Abstract
In chronic hypoxia, pulmonary hypertension (PH) induces right ventricular hypertrophy (RVH). Evidence indicates that protein kinase C (PKC) serves a crucial role in hypoxia-induced RVH. The present study investigated PKC isoform-specific expression and its involvement in RVH. Rats were exposed to normobaric hypoxia for a number of days to induce PH. PKC isoform-specific membrane translocation and protein expression in the myocardium were evaluated by western blotting and immunostaining. A total of six isoforms of conventional PKC (cPKC; α, βI and βII) and of novel PKC (nPKC; δ, ε and η), were detected in the rat myocardium. Hypoxic exposure (1–21 days) induced PH with RVH and vascular remodeling. nPKCδ membrane translocation at 3–7 days and cPKCβI expression at 1–21 days in the RV following hypoxic exposure were significantly decreased as compared with the normoxia control group. Membrane translocation of cPKCβII at 14–21 days and of nPKCη at 7–21 days in the left ventricle following hypoxic exposure was significantly increased when compared with the control. The results of the present study suggested that the alterations in membrane translocation, and nPKCδ and cPKCβI expression, are associated with RVH following PH, and the upregulation of cPKCβII membrane translocation is involved in left-sided heart failure.
Keywords: protein kinase C, chronic hypoxia, pulmonary hypertension, membrane translocation, protein expression, right ventricular hypertrophy
Introduction
Pulmonary hypertension (PH) is characterized by high pulmonary arterial pressure, increased pulmonary vascular resistance, right ventricular hypertrophy (RVH) and right-sided heart failure (1,2). Although PH is a lung disease, it frequently leads to right sided heart failure, a progressive condition associated with increased mortality in which initial compensatory RVH is overwhelmed by increased systolic requirements, while left ventricular systolic function is preserved. The predominant determinant of survival in PH is the response of the RV to the functional and structural alterations of the pulmonary circulation (3).
Hypoxic pulmonary artery hypertension (HPH) is a common complication of chronic lung disease, including chronic obstructive pulmonary disease, interstitial lung disease and chronic cor pulmonale. Chronic hypoxia is the primary factor driving pulmonary vasoconstriction, vascular remodeling and PH (4,5). The effects of HPH on the RV include early remodeling, hypertrophy, dilatation and eventual failure with increased mortality (6).
Despite the availability of drugs targeting the lung vessels, pulmonary microvascular obstruction usually progresses and imposes an increasingly larger load on the RV. Current drug therapies can further compromise the already ischemic, fibrotic, and impaired RV (7,8). As PH is a cardiopulmonary disease, drug trials should not only focus on the lung circulation but also consider potential positive and negative effects on the RV. Recently, the right side of the heart was identified as a direct treatment target in PH (9).
Cardiac hypertrophy is defined as an increase in heart mass, which may be either beneficial (physiological hypertrophy) or detrimental (pathological hypertrophy) (10). However, very little is known about the cellular and molecular mechanisms that underlie the transition from compensated RVH to dilatation and failure in HPH, and about direct interventions that could preserve RV function. Data on heart failure demonstrate that multiple signaling molecules (angiotensin II, transforming growth factor β1, tumor necrosis factor α and endothelin-1) can induce hypertrophic signaling pathways involving mitogen-activated protein kinases, protein kinase C (PKC) and p60-Src within cardiomyocytes (11). Among the various signaling pathways involved in promoting cardiac hypertrophy, PKC has been identified to be an important component in the response to a variety of hypertrophic stimuli. As crucial mediators, PKC isoforms may contribute to the growth and contractile performance of cardiomyocytes in PH-associated heart failure; therefore, they have gained attention as novel potential therapeutic targets for the treatment of heart disease (10).
PKCs are a group of serine/threonine kinases that regulate cellular effector responses. PKC activation leads to rapid changes in contractile performance and more long-term effects on ventricular remodeling in the heart (12). The PKC family comprises ~10 isozymes, which are broadly classified by their activation characteristics. The conventional PKC isozymes (cPKCα, βI, βII, and γ) are Ca2+ and lipid-activated, while the novel (nPKCε, θ, η and δ) and atypical (aPKCζ and ι/λ) isozymes are activated by distinct lipids but not by Ca2+ (13). Multiple PKC isoforms are expressed in cardiomyocytes (14,15), and membrane translocation of PKC or alterations in PKC level can affect cellular signaling pathways (16).
Koide et al (17) reported that the levels of cPKCα, cPKCβ, nPKCδ and nPKCε increase at the cardiac hypertrophy stage; however, at the heart failure stage, the expression of cPKCα, cPKCβ and nPKCδ remains elevated, while that of nPKCε tends to decline (17). PKC activation has been demonstrated to be associated with pulmonary artery banding-induced RVH, which was accompanied by increased expression of PKCα, PKCδ and PKCβI, but not PKCε and PKCβII, in the hypertrophied RV (18). Klein et al reported (19) that PKCε-knockout mice exhibit normal cardiac morphology and function, suggesting that PKCε is not required for the development of pressure overload-induced myocardial hypertrophy (19). Therefore, the regulation of PKCs in cardiac hypertrophy remains obscure.
Although PKC is known to be involved in cardiovascular functions, little is known about the individual roles of the various isoforms in hypoxia-induced PH, in which only the RV is exposed to pressure overload. Therefore, this study was undertaken to establish the role of different PKC isoforms in the development of HPH-associated RVH by observing their protein expression and membrane translocation.
Materials and methods
Materials
Anti-PKCα (mouse monoclonal; cat. no. sc-8393), anti-PKCβI (rabbit polyclonal; cat. no. sc-209), anti-PKCβII (rabbit polyclonal; cat. no. sc-210), anti-PKCγ (rabbit polyclonal; cat. no. sc-211), anti-PKCε (rabbit polyclonal; cat. no. sc-214), anti-PKCδ (rabbit polyclonal; cat. no. sc-213), anti-PKCθ (rabbit poyclonal; cat. no. sc-212), anti-PKCη (rabbit polyclonal; cat. no. sc-215), anti-PKCι/λ (rabbit polyclonal; cat. no. sc-11399), and anti-PKCζ (mouse monoclonal; cat. no. sc-17781) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Proteinase inhibitors (leupeptin, aprotinin, pepstatin A and chymostatin), phosphatase inhibitors (okadaic acid, sodium pyrophosphate and potassium fluoride), anti-β-actin antibody (mouse monoclonal; cat. no. A1978), as well as other reagents, including dithiothreitol (DTT), Nonidet™ P-40, EDTA, EGTA, and SDS, were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Bicinchoninic acid (BCA) protein assay kit, horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin (Ig) G (cat. no. AP307P), and goat anti-mouse IgG (cat. no. AP200P) were purchased from Merck KGaA.
Animals and hypoxic exposure
All procedures conducted in the present study were approved by the Animal Care and Use Committee of Capital Medical University (Beijing, China) and conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (20). Specific pathogen-free adult male Sprague-Dawley rats (n=72; age, 6–7 weeks; weight, 200–250 g), were randomly divided into 6 groups (n=12 rats/group): Hypoxic treated groups (1, 3, 7, 14 and 21 days) and normoxic control group. Rats in the hypoxic groups were exposed to normobaric hypoxia (10% oxygen) for specified periods (1, 3, 7, 14 and 21 days) in a ventilated Plexiglas chamber at room temperature, under a 12-h light/dark cycle, while age- and weight-matched rats of the normoxic control group were maintained in a 21% oxygen environment.
To establish the hypoxic conditions as previously reported (21), the chamber was flushed with a mixture of oxygen and nitrogen from high-pressure cylinders, and an oxygen analyzer was used to monitor the chamber environment. CO2 was removed with soda lime, excess humidity was prevented by Drierite granules and boric acid was used to maintain ammonia levels within the chamber to a minimum. The chamber was opened every other day for 30 min to clean the cages and to replenish food and water.
Hemodynamics and estimation of RVH
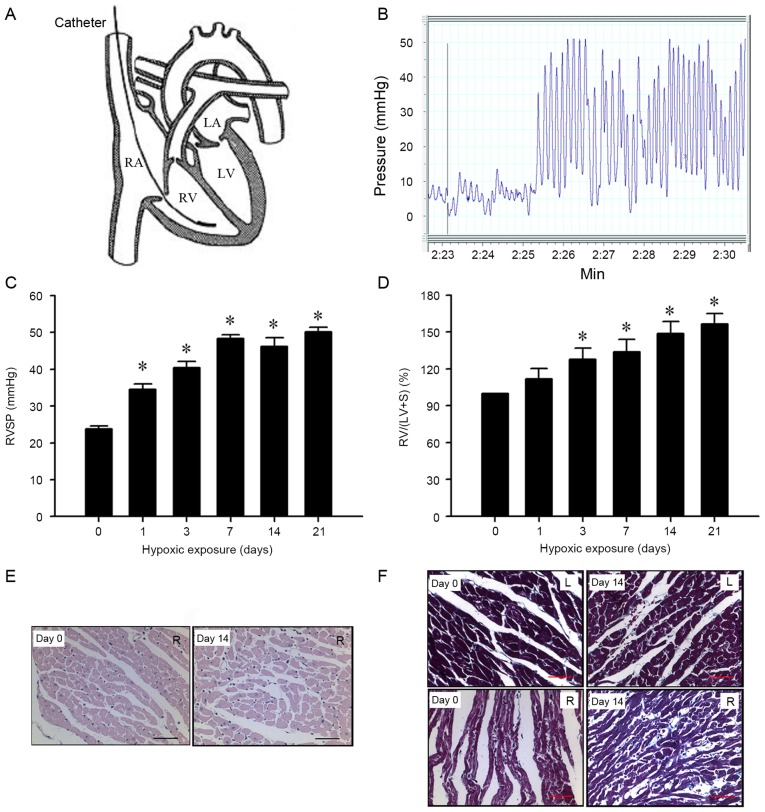
At the end of the hypoxic exposure, the animals were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneal) and a 1.4-F microtip pressure transducer (SPR-671; Millar Instruments, Houston, TX, USA) was inserted into the RV through the jugular vein for hemodynamic measurements (Fig. 1A and B). RV systolic pressure (RVSP) an indirect measure of pulmonary artery pressure, was measured with a polygraph system (PowerLab; AD Instruments, Sydney, Australia). The heart and lungs were excised and placed in ice-cold saline. The RV was dissected from the LV and the septum (S), and weighed separately. Then, the organs were shock-frozen in liquid nitrogen (22). RVH was determined as the RV/(LV+S) ratio.
Figure 1.
Assessment of hypoxia-induced PH in rats. (A) Schematic view of catheterization of the heart, the 1.4-Fr catheter was inserted through the right jugular vein into the RV. (B) Typical RV pressure curve indicating hypoxia-induced PH. Physiological data of RVH upon hypoxia-induced PH in rats. (C) RVSP and the (D) RV/(LV+S) weight ratio were increased under hypoxic exposure when compared with the normoxia control rats (n=12 per group). *P<0.05 vs. the control group. (E) Hematoxylin and eosin staining of RVH in PH rats (magnification, ×400; scale, 100 µm). (F) Masson staining of RVH in PH rats (magnification, ×400; scale, 100 µm). PH, pulmonary hypertension; RV, right ventricle; RVH, right ventricular hypertrophy; RVSP, right ventricular systolic pressure; LV, left ventricle; S, septum.
Subcellular fractionation and whole tissue homogenate preparations
The frozen samples were placed into 100 µl freshly-prepared homogenization buffer A [50 mM Tris-Cl, (pH 7.5), 2 mM DTT, 2 mM EDTA, 2 mM EGTA, 5 mg/ml leupeptin, 5 mg/ml aprotinin, 5 mg/ml pepstatin A, 5 mg/ml chymostatin, 50 mM potassium fluoride, 50 µM okadaic acid, and 5 mM sodium pyrophosphate] and homogenized. The homogenate was centrifuged at 300,000 × g for 30 min at 4°C and the supernatant was collected as the cytosolic fraction. The pellet was solubilized in 100 µl homogenization buffer B (buffer A containing 0.5% Nonidet P-40), prior to being sonicated 3 times for 10 sec at 4°C and centrifuged at 300,000 × g for 30 min at 4°C. The supernatant was taken as the particulate fraction. The two fractions were prepared for PKC isoform-specific membrane translocation analysis. To analyze PKC isoform-specific protein expression levels, frozen samples were homogenized in 250 µl homogenization buffer C (buffer A containing 2% SDS) and sonicated 3 times for 10 sec at 4°C to dissolve completely as the whole tissue homogenate. The protein concentration was determined by the bicinchoninic acid protein assay (Thermo Fisher Scientific Inc., Waltham, MA, USA) (16,23).
Western blot analysis
Aliquots of the cytosolic (40 µg) and particulate (50 µg) fractions were separated on 10% SDS-PAGE. Following separation, the proteins were transferred onto a polyvinylidene difluoride membrane (GE Healthcare Life Sciences, Little Chalfont, UK) at 4°C. The membrane was washed three times for 10 min with TBS-Tween-20 [TBST; 20 mM Tris-HCl (pH 7.5), 0.15 M NaCl and 0.05% Tween-20] and blocked with 10% non-fat milk in TBST for 1 h at room temperature. The membrane was incubated with primary antibodies against PKCα, βI, βII and γ, PKCδ, ε, η and θ, and PKCι/λ and ζ at 1:500 dilutions for 3 h at 4°C. To verify equal loading of protein, the blots were stripped by incubating the membranes for 45 min in stripping buffer containing 62.5 mM Tris-HCl (pH 6.7), 2% SDS and 100 mM 2-mercaptoethanol at 55°C, and re-probed with anti-β-actin antibody at a 1:1,000 dilution for 3 h at 4°C. HRP-conjugated goat anti-rabbit or anti-mouse IgG were used as secondary antibodies (1:5,000) for 1 h at 4°C. An enhanced chemiluminescence kit (GE Healthcare Life Sciences) was employed to visualize protein bands (24,25).
To quantify membrane translocation, the ratio of the PKC isoform (band density in particulate/band densities in the two particulate and cytosolic fractions) of the control group was normalized to 100%, and hypoxia groups were expressed as percentages of the control group. For the quantitative analysis of PKC isoform-specific protein expression, the optical density of each band corresponding to a PKC isoform (from whole tissue homogenate) was normalized to that of β-actin. The protein expression ratio in the control group was regarded as 100%, and data from the hypoxia groups were expressed as percentages of the control group. Quantitative analysis of the immunoblots was conducted with the Quantitative-one software version 4.6.6 (GelDoc 2000 imaging system; Bio-Rad Laboratories Inc., Hercules, CA, USA).
Hematoxylin and eosin (H&E) staining and immunohistochemistry
The tissues were fixed in 10% formaldehyde at 4°C for ≥24 h. Paraffin slices (5-µm thick) were stained with H&E and Masson's trichrome stains.
For H&E staining, the sliced sections were stained with hematoxylin (Mayer's hematoxylin solution; Sigma-Aldrich; Merck KGaA) for 10 min at room temperature, and differentiated in 1% acid alcohol for 3–5 sec until the nuclei turned blue. Subsequently, sections were counterstained with eosin (Sigma-Aldrich; Merck KGaA) for 1 min at room temperature.
For Masson's trichrome staining, paraffin sections were stained in hematoxylin solution for 5 min, Ponceau S solution (Sigma-Aldrich; Merck KGaA) for 5 min, and 0.1% Light Green SF yellowish (Sigma-Aldrich; Merck KGaA) for 1 min at room temperature. The slices were examined under a light microscope (BX51; Olympus, Tokyo, Japan) for structural changes of myocardial cells in response to hypoxic exposure.
To determine changes in PKC isoform-specific expression in the ventricles of rats following chronic hypoxia-induced PH, immunostaining with cPKCα, βI, βII and γ, and nPKCδ was carried out as reported (26). LV and RV tissues were fixed in 10% formaldehyde in PBS at 4°C for 24 h. The tissues were dehydrated through graded alcohol, cleared with xylene, embedded in paraffin, and sectioned at a thickness of 5 µm. Paraffin slices were deparaffinized with xylene at 60°C for 45 min, and rehydrated through graded alcohols. The slices were incubated with 0.3% H2O2 for 10 min to exhaust the endogenous peroxidase and washed in 0.1 M PBS (pH 7.4). Then, sections were pretreated with citrate buffer (0.01 mol/l citric acid, pH 6.0) for 20 min at 95°C, followed by blocking with 10% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The blocked slices were incubated with antibodies against PKCα, βI, βII, and γ at dilutions of 1:200 for 12 h at 4°C. Then, the specimens were incubated with HRP-conjugated goat anti-rabbit IgG, diluted at 1:300 at 4°C for 2 h. Finally, the slices were thoroughly washed in PBS, and a solution containing H2O2 (0.03%) and 3,3′-diaminobenzidine (60%) was added to visualize the slices. Control sections were prepared in the same way, but primary antibodies were omitted. Images were captured on a Leica microscope imaging system (DM4000B; Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Data are expressed as the mean ± standard error of at least 6 independent experiments. Statistical analysis was performed using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). The statistical significance of the differences between groups was assessed using one-way analysis of variance followed by pairwise multiple comparisons using the Bonferroni test. P<0.05 was considered to indicate a statistically significant difference.
Results
Assessment of hypoxia-induced PH in rats
To confirm the establishment of RVH in the hypoxia-exposed rats, RVSP (a marker of systolic pulmonary arterial pressure) and the RV/(LV+S) ratio (a marker of RVH) were measured. As presented in Fig. 1C, compared with day 0 (23.8±0.8 mmHg), RVSP increased significantly with hypoxic exposure time (day 1, 34.6±1.4 mmHg; day 3, 40.4±1.7 mmHg; day 7, 48.3±1.1 mmHg; day 14, 46.2±2.4 mmHg; and day 21, 50.1±1.3 mmHg; all P<0.05). Similarly, there was a significant increase in the RV/(LV+S) ratio compared with day 0 (100%) in rats following hypoxic exposure (day 1, 111.7±8.4%; day 3, 127.8±9.3%; day 7, 133.9±10.1%; day 14, 148.7±9.8%; and day 21, 156.5±8.6%; all P<0.05; Fig. 1D) in a time-dependent manner.
In addition, pathological changes were observed in a time dependent manner in the myocardium of rats following 14 days of hypoxic exposure (Fig. 1E and F). Myocardial cells were markedly hypertrophied and sparsely distributed when compared with the controls. In addition, myocardial fibers were thickened and discrete, and revealed fractures and a disordered arrangement. These results suggest that prolonged hypoxia can induce PH with RVH in rats.
PKC isoform-specific protein expression in myocardium of hypoxia-induced PH model rats
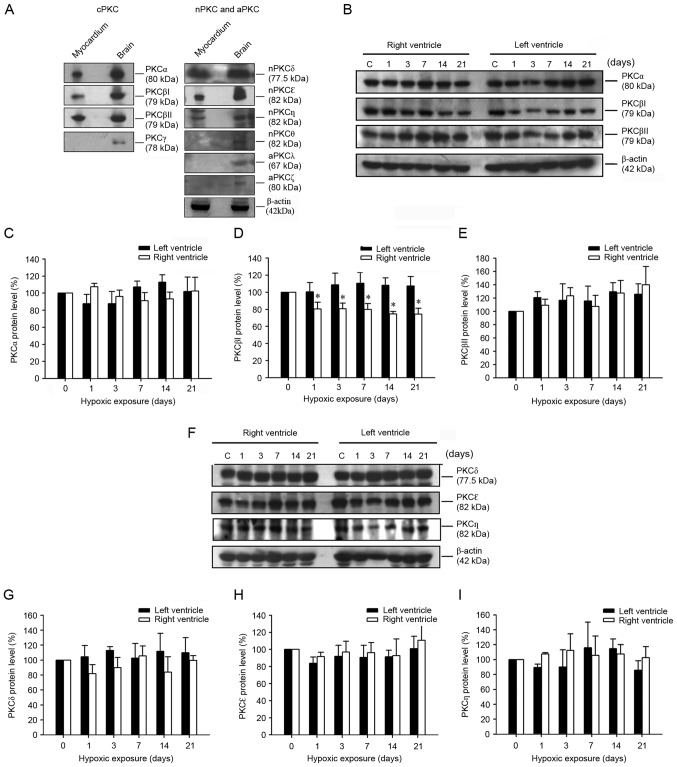
Western blot analysis demonstrated that cPKCα, cPKCβI, cPKCβII, nPKCδ, nPKCε and nPKCη, but not cPKCγ, nPKCθ, aPKCι/λ and aPKCζ are present in the rat myocardium (Fig. 2A). Expression of cPKCα, cPKCβI, cPKCβII, nPKCδ, nPKCε and nPKCη was further evaluated by western blotting (Fig. 2B-I). As demonstrated in Fig. 2B and D, the protein expression of cPKCβI in the RV decreased significantly under hypoxia (day 1, 80.3±7.9%; day 3, 80.7±6.5%; day 7, 79.8±6.9%; day 14, 74.5±2.9%; and day 21, 74.3±6.8%; all P<0.05 vs. day 0); however, the protein expression of the other PKC isoforms remained unchanged.
Figure 2.
PKC isoform-specific protein expression in the myocardium of hypoxia-induced PH model rats. (A) Representative western blots presenting the PKC isoform-specific protein expression in myocardium and brain. (B) A western blot presenting the expression of cPKCα, cPKCβI and cPKCβII, in the RV and LV following hypoxic exposure for 0, 1, 3, 7, 14 and 21 days. Protein levels of (C) cPKCα, (D) cPKCβI and (E) cPKCβII, in the RV and LV of rats following hypoxic exposure for 0, 1, 3, 7, 14 and 21 days. (F) Representative western blot presenting changes in nPKCδ, nPKCε and nPKCη protein expression in the RV and LV of rats following hypoxic exposure for 0, 1, 3, 7, 14 and 21 days. Alterations in (G) nPKCδ, (H) nPKCε and (I) nPKCη protein expression in the RV and LV of rats following hypoxic exposure for 0, 1, 3, 7, 14 and 21 days. Representative western blots and quantitative analysis demonstrating that cPKCβI protein expression was significantly decreased in the RV following hypoxic exposure for 1–21 days when compared with that of the normoxia control group (n=12 per group). *P<0.05 vs. day 0. PH, pulmonary hypertension; RV, right ventricle; LV, left ventricle; cPKC, conventional protein kinase C; nPKC, novel protein kinase C.
PKC isoform-specific membrane translocation in the myocardium of hypoxia-induced PH model rats
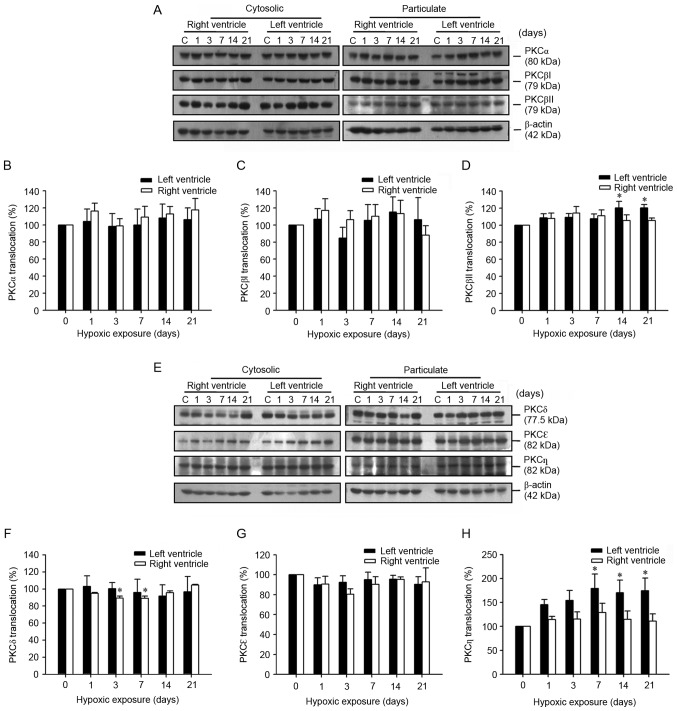
In the LV, the membrane translocation of cPKCβII and nPKCη, but not of the other isoforms, was increased. The translocation of cPKCα and cPKCβI from the cytosolic to the particulate fraction did not alter significantly over the course of the study, however a significant increase in cPKCβII was demonstrated at day 14 (120.2±7.5%) and day 21 (120.3±3.7%) of hypoxia compared with day 0 (Fig. 3A-D). A decrease in the membrane translocation of nPKCδ was observed in the RV of the rats with PH, but not in the other PKC isoforms (Fig. 3E and F). The translocation of nPKCδ from the cytosolic to the particulate fraction significantly decreased at day 3 (89.4±2.4%) and day 7 (88.9±2.8%) of hypoxia when compared with that of the normoxic control group. The translocation of nPKCδ and nPKCε did not alter significantly, however translocation of nPKCη from the cytosolic to the particulate fraction significantly (both P<0.05) increased at day 14 (170.2±26.3%) and day 21 (174.5±26.5%) of hypoxia compared with day 0 (Fig. 3E-H).
Figure 3.
Determination of PKC isoform-specific membrane translocation in the myocardium of hypoxia-induced PH model rats. (A) A western blot of cPKCα, cPKCβI and cPKCβII isoforms expression between the cytosolic and particulate fractions in the RV and LV of rats following hypoxic exposure for 0, 1, 3, 7, 14 and 21 days. Alterations of (B) cPKCα, (C) cPKCβI and (D) cPKCβII membrane translocation in the RV and LV of rats following hypoxic exposure for 0, 1, 3, 7, 14 and 21 days. (E) A representative western blot demonstrating nPKCδ, nPKCε and nPKCη expression between the cytosolic and particulate fractions in the RV and LV of rats following hypoxic exposure for 0, 1, 3, 7, 14, and 21 days. Alterations in (F) nPKCδ, (G) nPKCε and (H) nPKCη membrane translocation, in the RV and LV of rats following hypoxic exposure for 0, 1, 3, 7, 14 and 21 days. Representative western blot results and quantitative analysis indicating that the membrane translocation of nPKCδ decreased significantly in the RV after hypoxic exposure for 3–7 days, while cPKCβII and nPKCη membrane translocation increased in the LV of hypoxia-induced rats following hypoxic exposure compared with that of the normoxia C group (n=12 per group). *P<0.05 vs. day 0. PH, pulmonary hypertension; RV, right ventricle; LV, left ventricle; cPKC, conventional protein kinase C; nPKC, novel protein kinase C; C, control.
PKC isoform-specific cellular localization in the myocardium of chronic hypoxia-induced HP model rats
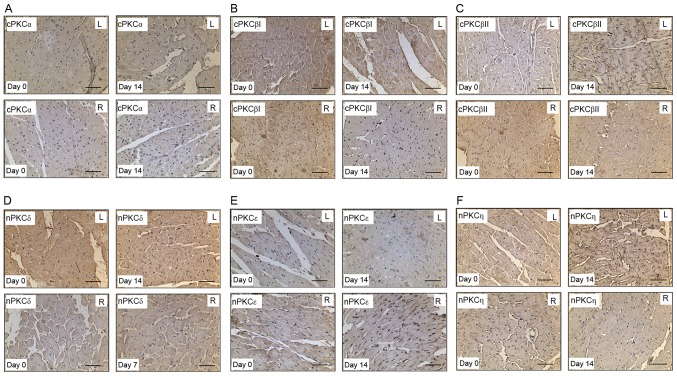
Immunostaining was used to further characterize the cellular localization of the PKC isoforms in the myocardium of the hypoxia-induced PH model rats. Immunolabeled cPKCα, cPKCβI, cPKCβII, nPKCδ, nPKCε and nPKCη proteins were diffusely located throughout the cytoplasmic areas of the cardiomyocytes (Fig. 4A-E). The membrane translocation of nPKCδ in the RV decreased following induction of hypoxia at day 7 (Fig. 4D). At day 14 of hypoxia, the protein expression of cPKCβI in the RV was decreased (Fig. 4B), and the membrane translocation of cPKCβII and nPKCη in the LV was increased (Fig. 4C and F, respectively).
Figure 4.
Immunostaining of cPKCα, β and βII, and nPKCδ, ε and η in the LV and RV of rats following hypoxic exposure. Heart sections of rats under normoxia and hypoxia exposure were stained with polyclonal antibodies against (A) cPKCα, (B) cPKCβI, (C) cPKCβII, (D) nPKCδ, (E) nPKCε and (F) nPKCη (n=12 per group). Scale, 100 µm. cPKC, protein kinase C; nPKC, novel protein kinase C.
Discussion
The present study reports the systematic description of the PKC isoform-specific protein expression pattern and membrane translocation in the RVs of rats with hypoxia-induced PH. It was demonstrated that six isoforms, cPKCα, cPKCβI, cPKCβII, nPKCδ, nPKCε and nPKCη, but not cPKCγ, nPKCθ, aPKCι/λ and aPKCζ, are expressed in cardiomyocytes in vivo by western blotting and immunohistochemistry. Similarly, cPKCα, cPKCβII, cPKCγ, nPKCδ, nPKCε and aPKCζ expression has been observed in the ventricles of hypobaric hypoxia-induced cardiac hypertrophy in rats (27). Braun et al (18) demonstrated the presence of cPKCα, cPKCβI, cPKCβII, nPKCδ and nPKCε in a pulmonary artery banding model of cardiac hypertrophy using western blot analysis.
PKC has been identified to be an important protein during cardiac hypertrophy (28). However, the isoform-specific roles of PKC during RVH associated with hypoxia-induced PH in vivo remain to be elucidated. Previous studies have indicated that the association between PKC isoforms and cardiac hypertrophy is ambiguous. Alterations in PKC isoform levels have been associated with hypertrophy, demonstrating that changes in protein expression of PKC isoforms can affect the activity and signaling, and act as a long-term regulatory mechanism (29). Increased PKC levels have been demonstrated to serve important roles in nuclear signaling, inducing immediate early response genes (Fos proto-oncogene AP-1 transcription factor subunit and MYC proto-oncogene bHLH transcription factor) and fetal gene programs (atrial natriuretic factor and skeletal α-actin), which are known to be involved in myocardial growth (30,31). However, while cPKCβI protein expression increased in RVH following pulmonary artery banding (18), it was observed to decrease in RVH in the hypoxia-induced PH model. These results implicate that cPKCβI may serve a role in the downregulation of hypoxia-induced RVH.
In addition to alterations in their expression levels, membrane translocation of PKCs provides another mechanism to regulate the development and/or maintenance of cardiac hypertrophy (32). Evaluation of the translocation of PKC isoforms from the cytosolic to the membrane fraction of cell or tissue homogenates has been used as a standard method to assess PKC activation. Once activated, PKC can transmit signals to the nucleus via different signal transduction pathways. Acute stimulation of PKC has been reported to be associated with intracellular translocation (33). Chen et al (34) demonstrated that activation of nPKCδ increased the damage induced by ischemia and caused cardiac hypertrophy. By contrast, it was observed that, while nPKCδ expression in the hypertrophied RV was unaltered, nPKCδ membrane translocation was decreased, suggesting increased expression in the cytosol. Similarly, Braun et al (18) observed increased expression of cytosolic nPKCδ in hypertrophied RV following pulmonary artery banding.
In the LV myocardium, no alterations in PKC isoform expression were revealed by immunostaining. The expression pattern of cardiac PKC isozymes in the hypoxia-exposed RV was not identical to that of the LV. Membrane translocation of cPKCβII and nPKCη increased in the LV in the present PH model. These results suggested that cPKCβI and nPKCδ serve a role in the development of LVH caused by hypoxia-induced HP. Previous studies in the heart demonstrated that the activity of cPKCβII increases in end-stage heart failure (35,36), which is in accordance with the results of the present study, suggesting that cPKCβII may be involved in left-sided heart failure of chronic hypoxia-induced PH in rats.
In conclusion, the present study systematically demonstrated PKC isoform-specific membrane translocation and protein expression in a rat model of hypoxia-induced PH with RVH. The results indicated that alterations in the membrane translocation and protein expression of nPKCδ and cPKCβI are involved in RVH in hypoxia-induced PH in rats, and that increased cPKCβII membrane translocation is involved in end-stage left-sided heart failure. Mechanical insights of the effects of expressional alterations and translocations of certain forms of PKCs in the context of RV hypertrophy remain to be further elucidated.
References
- 1.Liu XR, Liu Q, Chen GY, Hu Y, Sham JS, Lin MJ. Down-regulation of TRPM8 in pulmonary arteries of pulmonary hypertensive rats. Cell Physiol Biochem. 2013;31:892–904. doi: 10.1159/000350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, Binder T, Lang IM. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 3.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Poor HD, Girgis R, Studer SM. World Health Organization Group III pulmonary hypertension. Prog Cardiovasc Dis. 2012;55:119–127. doi: 10.1016/j.pcad.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Wang D, Yan G, Sun L, Tang C. TRPC6 is required for hypoxia-induced basal intracellular calcium concentration elevation, and for the proliferation and migration of rat distal pulmonary venous smooth muscle cells. Mol Med Rep. 2016;13:1577–1585. doi: 10.3892/mmr.2015.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangiabadi A, De Pasquale CG, Sajkov D. Pulmonary hypertension and right heart dysfunction in chronic lung disease. Biomed Res Int. 2014;2014:739674. doi: 10.1155/2014/739674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J. 2012;40:1555–1565. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 9.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, et al. Right ventricular function and failure: Report of a national heart, lung and blood institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 10.Naskar S, Datta K, Mitra A, Pathak K, Datta R, Bansal T, Sarkar S. Differential and conditional activation of PKC-isoforms dictates cardiac adaptation during physiological to pathological hypertrophy. PloS One. 2014;9:e104711. doi: 10.1371/journal.pone.0104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: Cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg SF. Cardiac actions of protein kinase C isoforms. Physiology (Bethesda) 2012;27:130–139. doi: 10.1152/physiol.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma P, Gao Q, Wang Z, Yu K. Expression of protein kinase C isoforms in cultured human Tenon's capsule fibroblast cells. Mol Med Rep. 2015;12:6025–6030. doi: 10.3892/mmr.2015.4205. [DOI] [PubMed] [Google Scholar]
- 14.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic cardiac disease: Possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 15.Han YS, Lan L, Chu J, Kang WQ, Ge ZM. Epigallocatechin gallate attenuated the activation of rat cardiac fibroblasts induced by angiotensin II via regulating beta-arrestin1. Cell Physiol Biochem. 2013;31:338–346. doi: 10.1159/000343371. [DOI] [PubMed] [Google Scholar]
- 16.Niu C, Li J, Cui X, Han S, Zu P, Li H, Xu Q. Changes in cPKC isoform-specific membrane translocation and protein expression in the brain of hypoxic preconditioned mice. Neurosci Lett. 2005;384:1–6. doi: 10.1016/j.neulet.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Koide Y, Tamura K, Suzuki A, Kitamura K, Yokoyama K, Hashimoto T, Hirawa N, Kihara M, Ohno S, Umemura S. Differential induction of protein kinase C isoforms at the cardiac hypertrophy stage and congestive heart failure stage in Dahl salt-sensitive rats. Hypertens Res. 2003;26:421–426. doi: 10.1291/hypres.26.421. [DOI] [PubMed] [Google Scholar]
- 18.Braun MU, Szalai P, Strasser RH, Borst MM. Right ventricular hypertrophy and apoptosis after pulmonary artery banding: Regulation of PKC isozymes. Cardiovasc Res. 2003;59:658–667. doi: 10.1016/S0008-6363(03)00470-X. [DOI] [PubMed] [Google Scholar]
- 19.Klein G, Schaefer A, Hilfiker-Kleiner D, Oppermann D, Shukla P, Quint A, Podewski E, Hilfiker A, Schröder F, Leitges M, Drexler H. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCepsilon. Circ Res. 2005;96:748–755. doi: 10.1161/01.RES.0000161999.86198.1e. [DOI] [PubMed] [Google Scholar]
- 20.Guide for the care and use of laboratory animals. Rev. NIH publication (USA) no 85–23. 1985 [Google Scholar]
- 21.Zhang E, Maruyama J, Yokochi A, Mitani Y, Sawada H, Nishikawa M, Ma N, Maruyama K. Sarpogrelate hydrochloride, a serotonin 5HT2A receptor antagonist, ameliorates the development of chronic hypoxic pulmonary hypertension in rats. J Anesthesia. 2015;29:715–723. doi: 10.1007/s00540-015-2015-y. [DOI] [PubMed] [Google Scholar]
- 22.Deten A, Millar H, Zimmer HG. Catheterization of pulmonary artery in rats with an ultraminiature catheter pressure transducer. Am J Physiol Heart Circ Physiol. 2003;285:H2212–H2217. doi: 10.1152/ajpheart.00315.2003. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Niu C, Han S, Zu P, Li H, Xu Q, Fang L. Identification of protein kinase C isoforms involved in cerebral hypoxic preconditioning of mice. Brain Res. 2005;1060:62–72. doi: 10.1016/j.brainres.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242:145–152. doi: 10.1023/A:1021106232511. [DOI] [PubMed] [Google Scholar]
- 25.Braun MU, LaRosée P, Simonis G, Borst MM, Strasser RH. Regulation of protein kinase C isozymes in volume overload cardiac hypertrophy. Mol Cell Biochem. 2004;262:135–143. doi: 10.1023/B:MCBI.0000038229.23132.9f. [DOI] [PubMed] [Google Scholar]
- 26.Ding J, Ding N, Wang N, Lu Q, Lu N, Yang D, Bu X, Han S, Li J. Determination of conventional protein kinase C isoforms involved in high intraocular pressure-induced retinal ischemic preconditioning of rats. Vision Res. 2009;49:315–321. doi: 10.1016/j.visres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Uenoyama M, Ogata S, Nakanishi K, Kanazawa F, Hiroi S, Tominaga S, Seo A, Matsui T, Kawai T, Suzuki S. Protein kinase C mRNA and protein expressions in hypobaric hypoxia-induced cardiac hypertrophy in rats. Acta Physiol (Oxf) 2010;198:431–440. doi: 10.1111/j.1748-1716.2009.02064.x. [DOI] [PubMed] [Google Scholar]
- 28.Deres L, Bartha E, Palfi A, Eros K, Riba A, Lantos J, Kalai T, Hideg K, Sumegi B, Gallyas F, et al. PARP-inhibitor treatment prevents hypertension induced cardiac remodeling by favorable modulation of heat shock proteins, Akt-1/GSK-3β and several PKC isoforms. PloS One. 2014;9:e102148. doi: 10.1371/journal.pone.0102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Molkentin JD. Protein kinase Cα as a heart failure therapeutic target. J Mol Cell Cardiol. 2011;51:474–478. doi: 10.1016/j.yjmcc.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmer HG. Catecholamine-induced cardiac hypertrophy: Significance of proto-oncogene expression. J Mol Med (Berl) 1997;75:849–859. doi: 10.1007/s001090050176. [DOI] [PubMed] [Google Scholar]
- 31.Zhu XX, Niu XL, Chen DZ, Zhou XD, Pei JM, Zhu MZ, Guo J, Zhu XL, Wang WQ. Inhibitory effects of rosiglitazone against endothelin-1-induced proliferation of rat cardiac myocytes: The role of PKC-c-fos pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1056–1060. [PubMed] [Google Scholar]
- 32.Sabri A, Steinberg SF. Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol Cell Biochem. 2003;251:97–101. doi: 10.1023/A:1025490017780. [DOI] [PubMed] [Google Scholar]
- 33.Chai J, Long B, Liu X, Li Y, Han N, Zhao P, Chen W. Effects of sevoflurane on tight junction protein expression and PKC-α translocation after pulmonary ischemia-reperfusion injury. Exp Mol Med. 2015;47:e167. doi: 10.1038/emm.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira JC, Brum PC, Mochly-Rosen D. βIIPKC and εPKC isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:479–484. doi: 10.1016/j.yjmcc.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz K, Stathopoulou K, Schmid E, Eder P, Cuello F. Heart failure-specific changes in protein kinase signalling. Pflugers Arch. 2014;466:1151–1162. doi: 10.1007/s00424-014-1462-x. [DOI] [PubMed] [Google Scholar]