Abstract
KEYNOTE-030 (ClinicalTrials.gov ID, NCT02083484) was a global expanded access program that allowed access to pembrolizumab, an antiprogrammed death 1 antibody, for patients with advanced melanoma before its regulatory approval. Patients with unresectable stage III/IV melanoma that progressed after standard-of-care therapy, including ipilimumab and, if BRAFV600 mutant, a BRAF inhibitor, were eligible to receive pembrolizumab 2 mg/kg every 3 weeks. Response was assessed by immune-related response criteria by investigator review. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. In the United States, 979 patients enrolled between April and September 2014. Of the 947 evaluable patients, 621 (65.6%) remained on treatment and transitioned to receive commercial pembrolizumab following approval by the Food and Drug Administration, whereas 326 (34.4%) discontinued, most commonly for disease progression (39.6%) or death (26.4%). Objective response rate was 14.5% (95% confidence interval, 12.2%–16.8%) in the treated population (n=947) and 22.1% (95% confidence interval, 18.8%–25.5%) in patients who had ≥1 response assessment reported (n=619). Twelve patients achieved complete response. One hundred eighty-one (19.1%) patients experienced ≥1 treatment-related AE, most commonly general disorders (8.0%), skin/subcutaneous tissue disorders (7.3%), and gastrointestinal disorders (6.4%); 29 (3.1%) patients experienced ≥1 grade 3/4 treatment-related AE. Immune-mediated AEs were also reported. There were no treatment-related deaths. The safety and efficacy observed in this expanded access program were consistent with those previously reported for similar populations and support the use of pembrolizumab for patients with advanced melanoma.
Key Words: expanded access program, immunotherapy, melanoma, PD-1, pembrolizumab
Melanoma incidence continues to rise both in the United States and globally, with 87,110 new cases and 9730 related deaths estimated in 2017 in the United States alone.1 The prognosis for patients with advanced disease has been historically poor; however, advances in immunotherapy as a therapeutic option for many tumors, including melanoma, have improved outcomes for this patient population.
Immune checkpoints are costimulatory or coinhibitory signals that maintain self-tolerance and limit collateral tissue damage during an immune response.2,3 Cytotoxic T-lymphocyte–associated antigen 4 is a coinhibitory molecule that inhibits T-cell proliferation.4–6 Another immune checkpoint, programmed death 1 (PD-1), acts within the tumor microenvironment to negatively regulate T-cell response at the effector stage.4,7 Tumors are able to co-opt immune checkpoint pathways to evade an antitumor immune response;4,8,9 thus, checkpoint blockade is an effective strategy for treating many tumors, including melanoma. The anticytotoxic T-lymphocyte–associated antigen 4 antibody ipilimumab was the first checkpoint inhibitor approved for the treatment of advanced melanoma in 2011 and was the first drug to demonstrate improved survival for this patient population.10,11 More recently, the PD-1–blocking drugs pembrolizumab12–15 and nivolumab16–19 have further improved outcomes in patients with metastatic melanoma.
The anti-PD-1 humanized monoclonal antibody pembrolizumab has demonstrated efficacy and manageable toxicity in patients with advanced melanoma enrolled in 3 large clinical trials. In the phase IKEYNOTE-001 study (ClinicalTrials.gov ID, NCT01295827; n=655), pembrolizumab demonstrated durable antitumor activity and a manageable safety profile in patients with ipilimumab-naive and ipilimumab-treated advanced melanoma.13 In the phase II KEYNOTE-002 study (ClinicalTrials.gov ID, NCT01704287; n=540), pembrolizumab demonstrated superior progression-free survival and objective response rate (ORR) and had less high-grade toxicity compared with investigator-choice chemotherapy in patients with ipilimumab-treated advanced melanoma.20 In addition, in the phase III randomized KEYNOTE-006 study (ClinicalTrials.gov, NCT01866319; n=834), pembrolizumab demonstrated superior overall survival, progression-free survival, and ORR, and less high-grade toxicity, compared with ipilimumab in patients with ipilimumab-naive advanced melanoma who received ≤1 prior therapy.21 Pembrolizumab is currently approved in more than 60 countries for 1 or more advanced malignancies, including unresectable or metastatic melanoma based on the results of the aforementioned studies, and is being evaluated in >30 additional solid tumors and hematologic malignancies.
KEYNOTE-030 (ClinicalTrials.gov, NCT02083484), the pembrolizumab multisite global expanded access program (EAP), was initiated in April 2014 based on the key melanoma clinical data described above to address the unmet medical need for patients with metastatic melanoma whose disease progressed on prior systemic treatments or for whom limited or no treatment options existed. The EAP was not designed as a clinical trial, but rather as a streamlined approach to provide patients who had no other therapeutic options access to pembrolizumab during the time between completion of enrollment of pembrolizumab clinical trials and its regulatory approval. As such, data collection and formal analyses were limited in scope. Here, we report the results for the cohort of patients enrolled in the EAP from the United States.
MATERIALS AND METHODS
Patients
Eligibility criteria for the EAP included age ≥12 years; unresectable (stage III) or metastatic (stage IV) melanoma with progression on standard systemic therapy including ipilimumab, and when indicated, a BRAF/MEK inhibitor; Eastern Cooperative Oncology Group performance status 0 or 1; adequate organ function; and provision of written informed consent. Patients were excluded if they had participated in or were eligible for a pembrolizumab clinical trial or for treatment with an approved BRAF/MEK inhibitor. Additional exclusion criteria included unresolved adverse events (AEs) from prior therapy; active central nervous system metastases (patients with previously treated brain metastases were eligible provided they were clinically stable as assessed by the treating physician); or history of pneumonitis, organ transplant, human immunodeficiency virus infection, active infection with hepatitis B or hepatitis C virus, or autoimmune disease requiring long-term immunosuppressive therapy. The EAP enrolled approximately 5800 patients worldwide.
Treatment and Assessments
Eligible patients received intravenous pembrolizumab 2 mg/kg every 3 weeks; treatment continued for 24 months or until confirmed disease progression per immune-related response criteria (irRC),22 unacceptable toxicity, confirmed positive pregnancy test, withdrawal of consent, physician decision, or approval of pembrolizumab in the country of treatment, whichever occurred first. The planned end of enrollment was designated as the time at which pembrolizumab became commercially available following regulatory approval. Patients enrolled at the time of approval could receive up to 2 additional treatment cycles through the EAP while transitioning to treatment with commercial pembrolizumab.
Per protocol, and similar to some other EAPs, extensive data collection and analyses were not required for this program. Clinical assessments were performed according to the standard of care at the treating institution or center. Tumor response was assessed per irRC by investigator review. Laboratory safety assessments were performed locally before the first pembrolizumab dose and before each treatment cycle thereafter. AEs were collected continuously until treatment discontinuation and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Prespecified immune-mediated AEs and serious AEs were reported within 24 hours. Treatment-related nonserious AEs were reported within 5 days.
Pembrolizumab was withheld for grade >2 treatment-related nonhematologic AEs (excluding fatigue). Upon recovery of the event to grade 0–1, dose modification (ie, increasing the dosing interval in subsequent cycles to 4 wk) was permitted. Treatment discontinuation was recommended for treatment-related AEs that did not resolve to grade 0–1 within 12 weeks after onset of the AE and for treatment-related severe or life-threatening AEs. Immune-mediated AEs were managed with the use of corticosteroids; discontinuation was recommended for grade 2 treatment-related AEs thought to be immune-mediated that persisted without improvement for >4 weeks and for the inability to reduce corticosteroid dose for the management of such AEs to the equivalent of ≤10 mg of prednisone daily. Additional information regarding the management of immune-mediated AEs was provided to all sites in the form of an Event of Clinical Interest and Immune-related Adverse Event Guidance Document.
Program Oversight
The EAP was conducted in accordance with the protocol, good clinical practice standards, and the Declaration of Helsinki. The protocol and all amendments were approved by the relevant institutional review boards or independent ethics committees at all participating sites in the United States. All patients provided written informed consent. Patient data and trial operations were monitored by the program sponsor through remote data monitors who were in contact with the sites.
Statistical Analysis
Efficacy and safety were evaluated in all enrolled patients who received ≥1 dose of pembrolizumab. Efficacy was also reported in the subset of patients with ≥1 postbaseline tumor assessment because tumor assessments were limited in the full treatment population by those patients who transitioned to commercial pembrolizumab before they had any response assessment. Patient demographics were reported using descriptive statistics. The ORR was calculated and reported as the number of complete or partial responses per irRC by investigator review, divided by the total number of evaluable patients in the efficacy population. Safety data were summarized using frequencies and percentages. The data cutoff for analysis was March 31, 2015. Data were not collected once patients transitioned to commercial pembrolizumab.
RESULTS
Patients
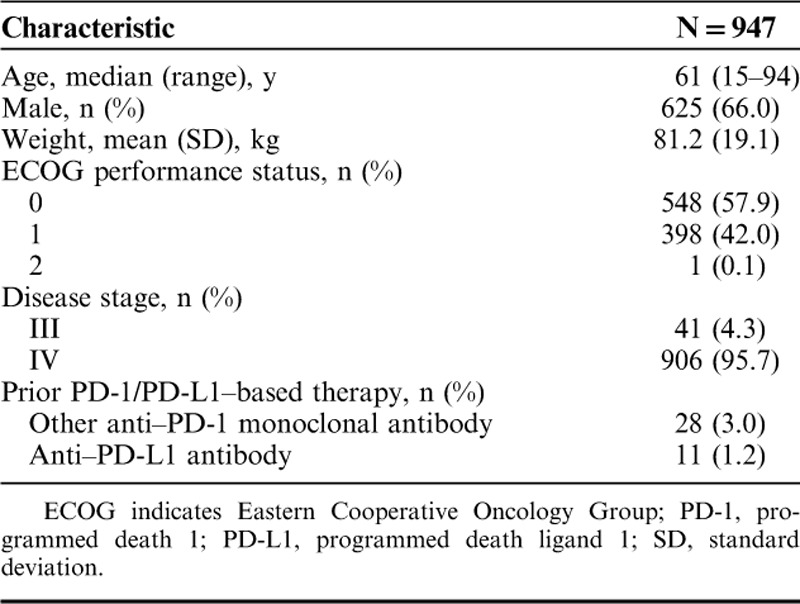
A total of 979 patients from 90 sites in the United States provided informed consent between April and September 2014 (Fig. 1). Of these, 947 patients received ≥1 dose of pembrolizumab (Fig. 1) and were included in the efficacy and safety analyses. Baseline patient characteristics were as expected for an advanced melanoma population with progression after ipilimumab and, if eligible, a BRAF/MEK inhibitor (Table 1). Median patient age was 61 years (range, 15–94 y), 66.0% were male, 57.9% had an Eastern Cooperative Oncology Group performance status of 0, and 95.7% had stage IV disease.
FIGURE 1.
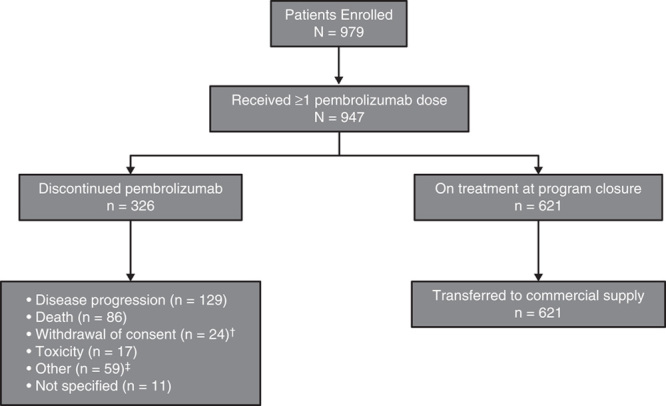
Patient disposition; †one patient who experienced a complete response and 2 patients who experienced a partial response; ‡clinical progression (n=20), transfer of care (n=15), decline in performance status (n=7), investigator decision (n=3), lost to follow-up (n=3), brain metastases (n=2), progressive disease per positron-emission tomography (n=2), complete response (n=1), lack of response (n=1), and treatment for a secondary malignancy [myelodysplastic syndrome (n=1)].
TABLE 1.
Baseline Demographics and Disease Characteristics of the Treated Population
The program was closed to enrollment in the United States as of September 4, 2014, the date of pembrolizumab approval by the Food and Drug Administration. At the time of completion of enrollment, the median duration of pembrolizumab treatment was 64 days (range, 1–190 d), and 621 (65.6%) patients remained on treatment and transitioned to receive commercial pembrolizumab (Fig. 1). The remaining 326 (34.4%) patients discontinued pembrolizumab treatment before enrollment was complete; the most common reasons for discontinuation were radiographic disease progression (39.6%) and death (26.4%; Fig. 1). Additional reported reasons for discontinuing pembrolizumab treatment before completion of enrollment were withdrawal of consent (7.4%), toxicity (5.2%), other reasons [18.1%; includes clinical progression, transfer of care, decline in performance status, investigator decision, lost to follow-up, brain metastases, progressive disease per positron-emission tomography, complete response, lack of response, treatment for a secondary malignancy (myelodysplastic syndrome in 1 patient)], and reason not specified (3.4%).
Efficacy
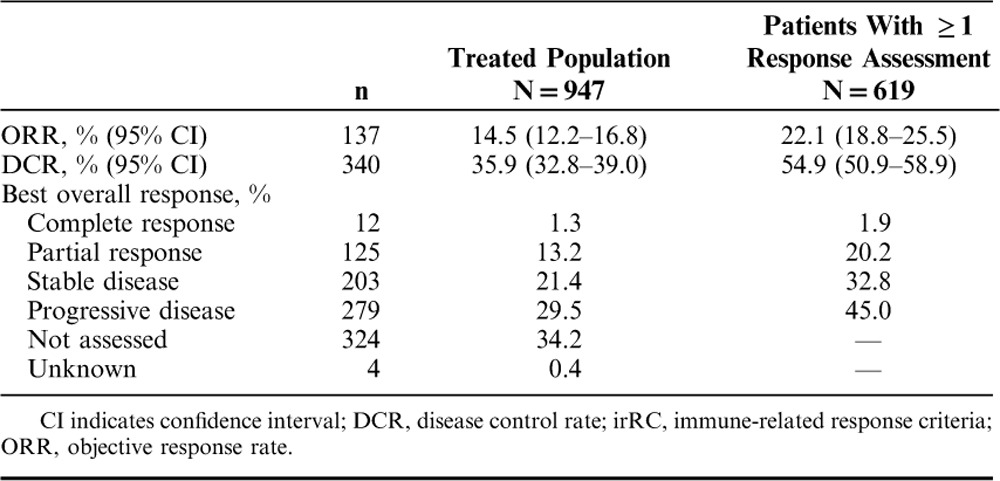
The ORR per irRC by investigator assessment was 14.5% (95% confidence interval, 12.2%–16.8%) in the entire treated population of 947 patients (regardless of whether response data were reported) and was 22.1% (95% confidence interval, 18.8%–25.5%) in the 619 patients who had ≥1 response assessment reported while enrolled in the program (Table 2). Best overall response in the 619 patients who had ≥1 response assessment was complete response in 12 (1.9%) patients and partial response in 125 (20.2%) patients; an additional 203 (32.8%) patients achieved stable disease (Table 2).
TABLE 2.
Best Overall Response per irRC by Investigator Assessment
Safety
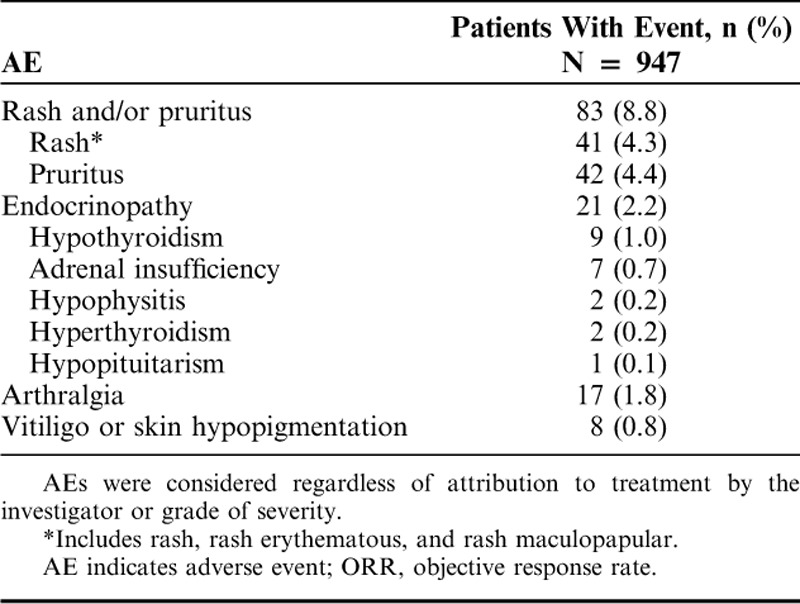
A total of 400 (42.2%) patients experienced ≥1 AE, regardless of grade or attribution to study treatment; treatment-related AEs were reported in 181 (19.1%) patients (Table 3). The most frequently reported treatment-related AEs of any grade fell under the system organ class categories of general disorders and administration site conditions (included fatigue; 8.0%), skin and subcutaneous tissue disorders (7.3%), and gastrointestinal disorders (6.4%; Fig. 2). A total of 29 (3.1%) patients experienced ≥1 grade 3–4 treatment-related AE (Table 3). The most common possibly immune-mediated AEs were rash (4.3%) and pruritus (4.4%; Table 4). There were no treatment-related deaths reported (Table 3), and only 17 (1.8%) patients discontinued therapy because of treatment-related toxicity (Fig. 1).
TABLE 3.
Summary of AEs in Patients Who Received ≥1 Dose of Pembrolizumab
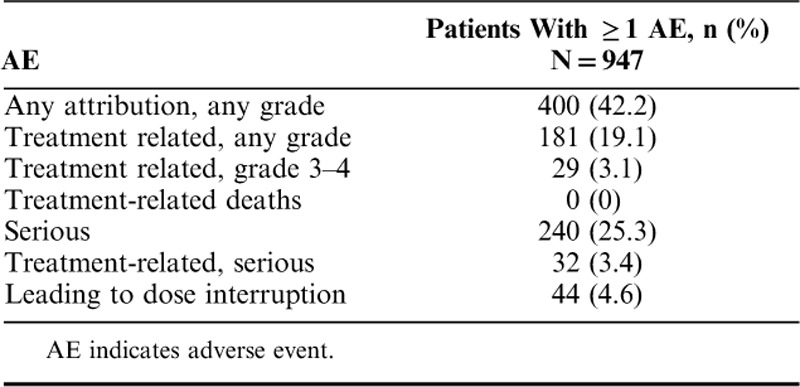
FIGURE 2.
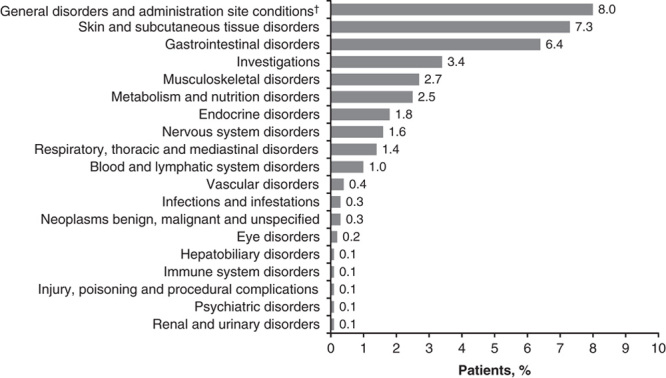
Incidence of treatment-related adverse events of any grade by system organ class. Patients could have experienced >1 treatment-related adverse event in >1 system organ class. There were no cardiac disorders, reproductive system and breast disorders, or surgical and medical procedures that were considered to be treatment related; †fatigue.
TABLE 4.
AEs of Interest Based on Possible Immune Etiology
DISCUSSION
In 3 key clinical trials,13,20,21 pembrolizumab demonstrated a favorable risk/benefit profile in both previously treated and treatment-naive patients with advanced melanoma. The KEYNOTE-030 global EAP for patients with stage III or IV melanoma whose disease progressed on ipilimumab and a BRAF/MEK inhibitor (when indicated) was designed to fill an unmet treatment need during the time between the completion of enrollment of pembrolizumab clinical trials and its commercial availability for those patients without other therapeutic options.
The 22.1% ORR observed in the 619 patients who had ≥1 response assessment was slightly lower than expected compared with clinical trials of pembrolizumab in mixed patient populations. In the KEYNOTE-001 trial, ORR as assessed per Response Evaluation Criteria In Solid Tumors, version 1.1 (RECIST v1.1) by central imaging vendor review was reported as 33% in the overall population, 29% among ipilimumab-treated patients, and up to 45% in the subset of treatment-naive patients.13 More recently, the ORR in the KEYNOTE-006 trial was reported as 33%–34%, depending on pembrolizumab dosing regimen, in patients who were ipilimumab naive.21 The patient populations in the KEYNOTE-001 and KEYNOTE-006 trials, however, were not closely matched to that of the EAP, whereas the KEYNOTE-002 trial included eligibility criteria similar to those of the EAP (eg, stage III or IV melanoma and previous treatment with ipilimumab and a BRAF inhibitor if BRAFV600 mutant). Although data on M substage were not collected for this EAP, we would expect a distribution similar to that of KEYNOTE-002, in which the majority of patients (82%–83%) treated with pembrolizumab had stage M1c disease.20 Accordingly, the ORR in KEYNOTE-002 was comparable to that observed in the EAP (21%–25% for the 2 pembrolizumab dose cohorts studied).20 Similar response rates have been reported for nivolumab, also in varying patient populations.16–19
Differences in ORR may be attributable to slight differences in the patient populations. Of note, unlike the clinical trials of pembrolizumab, this program included patients with uveal or mucosal melanoma, who may have lower response rates than patients with primary cutaneous melanoma.23,24 Although published data are limited on patients with uveal or mucosal melanoma treated with immunotherapies, lower rates of response for these populations are typically reported. Recent studies report an ORR of 0%–4% in patients with uveal melanoma23,25,26 and of 19%–23% in patients with mucosal melanoma.27,28 It is interesting to note that, in published case reports of a small subset of patients with uveal melanoma from this EAP, ORR was higher (37.5%) than in the overall population;29 however, an additional 3 patients, at least 1 of whom had a partial response, received commercial pembrolizumab outside of the EAP and were included in this response assessment, which might have contributed to the discrepancy in response rates between the case studies and the data reported here.29 In addition, post hoc analyses of outcomes in KEYNOTE-00130 and KEYNOTE-00631 found that patients with elevated lactate dehydrogenase (LDH) levels (>1× the upper limit of normal) had a lower ORR than patients with normal LDH levels. Although data on LDH level were not collected for the EAP, we would expect the distribution of LDH to be similar to that of KEYNOTE-002, in which 55%–58% of patients had normal LDH levels and 40%–43% had elevated LDH levels.20 Thus, inclusion of patient populations with uveal or mucosal melanoma and elevated LDH levels might have contributed to a lower overall ORR in this program.
The use of different response criteria, including the use of unconfirmed response by investigator assessment in the EAP, might also have contributed to differences in response rates between the EAP and clinical trials. Unlike irRC, conventional response criteria, such as RECIST v1.1, do not consider the fact that disease stabilization can occur after an initial increase in tumor burden, so response rates are typically lower than with irRC. Previous studies in melanoma have demonstrated that atypical response patterns (eg, disease stabilization after initial evidence of disease progression) were observed in 7% of patients treated with pembrolizumab32 and in 10% of patients treated with ipilimumab22 using irRC. However, in the EAP, response data were not submitted for all 947 evaluable patients because many of them (34.6%) had not yet reached their first response assessment when they transitioned to commercial drug supply at the time of program closure, and, as such, more patients with response might have been reported as having had clinical progression. Furthermore, responses to pembrolizumab are generally durable, as demonstrated in KEYNOTE-00133 and KEYNOTE-006,34 in which 97% and 93% of responses, respectively, were ongoing with 3 years of follow-up; we would therefore expect a similar outcome with the EAP if patients had continued the program. Thus, the results reported here must be interpreted within the context of the short treatment duration and follow-up times of the EAP because the potential for capturing atypical and durable responses was limited. Despite these differences, the response rate in the EAP is reassuringly similar to the response rates reported in the pembrolizumab clinical trial program.
In this EAP, pembrolizumab was well tolerated in patients with advanced melanoma whose disease progressed after standard-of-care therapy, with an observed safety profile consistent with that reported for similar populations in the KEYNOTE-001,13 KEYNOTE-002,20 and KEYNOTE-00621 studies. No new or unexpected AEs or treatment-related deaths were reported.
Similar to the pembrolizumab clinical trials, fatigue was the most frequent AE observed in the EAP. The lower rates of any grade (19.1%) and grade 3–4 (3.1%) treatment-related AEs reported in this EAP compared with the clinical trials are likely related in part to decreased reporting because the program was monitored remotely, with individual sites responsible for the collection and reporting of AE data for their patients. Thus, nonserious AEs were likely underreported. In addition, because this analysis included all patients who were enrolled up until the designated program end at the time of regulatory approval and commercial availability of pembrolizumab, median duration of treatment was only 64 days (range, 1–190 d) at the time of program closure. At this time, 621 (65.6%) patients remained on treatment and transitioned to receive commercial pembrolizumab. Had the median duration of treatment and follow-up period been longer for more patients, additional AEs would likely have been reported, with expected frequencies closer to those reported in the clinical trials. Therefore, the lower incidence of AEs reported here may be attributed to the underreporting of nonserious AEs in combination with the short follow-up times in the EAP.
This EAP was not designed to evaluate the feasibility and outcomes of treating patients with poor performance status, decreased organ function, uncontrolled brain metastases, or autoimmune disease with pembrolizumab; ongoing clinical trials will address PD-1 blockade in these subsets of patients. Despite the limitations inherent in the EAP, these data support the use of pembrolizumab for patients with advanced melanoma outside of the clinical trial setting. The administration of pembrolizumab was manageable across the broad scope of clinical treatment practices encompassed in this large EAP, demonstrating the feasibility of treating this population of patients in community practice.
ACKNOWLEDGMENTS
The authors thank the patients and their families and caregivers, as well as all investigators and site personnel. The authors also thank Roger Dansey, MD (Merck & Co. Inc., Kenilworth, NJ) for critical manuscript review. Medical writing and editorial assistance was provided by Tricia Brown, MS, and Payal Gandhi, PhD (the ApotheCom pembrolizumab team, Yardley, PA) and was funded by Merck & Co. Inc. The EAP was funded by Merck & Co. Inc.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
T.C.G. reports grants to her institution from Merck & Co. Inc., Kenilworth, NJ related to the submitted work; board membership from Merck & Co. Inc., Kenilworth, NJ; consulting fees from Bristol-Myers Squibb; grants from Merck & Co. Inc., Kenilworth, NJ, Bristol-Myers Squibb, Incyte, and Roche; and payment for development of educational presentations from Novartis and Medscape unrelated to the submitted work. W.-J.H. reports grants to her institution from Merck & Co. Inc., Kenilworth, NJ related to the submitted work; and grants to her institution from Bristol-Myers Squibb, Glaxo-SmithKline, and MedImmune unrelated to the submitted work. M.A.P. reports honoraria from Merck & Co. Inc., Kenilworth, NJ related to the submitted work; consulting fees from Bristol-Myers Squibb and Novartis; and grants from Bristol-Myers Squibb unrelated to the submitted work. O.H. reports consulting fees from Merck & Co. Inc., Kenilworth, NJ related to the submitted work; consulting fees from Amgen, Novartis, Roche, Bristol-Myers Squibb, and Merck & Co. Inc., Kenilworth, NJ; speaker fees from Bristol-Myers Squibb, Genentech, Novartis, and Amgen; and research fees from AstraZeneca, Bristol-Myers Squibb, Celldex, Genentech, Immunocore, Incyte, Merck & Co. Inc., Kenilworth, NJ, Merck-Serono, MedImmune, Novartis, Pfizer, Rinat, and Roche unrelated to the submitted work. A.D. reports grants to his institution from Merck & Co. Inc., Kenilworth, NJ related to the submitted work. R.D. reports provision of writing assistance, medicines, equipment, or administrative support to her institution from Merck & Co. Inc., Kenilworth, NJ related to the submitted work. R.J. reports consulting fees from Merck & Co. Inc., Kenilworth, NJ unrelated to the submitted work. S.J.O’D. reports grants to his institution from Merck & Co. Inc., Kenilworth, NJ related to the submitted work; consulting and speaker fees from Merck & Co. Inc., Kenilworth, NJ; and grants to his institution from Merck & Co. Inc., Kenilworth, NJ unrelated to the submitted work. F.S.H. reports grants to his institution from Merck & Co. Inc., Kenilworth, NJ related to the submitted work; consulting fees from Merck & Co. Inc., Kenilworth, NJ, Genentech, EMD Serono, Novartis, and Amgen; grants to his institution from Bristol-Myers Squibb; and pending patent-related royalties to his institution unrelated to the submitted work. A.C.P. reports no conflicts of interest. H.K. reports grants to her institution from Merck & Co. Inc., Kenilworth, NJ related to the submitted work; grants to her institution from Bristol-Myers Squibb; and consulting fees from Alexion, Genentech, and Regeneron unrelated to the submitted work. R.P.O. is an employee of Clinigen, a commercial company that runs EAPs for Merck & Co. Inc., Kenilworth, NJ both related and unrelated to the submitted work. A.Y. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ. M.G. was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ during the conduct of the study. D.A.K. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, and holds stock in the company. S.E. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, and holds stock in the company. A.K.S.S. reports grants to her institution from Bristol-Myers Squibb, Merck & Co. Inc., Kenilworth, NJ, Roche-Genentech, Celldex, Reata, and Immunocore; and consulting fees from Bristol-Myers Squibb unrelated to the submitted work.
Footnotes
Present address: Mihaela Gazdoiu, Novartis Pharmaceuticals, East Hanover, NJ.
T.C.G, W.-J.H., D.A.K., and S.E.: conceived, designed, or planned the study; T.C.G., W.-J.H., M.A.P., O.H., R.D., R.J., S.J.O’D., F.S.H., A.C.P., H.K., and D.A.K.: acquired the data; T.C.G., O.H., F.S.H., R.P.O., A.Y., M.G., S.E., and A.K.S.S.: analyzed the data; T.C.G., W.-J.H., M.A.P., O.H., A.D., R.D., S.J.O’D., F.S.H., A.C.P., H.K., M.G., S.E., and A.K.S.S.: interpreted the data; T.C.G., M.G., and S.E.: wrote the manuscript; T.C.G., W.-J.H., M.A.P., O.H., A.D., R.D., R.J., S.J.O’D., F.S.H., H.K., R.P.O, A.Y., D.A.K., S.E., and A.K.S.S.: critically reviewed the manuscript; all authors approved the final manuscript.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. [DOI] [PubMed] [Google Scholar]
- 3.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. [DOI] [PubMed] [Google Scholar]
- 9.Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. [DOI] [PubMed] [Google Scholar]
- 14.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. [DOI] [PubMed] [Google Scholar]
- 15.Robert C, Joshua AM, Weber JS, et al. Pembrolizumab (pembro; MK-3475) for advanced melanoma (MEL): randomized comparison of two dosing schedules. Ann Oncol. 2014;25:1–41. [Google Scholar]
- 16.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 23.Tsai KK, Shoushtari AN, Munhoz RR, et al. Efficacy and safety of programmed death receptor-1 (PD-1) blockade in metastatic uveal melanoma (UM). J Clin Oncol. 2016;34 (suppl):9507. [Google Scholar]
- 24.Larkin J, D'Angelo S, Sosman JA, et al. Efficacy and safety of nivolumab (NIVO) monotherapyin the treatment of advanced mucosal melanoma (MEL). Pigment Cell Melanoma Res. 2015;28:789. [Google Scholar]
- 25.Piperno-Neuman S, Servois V, Mariani P, et al. Activity of anti-PD1 drugs in uveal melanoma patients. J Clin Oncol. 2016;34 (suppl):9588. [Google Scholar]
- 26.Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoushtari AN, Munhoz RR, Kuk D, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122:3354–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamid O, Ribas A, Hodi FS, et al. Efficacy of pembrolizumab in patients with advanced mucosal melanoma: data from KEYNOTE-001, 002, and 006. Pigment Cell Res. 2017;30:102. [Google Scholar]
- 29.Kottschade LA, McWilliams RR, Markovic SN, et al. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res. 2016;26:300–303. [DOI] [PubMed] [Google Scholar]
- 30.Ribas A, Li XN, Daud A, et al. Elevated baseline serum LDH does not preclude durable responses with pembrolizumab. Pigment Cell Res. 2017;30:136. [Google Scholar]
- 31.Blank C, Ribas A, Long GV, et al. Impact of baseline lactate dehyrodgenase concentration on efficacy in the KEYNOTE-006 study of pembrolizumab v ipilimumab. Pigment Cell Res. 2017;30:85. [Google Scholar]
- 32.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C, Ribas A, Hamid O, et al. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. 2016;34(suppl):9503. [Google Scholar]
- 34.Robert C, Long GV, Schachter J, et al. Long-term outcomes in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in the phase 3 KEYNOTE-006 study who completed pembrolizumab (pembro) treatment. J Clin Oncol. 2017;35(suppl):9504. [Google Scholar]


