Abstract
Panax notoginseng saponins (PNS) are among the most important compounds extracted from Panax notoginseng root, and have long been used in traditional Chinese medicine to control bleeding. PNS have recently garnered attention for the treatment of circulatory system diseases. The present study aimed to evaluate the effects of PNS on angiogenesis in vitro and to explore the molecular mechanisms underlying their actions. The present results demonstrated that the proliferative ability of human umbilical vein endothelial cells (HUVECs) was augmented following treatment with PNS. In addition, wound healing and Boyden chamber assays indicated that PNS may enhance HUVEC motility and increase the number of capillary-like tube branches in HUVECs. These effects were suppressed by 5′ adenosine monophosphate-activated protein kinase (AMPK) and endothelial nitric oxide synthase (eNOS) inhibitors. Furthermore, western blot analysis demonstrated that PNS stimulated the phosphorylation of AMPK and eNOS at Thr-172 and Ser-1179, respectively. These results suggested that PNS may promote tube formation in endothelial cells through AMPK- and eNOS-dependent signaling pathways.
Keywords: Panax notoginseng saponins, angiogenesis, 5′ adenosine monophosphate-activated protein kinase-dependent pathway, endothelial nitric oxide synthase-dependent pathway
Introduction
Angiogenesis refers to the formation of new capillaries from existing vasculature and is a central process during normal embryonic development and wound healing (1,2). Angiogenesis serves a critical role during pathological neovascularization, which is characteristic of tumor growth and ischemic cardiovascular disease (2). Vascular stenosis and obstruction are among the main causes of ischemic cardiovascular disease, including myocardial infarction and angina, and can lead to reduction or depletion of blood flow to the heart. Enhanced angiogenic processes may serve as a compensatory mechanism to increase the compromised blood flow. Therefore, therapeutic angiogenesis has been considered as a supplementary strategy for the treatment of patients with vascular insufficiency (3). Recently, formulas used in traditional Chinese medicine have garnered attention in the search for novel proangiogenic agents (4).
Panax notoginseng (Burk.) F. H. Chen is a plant mainly cultivated in Yunnan, China (5). The root of Panax notoginseng, also known as Radix notoginseng and Sanqi, has been used as a tonic and hemostatic agent in traditional Chinese medicine for thousands of years. As the interest around traditional Chinese medicine increases, numerous studies have investigated the chemical constituents and the biological activity of Sanqi. It has been demonstrated that Sanqi exerts pharmacological effects on the immune, central nervous and cardiovascular systems, in diabetes mellitus and cancer (6). The chemical constituents of Panax notoginseng are complex and include saponins, flavonoids, carbenes, sterols, organic acids/esters, polysaccharides and amino acids (7). Panax notoginseng saponins (PNS) are considered the main bioactive ingredients.
5′ Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a heterotrimer composed of three subunits: A catalytic α subunit, and two regulatory β and γ subunits (8). AMPK has been reported to act as an energy sensor that participates in the maintenance of energy homeostasis (9). Stimuli that increase the AMP/adenosine triphosphate (ATP) ratio, including exercise, glucose deprivation, adiponectin, leptin, hypoxia and ischemia, are able to induce AMPK activation (10–13). When AMPK is activated, ATP-consuming pathways are inhibited and ATP-producing pathways are enhanced (8). A previous study demonstrated that under hypoxic conditions, the angiogenic properties of endothelial cells are potentiated, through the activation of AMPK signaling pathways (14). AMPK has been reported to phosphorylate endothelial nitric oxide synthase (eNOS) at Ser-1179 (15). The present study aimed to examine the effects of PNS during angiogenesis. The AMPK inhibitor 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-(13)-3-pyridin-4-yl-pyrrazolo [1, 5-a]-pyrimidine (Compound C) (16) and the eNOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) (17) were also used to investigate the molecular pathways underlying the effects of PNS during angiogenesis.
Materials and methods
Reagents
Total saponins extracted from Panax notoginseng were purchased from Yunnan Yuxi Wanfang Natural Medicine Co., Ltd. (Yuxi, China). L-NAME was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Anti-AMPK α-pan (cat. no. 2603), anti-phosphorylated (p)-AMPK (Thr-172; cat. no. 2535), anti-eNOS (cat. no. 9586) and anti-p-eNOS (Ser-1179; cat. no. 9570) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Fetal bovine serum (FBS) was purchased from HyClone (GE Healthcare Life Sciences, Logan, UT, USA). Cell culture media were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Vascular endothelial growth factor (VEGF) and Compound C were purchased from Merck KGaA. Anti-β-actin antibody (cat. no. M06209) was obtained from Yeasen Biological Technology Co., Ltd. (Shanghai, China) and horseradish peroxidase-conjugated anti-rabbit (cat. no. 7074) and anti-mouse (cat. no. 7076) secondary antibodies were from Cell Signaling Technology, Inc.
Cell culture
Primary human umbilical vein endothelial cells (HUVECs) were isolated from 7 neonatal umbilical cords in Shanghai Tenth People's Hospital between February and August 2015. Umbilical cords were isolated and rapidly placed in preheated PBS under aseptic conditions. Total blood was harvested from the umbilical cords, and a 20-cm clipping of the tissue was used for cell isolation. The tissue was thoroughly washed with PBS to remove all blood and treated with 0.1% collagenase II for 15 min at 37°C. Subsequently, the digested tissue was rinsed with RPMI-1640 medium and centrifuged at 181 × g for 5 min at 4°C. The supernatants were discarded and cells were resuspended in M200 medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with low serum growth supplement (Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and 10% heat-inactivated FBS. Cells (~1×106) were inoculated in 0.1% gelatin pre-coated culture flasks and cultured at 37°C in a humidified atmosphere containing 5% CO2. The following day, the medium was replaced to remove the non-adherent cells and cells were maintained at 37°C in a 5% CO2 atmosphere until further use. The medium was replaced every 2 days. HUVECs used in all experiments were between passages 2 and 8. The present study was approved by the ethics committee of Shanghai Tenth People's Hospital. Written informed consent was obtained from all human subjects providing umbilical cord samples.
Cell proliferation assay
HUVECs were seeded at a density of 4×103 cells/well in M200 medium, supplemented with low serum growth supplement, 1% penicillin-streptomycin and 10% heat-inactivated FBS into 96-well plates in triplicate. Following 24 h of attachment, cells were treated with increasing concentrations of PNS (0, 0.1, 0.2, 0.4, 0.6, 0.8, 1 and 2 mg/ml) for 48 h at 37°C. Cells were also treated with L-NAME (100 µmol/l) or Compound C (10 µmol/l) for 2 h, followed by incubation with PNS (1 mg/ml) for 48 h at 37°C. Cells treated with VEGF (20 ng/ml) served as positive controls. Cell proliferation was assessed using the Cell Counting Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Following further incubation for 48 h at 37°C, the medium in each well was discarded and replaced with fresh medium containing 10 µl CCK-8 solution and cells were incubated at 37°C for 2 h. Absorbance at 450 nm was measured using a microplate ELISA reader (BioTek Instruments, Inc., Winooski, VT, USA). A wavelength of 650 nm was used as reference.
Migration assay
Boyden chamber assay
A Boyden chamber assay was performed as previously described (18). Briefly, HUVECs were starved overnight in serum-free medium. Starved cells were resuspended in M200 medium supplemented with 0.5% FBS and diluted to a concentration of 2.0×104 cells in 200 µl/well. The cell suspension was then added into the upper chambers of Transwell inserts (8.0 µm pore size; BD Biosciences, Franklin Lakes, NJ, USA). Cell growth medium (600 µl), supplemented with bovine serum albumin (BSA; 30 µg/ml; Sangon Biotech Co., Ltd., Shanghai, China), PNS (1 mg/ml), Compound C (10 µM) or L-NAME (100 µM) in the absence or presence of PNS (1 mg/ml) or VEGF (20 ng/ml) was added to the lower chambers. Following 8 h of treatment at 37°C, the upper chambers were removed and washed with PBS. Cells attached to the upper surface of the inserts were discarded. Cells that had migrated to the lower surface of the inserts were fixed with methanol for 15 min and stained with crystal violet (Sigma-Aldrich; Merck KGaA). Stained cells were observed under an inverted microscope in five random fields per well and the number of cells was quantified using Image Pro Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Each experiment was performed in duplicate and repeated three times.
Scratch wound assay
HUVECs (1.0×105 cells) were seeded in 6-well plates in M200 medium, supplemented with low serum growth supplement, 1% penicillin-streptomycin and 10% heat-inactivated FBS. When cells had reached 90% confluence, a rectangular lesion was created by scratching the plate with a pipette tip. Cells were rinsed twice with PBS and incubated in M200 medium without FBS at 37°C. Photomicrographs were captured under an inverted phase-contrast microscope in 3 random fields and wound width was measured. Subsequently, cells were treated with PNS (1 mg/ml), Compound C (10 µM) or L-NAME (100 µM) in the absence or presence of PNS (1 mg/ml), or VEGF (20 ng/ml) for an additional 24 h. Photomicrographs were captured in the same fields. The migration distance was analyzed using Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.).
Tube formation assay
HUVECs suspended in M200 medium supplemented with 1% FBS were seeded into Matrigel (BD Biosciences) pre-coated 96-well plates at a density of 1×104 cells/100 µl. Cells were treated with 1 mg/ml PNS, Compound C (10 µM) or L-NAME (100 µM) in the absence or presence of PNS (1 mg/ml), 20 ng/ml VEGF or 0.1% dimethyl sulfoxide (DMSO) and were incubated for 8 h at 37°C. Photomicrographs were captured under an inverted phase-contrast microscope and the angiogenic ability was quantified via counting the number of branch points of formed tubes in three randomly selected fields per well using Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.). Each experiment was repeated three times.
Western blot analysis
HUVECs (1×105 cells) were seeded in 6-well plates and were pretreated with compound C (10 µmol/l) or L-NAME (100 µmol/l) for 40 min followed by PNS (1 mg/ml) for 1 h. Cells were then washed with cold PBS twice followed by incubation in lysis buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 30 min on ice. Cells were centrifuged at 13,523 × g for 10 min at 4°C. Protein concentration was quantified with a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, Haimen, China). Equal amounts of extracted protein samples (20 µg) were separated by 8% or 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% BSA at room temperature for 1 h and incubated with the following primary antibodies overnight at 4°C: Anti-AMPK α-pan (1:1,000), anti-p-AMPK (Thr-172; 1:1,000), anti-eNOS (1:1,000), anti-p-eNOS (Ser-1179; 1:1,000) and anti-β-actin (1:1,000). Subsequently, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000) for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence detection kit (Thermo Fisher Scientific, Inc.). Blots were semi-quantified by densitometry analysis using ImageJ software version 1.6.0 (National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard error of the mean of at least 3 independent experiments. The statistical significance of the difference between groups was assessed by Student's t-test for pair-wise comparisons or one-way analysis of variance followed by a post hoc Student-Newman-Keuls test for multiple comparisons. The analysis was performed using GraphPad Prism software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
PNS promote HUVEC proliferation
The proliferative capabilities of HUVECs following PNS treatment were assessed using the CCK-8 assay. The proliferation of PNS-treated HUVECs increased in a dose-dependent manner; PNS treatment reached its maximum effect at a concentration of 2.0 mg/ml (Fig. 1). Treatment with VEGF was used as a positive control. These results suggested that PNS may stimulate the proliferation of HUVECs.
Figure 1.

PNS stimulates HUVEC proliferation. HUVECs were incubated with various concentrations of PNS for 48 h. Treatment with VEGF was used as a positive control. Control cells received no treatment. Cell proliferation was assessed using the Cell Counting Kit-8 assay. Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 vs. the control group. PNS, Panax notoginseng saponins; HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor.
PNS promote HUVEC migration
Neovascularization requires proliferating endothelial cells to migrate through the basement membrane to distant sites. Therefore, the migratory capabilities of endothelial cells are critical for angiogenesis. To elucidate the effects of PNS on endothelial cell mobility, Boyden chamber and scratch wound assays were performed (Fig. 2). The number of migrated cells in the PNS- and VEGF-treated groups was significantly higher compared with in the control group (Fig. 2A and C). The scratch assay demonstrated that cells in the PNS- and VEGF-treated groups exhibited significantly increased motility compared with in the control group (Fig. 2B and D). These results suggested that PNS may enhance HUVEC migration and exert similar effects to VEGF stimulation.
Figure 2.

PNS stimulates HUVEC migration. Cells were treated with bovine serum albumin (30 µg/ml; control), PNS (1 mg/ml), or VEGF (20 ng/ml; positive control). (A) Invasive capabilities of HUVECs were assessed using the Boyden chamber assay. Magnification, ×10. (B) Migratory capabilities of HUVECs were assessed using the scratch assay. Magnification, ×10. (C and D) Quantitative evaluation of HUVEC mobility following various treatments. Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 vs. the control group. PNS, Panax notoginseng saponins; HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor.
PNS promote capillary-forming capabilities of HUVECs
The effects of PNS on the vessel-forming capabilities of HUVECs were assessed using the tube formation assay. Matrigel is a basement membrane matrix that facilitates the formation of network-like structures by endothelial cells. HUVECs were seeded onto the Matrigel and were treated with 1 mg/ml PNS, 20 ng/ml VEGF or 0.1% DMSO. Cells treated with PNS or VEGF formed more capillary-like networks compared with DMSO-treated control cells (Fig. 3A). In addition, the proangiogenic effects of PNS were confirmed using quantitative measurements. PNS- and VEGF-treated cells exhibited significantly more branch points compared with control cells (Fig. 3B).
Figure 3.

PNS stimulates capillary-forming capabilities of HUVECs. (A) Photomicrographs of HUVECs seeded on Matrigel-coated plates following incubation with vehicle (control group), PNS or VEGF (positive control) for 8 h. Magnification, ×10. (B) Cells treated with PNS or VEGF exhibited significantly more branch points compared with cells in the control group. Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 vs. the control group. PNS, Panax notoginseng saponins; HUVECs, human umbilical vein endothelial cells; VEGF, vascular endothelial growth factor.
PNS induce AMPK and eNOS phosphorylation in HUVECs
HUVECs were incubated with PNS (1 mg/ml), and phosphorylation of the AMPK α-subunit at Thr-172 was assessed using western blot analysis. Treatment with PNS enhanced AMPK phosphorylation in a time-dependent manner, without affecting the levels of total AMPK protein (Fig. 4A and B). The maximum effect was demonstrated following 60 min of treatment.
Figure 4.
PNS enhances AMPK and eNOS phosphorylation in HUVECs. (A and B) Time-dependent alterations were detected in AMPK and eNOS phosphorylation following PNS treatment. (C and D) Compound C, an AMPK inhibitor, and L-NAME, an eNOS inhibitor, suppressed PNS-induced increases in AMPK and eNOS phosphorylation. HUVECs were pretreated with Compound C (10 µmol/l) or L-NAME (100 µmol/l) for 40 min, followed by treatment with PNS (1 mg/ml) for 1 h. Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 vs. the control group; #P<0.05 vs. the PNS group. PNS, Panax notoginseng saponins; AMPK, 5′ adenosine monophosphate-activated protein kinase; eNOS, endothelial nitric oxide synthase; HUVECs, human umbilical vein endothelial cells; Compound C, 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)](13)-3-pyridin-4-yl-pyrrazolo [1,5-a]-pyrimidine; L-NAME, Nω-nitro-L-arginine methyl ester.
AMPK is able to activate eNOS via phosphorylation at Ser-1179 (19), which can trigger endothelial cell growth and migration due to increased NO synthesis (17,20,21). Therefore, eNOS phosphorylation was also assessed. PNS was demonstrated to enhance eNOS phosphorylation without altering total protein levels (Fig. 4A and B). To examine whether AMPK may be involved in the regulation of PNS-induced eNOS phosphorylation, the AMPK inhibitor Compound C and the eNOS inhibitor L-NAME were used. AMPK phosphorylation induced by PNS was inhibited by Compound C, but was not affected by L-NAME. Conversely, eNOS phosphorylation was inhibited by Compound C and L-NAME (Fig. 4B and C). These results suggested that the proangiogenic effects exerted by PNS may depend on AMPK- and eNOS-mediated signaling pathways.
Role of AMPK and eNOS pathways in PNS-induced HUVEC proliferation
To investigate whether AMPK- and eNOS-dependent pathways may be involved in PNS-stimulated HUVEC proliferation, the inhibitors L-NAME and Compound C were use Compound C and L-NAME abolished the PNS-induced stimulation of HUVEC proliferation (Fig. 5). These results suggested that PNS may promote HUVEC proliferation through the activation of AMPK- and eNOS-mediated signaling pathways.
Figure 5.
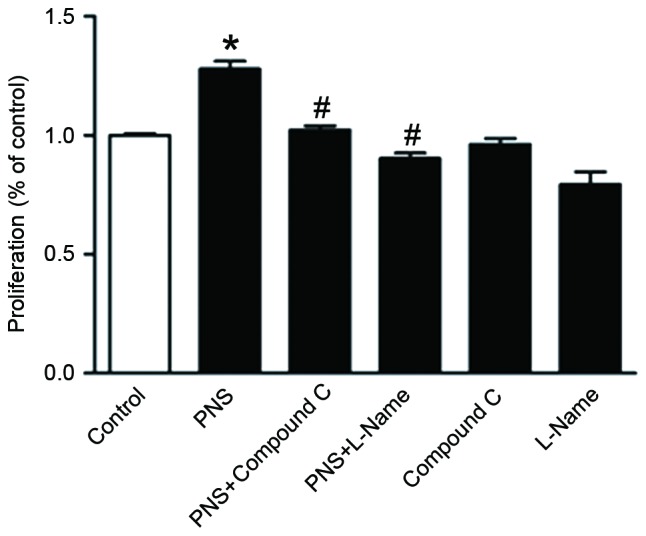
AMPK- and eNOS-mediated pathways are implicated in PNS-stimulated HUVEC proliferation. HUVECs were pretreated with Compound C (10 µM) or L-NAME (100 µM) for 2 h, followed by incubation with PNS (1 mg/ml) for 48 h. Compound C and L-NAME suppressed PNS-stimulated cellular proliferation. Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 vs. the control group; #P<0.05 vs. the PNS group. AMPK, 5′ adenosine monophosphate-activated protein kinase; eNOS, endothelial nitric oxide synthase; PNS, Panax notoginseng saponins; HUVECs, human umbilical vein endothelial cells; Compound C, 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)](13)-3-pyridin-4-yl-pyrrazolo [1,5-a]-pyrimidine; L-NAME, Nω-nitro-L-arginine methyl ester.
Role of AMPK and eNOS signaling pathways in PNS-stimulated HUVEC migration and capillary formation. The implication of AMPK and eNOS signaling pathways in PNS-induced endothelial cell migration and angiogenesis were investigated. HUVECs were treated with PNS and the inhibitors Compound C and L-NAME. Results of the Boyden chamber assay indicated that Compound C and L-NAME significantly suppressed the PNS-stimulated HUVEC migration (Fig. 6A and B). The scratch assay revealed that PNS-treated HUVECs migrated longer distances compared with vehicle-treated cells, whereas the effects of PNS were reversed following treatment with the AMPK and eNOS inhibitors (Fig. 6C and D). In addition, a Matrigel assay demonstrated that in HUVECs treated with the inhibitors, PNS-induced tube formation was abolished (Fig. 6E and F). These results suggested that PNS may promote endothelial cell migration and tube formation through AMPK- and eNOS-mediated signaling pathways.
Figure 6.
AMPK- and eNOS-mediated pathways are implicated in PNS-stimulated HUVEC migration and angiogenesis. HUVECs were treated with compound C (10 µmol/l) or L-NAME (100 µmol/l) in the presence or absence of PNS (1 mg/ml). (A and B) Boyden chamber assay; (C and D) scratch wound assay; and (E) Matrigel assay. (F) Number of branch points was detected during HUVEC capillary formation. Magnification for all images, ×10. Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 vs. the control group; #P<0.05 vs. the PNS group. AMPK, 5′ adenosine monophosphate-activated protein kinase; eNOS, endothelial nitric oxide synthase; PNS, Panax notoginseng saponins; HUVECs, human umbilical vein endothelial cells; Compound C, 6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)](13)-3-pyridin-4-yl-pyrrazolo [1,5-a]-pyrimidine; L-NAME, Nω-nitro-L-arginine methyl ester.
Discussion
The present study demonstrated that PNS promoted angiogenesis in vitro. PNS were revealed to enhance several features of angiogenesis, including the proliferation, migration and differentiation of endothelial cells into capillary-like structures. Western blot analysis suggested that the mechanism underlying their proangiogenic effects may involve AMPK- and eNOS-dependent signaling.
Ischemic cardiovascular diseases are one of main causes of mortality worldwide and their prevalence increases with age (22). Two major revascularization strategies have garnered attention: Surgical and catheter-based revascularization. However, a large number of patients are not appropriate candidates for these therapies, or remain symptomatic despite receiving revascularization therapy and drug treatment. In addition, the cost of revascularization surgery limits its use. Therefore, the development of alternative treatment strategies, including those aimed at promoting angiogenesis, are mandatory for the effective treatment of patients that are not successfully treated by these revascularization strategies (23).
Angiogenesisis an essential process during vessel formation in adult tissue, and involves complex interactions between endothelial cells, angiogenic growth factors and extracellular matrix components (24). Numerous diseases stem from persistent unregulated angiogenesis; however, therapeutic angiogenesis has garnered attention for its use in the treatment of ischemic diseases (25–28). Chemical agents with proangiogenic effects hold potential as an alternative strategy for the treatment of ischemic cardiovascular diseases (29). Radix notoginseng has long been used in traditional Chinese medicine and has been demonstrated to interfere with angiogenic processes. Radix notoginseng has been reported to increase the expression of VEGF receptor 2 (VEGFR2), as well as the secretion of VEGF in HUVECs (30). The predominant ginsenoside isolated from radix notoginseng, Rg1, has previously exhibited strong proangiogenic actions, exerted through VEGFR2 activation and thephosphatidylinositol-4,5-bisphosphate 3-kinase/Akt/eNOS pathway (31). The present study aimed to investigate the proangiogenic effects of PNS on endothelial cells and explore the molecular mechanisms underlying these actions.
AMPK is a cellular metabolic sensor, which can be activated by increasing the intracellular AMP/ATP ratio, and in turn can inhibit anabolic pathways and stimulate catabolic processes (32). In vascular endothelium, AMPK has been demonstrated to serve a role in the regulation of angiogenesis, particularly under hypoxic conditions (14). A previous study reported that 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK stimulator, promoted angiogenesis in mouse myoblasts, via enhancing VEGF production and AMPK phosphorylation (33). Another study demonstrated that AICAR improved angiogenesis via upregulating VEGF gene expression, promoting AMPK phosphorylation and activating mitogen-activated protein kinase/extracellular signal-regulated kinase pathways in myocardial microvascular endothelial cells (34). eNOS is a substrate for AMPK, and is critical for cardiovascular homeostasis, vascular remodeling and angiogenesis (19). Therefore, the present study investigated whether the activation of AMPK- and eNOS-dependent pathways may participate in the proangiogenic actions of PNS.
To investigate the angiogenic capabilities of PNS, angiogenesis was assessed in vitro in HUVECs, using proliferation, migration and capillary formation assays. The present results demonstrated that endothelial cell proliferation was enhanced by PNS in a dose-dependent manner. HUVEC migration was also increased by PNS treatment. Furthermore, PNS increased the number of branch points in the tube formation assay. These results suggested that treatment with PNS promoted angiogenesis in HUVECs in vitro. Conversely, PNS-stimulated proangiogenic processes were abolished following treatment of HUVECs with AMPK and eNOS inhibitors. In conclusion, these results suggested that PNS may hold potential for the development of alternative strategies aiming to promote therapeutic angiogenesis in patients with ischemic cardiovascular diseases.
Limitations of the present study should be noted: The study was performed using only in vitro experimental systems, and further in vivo experiments are required to confirm the proangiogenic effects of PNS. Further research is also required to elucidate the complex molecular mechanisms underlying the involvement of AMPK and eNOS, as well as their modulation by PNS, in angiogenic processes.
In conclusion, the present study demonstrated that PNS stimulated angiogenesis in endothelial cells in vitro. The present results suggested that the mechanisms underlying the observed proangiogenic effects may involve the activation of AMPK- and eNOS-dependent pathways. These findings suggested that PNS may hold potential for the development of alternative therapeutic strategies to be used in clinical practice for the treatment of patients with ischemic diseases.
Acknowledgements
The present study was supported by the Natural Science Foundation of Shanghai Science and Technology Commission (grant no. 12ZR1422900) and the National Natural Science Foundation of China (grant nos. 30800466 and81270193).
References
- 1.Breier G, Damert A, Plate KH, Risau W. Angiogenesis in embryos and ischemic diseases. Thromb Haemost. 1997;78:678–683. [PubMed] [Google Scholar]
- 2.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: Mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 3.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis: A single intra-arterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hindlimb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: From plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Dong TT, Cui XM, Song ZH, Zhao KJ, Ji ZN, Lo CK, Tsim KW. Chemical assessment of roots of Panax notoginseng in China: Regional and seasonal variations in its active constituents. J Agric Food Chem. 2003;51:4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- 6.Ru W, Wang D, Xu Y, He X, Sun YE, Qian L, Zhou X, Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 7.Yang WZ, Hu Y, Wu WY, Ye M, Guo DA. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carling D. The AMP-activated protein kinase cascade-a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 11.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 12.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcCc, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 14.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 15.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 16.Fryer LG, Parbu-Patel A, Carling D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 2002;531:189–192. doi: 10.1016/S0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]
- 17.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzenbichler B, Kureishi Y, Luo Z, Le Roux A, Branellec D, Walsh K. Regulation of smooth muscle cell migration and integrin expression by the Gax transcription factor. J Clin Invest. 1999;104:1469–1480. doi: 10.1172/JCI7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, de Montellano Ortiz PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/S0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 20.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 21.Morales-Ruiz M, Lee MJ, Zöllner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine1-phosphate activates Akt, nitric oxide production, and chemotaxis though a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 22.Richard K. Primary prevention of coronary heart disease: Integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des Devel Ther. 2011;5:325–380. doi: 10.2147/DDDT.S14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman SB, Isner JM. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol. 2001;33:379–393. doi: 10.1006/jmcc.2000.1329. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 25.Rosengart TK, Patel SR, Crystal RG. Therapeutic angiogenesis: Protein and gene therapy delivery strategies. J Cardiovasc Risk. 1999;6:29–40. doi: 10.1177/204748739900600106. [DOI] [PubMed] [Google Scholar]
- 26.Horvath KA, Cohn LH, Cooley DA, Crew JR, Frazier OH, Griffith BP, Kadipasaoglu K, Lansing A, Mannting F, March R, et al. Transmyocardial laser revascularization: Results of a multicenter trial with transmyocardial laser revascularization used as sole therapy for end-stage coronary artery disease. J Thorac Cardiovasc Surg. 1997;113:645–654. doi: 10.1016/S0022-5223(97)70221-6. [DOI] [PubMed] [Google Scholar]
- 27.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy formyocardial angiogenesis: Initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.CIR.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 28.Silvestre JS, Lévy BI. Angiogenesis therapy in ischemic disease. Arch Mal Coeur Vaiss. 2002;95:189–196. [PubMed] [Google Scholar]
- 29.Feng JH, Shi Q, Wang Y, Cheng YY. Effects of radix ginseng and radix ophiopogonisextract (SMF) on protein S-nitrosylation in ischemic myocardial tissue. Zhongguo Zhong Yao Za Zhi. 2008;33:1894–1897. (In Chinese) [PubMed] [Google Scholar]
- 30.Lei Y, Tian W, Zhu LQ, Yang J, Chen KJ. Effects of radix ginseng and radix notoginseng formula on secretion of vascular endothelial growth factor and expression of vascular endothelial growth factor receptor-2 in human umbilical vein endothelial cells. Zhong Xi Yi Jie He Xue Bao. 2010;8:368–372. doi: 10.3736/jcim20100412. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 31.Yang BR, Hong SJ, Lee SM, Cong WH, Wan JB, Zhang ZR, Zhang QW, Zhang Y, Wang YT, Lin ZX. Pro-angiogenic activity of notoginsenoside R1 in human umbilical vein endothelial cells in vitro and in a chemical-induced blood vessel loss model of zebrafish in vivo. Chin J Integr Med. 2016;22:420–429. doi: 10.1007/s11655-014-1954-8. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 34.Ahluwalia A, Tarnawski AS. Activation of the metabolic sensor-AMP activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. Implications for the aging heart. J Physiol Pharmacol. 2011;62:583–587. [PubMed] [Google Scholar]




