Article first published online 14 June 2017.
Key Words: inflammatory bowel disease, thiopurines, optimization, therapeutic drug monitoring, allopurinol, azathioprine, mercaptopurine, thioguanine
Abstract
Background:
Thiopurines (azathioprine and mercaptopurine) are frequently used immunosuppressive drugs to maintain remission in patients with inflammatory bowel disease. Half of the conventional thiopurine-derivative users have to discontinue treatment within 5 years, mainly because of intolerable adverse events. Over recent years, different strategies to optimize thiopurine treatment were suggested, yet, studies describing the clinical effectiveness of these strategies remain scarce. The aims of this study were to compare tolerability and sustained clinical benefit of conventional thiopurine derivatives therapy among two 5-year real-life intercept cohorts and to assess the clinical value of specifically allopurinol cotherapy.
Methods:
In this retrospective single-center cohort study, we analyzed data from patients in whom weight-based thiopurine monotherapy was initiated between 2005 and 2009 (cohort 1) or between 2010 and 2014 (cohort 2). The initiation of the second cohort was synchronic to the start of allopurinol-based optimization in our center. Optimization strategies were extracted from patient charts.
Results:
In total, 105 patients were included (60 in cohort 1, and 45 in cohort 2). Metabolite measurement was performed in 37% versus 84% of the patients (P < 0.001). Subsequent optimization strategies were applied in 33% versus 58% of the patients because of inadequate metabolite concentrations, intolerance, or ineffectiveness (P = 0.01). Allopurinol was coadministered to therapy in 18 patients (40%) in the second cohort. Therapy was switched to thioguanine in 11 versus 6 patients (P > 0.05). Overall, total duration was longer in the second cohort (10.8 versus 34.1 months, P < 0.001). The number of ongoing thiopurine users (20% versus 49%) and sustained clinical benefit (13% versus 38%) were higher in the second cohort (both P < 0.05). This was mainly because of a decrease in hepatotoxicity after optimization (P < 0.01).
Conclusions:
Optimization of thiopurine therapy by the use of therapeutic drug monitoring with subsequent administration of allopurinol cotherapy successfully enhanced sustained clinical benefit and tolerability in patients with inflammatory bowel disease.
Crohn's disease and ulcerative colitis, together known as inflammatory bowel diseases (IBDs), are chronic inflammatory disorders of the gastrointestinal (GI) tract accounting for substantial morbidity with hospital admissions and associated costs.1,2 Especially in maintaining remission, conventional thiopurines (azathioprine [AZA], mercaptopurine [MP]) play an important role as first-line immunosuppressive treatment.3–5 Unfortunately, up to 60% of thiopurine users have to discontinue treatment within 5 years after initiation because of side effects or ineffectiveness, frequently related to a disadvantageous thiopurine metabolism.6
Over recent years, different strategies to optimize thiopurine therapy have been proposed. Rechallenge of dose-adjusted thiopurine monotherapy, split-dose administration and measurement of thiopurine metabolites, therapeutic drug monitoring (TDM) are currently integrated in daily practice of IBD care.3,7,8 In those patients with an aberrant thiopurine metabolism, the so-called skewers with preferential 6-methylmercaptopurine (6-MMP) formation, addition of allopurinol to low-dose thiopurine therapy leads to less 6-MMP formation, which contributes to, among other, a lower incidence of hepatotoxicity.9–12 In addition, the introduction of thioguanine (TG), a thiopurine analog with a less complex metabolism without 6-MMP formation, to patients intolerant to AZA or MP avoids the development of 6-MMP–induced side effects, and which was, therefore, associated with better tolerability.13,14
In this study, we aimed to compare tolerability with thiopurine therapy among two 5-year intercept cohorts. We hypothesized that thiopurine therapy is better tolerated because the introduction of allopurinol cotherapy as a new strategy to optimize IBD care in daily practice. This beneficiary effect was demonstrated in several clinical trials (10, 11), yet, real-life studies in which the clinical importance of TDM-based optimization is described are scarce. In addition, we wanted to determine whether optimizing thiopurine treatment improved effectiveness of thiopurine-based immunosuppressive therapy in patients with IBD. Finally, we intended to observe if nonoptimized continued users (i.e., patients with good response to weight-based monotherapy) were as effective as TDM-based optimized users (i.e., patients with good response to optimized therapy).
MATERIALS AND METHODS
Data Selection
All consecutive thiopurine-naive adult patients with IBD who initiated weight-based (AZA 2.0–2.5 mg/kg and MP 1.0–1.5 mg/kg) thiopurine therapy in the VU University medical center (VUmc) between January 1, 2005 and December 31, 2014 were eligible for this study. The patients with IBD were selected from a prospectively maintained database of all patients with IBD treated at the VUmc since 1998.6 All patients were treated and supervised by the same clinician (A.v.B.). Diagnosis of IBD was ascertained by standard clinical, radiological, histological, and endoscopical criteria.15 Thiopurines were prescribed according to a strict step-up approach with frequent therapy effect assessment, as recommended in current IBD guidelines.3,4 Exclusion criteria for this study were previous immunosuppressive treatment (thiopurines, methotrexate, or anti–tumor necrosis factor α therapy). Patient characteristics, thiopurine characteristics, tolerability, and effectiveness of thiopurine therapy were determined. The patients were subdivided into 2 real-life cohorts; the first cohort included all patients who initiated thiopurine therapy between the first of January 1, 2005 and December 31, 2009. The second cohort consisted of patients who initiated thiopurine therapy between January 1, 2010 and December 31, 2014. These cohorts were synchronic with the introduction of allopurinol cotherapy to thiopurines in our center from 2010.
Patient and Thiopurine Characteristics
Demographic patient characteristics, which we collected were sex, age, weight, smoking, type of IBD, and IBD related abdominal surgery (i.e., ileocecal resection or [procto]colectomy). The Montreal classification was used for classification of the underlying disease.16 In addition, thiopurine therapy characteristics were collected, including types of thiopurine (AZA and MP), dosage of thiopurine, age at initiation of thiopurine treatment, duration of thiopurine therapy, and reason for discontinuation (if applicable, subdivided into adverse effects, ineffectiveness, pregnancy, or prolonged remission). Optimization strategies (e.g., coadministration allopurinol, treatment with TG) and the addition of anti–tumor necrosis factor or steroids (if applicable) during the first year of follow-up were documented. All patients had a minimal follow-up of 1 year. When thiopurine metabolites were determined, the time of measurement and concentrations of 6-MMP and 6-TGN were extracted from the patient charts.
Thiopurine Metabolites
The thiopurine metabolites, 6-MMP and 6-TGN, were determined in red blood cells (RBCs) using a slightly modified method described by Dervieux.17 To compare these values with the internationally common method described by Lennard and Singleton,18 we divided 6-TGN values by 2.6, as previously shown by Shipkova et al.19 Because 6-MMP values are comparable between both methods, these values were not modified. As defined by Dubinsky et al, we considered 6-TGN concentrations between 235 and 450 pmol/8 × 108 RBC effective. Furthermore, 6-MMP concentrations were aimed to be below 5700 pmol/8 × 108 RBC.20 Optimization strategies (e.g., switch to TG, dose adjustment or allopurinol coadministration) were subsequently applied based on metabolite concentrations, according to the evaluation of one experienced gastroenterologist (A.v.B.). Coadministration of allopurinol was only applied in the second cohort.
Sustained Clinical Benefit of Thiopurine Therapy
Sustained clinical benefit was defined as an ongoing use of thiopurine treatment after at least 1 year of follow-up without either the addition of corticosteroids (≥10 mg/d) or anti–tumor necrosis factor α therapy or surgical intervention.6
Tolerability
An adverse event (AE) was defined as any reaction or medical event that occurred during the course of treatment, resulting in discontinuation of thiopurine therapy. AEs were subdivided into myelosuppression, hepatotoxicity (i.e., elevation of liver tests), pancreatitis, GI complaints, flu-like illness, arthralgia, or other complaints (e.g., alopecia, dizziness, or neurological symptoms). Myelosuppression was defined as leukocyte count below 3.5 × 109/L. Elevation of liver tests was defined according to the WHO toxicity score version 3.0.21 Grade 1 was defined as liver test values between the upper limit of normal (ULN) and 2.5 × ULN, grade 2 as 2.5 to 5.0 × ULN, and grade 3 as 5.0 ULN or higher. When one patient experienced more than one AE on thiopurine therapy, all AEs were separately counted, because of which the summed percentages of singular AE groups may exceed 100%.
Optimizing Strategies of Thiopurine Therapy
When thiopurine therapy was either optimized using treatment strategies (i.e., allopurinol coadministration or TG therapy) or reintroduced within 3 months after previous discontinuation, this was classified as optimization of therapy, and subsequent duration was added to the total duration of therapy. When thiopurine therapy was reintroduced after 3 months, this second admission was excluded from our analysis, and patients were classified as discontinuers of therapy.
Duration of Thiopurine Therapy and Continued Use
Duration of thiopurine therapy was calculated for both groups. In the first cohort, which included patients between 2005 and 2009 with at least 1 year follow-up, patients were classified as continuing users when still using therapy at January 1, 2011. For the calculation of therapy duration, the time from initiation to January 1, 2011 was computed.
In the second cohort, which included patients between 2010 and 2014 with 1 year follow-up, patients were classified as continued users when still using therapy at January 1, 2016. For the calculation of therapy duration, the time from initiation to January 1, 2016 was calculated.
Data Analysis
Descriptive data were presented as numbers with percentages. Continuous data were presented as median with (interquartile) range or mean with SD, according to distribution. Comparisons between the 2 cohorts were performed using the Chi-square test. Differences in metabolite levels before and after therapy optimization were calculated using the paired Wilcoxon ranks test. Time-to-event analysis was executed using the Kaplan–Meier method. Statistical analyses were performed using SPSS statistics (version 22.0; IBM, New York, NY). P-Values under 0.05 were considered statistically significant.
Post hoc Analysis
Our main target of this study was to assess the clinical value of allopurinol coadministration in our IBD cohort. Since we started the coadministration of allopurinol in 2010, we chose to initiate the second cohort at this point. However, because our protocol involved a minimum of 1 year follow-up, there were some patients in the first cohort with allopurinol cotherapy. To robustly answer our research question and hypothesis, we excluded the patients using allopurinol during the follow-up phase of the first cohort. These patients were classified as noncontinuing, nonoptimized users for our post hoc analysis.
Ethical Considerations
This study was approved by the Medical Ethics Review Committee (METC) of the VU University Medical Center with file-number 2016 to 400.
RESULTS
Patient Characteristics
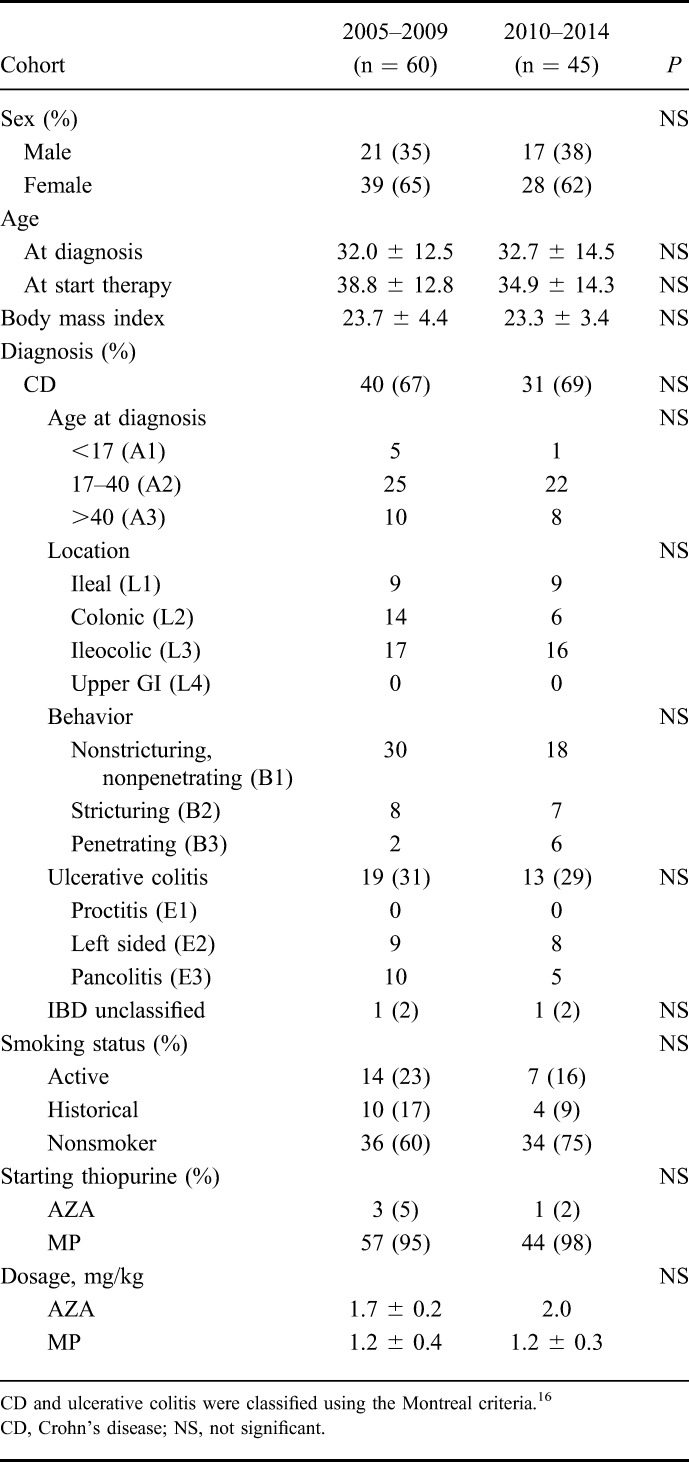
In total, 105 thiopurine-naive patients were included, of which 60 patients (cohort 1) initiated weight-based thiopurine monotherapy between 2005 to 2009 and 45 patients (cohort 2) between 2010 and 2014 (Fig. 1). Crohn's disease was diagnosed in 71 patients (68%), mainly with an (ileo)colonic disease localization (75%). The mean age at diagnosis was 32.0 ± 12.5 versus 32.7 ± 14.5 years. There were no differences in patient characteristics between the 2 cohorts. Demographic characteristics were depicted in Table 1.
FIGURE 1.
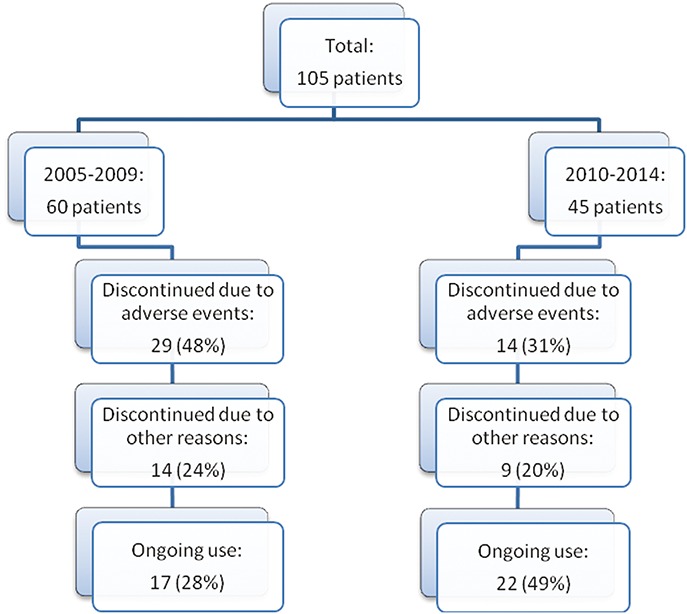
Flowchart of patient selection. In total, 105 patients were included, of which 60 patients received first thiopurine treatment in the 2005 to 2009 cohort and 45 patients in the 2010 to 2014 cohort. Reasons for discontinuation were intolerable AEs or other reasons, such as ineffectiveness, loss to follow-up, or at patient's wish.
TABLE 1.
Patient Characteristics
Tolerability of Thiopurine Therapy
Of the 60 patients in the first cohort, 17 were able to continue therapy (28%). Twenty-nine patients (48%) had to discontinue therapy within 1 year after initiation because of intolerable AEs, mainly hepatotoxicity or unspecific AEs (e.g., GI complaints or flu-like illness). The other 14 patients (24%) discontinued treatment after at least 1 year of treatment because of various reasons (Table 2).
TABLE 2.
Tolerability and Sustained Clinical Benefit of Thiopurines and Applied Optimization Strategies, Excluding Patients Receiving Allopurinol During the Follow-up of the First Cohort
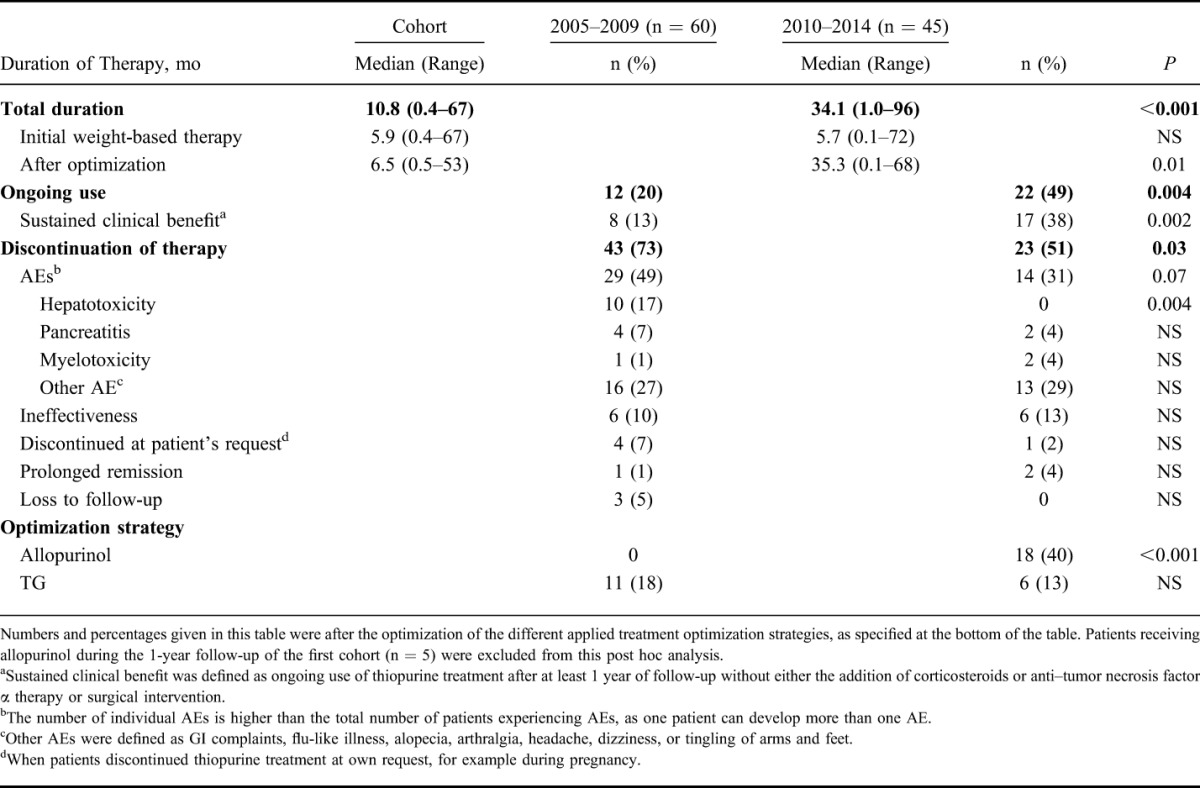
In the second (optimization) cohort, 22 out of 45 patients (49%) were able to continue therapy. This proportion was larger compared with the first cohort (P = 0.03). Of the 23 patients who had to discontinue treatment, 11 (24%) had to stop within the first year of treatment because of intolerable, mainly aspecific, AEs. These results are visualized in Figure 2.
FIGURE 2.
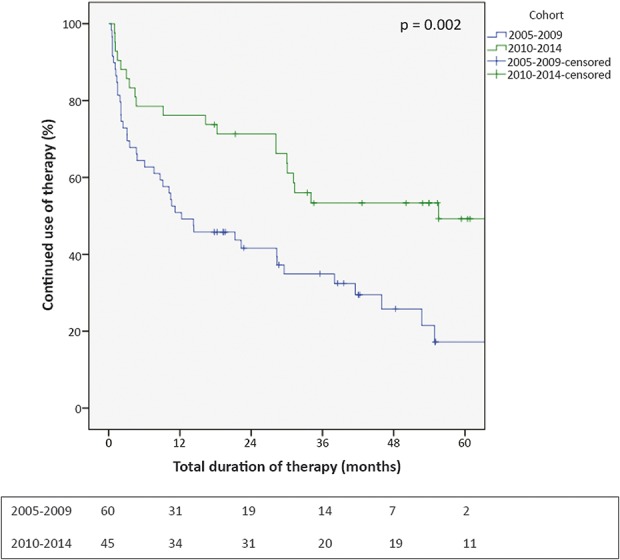
Kaplan–Meier curve of therapy continuation. Kaplan–Meier curve showing the percentage of continued thiopurine users over time. Cases discontinuing therapy for other reasons than intolerable AEs were censored. Absolute numbers of continuing users were depicted under the figure.
AEs Leading to Discontinuation of Thiopurine Therapy
In the first cohort, 29/60 (48%) of the patients experienced one or more AEs of therapy compared with 14/45 (31%) of the patients in the second cohort (P = 0.07). The heterogeneity of almost all AEs was comparable between the groups, the only difference was seen in the development of hepatotoxicity, which was more common in the first cohort (10/60, 17%) compared with the second cohort, after applying treatment optimization (0/45, P = 0.004).
Duration of Thiopurine Therapy
Overall, the use of thiopurine therapy was longer in the second cohort (median duration 34.1 versus 11.7 months, P < 0.001). This difference was not seen in the initial weight-based therapy phase (P > 0.05) but was mainly because of a longer duration after applying optimization strategies (33 versus 58% of the patients in cohort 1 and 2, respectively (P = 0.01); median duration of optimization phase 7.4 versus 35.3 months, P = 0.004).
Sustained Clinical Benefit of Thiopurine Therapy
In the first cohort, 13 of 60 patients (22%) experienced clinical benefit from thiopurine therapy, compared with 17 of 45 patients (38%) in the second cohort. This difference did not reach statistical significance; however, there was a statistical trend toward better effectiveness in the second cohort (P = 0.07). When we solely look into the effect of optimizing therapy, which was initiated in 2010 in our hospital, thus excluding all patients from the first cohort receiving an optimized thiopurine treatment, which was TG or allopurinol addition, there were 11 patients with clinical effectiveness of therapy in the first cohort compared with 17 in the second cohort, being statistically significant (P = 0.03).
Measuring Thiopurine Metabolites
Overall, in 60 patients (57%), thiopurine metabolites were determined during weight-based thiopurine therapy. In 22 patients of the first cohort (22/60, 37%), TDM was performed after a median period of 14 weeks (interquartile 9–35 wk). The median 6-TGN concentration was 171 pmol/8 × 108 RBC (range 63–885), and median 6-MMP value was 9200 pmol/8 × 108 RBC (range 410–48,000). Adequate 6-TGN concentrations were measured in 6 patients (6/22, 27%), whereas 14 patients (64%) had low levels and 2 patients (9%) had high 6-TGN concentrations. Follow-up metabolite measurement was performed in one optimized patient receiving allopurinol alongside MP with 6-TGN and 6-MMP concentrations of 288 and 250 pmol/8 × 108 RBC, respectively.
In the second cohort, more patients (38/45, 84%, P < 0.001) had metabolite measurement after a median duration of thiopurine therapy of 19 weeks (interquartile 8–56 wk). Median 6-TGN and 6-MMP concentrations during weight-based thiopurine therapy were 169 pmol/8 × 108 RBC (range 50–988) and 9100 (range 270–40,000), respectively. In 11 patients (11/38, 29%), 6-TGN concentrations were therapeutic, in 23 patients (60%), subtherapeutic, and in the remaining 4 patients (11%), concentrations were above 450 pmol/8 × 108 RBC. This distribution was comparable with the first cohort (P = 0.97).
After optimization, metabolite levels were determined in 16 patients (42%). Concentrations of 6-TGN were higher after optimization with a median concentration of 312 pmol/8 × 108 RBC (range 32–538; P = 0.03), whereas 6-MMP concentrations were lower with a median concentration of 325 pmol/8 × 108 RBC (range 130–740; P < 0.001).
Optimization of Therapy
In the first cohort, 5 patients (8%) received allopurinol cotherapy based on a skewed thiopurine metabolism during the 1-year follow-up of this cohort. In 4 patients, hepatotoxicity was present; the other patient was having complaints of general malaise. The number of allopurinol cousers was higher in the second cohort (18/45, 40%; P < 0.001). Seven patients were having laboratory signs of elevated liver enzymes, 4 patients experienced therapy ineffectiveness and the remaining 7 patients were having other AEs (e.g., flu-like illness, GI symptoms, or general malaise).
This difference between the 2 cohorts was not observed in patients who had TG as optimized thiopurine treatment, which was received by 11 patients in the first cohort (18%) and by 6 patients (13%) in the second cohort (Table 2).
Post hoc Analysis Excluding Patients Receiving Allopurinol from the First Cohort
Median total duration of therapy in the first cohort after reclassification of patients was 10.8 months, which was lower as compared to the second cohort (34.1 months, P < 0.001). The number of patients successfully continuing therapy was lower in the first cohort (20% versus 49%, P = 0.002), and the number of patients experiencing sustained clinical benefit (13% versus 38%, P = 0.004).
DISCUSSION
In this study comprising two 5-year real-life cohorts, we described the effect of a TDM-based optimization strategy of conventional thiopurine derivatives in patients with IBD. This is the first study describing the effect of optimizing thiopurine therapy in a real-life intercept cohort of 105 thiopurine using patients with IBD from a referral teaching hospital. We observed that optimization of thiopurine therapy, especially by adding allopurinol alongside thiopurine therapy, improved tolerability of therapy, as a greater proportion of patients was able to continue thiopurine therapy over time. In addition, patients treated with optimized thiopurine therapy experienced sustained clinical benefit in a larger proportion compared with nonoptimized patients.
The beneficial effects of optimization in the second cohort were mainly caused by a higher percentage of patients receiving TDM (37% versus 84%) and subsequent administration of allopurinol (8% versus 40%). Because the design of our study consisted of a 1-year follow-up, there were some patients receiving allopurinol in the first cohort as well. To correct for these patients, thus answering our research question and hypothesis, we performed a post hoc analysis which showed fairly similar results, yet significantly stronger. Other TDM-based optimization strategies had less significant effects on the primary outcome. Therapy switch to the less conventional thiopurine-derivative TG, for example, was comparable between the cohorts (18% versus 13%, P > 0.05).
Optimization of the tolerability of thiopurine therapy was most effective in reducing the number of hepatotoxic AEs, as the number of patients discontinuing therapy because of hepatotoxicity was reduced to zero in the second cohort. This steep decrease was also reported in several clinical studies observing the effect of allopurinol addition to thiopurine therapy. In these studies, incidence of hepatotoxicity decreased from up to 46% to 11% after the addition of allopurinol, which was underlined in this real-life cohort.9,10,22 One of the possible explanations for this difference is the proposed underlying mechanism for the development of hepatotoxicity because allopurinol inhibits the formation of the hepatotoxic metabolite 6-MMP.5,23
The incidence of myelosuppression during allopurinol cotherapy (i.e., leukocytopenia with leukocyte count below 3.5 ×109/L) was 2% to 5% in our cohorts, which is low compared with incidence rates up to 40% in early clinical trials examining the use of allopurinol alongside thiopurines, also depending on applied definitions.24 This feared AE, which occurs more frequent in tightly monitored settings as more blood samples are drawn (i.e., clinical trials), is often asymptomatic, and therefore, rarely leads to cessation of therapy in daily practice.11,25
Clinical benefit of (optimized) thiopurine therapy was observed in 38% of the patients in the second cohort, compared with 18% in the first cohort. This 2-fold higher proportion was also observed in clinical trials studying the benefit of allopurinol; however, absolute numbers were lower in this study. An explanation for this could be that sustained clinical benefit was strictly defined as ongoing use without the addition of steroids or biologicals.9,10,22 Using therapy optimization strategies, patients are able to gain clinical beneficiary effects of conventional immunomodulators, thus optimizing step-up treatment strategies, including well-documented cost savings.1 Solid statements about efficacy, however, have to be interpreted with caution, because of the retrospective nature of this study.
The major limitation of this study is the lack of a standardized protocol, as in this study, we wanted to assess the clinical relevance of optimization strategies in a real-life, nonstandardized, noncontrolled cohort. Accordingly, patients between the 2 cohorts were not matched, which might have influenced the outcomes of our study. Another limitation, probably leading to selection bias, was the recruitment of patients from a tertiary referral hospital. Because of the complex nature of IBD in patients treated in a tertiary center, the number of AEs and patients experiencing therapy ineffectiveness was slightly higher in our cohort as compared to data from other studies.26 The treatment of patients by one practitioner might account for an inherent limitation for the generalizability of this study. However, we believe that the treatment by one practitioner also accounts for uniformity in applied treatment strategies, which strengthens the methodology of our study. Finally, as thiopurine metabolite measurement was not performed per protocol throughout this whole cohort, some AEs could probably have been prevented when early metabolite assessment was performed.27
CONCLUSIONS
With these two 5-year intercept cohorts, we observed that optimization of thiopurine therapy in real-life, clinical practice successfully enhanced tolerability (28% versus 49%) and effectiveness (22% versus 38%) of thiopurine derivatives in patients with IBD. In these series, this was mainly because of the TDM-based coadministration of allopurinol. Future randomized trials, in which the use of TDM (and subsequently applied optimization strategies) is randomized between groups, could assess the real-life clinical role of TDM-based optimization more thoroughly.
Footnotes
The authors have no conflict of interest to disclose.
REFERENCES
- 1.van der Valk ME, Mangen MJ, Severs M, et al. Evolution of costs of inflammatory bowel disease over two years of follow-up. PLoS One. 2016;11:e0142481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Heuvel TR, Jonkers DM, Jeuring SF, et al. Cohort profile: the inflammatory bowel disease south limburg cohort (IBDSL). Int J Epidemiol. [Published online ahead of print June 4, 2015]. 10.1093/ije/dyv088. [DOI] [PubMed] [Google Scholar]
- 3.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. [DOI] [PubMed] [Google Scholar]
- 4.Chande N, Patton PH, Tsoulis DJ, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;10:CD000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer NK, van Bodegraven AA, Jharap B, et al. Drug insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:686–694. [DOI] [PubMed] [Google Scholar]
- 6.Jharap B, Seinen ML, de Boer NK, et al. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541–1549. [DOI] [PubMed] [Google Scholar]
- 7.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010;4:28–62. [DOI] [PubMed] [Google Scholar]
- 8.Haines ML, Ajlouni Y, Irving PM, et al. Clinical usefulness of therapeutic drug monitoring of thiopurines in patients with inadequately controlled inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1301–1307. [DOI] [PubMed] [Google Scholar]
- 9.Ansari A, Patel N, Sanderson J, et al. Low-dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31:640–647. [DOI] [PubMed] [Google Scholar]
- 10.Hoentjen F, Seinen ML, Hanauer SB, et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:363–369. [DOI] [PubMed] [Google Scholar]
- 11.Leung Y, Sparrow MP, Schwartz M, et al. Long term efficacy and safety of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohns Colitis. 2009;3:162–167. [DOI] [PubMed] [Google Scholar]
- 12.Min MX, Weinberg DI, McCabe RP. Allopurinol enhanced thiopurine treatment for inflammatory bowel disease: safety considerations and guidelines for use. J Clin Pharm Ther. 2014;39:107–111. [DOI] [PubMed] [Google Scholar]
- 13.van Asseldonk DP, Seinen ML, de Boer NK, et al. Hepatotoxicity associated with 6-methyl mercaptopurine formation during azathioprine and 6-mercaptopurine therapy does not occur on the short-term during 6-thioguanine therapy in IBD treatment. J Crohns Colitis. 2012;6:95–101. [DOI] [PubMed] [Google Scholar]
- 14.Seinen ML, van Asseldonk DP, Mulder CJ, et al. Dosing 6-thioguanine in inflammatory bowel disease: expert-based guidelines for daily practice. J Gastrointestin Liver Dis. 2010;19:291–294. [PubMed] [Google Scholar]
- 15.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6; discussion 16–19. [DOI] [PubMed] [Google Scholar]
- 16.Satsangi J, Silverberg MS, Vermeire S, et al. The montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dervieux T, Meyer G, Barham R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005;51:2074–2084. [DOI] [PubMed] [Google Scholar]
- 18.Lennard L, Singleton HJ. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr. 1992;583:83–90. [DOI] [PubMed] [Google Scholar]
- 19.Shipkova M, Armstrong VW, Wieland E, et al. Differences in nucleotide hydrolysis contribute to the differences between erythrocyte 6-thioguanine nucleotide concentrations determined by two widely used methods. Clin Chem. 2003;49:260–268. [DOI] [PubMed] [Google Scholar]
- 20.Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. [DOI] [PubMed] [Google Scholar]
- 21.Program CTE. Common Terminology Criteria for Adverse Events (CTCAE). CTEP [Internet]. 2006. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed May 19, 2017. [Google Scholar]
- 22.Kiszka-Kanowitz M, Theede K, Mertz-Nielsen A. Randomized clinical trial: a pilot study comparing efficacy of low-dose azathioprine and allopurinol to azathioprine on clinical outcomes in inflammatory bowel disease. Scand J Gastroenterol. 2016;51:1470–1475. [DOI] [PubMed] [Google Scholar]
- 23.Quemeneur L, Gerland LM, Flacher M, et al. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol. 2003;170:4986–4995. [DOI] [PubMed] [Google Scholar]
- 24.Govani SM, Higgins PD. Combination of thiopurines and allopurinol: adverse events and clinical benefit in IBD. J Crohns Colitis. 2010;4:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijer B, Kreijne JE, van Moorsel SA, et al. 6-methylmercaptopurine induced leukocytopenia during thiopurine therapy in IBD patients. J Gastroenterol Hepatol. [Published online ahead of print June 2017]. 10.1111/jgh.13656. [DOI] [PubMed] [Google Scholar]
- 26.Coenen MJ, de Jong DJ, van Marrewijk CJ, et al. Identification of patients with variants in TPMT and dose reduction reduces hematologic events during thiopurine treatment of inflammatory bowel disease. Gastroenterology. 2015;149:907–917 e907. [DOI] [PubMed] [Google Scholar]
- 27.Wong DR, Coenen MJ, Vermeulen SH, et al. Early assessment of thiopurine metabolites identifies patients at risk of thiopurine-induced leukopenia in inflammatory bowel disease. J Crohns Colitis. 2017;11:175–184. [DOI] [PubMed] [Google Scholar]


