Abstract
Glioblastoma (GBM) is the most common and aggressive type of primary human brain tumor in China. Dysregulated microRNA (miRNA/miR) expression has been hypothesized to serve a role in the tumorigenesis and progression of human GBM. To explore the potential mechanisms affecting GBM tumorigenesis, the function of miR-518b in regulating GBM cell proliferation and angiogenesis was examined in vitro by CCK-8 and tube formation assay and in vivo by xenograft assay. The present study demonstrated that the expression of miR-518b was downregulated in GBM tissues and in GBM cell lines (U87 and U251). In addition, the expression levels of miR-518b were highly associated with tumor size, World Health Organization grade and prognosis. Furthermore, overexpression of miR-518b suppressed GBM cell proliferation and angiogenesis, and induced GBM cell apoptosis in vitro and in vivo. Overexpression of miR-518b also inhibited the expression of platelet-derived growth factor receptor β (PDGFRB), and the present study confirmed that the 3′ untranslated region (3′UTR) of PDGFRB was a direct target of miR-518b. In conclusion, to the best of our knowledge, the present study is the first to present evidence suggesting that miR-518b may serve as a potential marker and target in GBM treatment.
Keywords: microRNA-518b, platelet-derived growth factor receptor β, glioblastoma, angiogenesis
Introduction
Glioma, which is the most aggressive type of primary human brain tumor, accounts for >44% of all brain cancers in China and 50% of all malignant brain cancers in the US (1,2). In particular, glioblastoma (GBM), which is also referred to as World Health Organization (WHO) grade IV glioma, is the most common form of glioma. In addition, GBM has the worst survival rate, with an average survival of 12–15 months despite surgical, radio- and chemotherapeutic treatment (3). Although novel therapeutic strategies, including surgical resection, chemotherapy and radiotherapy, have made great progress, the survival rate and prognosis of GBM remain poor (4). Therefore, it is essential to identify potential novel treatments for patients with GBM. It has previously been reported that microRNAs (miRNAs/miR) may be used as biomarkers to estimate GBM prognosis, and may be used as targets in novel therapeutic strategies for the treatment of human GBM (5).
miRNAs are small noncoding RNAs, 16–22 nucleotides in length, which serve an important role in post-transcriptional modification of gene expression by targeting complementary mRNA sequences (6,7). miRNAs are involved in various oncogenic processes, including apoptosis, proliferation, angiogenesis and survival. In recent years, an increasing amount of studies have focused on the roles of miRNAs in human GBM. It has been reported that aberrant expression of miRNAs is closely associated with GBM tumorigenesis, and ectopic expression of specific miRNAs may modulate GBM biological function (7–9). Although these miRNAs have been reported to serve a role in GBM, the potential mechanisms underlying GBM tumorigenesis require further investigation. miR-518b has been reported to be a possible tumor suppressor, which may be used to predict the prognosis of GBM (10). However, information regarding the function and underlying molecular mechanism of miR-518b in GBM angiogenesis is limited.
In order to assess the potential mechanisms of miR-518b in GBM, the present study confirmed that miR-518b expression is downregulated in GBM, and miR-518b overexpression significantly inhibited GBM cell growth and angiogenesis in vitro and in vivo. In addition, the present study indicated that miR-518b exerts its effect on GBM angiogenesis by inhibiting the expression of platelet-derived growth factor receptor β (PDGFRB). The present study, to the best of our knowledge, is the first to reveal a molecular association between miR-518b and PDGFRB, thus suggesting a novel therapeutic target for GBM.
Materials and methods
Patient samples and cell lines
Glioma specimens and matched adjacent normal tissues (n=68) were obtained from patients who underwent curative resection at the Department of Neurosurgery, The Tenth People's Hospital Affiliated to Tongji University (Shanghai, China). The specimens were snap-frozen in liquid nitrogen and were then stored at −80°C for RNA extraction and further experiments. None of the patients received interventions or treatments prior to surgery, and all samples were obtained with informed consent. The histopathological diagnoses of these clinical samples were confirmed by two established neuropathologists. The present study was approved by the Ethics Committee of The Tenth People's Hospital Affiliated to Tongji University. Normal human astrocytes (NHAs) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Human GBM cell lines, U87 and U251, were obtained from the American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator containing 5% CO2 at 37°C.
miRNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR analysis of miRNAs was conducted as previously described (11). Total RNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), and 1 ng RNA was reverse transcribed into cDNA using reverse transcriptase (Applied Biosystems; Thermo Fisher Scientific, Inc.). The RT protocol was as follows: 30 min at 16°C, 30 min at 42°C, 5 min at 85°C. miRNA expression was detected by qPCR using the SYBR PCR Master Mix (Qiagen China Co., Ltd., Shanghai, China) on the ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR was performed for 25 cycles of: 10 sec at 98°C, 10 sec at 55°C, 20 sec at 72°C. U6 served as a normalization control gene. The 2−ΔΔCq method (12) was used to calculate the relative expression of each gene.
RNA oligonucleotide and cell transfection
miR-518b (5′-CAAAGCGCTCCCCTTTAGAGGT-3′) and control miRNA (miR-ctrl) mimics (5′-CATGCCGGGTAAACCGTAGA-3′) were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). GBM cells were transfected with the miRNA mimics (50 nM) using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol, once the cell density reached 70% confluence. All subsequent assays were performed 48 h post-transfection.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) colorimetric assay was conducted to determine cell proliferative capacity. The cells were seeded onto 96-well plates (5×103 cells/well). Subsequently, 10 µl CCK-8 was added to each well at the indicated time-points (1, 2, 3, 4, 5 and 6 days), and the plates were incubated at 37°C for 2 h. The data were measured at 560 nm using a Benchmark Microplate Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell cycle and apoptosis analysis
The role of miR-518b in cell cycle progression and apoptosis was detected by flow cytometry. According to the manufacturer's protocol, cells were cultured for 48 h, harvested and rinsed with phosphate-buffered saline (PBS), before being fixed in 70% ethanol at 4°C overnight. Subsequently, cells were rinsed with PBS again, and were treated with PBS containing 100 mg/l bovine pancreatic RNase A (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 2 h, followed by incubation with propidium iodide (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. Cell apoptosis was detected using the Annexin V fluorescein isothiocyanate Apoptosis kit (Calbiochem; EMD Millipore, Billerica, MA, USA) according to the manufacturer's protocol. Cell cycle progression and apoptosis were analyzed on a BD FACSCalibur flow cytometer with ModFit 3.0 software (both from BD Biosciences, Franklin Lakes, NJ, USA).
Tube formation assay and xenograft assay
U87 and U251 cells transfected with miR-518b or miR-ctrl mimics were grown in Dulbecco's modified Eagle's medium for 6 h at 37°C in a 24-well plate coated with Matrigel (Sigma-Aldrich; Merck KGaA). For the tube formation assay, human umbilical vein endothelial cells (HUVECs; 5×103 cells/well) were cultured in Ham's F12K basal medium (Gibco; Thermo Fisher Scientific, Inc.) with 200 ml transfected U87 or U251 cell-conditioned medium. Tube formation was analyzed to determine the capability of transfected cells to develop a vascular phenotype under a light microscope. The branch points of the formed tubes, which represent the degree of in vitro angiogenesis, were quantified by eye in 5 high-power fields using a light microscope. For the xenograft assay, the indicated cells (2×106) were subcutaneously injected into the flanks of female athymic nude mice (5 per group; 8-weeks old; weight, 18–22 g; obtained from Tongji University Animal Centre). All mice were allowed ad libitum access to an autoclaved chow diet and water in filter-topped cages in an SPF animal room (12-h light: Dark cycle; temperature, 25°C; humidity, 40–60%). A total of 30 days after injection, the nude mice were sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde for 24 h at room temperature and embedded in paraffin. Tissue sections (5 µm) were then dewaxed, rehydrated in graded alcohol, and incubated with 0.3% hydrogen peroxide to block endogenous peroxidase activity. Subsequently, the sections were treated with rabbit monoclonal anti-PDGFRB (cat no. 3169, 1:500; Cell Signaling Technology, Inc., Danvers, MA, USA) and anti-cluster of differentiation (CD) 34 (cat no. 3569, 1:500; Cell Signaling Technology, Inc.) antibodies at 4°C overnight, followed by incubation with a secondary anti-mouse immunoglobulin G antibody (cat no. sc-3749, 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 15 min at room temperature, followed by diaminobenzidine staining. Each step was conducted according to the manufacturers' protocols. Microvessel density (MVD) was assessed using light microscopy by counting vessel density in 5 high-power fields.
Western blot analysis
Total protein was isolated from the cultured cells and lysed with radioimmunoprecipitation assay lysis buffer. Protein concentration was determined using the bicinchoninic acid method, after which equal protein samples (50 µg) were separated by 10–15% SDS-PAGE and were transferred onto polyvinylidene fluoride membranes. After blocking the membranes with 5% skim milk at room temperature for 1 h, the membranes were incubated with primary antibodies against PDGFRB (cat no. 3169, 1:2,000; Cell Signaling Technology, Inc.) and GAPDH (cat no. sc-25778, 1:2,000; Santa Cruz Biotechnology, Inc.) at 4°C overnight. The membranes were subsequently incubated with secondary goat anti-rabbit antibody (cat no. 1662408; 1:3,000; Bio-Rad Laboratories Inc., Hercules, CA, USA) at 37°C for 1 h. Protein bands were analyzed using an enhanced chemiluminescence system (Bio-Rad Laboratories, Inc.).
Plasmid construction
Sequences containing wild-type and mutant 3′ untranslated regions (3′UTRs) of PDGFRB (2,671 bp; NCBI Reference Sequence, XM_005268464.2) were amplified by PCR to construct the reporter vectors. The PCR amplicons were then cloned downstream of the luciferase open reading frame in the pMIR-REPORT vector (Ambion; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The correct clones were confirmed by sequencing.
Dual-luciferase reporter assay
For the luciferase assay, U87 and U251 cells were grown to 70–80% confluence in 24-well plates and were co-transfected with miR-518b/miR-ctrl and wild type/mutant recombinant luciferase reporter plasmids using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. After 24 h, luciferase activity was measured using the Dual-Luciferase Reporter Assay system (Beyotime Institute of Biotechnology, Beijing, China) according to the manufacturer's protocol. Values were normalized against Renilla luciferase gene activity.
Statistical analysis
SPSS 15.0 software (SPSS Inc., Chicago, IL, USA) was used to analyze the data and results are presented as the mean ± standard deviation. Student's t-test was used to analyze the statistical differences between two groups. Following one-way analysis of variance, the Tukey test was used to compare the differences between three groups. Fisher's exact test was performed to identify the differences between categorical variables. Kaplan-Meier method and log-rank test were used to conduct a univariate survival analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-518b expression and clinicopathological features of GBM
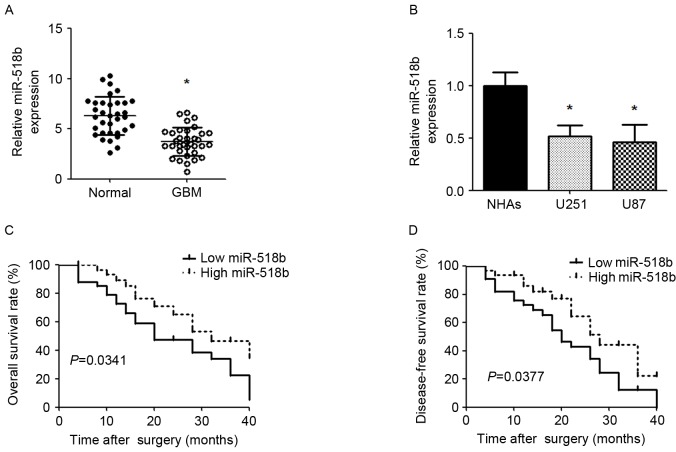
The expression levels of miR-518b were lower in GBM tissues compared with in adjacent nontumor tissues (Fig. 1A). The expression levels of miR-518b were also significantly reduced in U87 and U251 cell lines compared with in NHAs (Fig. 1B). miR-518b expression levels were classified low or high based on the median expression level of the cohort. As presented in Table I, a clinical characteristics analysis revealed that low miR-518b expression was associated with larger tumor size (P=0.0146) and a higher WHO grade (P=0.0071); however, miR-518b expression was not significantly associated with other characteristics, including age, gender, differentiation and Karnofsky performance status. Kaplan-Meier analysis revealed that low miR-518b expression was significantly associated with worse overall survival and disease-free survival in patients with GBM (P=0.0341 and P=0.0377, respectively; Fig. 1C and D).
Figure 1.
miR-518b expression in GBM and its association with survival. (A) Mature miR-518b expression was determined by qPCR in human GBM tissues and adjacent noncancerous tissues. Data are presented as the mean ± standard deviation. *P<0.05 vs. normal group (n=34). (B) Expression of miR-518b was detected in cell lines by qPCR. Data are expressed as the mean ± standard deviation. *P<0.05 vs. NHAs. All experiments were repeated 5 times. Kaplan-Meier analyses of correlations between miR-518b expression and (C) overall survival rate or (D) disease-free survival rate. GBM, glioblastoma; miR-518b, microRNA-518b; qPCR, quantitative polymerase chain reaction; NHA, normal human astrocytes.
Table I.
Correlation between miR-518b expression and clinical characteristics.
| Relative miR-518b expression | ||||
|---|---|---|---|---|
| Characteristic | No. | Low | High | P-value |
| Age (years) | ||||
| ≤50 | 31 | 13 | 18 | 0.3302 |
| >50 | 37 | 21 | 16 | |
| Sex | ||||
| Male | 44 | 20 | 24 | 0.4469 |
| Female | 24 | 14 | 10 | |
| Diameter (mm) | ||||
| ≤3 | 35 | 12 | 23 | 0.0146a |
| >3 | 33 | 22 | 11 | |
| Differentiation | ||||
| Well and moderate | 35 | 15 | 20 | 0.3319 |
| Poor | 33 | 19 | 14 | |
| WHO grade | ||||
| I–II stage | 32 | 10 | 22 | 0.0071a |
| III–IV stage | 36 | 24 | 12 | |
| KPS | ||||
| ≤90 | 27 | 13 | 14 | 0.8043 |
| >90 | 41 | 21 | 20 | |
P<0.05. WHO, World Health Organization; KPS, Karnofsky performance status; miR, microRNA.
miR-518b affects GBM cell proliferation, apoptosis and angiogenesis in vitro and in vivo
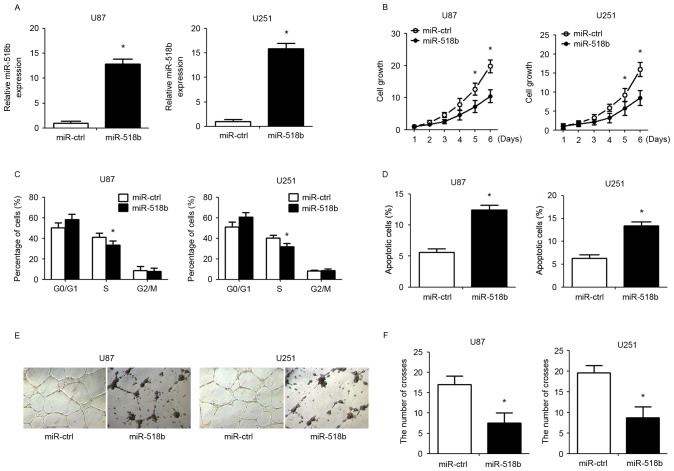
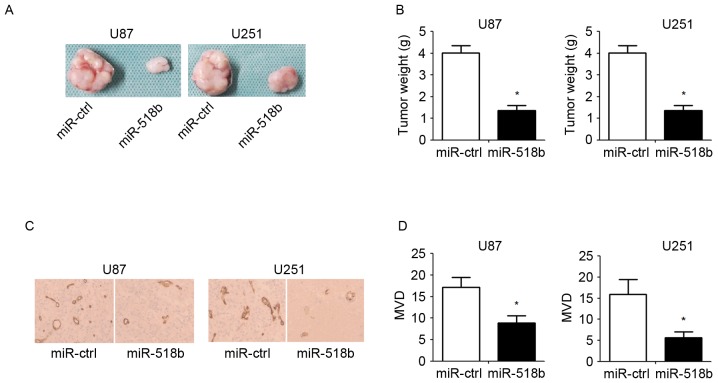
To investigate the role of miR-518b in GBM cells, miR-518b overexpression was induced in human GBM cells (U87 and U251) by transfection with miR-518b mimics. The transfection efficiency was confirmed qPCR (Fig. 2A). CCK-8 assay demonstrated that overexpression of miR-518b suppressed the growth of U87 and U251 cells in a time-dependent manner (Fig. 2B). Compared with the control group, flow cytometry revealed that transfection with miR-518b mimics markedly reduced the percentage of cells in S phase and enhanced the percentage of apoptotic cells (Fig. 2C and D). To determine whether miR-518b may regulate angiogenesis in GBM, human umbilical vein endothelial cells were resuspended in GBM cell-conditioned medium and were cultured in Matrigel to allow for tube formation. The results indicated that overexpression of miR-518b significantly reduced the formation of tube-like structures (Fig. 2E and F). To clarify the effects of miR-518b on GBM cell proliferation and angiogenesis in vivo, nude mice were inoculated with GBM cells post-transfection. The results demonstrated that overexpression of miR-518b significantly reduced tumor growth, as reflected by decreased tumor weight (Fig. 3A and B). In addition, CD34 immunohistochemical staining indicated that miR-518b overexpression significantly decreased MVD compared with in the control group (Fig. 3C and D).
Figure 2.
Effects of miR-518b on cell proliferation, apoptosis and angiogenesis. U87 and U251 cells were transfected with miR-518b or miR-ctrl mimics for 48 h. (A) miR-518b overexpression in U87 and U251 cells was validated by quantitative polymerase chain reaction. (B) Cell Counting Kit-8 assays were performed to evaluate cell proliferation at the indicated time-points. (C) Proportion of cells at each stage of the cell cycle. (D) The rate of apoptosis was detected by flow cytometry. (E and F) Capillary tube formation ability was determined by calculating the branch points of the newly formed tubes. Data are presented as the mean ± standard deviation (n=5). *P<0.05 vs. miR-ctrl group. miR, microRNA; ctrl, control.
Figure 3.
miR-518b inhibits the growth and angiogenesis of glioblastoma cell lines in vivo. Transfected cells were subcutaneously injected into the flanks of athymic mice for 30 days. (A) Representative images of xenografts from nude mice are presented. (B) Tumor weight of the excised tumors. (C) Immunohistochemical staining of CD34 in xenograft tumors (magnification, 200x). (D) MVD analysis was performed according to CD34 staining. Data are presented as the mean ± standard deviation (n=5). *P<0.05 vs. miR-ctrl group. miR, microRNA; ctrl, control; CD, cluster of differentiation; MVD, microvessel density.
miR-518b directly inhibits PDGFRB expression via targeting its 3′UTR
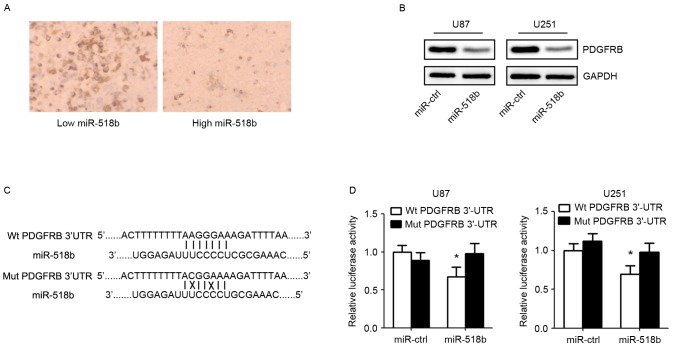
To identify the target gene of miR-518b, putative protein-coding gene targets of miR-518b were searched for using bioinformatics prediction software. PDGFRB was identified as a potential target by TargetScan (http://www.targetscan.org/vert_71/) and miRBase (http://www.mirbase.org/) algorithms. Immunohistochemistry demonstrated that the expression of PDGFRB was markedly reduced in GBM samples with high miR-518b expression compared with in GBM samples with low miR-518b expression (Fig. 4A). Western blot analysis indicated that the expression of PDGFRB was decreased in cells transfected with miR-518b mimics (Fig. 4B). Furthermore, to investigate whether PDGFRB was a direct target of miR-518b, PDGFRB wild-type or mutant 3′UTRs were cloned into a dual-luciferase reporter assay system (Fig. 4C). The results suggested that miR-518b mimics transfection, in U87 and U251 cell lines, significantly suppressed the luciferase activity of wild-type PDGFRB 3′UTR but not that of mutant PDGFRB3′UTR (Fig. 4D).
Figure 4.
PDGFRB is a direct target gene of miR-518b. (A) Inverse relationship between the expression of miR-518b and PDGFRB was detected in GBM specimens (magnification, 200x). (B) Western blot analysis of PDGFRB expression in U87 and U251 cells 48 h post-transfection with miR-518b and miR-ctrl. (C) PDGFRB mRNA 3′UTR contains binding sites for miR-518b. (D) U87 and U251 cells were co-transfected with the dual-luciferase reporter plasmid carrying the Wt or Mut 3′UTR sequences of PDGFRB and miR-518b or miR-ctrl mimics. A luciferase reporter system analysis was performed. Data are presented as the mean ± standard deviation (n=5). *P<0.05 vs. miR-ctrl group. miR, microRNA; ctrl, control; PFGFRB, platelet-derived growth factor receptor β; Wt, wild-type; Mut, mutant; 3′UTR, 3′ untranslated region.
Discussion
GBM is a common type of tumor that affects children and adults, which is associated with poor prognosis, high relapse frequency and unsatisfactory therapeutic effects in response to clinical comprehensive treatment (13). Previous studies have indicated that miRNAs serve an important role in the occurrence and progression of GBM (14), and various miRNAs exert their impact on GBM carcinogenesis (15,16). The present study focused on the aberrant expression of miR-518b in GBM and its newly identified relationship with PDGFRB in GBM.
miR-518b has been deemed a tumor suppressor in carcinogenesis, as demonstrated by the observation that downregulation of miR-518b has frequently been detected in esophageal squamous cell carcinoma (17) and chondrosarcoma (18). In a recent study, a miRNA microarray demonstrated that miR-518b, which targets PDGFRB, is downregulated in microvascular proliferation, which may be the mechanism underlying the high expression of PDGFRB in GBM microvascular proliferation (19). The present study demonstrated that the expression of miR-518b was reduced in GBM tissues and cell lines. Low miR-518b expression was also associated with unfavorable clinical characteristics, including tumor size and WHO grade, suggesting that miR-518b may be closely associated with the occurrence and development of GBM. In addition, the patients with GBM and low miR-518b expression exhibited a worse survival rate, which indicated that miR-518b may be a prognostic marker in patients with GBM. Furthermore, overexpression of miR-518b in vitro markedly inhibited cell growth by regulating cell cycle progression and apoptosis. Tube formation and xenograft assays provided direct evidence to suggest that miR-518b suppressed the development of GBM by inhibiting angiogenesis. Therefore, in vitro and in vivo findings indicated that overexpression of miR-518b in GBM inhibited tumor proliferation and angiogenesis. These results were consistent with the findings of previous studies (17,18,20), which indicated that miR-518b suppressed the tumorigenesis of various cancer cells. The results of the present study revealed that miR-518b acted as a tumor suppressor gene in GBM pathogenesis.
PDGFRB, which is a cell-surface tyrosine kinase receptor, is an important target for anticancer therapeutics (19,21). It has been reported that PDGFRB is an angiogenic factor, which is implicated in numerous cellular processes, including angiogenesis, proliferation and migration (22,23). Previous studies have suggested that PDGFRB may induce GBM tumorigenesis, and enhance tumor proliferation by modulating the expression of tumor suppressor miRNAs in U87 human GBM cells (21,24). Furthermore, the expression levels of PDGFRB are significantly increased in GBM microvascular proliferation than in GBM tumor cells indicating that PDGFRB serves a critical role in GBM microvascular proliferation (19). In the present study, dual-luciferase reporter assay and western blotting demonstrated that PDGFRB is a direct target of miR-518b, and PDGFRB is negatively regulated by miR-518b in GBM tissues. These findings indicated that miR-518b may inhibit GBM proliferation and angiogenesis by suppressing PDGFRB expression. PDGFRB overexpression is a notable feature that may be considered a critical event in GBM tumorigenesis. The present study confirmed that PDGFRB is a target gene of miR-518b in GBM; however, more relevant functions and downstream regulatory mechanisms remain to be explored.
In conclusion, the present study revealed that miR-518b expression is decreased in GBM, and its reduced expression is associated with tumor size and WHO grade. Furthermore, miR-518b exerts critical effects on prognosis, proliferation, apoptosis and angiogenesis in GBM by directly targeting PDGFRB. These findings indicated that miR-518b may function as a tumor suppressor by targeting PDGFRB in the occurrence and development of GBM. Taken together, this newly identified target of miR-518b may also be considered a prognostic marker and therapeutic target for patients with GBM.
References
- 1.Fuller GN, Scheithauer BW. The 2007 Revised World Health Organization (WHO) classification of tumours of the central nervous system: Newly codified entities. Brain Pathol. 2007;17:304–307. doi: 10.1111/j.1750-3639.2007.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang F, Wang W, Zhou C, Xi W, Yuan L, Chen X, Li Y, Yang A, Zhang J, Wang T. miR-221/222 promote human glioma cell invasion and angiogenesis by targeting TIMP2. Tumour Biol. 2015;36:3763–3773. doi: 10.1007/s13277-014-3017-3. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Li C, Shen C, Yin F, Wang K, Liu Y, Zheng B, Zhang W, Hou X, Chen X, et al. miR-212-3p inhibits glioblastoma cell proliferation by targeting SGK3. J Neurooncol. 2015;122:431–439. doi: 10.1007/s11060-015-1736-y. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Zheng G, Gu Z, Guo Z. miR-137 inhibits proliferation and angiogenesis of human glioblastoma cells by targeting EZH2. J Neurooncol. 2015;122:481–489. doi: 10.1007/s11060-015-1753-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao D, Wang R, Fang J, Ji X, Li J, Chen X, Sun G, Wang Z, Liu W, Wang Y, et al. miR-154 functions as a tumor suppressor in glioblastoma by targeting Wnt5a. Mol Neurobiol. 2017;54:2823–2830. doi: 10.1007/s12035-016-9867-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Han D, Wei W, Cao W, Zhang R, Dong Q, Zhang J, Wang Y, Liu N. miR-218 inhibited growth and metabolism of human glioblastoma cells by directly targeting E2F2. Cell Mol Neurobiol. 2015;35:1165–1173. doi: 10.1007/s10571-015-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alrfaei BM, Vemuganti R, Kuo JS. MicroRNA-100 targets SMRT/NCOR2, reduces proliferation, and improves survival in glioblastoma animal models. PLoS One. 2013;8:e80865. doi: 10.1371/journal.pone.0080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Li Z, Tian N, Han L, Fu Y, Guo Z, Tian Y. miR-148b-3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expression. Oncol Lett. 2016;12:879–886. doi: 10.3892/ol.2016.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Zhang J, Yan W, You G, Bao Z, Li S, Kang C, Jiang C, You Y, Zhang Y, et al. Whole-genome microRNA expression profiling identifies a 5-microRNA signature as a prognostic biomarker in Chinese patients with primary glioblastoma multiforme. Cancer. 2013;119:814–824. doi: 10.1002/cncr.27826. [DOI] [PubMed] [Google Scholar]
- 11.Zhu K, Ding H, Wang W, Liao Z, Fu Z, Hong Y, Zhou Y, Zhang CY, Chen X. Tumor-suppressive miR-218-5p inhibits cancer cell proliferation and migration via EGFR in non-small cell lung cancer. Oncotarget. 2016;7:28075–28085. doi: 10.18632/oncotarget.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Liao H, Liu T, Zeng X, Xiao F, Luo L, Guo H, Guo L. miR-296-3p regulates cell growth and multi-drug resistance of human glioblastoma by targeting ether-à-go-go (EAG1) Eur J Cancer. 2013;49:710–724. doi: 10.1016/j.ejca.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Abba ML, Patil N, Leupold JH, Moniuszko M, Utikal J, Niklinski J, Allgayer H. MicroRNAs as novel targets and tools in cancer therapy. Cancer Lett. 2017;387:84–94. doi: 10.1016/j.canlet.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Wu N, Xiao L, Zhao X, Zhao J, Wang J, Wang F, Cao S, Lin X. miR-125b regulates the proliferation of glioblastoma stem cells by targeting E2F2. FEBS Lett. 2012;586:3831–3839. doi: 10.1016/j.febslet.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Shi M, Hou S, Ding B, Liu L, Ji X, Zhang J, Deng Y. miR-483-5p suppresses the proliferation of glioma cells via directly targeting ERK1. FEBS Lett. 2012;586:1312–1317. doi: 10.1016/j.febslet.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Zhou S, Zhang L, Zhang J, Cai H, Zhu J, Huang C, Wang J. miR-518b is down-regulated and involved in cell proliferation and invasion by targeting Rap1b in esophageal squamous cell carcinoma. FEBS Lett. 2012;586:3508–3521. doi: 10.1016/j.febslet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Liang W, Li X, Li Y, Li C, Gao B, Gan H, Li S, Shen J, Kang J, Ding S, et al. Gallic acid induces apoptosis and inhibits cell migration by upregulating miR-518b in SW1353 human chondrosarcoma cells. Int J Oncol. 2014;44:91–98. doi: 10.3892/ijo.2013.2155. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Z, Qiao Z, Chen W, Gong R, Wang Y, Xu L, Ma Y, Zhang L, Lu Y, Jiang B, et al. CIP2A regulates proliferation and apoptosis of multiple myeloma cells. Mol Med Rep. 2016;14:2705–2709. doi: 10.3892/mmr.2016.5553. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Li JY. Differential expression of PDGFRB and EGFR in microvascular proliferation in glioblastoma. Tumour Biol. 2016;37:10577–10586. doi: 10.1007/s13277-016-4968-3. [DOI] [PubMed] [Google Scholar]
- 21.Camorani S, Esposito CL, Rienzo A, Catuogno S, Iaboni M, Condorelli G, de Franciscis V, Cerchia L. Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRβ aptamer. Mol Ther. 2014;22:828–841. doi: 10.1038/mt.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Chintalgattu V, Shih T, Ai D, Xia Y, Khakoo AY. MicroRNA-9 is an activation-induced regulator of PDGFR-beta expression in cardiomyocytes. J Mol Cell Cardiol. 2011;51:337–346. doi: 10.1016/j.yjmcc.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Moreno L, Popov S, Jury A, Al Sarraj S, Jones C, Zacharoulis S. Role of platelet derived growth factor receptor (PDGFR) over-expression and angiogenesis in ependymoma. J Neurooncol. 2013;111:169–176. doi: 10.1007/s11060-012-0996-z. [DOI] [PubMed] [Google Scholar]
- 24.Costa PM, Cardoso AL, de Almeida Pereira LF, Bruce JN, Canoll P, de Lima Pedroso MC. PDGF-B-mediated downregulation of miR-21: New insights into PDGF signaling in glioblastoma. Hum Mol Genet. 2012;21:5118–5130. doi: 10.1093/hmg/dds358. [DOI] [PMC free article] [PubMed] [Google Scholar]