Abstract
Tetrahydroxy stilbene glucoside (TSG) is one of the main active ingredients of Polygonum multiflorum and performs various types of biological activity, particularly anti-inflammatory and anti-oxidative activities. However, the beneficial effect of TSG in H2O2-induced human brain microvascular endothelial cell (HBMEC) dysfunction has not been fully elucidated. In the present study, H2O2-induced oxidative stress and inflammatory responses, and the pharmacological effect of TSG were investigated. The results demonstrated that H2O2 appeared to exert a cytotoxic effect on HBMECs, as the cell viability was significantly inhibited in H2O2-treated HBMECs. Conversely, TSG did not exert a toxic effect on HBMECs, and TSG inhibited H2O2-induced HBMEC cytotoxicity in a dose-dependent manner. Furthermore, the findings indicated that TSG restricted the oxidative stress caused by H2O2 via inhibition of malondialdehyde and reactive oxygen species, and upregulation of superoxide dismutase and glutathione. H2O2-induced injury was associated with enhancing the levels of inflammatory cytokines, tumor necrosis factor-α, interleukin (IL)-6 and IL-1β in the cultured HBMECs, which were attenuated by TSG treatment. Furthermore, the findings demonstrated that TSG inhibited necrosis factor-κB protein expression levels, which, as an upstream transcription factor, may regulate inflammatory responses. Thus, TSG protected HBMECs from H2O2-induced dysfunction by inhibiting oxidative stress and inflammatory responses.
Keywords: stilbene glucoside, human brain microvascular endothelial cell, oxidative, inflammatory
Introduction
Oxidative stress is usually defined as an imbalance between the generation and degradation of reactive oxygen species (ROS) by the various antioxidant defense mechanisms (1). Excessive levels of ROS significantly contribute to the pathogenesis of numerous types of vascular disease, including cerebrovascular-associated diseases (2). Notably, there is increasing evidence that oxidative stress often negatively impacts the vessel wall and regulates cerebral blood flow, which controls permeability of the blood-brain barrier (BBB) (3). Aberrant BBB permeability results in secondary damage to neurons (4). In this process, cerebral endothelial cells are equipped with a defense system against oxidative stress by upregulating glutathione (GSH), GSH peroxidase, GSH reductase and catalase (5). A high concentration of mitochondria in endothelial cells provides an energy-dependent transport mechanisms for BBB and is proposed to be vital in BBB maintenance; however, a decrease in mitochondrial number results in the loss of BBB integrity (6,7). Therefore, normal endothelial cell function is beneficial for maintaining the integrity of BBB.
Tetrahydroxy stilbene glucoside (TSG; 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside) is one of the main active ingredients of Polygonum multiflorum, which is widely administered as a Chinese Herbal Medicine (8). TSG has been reported to perform multiple types of biological activity, particularly anti-inflammatory (9) and anti-oxidative in the various types of cell (10). In PC12 cells, TSG exhibits a cytoprotective effects against H2O2-induced oxidative stress via the regulation of heme oxygenase-1 expression and the translocation of nuclear factor E2-related factor 2 (11). TSG inhibits inflammatory cytokines, intracellular ROS and malondialdehyde (MDA) in osteoblastic MC3T3-E1 cells induced by H2O2 and may be effective in providing protection against osteoporosis associated with oxidative stress (12). Li et al (10) proposed that TSG may attenuate the 1-methyl-4-phenylpyridinium-induced apoptosis in PC12 cells by inhibiting ROS generation. Recently, TSG has been shown to reduce cognitive impairment in rats (13). Furthermore, TSG attenuates injury to endoplasmic reticulum functions and improves learning, memorizing and spatial orientation behavior in transgenic mice by inhibiting the autophagy signaling pathway (14). However, the beneficial effect and underlying mechanisms of TSG in H2O2-induced human brain microvascular endothelial cell (HBMEC) dysfunction have not been clearly delineated.
Thus, the aim of the present study, was to demonstrate the protective effect of TSG on H2O2-induced injury in the cultured HBMECs and identify the potential underlying mechanisms, which are responsible for the protective action.
Materials and methods
Cell culture
The primary HBMECs (cat. no. ACBRI 376) were purchased from Cell Systems Corporation (Kirkland, WA, USA) and maintained in CSC Complete Medium (cat. no. 4Z0-500; Cell Systems Corporation) according to the manufacturer's instructions. TSG (dissolved in distilled water; relative molecular weight, 406 g/mol; purity, >98%) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Cell viability detection by MTT
HBMECs were incubated with H2O2 or TSG at different concentrations (0–200 µM) for 24 h at 37°C, and the cell viability was examined using an MTT Cell Proliferation/Viability Assay kit (R&D Systems, Inc., Minneapolis, MN, USA). The purple formazan was dissolved in dimethyl sulfoxide, according to the manufacturer's instructions. Optical density (OD) was read at a wavelength of 490 nm on a microplate reader (MD SpectraMax M5; Molecular Devices, LLC, Sunnyvale, CA, USA).
Lactate dehydrogenase (LDH) activity
HBMECs were plated and treated with TSG (0–200 µM) in 96-well plates, and stimulated with H2O2. Subsequently, (24 h later) the cells were centrifuged (10,000 × g for 15 min at 4°C) to obtain the supernatant and the level of LDH (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) was measured. Briefly, LDH activity was measured by a redox reaction that couples the oxidation of lactate iodotetrazolium chloride to a colored formazan salt, using NADH as the electron transfer agent and NADH diaphorase as the catalyst. The absorbance at a wavelength of 490 nm was read using an ELISA reader (SpectraMax M5; Molecular Devices, LLC, Sunnyvale, CA, USA) according to the manufacturer's instructions.
Detection of MDA, superoxide dismutase (SOD) and GSH
HBMECs were incubated with H2O2 or TSG at different concentration for 24 h at 37°C, cells were lysed with extraction buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM glycerophosphate, 1 mM NaVO, 1 µg/ml leupeptin and 1 mM phenylmethylsulfonyl fluoride]. Cell lysates were collected and used in the following kits: MDA assay kit [thiobarbituric acid (TBA) method; catalog no. A003-1], SOD assay kit (WST-1 method; catalog no. A001-3) and GSH assay kit (catalog no. A006-2); the kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Measurement of ROS
The generation of ROS of cells was evaluated by a fluorometric assay using intracellular oxidation of dichlorodihydrofluorescein diacetate (DCFH-DA). The cells were incubated in 6-well plates for 24 h for stabilization at 37°C, and the cells were detected and analyzed using a LSRFortessa flow cytometric instrument (BD Biosciences, Franklin Lakes, NJ, USA) and the CellQuest program (version 5.1; BD Biosciences) with an excitation wavelength of 300 nm and emission wavelength of 610 nm. Briefly, cells were treated with or without H2O2 or TSG. The cells were washed twice with phosphate-buffered saline (PBS) and incubated with 10 µM DCFH-DA in PBS at 37°C for 30 min, in the dark, using the fluorescent probe dihydroethidium (catalog no. S0063; Beyotime Institute of Biotechnology, Haimen, China). Following incubation, the cells were washed with PBS, harvested with trypsin/EDTA and evaluated by flow cytometry.
Comet assay
Briefly, fully frosted slides were precoated on each side with 0.8% agarose (100 ml) in PBS (pH 7.4), covered with a 22×22 mm glass coverslip and left at room temperature for 20 min. Subsequently, 30 ml cell culture was combined with 70 ml of 1% low-melting point agarose in PBS and maintained at 42°C on a dry-bath incubator. The mixture was immediately spread onto each end of a precoated slide and covered with a fresh glass coverslip, and the comets were captured with an Olympus microscope equipped with a charge-coupled device camera connected to a fluorescent microscope.
Measurement of 8-hydroxy-2′-deoxyguanosine (8-OHdG)
Genomic DNA was extracted using an QIAamp DNA isolation kit (cat. no. 12830-50; Qiagen) and 10 µg DNA from cells was incubated with a 8-OHdG ELISA kit (cat. no. ALX-480-090-M001; Enzo Life Sciences, Inc., Farmingdale, NY), according to the manufacturer's instruction.
Measurement of inflammatory cytokines
The levels of TNF-α (cat. no. E-EL-H0109c), IL-6 (catalog no. E-EL-H0102c) and IL-1β (cat. no. E-EL-H0149c) were detected using a bioactive ELISA assay in supernatant according to the manufacturer's instructions (Elabscience Biotechnology Co., Ltd., Wuhan, China).
Western blot analysis
Protein was extracted using NP-40 buffer (Beyotime Institute of Biotechnology). Following 5–10 min boiling, homogenates were centrifuged at 10,000 × g, at 4°C for 10 min to obtain the supernatant. Protein samples (50 µg) were separated by 10% sodium dodecyl sulfate-polyacrylimide gel electrophoresis and transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA) at 80 V for 20 min in spacer gel and 120 V for 90 min in separation gel. Membranes were blocked with 5% (w/v) non-fat milk powder in Tris-buffered saline and 0.1% (w/v) Tween-20, and incubated with the following primary antibodies: TNF-α (cat. no. sc-515765; dilution, 1:1,000), IL-1β (cat. no. sc-1250; dilution, 1:1,000), IL-6 (cat. no. sc-130326; dilution, 1:1,000), nuclear factor (NF)-κB (cat. no. sc-8008; dilution, 1:500) and β-actin (cat. no. sc-130065; dilution, 1:2,000), which were obtained from Santa Cruz Biotechnoogy, Inc. (Dallas, TX, USA; dilution) at 4°C overnight. After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-IgG at room temperature for 2 h. Signal detection was performed using an ECL system (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA).
Statistical analysis
The data from these experiments were reported as means ± standard deviation (SD) for each group. All statistical analyses were performed using PRISM version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey's multiple comparison test as a post-test to compare the group means when the overall P-value was <0.05. P<0.05 was considered to indicate a statistically significant difference.
Results
TSG suppresses H2O2-induced HBMEC growth inhibition
First, the cytotoxicity of H2O2 and TSG were investigated when HBMECs were exposed to H2O2 and TSG at different concentrations for 24 h. As shown in Fig. 1A, a dose-dependent decrease in cell viability was observed when HBMECs were exposed to H2O2. The median lethal concentration of H2O2 was ~300 µM in HBMECs (24-h incubation). Furthermore, HBMEC exposure to various concentrations of TSG alone for 24 h demonstrated no obvious effect on cell viability (Fig. 1B). Conversely, the decrease in HBMEC viability incubation with H2O2 was significantly reversed by TSG treatment at concentrations of 50 and 100 µM (Fig. 1C). These findings indicate that TSG exerts a beneficial effect on H2O2-induced HBMEC growth inhibition in a dose-dependent manner.
Figure 1.
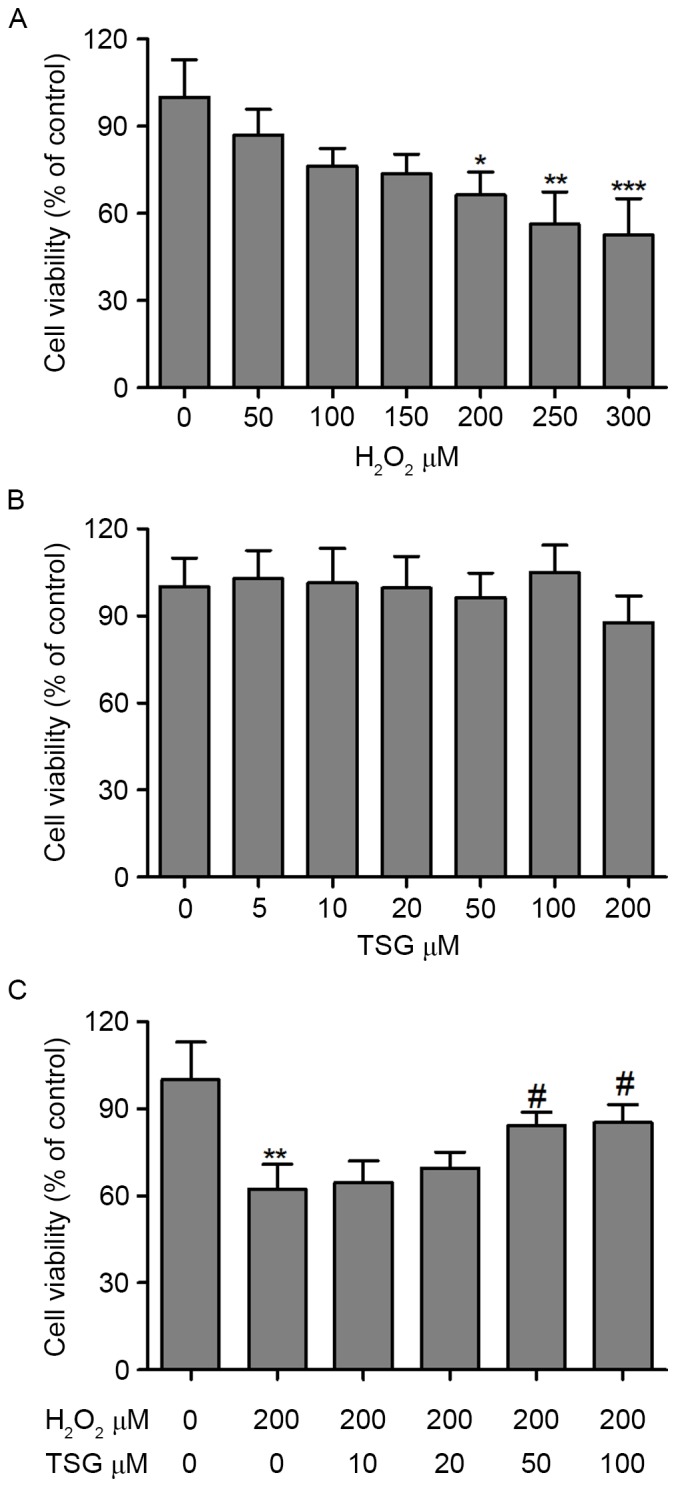
Effects of TSG and H2O2 on cell viability. HBMECs were incubated with (A) H2O2 or (B) TSG at various concentrations for 24 h, and the cell viability was examined using an MTT assay. (C) Cells were incubated with different concentrations of TSG or 200 µM H2O2. Values are expressed as means ± standard deviation (n=3 per group). *P<0.05, **P<0.01 and ***P<0.001 vs. the untreated group; #P<0.05 vs. H2O2 (200 µM) single treatment. TSG, tetrahydroxy stilbene glucoside; HBMEC, human brain microvascular endothelial cell.
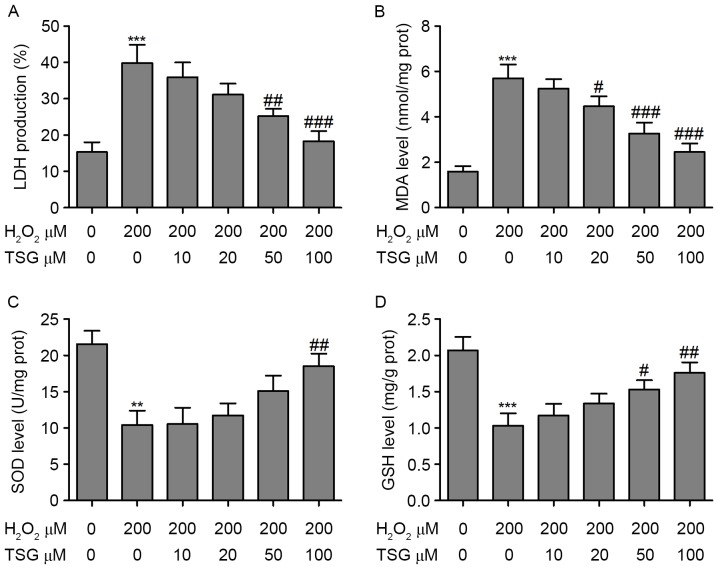
TSG inhibits H2O2-induced oxidative stress in HBMECs
LDH, which is expressed in the cytoplasm, is released when the cell membrane is damaged (15). As shown in Fig. 2A, in the TSG treatment groups with H2O2, supernatant LDH levels significantly decreased with the increase of TSG, which indicated that TSG protects against H2O2-induced membrane damage. MDA is the product of lipid peroxidation of the cell membrane and reflects the severity of oxidative stress (16). In the present study, supernatant MDA levels were detected using the TBA reaction method. The results demonstrated that H2O2 induced the upregulation of MDA in HBMECs; however, TSG treatment markedly reversed the H2O2-induced upregulation of MDA in HBMECs in a dose-dependent manner (Fig. 2B). Furthermore, anti-oxidative markers, SOD and reduced GSH, were measured in HBMECs with or without H2O2 and TSG treatment. Incubation of HBMECs with H2O2 (200 µM) resulted in a significant decrease in SOD and GSH. However, TSG treatment significantly increased the levels of SOD and GSH in H2O2-treated HBMECs (Fig. 2C and D). To identify the role of ROS in HBMEC injury, the ROS concentration was measured by flow cytometry using DCFH-DA. The results demonstrated that H2O2 incubation resulted in a significant increase in the ROS concentration in HBMECs. Notably, the ROS level demonstrated a dose-dependent decline in the TSG treatment groups (Fig. 3).
Figure 2.
Oxidative stress in HBMECs following intervention with H2O2 or TSG. (A) LDH, (B) MDA, (C) SOD and (D) GSH release was measured in HBMECs following incubation with H2O2 or TSG at different concentrations. Values are expressed as means ± standard deviation (n=3 per group). **P<0.01, ***P<0.001 vs. the untreated group; #P<0.05, ##P<0.01, ###P<0.001 vs. H2O2 (200 µM) single treatment. HBMEC, human brain microvascular endothelial cell; TSG, tetrahydroxy stilbene glucoside; LDH, lactate dehydrogenase; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Figure 3.
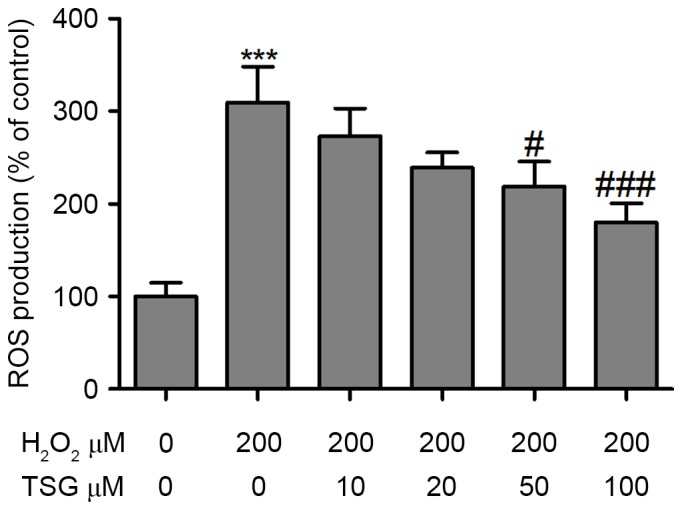
Effect of TSG on H2O2-induced ROS accumulation in HBMECs. HBMECs were incubated with H2O2 or TSG at different concentrations for 24 h, and the ROS concentration was measured by flow cytometry using dichlorodihydrofluorescein diacetate. Values are expressed as means ± standard deviation (n=3 per group). ***P<0.001 vs. the untreated group; #P<0.05, ###P<0.001 vs. H2O2 (200 µM) single treatment. TSG, tetrahydroxy stilbene glucoside; ROS, reactive oxygen species; HBMEC, human brain microvascular endothelial cell.
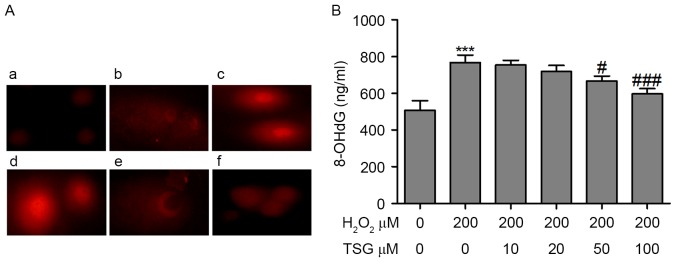
TSG attenuates oxidative stress-mediated DNA damage in HBMECs
A comet assay was performed to determine the oxidative stress-mediated genotoxicity of H2O2 in HBMECs. As shown in Fig. 4A, the olive tail moment (OTM), as the parameter to reflect DNA damage, was significantly greater in the H2O2-treated group than in the blank control group. However, TSG treatment attenuated H2O2-induced DNA damage at concentrations of 50 and 100 µM. A specific product of DNA oxidative damage, 8-OHdG, serves as a marker to evaluate DNA oxidative damage. The 8-OhdG levels in the supernatant were evaluated to reflect the degree of DNA oxidative damage induced by H2O2 treatment. The results demonstrated that H2O2 treatment significantly increased 8-OhdG levels and TSG treatment reduced the content of 8-OhdG in the supernatant of H2O2-treated HBMECs, which indicates that TSG may inhibit oxidative stress-mediated DNA damage (Fig. 4B).
Figure 4.
TSG attenuated oxidative stress-mediated DNA damage in HBMECs. The cell DNA damage was measured using the (A) comet assay; (a) control group, (b) HBMECs treated with H2O2 (200 µM), (c) HBMECs treated with H2O2 (200 µM) and TSG (10 µM), (d) HBMECs treated with H2O2 (200 µM) and TSG (20 µM), (e) HBMECs treated with H2O2 (200 µM) and TSG (50 µM), (f) HBMECs treatment with H2O2 (200 µM) and TSG (100 µM). Magnification, ×400. (B) The 8-OhdG levels in the supernatant of the HBMECs were measured using a DNA damage ELISA kit. Values are expressed as means ± standard deviation (n=3 per group). ***P<0.001 vs. the untreated group; #P<0.05, ###P<0.001 vs. H2O2 (200 µM) single treatment. TSG, tetrahydroxy stilbene glucoside; HBMEC, human brain microvascular endothelial cell; 8-OhdG, 8-hydroxy-2′-deoxyguanosine.
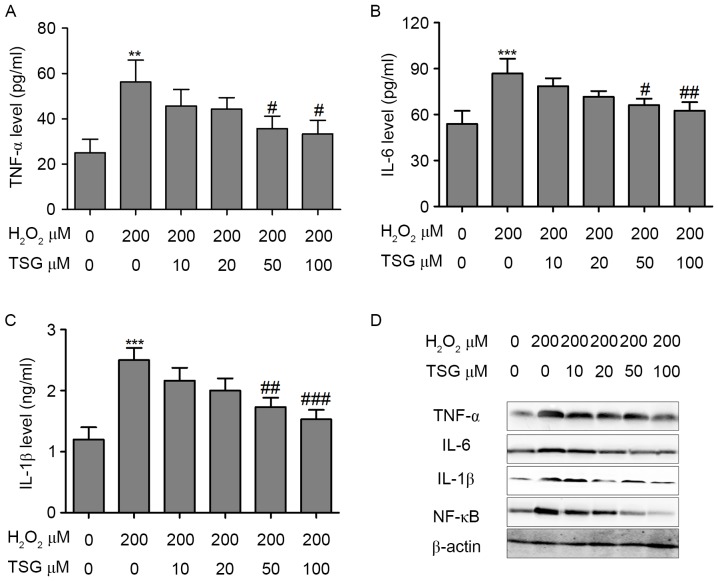
TSG inhibites the H2O2-induced inflammatory response in HBMECs
Previous studies indicate that oxidative stress directly or indirectly induces inflammation response (17,18). TNF-α, IL-6 and IL-1β expression levels in the supernatant were evaluated to reflect the oxidative stress-induced inflammatory response. As shown in Fig. 5A-C, expression levels of TNF-α, IL-6 and IL-1β were significantly increased in the supernatant of H2O2-treated HBMECs. However, TSG induced a dose-dependent decline of TNF-α, IL-6 and IL-1β expression levels in H2O2-treated HBMECs. Furthermore, treatment with TSG efficiently suppressed the H2O2-induced increase in TNF-α, IL-6 and IL-1β protein expression levels (Fig. 5D). Furthermore, whether upstream NF-κB signaling was involved in H2O2-induced inflammatory responses was investigated in the HBMECs. NF-κB, as a transcription factor, is considered to be a major mediator of the inflammatory response, and the inhibition of NF-κB has been shown to alleviate oxidative stress-induced inflammatory responses (19). Therefore, the effect of TSG on the nuclear translocation of NF-κB was evaluated. The results demonstrated that nuclear localized NF-κB was markedly increased in H2O2-treated HBMECs. Conversely, treatment with TSG efficiently inhibited H2O2-induced increase in NF-κB protein expression (Fig. 5D).
Figure 5.
TSG inhibited H2O2-induced inflammatory response in HBMECs. HBMECs were incubated with H2O2 or TSG at different concentrations for 24 h, and (A) TNF-α, (B) IL-6 and (C) IL-1β expression levels in the supernatant of HBMECs were measured using an ELISA kit. (D) The protein expression levels of TNF-α, IL-6, IL-1β and NF-κB were analyzed by western blotting. Values are expressed as means ± standard deviation (n=3 per group). **P<0.01, ***P<0.001 vs. the untreated group; #P<0.05, ##P<0.01, ###P<0.001 vs. H2O2 (200 µM) single treatment. TSG, tetrahydroxy stilbene glucoside; HBMEC, human brain microvascular endothelial cell; TNF, tumor necrosis factor; IL, interleukin; NF-κB, nuclear factor-κB.
Discussion
In the present study, an experimental cell model was used to observe the effects of TSG on H2O2-induced HBMEC dysfunction. It was found that H2O2-induced injury was associated with ROS accumulation and enhanced expression levels of inflammatory cytokines, including TNF-α, IL-6 and IL-1β, in the cultured HBMECs, which were attenuated by TSG treatment. Furthermore, TSG may inhibit oxidative stress-mediated DNA damage in HBMECs. In addition, the findings indicated that TSG may inhibit NF-κB protein expression levels, which, as an upstream transcription factor, may regulate the inflammatory response.
It is well known that neurodegeneration is associated with upregulated levels of oxidative stress (20). Localized increases in the levels of oxidative stress in the cerebrovasculature may be sufficient to promote deleterious changes in blood-flow, BBB integrity and serve as an initiator of numerous types of pathogenesis in the brain (5,21). A previous study indicates that cerebral vascular endothelial cells may exert a protective role in various brain injuries by releasing antioxidants, such as SOD and GSH (22). The present study observed the cytotoxicity and genotoxicity of H2O2 in HBMECs, and investigated whether TSG decreased endothelial cell injury, with the aim of protecting people from neurodegeneration. In order to observe the unknown cytotoxicity of H2O2 and TSG on HBMECs, the MTT assay was conducted to determine cell viability. The findings demonstrated that H2O2 exerted a cytotoxic effect on HBMECs, whereas the effect of TSG was nontoxic. Notably, TSG inhibited H2O2-induced HBMEC cytotoxicity in a dose-dependent manner. Furthermore, the findings indicated that TSG restricted the oxidative stress caused by H2O2 by inhibition of MDA and ROS, and upregulation of SOD and GSH. MDA is excessively generated following ROS-mediated lipid peroxidation, which induces cell membrane damage by accelerating MDA-low density lipoprotein adduct production (15). SOD is a specific antioxidant enzyme that is critical to inhibiting oxidative stress (23). Cellular SOD reduces O2− to H2O2, then catalase reduces H2O2 to water and molecular oxygen in order to minimize oxidative damage (15,24). Based on these studies, the present study concluded that TSG exhibited a significant antioxidant capacity in H2O2-treated HBMECs.
A previous study indicates that H2O2-induced chromatin degradation efficiently dismantles the genome leading to cell death (25). In the present study, the level of DNA damage was assessed as a biomarker of H2O2-induced oxidative stress in HBMECs. Collectively, these data indicated that H2O2 induced DNA damage in HBMECs; however, TSG treatment reduced the content of 8-OhdG and OTM in H2O2-treated HBMECs, which indicated that TSG inhibits oxidative stress-mediated DNA damage.
TSG, as a natural active component, exerts anti-inflam-matory effects on acute edema inflammation by downregulating cyclooxygenase-2 expression (26). In experimental colitis mice, TSG alleviated the oxygen and nitrogen free radical levels, downregulated inducible nitric oxide synthase expression and suppressed the inflammatory response (27). A previous study demonstrated that TSG improves the inflammatory reactions of the brain in dementia mice by reducing IL-6 expression levels (26). This finding indicated that TSG may exert protective effects on inflammatory disease. In the present study, the results demonstrated that TSG induced a dose-dependent decline of TNF-α, IL-6 and IL-1β expression levels in H2O2-treated HBMECs. In addition, TSG inhibited NF-κB protein expression levels, which, as an upstream transcription factor, may regulate the inflammatory response. Therefore, it was hypothesized that the protective effects of TSG were associated with inhibiting oxidative stress and inflammatory responses in H2O2-treated HBMECs.
In conclusion, TSG may be present as a suppressor of oxidative stress and inflammatory responses in HBMECs; thus, the appropriate dosage of TSG may contribute to preventing the H2O2-induced endothelial cell dysfunction. Further studies may be required to assess the therapeutic effect of TSG in a human brain microvascular endothelial dysfunction-associated animal model.
References
- 1.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YF, Gu YT, Qin GH, Zhong L, Meng YN. Curcumin ameliorates the permeability of the blood-brain barrier during hypoxia by upregulating heme oxygenase-1 expression in brain microvascular endothelial cells. J Mol Neurosci. 2013;51:344–351. doi: 10.1007/s12031-013-9989-4. [DOI] [PubMed] [Google Scholar]
- 5.Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: Regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta. 2012;1822:822–829. doi: 10.1016/j.bbadis.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AA, Dusting GJ, Roulston CL, Sobey CG. NADPH-oxidase activity is elevated in penumbral and non-ischemic cerebral arteries following stroke. Brain Res. 2006;1111:111–116. doi: 10.1016/j.brainres.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 7.Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK, Sobey CG. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci (Landmark Ed) 2011;16:1733–1745. doi: 10.2741/3816. [DOI] [PubMed] [Google Scholar]
- 8.Tao L, Li X, Zhang L, Tian J, Li X, Sun X, Li X, Jiang L, Zhang X, Chen J. Protective effect of tetrahydroxystilbene glucoside on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. PLoS One. 2011;6:e26055. doi: 10.1371/journal.pone.0026055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J, Zheng L, He YS, Li HJ. Hepatotoxic assessment of Polygoni Multiflori Radix extract and toxicokinetic study of stilbene glucoside and anthraquinones in rats. J Ethnopharmacol. 2015;162:61–68. doi: 10.1016/j.jep.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Li Y, Chen J, Sun J, Li X, Sun X, Kang X. Tetrahydroxystilbene glucoside attenuates MPP+-induced apoptosis in PC12 cells by inhibiting ROS generation and modulating JNK activation. Neurosci Lett. 2010;483:1–5. doi: 10.1016/j.neulet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Hur J, Kim S, Lee P, Lee YM, Choi SY. The protective effects of oxyresveratrol imine derivative against hydrogen peroxide-induced cell death in PC12 cells. Free Radic Res. 2013;47:212–218. doi: 10.3109/10715762.2012.762769. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JK, Yang L, Meng GL, Fan J, Chen JZ, He QZ, Chen S, Fan JZ, Luo ZJ, Liu J. Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur J Pharmacol. 2012;689:31–37. doi: 10.1016/j.ejphar.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Luo HB, Yang JS, Shi XQ, Fu XF, Yang QD. Tetrahydroxy stilbene glucoside reduces the cognitive impairment and overexpression of amyloid precursor protein induced by aluminum exposure. Neurosci Bull. 2009;25:391–396. doi: 10.1007/s12264-009-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo H, Li Y, Guo J, Liu Z, Zhang Z, Wang Y, Liu Z, Shi X. Tetrahydroxy stilbene glucoside improved the behavioral disorders of APP695V717I transgenic mice by inhibiting the expression of Beclin-1 and LC3-II. J Tradit Chin Med. 2015;35:295–300. doi: 10.1016/S0254-6272(15)30100-X. [DOI] [PubMed] [Google Scholar]
- 15.Bo L, Jiang S, Xie Y, Kan H, Song W, Zhao J. Effect of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS One. 2016;11:e0152216. doi: 10.1371/journal.pone.0152216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Zhang YJ, Liu WW, Shi AW, Gu N. Salidroside suppresses HUVECs cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules. 2016;21 doi: 10.3390/molecules21081033. pii: E1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94:1167–1184. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aristoteles LR, Righetti RF, Pinheiro NM, Franco RB, Starling CM, da Silva JC, Pigati PA, Caperuto LC, Prado CM, Dolhnikoff M, et al. Modulation of the oscillatory mechanics of lung tissue and the oxidative stress response induced by arginase inhibition in a chronic allergic inflammation model. BMC Pulm Med. 2013;13:52. doi: 10.1186/1471-2466-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu Y, Tao L, Lei C, Wang J, Yang P, Li Q, Lei B. Downregulating p22phox ameliorates inflammatory response in Angiotensin II-induced oxidative stress by regulating MAPK and NF-κB pathways in ARPE-19 cells. Sci Rep. 2015;5:14362. doi: 10.1038/srep14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- 21.Favit A, Grimaldi M, Alkon DL. Prevention of beta-amyloid neurotoxicity by blockade of the ubiquitin-proteasome proteolytic pathway. J Neurochem. 2000;75:1258–1263. doi: 10.1046/j.1471-4159.2000.0751258.x. [DOI] [PubMed] [Google Scholar]
- 22.Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 23.Perveen S, Patel H, Arif A, Younis S, Codipilly CN, Ahmed M. Role of EC-SOD overexpression in preserving pulmonary angiogenesis inhibited by oxidative stress. PLoS One. 2012;7:e51945. doi: 10.1371/journal.pone.0051945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bivalacqua TJ, Armstrong JS, Biggerstaff J, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, Champion HC. Gene transfer of extracellular SOD to the penis reduces O2-* and improves erectile function in aged rats. Am J Physiol Heart Circ Physiol. 2003;284:H1408–H1421. doi: 10.1152/ajpheart.00770.2002. [DOI] [PubMed] [Google Scholar]
- 25.Konat GW. H2O2-induced higher order chromatin degradation: A novel mechanism of oxidative genotoxicity. J Biosci. 2003;28:57–60. doi: 10.1007/BF02970132. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YZ, Shen JF, Xu JY, Xiao JH, Wang JL. Inhibitory effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside on experimental inflammation and cyclooxygenase 2 activity. J Asian Nat Prod Res. 2007;9:355–363. doi: 10.1080/10286020600727772. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Zhao L, Han T, Chen S, Wang J. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur J Pharmacol. 2008;578:339–348. doi: 10.1016/j.ejphar.2007.09.013. [DOI] [PubMed] [Google Scholar]