Abstract
Cannabinoids, as multi-target mediators, activate cannabinoid receptors and transient receptor potential vanilloid (TRPV) channels. There is evidence to support a functional interaction of cannabinoid receptors and TRPV channels when they are coexpressed. Human conjunctiva demonstrates widespread cannabinoid receptor type 1 (CB1), CB2 and TRPV channel localization. The aim of the present study was to investigate the expression profile for cannabinoid receptors (CB1 and CB2) and TRPV channels in pterygium, an ocular surface lesion originating from the conjunctiva. Semi-serial paraffin-embedded sections from primary and recurrent pterygium samples were immunohistochemically examined with the use of specific antibodies. All of the epithelial layers in 94, 78, 96, 73 and 80% of pterygia cases, exhibited CB1, CB2, TRPV1, TRPV2 and TRPV3 cytoplasmic immunoreactivity, respectively. The epithelium of all pterygia cases (100%) showed strong, mainly nuclear, TRPV4 immunolocalization. In the pterygium stroma, scattered cells demonstrated intense CB2 immunoreactivity, whereas vascular endothelial cells were immunopositive for the cannabinoid receptors and all TRPV channels. Quantitative analyses of the immunohistochemical findings in epithelial cells demonstrated a significantly higher expression level in conjunctiva compared with primary pterygia (P=0.04) for CB1, but not for CB2 (P>0.05). Additionally, CB1 and CB2 were significantly highly expressed in primary pterygia (P=0.01), compared with recurrent pterygia. Furthermore, CB1 expression levels were significantly correlated with CB2 expression levels in primary pterygia (P=0.005), but not in recurrent pterygia (P>0.05). No significant difference was detected for all TRPV channel expression levels between pterygium (primary or recurrent) and conjunctival tissues (P>0.05). A significant correlation between the TRPV1 and TRPV3 expression levels (P<0.001) was detected independently of pterygium recurrence. Finally, TRPV channel expression was identified to be significantly higher than the expression level of cannabinoid receptors in the pterygium samples (P<0.001). The differentiated expression of cannabinoid receptors in combination with the presence of TRPV channels, in primary and recurrent pterygia, imply a potential role of these cannabinoid targets in the underlying mechanisms of pterygium.
Keywords: cannabinoid receptor type 1, cannabinoid receptor type 2, transient receptor potential cation channel subfamily V member 1, transient receptor potential cation channel subfamily V member 2, transient receptor potential cation channel subfamily V member 3, transient receptor potential cation channel subfamily V member 4, pterygium
Introduction
Human pterygium, a common ocular surface disease, is characterized by chronic proliferation of limbal epithelial cells and fibrovascular tissue, forming a wing-like shape, which obstructs the visual axis (1). Pathobiology of this lesion includes various mechanisms, such as stem cell dysfunction, cellular proliferation and aberration of apoptosis, epithelial-mesenchymal transition, inflammatory influence, genetic instability, angiogenesis, redox related toxicity and hypoxia (2–4).
The cannabinoid receptor family includes cannabinoid receptor type 1 (CB1) and CB2, which belong to the seven transmembrane-spanning superfamily of G protein-coupled receptors and are the targets of naturally, synthetic or endogenous cannabinoid ligands. CB1 receptor has the highest expression level in the brain (5,6) whereas CB2 receptor expression is predominantly found in the immune system (B lymphocyte enriched areas) (7). The cannabinoid system modulates various physiological processes of the nervous and immune systems, and is key in cell proliferation, differentiation and survival (6,8,9). Thus, cannabinoids have been reported as putative therapeutic agents for the treatment of neurodegenerative diseases, multiple sclerosis, glaucoma, neuropathic and inflammatory pain, as well as cancer (10–12). It has been discovered that members of the transient receptor potential (TRP) protein superfamily of cation channels are activated by cannabinoids (13), whereas the two cannabinoid receptors and the TRP channels have more than one cannabinoid ligand (14). TRP vanilloid (TRPV) channels belong to the TRP superfamily and are also activated by various stimuli (15). Notably, TRPV1 was originally discovered as the pharmacological site of action of pungent vanilloid compounds, such as capsaicin. In addition, noxious heat (>43°C) and high temperatures (>52°C) activate TRPV1 and TRPV2, respectively. Warm temperatures in the range of 32–39°C activate TRPV3 and moderate heat (>27°C) activates TRPV4. Furthermore, chemical stimuli that include diphenyl-containing compounds, which activate TRPV2, and plant-derived compounds, such as camphor, carvacol and incensole acetate, which activate TRPV3 (16–25). TRPV4 was originally identified as a channel that could be gated by changes in osmolarity (26), but it also elicits responses to a variety of endogenous and exogenous agonists, such as the phytochemical, bisandrographolide A and the phorbol ester, 4α-phorbol 12,13-didecanoate, and the small molecule TRPV4 channel activator, GSK1016790A (27–29). Thus, TRP channels are important mediators of nociception, mechano-, osmo- and thermosensation, regulating cellular functions and signaling pathways (13). Inherited (channelopathy) or acquired dysfunction of TRP channels has been implicated in various diseases, providing these channels as targets for many currently prescribed therapeutic agents (30).
Growing evidence demonstrates that cannabinoid receptors and TRP channels are coexpressed in certain tissue samples suggesting that there is a functional interaction by which cannabinoids may regulate physiological processes. Yang et al (31) reported that CB1 activation suppressed TRPV1-induced increases in interleukin (IL)-6 and IL-8 in corneal epithelial cells, implying a novel drug strategy to reduce TRPV1-induced proinflammatory cytokine release. Furthermore, as various cell types in synovial tissue express CB1 and TRPs, cannabinoid-based therapeutic agents targeting CB1 and TRPV1 have been proposed to reduce inflammation in rheumatoid arthritis (32,33). Together with the above findings, CB1 activation has been shown to produce analgesia by preventing nerve growth factor-induced sensitization of TRPV1 in adult mouse afferent nociceptor nerve endings (34,35). Finally, experimental data in the skin support a model in which cannabinoids indirectly suppress TRPV1 effects on pain and inflammation by acting at CB1, but directly activate TRPV1 at higher concentrations (36). However, primarily with respect to nociceptive responses, the effect of CB1 on TRPV1 may not always be inhibitory (37).
Human and rat conjunctiva demonstrate widespread tissue localization of cannabinoid receptors. It has been reported that conjunctival cannabinoid receptors are important in the regulation of epithelial renewal and inflammatory processes at the ocular surface (38). Previously, TRPV1, TRPV2 and TRPV4 gene expression, and cellular localization was detected and functionally characterized, with regard to thermosensitivity and regulatory volume behavior, in human conjunctival epithelial cells and ex vivo human conjunctivas (39). The aim of the present study was to investigate the distribution patterns of cannabinoid receptors (CB1 and CB2) and TRPV channels in the human conjunctival lesion, pterygium.
Materials and methods
Patients
A total of 32 patients of Greek origin (22 males and 10 females; mean age, 72.5 years) with primary (n=27) or recurrent (n=5) pterygia, who underwent routine pterygium excision surgery at the Department of Ophthalmology, University Hospital of Patras (Rio, Greece) between January 2008 and February 2010, were included in the present study. Bulbar conjunctival tissues (n=8; mean age, 72 years) from patients undergoing glaucoma or cataract surgery were collected from the same department, during the same period. The use of the human specimens was in accordance with the University Hospital of Patras Ethics Commission. All research protocols were conducted and patients were treated in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Immunohistochemistry
All tissues for immunohistochemistry were fixed in formalin and embedded in paraffin. Consecutive (semi-serial) 4-µm sections of ocular pterygium and normal conjunctival tissue samples were collected on poly-L-lysine-coated slides. Pterygia were oriented such that sections were cut longitudinally through the head and the body of the pterygium. One section for each sample was stained with hematoxylin and eosin. For immunohistochemical studies, the histological sections were deparaffinized in xylene and rehydrated through a graded series of alcohols to water. Antigen retrieval was performed by microwaving the slides in 0.01 M citrate buffer (pH 6). Endogenous peroxidase activity was quenched by treatment with 1% hydrogen peroxide for 20 min. Incubation at room temperature with 1% bovine serum albumin (SERVA, Heidelberg, Germany) in Tris-HCl-buffered saline was performed for 10 min. Sections were subsequently incubated with the following primary antibodies: Anti-CB1 (cat no. C2866; dilution, 1:100; Merck KGaA, Darmstadt, Germany) and anti-CB2 (cat no. 101550; dilution, 1:100; Cayman Chemical Co., Ann Arbor, MI, USA). In addition, a rabbit polyclonal antibody, phosphorylated-CB1 [p-CB1 (Ser316)], raised against a short amino acid sequence containing phosphorylated Ser316 of CB1 of human origin (cat no. sc-17555; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used. TRPV channel expression was detected using polyclonal rabbit anti-TRPV1 antibody (cat no. NBP1-71774; dilution, 1:200) manufactured by Novus Biologicals, Ltd. (Cambridge, UK), polyclonal rabbit anti-TRPV2 (cat no. TA317464; dilution 1:200) and monoclonal mouse anti-TRPV3 antibody (cat no. AM20072PU-N; dilution 1:300) produced by Acris Antibodies GmbH (Herford, Germany) and rabbit polyclonal anti-TRPV4 antibody from Abcam (cat no. ab39260; dilution 1:200 Cambridge, UK). Detection was performed using the EnVision Plus Detection System kit, according to the manufacturer's instructions (Dako Cytomation; Agilent Technologies, Inc., Santa Clara, CA, USA), with 3,3′-diaminobenzidine (DAB) as a chromogen (which yielded brown reaction products). Sections were counterstained with Mayer's hematoxylin solution, dehydrated and mounted. To ensure antibody specificity, negative controls included the omission of primary antibody and substitution with non-immune serum. Control slides were invariably negative for immunostaining. Positive normal brain and high grade astrocytomas (glioblastoma multiforme; World Health Organization grade IV) for CB1 and CB2 (40), respectively, and human inflammatory bowel samples for TRPV channels, were also included (41) as positive controls.
Two observers independently evaluated the results of immunohistochemistry in the epithelium followed by resolution of any differences by joint review. To determine the labeling index (LI; % labelled cells) for each antibody, two observers independently assessed 10 non-overlapping, random fields (total magnification, ×400) for each case and manually counted 100 epithelial cells in each field with the aid of an ocular grid. Immunopositive endothelial and stromal cells were excluded from the cell counts. Expression of proteins included in the current study was examined in adjacent (semi-serial) sections of each sample. Microphotographs were obtained using a Nikon DXM 1200C digital camera mounted on a Nikon Eclipse 80i microscope and ACT-1C software (Nikon Instruments Inc., Melville, NY, USA) was used.
Statistical analysis
Non-parametric methods were used for statistical analysis of the results. Median comparisons were performed with Wilcoxon's rank-sum test (equivalent to the Mann-Whitney U test) and the Kruskal-Wallis test. Spearmans correlation was used to assess the significance of associations between LIs. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using the SPSS package (version 17.0; SPSS, Inc., Chicago, IL, USA).
Results
Immunolocalization of CB1 and CB2 cannabinoid receptors in pterygium and normal conjunctival tissue samples
Strong cytoplasmic immunoreactivity for CB1 and CB2 receptors was detected in almost every epithelial layer of 30/32 (94%) and 25/32 (78%) pterygium tissue samples, respectively. Immunostaining usually exhibited a granular pattern. Polygonal epithelial cells were strongly immunostained by CB1 and CB2 antibodies, whereas goblet cells intermingled in the superficial layer presented weak immunostaining for CB1 and strong immunostaining for CB2 (Fig. 1). In certain cases, CB1 immunoreactivity was also observed in the membrane of the epithelial cells. Considerable staining heterogeneity was observed among the different pterygium tissue samples included in the current study. Furthermore, variability of epithelial positive cells was also common within individual tissues (Fig. 2). In the pterygium stroma, scattered cells with intense CB2 immunoreactivity (Fig. 1B) and vascular endothelial cells with CB1 and CB2 immunoreactivity (Fig. 2A and C) were observed. In conjunctival epithelium, all epithelial cell layers displayed intense CB1 immunoreactivity, although the strongest immunopositivity was detected in the basal and outermost layers; a similar pattern was observed for CB2 immunoreactivity. CB1 expression was not detected in endothelial cells. By contrast, stromal scattered cells and vascular endothelium exhibited intense CB2 expression (Fig. 1). Immunoreactivity of p-CB1 (Ser316) demonstrated the same distribution patterns as CB1.
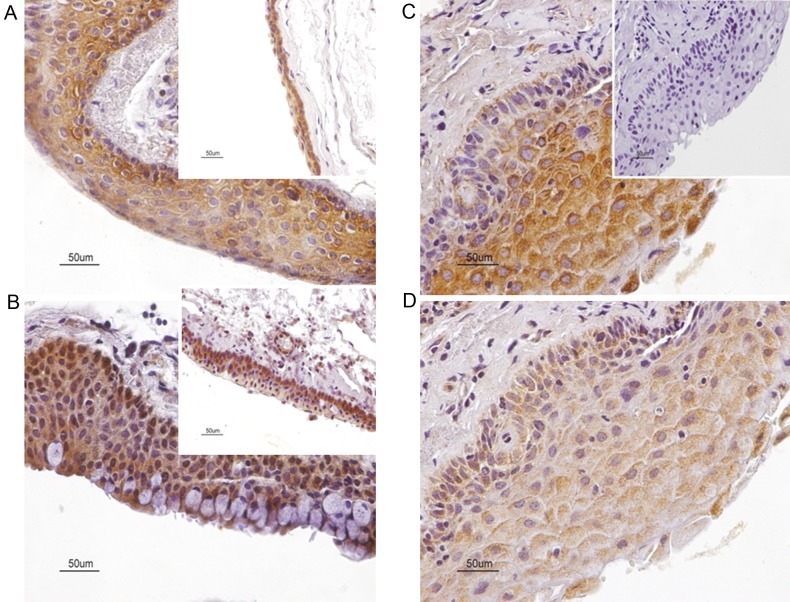
Figure 1.
Panel depicting the cellular distribution of cannabinoid receptors, CB1 and CB2 inhuman primary pterygia. (A) Strong diffuse cytoplasmic immunoreactivity in numerous epithelial cells for CB1. (A, insert) In human conjunctiva, all epithelial cells, but predominantly epithelial cells localized in basal and suprabasal layers, demonstrate CB1 immunoreactivity. (B) Cytoplasmic CB2 immunolocalization in all epithelial layers. Note that goblet cells demonstrate strong CB2 immunopositivity. Certain cells also exhibit nuclear CB2 immunoreactivity. Furthermore, CB2 immunolocalization is distributed in the cytoplasm of scattered stromal cells. (B, insert) In human conjunctiva, strong CB2 immunoreactivity is primarily localized in basal and suprabasal layers. Strong CB2 immunoreactivity is also present in vascular endothelium and stromal cells. (C and D) Strong and moderate granular cytoplasmic immunoreactivity for CB1 and CB2, respectively, in homologous fields of immediately adjacent sections of a pterygium sample. (C, insert) Immunostaining is absent in negative controls sections. Counterstain, hematoxylin; original magnification, ×400; scale bar, 50 µm. CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2.
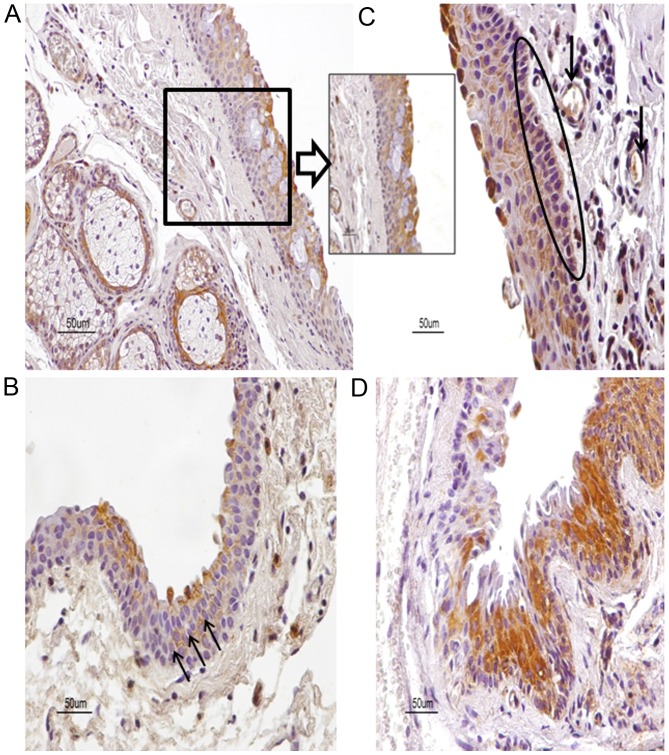
Figure 2.
Expression patterns for CB1 in human pterygia with staining patterns demonstrating heterogeneity. (A) CB1 is distributed in epithelium, stromal vessels, and a gland of a primary pterygium sample. (A, insert) Note CB1 immunolocalization predominantly in the upper layers of epithelium. (B) The membrane of epithelial cells is strongly immunostained for CB1 (arrows) in a primary pterygium sample. (C) Basal cells (oval) and vessels (arrows) in pterygium stroma are immunostained for CB1 in another primary pterygium sample. (D) Variability of CB1 immunopositive cells is detected in a recurrent pterygium sample. Counterstain, hematoxylin; original magnification, ×400; scale bar, 50 µm. CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2.
Immunolocalization of transient receptor potential vanilloid channels in pterygium and normal conjunctival tissue samples
All of the epithelial layers in 96, 73 and 80% of pterygia cases, exhibited TRPV1 TRPV2 and TRPV3 cytoplasmic immunoreactivity, respectively. The epithelium of all the pterygia cases (100%) exhibited strong, predominantly nuclear, TRPV4 immunolocalization. Similarly, the conjunctival tissue samples showed cytoplasmic TRPV1, TRPV2, TRPV3 and TRPV4 expression in a large number of epithelial cells. TRPV4 nuclear immunoreactivity was also noticed in certain epithelial cells. TRPV immunopositive vascular endothelial cells were observed in the pterygium and conjunctival samples (Fig. 3).
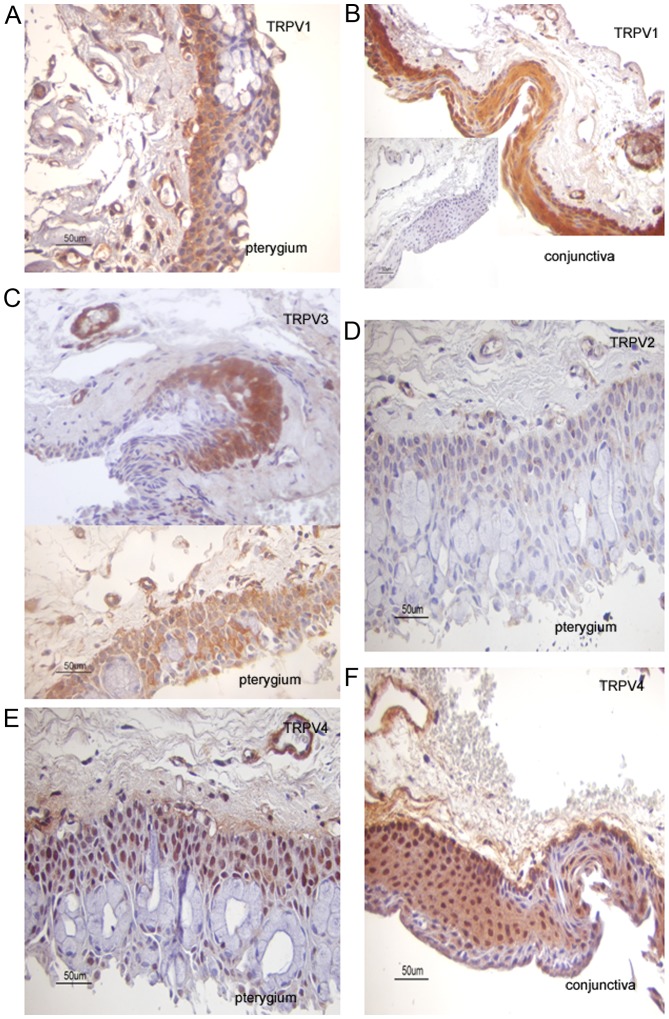
Figure 3.
TRPV immunolocalization in pterygium and conjunctival tissue samples. Cytoplasmic TRPV1 expression in (A) primary pterygium and (B) conjunctiva samples. Endothelial cells are TRPV1 immunopositive. (B, insert) Immunostaining is absent in negative controls sections. (C) Photomicrographs from the same recurrent pterygium sample demonstrating immunostaining heterogeneity for TRPV3 expression in (top) epithelial cells and (bottom) epithelial cells with strong TRPV3 cytoplasmic immunoexpression with wide distribution. Endothelial cells exhibit TRPV3 immonopositivity. Immunostaining was weak for CB1 (LI=5) and absent for CB2 (LI=0) cannabinoid receptors. Photomicrographs from the same primary pterygium sample displaying (D) weak TRPV2 (LI=8) and (E) predominantly nuclear TRPV4 (LI=80) immunoreactivity in epithelial cells. Note that TRPV4 expression is visible in all layers of the pterygium sample, including a few goblet cells. Vessels demonstrate TRPV2 and TRPV4 immunoreactivity. In addition, immunostaining for CB1 (LI=60) and CB2 (LI=50) cannabinoid receptors was detected. (F) TRPV4 immunostaining is localized in the cytoplasm and nucleus of basal and suprabasal epithelial cells, as well as in certain lamina propria cells in this conjunctival sample. Counterstain, hematoxylin; original magnification, ×400; scale bar, 50 µm. TRPV, transient receptor potential cation channel subfamily V member; CB1, cannabinoid receptor type 1; LI, labeling index; CB2, cannabinoid receptor type 2.
Quantitative analyses of the immunohistochemical findings
Quantitative analyses of the immunohistochemical LIs in epithelial cells revealed no significant differences based on age and gender of patients or grade of pterygium (P≥0.05). However, significant correlations were noted between the LIs for CB1 and CB2 in primary pterygia (Spearman correlation=0.522; P=0.005), but not in recurrent pterygia (Spearman correlation=0.28; P=0.6) (Fig. 4). Comparison of median LIs between primary and recurrent pterygia showed a significantly higher expression level of CB1 and CB2 in primary pterygia (P=0.01). LIs for CB1 in conjunctiva were significantly higher compared with primary pterygia (P=0.04) and recurrent pterygia (P=0.03). By contrast, no significant difference was identified for CB2 LIs between conjunctiva and primary pterygia (P=0.1; Table I). Furthermore, differences for TRPV channel expression levels between pterygium (primary or recurrent) and conjunctival tissue samples were identified, although these were not significant (P>0.05; Table II). A significant correlation was detected between the TRPV1 and TRPV3 expression levels (Spearman correlation=0.783; P<0.001) independently of pterygium recurrence. Finally, TRPV channel expression was significantly higher when compared with cannabinoid receptor expression in the pterygium samples (P<0.001).
Figure 4.
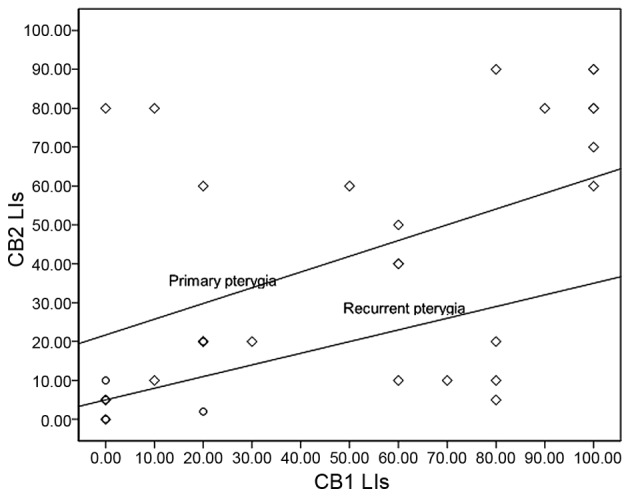
Spearman correlation analysis revealed a highly significant association between CB1 and CB2 LIs in primary pterygia (r2=0.522; P=0.005), although not in recurrent pterygia (r2=0.28; P=0.6). The scatter plot graph presents the comparison of LIs for CB1 and CB2 in primary and recurrent pterygia. P<0.05 was considered to indicate a statistically significant difference. Primary and recurrent pterygia are represented by diamond and circular symbols, respectively. CB2, cannabinoid receptor type 2; LI, labeling index; CB1, cannabinoid receptor type 1.
Table I.
Immunohistochemical expression of CB1 and CB2 receptors in human conjunctiva and pterygium epithelium.
| Sample | CB1 LIs mean ± SD, % (range) | CB2 LIs mean ± SD, % (range) |
|---|---|---|
| Primary pterygia (n=27) | 43.88±32.67 (0–90)a | 54.81±37.55 (0–100)b |
| Recurrent pterygia (n=5) | 7.40±7.98 (0–20) | 8.00±10.95 (0–20) |
| Normal conjunctiva (n=8) | 86.66±11.54 (80–100)c,d | 90±17.32 (70–100)e |
LI, the percentage of positively-labeled cells from the total number of epithelial cells counted.
P=0.01 vs. recurrent pterygia
P=0.01 vs. recurrent pterygia
P=0.04 vs. primary pterygia
P=0.03 vs. recurrent pterygia
P=0.03 vs. recurrent pterygia. CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; LI, labeling index; SD, standard deviation.
Table II.
Immunohistochemical expression of TRPV channels in human conjunctiva and pterygium epithelium.
| LIs mean ± standard deviation, % (range) | Pterygia (n=32) | Normal conjunctiva (n=8) |
|---|---|---|
| TRPV1 | 84.79±22.91 (0–100) | 100.00±0.00 (100–100) |
| TRPV2 | 23.57±27.23 (0–100) | 53.33±50.33 (0–100) |
| TRPV3 | 61.00±38.64 (0–100) | 33.33±27.74 (0–100) |
| TRPV4 | 54.50±27.23 (10–90) | 80.00±0.00 (80–80) |
LI, labeling index; TRPV, transient receptor potential cation channel subfamily V member.
Discussion
Increasing evidence reveals that cannabinoid receptors and their endogenous ligands, termed the endocannabinoid system, are involved in a variety of physiological functions of the human eye (38,42–45). Experimental data provide information regarding the functional contribution of CB1 in eliciting responses underlying wound healing in human corneal epithelial cells (31,46). Furthermore, cannabinoid receptors are important in the maintenance of conjunctival epithelium and in ocular surface modulation of inflammation (38). According to Zheng et al (47) activation of CB1 and CB2 receptors is required for ultraviolet (UV)-induced inflammation in skin, whereas the absence of CB1/2 receptors results in reduced UV-induced skin carcinogenesis. In addition, it is known that CB1 and CB2 activation increases cell proliferation and expands the available stem cell population (48). Specifically, it has been shown that anandamide inhibits differentiation of keratinocytes and this effect is mediated by epigenetic gene methylation of keratins by CB1 (49,50). A previous study demonstrated the novel activity of cannabinoids as transcriptional repressors via epigenetic mechanisms (51). In addition to this, pterygium demonstrates abundant expression of different keratins, which have been detected as conjunctival markers (52,53). In the present study, the expression levels of CB1 and CB2 in pterygium epithelial cells was decreased as compared to conjunctiva, and the reduction was significant with regard to CB1 expression. Based on the above-mentioned data, the reduction of cannabinoid receptors in pterygium may indicate an association of these receptors with cell differentiation and provides a tool for investigating drugs that reverse possible methylation abnormalities in pterygial epithelial cells. Thus, considering the most prevalent hypothesis for the pathogenesis of pterygium (which involves UV radiation as the principal environmental factor that affects epithelial stem cells residing at the nasal limbus (1)), it is logical to speculate that CB1 and CB2 may be involved in the pathogenesis of pterygium. However, further investigation is required to clarify whether the decreased expression level of cannabinoid receptors in pterygium epithelium may reflect a cytoprotective mechanism against UV or promotes the deregulation of cell proliferation at the ocular surface.
Notably, CB1 was immunolocalized in the membrane of certain pterygial epithelial cells. Recent data demonstrated that the palmitoylation of Cys415 in helix 8 of CB1 is critical for the membrane localization of this receptor (54). Previous findings highlight the regulation of CB1 by its association with membrane microdomains and consequently its coupling with G-proteins (55). Furthermore, only a proportion of epithelial cells demonstrated CB1 membrane immunoreactivity. Further studies on the concept of the membrane environment of pterygial cells are necessary for decoding the regulation of cannabinoid receptors in these cells. However, the intracellular CB1 receptors located in the majority of conjunctival and pterygial epithelial cells may also be functional. Previous data indicate that the intracellular CB1 receptor mediates signal transduction by stimulating extracellular regulated kinase (ERK) phosphorylation (56). CB2 cytoplasmic expression was detected in conjunctival and pterygial epithelial cells. He et al (57) demonstrated the involvement of CB2 in cell migration via the Gi-Ras-related C3 botulinum toxin substrate 1 signaling pathway with the support of heat shock protein90 (Hsp90), which may serve as a scaffold to maintain CB2 and its signaling components proximal to the cell membrane. Using geldamycin, an inhibitor of Hsp90, the authors demonstrated that Hsp90 is important as a molecular chaperone in CB2 receptor-mediated cell signaling and actin cytoskeleton rearrangement in trabecular meshwork cells (58). In our previous study (59) the abundant expression of Hsp90 in pterygial epithelial cells was displayed in contrast to that in conjunctival cells. In addition, correlation analysis revealed that Hsp90 expression is significantly correlated with the expression of CB2 receptor (Spearman's rho=0.660; P<0.01) in pterygium samples, but not in conjunctiva (P>0.05). These results imply that Hsp90 may be involved in CB2 receptor signaling in the epithelium of pterygium.
Previous studies have shown that although the tissues of recurrent and primary pterygia are very similar histopathologically (60), the biology of recurrent pterygia is quite different to that of primary pterygium and the prognosis is worse (61). In the current study, it was immunohistochemically demonstrated that, compared with primary pterygium, cannabinoid receptor expression levels were decreased in recurrent pterygium. Notably, the expression level of CB1 was correlated with the expression of CB2 in primary pterygial epithelial cells, but not in recurrent pterygia, although the number of recurrent pterygia was particularly low in the current study and further experiments with a larger series of recurrent pterygia are required. These findings indicate the presence of different pathological mechanisms between primary and recurrent pterygia. Thus, in recurrent pterygium, there may be a loss of epithelial cells containing cannabinoid receptors and/or differentially regulated expression of these receptors in the epithelium.
TRPV channels are abundantly expressed in skin epithelial cells (62). This expression has been linked to normal pain and temperature sensation (36). In the current study, TRPV1, TRPV2, TRPV3 and TRPV4 wide expression was identified in pterygial and conjunctival epithelial cells based on immunohistochemical evaluation. No significant difference in TRPV channel expression was identified between pterygium and conjunctival epithelial cells although a reduction of TRPV1, TRPV2 and TRPV4 expression levels and an increased level of TRPV3 expression was detected in pterygial epithelium. Previously, Mergler et al (39) established the functional activity of TRPV1, TRPV2 and TRPV4 channels in human conjunctival epithelial cells, which includes cell-volume control and thermosensitivity supporting conjunctival tissue homeostasis. Furthermore, functional studies in corneal epithelial cells demonstrated the involvement of TRPV3 in thermosensation and wound healing (63), as well as the mediatory role of TRPV1 in pro-inflammatory cytokine secretion (64). These data support the hypothesis that the expression of TRPV channels in epithelial pterygial and conjunctival cells is essential for the maintenance of epithelium homeostasis for pterygium and conjunctiva.
TRPV4 was shown with an unusual distribution profile in the nucleus of the majority of epithelial pterygial cells. Notably, a previous study demonstrated that TRPV4 is predominantly located in the nucleus of cultured neonatal ventricular myocytes; however, following hypotonic stimulation, the TRPV4 protein was translocated out of the nucleus (65). Indeed, in the present study, primarily nuclear TRPV4 immunoreactivity was identified in pterygium epithelium, compared with the conjunctiva, where the TRPV4 was cytoplasmic and only identified in the nucleus in certain cells. The above findings provide evidence of the involvement of TRPV4 in additional functions, including in the nucleus of pterygial epithelial cells.
In the pterygium stroma, a variety of endothelial cells exhibited cytoplasmic immunoreactivity for CB1 and CB2. It is noteworthy that CB1 expression was not observed in conjunctival stromal vessels in the present study. These findings reveal an induction of CB1 expression in pterygium endothelial cells, which indicates a role of CB1 in pterygium angiogenesis. There is evidence for the existence of endothelial receptors, termed endothelial cannabinoid receptors, which mediate endocannabinoid-induced vasodilation (66) and improve tissue oxygenation in hypoxic conditions. These endothelial receptors were proposed to act independently of CB1 and CB2 receptors. However, the presence of CB1 and CB2 receptors in pterygium vessels highlights the vascular responsiveness of pterygium to cannabinoids. It is well known that cannabinoids affect gap junctions, a critical aspect of vascular electrical and mechanical responses (67). Previous data have shown that CB1 receptors located in human vascular endothelium are functionally coupled to the mitogen-activated protein kinase cascade, and activation of these protein kinase cascades by anandamide may contribute to the modulation of endothelial cell growth and proliferation (68).
Additionally, TRPV channel expression was observed in pterygium and conjunctival endothelial cells. In human corneal endothelial cells, functional TRPV4 expression was reported (69), and TRPV1, TRPV2 and TRPV3 activity was modulated by temperature providing an essential homeostatic mechanism of corneal endothelial function under different ambient conditions (70). Furthermore, cannabinoid ligands abate the inflammatory activation of human endothelial cells, but paradoxically, TRPV1 inhibition augments inflammation in the absence of the cannabinoids (71). TRPV channels are also expressed in smooth muscle and perivascular cells indicating their role in modulating vascular function, perceiving and responding to local environmental changes (72). In the present study, cannabinoid receptors and TRPV channel immunoreactivity was identified in stromal cells. Thus, these results indicate that the endothelial endocannabinoid system may represent a useful tool for anti-angiogenic therapeutic strategies in pterygium.
Previous findings have established an interaction between TRPVs, particularly TRPV1 and CB1, when they are co-expressed in the same cells. Indeed, crosstalk between CB1 and TRPV1 modulates pain and inflammation in arthritis (33). CB1 and TRPV1 may function as constitutively and/or continuously activated receptors via endocannabinoids for proliferation and survival of epidermal keratinocytes (73,74) and human corneal epithelial cells (46). Suppression of TRPV1-induced inflammatory responses to corneal injury by CB1 activation has been reported (31,46).
In conclusion, in the present study, the majority of epithelial cells in conjunctiva demonstrate CB1 and TRPV1 expression co-localization based on overlapping immunostaining. Taken together, injury (e.g. endogenous ligand release, environmental stresses and/or infections) to epithelial cells activates: i) TRPV1 inducing proinflammatory cytokine and chemoattractant expression (75); ii) CB1 leading to reduction of inflammation (76); and iii) the protein-protein interaction between TRPV1 and CB1, which downregulates TRPV1-induced inflammation (31,46). Thus, it appears that there is an adaptive mechanism, which sustains the epithelial protective barrier function against infection and injury in conjunctiva. However, it is likely that this balance is disturbed in pterygium epithelium due to the reduction of cannabinoid receptor expression. However, there are a proportion of cells that express CB1 (and CB2) in addition to TRPV channels in pterygium. Therefore, developing strategies to either block injury-induced TRPV1 activation or promote CB1 activation may provide novel therapeutic approaches for pterygium. Further studies may clarify how the differential expression and regulation of these receptors correlates with the severity of pterygium pathogenesis and demonstrate the effects of the cannabinoids in this type of conjunctival lesion.
References
- 1.Coroneo MT, Di Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 1999;10:282–288. doi: 10.1097/00055735-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: Role of cytokines, growth factors and matrix metalloproteinases. Prog Retin Eye Res. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Detorakis ET, Spandidos DA. Pathogenetic mechanisms and treatment options for ophthalmic pterygium: Trends and perspectives (Review) Int J Mol Med. 2009;23:439–447. doi: 10.3892/ijmm_00000149. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JC, Yang W, Bradley RH, Reid TW, Schwab IR. The science of pterygia. Br J Ophthalmol. 2010;94:815–820. doi: 10.1136/bjo.2008.151852. [DOI] [PubMed] [Google Scholar]
- 5.De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: A general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demuth DG, Molleman A. Cannabinoid signaling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 8.Guzmán M, Sánchez C, Galve-Ropeth I. Cannabinoids and cell fate. Pharmacol Ther. 2002;95:175–184. doi: 10.1016/S0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 9.Pazos MR, Núñez E, Benito C, Tolón RM, Romero J. Functional neuroanatomy of the endocannabinoid system. Pharmacol Biochem Behav. 2005;81:239–247. doi: 10.1016/j.pbb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Perwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 2006;30(Suppl 1):S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarti B, Ravi J, Ganju RK. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget. 2014;5:5852–5872. doi: 10.18632/oncotarget.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhopeshwarkar A, Mackie K. CB2 cannabinoid receptors as a therapeutic target-What does the future hold? Mol Pharmacol. 2014;86:430–437. doi: 10.1124/mol.114.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: A clinical perspective. Br J Pharmacol. 2014;171:2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 17.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 19.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2 and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 20.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- 21.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al. TRPV3 is a temperatsure-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 23.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clone-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 25.Moussaieff A, Rimmermann N, Bregman T, Straiker A, Felder CC, Shoham S, Kashman Y, Huang SM, Lee H, Shohami E, et al. Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 2008;22:3024–3034. doi: 10.1096/fj.07-101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog Biophys Mol Biol. 2010;103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 28.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 30.Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE, Stevens EB, Storer RI, Swain NA. Ion channels as therapeutic targets: A drug discovery perspective. J Med Chem. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Yang H, Wang Z, Varadaraj K, Kumari SS, Mergler S, Okada Y, Saika S, Kingsley PJ, Marnett LJ, Reinach PS. Cannabinoid receptor 1 suppresses transient receptor potential vanilloid 1-induced inflammatory responses to corneal injury. Cell Signal. 2013;25:501–511. doi: 10.1016/j.cellsig.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler A, Aeschlimann A, Simmen BR, Michel BA, Gay RE, Gay S, Sprott H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem Biophys Res Commun. 2007;359:884–888. doi: 10.1016/j.bbrc.2007.05.178. [DOI] [PubMed] [Google Scholar]
- 33.Lowin T, Straub RH. Cannabinoid-based drugs targeting CB1 and TRPV1, the sympathetic nervous system, and arthritis. Arthritis Res Ther. 2015;17:226. doi: 10.1186/s13075-015-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDowell TS, Wang ZY, Singh R, Bjorling D. CB1 cannabinoid receptor agonist prevents NGF-induced sensitization of TRPV1 in sensory neurons. Neurosci Lett. 2013;551:34–38. doi: 10.1016/j.neulet.2013.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ZY, McDowell T, Wang P, Alvarez R, Gomez T, Bjorling DE. Activation of CB1 inhibits NGF-induced sensitization of TRPV1 in adult mouse afferent neurons. Neuroscience. 2014;277:679–689. doi: 10.1016/j.neuroscience.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caterina MJ. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem Neurosci. 2014;5:1107–1116. doi: 10.1021/cn5000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fioravanti B, De Felice M, Stucky CL, Medler KA, Luo MC, Gardell LR, Ibrahim M, Malan TP, Jr, Yamamura HI, Ossipov MH, et al. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: Antinociceptive actions of CB1 inverse agonists. J Neurosci. 2008;28:11593–11602. doi: 10.1523/JNEUROSCI.3322-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iribarne M, Torbidoni V, Julián K, Prestifilippo JP, Sinha D, Rettori V, Berra A, Suburo AM. Cannabinoid receptors in conjunctival epithelium: Identification and functional properties. Invest Ophthalmol Vis Sci. 2008;49:4535–4544. doi: 10.1167/iovs.07-1319. [DOI] [PubMed] [Google Scholar]
- 39.Mergler S, Garreis F, Sahlmüller M, Lyras EM, Reinach PS, Dwarakanath A, Paulsen F, Pleyer U. Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells. Histochem Cell Biol. 2012;137:743–61. doi: 10.1007/s00418-012-0924-5. [DOI] [PubMed] [Google Scholar]
- 40.Held-Feindt J, Dörner L, Sahan G, Mehdorn HM, Mentlein R. Cannabinoid receptors in human astroglial tumors. J Neurochem. 2006;98:886–893. doi: 10.1111/j.1471-4159.2006.03911.x. [DOI] [PubMed] [Google Scholar]
- 41.Dömötör A, Peidl Z, Vincze A, Hunyady B, Szolcsányi J, Kereskay L, Szekeres G, Mózsik G. Immunohistochemical distribution of vanilloid receptor, calcitonin-gene related peptide and substance P in gastrointestinal mucosa of patients with different gastrointestinal disorders. Inflammopharmacology. 2005;13:161–177. doi: 10.1163/156856005774423737. [DOI] [PubMed] [Google Scholar]
- 42.He F, Song ZH. Molecular and cellular changes induced by the activation of CB2 cannabinoid receptors in trabecular meshwork cells. Mol Vis. 2007;13:1348–1356. [PubMed] [Google Scholar]
- 43.McIntosh BT, Hudson B, Yegorova S, Jollimore CA, Kelly ME. Agonist-dependent cannabinoid receptor signalling in human trabecular meshwork cells. Br J Pharmacol. 2007;152:1111–1120. doi: 10.1038/sj.bjp.0707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Middleton TP, Protti DA. Cannabinoids modulate spontaneous synaptic activity in retinal ganglion cells. Vis Neurosci. 2011;28:393–402. doi: 10.1017/S0952523811000198. [DOI] [PubMed] [Google Scholar]
- 45.Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci. 1999;40:2442–2448. [PubMed] [Google Scholar]
- 46.Yang H, Wang Z, Capó-Aponte JE, Zhang F, Pan Z, Reinach PS. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp Eye Res. 2010;91:462–71. doi: 10.1016/j.exer.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng D, Bode AM, Zhao Q, Cho YY, Zhu F, Ma WY, Dong Z. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res. 2008;68:3992–3998. doi: 10.1158/0008-5472.CAN-07-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, Bari M, Guzmán M, Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013;52:633–650. doi: 10.1016/j.plipres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Paradisi A, Pasquariello N, Barcaroli D, Maccarrone M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J Biol Chem. 2008;283:6005–6012. doi: 10.1074/jbc.M707964200. [DOI] [PubMed] [Google Scholar]
- 50.Maccarrone M, Di Rienzo M, Battista N, Gasperi V, Guerrieri P, Rossi A, Finazzi-Agrò A. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J Biol Chem. 2003;278:33896–33903. doi: 10.1074/jbc.M303994200. [DOI] [PubMed] [Google Scholar]
- 51.D'Addario C, Di Francesco A, Pucci M, Finazzi Agrò A, Maccarrone M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013;280:1905–1917. doi: 10.1111/febs.12125. [DOI] [PubMed] [Google Scholar]
- 52.Kasper M, Moll R, Stosiek P, Karsten U. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry. 1988;89:369–377. doi: 10.1007/BF00500639. [DOI] [PubMed] [Google Scholar]
- 53.Jaworski CJ, Aryankalayil-John M, Campos MM, Fariss RN, Rowsey J, Agarwalla N, Reid TW, Dushku N, Cox CA, Carper D, Wistow G. Expression analysis of human pterygium shows a predominance of conjunctival and limbal markers and genes associated with cell migration. Mol Vis. 2009;15:2421–2434. [PMC free article] [PubMed] [Google Scholar]
- 54.Oddi S, Dainese E, Sandiford S, Fezza F, Lanuti M, Chiurchiù V, Totaro A, Catanzaro G, Barcaroli D, De Laurenzi V, et al. Effects of palmitoylation of Cys (415) in helix 8 of the CB (1) cannabinoid receptor on membrane localization and signalling. Br J Pharmacol. 2012;165:2635–2651. doi: 10.1111/j.1476-5381.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn KH, Nishiyama A, Mierke DF, Kendall DA. Hydrophobic residues in helix 8 of cannabinoid receptor 1 are critical for structural and functional properties. Biochemistry. 2010;49:502–511. doi: 10.1021/bi901619r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008;22:2311–2322. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He F, Qiao ZH, Cai J, Pierce W, He DC, Song ZH. Involvement of the 90-kDa heat shock protein (Hsp-90) in CB2 cannabinoid receptor-mediated cell migration: A new role of Hsp-90 in migration signaling of a G protein-coupled receptor. Mol Pharmacol. 2007;72:1289–1300. doi: 10.1124/mol.107.036566. [DOI] [PubMed] [Google Scholar]
- 58.He F, Kumar A, Song ZH. Heat shock protein 90 is an essential molecular chaperone for CB2 cannabinoid receptor-mediated signaling in trabecular meshwork cells. Mol Vis. 2012;18:2839–2846. [PMC free article] [PubMed] [Google Scholar]
- 59.Pagoulatos D, Pharmakakis N, Lakoumentas J, Assimakopoulou M. Ηypoxia-inducible factor-1α, von Hippel-Lindau protein and heat shock protein expression in ophthalmic pterygium and normal conjunctiva. Mol Vis. 2014;20:441–457. [PMC free article] [PubMed] [Google Scholar]
- 60.Nuhoglu F, Turna F, Uyar M, Ozdemir FE, Eltutar K. Is there a relation between histopathologic characteristics of pterygium and recurrence rates? Eur J Ophthalmol. 2013;23:303–308. doi: 10.5301/ejo.5000231. [DOI] [PubMed] [Google Scholar]
- 61.Tong L, Chew J, Yang H, Ang LP, Tan DT, Beuerman RW. Distinct gene subsets in pterygia formation and recurrence: Dissecting complex biological phenomenon using genome wide expression data. BMC Med Genomics. 2009;2:14. doi: 10.1186/1755-8794-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, Caterina MJ. TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch. 2005;451:160–167. doi: 10.1007/s00424-005-1438-y. [DOI] [PubMed] [Google Scholar]
- 63.Yamada T, Ueda T, Ugawa S, Ishida Y, Imayasu M, Koyama S, Shimada S. Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: Involvement in thermosensation and wound healing. Exp Eye Res. 2010;90:121–129. doi: 10.1016/j.exer.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F, Yang H, Wang Z, Mergler S, Liu H, Kawakita T, Tachado SD, Pan Z, Capó-Aponte JE, Pleyer U, et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol. 2007;213:730–739. doi: 10.1002/jcp.21141. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Huang H, Jiang Y, Wei H, Liu P, Wang W, Niu W. Unusual localization and translocation of TRPV4 protein in cultured ventricular myocytes of the neonatal rat. Eur J Histochem. 2012;56:e32. doi: 10.4081/ejh.2012.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kunos G, Bátkai S, Offertáler L, Mo F, Liu J, Karcher J, Harvey-White J. The quest for a vascular endothelial cannabinoid receptor. Chem Phys Lipids. 2002;121:45–56. doi: 10.1016/S0009-3084(02)00145-7. [DOI] [PubMed] [Google Scholar]
- 67.Bondarenko AI. Endothelial atypical cannabinoid receptor: Do we have enough evidence? Br J Pharmacol. 2014;171:5573–5588. doi: 10.1111/bph.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, Kunos G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346:835–840. doi: 10.1042/bj3460835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mergler S, Valtink M, Taetz K, Sahlmüller M, Fels G, Reinach PS, Engelmann K, Pleyer U. Characterization of transient receptor potential vanilloid channel 4 (TRPV4) in human corneal endothelial cells. Exp Eye Res. 2011;93:710–719. doi: 10.1016/j.exer.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 70.Mergler S, Valtink M, Coulson-Thomas VJ, Lindemann D, Reinach PS, Engelmann K, Pleyer U. TRPV channels mediate temperature-sensing in human corneal endothelial cells. Exp Eye Res. 2010;90:758–770. doi: 10.1016/j.exer.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Wilhelmsen K, Khakpour S, Tran A, Sheehan K, Schumacher M, Xu F, Hellman J. The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55,212–2 abate the inflammatory activation of human endothelial cells. J Biol Chem. 2014;289:13079–13100. doi: 10.1074/jbc.M113.536953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 2011;203:99–116. doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pucci M, Pirazzi V, Pasquariello N, Maccarrone M. Endocannabinoid signaling and epidermal differentiation. Eur J Dermatol. 2011;21(Suppl 2):S29–S34. doi: 10.1684/ejd.2011.1266. [DOI] [PubMed] [Google Scholar]
- 74.Tóth BI, Dobrosi N, Dajnoki A, Czifra G, Oláh A, Szöllosi AG, Juhász I, Sugawara K, Paus R, Bíró T. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol. 2011;131:1095–1104. doi: 10.1038/jid.2010.421. [DOI] [PubMed] [Google Scholar]
- 75.Straub RH. TRPV1, TRPA1 and TRPM8 channels in inflammation, energy redirection and water retention: Role in chronic inflammatory diseases with an evolutionary perspective. J Mol Med (Berl) 2014;92:925–937. doi: 10.1007/s00109-014-1175-9. [DOI] [PubMed] [Google Scholar]
- 76.Brown I, Cascio MG, Rotondo D, Pertwee RG, Heys SD, Wahle KW. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog Lipid Res. 2013;52:80–109. doi: 10.1016/j.plipres.2012.10.001. [DOI] [PubMed] [Google Scholar]