Abstract
The present study aimed to investigate the effect of exogenous nerve growth factor (NGF) pretreatment on demyelination in the spinal cord of lidocaine-treated rats, and explored the potential neuroprotective mechanisms of NGF. A total of 36 rats were randomly assigned to three groups (n=12 per group): Sham group; Lido group, received intrathecal injection of lidocaine; NGF group, received intrathecal injection of NGF followed by intrathecal injection of lidocaine. Tail-flick tests were used to evaluate neurobehavioral function. Ultrastructural alternations were analyzed by transmission electron microscopy. Immunofluorescence was used to examine the expression of myelin basic protein (MBP) and brain-derived neurotrophic factor (BDNF). ELISA was used to determine serum levels of MBP and proteolipid protein (PLP). Western blotting was used to detect the expression of phosphorylated mitogen activated protein kinase (MAPK). NGF pretreatment reduced lidocaine-induced neurobehavioral damage, nerve fiber demyelination, accompanied by a decrease in MBP expression in the spinal cord and an increase in MBP and PLP in serum. In addition, NGF pretreatment increased BDNF expression in the spinal cord of lidocaine-treated rats. Furthermore, NGF pretreatment reduced p38 MAPK phosphorylation in the spinal cord of lidocaine-treated rats. NGF treatment reduces lidocaine-induced neurotoxicity via the upregulation of BDNF and inhibition of p38 MAPK. NGF therapy may improve the clinical use of lidocaine in intravertebral anesthesia.
Keywords: lidocaine, neurotoxicity, nerve growth factor, brain-derived neurotrophic factor, p38 mitogen activated protein kinase
Introduction
Local anesthetics are frequently used in intravertebral anesthesia, however there are increasing concerns regarding effects of neurotoxicity. Numerous studies have previously demonstrated that local anesthetics used for intravertebral anesthesia result in neurotoxicity, and eventually lead to neuronal dysfunction (1–3). Of the frequently-used local anesthetics, lidocaine has been revealed to produce high levels of neurotoxicity (4,5). The underlying mechanisms of lidocaine-induced neurotoxicity remain to be elucidated, however high concentrations of lidocaine have been reported to lead to neurotoxicity via the promotion of mitochondrial dysfunction, induction of neuronal apoptosis and activation of p38 mitogen-activated protein kinase (p38 MAPK) (6–9). In addition, it was demonstrated that the neurotoxicity of lidocaine increased when combined with ropivacaine (10). Therefore, it is important to identify factors that reverse lidocaine-induced neurotoxicity; and thus, improve the clinical use of lidocaine in intravertebral anesthesia.
Nerve growth factor (NGF), a primary member of the family of neurotrophic factors, has been used as a neuroprotective drug in the treatment of various neurological diseases, including brain injury and stroke (11,12). The authors previously reported that intrathecal injection of exogenous NGF reduces lidocaine-induced neurotoxicity in the spinal cord, potentially via the inhibition of neuronal apoptosis (10). However, the exact neuroprotective mechanisms of NGF in lidocaine-induced neurotoxicity remain unclear. The p38 MAPK signal transduction pathway is important in the regulation of cell proliferation, apoptosis and differentiation (13,14). Lirk et al (15) demonstrated that p38 MAPK inhibitors reduced lidocaine-induced neurotoxicity in cultured dorsal root ganglia cells and in a rat sciatic nerve model, suggesting that the activation of p38 MAPK may contribute to lidocaine-induced neurotoxicity and p38 MAPK inhibition may protect neurons from lidocaine-induced damage (15). However, it remains to be determined whether exogenous NGF pretreatment may effectively reduce lidocaine-induced neurotoxicity via the p38 MAPK signal transduction pathway.
The present study aimed to investigate the effect of NGF pretreatment on demyelination in the spinal cord of lidocaine-treated rats, and explore the potential neuroprotective mechanisms of NGF in lidocaine-induced neurotoxicity via p38 MAPK inhibition.
Materials and methods
Animals
The Institutional Animal Care and Use Committee of China Medical University (Shenyang, China) approved the experimental protocols of the present study. All procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Animal Welfare Act (16). A total of 36 10-week-old adult Sprague-Dawley rats (male; weight, 260–280 g) were used, obtained from the Animal Care Center of China Medical University. Animals were housed at room temperature (25±1°C) with a relative humidity of 40–60% and a 12 h light/dark cycle. Animals were housed alone and fed standard rat chow and water ad libitum. The animals were randomly assigned to three groups (n=12 for each group): Sham group, Lido group receiving intrathecal injection of lidocaine and NGF group receiving intrathecal injection of NGF followed by intrathecal injection of lidocaine.
Intrathecal catheter insertion
An intrathecal catheter was inserted, as previously described (10). Briefly, animals were anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg). Rats were placed in the prone position following anesthetization. Following exposure of the L4-L5 interspinous space and the ligamentum flavum, a heat-connected polyethylene catheter (PE-10; An Lai Software Technology Co., Ltd., Ningbo, China) was inserted into the subarachnoid space at the L4-L5 intervertebral space. The catheter was successfully inserted into the subarachnoid space when the animal exhibited a side tail swing or a hind leg twitch. The catheter was caudally advanced by 2 cm, and the outflow of cerebrospinal fluid indicated the success of the catheter insertion. The catheter was then flushed with normal saline (10 µl), the distal end of the catheter was closed and fixed subcutaneously. The incision was sutured, and an intramuscular injection of penicillin (Huabei Pharmaceutical Co., Ltd., Huabei, China) was administered. A total of 10% lidocaine (20 µl) was injected through the catheter one day following catheter insertion. The present study only included lidocaine-positive rats that exhibited obvious hind limb paralysis in the two hind limbs within 30 sec following the lidocaine injection. Rats were excluded when obvious limb paralysis or movement disorders following catheter insertion were present, or unilateral limb paralysis occurred following lidocaine injection.
Drug administration
In rats in the Lido group, 10% lidocaine hydrochloride (20 µl; 0219011125; MP Biomedicals, LLC, Santa Ana, CA, USA) was intrathecally administered, one day following the intrathecal catheter insertion. Rats in the NGF group were intrathecally administered with NGF (20 µg/20 µl; N2513 Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 1 h prior to intrathecal administration of 10% lidocaine hydrochloride (20 µl). Rats in the Sham group were sham operated and intrathecally administered with normal saline (20 µl) without intrathecal injection of lidocaine or NGF.
Behavioral tests
Sensory function was evaluated at postoperative days 1, 2 and 3 by the tail-flick test; in which the tail was irradiated by a radiant heat source (BME-410 thermal radiator; Chinese Academy of Medical Sciences, Beijing, China). Tail-flick latency was determined by measuring the time (in sec) the rats took to withdraw the tail from the heat source. In order to avoid tissue damage, a cut-off time was set at 12 sec. Tail-flick latency was determined at the proximal, middle and distal portion of the tail at 5 min intervals; and the average of 3 determinations was used. The percentage maximal possible effect (%MPE) was used to evaluate tail-flick latency, and was calculated as follows: (T1−T0)/(T2−T0) ×100; where T0 and T1 are the tail-flick latencies obtained prior to and following drug application, and T2 is the cut-off time. The standard neurological disability scoring was used for the behavioral assessments of myelin damage. Rats were evaluated daily and graded according to the following scale: Grade 1=limp tail; grade 2=hind limb weakness sufficient to impair righting behavior; grade 3=one limb plegic; grade 4=two limbs plegic; grade 5=3 or 4 limbs plegic or the animal was moribund. All neurobehavioral measurements were conducted by an investigator blinded to the experimental conditions.
Tissue preparation
Following completion of the behavioral tests, 4 rats were randomly selected from each group and anesthetized by intraperitoneal injection of sodium pentobarbital (40 mg/kg). The rats were transcardially perfused with normal saline (200 ml). The spinal cord between the L4 and L6 segments was removed. The tissues were stored at −80°C, and were used for western blotting (n=2 rats per group). The tissues were post-fixed and dehydrated in 30% sucrose in PBS at 4°C for 24 h, and embedded in paraffin. These tissue blocks were used for immunofluorescence (n=2 rats per group).
Transmission electron microscopy (TEM)
For ultrastructural analysis, white matter tissues (1 mm) in the dorsal horn of the spinal cord were removed and fixed with 2.5% glutaraldehyde for 24 h at 4°C. Following 3 10-min washes with 0.1 M PBS, tissue samples were postfixed in 1% OsO4 for 1 h, dehydrated in a graded series of ethanol (50, 70, 90 and 100% for 10 min each) and then embedded in a 1:1 mixture of Epon812 and acetone for 2–3 h, followed by embedding in Epon812 for 2 h and subsequent heating at 35°C for 12 h, 45°C for 12 h and then at 60°C for 24 h. Ultrathin sections were stained with lead citrate and uranyl acetate for 24 h at 4°C, viewed and imaged with a scanning electron microscope.
Immunofluorescence
Sections (5 µm thick) from the L4-L6 spinal cord tissue were obtained. The sections were incubated at 4°C overnight with a mouse anti-rat primary antibody against myelin basic protein (MBP; ab62631; 1:500; Abcam, Cambridge, MA, USA) or a sheep anti-rat monoclonal primary antibody against brain-derived neurotrophic factor (BDNF; ab75040; 1:500; Abcam), followed by incubation with biotinylated donkey anti-mouse or anti-sheep IgG (A10036 and A-11015; 1:200; Vector Laboratories, Burlingame, CA) in 1.5% normal donkey serum (Jackson Immuno Research Laboratories Inc., West Grove, PA) for 20 min at 37°C. Then, a mixture of streptavidin-conjugated Alexa Fluor 546 (SV1632 Beijing Baolaibo Biological Technology Co., Ltd., Beijing, China) and 488 (SV1634 Beijing Baolaibo Biological Technology Co., Ltd., Beijing, China)-labelled secondary antibodies was added for 1 h at room temperature and the sections were counterstained with DAPI (C1005; Beyotime Institute of Biotechnology, Shanghai, China) for 5 min at room temperature. The specificity of the immunostaining was verified by omitting the primary antibodies, and the specificity of the primary antibodies was verified by preabsorption experiments. Images were analyzed using ImageJ software version 11.0 (National Institutes of Health, Bethesda, MD, USA). For each animal, 8 sections of spinal L4-L5 were randomly selected for quantitative evaluation under a light microscope. A total of 5 sections that were not damaged, folded, or torn were selected from each group for the analysis.
ELISA
Serum levels of MBP and proteolipid protein (PLP) were measured using ELISA, as previously described (10). Briefly, blood samples were obtained from rats in each group and centrifuged at 10,000 × g at 4°C for 10 min. The supernatant was collected, and the concentrations of MBP and PLP were evaluated using ELISA kits (BA0094 and BA3695 respectively, both from Wuhan Boster Biological Technology Co., Ltd., Wuhan, China), according to manufacturer's protocol. The absorbance of each sample was measured at a wavelength of 490 nm using a microplate reader (MK3; Labsystems Diagnostics, Vantaa, Finland).
Western blotting
Spinal cord tissues were homogenized in ice-cold lysis buffer (P0013G; Beyotime Institute of Biotechnology) and centrifuged at 4°C. The concentration of proteins in the supernatant was determined using the bicinchoninic acid method. Proteins (80 µg) were resolved by 8% SDS-PAGE and transferred onto polyvinylidene fluoride membranes by electroblotting. Membranes were blocked in bovine serum albumin (A8010-10; Solarbio Biological Technology Co., Ltd., Beijing, China) in a blocking solution consisting of TBS + 0.05% Tween-20 at room temperature for 1 h. Membranes were incubated with primary antibodies against p38 MAPK (rabbit anti-rat p38 MAPK; sc-7975-R; 1:1,000; Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 4°C overnight. β-actin was used as a loading control. Then, the membranes were incubated with horseradish peroxidase-linked goat anti-rabbit secondary antibodies (sc-2007; 1:1,000; Santa Cruz Biotechnology, Inc.) at room temperature for 2 h. Bands were visualized using a chemiluminescence detection system (Amersham; GE Healthcare Life Sciences, Little Chalfont, UK) and analyzed with Scion Image Analysis Software version 4.0.3.2 (Scion Corporation, Walkersville, MD, USA).
Statistical analysis
Analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Numerical data with a normal distribution were presented as the mean ± standard deviation. Data without a normal distribution were presented as the median ± percentiles. One-way analysis of variance (ANOVA) was used to compare differences among groups, followed by Bonferroni correction. For tail-flick latency, one-way ANOVA followed by Scheffé and Dunnett tests were used. P<0.05 was considered to indicate a statistically significant difference.
Results
NGF pretreatment reduces lidocaine-induced neurotoxicity in the spinal cord of rats
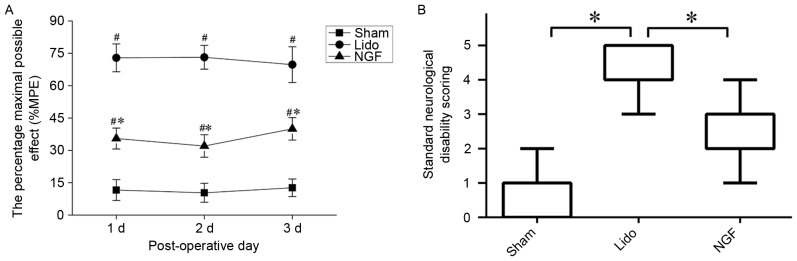
The effect of NGF on lidocaine-induced neurotoxicity in the spinal cord was investigated using tail-flick tests and the standard neurological disability scoring. Tail-flick latencies measured at 1–3 days following lidocaine administration were significantly increased in the Lido group compared with the Sham group (P<0.05). Tail-flick latencies in lidocaine-treated rats were significantly reduced by NGF pretreatment (P<0.05; Fig. 1A). The standard neurological disability was scored 3 days following lidocaine administration. As presented in Fig. 1B, the neurological disability score was significantly increased in the Lido group compared with the Sham group (P<0.05), which was then reversed by NGF pretreatment (P<0.05 compared with Lido group).
Figure 1.
Effects of NGF treatment detected via behavioral tests. (A) Effect of NGF pretreatment on tail-flick latencies in rats from the Sham, Lido and NGF groups at 1–3 days following operation; n=4. *P<0.05 vs. Lido group, #P<0.05 vs. Sham group. (B) Standard neurological disability was scored three days following lidocaine administration. *P<0.05. Lido, rats receiving intrathecal injection of lidocaine; NGF, NGF, nerve growth factor; MPE, maximal possible effect.
NGF reduces demyelination in the spinal cord of lidocaine-treated rats
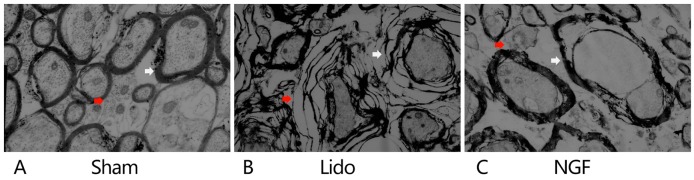
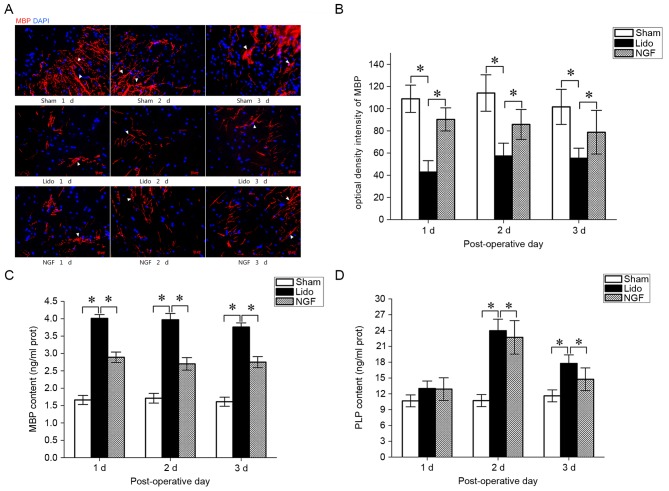
TEM analysis revealed a normal ultrastructure of the spinal cord, with intact axons and myelin lamellae of myelinated fibers, in Sham group (Fig. 2). In the Lido group, severe edema and splitting of myelin lamellae of the myelinated fibers were detected. Notably, administration of lidocaine resulted in the appearance of unmyelinated fibers with unclear boundaries. In the NGF group, local edema and splitting of myelin lamellae in the myelinated fibers were alleviated, when compared with the Lido group. The effect of NGF on the expression of MBP, a marker for demyelination in the central nervous system, was examined. On postoperative days 1–3, MBP expression levels in the spinal cord were significantly decreased in the Lido group, compared with the Sham group (P<0.05; Fig. 3A and B). NGF pretreatment significantly increased the expression of MBP in the spinal cord of lidocaine-treated rats (Fig. 3A and B). In contrast, MBP serum levels were significantly higher in the Lido group compared with the Sham group (P<0.05; Fig. 3C). Furthermore, MBP serum levels were significantly decreased in the NGF group compared with the Lido group (P<0.05; Fig. 3C). Serum levels of PLP were significantly increased in the Lido group compared with the Sham group on the postoperative days 2–3 (P<0.05), which were then reversed by the administration of NGF (P<0.05 compared with Lido; Fig. 3D).
Figure 2.
Effect of NGF pretreatment on ultrastructural alternations of spinal cord. Transmission electron microscopy analysis of the spinal cord in the (A) Sham, (B) Lido and (C) NGF groups, 3 days following the operation. Mild edema in the myelinated (indicated by white arrows) and unmyelinated (indicated by red arrows) fibers was seen. (A) Axons and myelin lamellae of myelinated fibers were intact, and unmyelinated fibers had a clear boundary. (B) Disintegrated myelin lamellae and edema was observed in myelinated fibers (indicated by white arrows), and unmyelinated fibers demonstrated unclear boundaries (indicated by red arrows). (C) Axonal swelling and degeneration, separation between axon and myelin sheath, local edema and splitting of myelin lamellae in the myelinated fibers were alleviated in the NGF group (indicated by white arrow). The boundaries of unmyelinated fibers disappeared (indicated by red arrow). Magnification, ×8,000. Lido, rats receiving intrathecal injection of lidocaine; NGF, NGF, nerve growth factor.
Figure 3.
The effect of NGF pretreatment on the expression of MBP and PLP in the Sham, Lido and NGF groups, 1–3 days following operation. (A) Representative immunofluorescence staining of MBP in the spinal cord of rats in the Sham, Lido and NGF groups. Red, MBP; Blue, DAPI. (B) Optical density intensity of MBP in the Sham, Lido and NGF groups; n=2. *P<0.05. ELISA results demonstrate the serum levels of (C) MBP and (D) PLP in the Sham, Lido and NGF groups; n=4. *P<0.05. Lido, rats receiving intrathecal injection of lidocaine; NGF, NGF, nerve growth factor; MBP, myelin basic protein; PLP, proteolipid protein.
NGF pretreatment increases the expression of BDNF in the spinal cord of lidocaine-treated rats
The effect of NGF on the expression of BDNF in the spinal cord in lidocaine-treated rats was tested. On postoperative days 1–3, spinal BDNF expression in the Lido group slightly increased compared with the Sham group, however no statistical differences were observed (Fig. 4). Compared with the Lido group, spinal BDNF expression significantly increased in the NGF group; suggesting that NGF pretreatment increased the expression of BDNF in the spinal cord of lidocaine-treated rats.
Figure 4.
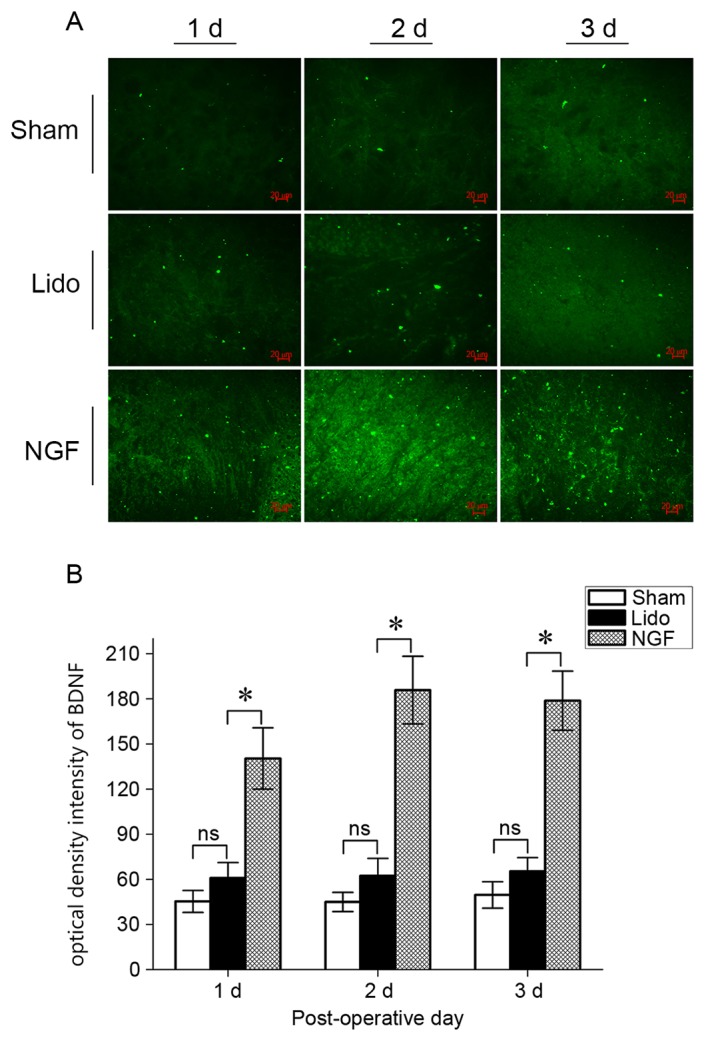
Immunofluorescence staining for BDNF expression in the spinal cord in rats in the Sham, Lido and NGF groups at 1–3 days following operation. (A) Representative images of BDNF staining. Magnification: ×400. (B) Optical density intensity; n=2. *P<0.05 vs. Lido. ns, not significant; Lido, rats receiving intrathecal injection of lidocaine; NGF, NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor.
NGF pretreatment reduces p38 MAPK phosphorylation in the spinal cord
Western blotting results revealed that the expression level of phosphorylated p38 MAPK was significantly increased in the spinal cord of rats in the Lido group, compared with the Sham and NGF groups (P<0.05; Fig. 5). The level of phosphorylated p38 MAPK was significantly decreased in the NGF group compared with the Lido group (P<0.05), suggesting that NGF pretreatment inhibited p38 MAPK activation in the spinal cord of lidocaine-treated rats.
Figure 5.
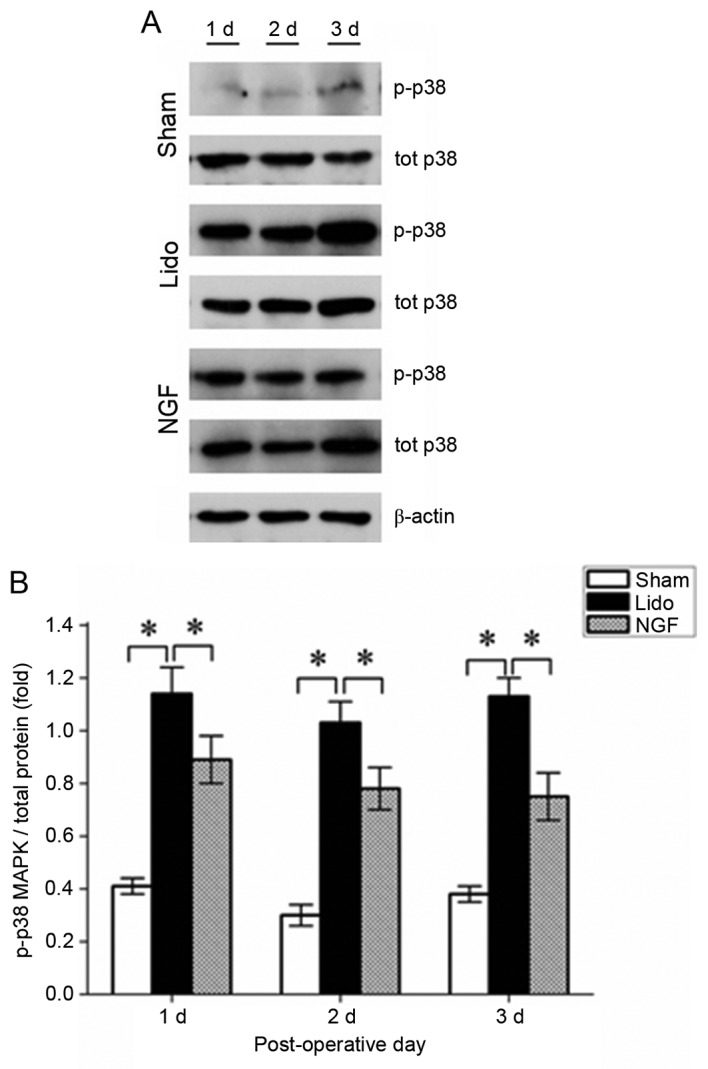
Effect of NGF pretreatment on p38 MAPK phosphorylation in the spinal cord in rats in the Sham, Lido and NGF groups at 1–3 days following operation. (A) Representative western blotting results demonstrating the expression of phosphorylated p38 MAPK in the spinal cord of rats in the Sham, Lido and NGF groups. (B) The relative expression of p38 MAPK was normalized to the expression of β-actin; n=2. *P<0.05. Lido, rats receiving intrathecal injection of lidocaine; NGF, NGF, nerve growth factor; p38 MAPK, p38 mitogen activated protein kinase; p, phosphorylated.
Discussion
Local anesthetics including lidocaine have been used for intravertebral anesthesia, however there are currently concerns regarding the neurotoxicity associated with their use. The identification of neurotrophic factors that prevent lidocaine-induced neurotoxicity may facilitate its clinical use in spinal anesthesia. NGF may promote growth and regeneration of neurons (17), and is a potential neurotrophic factor that may be used to reduce lidocaine-induced neurotoxicity. The present study investigated the effect of exogenous NGF pretreatment on lidocaine-induced neurotoxicity in the spinal cord of rats. NGF pretreatment significantly reduced tail-flick latencies in lidocaine-treated rats, suggesting that NGF pretreatment may reduce lidocaine-induced neurotoxicity in the spinal cord. It was additionally observed that lidocaine treatment resulted in a decrease in the expression of the demyelination marker, MBP, in the spinal cord; which was accompanied by an increase in MBP and PLP serum levels. NGF pretreatment significantly reduced the effect of lidocaine, suggesting that NGF effectively reduced lidocaine-induced demyelination in the spinal cord. In addition, NGF pretreatment increased the expression of BDNF in lidocaine-treated rats, suggesting that BDNF may mediate the beneficial effect of NGF in reducing lidocaine-induced toxicity. Furthermore, NGF pretreatment significantly inhibited p38 MAPK activation in the spinal cord of lidocaine-treated rats, suggesting that NGF may reduce lidocaine-induced neurotoxicity via the inhibition of p38 MAPK.
The intrathecal injection of lidocaine is associated with irreversible neurological complications including transient neural symptoms (5). It has been reported that lidocaine may induce nerve demyelination, and contribute to the neurotoxicity of lidocaine (15). The present study demonstrated that the intrathecal injection of lidocaine significantly increased tail-flick latencies and neurological disability, accompanied by a significant decrease in the spinal expression of MBP, a major constituent of myelin. The serum levels of MBP and PLP significantly increased following intrathecal injection of lidocaine. These findings are in accordance with the hypothesis that lidocaine may induce neurotoxicity via nerve demyelination. NGF has previously been demonstrated to be important in neuroprotection and neuronal repair (18). Various studies have reported that NGF may prevent demyelination in animal models of numerous demyelinating diseases, including allergic encephalomyelitis and multiple sclerosis (19,20). The present study demonstrated that NGF pretreatment significantly reduced the lidocaine-induced increase in tail-flick latencies and neurological disability, increased the expression of MBP in the spinal cord and decreased MBP and PLP serum levels, suggesting that NGF may inhibit demyelination and reduce lidocaine-induced neurotoxicity.
The beneficial effect of NGF in reducing lidocaine-induced neurotoxicity may not be due to its direct effect on spinal neurons, as it has been reported that NGF, unlike BDNF, does not reduce lidocaine-induced damage on the growth cone of dorsal root ganglion neurons (21). It is possible that BDNF, a neurotrophic factor that is important in neuronal survival, differentiation, and growth (22), may mediate the beneficial effect of NGF; since NGF may increase the expression of BDNF in intact and injured trkA-positive neurons (23). Furthermore, it has been reported that NGF may promote BDNF expression by activating the BDNF promoter via the extracellular regulated kinase 1/2 pathway in PC12 cells (24). BDNF has been demonstrated to produce neuroprotective effects in animal models of numerous diseases, including hypoxic ischemic brain injury (25), Huntington's disease (26) and Parkinson's disease (27). It has been reported that NGF promotes the release of BDNF from the primary afferents of dorsal horn neurons (28). In the present study, it was demonstrated that exogenous NGF treatment reduced lidocaine-induced neurobehavioral damage, accompanied with a significant increase in BDNF expression. This suggests that NGF may protect spinal neurons from lidocaine-induced neurotoxicity via upregulation of BDNF.
The p38 MAPK signal transduction pathway has been demonstrated to be activated following nerve injury in the spinal cord (29–31). Lidocaine induces p38 MAPK activation in human thyroid cancer cells (32). Furthermore, Haller et al (33) reported that the specific activation of the p38 MAPK signaling pathway is involved in lidocaine-induced neurotoxicity (34). Consistent with these previous studies, the present study revealed that the intrathecal injection of lidocaine significantly increased the expression of phosphorylated p38 MAPK, suggesting that lidocaine induced activation of the p38 MAPK pathway. Various studies have demonstrated that spinal p38 MAPK inhibition reduces inflammation and neuropathic pain in animal models (30,34,35). In addition, it has been reported that p38 MAPK inhibition reduces lidocaine-induced neurotoxicity in cultured dorsal root ganglia cells and in a rat sciatic nerve model (36). In the present study, NGF treatment reduced lidocaine-induced behavioral damage, accompanied by a decrease in the expression of phosphorylated p38 MAPK; suggesting that NGF reduces lidocaine-induced neurotoxicity via the inhibition of p38 MAPK.
The mechanisms underlying the neuroprotective role of NGF in lidocaine-induced neurotoxicity remain unclear. The authors previously reported that intrathecal injection of NGF reduces lidocaine-induced neurotoxicity via the inhibition of neuronal apoptosis (10). Various studies have demonstrated that the inhibition of p38 MAPK protects spinal neurons from apoptosis (34,37,38). In addition, Lirk et al (15) reported that p38 MAPK inhibition reduced lidocaine-induced apoptosis in vitro and in vivo. Furthermore, Torcia et al (39) reported that NGF inhibits apoptosis in memory B lymphocytes via the inactivation of p38 MAPK (39). The present study further demonstrated that NGF treatment inhibited p38 MAPK in the spinal cord of lidocaine-treated rats, suggesting that NGF may inhibit lidocaine-induced neurotoxicity via the inhibition of p38 MAPK-mediated apoptosis.
In conclusion, the present study demonstrated that NGF pretreatment significantly reduced lidocaine-induced neurobehavioral damage, accompanied by an increase in BDNF expression; suggesting that the neuroprotective effect of NGF may be associated with its inhibition of demyelination and the upregulation of BDNF. Furthermore, it was revealed that NGF treatment resulted in p38 MAPK inhibition in lidocaine-treated rats, suggesting that p38 MAPK inhibition may be involved in the neuroprotective effects of NGF. Increased levels of NGF have been demonstrated to contribute to generation of pain (40), and therefore the dosage of NGF should be carefully adjusted. However, the findings suggest that NGF therapy may be useful in preventing lidocaine-induced neurotoxicity, which may improve the clinical use of local anesthetics in intravertebral anesthesia.
Acknowledgements
The present study was supported by the Technology Research and Development Program of Liaoning Province (grant no. 2011408004). The authors would like to thank Dr. Lingxin Meng at the Department of Anesthesiology, Shengjing Hospital of China Medical University (Shenyang, China) for supporting the study.
Glossary
Abbreviations
- BDNF
brain-derived neurotrophic factor
- MBP
myelin basic protein
- MPE
maximal possible effect
- NGF
nerve growth factor
- p38 MAPK
p38 mitogen-activated protein kinase
- PLP
proteolipid protein
References
- 1.Onizuka S, Yonaha T, Tamura R, Kasiwada M, Shirasaka T, Tsuneyoshi I. Lidocaine depolarizes the mitochondrial membrane potential by intracellular alkalization in rat dorsal root ganglion neurons. J Anesth. 2011;25:229–239. doi: 10.1007/s00540-010-1079-y. [DOI] [PubMed] [Google Scholar]
- 2.Takenami T, Yagishita S, Murase S, Hiruma H, Kawakami T, Hoka S. Neurotoxicity of intrathecally administered bupivacaine involves the posterior roots/posterior white matter and is milder than lidocaine in rats. Reg Anesth Pain Med. 2005;30:464–472. doi: 10.1097/00115550-200509000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Werdehausen R, Fazeli S, Braun S, Hermanns H, Essmann F, Hollmann MW, Bauer I, Stevens MF. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103:711–718. doi: 10.1093/bja/aep236. [DOI] [PubMed] [Google Scholar]
- 4.Hampl KF, Heinzmann-Wiedmer S, Luginbuehl I, Harms C, Seeberger M, Schneider MC, Drasner K. Transient neurologic symptoms after spinal anesthesia: A lower incidence with prilocaine and bupivacaine than with lidocaine. Anesthesiology. 1998;88:629–633. doi: 10.1097/00000542-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Zaric D, Pace NL. Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics. Cochrane Database Syst Rev. 2009;15:CD003006. doi: 10.1002/14651858.CD003006.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Johnson ME, Uhl CB, Spittler KH, Wang H, Gores GJ. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology. 2004;101:1184–1194. doi: 10.1097/00000542-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya Y, Ohta K, Kaneko Y. Lidocaine-induced apoptosis and necrosis in U937 cells depending on its dosage. Biomed Res. 2005;26:231–239. doi: 10.2220/biomedres.26.231. [DOI] [PubMed] [Google Scholar]
- 8.Lirk P, Haller I, Colvin HP, Frauscher S, Kirchmair L, Gerner P, Klimaschewski L. In vitro, lidocaine-induced axonal injury is prevented by peripheral inhibition of the p38 mitogen-activated protein kinase, but not by inhibiting caspase activity. Anesth Analg. 2007;105:1657–1664. doi: 10.1213/01.ane.0000286171.78182.e2. [DOI] [PubMed] [Google Scholar]
- 9.Werdehausen R, Braun S, Essmann F, Schulze-Osthoff K, Walczak H, Lipfert P, Stevens MF. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology. 2007;107:136–143. doi: 10.1097/01.anes.0000268389.39436.66. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G, Ding X, Guo Y, Chen W. Intrathecal lidocaine neurotoxicity: Combination with bupivacaine and ropivacaine and effect of nerve growth factor. Life Sci. 2014;112:10–21. doi: 10.1016/j.lfs.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Radwan IA, Saito S, Goto F. The neurotoxicity of local anesthetics on growing neurons: A comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth Analg. 2002;94:319–324. doi: 10.1213/00000539-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Zareen N, Biswas SC, Greene LA. A feed-forward loop involving Trib3, Akt and FoxO mediates death of NGF-deprived neurons. Cell Death Differ. 2013;20:1719–1730. doi: 10.1038/cdd.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Chang F, Li F, Fu H, Wang J, Zhang S, Zhao J, Yin D. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun. 2015;463:262–267. doi: 10.1016/j.bbrc.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 14.Mishra R, Karande AA. Endoplasmic reticulum stress-mediated activation of p38 MAPK, Caspase-2 and Caspase-8 leads to abrin-induced apoptosis. PLoS One. 2014;9:e92586. doi: 10.1371/journal.pone.0092586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lirk P, Haller I, Myers RR, Klimaschewski L, Kau YC, Hung YC, Gerner P. Mitigation of direct neurotoxic effects of lidocaine and amitriptyline by inhibition of p38 mitogen-activated protein kinase in vitro and in vivo. Anesthesiology. 2006;104:1266–1273. doi: 10.1097/00000542-200606000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Laboratory Animal Resources (US) Committee on Care Use of Laboratory Animals National Institutes of Health (US) Division of Research Resources: Guide for the care and use of laboratory animals. 8th. National Academies Press; Washington, DC: 2011. [Google Scholar]
- 17.Manni L, Rocco ML, Bianchi P, Soligo M, Guaragna M, Barbaro SP, Aloe L. Nerve growth factor: Basic studies and possible therapeutic applications. Growth Factors. 2013;31:115–122. doi: 10.3109/08977194.2013.804073. [DOI] [PubMed] [Google Scholar]
- 18.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 19.Acosta CM, Cortes C, MacPhee H, Namaka MP. Exploring the role of nerve growth factor in multiple sclerosis: Implications in myelin repair. CNS Neurol Disord Drug Targets. 2013;12:1242–1256. doi: 10.2174/18715273113129990087. [DOI] [PubMed] [Google Scholar]
- 20.Tafreshi Parvaneh A. Nerve growth factor prevents demyelination, cell death and progression of the disease in experimental allergic encephalomyelitis. Iran J Allergy Asthma Immunol. 2006;5:177–181. [PubMed] [Google Scholar]
- 21.Radwan IA, Saito S, Goto F. Growth cone collapsing effect of lidocaine on DRG neurons is partially reversed by several neurotrophic factors. Anesthesiology. 2002;97:630–635. doi: 10.1097/00000542-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karchewski LA, Gratto KA, Wetmore C, Verge VM. Dynamic patterns of BDNF expression in injured sensory neurons: Differential modulation by NGF and NT-3. Eur J Neurosci. 2002;16:1449–1462. doi: 10.1046/j.1460-9568.2002.02205.x. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Lee JY, Choi JY, Park MJ, Kim DS. Nerve growth factor activates brain-derived neurotrophic factor promoter IV via extracellular signal-regulated protein kinase 1/2 in PC12 cells. Mol Cells. 2006;21:237–243. [PubMed] [Google Scholar]
- 25.Chen A, Xiong LJ, Tong Y, Mao M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep. 2013;1:167–176. doi: 10.3892/br.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B. AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther. 2004;9:682–688. doi: 10.1016/j.ymthe.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Singh S, Ahmad R, Mathur D, Sagar RK, Krishana B. Neuroprotective effect of BDNF in young and aged 6-OHDA treated rat model of Parkinson disease. Indian J Exp Biol. 2006;44:699–704. [PubMed] [Google Scholar]
- 28.Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 30.Qu WS, Tian DS, Guo ZB, Fang J, Zhang Q, Yu ZY, Xie MJ, Zhang HQ, Lü JG, Wang W. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J Neuroinflammation. 2012;9:178. doi: 10.1186/1742-2094-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terayama R, Omura S, Fujisawa N, Yamaai T, Ichikawa H, Sugimoto T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience. 2008;153:1245–1255. doi: 10.1016/j.neuroscience.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 32.Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014;9:e89563. doi: 10.1371/journal.pone.0089563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haller I, Hausott B, Tomaselli B, Keller C, Klimaschewski L, Gerner P, Lirk P. Neurotoxicity of lidocaine involves specific activation of the p38 mitogen-activated protein kinase, but not extracellular signal-regulated or c-jun N-terminal kinases and is mediated by arachidonic acid metabolites. Anesthesiology. 2006;105:1024–1033. doi: 10.1097/00000542-200611000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi H, Ogata T, Morino T, Chuai M, Yamamoto H. Continuous intrathecal infusion of SB203580, a selective inhibitor of p38 mitogen-activated protein kinase, reduces the damage of hind-limb function after thoracic spinal cord injury in rat. Neurosci Res. 2003;47:209–217. doi: 10.1016/S0168-0102(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 35.Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji RR. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lirk P, Haller I, Colvin HP, Lang L, Tomaselli B, Klimaschewski L, Gerner P. In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesth Analg. 2008;106:1456–1464. doi: 10.1213/ane.0b013e318168514b. [DOI] [PubMed] [Google Scholar]
- 37.Huang ZZ, Li D, Liu CC, Cui Y, Zhu HQ, Zhang WW, Li YY, Xin WJ. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun. 2014;40:155–165. doi: 10.1016/j.bbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Xu Z, Wang BR, Wang X, Kuang F, Duan XL, Jiao XY, Ju G. ERK1/2 and p38 mitogen-activated protein kinase mediate iNOS-induced spinal neuron degeneration after acute traumatic spinal cord injury. Life Sci. 2006;79:1895–1905. doi: 10.1016/j.lfs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Torcia M, De Chiara G, Nencioni L, Ammendola S, Labardi D, Lucibello M, Rosini P, Marlier LN, Bonini P, Dello Sbarba P, et al. Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation, and cytochrome c release. J Biol Chem. 2001;276:39027–39036. doi: 10.1074/jbc.M102970200. [DOI] [PubMed] [Google Scholar]
- 40.McKelvey L, Shorten GD, O'Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem. 2013;124:276–289. doi: 10.1111/jnc.12093. [DOI] [PubMed] [Google Scholar]