPB model was established as described above;mice in the sham and CPB groups were injected with a vehicle (Control shRNA Lentiviral Particles-A, sc-108080, Santa, USA); in the PHA group, intraperitoneal injection of 0.8 mg/kg PHA568487 (Tocris Bioscience, Ellisville, MO, USA) was performed for 30 min prior to the CPB establishment; in the MLA group, intraperitoneal injection of 6 mg/kg MLA (Sigma, St Louis, MO, USA) was performed for 30 min prior to the CPB establishment; in the CPT group, rats were also pretreated with PHA568487 (0.8 mg/kg), followed by the intraperitoneal injection of 3 mg/kg TLR4 shRNA (sc-40260-V, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and finally performed with CPB (Fig. 1-B).
Specimen collection and processing
Arterial and venous blood samples were respectively collected at CPB for 6 h after rats were sacrificed by administration of an overdose of anesthesia of sodium pentobarbital. After that, the hippocampus was isolated from the rat, the one side was fixed in 4% paraformaldehyde (PFA), and the other side was stored at −80°C for western blot and PCR analysis. The sera were separated by centrifugation at 1,000 × g for 10 min, stored at −80°C.
HE staining of histopathological changes
Hippocampal tissues were taken at 6 h of CPB and fixed with neutral formalin. 48 h later, brain tissue was dehydrated with 70, 80, 90, 95, 100% ethanol, xylene transparent, embedded, sliced and detected with HE staining.
Tunel staining of hippocampal neurons
The apoptosis of hippocampal neurons was detected according to the instructions of TUNEL Apoptosis Assay Kit (NO. 11684817, Roche, Mannheim, Germany). Hippocampal tissue was dehydrated, embedded, sliced and incubated with 0.9% NaCl for 5 min, then rinsed twice with PBS, mixed with biotinylated nucleotides and terminal deoxynucleotidyl transferase, covered with plastic coverslips and incubated at 37°C for 60 min. After another PBS wash, brain tissue was blocked with 0.3% hydrogen peroxide, and incubated with HRP-labeled streptavidin at room temperature for 30 min, followed by wash, DAB staining, mounting and observed under a microscope.
ELISA detection of serum levels of IL-1β, IL-6, TNF-α, S-100β and NSE. Inflammatory factors such as IL-1β (SEA563Ra, CCC, Compressor Controls Corporation, Des Moines, IA, USA), IL-6 (SEA079Ra, CCC, USA), TNF-α (SEA133Ra, CCC, USA), S-100β (SEA567Ra, CCC, USA) and NSE (SEA537Ra, CCC, USA) in the plasma of rats were measured by ELISA kit. After the reagents were equilibrating to room temperature (20–25°C), reaction plate was taken out and added with 100 µl standard sample and l00 ul diluted sample, gently shaking for 30 sec and incubating at 20–25°C for 20 min; each hole within the reaction plate was added with 100 µl serum sample and incubated at 37°C for 2 h; then, 100 µl HRP-labeled antibody was added to each hole and incubated at 37°C for 30 min; after another wash, color liquids A and B, each 50 µl, was added for developing and the reaction was terminated with 50 µl solution. The optical density (OD) at 450 nm was measured with microplate reader; standard curve was plotted taking the OD value as the ordinate and standard sample concentration as the abscissa, the curve equation and r value were calculated, thus the sample concentration value was measured.
Western blot analysis
The hippocampal tissue was grid and centrifuged in a pre-chilled tissue lysate at 12,000 rpm for 30 min. The supernatant of total protein was extracted for SDS-PAGE and the protein was semi-dried for membrane transfer. The cells were blocked 2 h and incubated overnight at 4°C with TLR4 (ab22048, Abcam, Cambridge, MA, USA), Myd88 (ab2068, Abcam, USA), NF-κB antibody (ab32360, Abcam, USA), followed three washes and incubation with secondary antibody for 1 h. After four washes using TBST, cells were developed with ECL Western Blotting Substrate kit (32109, Pierce™, Thermo Fisher Scientific, Waltham, MA, USA) and gray value was measured by using Quantity One software.
Realtime-PCR detection
Primers were designed according to the sequences of TLR4, Myd88, NF-κB and GAPDH reported in Genbank, and were synthesized in Shanghai Biomedical Biotechnology Co., Ltd., (Shanghai, China) (Table I). Rat hippocampus total RNA was isolated with TRIzol reagent (15596018, Invitrogen, Carlsbad, CA, USA).and reversely transcribed into cDNA (4387406, Invitrogen, USA). Real-time PCR kit (RR820A, Takara Bio, Dalian, China) was used for the detection.
Table I.
Primer sequences.
| ITEAM | PRIMER SEQUENCES |
|---|---|
| Myd88 | P1:agatccgcga gtttgagacg cg |
| P2:ctttctacca accttctgta cg | |
| TLR9 | P1:ctctcctggt acaggctgca gt |
| P2:cctggcaagt tccttcaaga gc | |
| NF-κB | P1:cggcctcatc cacatgaact tgt |
| P2:ctaaggcact gatggtgacc ct | |
| GAPDH | P1:aactttggcattgtggaagg |
| P2:cacattgggggtaggaacac |
Statistical analysis
Data were analyzed using SPSS 19.0 statistical software, measurement data were expressed as mean ± standard deviation. Comparisons between groups were made using the Student's t-test (two-tailed) or ANOVA with Tukey's post-test analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Hippocampal pathological detection results
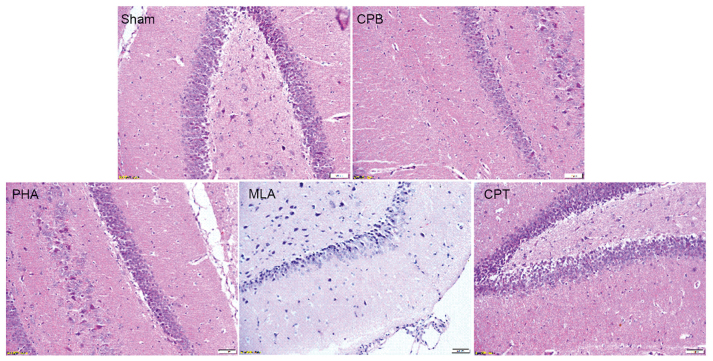
HE staining showed that hippocampal neurons in Sham group were arranged in a regular and close manner, with clear boundary and complete cell band, and no abnormal cell structure was found. The hippocampus in CPB, MLA and CPT group were severely damaged, the damage in the PHA group was lighter than that of the CPB group, cells were tightly arranged and cell band was not complete (Fig. 2).
Figure 2.
α-7nAchR agonist alleviates pathological injury in hippocampus. Sham group: There was no evidence of morphological damage; CPB group: Obvious cellular degeneration and abnormal cell arrangements; PHA group: Only a slight morphological; MLA and CPT group: The typical vacuolated degenerations in hippocampal neurons.
Hippocampal neuronal apoptosis
Compared with Sham group, the number of TUNEL-positive cells in CPB, MLA and CPT group were significantly increased (P<0.05); compared with CPB group, the number of TUNEL-positive cells in the hippocampus of PHA group was significantly decreased (P<0.05) (Fig. 3).
Figure 3.
α-7nAchR agonist inhibits neuronal apoptosis in hippocampus. Sham group: There was no evidence of neuronal apoptosis; CPB group: The neurons exhibited the typical apoptosis; PHA group: Only a slight neuronal apoptosis; MLA and CPT group: The neuronal apoptosis in hippocampal neurons. Compared with Sham group, *P<0.05, Compared with CPB group, **P<0.05.
Changes of IL-1β, IL-6 and TNF-α in serum
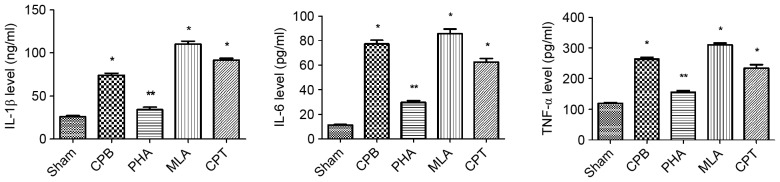
Compared with Sham group, the levels of IL-1β, IL-6 and TNF-α in CPB, MLA and CPT group were significantly increased (P<0.05); compared with CPB group, IL-1β, IL-6 and TNF-α levels in PHA group were significantly decreased (P<0.05) (Fig. 4).
Figure 4.
α-7nAchR agonist reduces the serum levels of IL-1β, TNFα and IL6 in CPB-injured rats. Compared with S group, *P<0.05; Compared with CPB group **P<0.05.
Changes of S-100β and NSE levels
The expression of S-100β and NSE in CPB, MLA and CPT group were significantly higher than those in Sham group (P<0.05). Compared with CPB group, the expressions of S-100β and NSE in PHA group were significantly decreased (P<0.05) (Fig. 5).
Figure 5.
α-7nAchR agonist reduces the levels of S-100β and NSE in CPB-injured rats. Changes of S-100β and NSE in brain tissue were detected by ELISA. Compared with Sham group, *P<0.05; Compared with CPB group **P<0.05.
Detection of TLR4, Myd88, NF-κB mRNA and protein. The expressions of TLR4, Myd88, NF-κB mRNA and protein in CPB, MLA and CPT group were significantly higher than those in Sham group (P<0.05). Compared with CPB group, TLR4, Myd88, NF-κB expression in brain tissue of PHA group were significantly decreased (P<0.05) (Fig. 6).
Figure 6.
α-7nAchR agonist promotes expression of TLR4, Myd88, NF-κB. (A and B) The expression of TLR4, Myd88, NF-κB were detected by Western blot; (C) The expression of TLR4, Myd88, NF-κB were detected by Realtime-PCR. Compared with Sham group, *P<0.05, Compared with CPB group **P<0.05.
Discussion
In the present study, we observed the changes of neuronal morphology, the expression of inflammatory factor and TLR4, Myd88, NF-κB pathway in rat hippocampus by HE and TUNEL staining, ELISA assay and western blot analysis. Results showed that PHA568487 could decrease the morphological changes of hippocampus, downregulate the expression of inflammatory factors and the apoptosis. In addition, PHA568487 contribute to reduce CPB-induced TLR4, Myd88, NF-κB pathway protein expression. This evidence suggests that PHA568487 can attenuate CPB-induced brain injury in rats, the underlying mechanism may be mediated through the regulation of TLR4, Myd88, NF-κB pathway. The incidence of CPB-induced brain injury is increasing year by year, becoming an urgent problem, the present study provides experimental and theoretical evidence for the modification of perioperative brain protection of heart surgery.
At present, CPB is the main means of clinical open heart surgery, as CPB devices are continuously developing, anesthesia techniques and surgical skills gradually increase, heart surgery complications and mortality are significantly reduced (11). However, increasing number of previous studies have reported the CPB-induced brain injury and related inflammatory response, leaving its pathogenesis unclear (12,13). A good CPB model is essential for the study of therapeutic and prophylactic measures. In the present study, CPB model was established by injecting 6% hydroxyethyl starch into SD rats through right venous drainage and right femoral arterial fusion. HE staining and ELISA assay showed that, CPB caused brain tissue injury characterized by neuronal apoptosis, increased brain injury markers significantly, especially serum inflammatory factors were increased significantly at postoperative 2 h. This finding suggested that CPB can induce brain neuron injury and systemic inflammatory response. On the other hand, the model is established successfully, which can reflect clinical features and can be used as experimental tool to study systemic inflammatory response, organ protection mechanism and related protection measures.
CAP is an endogenous neurofeedback regulation, studies have shown that cholinergic agonists acting on α7nAChR-knockout macrophages can not produce the desired anti-inflammatory effect, suggesting that α7nAchR is the necessary receptor for CAP (14). Under the stimuli of infection, injury or embolism, vagus nerve is activated, a large number of Ach release into peripheral tissue and bind with macrophages, microglia and other antibody on the surface of immune cells (14,15). Activation of α7nAchR, cholinergic anti-inflammatory signal can inhibit the release of IL-1β, IL-6, TNF-α and other inflammatory cytokines and endotoxin through the various signal transduction pathways in the cells, increase the formation of anti-inflammatory factors, thus producing a rapid and efficient regulation of inflammatory response (16,17). Previous studies have found that, α7nAChR agonists can not only exert clear anti-inflammatory effect for cerebral embolism-caused neuropathic inflammatory response, fracture-caused inflammatory brain injury and neonatal encephalitis, but also improve postoperative cognitive dysfunction and Alzheimer's disease (18,19). Activation of α7nAChR induces the upregulation of its function, antagonizes the toxicity of β-amyloid (Aβ), modulates downstream signal transduction and thus improves cognitive memory impairment (20). Α7nAChR agonist PHA568487 can improve cognitive impairment such as schizophrenic symptoms in the MK801 mouse model of spatial memory impairment (21). In the ischemic stroke model, the protection effect of α7nAChR agonist PHA568487 is achieved by reducing the macrophage release of inflammatory factors and the body's oxidative stress. Furthermore, α7nAchR agonist could protect primary brain cortical neurons from oxygen-glucose deprivation injury model after culture for 7 days in vitro (22). The protection effect was associated with anti-oxidative stress. In the present study, we found that compared with the CPB group, brain injury was attenuated, neuronal apoptosis rate was decreased, the levels of IL-1β, IL-6 and TNF-α was lower, the concentration of S100β and NSE in the brain tissue was also lower in the α7nAChR agonist group, suggesting that α7nAChR agonists protect against CPB-induced brain injury.
Previous studies have demonstrated that TLR4/Myd88/NF-κB signaling pathway is involved in the damage of neuronal cells during cerebral ischemia (23,24). TLRs is expressed on various kinds of immune cells, and TLR4 is mainly expressed on monocytes and macrophages and dendritic cells. TLR4 through the selective recognition of PAMPs such as lipopolysaccharide, flagellin and microbial nucleic acid and endogenous molecules damage associated molecular patterns such as tissue injury necrosis by the release of hyaluronic acid, high mobility group protein 1, heat shock protein, triggered Myd88 dependent and independent pathways, the final activation of NF-κB, leading to TNF-α, IL-6, IL-1β and other inflammatory cytokines and chemokine release induced inflammation (25). Kong et al (26) found in macrophage research, TLR4/Myd88/NF-κB and the expression of many inflammatory factors regulation and control, curcumin can inhibit the inflammatory reaction through the TLR4/Myd88/NF-κB. Zhu et al (27) in the acute brain injury research shows that Curcumin can inhibit the inflammatory response through the TLR4/Myd88/NF-κB signaling pathway.
In summary, the expression of TLR4, Myd88, NF-κB protein in the hippocampus of CPB rats were significantly increased compared with the control group, and the expression of TLR4, Myd88, NF-κB was significant decreased after the intervention of α7nAChR agonist. The results suggest that α7nAChR agonists protect brain neurons against CPB-induced injury in rats by activating TLR4, Myd88, NF-κB pathway.
Acknowledgements
The present study was supported by the Natural Science Foundation of China (grant no. 81471121 and no. 3120175). New teacher foundation of China Medical University (XZR20160036).
References
- 1.Boshes B, Priest WS, Yacorzynski GK, Zaks MS. The neurologic, psychiatric and psychologic aspects of cardiac surgery. Med Clin North Am. 1957;41:155–169. doi: 10.1016/S0025-7125(16)34473-X. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, Racz MJ, Walford G, Jones RH, Ryan TJ, Bennett E, Culliford AT, Isom OW, Gold JP, Rose EA. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med. 2005;352:2174–2183. doi: 10.1056/NEJMoa040316. [DOI] [PubMed] [Google Scholar]
- 3.Knipp SC, Matatko N, Wilhelm H, Schlamann M, Thielmann M, Lösch C, Diener HC, Jakob H. Cognitive outcomes three years after coronary artery bypass surgery: Relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2008;85:872–879. doi: 10.1016/j.athoracsur.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 4.Plicner D, Stoliński J, Wasowicz M, Gawęda B, Hymczak H, Kapelak B, Drwiła R, Undas A. Preoperative values of inflammatory markers predict clinical outcomes in patients after CABG, regardless of the use of cardiopulmonary bypass. Indian Heart J. 2016;68(Suppl 3):S10–S15. doi: 10.1016/j.ihj.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehne M, Sasse M, Karch A, Dziuba F, Horke A, Kaussen T1, Mikolajczyk R, Beerbaum P, Jack T. Systemic inflammatory response syndrome after pediatric congenital heart surgery: Incidence, risk factors, and clinical outcome. J Card Surg. 2017;32:116–125. doi: 10.1111/jocs.12879. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Yang J, Bao J, Li X, Ye A, Zhang G, Liu H. Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta. 2017;49:23–32. doi: 10.1016/j.placenta.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Xiong J, Yuan YJ, Xue FS, Wang Q, Cheng Y, Li RP, Liao X, Liu JH. Postconditioning with α7nAChR agonist attenuates systemic inflammatory response to myocardial ischemia-reperfusion injury in rats. Inflammation. 2012;35:1357–1364. doi: 10.1007/s10753-012-9449-2. [DOI] [PubMed] [Google Scholar]
- 8.Frasch MG, Szynkaruk M, Prout AP, Nygard K, Cao M, Veldhuizen R, Hammond R, Richardson BS. Decreased neuroinflammation correlates to higher vagus nerve activity fluctuations in near-term ovine fetuses: A case for the afferent cholinergic anti-inflammatory pathway? J Neuroinflammation. 2016;13:103. doi: 10.1186/s12974-016-0567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghijselings I, Himpe D, Rex S. Safety of gelatin solutions for the priming of cardiopulmonary bypass in cardiac surgery: A systematic review and meta-analysis. Perfusion. 2017;32:350–362. doi: 10.1177/0267659116685418. [DOI] [PubMed] [Google Scholar]
- 10.Cao HJ, Sun YJ, Zhang TZ, Zhou J, Diao YG. Penehyclidine hydrochloride attenuates the cerebral injury in a rat model of cardiopulmonary bypass. Can J Physiol Pharmacol. 2013;91:521–527. doi: 10.1139/cjpp-2012-0329. [DOI] [PubMed] [Google Scholar]
- 11.Lomivorotov VV, Shmyrev VA, Ponomarev DN, Efremov SM, Shilova AN, Postnov VG. Influence of remote ischemic preconditioning on brain injury markers dynamics during cardiopulmonary bypass. Anesteziol Reanimatol. 2015;60:33–38. (In Russian) [PubMed] [Google Scholar]
- 12.von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 13.Ni M, Fu H, Huang F, Zhao T, Chen JK, Li DJ, Shen FM. Vagus nerve attenuates hepatocyte apoptosis upon ischemia-reperfusion via α7 nicotinic acetylcholine receptor on kupffer cells in mice. Anesthesiology. 2016;125:1005–1016. doi: 10.1097/ALN.0000000000001309. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Chen H, Zhu W, Xu Y, Liu M, Zhu L, Yang F, Zhang L, Liu X, Zhong Z, et al. Nicotine accelerates atherosclerosis in apolipoprotein E-deficient mice by activating α7 nicotinic acetylcholine receptor on mast cells. Arterioscler Thromb Vasc Biol. 2017;37:53–65. doi: 10.1161/ATVBAHA.116.307264. [DOI] [PubMed] [Google Scholar]
- 15.Xu ZQ, Shao BZ, Ke P, Liu JG, Liu GK, Chen XW, Su DF, Liu C. Combined administration of anisodamine and neostigmine rescued acute lethal crush syndrome through α7nAChR-dependent JAK2-STAT3 signaling. Sci Rep. 2016;6:37709. doi: 10.1038/srep37709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XM, Li FQ, Yan S, Wu XC, Tang CL. Nicotine alleviates the liver inflammation of non-alcoholic steatohepatitis induced by high-fat and high-fructose in mice. Beijing Da Xue Xue Bao. 2016;48:777–782. (In Chinese) [PubMed] [Google Scholar]
- 17.Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, Fardella S, Carnevale L, Carnevale R, De Lucia M, Cifelli G, Lembo G. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat Commun. 2016;7:13035. doi: 10.1038/ncomms13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasch MG, Szynkaruk M, Prout AP, Nygard K, Cao M, Veldhuizen R, Hammond R, Richardson BS. Decreased neuroinflammation correlates to higher vagus nerve activity fluctuations in near-term ovine fetuses: A case for the afferent cholinergic anti-inflammatory pathway? J Neuroinflammation. 2016;13:103. doi: 10.1186/s12974-016-0567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arèvalo-Serrano J, Sanz-Anquela JM, Gonzalo-Ruiz A. Beta-amyloid peptide-induced modifications in alpha7 nicotinic acetylcholine receptor immunoreactivity in the hippocampus of the rat: Relationship with GABAergic and calcium-binding proteins perikarya. Brain Res Bull. 2008;75:533–544. doi: 10.1016/j.brainresbull.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Ge J, Tian J, Yang H, Hou L, Wang Z, He Z, Wang X. ALPHA7 nicotine acetylcholine receptor agonist PNU-282987 attenuates acute lung injury in a cardiopulmonary bypass model in rats. Shock. 2017;47:474–479. doi: 10.1097/SHK.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 22.Feng H, Su R, Song Y, Wang C, Lin L, Ma J, Yang H. Positive correlation between enhanced expression of TLR4/MyD88/NF-κB with insulin resistance in placentae of gestational diabetes mellitus. PLoS One. 2016;11:e0157185. doi: 10.1371/journal.pone.0157185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SH, Bang J, Son CN, Baek WK, Kim JM. Grape seed proanthocyanidin extract ameliorates murine autoimmune arthritis through regulation of TLR4/MyD88/NF-κB signaling pathway. Korean J Intern Med. 2016 Jun 3; doi: 10.3904/kjim.2016.053. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han LP, Li CJ, Sun B, Xie Y, Guan Y, Ma ZJ, Chen LM. Protective effects of celastrol on diabetic liver injury via TLR4/MyD88/NF-κB signaling pathway in type 2 diabetic rats. J Diabetes Res. 2016;2016:2641248. doi: 10.1155/2016/2641248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Xie G, Li L, Jiang Z, Yue Z, Pan Z. The effect of TLR4/MyD88/NF-κB signaling pathway on proliferation and apoptosis in human nasopharyngeal carcinoma 5–8F cells induced by LPS. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29:1012–1015. [PubMed] [Google Scholar]
- 26.Kong F, Ye B, Cao J, Cai X, Lin L, Huang S, Huang W, Huang Z. Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced macrophages. Front Pharmacol. 2016;7:369. doi: 10.3389/fphar.2016.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, Wang CS, Feng H, Lin JK. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J Neuroinflammation. 2014;11:59. doi: 10.1186/1742-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]