Abstract
Endothelial progenitor cells (EPCs) are decreased in cardiac dysfunction morbidity associated with acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Therefore, the present study aimed to assess the role of EPCs in AECOPD. Patients with AECOPD (n=27) or stable COPD (n=26) were enrolled. Systemic inflammatory markers (high-sensitivity C-reactive protein) were measured. In addition, EPCs were counted, isolated and cultured, and their proliferative, migratory, adhesive and tube-forming capabilities were determined, in cells from patients with AECOPD and stable COPD. EPC number was lower in patients with AECOPD (5.1±2.6×103/ml) compared with patients with stable COPD (6.0±3.2×103/ml). Migration assay indicated that the early-EPCs isolated from patients with AECOPD were significantly less mobile than EPCs derived from stable COPD subjects, at a stromal-cell derived factor-1α concentration of 100 ng/ml (3,550/30,000 vs. 7,853/30,000, P<0.05). C-X-C chemokine receptor-4 positivity was significantly reduced in AECOPD patients (16.1±9.9 vs. 56.33±6.3%, P<0.05). Furthermore, fewer early-EPC clusters were formed by EPCs derived from AECOPD, compared with those derived from stable COPD (8.2±0.86 vs. 14.4±1.36, P=0.027). Stable COPD late-EPCs were markedly deficient in intact tubule formation, however AECOPD late-EPCs formed no tubules. The number of AECOPD- and stable COPD-derived late-EPCs adhering to Matrigel-induced tubules was 36.8±1.85 and 20.6±1.36 (P<0.05) respectively, and the cluster of differentiation 31 positivity in late-EPCs was 79.69±1.3 and 29.1±2.47%, in AECOPD and stable COPD patients, respectively (P<0.001). The findings demonstrated that early-EPCs are decreased and dysfunctional in AECOPD patients, which may contribute to the altered vascular endothelium in this patient population.
Keywords: endothelial progenitor cells, acute exacerbation of chronic obstructive pulmonary disease, cardiovascular disease, dysfunction, C-X-C chemokine receptor-4
Introduction
Chronic obstructive pulmonary diseases (COPD) are prevalent worldwide and occur from environmental challenges, particularly cigarette smoke, which ultimately results in the development of airflow obstruction. Acute exacerbations of COPD (AECOPD), to a very large extent, increases the total number of visits to the emergency department or hospitalizations and expands healthcare costs. Patients with AECOPD are generally treated with systemic glucocorticoids, antibiotics and controlled oxygen therapy. Previous research has indicated that the most common cause of mortality in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) results from cardiac, rather than respiratory, complications. The presentation of cardiovascular comorbidities is a risk factor for acute exacerbation (1) and poor prognosis (2) of COPD. Several mechanisms have been proposed to explain the occurrence of cardiac comorbidities in AECOPD, including systemic inflammation (3), oxidative stress (4), endothelial cell apoptosis (5) and anti-endothelial cell antibodies (6) that induce endothelial cell dysfunction.
Endothelial progenitor cells (EPCs) expressing cluster of differentiation (CD) 34, CD133 and vascular endothelial growth factor receptor 2 (VEGFR2) (7) are a subtype of bone marrow-derived progenitor cells that are able to maintain vascular integrity. EPCs are mobilized from bone marrow and recruited to sites of vascular injury during neoangiogenesis (8). Notably, reduced number and function of circulating EPCs predicts the occurrence of cardiovascular events, and associated mortality (9,10).
Recent research has indicated that EPCs are significantly reduced in patients with AECOPD, with levels demonstrating correlation with classic cardiac biomarkers, including N-terminal pro-B-type natriuretic peptide, left ventricular ejection fraction, pulmonary artery systolic pressure and resting heart rate (11). However, data pertaining to the function of circulating progenitors in AECOPD are limited. The present study hypothesized that EPC number, proliferation, migration, adhesion and tube formation may be significantly decreased in patients with AECOPD, which may contribute to an altered vascular endothelium, and increased cardiovascular risk.
Materials and methods
Study participants
Ex-smokers with AECOPD (n=27) and patients with clinically stable COPD (n=26) were enrolled from the first affiliated hospital of Sun Yat-Sen University (Guangzhou, China). The inclusion criteria for recruitment into the AECOPD group were: i) COPD stages III and IV according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (12); ii) Treatment with anticholinergics, long-acting beta-2 agonists (LABA), and inhaled corticosteroids for ≥3 months prior to inclusion; and iii) an acute change in baseline dyspnea, cough, and/or sputum beyond the day-to-day variability as defined by the American Thoracic Society/European Respiratory Society consensus (13), including the use of systemic glucocorticoids, antibiotics and controlled oxygen therapy. The inclusion criteria for patients with stable COPD were: i) Moderate to very severe COPD (GOLD stages III and IV); ii) clinical stability with no requirement for increased treatment above maintenance therapy, other than bronchodilators, for 12 weeks; and iii) treatment with anticholinergics, LABA and inhaled corticosteroids for ≥3 months prior to inclusion.
Patients that demonstrated evidence of: Pneumonia, pulmonary embolism, chronic kidney disease, liver function abnormalities, malignant diseases, cardiovascular, cerebrovascular or metabolic disorders, or had undergone surgery within the previous 6 months prior to study initiation, were excluded. Systemic hypertension, diabetes mellitus and hypercholesterolemia were considered cardiovascular risk factors and were ruled out. The study protocol was approved by the Human Experimentation and Ethics Committees of the Sun Yat-Sen University (Guangzhou, China), and all subjects signed a written informed consent form. All experiments were conducted in accordance with the Declaration of Helsinki.
Blood collection
All subjects underwent clinical assessment, including pulmonary function tests and a 12-lead electrocardiogram. Fasting venous blood samples (20 ml) were collected into heparinized tubes during the first 24 h of hospital admission, to allow fora CD34+ assay. The plasma was then immediately recovered via centrifugation (4°C, 2,000 × g, 20 min) and frozen at −80°C. C-reactive protein (CRP) was assessed using a high sensitivity CRP (hsCRP) test, using latex immunoturbidimetry according to the manufacturer's protocol (Orion Diagnostica Ltd., Espoo, Finland).
EPC isolation and purification
Total blood cell counts were measured by hematocytometry, and CD34+cells were isolated and characterized, as previously described. (14,15). Briefly, peripheral blood mononuclear cells (MNCs) were extracted by Ficoll gradient centrifugation over Ficoll-Hypaque 1.077 (Tianjin Haoyang Biological Products Technology Co., Ltd., Tianjin, China) and then washed with Hank's Balanced Salt solution (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) and incubated with a CD34+ positive cocktail (100 µl/ml; EasySep®; Stemcell Technologies, Inc., Vancouver, BC, Canada) at room temperature for 15 min, followed by incubation with EasySep® magnetic nanoparticles (cat. no. 130-098-142; 50 µl/ml, StemCell Technologies, Inc.) at room temperature for 10 min. The cells were then resuspended in 2.5 ml RoboSep®buffer (cat. no. 20104; Stemcell Technologies, Inc.) and transferred to a 5 ml polystyrene tube for 5 min. The resulting supernatant fraction was discarded, leaving behind a pellet of magnetically-labeled cells. The previous two steps were repeated three times. Subsequently, the CD34+ cells were collected and counted under a light microscope by two independent investigators and the average value was recorded.
The purity of the extracted CD34+ cells was assessed by flow cytometry as described in the author's previous study (15). A total of 8 samples (n=4/group) were labeled with anti-CD34-fluorescein isothiocyanate (FITC; cat. no. 130-098-142; 1:100; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Isotypic immunoglobulin G (cat. no. 348050; BD Biosciences, Franklin Lakes, NJ, USA) served as the non-binding control. The population of CD34+ cells was determined by flow cytometry using a FACSCalibur system equipped with CellQuest software version 6.1.3 (BD Biosciences). The purity of the extracted CD34+ cells was determined to be 93%.
CD34+ cell characterization
The purified CD34+cells were seeded onto 6-well plates (2×106 cells/well) coated with 3% recombinant human gelatin (Prospec-Tany TechnoGene Ltd., Rehovot, Israel) and cultivated in endothelial growth medium:EBM-2 (Lonza Group Ltd., Basel, Switzerland) containing 10% heat-inactivated fetal calf serum (FCS; Invitrogen; Thermo Fisher Scientific, Inc.), 10% horse serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 10 ng/ml stem-cell growth factor (TEBU GmbH, Frankfurt, Germany), 2 mM L-glutamine and 5 ng/ml VEGF (Sigma- Aldrich; Merck KGaA) and 10 ng/ml SCGF (Sigma-Aldrich; Merck KGaA). Cells were maintained at 37°C in an atmosphere containing 5% CO2. Nonadherent cells were removed after 48 h, and adherent cells were resuspended. A total of 25% of the cells were incubated for 30 min at room temperature, with: Anti-human CD34-FITC (1:50; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), anti-human CD133-phycoerythrin (1:50; Miltenyi Biotec GmbH), mouse anti-human VEGFR2-allophycocyanin (APC) primary (1:50) and rabbit anti-mouse-APC secondary (1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies, and subsequently analyzed by flow cytometry as previously described (15). Cell suspensions were prepared in PBS containing 1% (vol/vol) FCS, using 2% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to block non-specific antigens on the cells.
Human EPC cultivation
Purified CD34+MNCs (2×106) were plated in 2 ml endothelial growth medium on recombinant human gelatin (Prospec-Tany TechnoGene Ltd.) -coated 6-well plates. After ~10 days incubation, the medium was changed and nonadherent cells were removed. The attached early-EPCs appeared elongated and spindle-shaped. Early-EPC characteristics and phenotype were assessed by flow cytometry as previously described (11).
EPC characterization
Early EPCs were characterized as adherent cells that were double positive for Dil-acetylated low density lipoprotein (cat. no. YB-0010; Yiyuan Biotech., Guangzhou, China) diluted to 10–30 µg/ml and FITC-oxytropis lectin 1 (Dil-acLDL and FITC-UEA-1; Molecular Probes; Thermo Fisher Scientific, Inc.) binding by direct fluorescent staining, as previously described (15). The fluorescent images were recorded using a laser scanning confocal microscope. A specific number of MNCs were allowed to grow into colonies of late-EPCs; these developed following MNC culture for 2–4 weeks. Late-EPCs exhibited a ‘cobblestone’ morphology and monolayer growth pattern that is typical of mature endothelial cells at confluence. Both early- and late-EPCs were collected and used for the functional assays in this study.
Chemotaxis
Chemotaxis was studied by Transwell® assay. Cultured early-EPCs (3×104) were resuspended in 200 µl 0.1% FCS medium and plated in the upper Transwell®chamber (6.5-mm diameter and 5 µm pore; Corning Incorporated, Corning, NY, USA). The lower chamber was filled with 500 µl of the same medium supplemented with 100 ng/ml stromal-cell derived factor-1α (SDF-1α; R&D systems, Inc., Minneapolis, MN, USA). In control group, the lower chamber was filled with 500 µl of the same medium supplemented with 5 ng/ml VEGF (Sigma-Aldrich; Merck KGaA). Following incubation at 37°C for 2 h, migrated cells were harvested from the lower chamber, and the number of migrating cells was counted under a light microscope.
Colony forming and MTT assays
CD34+ progenitor cells were seeded (2×106 cells/well) onto 6-well plates coated with 3% recombinant human gelatin, and cultivated in EBM-2 medium, supplemented as aforementioned, containing 10% heat-inactivated fetal calf serum (FCS, Invitrogen; Thermo Fisher Scientific, Inc.), 10% horse serum (Sigma-Aldrich; Merck KGaA), 5 ng/ml VEGF (Sigma-Aldrich; Merck KGaA) and 10 ng/ml SCGF (Sigma-Aldrich; Merck KGaA). Nonadherent cells were removed after 48 h. Colony-forming units were counted in 10 random fields under a light microscope.
Early-EPCs (2×104 cells/well) were plated in 100 µl EBM-2 medium, in 96-wellplates, and incubated in a humidified atmosphere (37°C, 5% CO2) for 0, 24, 48, 72, 96, and 120 h. Subsequently, 20 µl MTT reagent (0.5 mg/ml) was added to each well, and the plate was incubated for 4 h under similar conditions (37°C, 5% CO2). Plates were supplemented with 150 µl dimethyl sulfoxide in each well and oscillated gently for 10 min, for solubilization of MTT to occur, and the plates were then read at a wavelength of 450 nm using a microtiter plate reader.
Late-EPCs tube formation
The spontaneous formation of capillary-like structures by late-EPCs on a Matrigel™ Basement Membrane Matrix (BD Biosciences) preparation was used to assess angiogenic potential. Culture plates (24-well) were coated with Matrigel™ (3 mg/ml), late-EPCswere subsequently seeded (1×105 cells/well) and the plates were incubated at 37°C. Following 72 h of cell stretching, endothelial networks were imaged using phase-contrast microscopy.
Late-EPC adhesion to Matrigel™-induced tubules and CD31 assay
Culture plates (24-well) were coated with 250 µl cold Matrigel™, diluted to 4 mg/ml with EBM-2 to reduce the viscosity. Plates were incubated for 12 h at 37°C and 5% CO2 to solidify the Matrigel™. Human umbilical vein endothelial cells (HUVECs; 1×105; Sun Yat-sen University, Guangzhou, China) were pretreated for 12 h with 1 ng/ml tumor necrosis factor-α (Peprotech China, Suzhou, China), and seeded onto the Matrigel™. HUVECs were subsequently incubated for 12 h at 37°C in a humidified atmosphere containing 5% CO2. Late EPCs labeled with Dil-acLDL were subsequently seeded (2×104/well) onto the plates and co-incubated for 6 h, to determine their adhesion to Matrigel™-induced HUVEC tubules. Adherent EPCs were counted in 10 random fields under an inverted-fluorescence microscope.
To determine CD31+ positivity, late-EPCs (1×105) were blocked with 2% bovine serum albumin for 30 min, and incubated with monoclonal-CD31-FITC (1:100; cat.ue no. 557508; BD Biosciences) for 30 min at room temperature, and analyzed by flow cytometry as previously described (15).
Statistical analysis
Data were presented as the mean ± standard deviation for variables with normal distribution (n=6). Parametric variables among the groups were compared usingone-way analysis of variance (ANOVA); significant differences were further explored using the least significant difference post hoc test. The Kruskal-Wallis ANOVA and the Mann-Whitney U test for pair-wise analyses were used for non-parametric variables. P<0.05 was considered to indicate a statistically significant difference. Analyses were performed using SPSS 16.0. (SPSS, Inc., Chicago, IL, USA).
Results
Clinical characteristics of patients
There were no significant differences between the two patient groups in terms of age, sex, body mass index, and blood glucose and cholesterol levels (Table I).
Table I.
Clinical profile of patients.
| Characteristic | AECOPD (n=27) | Stable COPD (n=26) | P-value |
|---|---|---|---|
| Age (years) | 70±9 | 65±8 | NS |
| Sex (M/ F) | 24/3 | 24/2 | NS |
| BMI (kg/m2) | 19.3±3.6 | 20.2±2.4 | NS |
| LDL (mmol/l) | 3.03±0.90 | 3.42±0.70 | NS |
| FPG (mmol/l) | 6.3±2.2 | 5.8±1.3 | NS |
AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; M, male; F, female; BMI, body mass index; LDL, low-density lipoprotein; FPG, fasting plasma glucose; NS, not significant.
EPC and hsCRP level
The number of CD34+EPCs was lower in the patients with AECOPD (5.1±2.6×103/ml) compared with stable COPD patients (6.0±3.2×103/ml), however this was not significantly different (Table II). The hsCRP plasma level was significantly higher in the patients with AECOPD [4 mg/l (range 1–35)], compared with the stable COPD patients [2 mg/l (range 1–9)] indicating a higher level of systemic inflammation.
Table II.
Comparison of EPCs and hsCRP.
| Variable | AECOPD(n=27) | Stable COPD (n=26) | P-value |
|---|---|---|---|
| EPCs (×103/ml) | 5.1±2.6 | 6.0±3.2 | NS |
| hsCRP (mg/l) [range] | 4 [1-35] | 2[1-9] | <0.05 |
AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; EPCs, endothelial progenitor cells; hsCRP, hyper-sensitivity C-reactive protein; NS, not significant.
Characterization of human EPCs
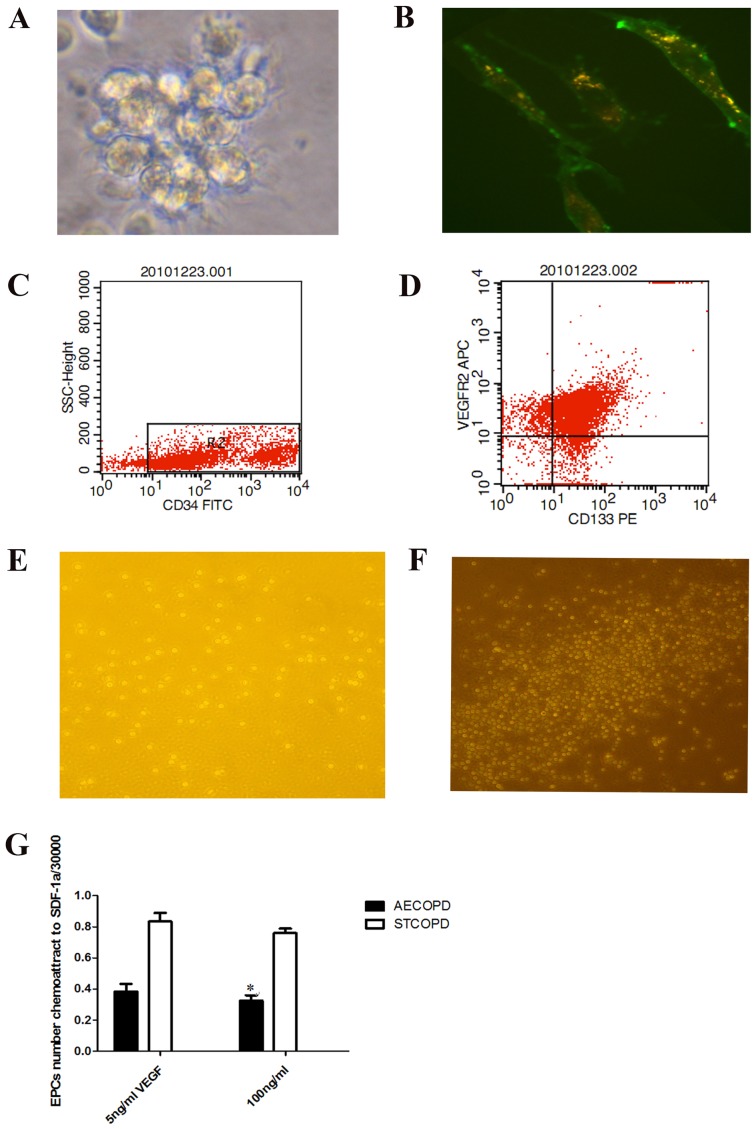
Early-EPCs were isolated from peripheral blood MNCs of AECOPD and stable COPD patients. Following only a 48-h incubation, the peripheral blood MNCs seeded onto fibronectin-coated wells were round (Fig. 1A). After the medium was changed on day 10, attached early-EPCs appeared to be elongated with a spindle shape (Fig. 1B). Early-EPC characterization was performed by flow cytometry (CD34+; Fig. 1C) and immunofluorescence. The majority of early-EPCs expressed endothelial and hematopoietic stem cell markers including CD34, CD133 and VEGFR2 (Fig. 1D).
Figure 1.
Characterization and chemotaxis of human EPCs. (A) Early-EPC clusters from patients with COPD following a 48-h incubation (magnification, ×20). (B) Early EPCs (10-day incubation) were labeled with low density lipoprotein (red) and FITC-oxytropis lectin (green) (magnification, ×40). (C) CD34+ overexpressing cells were isolated. (D) CD34+, CD133+, and VEGFR2+events were measured. Migrating early-EPCs isolated from patients with (E) AECOPD and (F) STCOPD, as observed using chemotaxis analysis. (G) Number of migrated early-EPCs isolated from patients with AECOPD were significantly fewer in number compared with patients with STCOPD (3,550/30,000 vs. 7,853/30,000, *P<0.05). EPCs, endothelial progenitor cells; COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbated COPD; STCOPD, stable COPD; CD, cluster of differentiation; VEGFR, vascular endothelial growth factor receptor; SSC, side scatter; FITC, fluorescein isothiocyanate; PE, phycoerythrin; SDF-1α, stromal-cell derived factor 1α; APC, allophycocyanin.
EPC chemotaxis
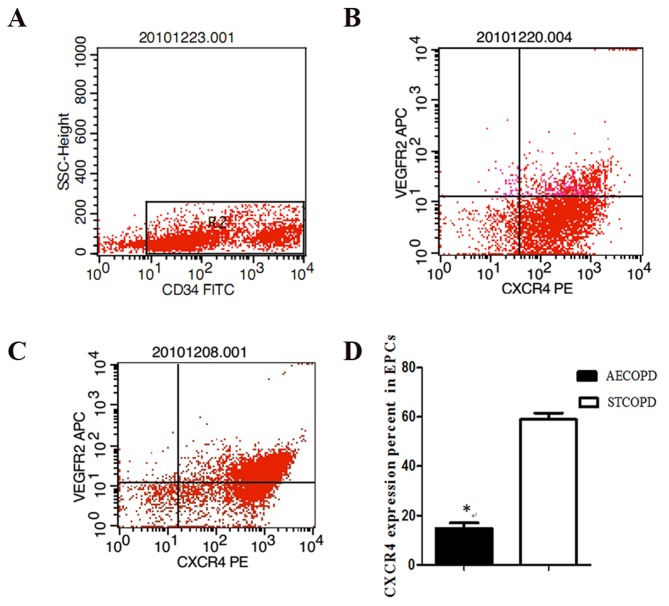
Significantly fewer early-EPCs isolated from patients with AECOPD had migrated across the Transwell® membrane, compared with EPCs isolated from patients with stable COPD, at an SDF-1α concentration of 100 ng/ml (3,550/30,000 vs. 7,853/30,000, P<0.05; Fig. 1E-G). To investigate the impaired migratory activity of EPCs in response to SDF-1α, the EPC expression of C-X-C chemokine receptor type 4 (CXCR4, mouse-anti-human CXCR4-PE, cat. no. 551510, BD Biosciences) was diluted 1:100 with PBS and, mouse-anti-human VEGFR2-APC (cat. no. 560495; BD Biosciences) diluted 1:50 with PBS, then incubated with EPCs for 30 min at room temperature in polystyrene round-bottom tubes (BD BioSciences; cat. no 352057). This procedure was used for processing 1 ml of the sample, which was then analyzed by flow cytometry. A gate was drawn around mononuclear cells. Primitive cells characteristically expressed CD34 at low levels (Fig. 2A). Double staining Cells:CD309 (VEGFR2) -APC and CD184 (CXCR4) -PE, were quantitated in the first quadrant (Fig 2 B and C). CXCR4 positivity was significantly reduced in AECOPD patients (16.1±9.9 vs. 56.33±6.3%, P<0.05; Fig. 2D).
Figure 2.
CXCR4 expression on EPCs from patients with AECOPD and STCOPD. CXCR4 expression in (A) CD34+EPCs isolated from (B) AECOPD and (C) STCOPD patients. (D) CXCR4 expression was significantly reduced in AECOPD patients (16.1±9.9 vs. 56.33±6.3%, *P<0.05). CXCR4, C-X-C chemokine receptor type 4; EPCs, endothelial progenitor cells; COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbated chronic obstructive pulmonary disease; STCOPD, stable COPD; VEGFR2, vascular endothelial receptor 2; APC, allophycocyanin; PE, phycoerythrin.
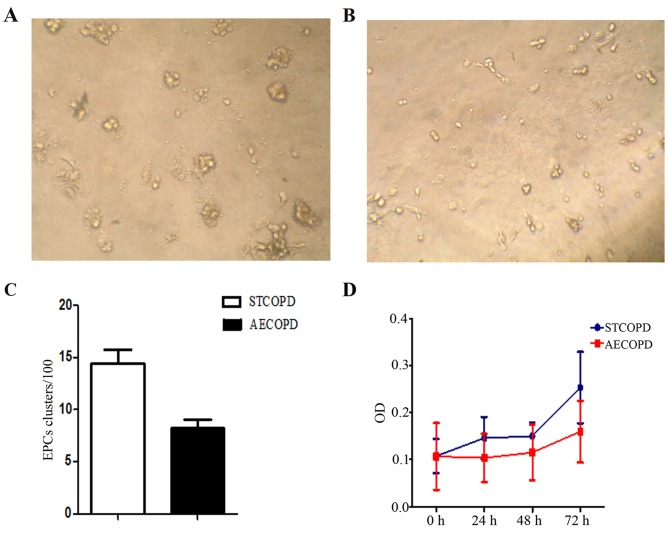
Colony forming unit assay and EPC proliferation
Analysis of EPC cluster formation (Fig. 3) indicated that the average number of colonies formed by early-EPCs from stable COPD and AECOPD was 14.4±1.36 and 8.2±0.86, respectively (P=0.027). Furthermore, the AECOPD clusters were smaller than the stable COPD clusters. Isolated early EPCs from patients with AECOPD demonstrated a hypo-proliferation compared with STCOPD at 48–72 h, which indicated impaired proliferation of AECOPD-derived EPCs.
Figure 3.
Proliferation of EPCs from patients with AECOPD and STCOPD. Early-EPC clusters from (A) STCOPD and (B) AECOPD patients after 72 h (magnification, ×20). (C) Quantification of the number of EPC clusters from the two groups indicated that AECOPD EPCs demonstrated impaired proliferation. (D) Proliferation of EPCs (n=6). *P<0.05 vs. STCOPD cells. EPCs, endothelial progenitor cells; AECOPD, acute exacerbated COPD; STCOPD, stable COPD; COPD, chronic obstructive pulmonary disease; OD, optical density.
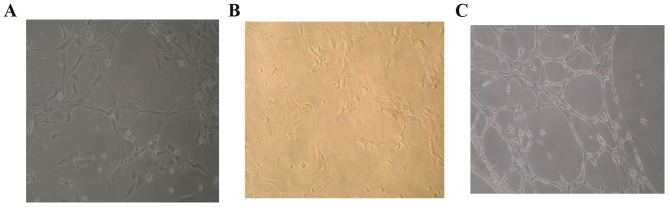
Late-EPCs form tubules
Stable COPD-derived late-EPCs were markedly deficient in their ability to form intact tubules; however, AECOPD-derived late-EPCs formed no tubules (Fig. 4).
Figure 4.
Phenotypic analysis of tubule formation in late-EPCs. There were many circle tubules formed by late-EPCs in different groups just like capillary network. (A) Stable COPD-derived late-EPCs were markedly deficient in forming intact tubules; (B) acute exacerbated COPD-derived late-EPCs formed no tubules. (C) Non-COPD derived late-EPCs form intact tubuls. Experiments were performed four times (n=6). EPCs, endothelial progenitor cells; COPD, chronic obstructive pulmonary disease.
Late-EPC adhesion to Matrigel™-induced tubules and CD31 expression
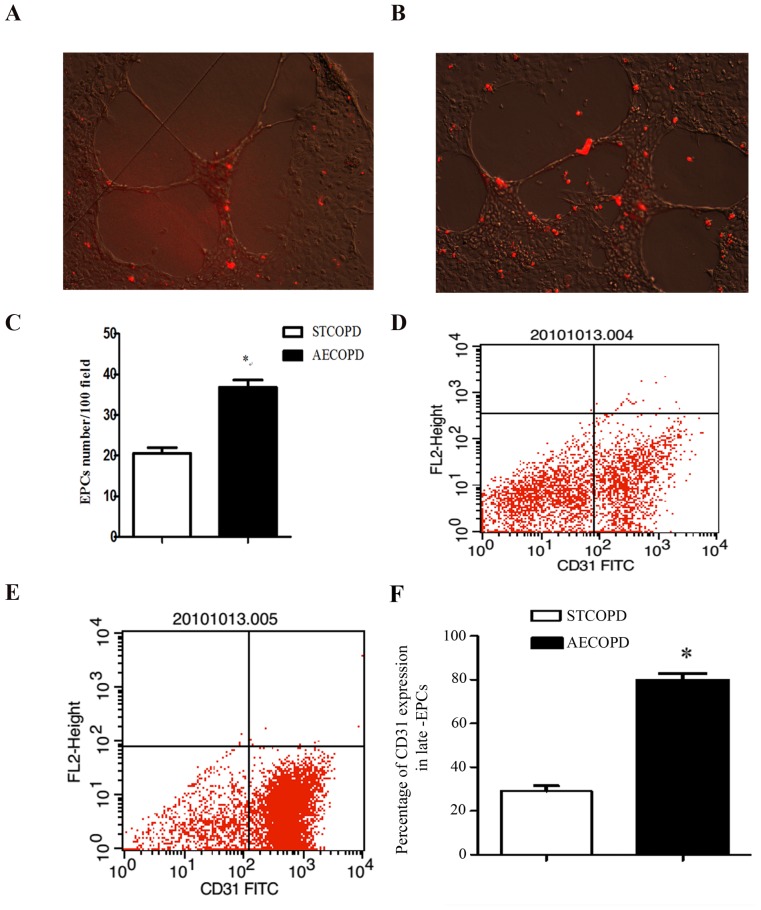
The number of AECOPD- and stable COPD-derived late-EPCs adhering to Matrigel™-induced HUVEC tubules was 36.8±1.85 and 20.6±1.36, respectively (P<0.05; Fig. 5A-C). The population of CD31-expressing cells in stable COPD- and AECOPD-derived late-EPCs was 29.1±2.47 and 79.69±1.3%, respectively (P<0.001; Fig. 5D-F).
Figure 5.
Late-EPC tubule formation. (A and B) HUVECs (105/well) were seeded onto Matrigel™-coated 24-well plates to form tubules. After a 12-h incubation, low density lipoprotein-labeled late-EPCs (4×104, red) were co-incubated with HUVEC tubules (magnification, ×10). There were less Late-EPC labeled with Dil-LDL (red) adhering to Matrigel™-induced HUVEC tubules depicted in (A) compared with (B). (C) A higher number of AECOPD late-EPCs were incorporated into tubules. CD31+ expression of late-EPCs from patients with (D) STCOPD and (E) AECOPD. (F) Quantification of CD31+ expression in late-EPCs. Data are presented as the mean ± standard deviation. *P<0.05 vs. STCOPD. Experiments were repeated four times (n=6). HUVECs, human umbilical vein endothelial cells; AECOPD, acute exacerbated chronic obstructive pulmonary disease; STCOPD, stable COPD; COPD, chronic obstructive pulmonary disease; FITC, fluorescein isothiocyanate; CD, cluster of differentiation.
Discussion
COPD is a highly prevalent chronic inflammatory condition associated with significant extrapulmonary manifestations. In addition, an increased risk of cardiovascular disease has been observed in patients with COPD (16,17). The Lung Health Study, which examined ~6,000 smokers with a forced expiratory volume ranging between 55 and 90%, predicted that cardiovascular diseases were the leading cause of COPD-based hospitalizations, accounting for ~50% of all hospital admissions, and the second leading cause of mortality, accounting for a quarter of all cases (18). Subsequent studies have confirmed that patients with COPD have an average 2–3 times higher risk of hospitalization for cardiovascular conditions, such as ischemic heart disease, stroke and heart failure, compared with patients without COPD (19,20). However, the pathophysiological mechanisms underlying the progression from COPD to atherosclerosis and development of cardiovascular events remain to be elucidated.
The integrity and functional activity of the endothelial monolayer serves a critical role in atherogenesis (21). Extensive endothelial cell damage, due to cardiovascular risk factors, results in endothelial cell apoptosis and subsequent loss of integrity of the endothelium. Bone marrow-derived circulating EPCs provide novel insights into these processes and have indicatedthe pivotal role thatcirculating EPCs serve in endothelial integrity, function and postnatal neovascularization (22). Early EPCs express CD34, CD133 and VEGFR2.Late-EPCs lose CD133 expression, and instead express endothelial lineage cell markers (23). Under normal physiological conditions, EPCs are mobilized from the bone marrow to the peripheral blood to actively repair the endothelial layer by forming a patch at the site of intimal damage. AECOPD, in conjunction with endothelial dysfunction due to impaired vascular repair, may contribute to the higher incidence of cardiovascular events (24,25). Bone marrow is greatly depressed in AECOPD patients and this results in a reduced number of circulating progenitors, which has been correlated with alterations in cardiac function and pulmonary arterial pressure (11,15,26). The present study extended our previous data (11,15), and demonstrated that the function of EPCs is also attenuated. Furthermore, patients with AECOPD displayed enhanced inflammation. These findings suggested that attenuated endothelial repair may contribute to atherosclerotic disease progression and increased risk of cardiovascular events in patients with AECOPD.
The present study demonstrated a decreased expression of CXCR4 in early-EPCs of AECOPD patients, in addition to impaired SDF-1α-induced migration, compared with early-EPCs from patients with stable COPD. These findings indicated that early-EPCs from AECOPD patients may not be able to adequately repair the injured endothelium. EPC production by the bone marrow is initiated by increased sympathetic nerve activity and the presence of cytokines in the peripheral blood circulation (27). Recent evidence (28,29) has demonstrated that the chemokine SDF-1, also known as C-X-C motif chemokine 12, serves a major role in the recruitment and retention of CXCR4+bone marrow-derived cells to the neoangiogenic sites, therefore supporting revascularization of ischemic tissue. EPCs express CXCR4, which is a receptor for SDF-1 thatis normally expressed in stromal and injured tissue. EPCs attracted by the SDF-1 gradient are therefore ‘anchored’ to the injury site by the association of SDF-1 with CXCR4 (28). The present study revealed that early-EPC migration was impaired, and CXCR4 expression was also reduced in patients with AECOPD, thus leading to a reduced number of available EPCs that may migrate to injured vascular sites, as previously described (29).
Circulating EPCs from patients with AECOPD also exhibited a reduced capacity to form tubules. CD31 has a distinct role in the transendothelial migration of leukocytes. Deletion of CD31 expression disrupts junction integrity, increases vascular permeability and impairs transendothelial cell migration (30,31). In the present study, CD31 expression was increased in late-EPCs from AECOPD, which revealed greater adhesion to Matrigel™-induced HUVEC tubules, the adhesion process was increased by inflammation. Notably, AECOPD-derived late-EPCs formed no tubules in vitro, thus contributing to a decrease in angiogenesis.
The mechanisms underlying the relationship between AECOPD and reduced number and function of EPCs remain to be determined, howeverthey may be related to enhanced inflammation and associated endothelial dysfunction, thus serving a key role in atherogenesis. Recombinant human CRP directly inhibits EPC differentiation, survival and function, at concentrations known to predict adverse vascular outcomes (32). In the present study, hsCRP levels were significantly increased in patients with AECOPD. The enhanced inflammation observed in patients with AECOPD may therefore suppress the number and function of circulating EPCs, thus resulting in attenuated vascular repair.
Collectively, the present study detected hypoproliferation and depressed migration, adhesion and tube formation, indicating decreased EPC number and increased dysfunction in AECOPD patients. The results demonstrated that the EPC-based capacity for repair was affected in patients with AECOPD, which may contribute to an altered vascular endothelium in this patient population.
The study limitations are focused on the limited patient population included in the analysis. Furthermore, the patients were all GOLD III–IV, and therefore are not representative of the entire clinical spectrum of patients with COPD. This was a cross-sectional study, and included AECOPD patients with altered circulating EPC number and function; however, whether the decrease in circulating EPCs was the cause or the result of AECOPD could not be determined. Further prospective studies are required to elucidate the potential cause-and-effect relationship.
Acknowledgements
The authors would like to thank Dr. Tan WP and the staff nurses at the Respiratory Department of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China), for collecting the clinical cases, and also Dr. Chen DB (Sun Yat-sen University, Guangzhou, China) for their technical assistance. The present study was partly supported by grants from the National Science Foundation of China (grant nos. 81260010 and 81460006).
References
- 1.Niewoehner DE, Lokhnygina Y, Rice K, Kuschner WG, Sharafkhaneh A, Sarosi GA, Krumpe P, Pieper K, Kesten S. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131:20–28. doi: 10.1378/chest.06-1316. [DOI] [PubMed] [Google Scholar]
- 2.Chang CL, Robinson SC, Mills GD, Sullivan GD, Karalus NC, McLachlan JD, Hancox RJ. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66:764–768. doi: 10.1136/thx.2010.155333. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.CIR.0000056767.69054.B3. [DOI] [PubMed] [Google Scholar]
- 4.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voelkel N, Taraseviciene-Stewart L. Emphysema: An autoimmune vascular disease? Proc Am Thorac Soc. 2005;2:23–25. doi: 10.1513/pats.200405-033MS. [DOI] [PubMed] [Google Scholar]
- 6.Karayama M, Inui N, Suda T, Nakamura Y, Nakamura H, Chida K. Antiendothelial cell antibodies in patients with COPD. Chest. 2010;138:1303–1308. doi: 10.1378/chest.10-0863. [DOI] [PubMed] [Google Scholar]
- 7.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 8.Fadini GP, Agostini C, Sartore S, Avogaro A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis. 2007;194:46–54. doi: 10.1016/j.atherosclerosis.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Liu X, Lin G, Sun L, Li H, Xie C. Decreased CD34+ cell number is correlated with cardiac dysfunction in patients with acute exacerbation of COPD. Heart Lung Circ. 2014;23:875–882. doi: 10.1016/j.hlc.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 13.Celli BR, MacNee W. ATS/ERS Task Force: Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 14.de Wynter EA, Ryder D, Lanza F, Nadali G, Johnsen H, Denning-Kendall P, Thing-Mortensen B, Silvestri F, Testa NG. Multicentre European study comparing selection techniques for the isolation of CD34+ cells. Bone Marrow Transplant. 1999;23:1191–1196. doi: 10.1038/sj.bmt.1701789. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Xie C. Human endothelial progenitor cells isolated from COPD patients are dysfunctional. Mol Cell Biochem. 2012;363:53–63. doi: 10.1007/s11010-011-1157-y. [DOI] [PubMed] [Google Scholar]
- 16.Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E, Jr, She D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Lee HM, Lee J, Lee K, Luo Y, Sin DD, Wong ND. Relation between COPD severity and global cardiovascular risk in US adults. Chest. 2012;142:1118–1125. doi: 10.1378/chest.11-2421. [DOI] [PubMed] [Google Scholar]
- 18.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, Enright PL, Kanner RE, O'Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. Jama. 1994;272:1497–1505. doi: 10.1001/jama.272.19.1497. [DOI] [PubMed] [Google Scholar]
- 19.Zvezdin B, Milutinov S, Kojicic M, Hadnadjev M, Hromis S, Markovic M, Gajic O. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136:376–380. doi: 10.1378/chest.08-2918. [DOI] [PubMed] [Google Scholar]
- 20.Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser permanente medical care program. Chest. 2005;128:2068–2075. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 21.Chiang CH, Huang PH, Chung FP, Chen ZY, Leu HB, Huang CC, Wu TC, Chen JW, Lin SJ. Decreased circulating endothelial progenitor cell levels and function in patients with nonalcoholic fatty liver disease. PLoS One. 2012;7:e31799. doi: 10.1371/journal.pone.0031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 23.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palange P, Testa U, Huertas A, Calabrò L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J. 2006;27:529–541. doi: 10.1183/09031936.06.00120604. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Suzuki S, Kubo H, Yamaya M, Kurosawa S, Kato M. Impaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary disease. Respirology. 2011;16:680–687. doi: 10.1111/j.1440-1843.2011.01959.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Tan W, Liu Y, Lin G, Xie C. The role of the β2 adrenergic receptor on endothelial progenitor cells dysfunction of proliferation and migration in chronic obstructive pulmonary disease patients. Expert Opin Ther Targets. 2013;17:485–500. doi: 10.1517/14728222.2013.773975. [DOI] [PubMed] [Google Scholar]
- 27.Aguila HL. Regulation of hematopoietic niches by sympathetic innervation. Bioessays. 2006;28:687–691. doi: 10.1002/bies.20427. [DOI] [PubMed] [Google Scholar]
- 28.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 30.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: New roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 31.Zocchi MR, Poggi A. PECAM-1, apoptosis and CD34+ precursors. Leuk Lymphoma. 2004;45:2205–2213. doi: 10.1080/10428190410001724312. [DOI] [PubMed] [Google Scholar]
- 32.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]