Abstract
Laryngeal squamous cell carcinoma (LSCC), the most common form of laryngeal carcinoma, is an aggressive malignancy that demonstrates the second highest rate of morbidity of all head and neck squamous cell carcinomas. The abnormal expression of microRNAs (miRs) has been demonstrated in a number of types of human cancer, and they have been demonstrated to be oncogenes or tumour suppressor genes. miR-503 has been studied in various types of human cancer; however, the expression level, roles and underlying mechanisms in LSCC remain unknown. In the present study, it was demonstrated that miR-503 was significantly upregulated in LSCC tissues and cell lines. The level of miR-503 in LSCC tissues was correlated with thyroid cartilage invasion, lymph node metastasis, and tumour, node and metastasis stage. In addition, down-regulation of miR-503 inhibited cell proliferation and invasion in LSCC. Programmed cell death protein 4 (PDCD4) was identified to be a direct target gene of miR-503. PDCD4 overexpression could mimic the roles of miR-503 underexpression in LSCC. Furthermore, PDCD4 was down-regulated in LSCC tissues and this correlated with the miR-503 expression level. In conclusion, these results suggested that miR-503 promotes tumour growth and invasion by directly targeting PDCD4. The identification of the miR-503/PDCD4 axis may provide novel targets for LSCC treatment and improve prognosis.
Keywords: laryngeal squamous cell carcinoma, programmed cell death protein 4, microRNA-503, prognosis, therapy
Introduction
Head and neck cell carcinoma represents the seventh most common type of cancer in the world, which is comprised of oral cavity, oropharyngeal, hypopharyngeal and laryngeal carcinomas (1,2). Laryngeal squamous cell carcinoma (LSCC), the most common form of laryngeal carcinoma, accounts for ~25% of all head and neck squamous cell carcinomas (3). LSCC is an aggressive malignancy that has the second highest rate of morbidity of all head and neck squamous cell carcinomas, particularly in the northern area of China (4). Currently, the aetiology of LSCC remains poorly understood. It is hypothesised that smoking, drinking alcohol, exposure to harmful dust and human papilloma virus infection is involved in the initiation and progression of LSCC (5). Despite the development of therapeutic treatments for LSCC, including surgery, chemotherapy and radiotherapy, the 5-year overall survival rate of patients with this disease has not significantly improved over the past 10 years (6).
Therefore, a complete understanding of the mechanisms underlying carcinogenesis and progression in LSCC may provide novel therapeutic strategies to improve the prognosis of patients with LSCC.
MicroRNAs (miRs) are a group of endogenous, short and noncoding RNAs that negatively regulate gene expression through base pairing with the 3′-untranslated regions (3′UTRs) of their target genes forming a stable duplex through Watson-Crick complementarities (7,8). By recruiting and incorporating with the RNA-induced silencing complex, translation of the target mRNA transcript is inhibited and/or the mRNA is subject to degradation (9,10). Thus far, >900 mature miRs have been identified in the human genome and may modulate the expression level of >30% protein-coding genes in humans (11). Numerous studies have demonstrated that miR serves a significant role in diverse biological processes, including cell proliferation, cell cycle progression, apoptosis, survival, motility, invasion, metastasis, angiogenesis and morphogenesis (12–14). In addition, the abnormal expression of miRs has been demonstrated in a number of types of human cancer, as they have been demonstrated to be oncogenes or tumour suppressor genes (15,16). These results suggested that miRs may be novel therapeutic targets for cancer therapy.
miR-503 has been studied in various types of human cancer (17–20); however, the expression level, role and underlying mechanism in LSCC remains unknown. The purpose of the present study is to investigate the expression level and biological roles of miR-503 in regulation of LSCC cell proliferation and invasion. Another aim is to investigate the underlying mechanism of miR-503 in LSCC.
Materials and methods
Tissue samples
The present study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). Informed consent was also obtained from all patients with LSCC prior to tissue sample collection. LSCC tissues and adjacent normal tissues were obtained from 48 patients who were treated with total or partial laryngectomy at Tianjin Medical University Cancer Institute and Hospital between February 2012 and November 2014. The age and sex distribution of patients involved in the present study is illustrated in Table I. None of these patients received chemotherapy or radiotherapy prior to surgery. All tissues were immediately frozen in liquid nitrogen and then stored at −80°C.
Table I.
Association between miR-503 expression level and clinicopathological features of laryngeal squamous cell carcinoma.
| miR-503 expression level | ||||
|---|---|---|---|---|
| Clinical features | Cases | High | Low | P-value |
| Age, years | 0.776 | |||
| <60 | 22 | 13 | 9 | |
| ≥60 | 26 | 14 | 12 | |
| Sex distribution | 0.560 | |||
| Male | 20 | 10 | 10 | |
| Female | 28 | 17 | 11 | |
| Primary location | 0.383 | |||
| Supraglottic | 23 | 11 | 12 | |
| Glottic | 25 | 16 | 9 | |
| Thyroid cartilage invasion | 0.023 | |||
| No | 25 | 10 | 15 | |
| Yes | 23 | 17 | 6 | |
| Pathological differentiation | 0.771 | |||
| Moderate-high | 28 | 15 | 13 | |
| Poor | 20 | 12 | 8 | |
| Lymph node metastasis | 0.019 | |||
| Negative | 24 | 9 | 15 | |
| Positive | 24 | 18 | 6 | |
| TNM stage | 0.040 | |||
| I–II | 21 | 8 | 13 | |
| III–IV | 27 | 19 | 8 | |
TMN, tumour node and metastasis.
Cell lines and culture condition
LSCC (AMC-HN-8 and Tu-177), normal human keratinocyte (HaCaT) and human embryonic kidney (HEK293T) cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% foetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin) at 37°C in a humidified atmosphere with 5% CO2.
Transient transfection
Cells were seeded into 6-well plates at a density of 60–70% confluence in DMEM containing 10% FBS without antibiotics at 37°C. Following overnight incubation, cells were transfected with an miR-503 inhibitor (100 pmol), an miR inhibitor negative control (NC) inhibitor (100 pmol), pcDNA3.1-programmed cell death protein 4 (PDCD4; 2,500 ng) or pcDNA3.1 (all Chinese Academy of Sciences, Changchun, Jilin, China) using the Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The miR-503 inhibitor sequence was 5′-CUGCAGAACUGUUCCCGCUGCUA-3′ and the NC inhibitor sequence was 5′-ACUACUGAGUGACAGUAGA-3′. Following 6–8 h incubation, the medium was replaced with a culture medium containing 10% FBS. The Cell Counting kit-8 (CCK8) assay, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), Transwell invasion assay and western blot analysis were performed at 24, 48, 48 and 72 h post-transfection, respectively.
RT-qPCR
Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) from cells and tissues. The total concentration of RNA was detected using an ND-2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA), according to the manufacturer's protocol. The relative expression of miR-503 was determined using the One Step SYBR® PrimeScript™ miRNA RT-PCR kit (Takara Bio, Inc., Otsu, Japan). The thermocycling conditions for qPCR were as follows: 42°C for 5 min, 95°C for 10 sec, followed by 40 cycles of 95°C for 5 sec, 55°C for 30 sec and 72°C for 30 sec. For PDCD4 mRNA expression, reverse transcription was performed using the Moloney murine leukaemia virus reverse transcriptase (M-MLV RT; Promega Corporation, Madison, WI, USA). The temperature protocol for reverse transcription was as follows: 95°C for 2 min, followed by 20 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 2 min, then a final extension at 72°C for 5 min. This reaction included 1 µl Oligo (dT)12–18 (500 µg/ml), 2 µl total RNA, 1 µl 10 mM dNTP Mix, 4 µl 5X First-Strand Buffer, 2 µl 0.1 M DTT, 1 µl RNaseOUT™ Recombinant Ribonuclease Inhibitor (40 units/µl), 1 µl (200 units) of M-MLV RT and 7 µl distilled water. Relative expressions of PDCD4 mRNA were measured using SYBR Premix Ex Taq™ kits (Takara Bio Inc.). Amplification was performed with the following thermocycling conditions: 5 min at 95°C, followed by 40 cycles of 95°C for 30 sec and 65°C for 45 sec. The expressions of miR-503 and PDCD4 mRNA were normalised against the relative expression of RNU6-1 and GAPDH, respectively. The primers were designed as follows: miR-503, 5′-GCGTAGCAGCGGGAACAGT-3′ (forward) and 5′-CCAGTGCGTGTCGTGGAGT-3′ (reverse); U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse); PDCD4, 5′-AAAGGCGACTAAGGAAAAACTCATC-3′ (forward) and 5′-GCCTATCCAGCAACCTTCCCT-3′ (reverse); and GAPDH, 5′-AGTGCCAGCCTCGTCTCATAG-3′ (forward) and 5′-CGTTGAACTTGCCGTGGGTAG-3′ (reverse). Relative expression levels were calculated according to the 2−∆∆Cq method (21).
CCK8 assay
Transfected cells were collected and re-seeded into 96-well plates (1×103 cells/well). The CCK8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay was performed at various time points (24, 36, 48 and 72 h). In total, 10 µl CCK8 solution was added to each well and incubated at 37°C for an additional 4 h. The absorbance values at 450 nm were detected using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transwell® invasion assay
The invasion assay was performed using Transwell chambers (8-µm pore size; BD Biosciences, Franklin Lakes, NJ, USA) pre-coated with a Matrigel® matrix (BD Biosciences). Transfected cells were harvested at 48 h post transfection. The upper chamber was seeded with 5×104 cells in 300 µl FBS-free culture medium. Then 500 µl culture medium containing 20% FBS was added into the lower chamber. Following 48 h incubation at 37°C, cells remaining on the top of the chamber were wiped away with cotton wool. The invading cells were fixed in 4% paraformaldehyde for 20 min at room temperature, stained with 0.5% crystal violet for 10 min at room temperature, washed with PBS and counted under an inverted microscope (5 fields/chamber). Each assay was repeated in three independent experiments.
Bioinformatic analysis
The potential target genes of miR-503 were analysed using the algorithms from TargetScan (targetscan.org), PicTar (pictar.mdc-berlin.de) and miRanda (www.microrna.org/microrna/home.do).
Western blot analysis
Total protein was isolated using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China). A BCA assay (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) was used to measure total protein concentration. Equal amounts of protein (20 µg/lane) were separated via SDS-PAGE on 10% gels, transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA) and then blocked in 5% non-fat milk for 1 h at room temperature. The membranes were incubated with mouse anti-human monoclonal PDCD4 antibody (1:1,000; cat. no. sc-376430; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse anti-human monoclonal GADPH antibody (1:1,000; cat. no. sc-166574; Santa Cruz Biotechnology, Inc.), at 4°C overnight. Following washing, the membranes were incubated with goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. sc-2005; 1:2,000; Santa Cruz Biotechnology, Inc.) at room temperature for 2 h. Protein bands were visualised using the EMD Millipore Immobilon™ Western Chemiluminescent HRP Substrate (EMD Millipore).
Luciferase reporter assay
HEK293T cells were seeded into 24-well plates and co-transfected with pmirGLO-PDCD4-3′UTR wild type (Wt) or pmirGLO-PDCD4-3′UTR mutant (Mut) (both Chang Jing Bio-Tech, Ltd., Changsha, China), and an miR-503 or NC inhibitor, using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Cells were harvested 48 h post-transfection. Luciferase activity was determined using Dual-Luciferase® Reporter Assay system (Promega Corporation) according to the manufacturer's protocol. Firefly luciferase activity was normalised to the Renilla luciferase activity.
Statistical analysis
Data were expressed as the mean ± standard deviation and analysed using SPSS (version 15.0; SPSS Inc., Chicago, IL, USA). Statistical comparisons between groups were analysed using Student's t-test or one-way analysis of variance (ANOVA) plus multiple comparisons. Student-Newman-Keuls test was used as a post hoc test following the ANOVA. Spearman's rank correlation coefficient analysis was used to investigate the association between miR-503 and PCDC4 in LSCC tissues. P<0.05 was considered to indicate a statistically significance difference.
Results
miR-503 is upregulated in LSCC tissues and cell lines
miR-503 expression was measured in LSCC and the adjacent normal tissues by RT-qPCR. The results demonstrated that miR-503 was significantly upregulated in the LSCC tissues compared with the adjacent normal tissues (P<0.05; Fig. 1A). The expression level of miR-503 in the LSCC cell lines was significantly increased compared with the HaCaT cell line (P<0.05; Fig. 1B). These results suggested that miR-503 serves an important role in LSCC carcinogenesis and progression.
Figure 1.
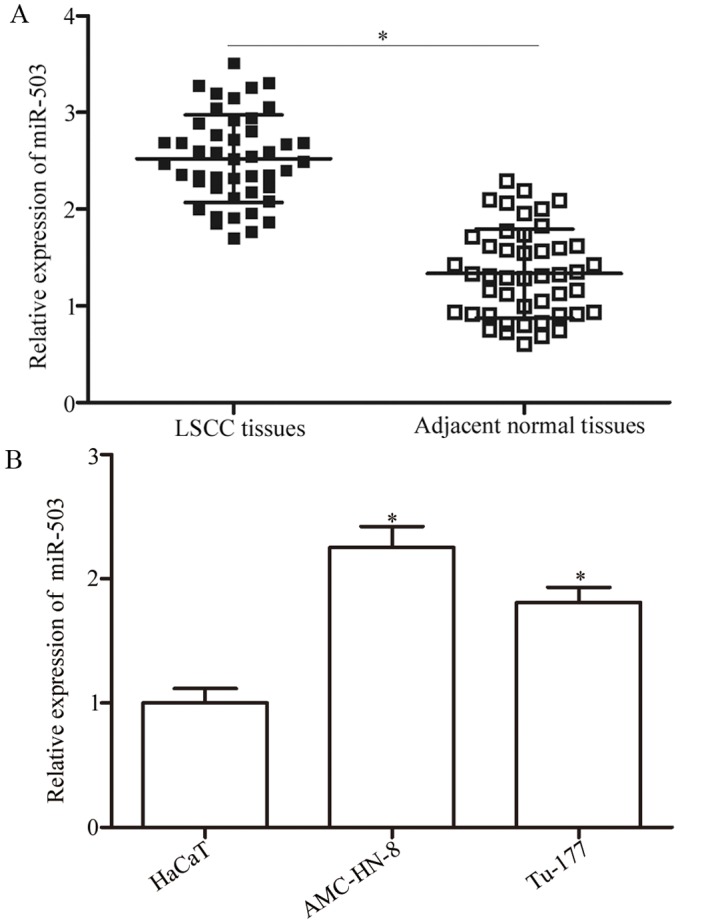
Analysis of the expression levels of miR-503 in LSCC tissues and cell lines. (A) The relative expression levels of miR-503 in LSCC tissues and adjacent normal tissues measured using the reverse transcription-quantitative polymerase chain reaction. (B) miR-503 expression in HaCaT, AMC-HN-8 and Tu-177 cell lines. *P<0.05 vs. the respective control. LSCC, laryngeal squamous cell carcinoma; miR, microRNA.
Association between miR-503 expression and LSCC clinicopathological features
The association between miR-503 expression and the clinicopathological features of LSCC patients was investigated. As illustrated in Table I, miR-503 expression level in LSCC demonstrated a significant association with thyroid cartilage invasion (P=0.023), lymph node metastasis (P=0.019) and tumour, node and metastasis (TNM) stage (P=0.040). However, no significant association was identified between the expression of miR-503 and other clinical features including age (P=0.776), sex (P=0.560), primary location (P=0.383) and pathological differentiation (P=0.771).
miR-503 regulates cellular proliferation and invasion of LSCC in vitro
To evaluate the roles of miR-503 in LSCC, its expression was silenced in AMC-HN-8 cells. AMC-HN-8 cells were transfected with a miR-503 inhibitor or a NC inhibitor. As illustrated in Fig. 2A, there was decreased expression of miR-503 in the miR-503 inhibitor-transfected AMC-HN-8 cells (P<0.05). The results of the CCK8 assay indicated that down-regulation of miR-503 inhibited the proliferation of AMC-HN-8 cells at 72 h (P<005; Fig. 2B). In addition, significantly fewer invasive cells were observed in the AMC-HN-8 cells transfected with the miR-503 inhibitor (P<005; Fig. 2C). Therefore, miR-503 acts as an oncogene in LSCC.
Figure 2.
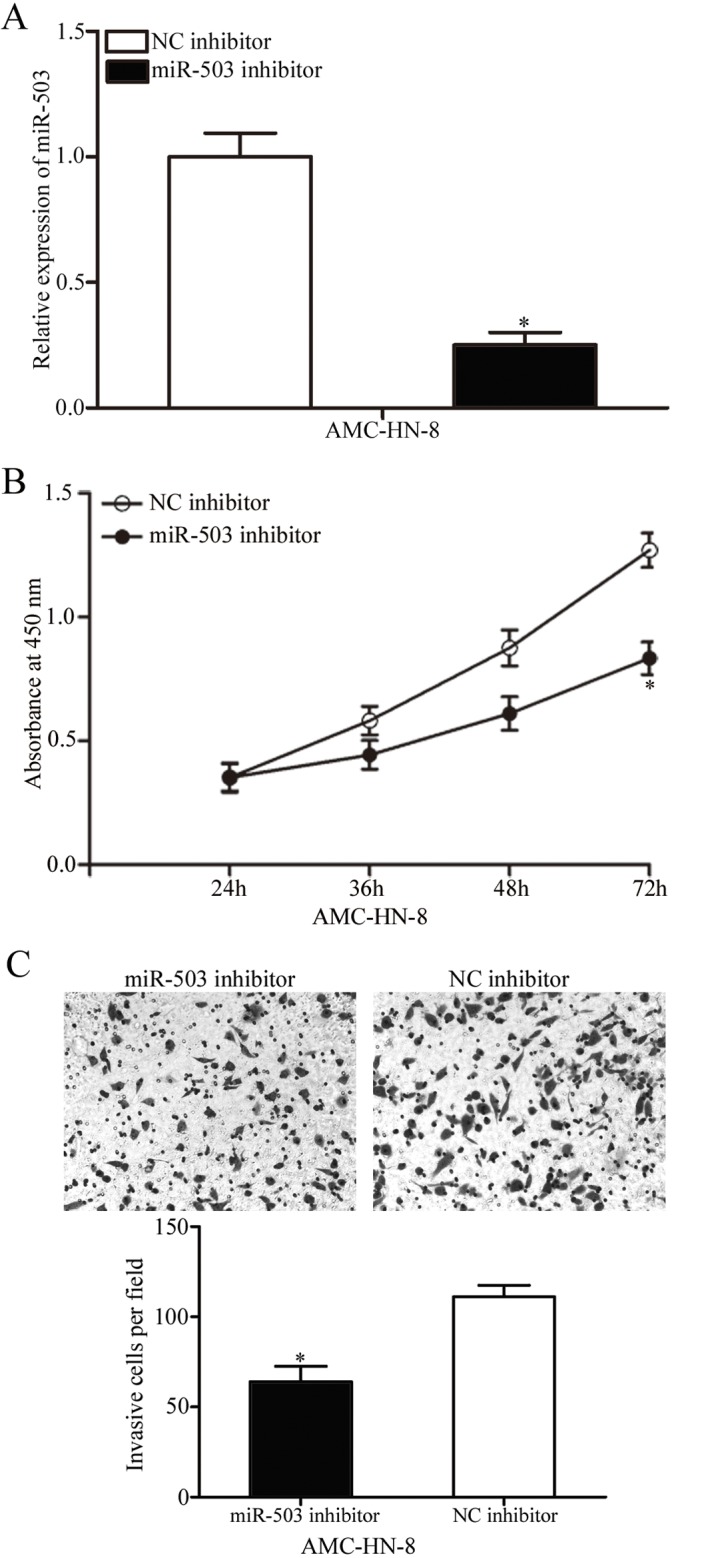
Investigation of miR-503 inhibition in AMC-HN-8 cells. (A) Relative expression of miR-503 in AMC-HN-8 cells transfected with a miR-503 or NC inhibitor. (B) Absorbance at 450 nm of AMC-HN-8 cells transfected with a miR-503 or NC inhibitor. (C) Cell invasive ability of AMC-HN-8 cells transfected with a miR-503 or NC inhibitor was evaluated using a Transwell invasion assay (magnification, ×200). *P<0.05 vs. the respective control. miR, microRNA; NC, negative control.
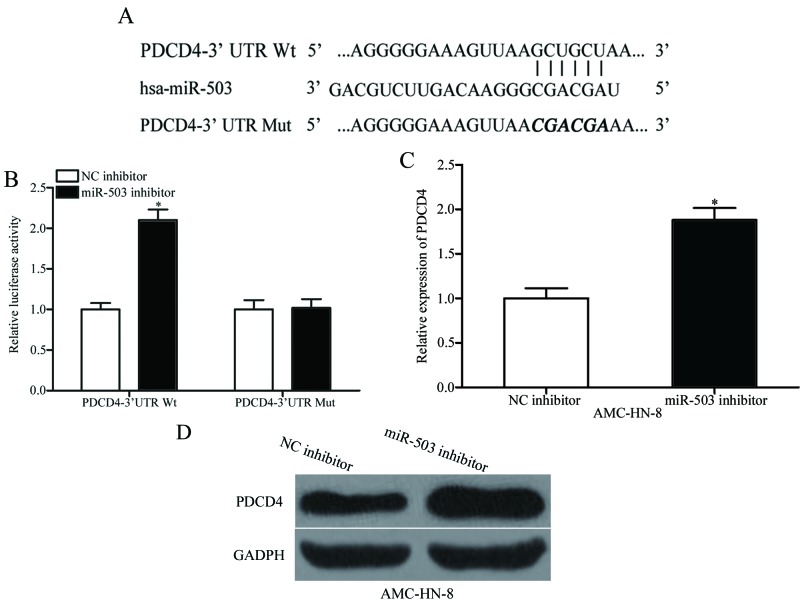
PDCD4 is a direct target of miR-503
The function of miRs in human cancer is dependent on their target genes, so it is important to identify the targets of miR-503. Bioinformatic analysis was used to analyse the potential targets. As illustrated in Fig. 3A, a highly-conserved miR-503 targeting sequence was predicted in the 3′UTR of PDCD4, which indicates that PDCD4 is a potential target of miR-503. To confirm this hypothesis, a luciferase reporter assay was performed in HEK293T cells transfected with pmirGLO-PDCD4-3′UTR Wt or pmirGLO-PDCD4-3′UTR Mut, along with a miR-503 or NC inhibitor. The results indicated that a significant upregulation of luciferase activity was observed in the presence of the miR-503 inhibitor in the HEK293T cells co-transfected with the pmirGLO-PDCD4-3′UTR Wt (P<0.05), but not with the pmirGLO-PDCD4-3′UTR Mut (Fig. 3B). RT-qPCR and western blotting were then performed to detect PDCD4 expression in AMC-HN-8 cells transfected with miR-503 or NC inhibitor. As demonstrated in Fig. 3C and D, down-regulation of miR-503 increased PDCD4 expression at the mRNA (P<0.05) and protein level in AMC-HN-8 cells. These results suggested that miR-503 negatively regulates PDCD4 expression through directly binding to the 3′UTR of PDCD4 gene.
Figure 3.
Investigation of the interaction between PDCD4 and miR-503. (A) The putative miR-503-binding sites in the 3′UTR of PDCD4 and the corresponding mutant binding sites. (B) The relative luciferase activity of luciferase reporter vectors containing Wt or Mut PDCD4-3′UTR in HEK293T cells, which were co-transfected with the miR-503 inhibitor or NC inhibitor. (C) The mRNA levels of PDCD4 in AMC-HN-8 cells transfected with miR-503 inhibitor or NC inhibitor. (D) The protein levels of PDCD4 determined by western blot analysis in AMC-HN-8 cells transfected with miR-503 inhibitor or NC inhibitor. *P<0.05 vs. the respective control. miR, microRNA; NC, negative control; PDCD4, programmed cell death protein 4; UTR, untranslated region; hsa, homo sapiens; Mut, mutant; Wt, wild type.
PDCD4 overexpression exhibits similar effects to that of miR-503 underexpression in LSCC cells
The above results indicated that miR-503 negatively regulated PDCD4 expression and was involved in LSCC progression. Therefore, it was hypothesised that the effects of the down-regulation of miR-503 on AMC-HN-8 cells would be exhibited by PDCD4 re-expression. To confirm this hypothesis, pcDNA3.1-PDCD4 was transfected into AMC-HN-8 cells to increase PDCD4 protein expression (Fig. 4A). The CCK8 and transwell invasion assays demonstrated that increasing expression of PDCD4 inhibited the proliferation at 72 h (P<0.05; Fig. 4B) and invasion (P<0.05; Fig. 4C) of AMC-HN-8 cells. These results further demonstrated that PDCD4 is a direct functional target of miR-503 in LSCC.
Figure 4.
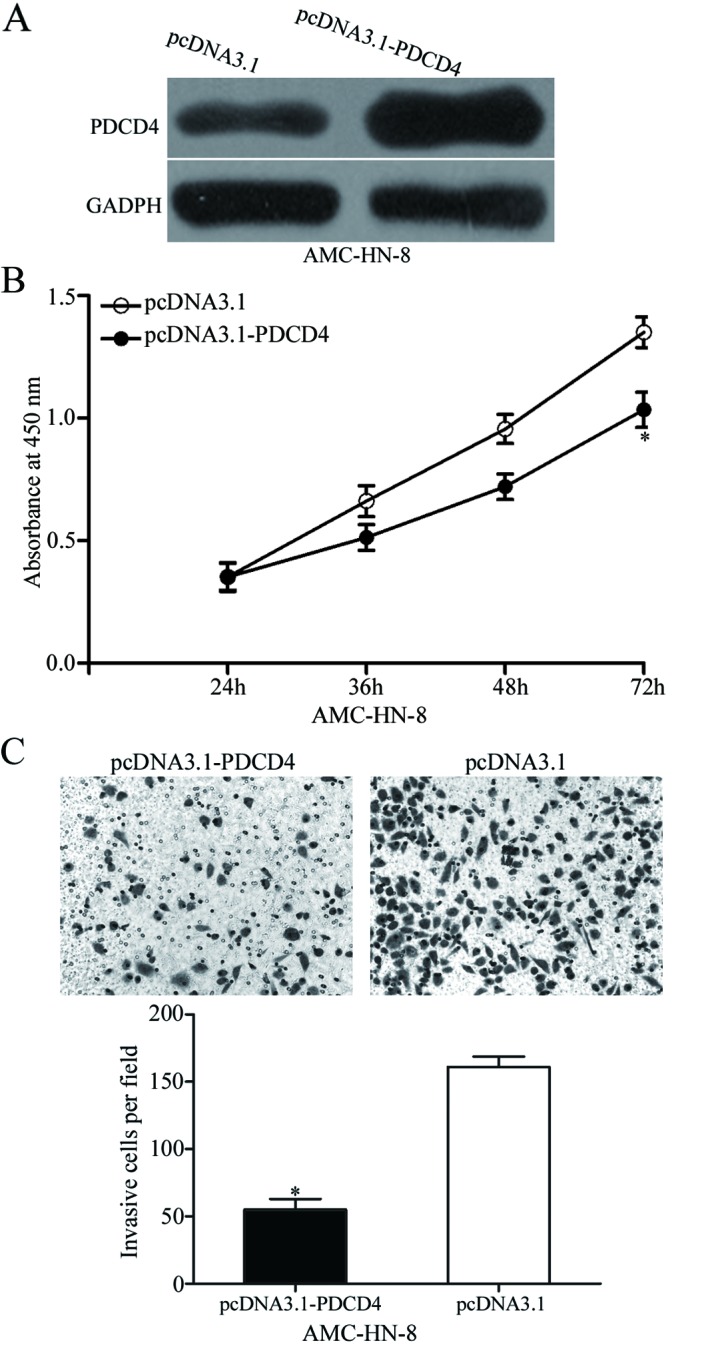
PDCD4 overexpression inhibits cell proliferation and invasion in laryngeal squamous cell carcinoma. (A) Western blot analysis was performed to measure PDCD4 protein expression in AMC-HN-8 cells transfected with pcDNA3.1-PDCD4 or pcDNA3.1. (B) Absorbance at 450 nm of AMC-HN-8 cells transfected with pcDNA3.1-PDCD4 or pcDNA3.1 (C) Transwell invasion assay to evaluate the invasion ability of AMC-HN-8 cells transfected with pcDNA3.1-PDCD4 or pcDNA3.1 (magnification, ×200). *P<0.05 vs. the respective control. miR, microRNA; PDCD4, programmed cell death protein 4.
PDCD4 is downregulated in LSCC tissues and is inversely correlated with miR-503 expression
PDCD4 mRNA in LSCC and adjacent normal tissues was detected using RT-qPCR. The results demonstrated that PDCD4 mRNA in LSCC tissues was significantly down-regulated compared with the adjacent normal tissues (Fig. 5A; P<0.05). Spearman's rank correlation coefficient analysis illustrated that that the expression level of PDCD4 was inversely correlated with miR-503 expression in LSCC tissues (r = −0.6021; P<0.001; Fig. 5B). Therefore, low PDCD4 mRNA expression in LSCC tissues was correlated with increased miR-503 expression.
Figure 5.
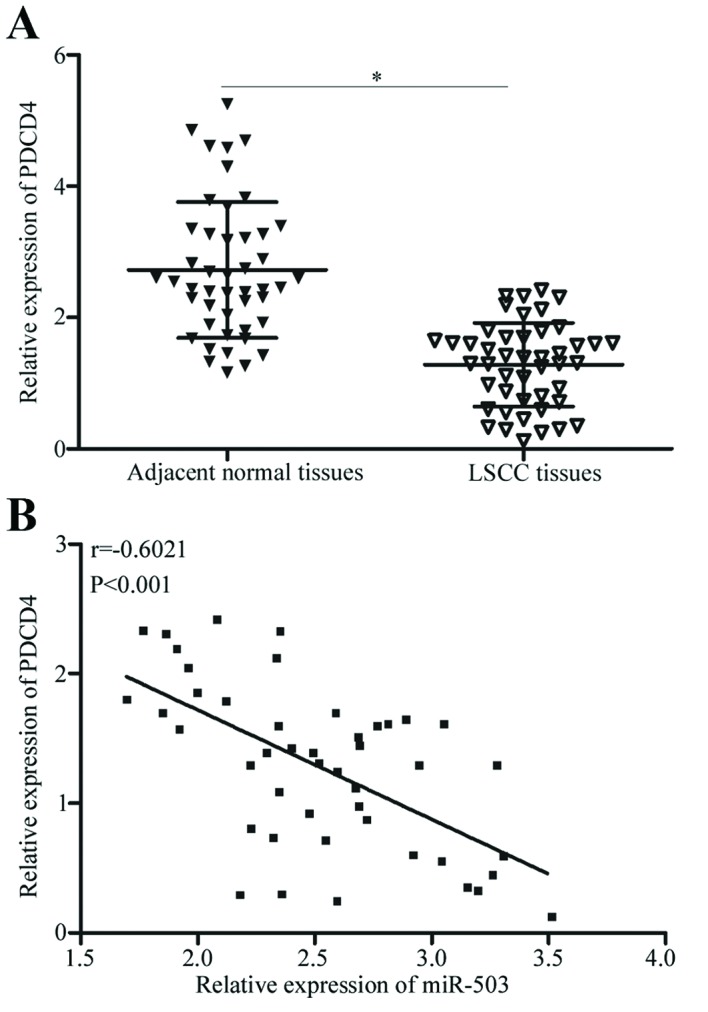
PDCD4 association with miR-503 expression in LSCC tissues. (A) The relative expression of PDCD4 mRNA in LSCC tissues and adjacent normal tissues was measured using reverse transcription-quantitative polymerase chain reaction. (B) The correlation between PDCD4 mRNA and miR-503 expression level was analysed in LSCC tissues by Spearman's rank correlation coefficient analysis. *P<0.05 vs. the respective control. LSCC, laryngeal squamous cell carcinoma; miR, microRNA; PDCD4, programmed cell death protein 4.
Discussion
In the past two decades, a number of miRNAs were demonstrated to serve important roles in cellular processes, including proliferation, differentiation, metastasis, tumourigenesis and morphogenesis (7,22). In the present study, miR-503 was observed to be upregulated in LSCC tissues and cell lines. miR-503 expression level in LSCC was correlated with thyroid cartilage invasion, lymph node metastasis and TNM stage. Transfection of LSCC cells with miR-503 inhibitor was demonstrated to inhibit cell proliferation and invasion by CCK8 and transwell invasion assay. Notably, PDCD4 was identified as a direct target gene of miR-503 in LSCC. These results demonstrated the expression level and roles of miR-503 in LSCC progression and suggested that miR-503 may serve as a prognostic and therapeutic target for LSCC.
A large number of studies have indicated that miR-503 was abnormally expressed in a number of types of human cancer. For example, in hepatocellular carcinoma, miR-503 expression was reduced in the primary tumour tissues and cell lines (23). In endometrioid endometrial cancer, the expression level of miR-503 was decreased and correlated with survival time of patients (24). In non-small cell lung cancer (NSCLC), miR-503 expression was down-regulated in tumour tissues and correlated with lymphatic invasion, distant metastasis, TNM stage, and tumour grade. In addition, Kaplan-Meier analysis revealed that patients with NSCLC with decreased expression of miR-503 exhibited a poor prognosis compared with patients with increased miR-503 expression. Additionally, multivariate analysis indicated that miR-503 expression in NSCLC was an independent prognostic factor for overall survival (25). miR-503 was also demonstrated to be down-regulated in glioma (26,27), gastric cancer (28), osteosarcoma (17) breast (18), prostate (19) and cervical cancer (20). However, in oesophageal (29) and colorectal cancer (30), expression levels of miR-503 were increased in tumour tissues and associated with a poor prognosis. These results suggested that the dysregulation of miR-503 was a frequent event in numerous types of human cancer and may be a prognostic marker.
miR-503 has been demonstrated to be involved in various biological processes associated with tumourigenesis and tumour development. Zhou et al (23) reported that, in hepatocellular carcinoma, miR-503 re-expression inhibited tumour angiogenesis in vitro as well as in vivo via direct targeting of fibroblast growth factor 2 and vascular endothelial growth factor A. Xiao et al (31) revealed that miR-503 overexpression reduced cell growth through directly targeting insulin-like growth factor 1 receptor (IGF-1R). Xu et al (24) demonstrated that the restoration of expression of miR-503 targeted G1/S-specific cyclin-D1 to decrease cell viability, colony formation efficiency, cell-cycle progression in vitro and cell-derived xenograft viability in vivo. Yang et al (32) demonstrated that enforced miR-503 expression inhibited cell proliferation and metastasis of NSCLC in vitro and in vivo via inhibition of phosphoinositol 3-kinase (PI3K), PI3K regulatory subunit α and inhibitor of nuclear factor κ-B kinase subunit β. Zhang et al (27) illustrated that abnormal expression of miR-503 attenuated cell proliferation by inducing G0/G1 cell cycle arrest and apoptosis, and decreased cell migration, and invasion through negative regulation of IGF-1R. In oesophageal carcinoma, down-regulation of miR-503 suppressed the proliferation and invasion of tumour cells by negative regulation of interleukin-2 and interferon-γ expression (33). These results suggested that miR-503 has a potential application in the treatment of a number of types of human cancer.
Identification of the miR target genes is important for understanding the role of miR in carcinogenesis. In the present study, an important molecular link between miR-503 and PDCD4 was observed in LSCC. Bioinformatic analysis predicted that PDCD4 is a potential target of miR-503. A luciferase reporter assay demonstrated that the 3′UTR of PDCD4 could be directly targeted by miR-503. RT-qPCR and western blot analysis indicated the regulatory effects of miR-503 on PDCD4 expression in LSCC. PDCD4 overexpression exhibits similar effects to that of miR-503 down-regulation in LSCC cells. PDCD4 is down-regulated in LSCC tissues and is inversely correlated with miR-503 expression level in LSCC tissues.
PDCD4 inhibits protein translation through binding to the translation eukaryotic initiation factor 4A-III, or via translation elongation by direct or indirect binding to the coding region of specific RNAs (34). Previous studies in tumour biology have demonstrated that PDCD4 expression was reduced in multiple types of human cancer, including hepatocellular carcinoma (35), gastric (36) and breast cancer (37). PDCD4 serves a pivotal role in the occurrence and development of LSCC. Wang et al (38) reported that PDCD4 expression was reduced in LSCC tissues compared with the normal laryngeal mucosa tissues. In addition, low PDCD4 expression was associated with different grading and lymphatic metastasis of patients with LSCC. In addition, PDCD4 expression was correlated with proliferation and apoptosis of LSCC cells (39). Li et al (40) also demonstrated that PDCD4 overexpression reduced the capacity of cellular metastasis in LSCC. These results suggested that targeting the miR-503/PDCD4 signaling pathway in LSCC may be promising in improving the efficacy of treatment and the clinical outcome.
In conclusion, the present study highlighted the regulatory mechanism by which miR-503-induced-loss of PDCD4 enhanced the proliferation and invasion of LSCC cells, and indicated that miR-503 could be investigated as a therapeutic target for the treatments of patients with this disease.
References
- 1.Kiadaliri Ahmad A, Jarl J, Gavriilidis G, Gerdtham UG. Alcohol drinking cessation and the risk of laryngeal and pharyngeal cancers: A systematic review and meta-analysis. PLoS One. 2013;8:e58158. doi: 10.1371/journal.pone.0058158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Cai K, Wang Y, Bao X. MiR-106b promotes cell proliferation via targeting RB in laryngeal carcinoma. J Exp Clin Cancer Res. 2011;30:73. doi: 10.1186/1756-9966-30-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji W, Guan C, Pan Z. Analysis of curative effects on laryngeal carcinoma patients in the northeast region of China. Acta Otolaryngol. 2008;128:574–577. doi: 10.1080/00016480701596104. [DOI] [PubMed] [Google Scholar]
- 5.Zhang K. Relation between air pollution and laryngeal cancer in Liaoning. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1990;25:240–242. (In Chinese) [PubMed] [Google Scholar]
- 6.Chu EA, Kim YJ. Laryngeal cancer: Diagnosis and preoperative work-up. Otolaryngol Clin North Am. 2008;41:673–695. doi: 10.1016/j.otc.2008.01.016. v. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Borel C, Deutsch S, Letourneau A, Migliavacca E, Montgomery SB, Dimas AS, Vejnar CE, Attar H, Gagnebin M, Gehrig C, et al. Identification of cis- and trans-regulatory variation modulating microRNA expression levels in human fibroblasts. Genome Res. 2011;21:68–73. doi: 10.1101/gr.109371.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 10.Mendell JT. MicroRNAs: Critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32:189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Bandrés E, Cubedo E, Agirre X, Malumbres R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M, García-Foncillas J. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu D, Zhou Y, Pan H, Zhou J, Fan Y, Qu P. microRNA-99a inhibiting cell proliferation, migration and invasion by targeting fibroblast growth factor receptor 3 in bladder cancer. Oncol Lett. 2014;7:1219–1224. doi: 10.3892/ol.2014.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang C, Zhang X, Wang HM, Liu XM, Zhang XJ, Zheng B, Qian GR, Ma ZL. MicroRNA-18a-5p functions as an oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis. 2017;8:e2764. doi: 10.1038/cddis.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JY, Lu JB, Xu Y. MicroRNA-153 inhibits the proliferation and invasion of human laryngeal squamous cell carcinoma by targeting KLF5. Exp Ther Med. 2016;11:2503–2508. doi: 10.3892/etm.2016.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng J, Liu Y, Jin Y, Tai J, Zhang J, Xiao X, Chu P, Yu Y, Wang SC, Lu J, et al. MicroRNA-365a-3p promotes tumor growth and metastasis in laryngeal squamous cell carcinoma. Oncol Rep. 2016;35:2017–2026. doi: 10.3892/or.2016.4617. [DOI] [PubMed] [Google Scholar]
- 17.Chong Y, Zhang J, Guo X, Li G, Zhang S, Li C, Jiao Z, Shao M. MicroRNA-503 acts as a tumor suppressor in osteosarcoma by targeting L1CAM. PLoS One. 2014;9:e114585. doi: 10.1371/journal.pone.0114585. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Long J, Ou C, Xia H, Zhu Y, Liu D. MiR-503 inhibited cell proliferation of human breast cancer cells by suppressing CCND1 expression. Tumour Biol. 2015;36:8697–8702. doi: 10.1007/s13277-015-3623-8. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Liu X, Wang M. miR-503 suppresses tumor cell proliferation and metastasis by directly targeting RNF31 in prostate cancer. Biochem Biophys Res Commun. 2015;464:1302–1308. doi: 10.1016/j.bbrc.2015.07.127. [DOI] [PubMed] [Google Scholar]
- 20.Yin ZL, Wang YL, Ge SF, Guo TT, Wang L, Zheng XM, Liu J. Reduced expression of miR-503 is associated with poor prognosis in cervical cancer. Eur Rev Med Pharmacol Sci. 2015;19:4081–4085. [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, Ma R, Si W, Li S, Xu Y, Tu X, Wang Q. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013;333:159–169. doi: 10.1016/j.canlet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Xu YY, Wu HJ, Ma HD, Xu LP, Huo Y, Yin LR. MicroRNA-503 suppresses proliferation and cell-cycle progression of endometrioid endometrial cancer by negatively regulating cyclin D1. FEBS J. 2013;280:3768–3779. doi: 10.1111/febs.12365. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Qu W, Zhong Z. Down-regulation of miR-503 expression predicate advanced mythological features and poor prognosis in patients with NSCLC. Int J Clin Exp Pathol. 2015;8:5609–5613. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Song Z, Liao D, Zhang T, Liu F, Zheng W, Luo K, Yang L. miR-503 inhibits cell proliferation and invasion in glioma by targeting L1CAM. Int J Clin Exp Med. 2015;8:18441–18447. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Chen X, Lian H, Liu J, Zhou B, Han S, Peng B, Yin J, Liu W, He X. MicroRNA-503 acts as a tumor suppressor in glioblastoma for multiple antitumor effects by targeting IGF-1R. Oncol Rep. 2014;31:1445–1452. doi: 10.3892/or.2013.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Y, Liu YM, Li LC, Wang LL, Wu XL. microRNA-503 inhibits gastric cancer cell growth and epithelial-to-mesenchymal transition. Oncol Lett. 2014;7:1233–1238. doi: 10.3892/ol.2014.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ide S, Toiyama Y, Shimura T, Kawamura M, Yasuda H, Saigusa S, Ohi M, Tanaka K, Mohri Y, Kusunoki M. MicroRNA-503 promotes tumor progression and acts as a novel biomarker for prognosis in oesophageal cancer. Anticancer Res. 2015;35:1447–1451. [PubMed] [Google Scholar]
- 30.Noguchi T, Toiyama Y, Kitajima T, Imaoka H, Hiro J, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Toden S, Kusunoki M. miRNA-503 promotes tumor progression and is associated with early recurrence and poor prognosis in human colorectal cancer. Oncology. 2016;90:221–231. doi: 10.1159/000444493. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Y, Tian Q, He J, Huang M, Yang C, Gong L. MiR-503 inhibits hepatocellular carcinoma cell growth via inhibition of insulin-like growth factor 1 receptor. Onco Targets Ther. 2016;9:3535–3544. doi: 10.2147/OTT.S106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Liu L, Zhang Y, Guan H, Wu J, Zhu X, Yuan J, Li M. MiR-503 targets PI3K p85 and IKK-beta and suppresses progression of non-small cell lung cancer. Int J Cancer. 2014;135:1531–1542. doi: 10.1002/ijc.28799. [DOI] [PubMed] [Google Scholar]
- 33.Zhao K, Chen BJ, Chen ZG, Zhang YJ, Xu D, Liu Q. Effect of miR-503 down-regulation on growth and invasion of esophagus carcinoma and related immune function. Med Sci Monit. 2015;21:3564–3569. doi: 10.12659/MSM.895518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Jiang Y, Song X, Guo C, Zhu F, Wang X, Wang Q, Shi Y, Wang J, Gao F, et al. Pdcd4 deficiency enhances macrophage lipoautophagy and attenuates foam cell formation and atherosclerosis in mice. Cell Death Dis. 2016;7:e2055. doi: 10.1038/cddis.2015.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K, Matsuhashi S. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25:6101–6112. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- 36.Liang H, Wang F, Chu D, Zhang W, Liao Z, Fu Z, Yan X, Zhu H, Guo W, Zhang Y, et al. miR-93 functions as an oncomiR for the downregulation of PDCD4 in gastric carcinoma. Sci Rep. 2016;6:23772. doi: 10.1038/srep23772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Zhang Y. Expression of programmed cell death 4 protein is closely correlated with laryngeal squamous cell carcinomas. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;25:539–541. (In Chinese) [PubMed] [Google Scholar]
- 39.Wang J, Zhang Y. Expression of programmed cell death 4 and its correlation with proliferation and apoptosis in laryngeal squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:266–269. (In Chinese) [PubMed] [Google Scholar]
- 40.Li JZ, Gao W, Lei WB, Zhao J, Chan JY, Wei WI, Ho WK, Wong TS. MicroRNA 744-3p promotes MMP-9-mediated metastasis by simultaneously suppressing PDCD4 and PTEN in laryngeal squamous cell carcinoma. Oncotarget. 2016;7:58218–58233. doi: 10.18632/oncotarget.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]