Abstract
Background:
Sleep disturbance and fatigue are common and distressing pediatric cancer-related outcomes. Sleep hygiene education and relaxation techniques are recommended to improve sleep in healthy children and adult cancer survivors. No studies have tested these interventions to improve sleep and fatigue for children with acute lymphoblastic leukemia (ALL) in the home setting.
Objectives:
The aim of this study is to establish the feasibility and acceptability of a sleep hygiene and relaxation intervention to improve sleep and fatigue for children receiving maintenance chemotherapy for ALL. The child's fatigue and sleep data were collected to inform sample size calculations for a future trial.
Methods:
In this pilot randomized controlled trial, 20 children were allocated randomly to the sleep intervention or control group. The sleep intervention group received a 60-minute educational session to discuss sleep and fatigue in children with cancer and strategies to improve sleep, including use of 2 storybooks to teach deep breathing and progressive muscle relaxation. Objective sleep data were collected using actigraphy and fatigue was measured using the Childhood Cancer Fatigue Scale.
Results:
The intervention was acceptable to families, and feasibility of the intervention and data collection was clearly established. Although not statistically significant, increases in mean nighttime sleep and decreases in mean wake time after sleep onset in the sleep intervention group represented clinically important improvements.
Conclusions:
This pilot study demonstrated the feasibility and acceptability of a sleep hygiene and relaxation intervention for children undergoing maintenance chemotherapy for ALL.
Implications for practice:
Given the clinically important improvements in sleep observed, replication in a larger, adequately powered randomized controlled trial is merited.
KEY WORDS: Actigraphy, Child, Fatigue, Leukemia, Parent, Randomized controlled trial, Relaxation, Sleep, Sleep hygiene
Background
Survival rates for pediatric acute lymphoblastic leukemia (ALL) currently exceed 85%.1 Given these excellent survival rates, the next challenge is to focus on improving the symptom experience and quality of life for pediatric patients with ALL. Children and adolescents have reported that fatigue is one of the most prevalent and distressing symptoms they experience during cancer therapy.2 Despite its reported significance, cancer-related fatigue is a rarely assessed and underreported clinical symptom, and there are calls for evidence-based clinical assessment of fatigue.3 Beyond assessment of cancer-related fatigue is the need to provide management of cancer-related fatigue with effective interventions.
Contemporary ALL therapy includes an intensive treatment phase lasting 6 to 10 months, followed by a longer maintenance phase that lasts 2 to 3 years. During the maintenance phase of ALL therapy it is hoped that children and adolescents resume regularly enjoyed activities, return to school, and recommence healthy lifestyle behaviors that may have been interrupted during more intensive phases of treatment. Sleep disruption during maintenance therapy for ALL represents an example of interrupted health behavior with multiple reported causes including medications, such as dexamethasone, and poor sleep habits.4,5 Disrupted and poor quality sleep in children with ALL is associated with distressing outcomes including fatigue and decreased quality of life.4,6,7
Independent of dexamethasone treatment, average sleep efficiency scores (time asleep/time in bed × 100) of children on maintenance therapy are lower than those of pediatric inpatients and healthy children,4 and number of nighttime awakenings (an average of 12 awakenings per night)8 well exceeds the reported average of 1 to 5 nighttime awakenings in healthy children of comparable ages.9 This raises the question of variables other than dexamethasone affecting sleep quantity and quality for children receiving at home maintenance chemotherapy for ALL.
Zupanec et al5 investigated the relationship between poor sleep habits and fatigue levels for children receiving ALL maintenance therapy. The results of this descriptive study found that 87% of children receiving ALL maintenance therapy scored above the established cutoff scores on the Children's Sleep Habits Questionnaire (CSHQ), indicating problematic sleep.5 Scores on the CSHQ were highly correlated (r = 0.69, P < .001) with fatigue,5 suggesting that improving sleep habits could potentially improve both sleep and fatigue for children receiving ALL therapy. Reasons for nighttime awakenings for children on maintenance therapy for ALL reported by parents included nightmares, worry, and fears, suggesting that anxiety may play more of a role than anticipated in disturbing sleep for pediatric patients receiving ALL maintenance therapy.5
An important role for pediatric oncology nurses is to manage symptoms and side effects of cancer and cancer therapy, including fatigue. Therefore, nurses are challenged to decrease levels of fatigue by exploring and utilizing interventions to improve the sleep quality of children receiving cancer therapy. There are currently no known trials aimed at improving sleep for children with ALL in the home setting. A pilot study of a home-based aerobic exercise program to decrease fatigue in children on maintenance chemotherapy for ALL found the 6-week program to be feasible but did not detect differences in fatigue and did not examine sleep outcomes.10
Sleep hygiene education is one intervention that has been recommended for various sleep disorders by sleep experts and has been successful in improving sleep quality in healthy children with sleep problems and adult cancer survivors.11,12 Sleep hygiene is a set of behaviors recommended for the development of healthy sleep habits. Examples of sleep hygiene behaviors include maintaining consistent bed and wake times, quiet activities before bedtime, a cool and dark sleep environment, avoiding caffeine, daily exposure to light and exercise, and a consistent bedtime routine. Given evidence that parents acknowledge that providing consistency, setting limits and providing discipline becomes more difficult following their child’s diagnosis,13 a set of structured sleep hygiene principles to follow might help guide parenting related to good sleep habits in this population.
Relaxation techniques are another sleep promoting intervention often provided in combination with sleep hygiene to promote feelings of calmness, decrease anxiety, and minimize distressing thoughts at bedtime that might interfere with both falling asleep and staying asleep.14,15 In children, diaphragmatic breathing has been used as a coping skill for painful medical procedures,16,17 as a method to reduce anxiety,18 and a technique used for symptom management in children with asthma and recurrent abdominal pain.19,20 A parent-focused educational program on sleep hygiene and relaxation is an intervention that could be administered by nurses and have a significant impact on the sleep quality of children receiving maintenance chemotherapy for ALL. Ideally, an empirical examination of these interventions would include parents to help coach their child when using relaxation skills as well as objective measures of sleep such as actigraphy.
The purpose of this study is to explore the feasibility and acceptability of a combined sleep hygiene and relaxation intervention to improve sleep and fatigue for children receiving maintenance chemotherapy for ALL in the home setting. To refine the intervention in preparation for a large-scale, definitive trial, elements of feasibility and acceptability21 to be examined were parent's views on the delivery, format, and content of the components of the intervention; components of the intervention used, frequency of use, and helpfulness; barriers to use of the intervention; parent/child compliance with data collection; and number of participants approached versus those consenting. The child's fatigue and objective and subjective sleep data were collected to inform sample size calculations for a future, larger-scale trial.
Methods
This study received approval from the hospital's research ethics board (REB 1000025162).
Participants
Participants and their parents were recruited from the outpatient leukemia/lymphoma clinic of a single urban Canadian, tertiary and quaternary care pediatric hospital. Eligible participants were children 4 to 10 years of age, with precursor B ALL in the maintenance phase of therapy beyond third course, within 1 year of an age-appropriate grade in school, and receiving intravenously administered chemotherapy (Vincristine) at the study hospital. Both child and at least 1 parent needed to understand and read English to participate. Children were excluded if they were receiving palliative care, had a physician-diagnosed mental health (eg, anxiety, depression) or sleep disorder (eg, insomnia, restless legs syndrome), had received radiation therapy, or were at risk for sleep disordered breathing as determined by the sleep and breathing subscale of the CSHQ.
Design and Procedures
The study was a pilot randomized controlled trial (RCT). Eligible children and parents were identified in advance by a clinic nurse practitioner or nurse and asked if they were interested in learning more about the study. For those interested, a research assistant (RA) then explained the study and sought consent from 1 parent and assent from the child.
Baseline data collection began during the regular clinic visit corresponding to day 57 of a maintenance treatment cycle (Figure), with all baseline data collection completed by day 77 of the cycle. Child sleep outcomes and fatigue levels were measured across 5 days and nights before randomization, using self-reported measures and actigraphy to collect objective sleep data as described below. Concurrent medication and routine complete blood count results were documented at baseline and follow-up to ensure compliance with usual maintenance therapy protocols and to identify any cases of anemia that might affect fatigue measures.
Figure.
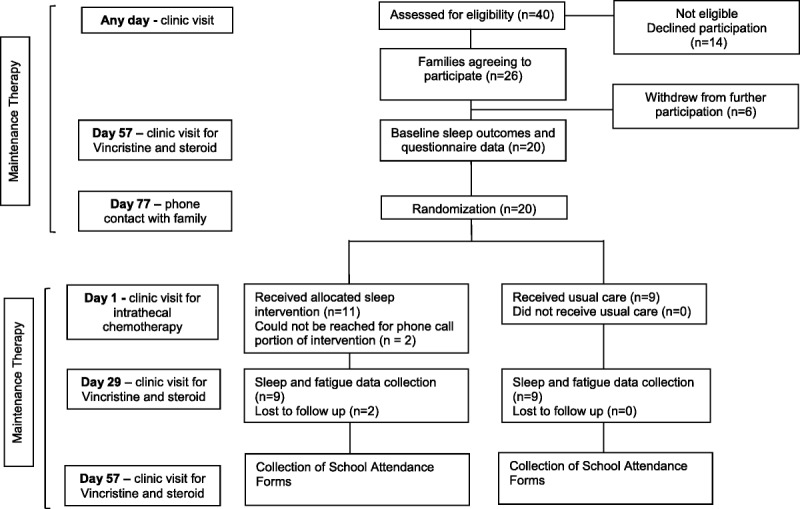
Flowchart of the randomized controlled trial.
Sealed, opaque, sequentially numbered envelopes containing a treatment allocation were produced using a computerized random numbers table by a research staff member external to the study. On day 77 of the treatment cycle (1 week before the next scheduled clinic visit), families were contacted by telephone to reconfirm their consent to participate. Also at this time, the RA opened the next envelope and the family was made aware of the group assignment. An audit trail was kept of the envelopes as group assignments were made. It was not possible to blind families to their group assignment, but objective measures were used where possible, and RAs who were analyzing actigraphy data and entering data from paper questionnaires were blinded to group assignment.
The intervention was administered to families in the intervention group, 1 week after randomization, during the first clinic visit of a new maintenance therapy cycle (ie, day 1). One parent was required to attend; attendance of the child, other parent, or other family member was optional. The intervention was administered during the typical wait times experienced by families at this clinic visit. Families were asked to implement the sleep hygiene and relaxation strategies discussed over the next 4 weeks. Families in the usual care group were also seen on day 1 but were not required to do anything beyond the usual clinic activities, which did not include any formal education related to sleep. Families in both groups were asked to record children's school attendance for 4 weeks postintervention.
At the follow-up clinic visit and data collection time, corresponding to day 29 of the maintenance cycle (4 weeks postintervention), the sleep and fatigue measures completed at baseline were repeated for both groups, with an actigraphy device applied to the child's wrist at that visit and worn for the following 5 days and nights.
Reminder calls were made 5 days after the baseline and follow-up data collection periods to encourage completion of data forms and return of the actigraphy equipment. Prepaid courier envelopes were supplied to facilitate return of the equipment and data collection tools so families were not burdened with extra clinic visits. Gift cards ($25 value) from a major retailer were given to children for each of 2 completed rounds of actigraphy measurement.
Intervention
The principal investigator (S.Z.) and coinvestigator (H.J.) completed an 8-hour training course on sleep hygiene and relaxation technique interventions provided by a senior investigator (R.S.) with expertise in behavioral sleep interventions for children and parents. The training course included role-play delivery of the intervention, including in-clinic sessions and follow-up telephone calls, and an intervention manual was provided. For the first 3 participants, the intervention nurses asked study participants for permission to audiotape their interaction so they could be reviewed for intervention fidelity and consistency.
The intervention was a one-to-one 60-minute educational session during a scheduled clinic visit in a private clinic room. The session included education about sleep in children and a description of what is known about sleep and fatigue issues in children with cancer. The session also addressed strategies to improve sleep hygiene in children (eg, consistent bed and wake times, bedtime routine). Relaxation strategies to promote sleep were outlined, and parents received 2 children's books designed to promote relaxation using the principles of deep breathing and progressive muscle relaxation, delivered in storybook format. The books (A Boy and a Bear [Specialty Press] and The Goodnight Caterpillar [Litebooks.Net]) were chosen based on feedback from children with ALL attending a follow-up clinic. At the conclusion of the session, a brochure containing all the information covered, along with a bedtime pass and a bedtime routine checklist, was given to families. The bedtime pass was a printed ticket that the child could use to leave bed 1 time for 1 final request after the agreed upon bedtime.22 The bedtime routine checklist was a visible schedule with Velcro-attached pictures that detailed the steps in the child's bedtime routine. A telephone call from the sleep intervention nurse was made within 1 week postintervention to review the content of the session and answer any questions. The telephone calls also served to provide support and encouragement to the parent(s) to persist with the interventions. The sleep intervention nurses completed an intervention fidelity checklist as a double check that all components of the intervention were delivered.
Measures
Feasibility and Acceptability Measures
After the intervention session, the parent was given a questionnaire to complete for each of the 4 weeks after the intervention. Questions asked parents to rate the frequency of use (range, never to ≥5 times a week) and degree of helpfulness (range, not helpful to very helpful) of each sleep recommendation (eg, consistent bedtimes, avoiding caffeine). There were also open-ended questions, where the parent was given space to write their views on: barriers to implementing the sleep advice and strategies given, the usefulness of the session with the nurse practitioner, the usefulness of the written handout, and the format of the intervention.
Other measures of feasibility and acceptability were tracked by the research team, including compliance with data collection procedures (eg, wearing the actigraph, completion of sleep diary, and other data forms), ability to deliver the intervention within the clinical setting, and number of participants approached versus those consenting.
Objective Sleep Measures
Sleep and wake times were collected using actigraphy. Actigraphs detect and record continuous motion data with a battery-operated wristwatch-size microprocessor. These detected movements are translated into digital counts across 1-minute intervals and stored in internal memory. Children participating in the study wore MiniMotionlogger actigraphs (Ambulatory Monitoring Inc, Ardsley, New York) on the wrist. Algorithms assessed the recorded activity for the previous 4 minutes and subsequent 2 minutes before making a determination of sleep or wake status for each 1-minute interval. Thus, brief movements in the middle of sleep periods were recorded as sleep and brief periods of no activity within time intervals of extensive wakeful movement were recorded as wake. The number of episodes of sleep and wake and their durations were abstracted so that amount of nighttime sleep, number of nighttime awakenings, number of minutes spent awake after initial sleep onset, and amount of daytime sleep could be reported. Congruence between polysomnography and actigraphy indicates adequate validity and reliability when sleep is assessed in toddlers, older children, and adolescents.23 Children wore the actigraphs for 5 consecutive nights and days, which is consistent with the Standards of Practice Committee of the American Academy of Sleep Medicine recommendations of at least 3 consecutive 24-hour periods of actigraph recording time.24 Sleep diary data were used to support actigraphy data and included the child's sleep times, wake times, naps, and times when the actigraph was removed for bathing. For example, sleep diaries confirmed that periods of complete inactivity on actigraphy corresponded to notes in the diary that actigraphs were removed. When sleep diary and actigraphy data indicated artefacts, data were recorded appropriately using Action4 actigraphy analysis software (Ambulatory Monitoring Inc).
Subjective Sleep and Fatigue Measures
Sleep disturbance was measured using the CSHQ, which asks about usual sleep habits (sleep duration and consistency of sleep and wake times during the week and between weekdays and weekends) as well as sleep problems in the most recent, typical week. The range of possible total scores is 33 to 99, with higher scores indicating greater sleep disturbance. Scores higher than 41 suggest a need for further evaluation of sleep problems. Satisfactory test-retest reliability scores have been reported for both normal and sleep-referred clinical populations (r = 0.62-0.79).25 After the baseline administration of the CSHQ, the sleep disordered breathing subscale was scored. Any child whose subscale score suggested potential sleep disordered breathing (>7/9) was referred to his/her primary oncologist for further investigations and excluded from further participation.
Fatigue levels were measured using the Childhood Cancer Fatigue Scale–Child (CCFS-C) and the Childhood Cancer Fatigue Scale–Parent (CCFS-P).26 Children older than 7 years completed the CCFS-C and parents completed the CCFS-P for all enrolled children. Scales were completed on the fifth day of data collection, which coincided with day 5 of the oral dexamethasone pulse. For the CCFS-C, total fatigue scores range from 0 to 40, with higher scores corresponding to greater levels of fatigue.27 Total scores for the CCFS-P range from 17 to 85, with higher scores corresponding to greater perceived fatigue. Internal consistency is .88 for the parent version and .76 for the child version. Construct validity for both versions was established using factor analysis, and the parent and child versions are strongly correlated to one another.28
The Family Inventory of Sleep Habits (FISH) is a validated 12-item scale used to assess families’ sleep and bedtime habits, along with a child's sleep environment. Total scores range from 12 to 60, with a higher number indicating closer-to-optimal sleep habits.29
To examine if improved sleep or decreased fatigue affected school attendance, parents were given a school attendance calendar and were asked to record if their child attended school for a full day, half day, or not at all, with reasons for absences.
Sample Size
A sample size of 30 families, with 15 in each of the control and intervention groups, was selected so that estimates of a between group effect size could inform power analyses for a future, large RCT.30 This sample size was also expected to provide representation across ages, ethnicity, and gender to best inform understanding of feasibility and acceptability of the intervention.
Data Analysis
Data from the actigraph were downloaded to a computer and interpreted using autoscoring programs in Action4 software (Ambulatory Monitoring Inc). Sleep variables were averaged across the 5 nights of actigraphy recording at each time point. For questionnaire data, an RA built a database using REDCap (Research Electronic Data Capture) software31 and subsequently performed data entry. Data were independently double checked by a second RA. Descriptive statistics and questionnaire scores were tabulated using REDCap. For all sleep variables, fatigue scores, and CSHQ scores, a 2-sample t test of the differences between baseline and postintervention scores was conducted using SAS Version 9.1 (SAS Institute, Cary, North Carolina). When a range of hours/minutes was reported, the mean of the low and high bounds of the range was used.
Results
Flow of Participants, Sample Characteristics and Compliance
As outlined in the Figure, between May 2011 and March 2013, 40 eligible families were approached to participate, and 26 consented (65% recruitment rate). After consent, but before randomization, 6 of the 26 families withdrew (eg, too busy to participate) or were unable to complete baseline measures (eg, child unable to tolerate wearing actigraph), leaving 20 families to be randomized. The original goal was to recruit a total of 30 families, with 15 in each of the control and intervention groups. This number proved difficult to reach for a number of reasons. Strict timing for time points 1 and 2 meant that if a potential participant was missed before time point 1 (day 57 of a maintenance cycle), it could be 3 months before the potential participant could be approached again, at which point several did not have sufficient time left on therapy. Many potential participants did not wish to alter their plan to receive maintenance therapy at a satellite clinic to complete study assessments. Finally, some potential participants were enrolled in a trial with vincristine and steroid pulses every 12 weeks compared with every 4 weeks, rendering them ineligible. Of the 9 families in the intervention group, 2 could not be reached for the intervention review telephone call. Of the 11 families randomized to the intervention group, 2 were lost to follow-up (1 missed her follow-up data collection and was then off treatment; 1 no longer wished to participate after baseline measures).
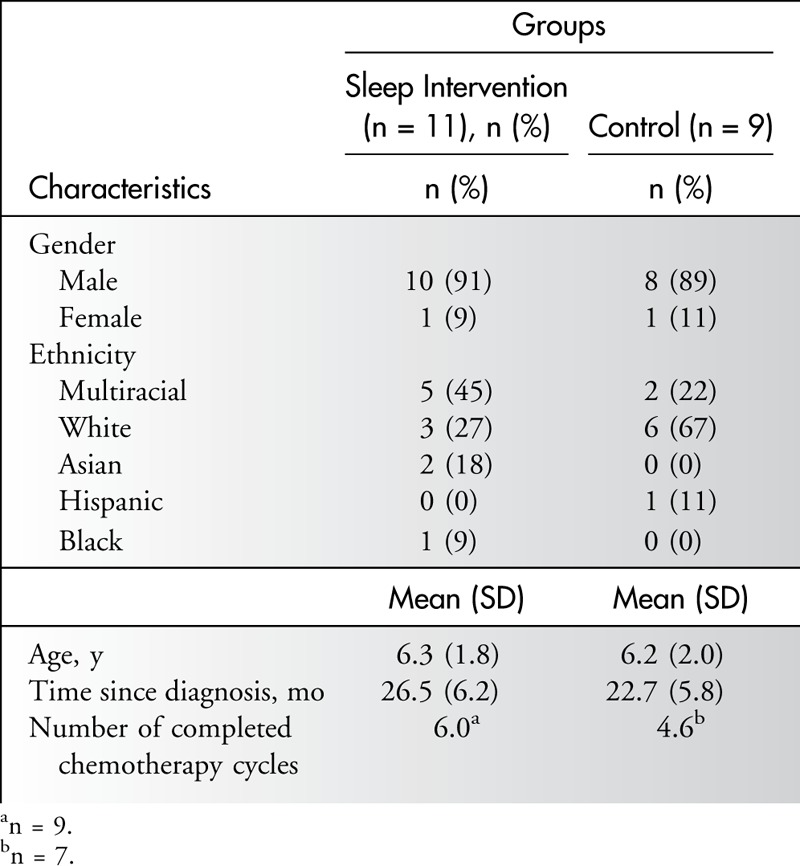
Child characteristics were similar across groups (see Table 1). Most of the children were male (90%) and white (45%), with a mean age of 6.2 years. All intervention group families (n = 11) received the one-to-one education session, the handout, and the books. Six children were present for the full education session, and 1 child was present for part of the session; the remaining 4 children did not attend the education session. Both mother and father were present for the intervention 45% (n = 5) of the time; otherwise, mothers alone received the educational session (n = 6). Most families (n = 9, 82%) received the 1 week postintervention follow-up telephone call.
Table 1.
Child Characteristics
Complete actigraphy data were received from 9 children in each group (n = 18); 2 children assigned to the intervention group completed baseline measures but follow-up measures were not completed. Two children in the intervention group began their follow-up measures on day 57 postintervention rather than on day 29 postintervention because of scheduling difficulties. Diary substitutions occurred for 1 child at baseline, when it was discovered that the actigraph failed to collect data. School attendance records were not completed for most families (n = 14 incomplete; n = 2 children not yet in school).
Feasibility and Acceptability of Sleep Intervention Strategies
All 8 families who completed the postintervention evaluation of the intervention rated the education session as somewhat or very useful. Of the 8 families, 2 reported that they were already practicing the recommended sleep hygiene tips, and the intervention served to affirm their current approaches rather than provide new strategies. The intervention components were viewed as informative by all families, although 1 family noted that a relaxation CD could have been a useful addition to the books.
Across weeks 1 to 4, the sleep tips used at least 5 times per week by most of the intervention group families (n = 7/8 families) and also rated as “very helpful” were as follows: child has same bedtime each night, child has same wake-up time each morning, child slept in a quiet room, child slept in a dark room, child slept in a cool room, child had no caffeine to eat/drink after lunch time, child did quiet activities in the hour before bedtime, and child avoided sleeping/napping during the daytime. Use of the relaxation books and their techniques was reported by most parents (n = 5/8 families) as at least 5 times per week. The bedtime pass and bedtime checklist remained unused by most families (n = 6/8 families).
Barriers to using the intervention strategies identified by families were fatigue levels so high that the child could not avoid napping, late-night medication administration that interfered with early bedtimes, and resistance from the child to reading the same book every day. Take-home materials were viewed as very useful as a reminder or review of information conveyed in the one-on-one session. All families appreciated the one-on-one delivery of the sleep intervention information, with respondents noting that the in-person session allowed the nurse to give detail and explanation when needed. Respondents also appreciated that while novel information about sleep was delivered, positive feedback and reinforcement of helpful strategies already being used by the family also occurred. The specific tailoring of information to children undergoing treatment for ALL was viewed positively. One respondent noted that it was difficult to gauge how much their child's current sleep habits needed to change and that review of the child's baseline sleep data with the intervention nurse would be helpful.
Objective Sleep Measures
Analysis of actigraphy data (see Table 2) revealed that children in the intervention group increased their mean nighttime sleep duration by 35 minutes compared with the control group; this difference did not reach statistical significance (P = .30). Wake time after sleep onset in the intervention group decreased by 44 minutes as compared with the control group; this difference almost reached statistical significance (P = .08). Change from baseline on other objectively measured sleep outcomes (eg, daytime sleep duration, longest stretch of daytime and nighttime sleep, number of nighttime awakenings) was similar across groups. For more than two-thirds of the sample, parent-reported bed and wake times did not allow enough time for the children to achieve recommended amounts of sleep.
Table 2.
Sleep and Fatigue Measures
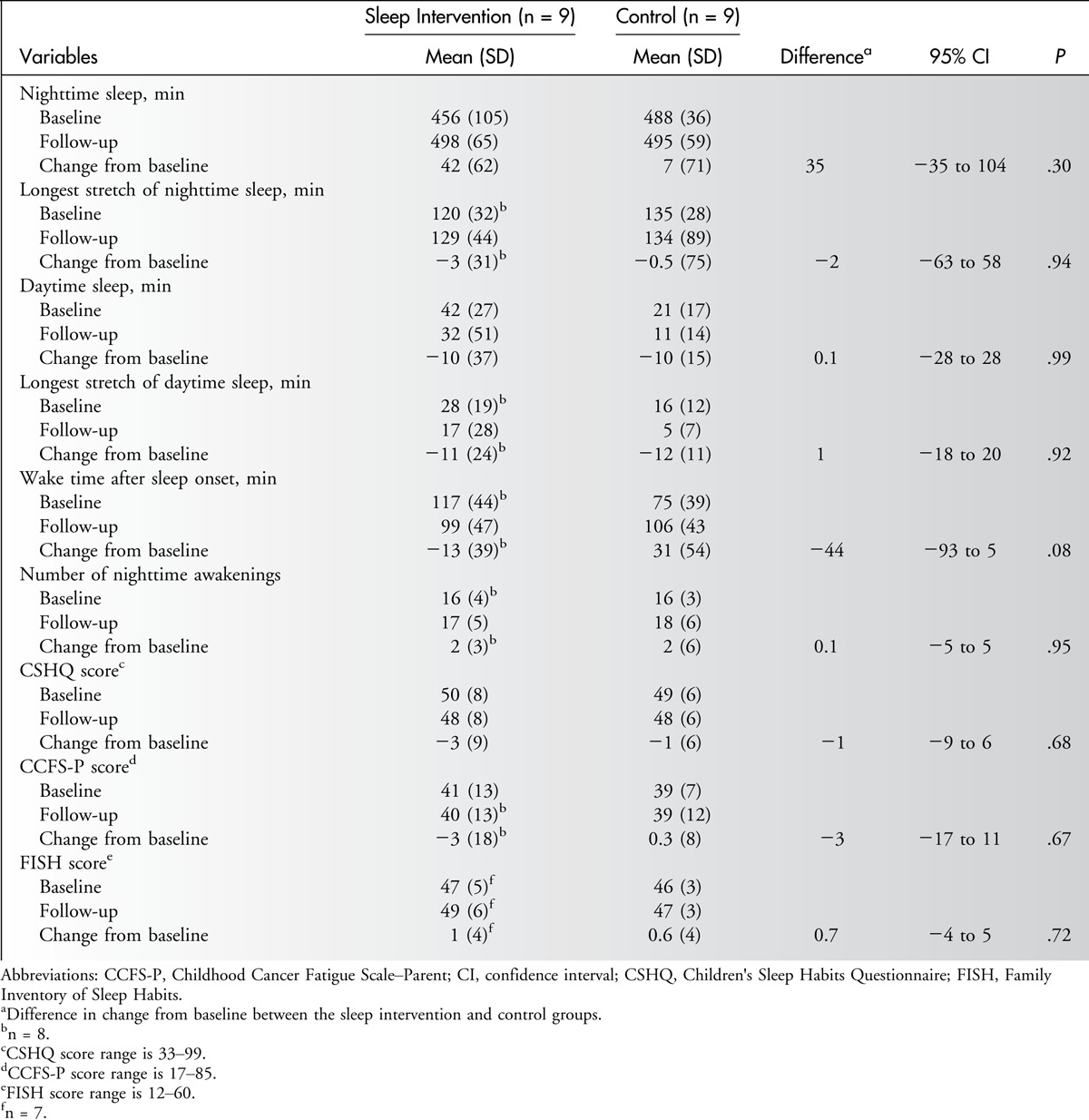
Subjective Sleep and Fatigue Measures
Most children (95% at baseline, 83% at follow-up) scored above the cutoff on the CSHQ, indicating clinically significant sleep disturbance (see Table 2). Preintervention and postintervention scores on the FISH measure were high (mean score >46 in both groups), indicating that families reported practicing good sleep habits before the intervention. Scores on the CCFS-P indicated moderate fatigue at both time points. Because few participants were old enough to complete the CCFS-C, those data were not summarized. There were no differences between groups in change from baseline on the CSHQ, FISH, or CCFS-P.
Discussion
This study is the first to evaluate the feasibility and acceptability of an innovative sleep hygiene and relaxation intervention aimed at improving 2 common and distressing pediatric cancer–related outcomes, sleep disturbance and fatigue. Use of actigraphy, an objective measure of sleep, along with valid and reliable subjective measures of sleep and fatigue, is a strength of this work.
The intervention was acceptable to families; most of those approached chose to participate, responded positively to the intervention, and found the sleep strategies useful. Families enjoyed the interactions with the sleep intervention nurse and appreciated the one-on-one delivery of the intervention and the take-home materials and books.
Feasibility of the intervention and data collection was also clearly established. Parents reported using many of the sleep strategies, including the books aimed at guiding relaxation breathing. It was feasible to deliver the intervention in the clinical setting during regularly scheduled appointments, although this was an additional demand on the sleep intervention nurses, who were also carrying out their usual clinic duties. Although completion rates for subjective sleep and fatigue and objective sleep data were high, not all parents used the available open text fields on data collection forms to describe barriers to implementing sleep tips. In future research, more detailed information and a better understanding of the barriers for changing sleep behaviors could be attained by semistructured interviews after delivery of the intervention. There was poor compliance with completion of the investigator-developed school attendance calendar. Future studies should consider use of text messaging at the end of each school day to survey parents regarding their child's attendance.
Feasibility of recruitment was limited by strict time points for data collection and intervention delivery. This design was intended to minimize burden of trial participation for patients and families by timing data collection with standard clinic visits but resulted in many eligible participants being missed for recruitment. Future trials should consider use of home visits and more flexible timing for data collection. Partnering with satellite clinics as data collection sites would also allow for increased recruitment in future trials so that participants are not limited to those attending only the main hospital site for treatment.
Given that this was a pilot study, not powered to detect differences on sleep and fatigue outcomes, statistically significant differences between groups were not expected. However, the observed 35-minute difference in nighttime sleep between the sleep intervention and control groups represents a clinically important improvement in sleep duration in a sleep-restricted population and, if maintained in a larger sample, would add more than 3 hours of additional sleep each week. Similarly, the observed 44-minute difference in wake time after sleep onset is a clinically important difference and suggests that the sleep intervention group benefited from the relaxation techniques, which were aimed at decreasing sleep onset latency at initiation of sleep and subsequent to any nighttime awakenings. Because there were no differences between the groups in the number of nighttime awakenings, it seems that the sleep intervention group was better able to return to sleep when waking did occur, perhaps as a result of their experience with the relaxation techniques. The observed differences in changes from baseline in sleep duration and the amount of wake time after sleep onset indicate that there is merit in studying this sleep intervention in a larger, full-scale RCT.
Children in both study arms slept significantly less than the recommended number of nighttime sleep hours for children aged 4 to 12 and had numerous nighttime awakenings, aligning with observational studies with similar samples and use of actigraphy.32–34 It is important to note that although the children were all on outpatient maintenance therapy, the number of nighttime awakenings was as frequent as those observed in children on active treatment, including those who are hospitalized,35,36 and considerably more frequent than the number of wakes expected in healthy, community samples of children.37 Scores on the CSHQ indicated that most children had clinically significant sleep disturbance, which is in line with previous observations,5,7 although there are concerns with the ability of the CSHQ to capture changes in sleep difficulties in acutely or chronically ill populations as the measure has not been validated in such groups.38 Similar to other studies in children undergoing maintenance chemotherapy, fatigue levels for children during maintenance therapy for ALL were high.4,7,8 Given the burden of fatigue in this population, it will be important to measure subjective reports of fatigue in larger trials of sleep interventions to determine if improvements in sleep outcomes translate to reduced fatigue.
Mean preintervention scores on the FISH measure were high in both groups, indicating that many families had good sleep habits before the intervention, which aligns with parent reports that the intervention served to affirm their current sleep-related practices. To maximize the benefit of sleep interventions in future trials with this population, baseline measures of sleep using actigraphy should be used for identification of families with poor sleep habits and significant sleep restriction so that interventions can be targeted at those who most need help. Parent and child readiness for addressing change in sleep behavior were not assessed, and 1 participant noted that it was difficult to know what degree of change in sleep patterns was needed. Sharing of the results of baseline, objective sleep outcomes by actigraphy and highlighting differences from optimal sleep duration and timing could serve to increase family motivation and commitment and facilitate a discussion of the child's specific sleep behaviors. With mean nighttime sleep at follow-up in both groups slightly over 8 hours, all children slept much less than the recommended 11 to 12 hours for children aged 4 to 7 years and 10 to 11 hours for children aged 8 to 12 years.39 Late bedtimes contributed to the truncation for sleep for many in this group of young children, and although earlier bedtimes were recommended as part of the sleep education session, this element seemed less amenable to change, in part because of current medication protocols requiring taking medication at bedtime without food for a determined period. Given the extreme sleep restriction observed in this group, and the known benefits of sleep for immune function, consideration of an altered medication protocol and planned administration at time other than bedtime could be explored to best maximize opportunities for sleep. Some parents reported that fatigue necessitated daytime naps for their child, and this likely contributed to later bedtimes, because daytime sleep decreases the homeostatic drive to sleep, thereby allowing the child to stay up later. Sleep hygiene advice in this population needs to take high levels of fatigue into account and acknowledge that napping may occur but that naps should not extend beyond 30 minutes and should not occur within 4 hours of the desired bedtime so as not to delay sleep onset at night.
Study limitations include recruitment from a single center and an overrepresentation of male participants. The greater proportion of male participants can be explained by the longer length of ALL therapy for boys (3.5 years) compared with girls (2.5 years) at the study site, allowing for more opportunity for recruitment of eligible boys.
Conclusions
In summary, this pilot study has demonstrated the feasibility and acceptability of a sleep hygiene and relaxation intervention for children undergoing maintenance chemotherapy for ALL. Given the clinically important improvements in sleep observed in the study, replication in an RCT adequately powered to detect differences on sleep and fatigue outcomes is merited. Future studies could include children with other cancer diagnoses and incorporate strategies to assess families’ readiness to change and create motivation for behavior change during a period when families experience additional stressors related to their child's cancer therapy.
ACKNOWLEDGMENT
The authors thank the families who so willingly gave their time.
Footnotes
Funding for the study was provided by an Alex’s Lemonade Stand Foundation Mentored Nurse Researcher Grant to Ms Zupanec. Dr Stremler was supported by a Canadian Institutes of Health Research New Investigator Award and an Ontario Ministry of Research and Innovation Early Researcher Award.
The authors have no conflicts of interest to disclose.
References
- 1.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2011;118(2):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hockenberry-Eaton M, Hinds PS, Alcoser P, et al. Fatigue in children and adolescents with cancer. J Pediatr Oncol Nurs. 1998;15(3):172–182. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue Version 1.2016. Fort Washington, PA: National Comprehensive Cancer Network; 2016. [Google Scholar]
- 4.Hinds PS, Hockenberry MJ, Gattuso JS, et al. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer. 2007;110(10):2321–2330. [DOI] [PubMed] [Google Scholar]
- 5.Zupanec S, Jones H, Stremler R. Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. J Pediatr Oncol Nurs. 2010;27(4):217–228. [DOI] [PubMed] [Google Scholar]
- 6.Gedaly-Duff V, Lee KA, Nail L, Nicholson HS, Johnson KP. Pain, sleep disturbance, and fatigue in children with leukemia and their parents: a pilot study. Oncol Nurs Forum. 2006;33(3):641–646. [DOI] [PubMed] [Google Scholar]
- 7.van Litsenburg RR, Huisman J, Hoogerbrugge PM, Egeler RM, Kaspers GJ, Gemke RJ. Impaired sleep affects quality of life in children during maintenance treatment for acute lymphoblastic leukemia: an exploratory study. Health Qual Life Outcomes. 2011;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanford SD, Okuma JO, Pan J, et al. Gender differences in sleep, fatigue, and daytime activity in a pediatric oncology sample receiving dexamethasone. J Pediatr Psychol. 2008;33(3):298–306. [DOI] [PubMed] [Google Scholar]
- 9.Riter S, Wills L. Sleep wars: research and opinion. Pediatr Clin North Am. 2004;51(1):1–13. [DOI] [PubMed] [Google Scholar]
- 10.Yeh CH, Man Wai JP, Lin US, Chiang YC. A pilot study to examine the feasibility and effects of a home-based aerobic program on reducing fatigue in children with acute lymphoblastic leukemia. Cancer Nurs. 2011;34(1):3–12. [DOI] [PubMed] [Google Scholar]
- 11.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. [DOI] [PubMed] [Google Scholar]
- 12.Mindell JA, Kuhn B, Lewin DS, Meltzer LJ, Sadeh A; American Academy of Sleep Medicine. Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep. 2006;29(10):1263–1276. [PubMed] [Google Scholar]
- 13.Williams LK, Lamb KE, McCarthy MC. Behavioral side effects of pediatric acute lymphoblastic leukemia treatment: the role of parenting strategies. Pediatr Blood Cancer. 2014;61(11):2065–2070. [DOI] [PubMed] [Google Scholar]
- 14.Morin C, Hauri P, Espie C, Spielman A, Buysse D, Bootzin R. Nonpharmacologic treatment of chronic insomnia. Sleep. 1999;22(8):1134–1156. [DOI] [PubMed] [Google Scholar]
- 15.Ritz T, Roth WT. Behavioral interventions in asthma: breathing training. Behav Modif. 2003;27(5):710–730. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez C, Marcos A. Psychological treatment of evoked pain and anxiety by invasive medical procedures in paediatric oncology. Psychol Spain. 1997;1(1):17–36. [Google Scholar]
- 17.McDonnell L, Bowden M. Breathing management: a simple stress and pain reduction strategy for use on a pediatric service. Issues Compr Pediatr Nurs. 1989;12:339–344. [DOI] [PubMed] [Google Scholar]
- 18.Day R, Sadek S. The effect of Benson's relaxation response on the anxiety levels of Lebanese children under stress. J Exp Child Psychol. 1982;34:350–356. [DOI] [PubMed] [Google Scholar]
- 19.Chiang L-C, Ma W-F, Huang J-L, Tseng L-F, Hsueh K-C. Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial. Int J Nurs Stud. 2009;46(8):1061–1070. [DOI] [PubMed] [Google Scholar]
- 20.Weydert J, Shapiro D, Acra SA, Monheim C, Chambers A, Ball T. Evaluation of guided imagery as treatment for recurrent abdominal pain in children: a randomized controlled trial. BMC Pediatr. 2006;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feeley N, Cossette S, Cote J, et al. The importance of piloting an RCT intervention. Can J Nurs Res. 2009;41(2):85–99. [PubMed] [Google Scholar]
- 22.Moore BA, Friman PC, Fruzzetti AE, MacAleese K. Brief report: evaluating the Bedtime Pass Program for child resistance to bedtime—a randomized, controlled trial. J Pediatr Psychol. 2007;32(3):283–287. [DOI] [PubMed] [Google Scholar]
- 23.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28(12):1568–1577. [DOI] [PubMed] [Google Scholar]
- 24.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. [DOI] [PubMed] [Google Scholar]
- 25.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- 26.Hinds PS, Hockenberry M, Tong X, et al. Validity and reliability of a new instrument to measure cancer-related fatigue in adolescents. J Pain Symptom Manage. 2007;34(6):607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinds PS, Yang J, Gattuso JS, et al. Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale-Child instrument. J Pain Symptom Manage. 2010;39(3):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockenberry MJ, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. J Pain Symptom Manage. 2003;25(4):319–328. [DOI] [PubMed] [Google Scholar]
- 29.Malow BA, Crowe C, Henderson L, et al. A sleep habits questionnaire for children with autism spectrum disorders. J Child Neurol. 2009;24(1):19–24. [DOI] [PubMed] [Google Scholar]
- 30.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–191. [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews EE, Neu M, Cook PF, King N. Sleep in mother and child dyads during treatment for pediatric acute lymphoblastic leukemia. Oncol Nurs Forum. 2014;41(6):599–610. [DOI] [PubMed] [Google Scholar]
- 33.Darezzo Rodrigues Nunes M, Jacob E, Adlard K, Secola R, Nascimento L. Fatigue and Sleep experiences at home in children and adolescents with cancer. Oncol Nurs Forum. 2015;42(5):498–506. [DOI] [PubMed] [Google Scholar]
- 34.Orsey AD, Wakefield DB, Cloutier MM. Physical activity (PA) and sleep among children and adolescents with cancer. Pediatr Blood Cancer. 2013;60(11):1908–1913. [DOI] [PubMed] [Google Scholar]
- 35.Hinds PS, Hockenberry M, Rai SN, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncol Nurs Forum. 2007;34(2):393–402. [DOI] [PubMed] [Google Scholar]
- 36.Linder LA, Christian BJ. Nighttime sleep disruptions, the hospital care environment, and symptoms in elementary school-age children with cancer. Oncol Nurs Forum. 2012;39(6):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Philadelphia, PA: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 38.Bloom BJ, Owens JA, McGuinn M, Nobile C, Schaeffer L, Alario AJ. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. J Rheumatol. 2002;29(1):169–173. [PubMed] [Google Scholar]
- 39.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]


