Abstract
Mutations of A-kinase anchoring protein 2 (AKAP2) have been reported to be associated with adolescent idiopathic scoliosis. However, its role in cancer is poorly understood. In the present study, the mRNA levels of AKAP2 in ovarian cancer tissues were examined using qPCR. The effects of AKAP2 on the growth and migration of cancer cells were examined using crystal violet and Boyden chamber assays. An in vivo image system was used to evaluate the effect of AKAP2 on the metastasis of ovarian cancer cells. The present study demonstrated that the expression of AKAP2 was elevated in ovarian cancer. Furthermore, overexpression of AKAP2 promoted the growth and migration of ovarian cancer cells, whereas knockdown of AKAP2 expression reduced the growth and migration of ovarian cancer cells. Analysis of the molecular mechanism indicated that AKAP2 activated β-catenin/T cell factor signaling and regulated the expression of several target genes. Furthermore, analysis of the in vivo metastatic capacity demonstrated that downregulation of AKAP2 inhibited the invasion of ovarian cancer cells. Taken together, the present study demonstrated an oncogenic role for AKAP2 in ovarian cancer, indicating that AKAP2 may be a therapeutic target for ovarian cancer.
Keywords: A-kinase anchoring protein 2, ovarian cancer, β-catenin/T cell factor signaling, cell growth, cell migration
Introduction
Ovarian cancer has very poor survival rates and is one of the most common malignances worldwide (1–3). Although progress has been made in the diagnosis and therapeutic management of ovarian cancer, the 5-year survival rate remains at <10% (4). Therefore, a greater understanding of the molecular mechanisms involved in this disease may facilitate the development of improved treatment options.
Activation of β-catenin/T cell factor (TCF) signaling has been observed in ~70% ovarian cancer cases (5,6). In normal ovarian epithelial cells, cytoplasmic β-catenin is degraded by the destruction complex which includes glycogen synthase kinase 3β, casein kinase 1 and axin (4), among others. Aberrant activation of receptor tyrosine kinase signaling and inactivating mutations or constitutive activation of β-catenin may lead to the cytoplasmic accumulation of β-catenin. The accumulated β-catenin translocalizes to the nucleus and forms a complex with TCF, which activates the expression of target genes (4). Activation of β-catenin in ovarian cancer promotes the growth, migration and metastasis of ovarian cancer cells (7,8). Furthermore, over-activation of β-catenin/TCF signaling may lead to drug resistance (2,9). These studies demonstrated the important role of the β-catenin/TCF signaling axis in the ovarian cancer. However, the molecular mechanisms in β-catenin/TCF signaling activation are not fully understood.
A-kinase anchoring protein (AKAP) binds to the regulatory subunit of cAMP-dependent protein kinase A (PKA), targeting the kinase to various intracellular locations (10). AKAP2 is a member of the AKAP family (4). Several studies have reported that AKAP2 has a role in carcinogenesis; AKAP2 was demonstrated to be upregulated in metastatic prostate cancer and was necessary for the invasion of cancer cells (11). Furthermore, whole genome and transcriptome sequencing revealed an association between AKAP2 and gastric cancer and ovarian cancer (12,13). However, the biological function of AKAP2 in ovarian cancer remains unknown. The present study examined the expression of AKAP2 in ovarian cancer tissues, investigated the role of AKAP2 in the progression of ovarian cancer and explored the underlying molecular mechanism.
Materials and methods
Cell culture
IOSE80 and IOSE144 ovarian epithelial cells and the OVCAR3, OVCA433, OVCA429 and HO-8910 ovarian cancer cells were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells were maintained at 37°C in a humidified incubator with an atmosphere containing 5% CO2.
Patients and specimens
The present study was approved by the Ethics Committee of the Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine. Written informed consent was obtained from all participants (age, 55-64; stage I, 6 patients; stage II, 10 patients; stage III, 9 patients) during recruitment. A total of 25 ovarian cancer tissues and 25 adjacent non-cancerous tissues were used in the present research. Tissues and paired non-cancerous tissues were stored at −80°C until use.
Plasmid construction and stable cell lines
The full length of the coding sequence for AKAP2 was amplified by polymerase chain reaction (PCR) and cloned into the expression vector pcDNA3.1-myc (Clontech Laboratories, Inc., Mountain View, CA, USA) using BamH1 and Pst1. The forward primer was 5′-ATGCGCTGGCCCCAGCCC-3′; and reverse primer was 5′-TCGTTGTCTTCTTCCTC-3′. The PCR mixture included 1.5 µl 10X buffer, 1.5 µl dNTP, 0.6 µl Mg2+, 1.5 µl forward primer, 1.5 µl reverse primer, 0.3 µl Taq, 8.1 µl cDNA. The PCR protocol was as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec, 68°C for 3 min. Final hold was performed at 68°C for 10 min and the samples were subsequently kept at 10°C. The expression vectors were transfected into OVCAR3 and OVCA433 cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Following selection with G418 (500 µg/ml), the resistant cells were pooled, and the expression of myc-tagged AKAP2 was confirmed by western blot analysis.
Knocking down the expression of AKAP2 in ovarian cancer cell lines
The lentivirus to knock down the expression of AKAP2 was purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China). The siRNA sequences were as follows: siRNA no. 1, 5′-aac ccc agga ctg cgc ccc cg-3′; siRNA no. 2, 5′-aac gta caa tgg aac atc ctc-3′; si con, 5′-cca cca cca ccc gag ggc tcg-3′. The siRNA and si con sequences were cloned into the pKO.1 plasmid (Addgene, LGS Standards, Teddington, UK). The ovarian cancer cells were seeded into a 6-well plate; the cells were infected 18 h later when they had reached 60% confluence. The virus (MOI=2) was incubated with the ovarian cancer cells overnight and selected with puromycine.
Reverse transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was isolated from ovarian cancer tissues and paired normal tissues using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription was performed according to the manufacturer's instructions, qPCR was performed using a SYBR Green PCR master mix (Takara Biotechnology Co., Ltd., Dalian, China) in a total volume of 8 µl (4 µl master mix and 4 µl cDNA) using a 7900 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the following conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The reference gene was 18S rRNA, and the relative levels of gene expression were calculated using the 2−ΔΔCq method (14). The sequences of the primers were as follows: AKAP2 forward primer, 5′-CACAAAAACATGGAAATTGA-3′; and reverse primer, 5′-TGGCAACATCATTATCCAGG-3′.
Western blot analysis
Proteins from cultured cells and ovarian tissues were extracted using radioimmunoprecipitation assay lysis buffer, containing protease inhibitors and phosphatase inhibitors (Sigma-Aldrich; Merck KGaA). Following centrifugation (10,000 × g for 20 min at 4°C), the protein concentration was determined using a Bradford assay. Proteins (20 µg/lane) were separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes (Merck KGaA) and probed with specific antibodies. The immunoreactive protein bands were visualized using unenhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.). The anti-AKAP2 (1:1,000, ab64904) antibody was purchased from Abcam (Cambridge, UK), and the anti-GAPDH (1:5,000, sc-25778) antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies against Snail (1:1,000, no. 3879), c-Myc (1:1,000, no. 13987), N-cadherin (1:1,000, no. 13116) and the mouse IgG (1:2,000, no. 7076) and rabbit IgG (1:2,000, no. 2985) secondary antibodies were all purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Immunohistochemistry
The paraffin-embedded tissue sections (5 µm) were deparaffinized and rehydrated by xylene and ethanol. Endogenous peroxidase activity was blocked with 0.35% H2O2. Antigen retrieval was performed using a microwave. Non-specific binding was blocked by 3% BSA solution. Sections were stained with AKAP2 antibody (1:200, ab64904) and visualized with a Dako EnVision Secondary antibody (1:100, K500711; Agilent Technologies, Inc. Santa Clara, CA, USA). Slides were then developed with DAB and counterstained with hematoxylin.
Reporter assay
For the luciferase assay, cells were seeded on a 24-well culture plate and grown overnight. The Topflash reporter plasmids were co-transfected with the Renilla construct (0.01 µg) and AKAP2 expression vector with Lipofectamine 2000 to assess the transcriptional activity of β-catenin/TCFcomplex. After incubation for 48 h, cells were treated with Wnt3a protein (10 ng/ml; R&D Systems, Inc., Minneapolis, MN, USA) for 8 h. Then, the transfected cells were washed twice with phosphate-buffered saline (PBS) and total proteins were lysed in passive lysis buffer (100 µl/well) provided by the Dual-Luciferase Reporter Assay system kit (Promega Corporation, Madison, WI, USA). Firefly and Renilla luciferase activities were measured from the lysate using a Centro LB 960 Microplate Luminometer (Berthold). Firefly luciferase values were normalized to Renilla luciferase values to control for transfection efficiency.
Crystal violet assay
The effects of AKAP2 on the growth of ovarian cancer cells were examined using a colony-forming crystal violet assay. Equal numbers of control cells and experimental cells (1,000 cells/well) were seeded in 12-well plates. For the ICG001 treatment, cells were incubated with the culture medium with ICG001 (5 µM). The medium was changed every two days. Following 14 days of culture under standard conditions, the medium was removed and the cells were stained with 0.5% crystal violet solution in 20% methanol at room temperaturefor 10 min. The fixed cells were washed with PBS and imaged using a light microscope. Four fields were assessed. The colonies were subsequently dissolved using 1% SDS solution and the optical density (OD) was measured at 600 nm. The experiments were repeated three times.
Boyden chamber assay
A Boyden chamber assay was performed to examine the effects of AKAP2 on the migration of the ovarian cancer cells. Cells (2×105) suspended in 50 µl culture medium containing 1% FBS were seeded in the upper chamber, and the lower chamber was loaded with 152 µl medium containing 10% FBS. Cells migrated to the lower surface of the filters after 12 h were detected using eosin staining. The migrated cells were counted using an inverted microscope. Four fields were assessed. The experiments were repeated three times.
In vivo metastasis assay
The pcDNA6 plasmid containing the luciferase gene was transfected into OVCAR3 cells using Lipofectamine 2000 and selected with G418 (500 µg/ml; OVCAR3-Luci). The Xenogen IVIS in vivo imaging system (PerkinElmer, Inc., Waltham, MA, USA) was used to measure the activity of luciferase following the administration of luciferin. OVCAR3-Luci cells were transfected with the lentivirus to knocking down the expression of AKAP2 and selected with puromycine. The resistant cells (1×106 cells in 200 µl PBS) were injected into the left ventricle of 5-week-old male nude mice (Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China). Each group included 6 mice, which were kept in a standard housing conditions with 12-h light/dark cycle, and provided with food and water ad libitum. The metastatic lesions were monitored every two weeks for seven weeks. Luciferin (150 mg/kg) was intraperitoneally injected into the mice 5 min prior to imaging. The nude mice were anaesthetized with 0.2–0.3 l/min Forane (Abbott Pharmaceutical, Co., Ltd., Lake Bluff, IL, USA), and placed into a light-tight chamber with a CCD camera system (PerkinElmer, Inc.); the photons emitted from the luciferase expressing cells within the mouse were quantified for 1 min, using the software program Living Image 2.0 (PerkinElmer, Inc.) as an overlay on Igor Pro 6.01 software (Wavemetrics, Inc., Portland, OR, USA). This study was carried out according to the recommendations of National Institutes of Health and approved by the Ethics Committee of Shangdong University of Traditional Chinese Medicine.
Statistical analysis
SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA) was used. Each experiment was repeated at least three times. The data were presented as the mean ± standard deviation, and statistical comparisons were performed using Student's t-test. Multiple comparisons about cell growth and migration were performed using one way analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
AKAP2 expression is elevated in ovarian cancer
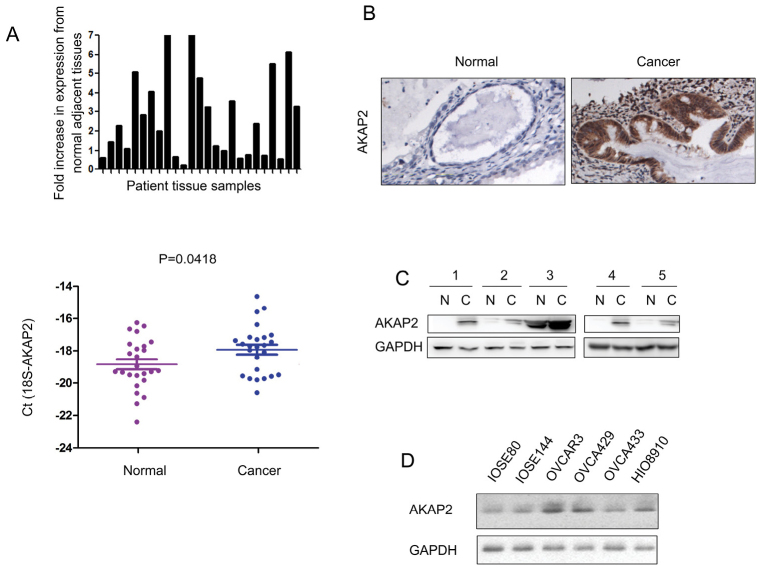
The expression pattern of AKAP2 was assessed in ovarian cancer. RT-qPCR analysis indicated that the mRNA level of AKAP2 was elevated in ovarian cancer tissues compared with the adjacent normal tissues (Fig. 1A). Furthermore, an upregulation of AKAP2 protein was observed in ovarian cancer tissues, when examined by immunohistochemistry and western blot analysis (Fig. 1B and C). The expression of AKAP2 was subsequently analyzed in a panel of ovarian cancer cell lines (OVCAR3, OVCA429, OVCA433 and HIO8910) and normal ovarian epithelial cells (IOSE80 and IOSE144). Elevated expression of AKAP2 protein level was demonstrated in most of the ovarian cancer cells (Fig. 1D). Taken together, these data indicated that AKAP2 was upregulated in ovarian cancer.
Figure 1.
The expression of AKAP2 is upregulated in ovarian cancer. (A) The mRNA level of AKAP2 in 25 ovarian cancer tissues and paired non-cancerous tissues was determined using reverse transcription-quantitative polymerase chain reaction. 18S served as an internal control. The results have been presented as 2−ΔΔCq values. (B) The protein level of AKAP2 in ovarian cancer tissues and paired non-cancerous tissues was examined using immunohistochemistry. (C) The protein level of AKAP2 in ovarian cancer tissues and paired non-cancerous tissues was examined using western blot analysis. (D) The expression of AKAP2 in normal ovarian epithelial cells (IOSE80 and IOSE144) and ovarian cancer cell lines (OVCAR3, OVCA429, OVCA433 and HIO8910). AKAP2, A-kinase anchoring protein 2; N, non-cancerous tissue; C, cancer tissue.
AKAP2 promotes the growth and migration of ovarian cancer cells
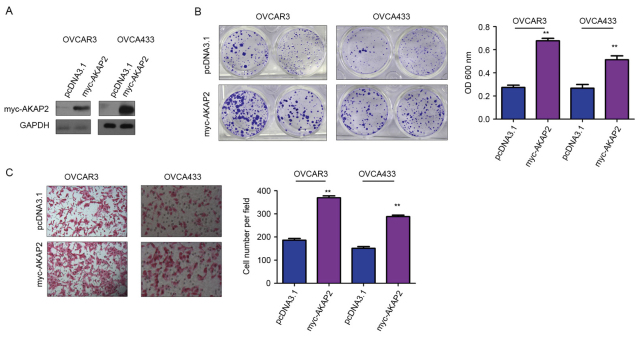
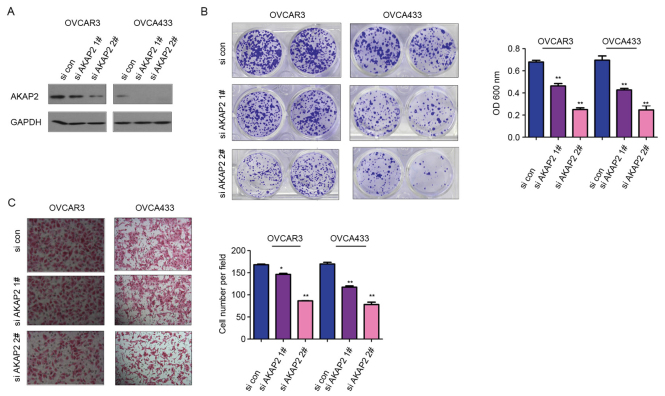
To study the biological functions of AKAP2, OVCAR3 and OVCA433 cells were transfected with AKAP2 (Fig. 2A). Crystal violet assay was used to examine the role of AKAP2 in the growth of ovarian cancer cells; the results indicated that forced overexpression of AKAP2 promoted the growth of OVCAR3 and OVCA433 cells (Fig. 2B). Furthermore, upregulation of AKAP2 enhanced the motility of ovarian cancer cells, observed using a modified Boyden chamber for a cell migration assay (Fig. 2C). To further clarify the function of endogenously expressed AKAP2 in the progression of ovarian cancer, the expression of AKAP2 was knocked down in OVCAR3 and OVCA433 using lentivirus containing two independent siRNA sequences (Fig. 3A). Consistent with earlier observations (Fig. 2B), downregulation of AKAP2 resulted in reduced cell growth and migration, assessed by crystal violet and Boyden chamber assay, respectively (Fig. 3B and C). Collectively, these data indicated AKAP2 may have oncogenic functions in the progression of ovarian cancer, by promoting cell growth and migration.
Figure 2.
AKAP2 promotes the growth and migration of ovarian cancer cells. (A) AKAP2 was overexpressed in OVCAR3 and OVCA433 cells. (B) Overexpression of AKAP2 promoted the growth of OVCAR3 and OVCA433 cells, assessed by colony formation and crystal violet staining. (C) Overexpression of AKAP2 promoted the migration of OVCAR3 and OVCA433 cells in the Boyden chamber assay. **P≤0.01 vs. pcDNA3.1. AKAP2, A-kinase anchoring protein 2; OD, optical density.
Figure 3.
Knockdown of AKAP2 expression inhibits the growth and migration of ovarian cancer cells. (A) AKAP2 was knocked down in OVCAR3 and OVCA433 cells. (B) Knocking down the expression of AKAP2 inhibited the growth of OVCAR3 and OVCA433 cells in the colony formation crystal violet assay. (C) Knocking down the expression of AKAP2 inhibited the migration of OVCAR3 and OVCA433 cells in the Boyden chamber assay. *P≤0.05 and **P≤0.01 vs. si con. AKAP2, A-kinase anchoring protein 2; si, small interfering RNA; con, control.
AKAP2 activates the β-catenin/TCF signaling pathway in ovarian cancer cells
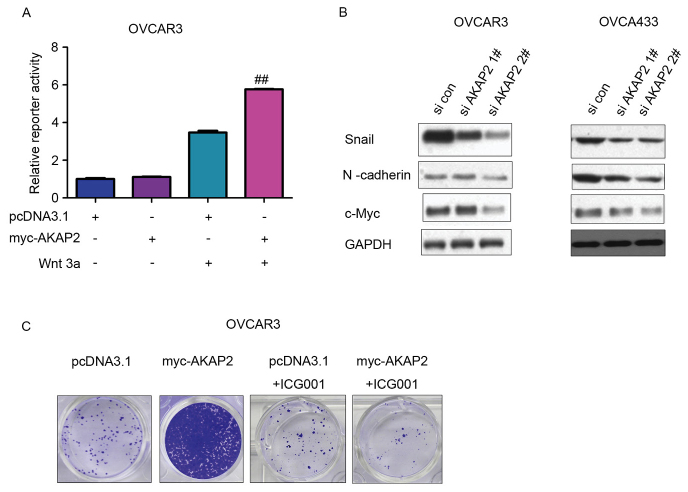
To explore the underlying molecular mechanism through which AKAP2 may promote the growth and migration of ovarian cancer cells, downstream signaling assessed by determining the expression levels of various target genes. Activation of the Topflash reporter, an indicator for β-catenin/TCF signaling, by AKAP2 was observed in OVCAR3 cells upon the stimulation of Wnt3a, suggesting β-catenin/TCF signaling is positively regulated by AKAP2 (Fig. 4A). Furthermore, knockdown of AKAP2 expression in OVCAR3 and OVCA433 cells resulted in decreased expression of several target proteins of the β-catenin/TCF transactivation complex, including Snail, c-Myc and N-cadherin, which may have been due to positive regulation of β-catenin/TCF signaling by AKAP2 (Fig. 4B). In addition, ICG001, an inhibitor of β-catenin/TCF signaling, reversed the increased growth of OVCAR3 cells overexpressing AKAP2, indicating that AKAP2 may promote the growth of ovarian cancer cells by activating β-catenin/TCF signaling (Fig. 4C).
Figure 4.
AKAP2 activate β-catenin/TCF signaling in ovarian cancer cells. (A) AKAP2 activated Topflash, the reporter gene for β-catenin/TCF signaling, in OVCAR3 cells. (B) Knockdown of AKAP2 expression inhibited the expression of Snail, c-Myc and N-cadherin in OVCAR3 and OVCA433 cells. (C) ICG001, an inhibitor of β-catenin/TCF signaling, reversed the promoting effects of AKAP2 on the growth of OVCAR3 cells in the crystal violet assay. ##P≤0.01 vs. pcDNA3.1 + Wnt3a. AKAP2, A-kinase anchoring protein 2; TCF, T cell factor; si, small interfering RNA; con, control.
AKAP2 knockdown reduces the invasion of ovarian cancer cells in an in vivo metastasis model
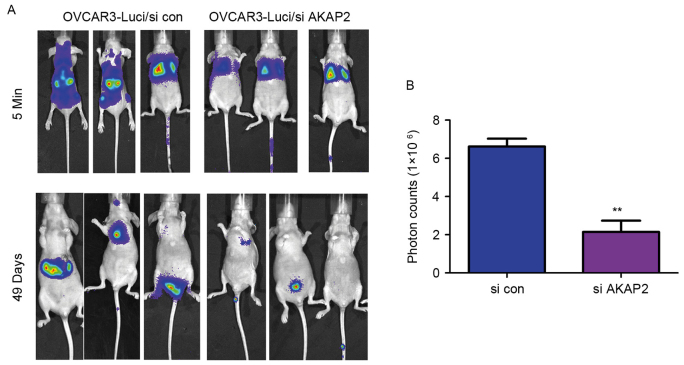
The observation that downregulation of AKAP2 inhibited cell migration in vitro prompted examination of whether AKAP2 served a role in the ability of ovarian cancer cells to invade in vivo. OVCAR3 cells were labeled with the luciferase gene, which enabled the tumor cells to be observed in vivo following the administration of luciferin, the substrate for luciferase. Knockdown of AKAP2 expression reduced the invasion of OVCAR3 cells, which was demonstrated by the intensity of the fluorescence (Fig. 5A and B). These data indicated that AKAP2 may serve a role in the metastatic ability of ovarian cancer cells.
Figure 5.
Knockdown of AKAP2 expression impaired the invasive ability of OVCAR3 cells. (A) In vivo imaging and (B) quantification of invasive ability of OVCAR3 cells with AKAP2 knockdown on day 49. **P<0.01 vs. si con. AKAP2, A-kinase anchoring protein 2; si, small interfering RNA; con, control; luci, luciferase.
Discussion
AKAP2 has previously been reported to regulate the activity of PKA (11), however, the expression pattern and the roles of AKAP2 in cancer are poorly understood. Consistent with the observation that the expression of AKAP2 was elevated in prostate cancer (11), upregulation of AKAP2 in the ovarian cancer tissues was also observed. AKAP2 promoted cancer cell growth and migration, potentially through activation of β-catenin/TCF signaling. Furthermore, the role of AKAP2 in the invasion of ovarian cancer cells was demonstrated using an in vivo imaging system.
The present study utilized a Topflash reporter system to investigate the ability of AKAP2 to activate β-catenin/TCF signaling. AKAP2 activated the Topflash reporter and upregulated target genes downstream of β-catenin/TCF signaling, indicating that AKAP2 may serve as a positive regulator of β-catenin/TCF signaling. PKA has been reported to phosphorylate Ser552 on β-catenin and activate β-catenin/TCF signaling (15). Notably, PKA activity and anchoring are required for the migration and invasion of ovarian cancer cells (16,17). Based on these studies, it may be speculated that AKAP2 could activate β-catenin/TCF signaling via PKA, however, further biochemical studies are required to test this hypothesis.
ICG00 is a small molecule inhibitor of β-catenin/TCF signaling (18,19). In the present study, OVCAR3 ovarian cancer cells treated with ICG001 exhibited a reversal of the increased growth induced by overexpression of AKAP2. Considering AKAP2 was overexpressed in ovarian cancer cell lines and tissue, these findings indicate that there may be cause to further investigate a potential clinical application of IGC001, in the treatment of ovarian cancer.
In addition, AKAP2 has been found as a mutated gene in the adolescent idiopathic scoliosis (20). However, whether AKAP2 was mutated in the cancer samples and the functions of the mutant AKAP2 remained unknown, which would be of great value in a further study.
In summary, the present study demonstrated an oncogenic role for AKAP2 in ovarian cancer by activating β-catenin/TCF signaling, suggesting the application of ICG001 as the potential drug for the cancer treatment. Due to the limitation of the ovarian cancer samples, we did not analyze the correlation between AKAP2 expression and clinical features. Further studies using AKAP2 knockout mice may provide novel insights into the function of AKAP2 in ovarian cancer.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;64:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Hu C, Dong T, Li R, Lu J, Wei X, Liu P. Emodin inhibits epithelial to mesenchymal transition in epithelial ovarian cancer cells by regulation of GSK-3β/β-catenin/ZEB1 signaling pathway. Oncol Rep. 2016;35:2027–2034. doi: 10.3892/or.2016.4591. [DOI] [PubMed] [Google Scholar]
- 5.Merritt MA, Cramer DW. Molecular pathogenesis of endometrial and ovarian cancer. Cancer Biomark. 2010;9:287–305. doi: 10.3233/CBM-2011-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanseverino F, D'Andrilli G, Petraglia F, Giordano A. Molecular pathology of ovarian cancer. Anal Quant Cytol Histol. 2005;27:121–124. [PubMed] [Google Scholar]
- 7.Hu J, Shao S, Song Y, Zhao J, Dong Y, Gong L, Yang P. Hepatocyte growth factor induces invasion and migration of ovarian cancer cells by decreasing the expression of E-cadherin, beta-catenin, and caveolin-1. Anat Rec (Hoboken) 2010;293:1134–1139. doi: 10.1002/ar.21147. [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka S, King ML, Ran S, Okuda H, MacLean JA, II, McAsey ME, Sugino N, Brard L, Watabe K, Hayashi K. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/β-catenin pathway. Mol Cancer Res. 2012;10:469–482. doi: 10.1158/1541-7786.MCR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barghout SH, Zepeda N, Xu Z, Steed H, Lee CH, Fu Y. Elevated β-catenin activity contributes to carboplatin resistance in A2780cp ovarian cancer cells. Biochem Biophys Res Commun. 2015;468:173–178. doi: 10.1016/j.bbrc.2015.10.138. [DOI] [PubMed] [Google Scholar]
- 10.Autenrieth K, Bendzunas NG, Bertinetti D, Herberg FW, Kennedy EJ. Defining A-kinase anchoring protein (AKAP) specificity for the protein kinase a subunit RI (PKA-RI) Chembiochem. 2016;17:693–697. doi: 10.1002/cbic.201500632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakkar A, Aljameeli A, Thomas S, Shah GV. A-kinase anchoring protein 2 is required for calcitonin-mediated invasion of cancer cells. Endocr Relat Cancer. 2016;23:1–14. doi: 10.1530/ERC-15-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Huang JY, Chen YN, Yuan F, Zhang H, Yan FH, Wang MJ, Wang G, Su M, Lu G, et al. Whole genome and transcriptome sequencing of matched primary and peritoneal metastatic gastric carcinoma. Sci Rep. 2015;5:13750. doi: 10.1038/srep13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn MC, Filali-Mouhim A, Provencher DM, Mes-Masson AM, Tonin PN. Reprogramming of the transcriptome in a novel chromosome 3 transfer tumor suppressor ovarian cancer cell line model affected molecular networks that are characteristic of ovarian cancer. Mol Carcinog. 2009;48:648–661. doi: 10.1002/mc.20511. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) methods. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie AJ, Campbell SL, Howe AK. Protein kinase A activity and anchoring are required for ovarian cancer cell migration and invasion. PLoS One. 2011;6:e26552. doi: 10.1371/journal.pone.0026552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheadle C, Nesterova M, Watkins T, Barnes KC, Hall JC, Rosen A, Becker KG, Cho-Chung YS. Regulatory subunits of PKA define an axis of cellular proliferation/differentiation in ovarian cancer cells. BMC Med Genomics. 2008;1:43. doi: 10.1186/1755-8794-1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alapati D, Rong M, Chen S, Hehre D, Hummler SC, Wu S. Inhibition of β-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2014;51:104–113. doi: 10.1165/rcmb.2013-0346OC. [DOI] [PubMed] [Google Scholar]
- 19.Ellerkamp V, Lieber J, Nagel C, Wenz J, Warmann SW, Fuchs J, Armeanu-Ebinger S. Pharmacological inhibition of beta-catenin in hepatoblastoma cells. Pediatr Surg Int. 2013;29:141–149. doi: 10.1007/s00383-012-3237-9. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Li Y, Zhang L, Guo H, Tian D, Li Y, Peng Y, Zheng Y, Dai Y, Xia K, et al. AKAP2 identified as a novel gene mutated in a Chinese family with adolescent idiopathic scoliosis. J Med Genet. 2016;53:488–493. doi: 10.1136/jmedgenet-2015-103684. [DOI] [PMC free article] [PubMed] [Google Scholar]