Abstract
A diagnosis of pancreatic cancer is devastating owing to its poor prognosis, with a 5-year survival rate of only 9%. Currently, most individuals are diagnosed at a late stage when treatment options are limited. Early detection of pancreatic cancer provides the greatest hope for making substantial improvements in survival. The Kenner Family Research Fund in partnership with the American Pancreatic Association has sponsored a series of fora to stimulate discussion and collaboration on early detection of pancreatic cancer. At the first forum in 2014, “Early Detection of Sporadic Pancreatic Cancer Summit Conference,” a strategic plan was set forth by an international group of interdisciplinary scientific representatives and subsequently The Strategic Map for Innovation was generated. The current conference report is the third forum in the series, “Early Detection of Pancreatic Cancer: The Role of Industry in the Development of Biomarkers,” which was held in Boston, Massachusetts, on October 27, 2016. This report provides an overview of examples of innovative initiatives by industry and confirms the critical need for collaboration among industry, government, research institutions, and advocacy groups in order to make pancreatic cancer more easily detectable in its earlier stages, when it is more treatable.
Key Words: early detection, PDAC, screening, biomarkers, high-risk group
Pancreatic cancer is an uncommon but deadly cancer. Its high mortality makes it the third leading cause of cancer-related death in the United States. It is the only major cancer with a rising mortality rate. Pancreatic cancer is on track to become the second leading cause of cancer-related deaths in the United States by 2030.1 A patient diagnosed with pancreatic cancer has a 5-year relative survival rate of 9%.2 In comparison, the survival rate for breast cancer is 90%, for colorectal cancer 67%, and for prostate cancer nearing 100%.3
Pancreatic cancer is characterized by late onset of symptoms, rapid progression, and death. However, no clinically useful test exists to identify early pancreatic cancer or high-grade pancreatic intraepithelial lesions. Currently, the only identified high-risk group for sporadic pancreatic cancer includes individuals with new-onset diabetes mellitus.4 Approximately 20% to 25% of people with pancreatic cancer develop diabetes within 6 to 36 months before their pancreatic cancer is diagnosed.5 With early detection, survival rate can increase 6-fold.6 Hence, the need for noninvasive, discriminatory biomarkers for early detection is urgent. Kenner Family Research Fund is dedicated to facilitating multidisciplinary approaches to early detection of pancreatic cancer. To further its goals, Kenner Family Research Fund in partnership with the American Pancreatic Association (APA) has sponsored a series of fora to stimulate discussion and collaboration on early detection of pancreatic cancer.
The first such forum held in 2014, “Early Detection of Sporadic Pancreatic Cancer Summit Conference”,6 gathered an international group of interdisciplinary scientific representatives to provide an in-depth summary of current efforts in the field, analyze gaps in specific areas of expertise, and identify challenges presented by pancreatic cancer. Ideas from this forum led to the development of a Strategic Map for Innovation7 (Fig. 1), which is an integrated process model with four congruent priorities: leadership, organizational structure and business planning, funding and partnerships, and research operations and initiatives. The core of the model is Facilitated Strategic Collaboration, which drives an accelerated pace of entrepreneurial organizational development, idea generation, significant research findings, and translation into clinical practice. The end goal is to develop an effective protocol for early detection of pancreatic cancer that can be used at the primary care level in health care systems.
FIGURE 1.

Strategic map for innovation (copyright Kenner Family Research Fund, 2015).
The second forum in 2015, “Early Detection of Pancreatic Cancer: Lessons Learned from Other Cancers”,8 built on this foundation with leading experts from breast, prostate, and colon cancers describing the development of early detection methods in their respective fields. A directive from the forum was that a major breakthrough in early detection of pancreatic cancer will be possible only through a definitive interdisciplinary collaborative effort involving a critical mass of committed academic research institutions, government agencies, industry leaders, and advocacy groups.
In line with the Strategic Map for Innovation, the 2016 forum “Early Detection of Pancreatic Cancer: The Role of Industry in the Development of Biomarkers” convened experts from government and industry to further examine the development of biomarkers. Sudhir Srivastava, PhD, PMH, MS, Chief of the Cancer Biomarkers Research Group of the National Cancer Institute and recipient of the APA Distinguished Service Award, provided an overview on biomarkers. Industry participants included Anne-Renee Hartman, MD, Medical Director and Head of Clinical Development at GRAIL; Laura Chirica, PhD, Chief Commercial Officer of Immunovia; Niven R. Narain, President and CEO of Berg Health; and A. James Moser, MD, Codirector, Associate Professor of Surgery at Harvard Medical School and Codirector of the Pancreas and Liver Institute, Beth Israel Deaconess Medical Center. Niven Narain and Dr. Moser are involved in a unique academic-industry collaborative research initiative called Project Survival. A robust discussion followed, which was facilitated by Suresh T. Chari, MD, and Stephen J. Pandol, MD. The audience of several hundred individuals was comprised of researchers from academia, government, and industry, clinicians, pharmaceuticals, and advocacy groups.
THE CURRENT STATE OF BIOMARKERS
To open the forum, Dr. Srivastava provided an overview on biomarkers. He discussed his concerns about researchers claiming biomarkers for clinical use before proven or selective reporting of findings. This practice makes it difficult for other researchers to work with those biomarkers or replicate findings. He advocated for open research on biomarkers that have not been validated. He noted that researchers must consider what the biomarker is going to be used for—biomarkers need to address a clinical question.
Collaboration is key, as the Pancreatic Cancer Detection Consortium and the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer are showing. Dr. Srivastava advocated for a team approach by industry, academia, and government scientists to systematically develop a biomarker. The focus of biomarker development should be for individuals who are considered to be at high risk for developing pancreatic cancer.
The Early Detection Research Network has developed and implemented systematic, comprehensive guidelines to develop, evaluate, and validate biomarkers, with rigor and reproducibility important for the scientific data.9,10 This 5-phase approach establishes both a standard and a road map for successfully translating research on biomarker applications from the laboratory to the bedside (Fig. 2).
FIGURE 2.
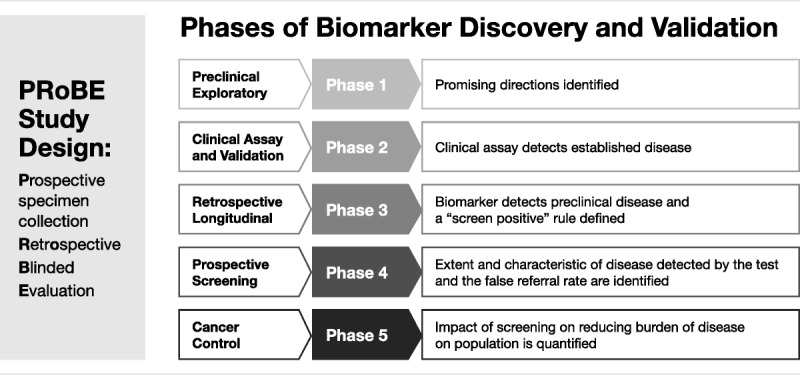
Phase 1 includes exploratory study to identify potentially useful biomarkers, which is the “discovery” phase.
Phase 2, the validation phase, is where biomarkers are studied to determine their capacity for distinguishing between people with cancer and those without.
Phase 3 determines the capacity of a biomarker to detect preclinical disease by testing the marker against tissues collected longitudinally from research cohorts.
Phase 4 includes prospective screening studies.
Phase 5 is when large-scale population studies evaluate not just the role of the biomarker for detection of cancer but the overall impact of screening on the population.
The Early Detection Research Network is primarily focused on biomarker discovery, development and validation for early detection of cancer. Biomarker research is actively funded through the traditional funding mechanisms, such as R01, R21, etc. In addition, there are a number of other National Cancer Institute (NCI)-supported programs that support biomarker research. Details on the various programs can be found on www.cancer.gov.
Dr. Srivastava concluded that the future looks brighter because of the current concerted efforts to find potential biomarkers for early detection.
FINDING TUMOR DNA IN THE BLOOD
One approach to early detection of cancer is to find circulating cell-free tumor DNA (cfDNA) in the blood. Anne-Renee Hartman, MD, explained that GRAIL was spun out of Illumina to develop a high-intensity sequencing approach to find tumor cfDNA before symptoms are apparent. The idea for pursuing noninvasive cancer screening grew out of Illumina's experience with noninvasive prenatal testing. Testing on 125,000 women found 10 whose blood showed evidence of chromosomal abnormalities and went on to deliver healthy babies—but who were subsequently diagnosed with cancer.
GRAIL’s mission is to develop technology that enables early detection of cancer before it presents at an advanced stage. The company is focusing on ensuring the technology will have high specificity to avoid false-positive results so that people do not undergo expensive or invasive follow-up procedures that are not needed.
Tumors shed cell-free nucleic acids into the blood but the levels are low early in cancer. To find the tumor DNA, GRAIL uses an approach that provides depth and breadth. The genomic regions are sequenced thousands of times to improve the signal-to-noise ratio, making the signal findable at very low levels. In addition, GRAIL sequences a large panel of genes, as the ability to detect a greater number of mutations makes it more likely to detect rare tumor DNA molecules.
In addition to nucleic acids shed from tumors, another source of mutations in plasma is white blood cells, a phenomenon called clonal hematopoiesis. The contribution of clonal hematopoiesis to signal in the plasma is currently being assessed.
Feasibility studies are being conducted through collaboration with Memorial Sloan Kettering on more than 1000 patients with various early-stage and late-stage cancers, including lung, prostate, colon, and breast cancers.
GRAIL will use tissue and plasma sequencing to look at concordance, address detection of early- versus late-stage disease, and changes in cell-free nucleic acid to determine correlation with tumor burden. The results of this collaborative effort will define the platform for GRAIL’s generation of larger-scale evidence.
GRAIL is initiating a study called the Circulating Cell-Free Genome Atlas, which involves some 10,000 participants including 7000 people with cancers across the 14 highest incidence types. Samples from early- and late-stage disease will be collected and compared with those of participants without cancer who have the same distribution of age, sex, and smoking history as the patients with cancer. With this information, GRAIL will develop predictive models for detection of cancer for clinical validation and utility studies.
Dr. Hartman noted that for pancreatic cancer, GRAIL’s test panel could possibly detect cancer at stage I or II, before it metastasizes.
AN ANTIBODY MICROARRAY-BASED BLOOD TEST
Immunovia has an antibody microarray-based blood test for early cancer diagnosis now in its validation phase. Called IMMray™ PanCan-d, it uses the IMMray technology platform to locate biomarkers in blood, which would indicate the presence of pancreatic cancer. Laura Chirica, PhD, explained that because Immunovia is in the process of publishing its results, it cannot provide information about the biomarker signatures they are using. The company aims to have IMMray™ PanCan-d on the market in 2017 or 2018.
Dr. Chirica outlined how this test was developed. They started with the hypothesis that the blood is the source of all information needed to understand, diagnose, and decipher cancers and autoimmune diseases. After analyzing their proprietary antibody library, Immunovia researchers decided that the platform will be based on the single-chain fragment variable of the antibody, as it is the most stable, active, and contains all the necessary information. They then focused to produce the single chain fragment variable of the antibodies and optimized the surface and the binding of the antibodies to the surface of the microarray. Starting with up to 2000 antibodies, they used the microarray to narrow it down to 450 antibody specificities, and then between 20 and 35 antibodies for the microarray slide for commercialization.
The next step was to test in blood samples. Over a decade, a total of 6 clinical studies were conducted with approximately 2500 to 2650 blood samples. To define the protein signature, 1500 samples were used to discriminate pancreatic cancer versus pancreatitis and healthy individuals. The test was found to have a high specificity and sensitivity of more than 95%. Tests conducted in China with a lower-resolution scanner produced similar results. The samples from China included stage I and stage II cancers. Further tests to validate the early stages results were conducted in Lund, Sweden using samples from all 4 stages as well as controls, and with Oregon Health & Science University, both with similar results.
Dr. Chirica explained that IMMray™ PanCan-d can be used for curable stage diagnosis in high-risk individuals, both asymptomatic and symptomatic. These high-risk groups include familial/hereditary pancreatic cancer asymptomatic individuals, patients presenting early/vague symptoms, and new-onset diabetes type II older than 50 years old. Immunovia is working on a prospective validation study for early diagnosis in asymptomatic individuals. This current study will include many different study centers conducting high-risk surveillance programs. They are also working on setting up a confirmatory diagnosis study with a reference center in Europe along with a retrospective and a subsequent prospective validation. Immunovia will be looking for additional partners, particularly for the prospective study validation. Agreements with Mount Sinai Medical Center, New York, NY; Oregon Health & Science University, Portland, Ore; Ramon Y Cajal University Hospital, Madrid, Spain; and with University Hospital in Liverpool, UK, are already completed and several others will be added during 2017 and 2018.11–13
PROJECT SURVIVAL
Patients with pancreatic cancer need data-driven tools to identify their disease earlier and to personalize treatment decisions.
Dr. A. James Moser, MD, serves as the national principal investigator for Project Survival. Project Survival is a unique cross sector collaboration between academia (Beth Israel Deaconess Medical Center, Boston, Mass) and industry (BERG, Framingham, Mass) created to discover and validate biomarkers to improve treatment decisions for patients with pancreatic cancer. This program works through an established clinical trial network (Pancreatic Cancer Research Team, Scottsdale, Ariz) with expert biostatistical support (Cancer Research and Biostatistics, Seattle, Wash). Project Survival incorporates a sector-leading discovery platform and bioinformatics effort, expertise in translational science, and real-time quality control metrics to accelerate treatment decisions for patients with pancreatic cancer.
Phase I of the project established a human tissue and data biorepository, central pathology mechanism with digital image archiving, and Web-based electronic data capture system to annotate and track urine, plasma, serum, tissue, and saliva samples. Phase II used existing samples in the Pancreatic Cancer Research Team serum bank to establish a biomarker training set for prospective validation. Phase II analyzed 115 pancreatic cancer and pancreatitis samples and ran them through the biology and artificial intelligence (AI) platforms. The result was an interactome of proteins, metabolites, and lipids with clinical outcome association leading to novel diagnostic findings. Project Survival is monitoring patients through their treatment experience through the serial collection of biofluids at various treatment stages: presurgery and postsurgery, chemotherapy, and radiation. This provides a dynamic assessment of biological changes that occur during the treatment paradigm leading to multiple diagnostic solutions for a range of molecularly informed treatment options for pancreatic cancer patients. To streamline this process, AI is engaged through this entire process to provide unbiased solutions.
Niven R. Narain outlined that there are critical unmet needs in diagnostics, prognostics, and theranostics in the diagnosis and treatment of pancreatic cancer. Clinicians need to be able to use biomarkers to stratify treatment decisions prior to surgery, chemotherapy, or clinical trial engagement. Biomarker research must account for factors beyond laboratory validation but also to determine the optimal dose for pharmaceuticals to minimize toxicity. He emphasized that cancer is more than just tumor. Beyond tumor genetics and molecular profiling, the tumor microenvironment and the systems microenvironment must also play a fundamental role. The most sustainable biomarkers may be from the environment around the cancer, and that information must be captured.
The important question for the biomarker researcher is how wide and how deep to go? Just because something is expressed in biology does not mean that it drives a substantive change in function. Project Survival performs an integrated phenomic assessment on every single tissue and biofluid sample capturing the full biological and physiological narrative of the patient at that given time. The expression data must be correlated with the functional or phenotypic data to appreciate the complexity of the underlying clinical phenotypes. Narain emphasized that precision medicine means understanding clinical phenotypes at all stages of cancer. Deep molecular profiling provides the comprehensive understanding of a patients’ health, which allows for informed decision making regarding treatment strategies.
DISCUSSION
The forum’s discussion session, facilitated by Suresh T. Chari, MD, and Stephen J. Pandol, MD, highlighted the government requirements for a test to be used in the clinic setting. Dr. Srivastava indicated that first of all, the clinical indication needs to be defined. For example, if the clinical indication is screening pancreatic cancer, the bar for approval is extremely high and by far the most difficult to achieve. Biomarkers need to be tested in an asymptomatic population to determine how they actually affect mortality. Early detection is the second highest bar, he explained further. If the focus of the biomarker is stage I disease, then the goal is to determine whether the biomarker is able to detect stage I. The sensitivity and specificity must be very high for that stage. The third lower bar is to detect lesion progression. Except for screening, randomized trials are not required for Food and Drug Administration approval.
Other discussion questions focused on sampling bias, levels of specificity and sensitivity required for a general population screening test versus a test targeted for a high-risk population, and the use of blood samples versus pancreatic fluid for discovery. One audience participant reminded those in attendance that there is no reason that [discovery] needs to be linear.
There was agreement that understanding tumor development is aligned with a whole body approach and learning about healthy individuals, which is often under the umbrella of preventive medicine. Within this context, the value of machine learning versus AI was discussed, with the former being defined as learning new information from existing data, whereas the latter is learning what is unknown.
Finally, the economic barrier inherent in collaborative efforts was examined, with the use of reservoirs such as the Department of Defense or the Genomics England Initiative as examples of opportunities to study larger populations.
CONCLUSION
The 2016 forum “Early Detection of Pancreatic Cancer: The Role of Industry in the Development of Biomarkers”, presented by Kenner Family Research Fund and the APA, highlighted examples of industry-driven approaches to early detection. Subsequent to the forum, the Alliance of Pancreatic Cancer Consortia for Early Detection met in December 2016 at the NCI. The objective of this meeting was to bring together investigators funded through NCI-supported programs on pancreatic cancer detection and stakeholders that are supporting biomarker research to discuss and debate existing or newly developed biomarkers that are likely to change the clinical management of pancreatic cancer. The enthusiastic support for the cause shown by the NCI, industry, advocacy groups, and academia was heartening to see and bodes well for the successful emergence of a strategy for early detection in the new future. The implementation of the Strategic Map for Innovation has now been initiated and organized, offering an earlier diagnosis, a better prognosis, improved quality of life and longer life expectancy for those individuals diagnosed with pancreatic cancer, which is our ultimate goal.8
ACKNOWLEDGMENTS
Presenters in the forum Early Detection of Pancreatic Cancer – The Role of Industry in the Development of Biomarkers were Sudhir Srivastava, PhD, MPH, MS, Chief of the Cancer Biomarkers Research Group, Division of Cancer Prevention, National Cancer Institute, Rockville, MD; Anne-Renee Hartman, MD, Medical Director and Head of Clinical Development at GRAIL; Laura Chirica, PhD, Chief Commercial Officer of Immunovia; Niven R. Narain, President and CEO of Berg Health; and A. James Moser, MD, Codirector, Associate Professor of Surgery at Harvard Medical School and Codirector of the Pancreas and Liver Institute, Beth Israel Deaconess Medical Center. Niven Narain and Dr. Moser are involved in a unique academic-industry collaborative research initiative called Project Survival. Suresh T. Chari, MD, from Mayo Clinic and Stephen J. Pandol, MD, from Cedars-Sinai served as facilitators. Forum-planning team members were Ann E. Goldberg, BA; Laura J. Rothschild, MBA; Suresh T. Chari, MD; Vay Liang W. Go, MD; Anirban Maitra, MBBS; Sudhir Srivastava, PhD, MPH, MS; Bruce Field; and Barbara J. Kenner, PhD. The authors thank Susan Randel for editing and review of the manuscript.
Footnotes
This work received no financial support.
The authors declare no conflict of interest.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 4.Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal G, Rabe KG, Petersen GM, et al. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology. 2012;12:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenner BJ, Chari ST, Cleeter DF, et al. Early detection of sporadic pancreatic cancer: strategic map for innovation—a white paper. Pancreas. 2015;44:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenner BJ, Chari ST, Maitra A, et al. Early detection of pancreatic cancer—a defined future using lessons from other cancers: a white paper. Pancreas. 2016;45:1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. [DOI] [PubMed] [Google Scholar]
- 10.Pepe MS, Feng Z, Janes H, et al. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100:1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerdtsson AS, Malats N, Säll A, et al. A multicenter trial defining a serum protein signature associated with pancreatic ductal adenocarcinoma. Int J Proteomics. 2015;2015:587250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delfani P, Dexlin Mellby L, Nordström M, et al. Technical advances of the recombinant antibody microarray technology platform for clinical immunoproteomics. PLoS One. 2016;11:e0159138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrebaeck CA. Precision diagnostics: moving towards protein biomarker signatures of clinical utility in cancer. Nat Rev Cancer. 2017;17:199–204. [DOI] [PubMed] [Google Scholar]


