Abstract
Aging is known to have deleterious effects on cerebral white matter, yet little is known about these white matter alterations in advanced age. In this study, 94 oldest-old adults without dementia (90–103 years) underwent diffusion tensor imaging to assess relationships between chronological age and multiple measures of integrity in 18 white matter regions across the brain. Results revealed significant age-related declines in integrity in regions previously identified as being sensitive to aging in younger-old adults (corpus callosum, fornix, cingulum, external capsule). For the corpus callosum, the effect of age on genu FA was significantly weaker than the relationship between age and splenium FA. Importantly, age-related declines in white matter integrity did not differ in cognitively normal and cognitively impaired-not demented (CIND) oldest-old, suggesting that they were not solely driven by cognitive dysfunction or preclinical dementia in this advanced age group. Instead, white matter in these regions appears to remain vulnerable to normal aging processes through the tenth decade of life.
Keywords: aging, white matter integrity, oldest-old, corpus callosum, fornix
1. Introduction
Aging is known to have deleterious effects on cerebral white matter (for reviews see Gunning-Dixon et al., 2009; Salat, 2011; Raz and Rodrigue, 2006). At the macroscopic level, the aging brain is characterized by shrinkage of white matter tissue and development of white matter lesions. These gross structural differences may be driven by age-related effects at the microscopic level, which includes loss or alterations to myelin (demyelination), loss or shrinkage of white matter axons (neuronal degeneration), expansion of perivascular spaces (Virchow-Robin spaces), and proliferation of glial cells (gliosis; Matsusue et al., 2006; Peters, 2007). Because neuropathology studies of normal brain aging have focused on younger-old adults, little is known about these white matter alterations in advanced age groups.
White matter microstructure can be studied in vivo using advanced neuroimaging techniques, such as diffusion tensor imaging (DTI). DTI measures the rate of molecular water diffusion (Beaulieu, 2002; Le Bihan, 2003), which moves more freely along the length of structures within white matter (neuronal cell membranes, myelin sheaths) relative to diffusion perpendicular to these restricting structures. Multiple diffusion indices can be calculated to assess the degree of restricted diffusion (fractional anisotropy, FA), rate of overall diffusion (mean diffusivity, MD), and the rate of diffusion parallel (axial diffusivity, AD) and perpendicular (radial diffusivity, RD) to the primary diffusion direction. These measures are thought to approximate the “integrity” of white matter because they are sensitive to numerous properties of the underlying microstructure (e.g., axonal size and density, degree of myelination, and coherence of fiber orientation) that differ across individuals and with aging.
DTI has been used extensively to assess age-related differences in white matter integrity (for reviews see Bennett and Madden, 2014; Madden et al., 2009; Madden et al., 2012; Gunning-Dixon et al., 2009; Sullivan and Pfefferbaum, 2006). Both longitudinal and cross-sectional studies of normal aging have revealed linear decreases in FA across the adult lifespan and quadratic increases in MD starting around age 60 years (e.g., Michielse et al., 2010; Kennedy and Raz, 2009; Hsu et al., 2010). These age-related declines in white matter integrity (decreased FA, increased MD) are most prominent in the genu of the corpus callosum, fornix, and external capsule (e.g., Burzynska et al., 2010; Bennett et al., 2010; Pfefferbaum et al., 2000; Sullivan and Pfefferbaum, 2006; Bucur et al., 2008; Michielse et al., 2010; Davis et al., 2009; Sala et al., 2012). To date, however, very few DTI aging studies have included sizeable samples of individuals over age 80 years (e.g., > 10; Kochunov et al., 2012; Westlye et al., 2010) and no studies have assessed oldest-old adults over age 90 years. Here, we hypothesize that if white matter integrity simply continues to decline linearly into advanced age, then similarly large age effects may also be expected in regions previously identified as being vulnerable to healthy aging (e.g., genu of the corpus callosum, fornix, and external capsule) in oldest-old adults without dementia.
Relative to younger-old adults, however, oldest-old adults are disproportionately affected by dementia (Corrada et al., 2008; Gardner et al., 2013; Yang et al., 2013). Alzheimer’s disease, in particular, has been linked to a number of white matter alterations that would directly influence measures of white matter integrity (demyelination, neuronal degeneration, gliosis; Brun and Englund, 1986; Zhan et al., 2014; Sachdev et al., 2013). Consistent with this view, DTI studies in younger-old adults diagnosed with mild cognitive impairment and Alzheimer’s disease have reported integrity declines in the fornix, cingulum, and splenium of the corpus callosum (Stebbins and Murphy, 2009). Importantly, similar dementia-related differences in white matter integrity have also been observed in cognitively normal younger-old adults at increased risk for Alzheimer’s disease (Gold et al., 2012; Rieckmann et al., 2016). Thus, any examination of white matter aging in the oldest-old will need to account for the potential contribution of preclinical dementia in this advanced age group. Here, we hypothesize that if white matter integrity declines in advanced age are primarily attributed to the increased prevalence of dementia-related pathology in this age group, then age effects in regions previously identified as being vulnerable to dementia (e.g., fornix, cingulum, and splenium of the corpus callosum) may differ as a function of cognitive status in oldest-old adults without dementia.
The current study is the first to assess age-related differences in white matter integrity in the oldest-old and the degree to which they may be driven by cognitive dysfunction associated with preclinical dementia. Ninety-four oldest-old adults without dementia (age 90–103 years) underwent DTI to assess relationships between chronological age and multiple measures of integrity (FA, MD, AD, RD) from 18 white matter regions across the brain. The effect of preclinical dementia was assessed by controlling for cognitive status and by comparing age effects in cognitively normal and cognitively impaired-not demented (CIND) oldest-old, the latter of whom are at increased risk of progressing to dementia, with incidence rates greater than 30% per year relative to only 8% for cognitively normal oldest-old (Peltz et al., 2011).
2. Methods
2.1 Participants
One hundred one oldest-old adults were recruited as part of a new neuroimaging component of The 90+ Study, a longitudinal study of aging and dementia in the oldest-old (see Kawas and Corrada, 2006 for additional details). Participants’ cognitive status were assessed by trained examiners who evaluated their neurologic, physical, and neuropsychological performance, the latter of which included the Mini-Mental State Examination (MMSE; Folstein et al., 1975) and modified MMSE (3MS; Teng and Chui, 1987). Seven participants who demonstrated cognitive and functional impairments consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; Association, 1994) criteria for dementia were excluded from further analysis. Thirty participants who did not meet DSM-IV criteria for dementia, but who demonstrated some degree of cognitive impairment (i.e., performed below age-specific norms in one or more cognitive domains), were diagnosed as cognitively impaired-not demented (CIND) and remained in the study. Demographic and neuropsychological data on the full sample of 94 oldest-old adults without dementia, and the cognitively normal and CIND subgroups, are provided in Table 1.
Table 1.
Demographic and Neuropsychological Data
| All Participants (n = 94) |
Normal (n = 64) |
CIND (n = 30) |
Group Difference (t or X2) |
|
|---|---|---|---|---|
| Age | 94.6 ± 3.3 | 94.4 ± 3.1 (90 – 103) | 95.0 ± 3.7 (90 – 103) | −0.9 |
| Sex (female) | 68 (72.3 %) | 44 (68.8 %) | 24 (80.0 %) | 1.3 |
| Education (> high school) | 76 (80.9 %) | 56 (87.5 %) | 20 (66.7 %) | 3.4 |
| MMSE | 27.6 ± 2.5 | 28.5 ± 1.4 (25 – 30) | 25.6 ± 3.0 (19 – 30) | 6.6 * |
| 3MS | 93.2 ± 7.0 | 96.2 ± 3.2 (84 – 100) | 86.4 ± 8.5 (68 – 100) | 9.1 * |
Note. Demographic and neuropsychological test data are presented as mean ± standard deviation (range) or n (%), separately for the full sample and the cognitively normal and cognitively impaired-not demented (CIND) subgroups. Group differences were assessed with independent sample t tests (t) or chi-square tests (X2), revealing significantly better performance in cognitively normal versus CIND oldest-old on the Mini-Mental State Examination (MMSE) and modified MMSE (3MS;
p< 0.001).
Prior to participation, individuals were screened for contraindications that would make it unsafe for them to undergo magnetic resonance imaging (MRI) scanning (e.g., having ferrous metal implants). Each participant provided informed consent, and the University of California, Irvine Institutional Review Board approved the experimental procedures. Participants were compensated for their time.
2.2 Imaging Data Acquisition
Participants were scanned using a GE Signa HD 3.0 Tesla MRI system. Fitted padding was used to minimize head movements.
One diffusion weighted echo planar imaging sequence was acquired using the following parameters: TR/TE = 12,850/72 ms, FOV = 256 × 256 mm, 59 axial slices, and 1.4 × 1.4 × 2.7 mm spatial resolution. Gradients (b = 1,000 s/mm2) were applied in 30 orthogonal directions, with 5 images having no diffusion weighting (b = 0).
A high-resolution T1-weighted fast-spoiled gradient recalled echo (TR/TE/IT = 7/3/400 ms, FOV = 256 × 256 mm, 160 sagittal slices, and 1.0 mm3 spatial resolution) and a fluid attenuation inversion recovery (FLAIR) sequence (TR/TE/IT = 11,000/151/2,250 ms, FOV = 256 × 256 mm, 42 axial slices, and 0.86 × 0.86 × 5 mm spatial resolution) were also acquired.
2.3 Imaging Data Analysis
2.3.1 FLAIR data
Our method for automatic segmentation of white matter hyperintensities (WMH) has been previously described in detail (DeCarli et al., 2005). Briefly, brain tissue was extracted from non-brain tissue and eroded to remove partial volume CSF effects, image intensity non-uniformities were removed before FLAIR image intensity was modeled with a Gaussian distribution, and voxels with image intensities greater than 3.5 standard deviations above the mean were identified as WMH.
Each participant’s segmented FLAIR image was aligned to their high-resolution T1-weighted image using a rigid-body transformation. The high-resolution T1-weighted image was then aligned to a T1-weighted template defined in MNI152 standard space using affine and nonlinear transformations. These transformations were then applied to the aligned segmented FLAIR image, allowing WMH to be placed in the same coordinate space as the normalized DTI-derived images.
2.3.2. Diffusion data
Diffusion-weighted data were pre-processed separately for each participant using the FMRIB Software Library (FSL) Diffusion Toolbox (Jenkinson et al., 2012; Smith et al., 2004). Brain tissue was extracted from non-brain tissue, gross head motion and eddy currents were corrected using affine transformations, and the diffusion tensor model was computed at each voxel. Output included a voxel-wise FA image that was aligned to a standard DTI template in MNI152 standard space (FMRIB58_FA_1mm.nii) using affine and nonlinear transformations. These transformations were then applied to the voxel-wise MD, AD (L1), and RD (L2+L3/2) images. To focus our analyses on age differences in integrity of normal appearing white matter, we excluded voxels labeled as WMH in each participant’s normalized segmented FLAIR from these normalized DTI-derived images.
Regional measures of each integrity metric (FA, MD, AD, RD) were obtained by superimposing the normalized DTI-derived images, corrected for WMH, onto a standard labeled atlas in the same MNI152 standard space (ICBM-DTI-81; Mori et al., 2005). A total of 18 white matter regions were identified from the labeled atlas, excluding brainstem and cerebellar regions: genu, body, and splenium of the corpus callosum, fornix body, fornix cres, cingulate gyrus, hippocampal cingulum, superior longitudinal fasciculus, inferior longitudinal fasciculus, superior fronto-occipital fasciculus, uncinate fasciculus, external capsule, corona radiata, corticospinal tract, posterior thalamic radiations, anterior limb of the internal capsule, posterior limb of the internal capsule, and retrolenticular part of the internal capsule. Bilateral regions included atlas labels from both hemispheres. The corona radiata included bilateral anterior, posterior, and superior atlas labels. Mean integrity values were calculated by multiplying each voxel-wise integrity image by each binarized white matter region, excluding voxels identified as WMH, and then averaging remaining values across voxels.
3. Results
3.1 Age-Related Differences in White Matter Integrity
Separate multiple regression analyses were conducted between chronological age and each integrity metric (FA, MD, AD, RD) from the 18 white matter regions of interest, controlling for sex and education. Significant effects survived the false discovery rate (FDR) correction for 72 comparisons (FDR-adjusted p < 0.012). These data are presented in Table 2. Descriptive statistics (mean, 95% confidence interval) for each integrity metric in each region of interest are presented in Supplementary Table 1.
Table 2.
Age-Related Differences in White Matter Integrity in the Oldest-Old
| FA [β (p)] | MD [β (p)] | AD [β (p)] | RD [β (p)] | |
|---|---|---|---|---|
| Corpus Callosum | ||||
| Genu | −0.18 (0.079) | 0.18 (0.085) | 0.14 (0.186) | 0.19 (0.068) |
| Body | −0.22 (0.037) | 0.17 (0.117) | 0.10 (0.337) | 0.18 (0.083) |
| Splenium | −0.30 (0.004) | 0.30 (0.004) | 0.22 (0.037) | 0.31 (0.003) |
| Limbic | ||||
| Fornix body | −0.23 (0.032) | 0.25 (0.019) | 0.24 (0.021) | 0.24 (0.020) |
| Fornix cres | −0.34 (0.001) | 0.33 (0.001) | 0.31 (0.003) | 0.34 (0.001) |
| Cingulate gyrus | −0.29 (0.004) | 0.04 (0.737) | −0.17 (0.104) | 0.13 (0.206) |
| Hippocampal cingulum | −0.19 (0.074) | 0.16 (0.116) | 0.15 (0.150) | 0.17 (0.107) |
| Association | ||||
| External capsule | −0.33 (0.001) | 0.24 (0.021) | 0.17 (0.119) | 0.28 (0.007) |
| Superior longitudinal fasciculus | −0.18 (0.085) | 0.16 (0.143) | 0.10 (0.342) | 0.17 (0.109) |
| Inferior longitudinal fasciculus | −0.30 (0.004) | 0.24 (0.023) | 0.18 (0.085) | 0.26 (0.012) |
| Superior fronto-occipital fasciculus | −0.28 (0.009) | 0.18 (0.085) | 0.13 (0.207) | 0.20 (0.051) |
| Uncinate fasciculus | −0.25 (0.015) | −0.05 (0.670) | −0.11 (0.291) | 0.01 (0.897) |
| Projection/Thalamic | ||||
| Corona radiata | −0.23 (0.033) | 0.20 (0.054) | 0.24 (0.021) | 0.22 (0.043) |
| Corticospinal | −0.22 (0.035) | 0.10 (0.360) | 0.25 (0.812) | 0.14 (0.184) |
| Posterior thalamic radiation | −0.27 (0.009) | 0.23 (0.028) | 0.17 (0.116) | 0.26 (0.015) |
| Anterior limb of internal capsule | −0.24 (0.021) | 0.20 (0.062) | 0.15 (0.146) | 0.22 (0.040) |
| Posterior limb of internal capsule | −0.14 (0.174) | 0.32 (0.002) | 0.30 (0.004) | 0.30 (0.005) |
| Retrolenticular part of internal capsule | −0.12 (0.248) | 0.28 (0.007) | 0.27 (0.010) | 0.28 (0.008) |
Note. Significant (bolded, FDR-adjusted p < 0.012) and trending (italics, p < 0.05) relationships [β (p)] between chronological age and white matter integrity, controlling for sex and education, are presented separately for each integrity metric (FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; RD, radial diffusivity) and each white matter region.
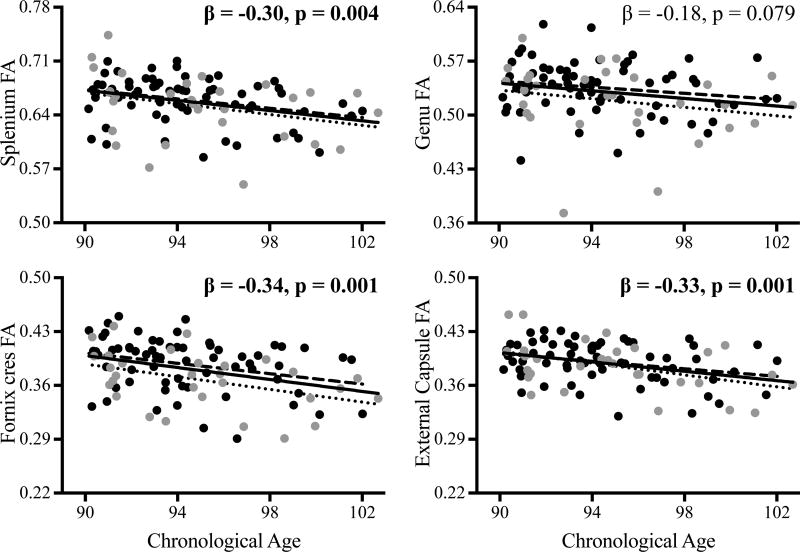
Results revealed significant age-related declines in white matter integrity (decreased FA; increased MD, AD, RD) in the splenium of the corpus callosum (FA, MD, AD, RD), fornix cres (FA, MD, AD, RD), cingulate gyrus (FA), external capsule (FA, RD), inferior longitudinal fasciculus (FA, RD), superior fronto-occipital fasciculus (FA), posterior thalamic radiation (FA), posterior limb of internal capsule (MD, AD, RD), and retrolenticular part of internal capsule (MD, AD, RD). Select data for FA are presented in Figure 1.
Figure 1.
Relationships between increased chronological age and decreased white matter integrity (fractional anisotropy, FA) in the splenium (upper left) and genu (upper right) of the corpus callosum, fornix cres (lower left), and external capsule (lower right) are presented across the full sample (black line; bolded, FDR-adjusted p < 0.012). These relationships did not differ between cognitively normal (black circles, dashed line) and cognitively impaired-not demented (CIND; gray circles, dotted line) subgroups. The effect of age on splenium FA was significantly stronger than the effect of age on genu FA.
Given the unexpected finding of significant age effects in the splenium, but not genu, of the corpus callosum, unplanned follow-up analyses compared regression coefficients in these regions using a procedure for correlated correlations (see Meng et al., 1992). Results revealed that the effect of age was significantly larger in splenium relative to the genu for FA (z = 2.4, p < 0.01), but not MD, AD, or RD (p’s > 0.73). Data for FA are presented in Figure 1.
3.2 Effects of Cognitive Status
3.2.1 Neuropsychological and Demographic Data
Independent sample t tests revealed that cognitively normal oldest-old performed significant better than CIND oldest-old on both the MMSE, t(92) = 6.6, p < 0.001, and 3MS, t(88) = 7.9, p < 0.001. These data are presented in Table 1.
An independent sample t test also showed that cognitively normal and CIND oldest-old did not differ in age (p > 0.08), and separate chi-square tests revealed that there were no significant relationships between oldest-old subgroups and sex (p > 0.25) or education (p > 0.06).
3.2.2 Age-related integrity differences are not moderated by cognitive status
Separate moderation analyses compared relationships between chronological age and white matter integrity in the cognitively normal and CIND subgroups for the 20 significant age effects observed across the full sample (see bolded effects in Table 2). For each region that demonstrated a significant decline in any integrity metric, the outcome variable was the corresponding integrity metric and the predictor variables were chronological age, group (cognitively normal, CIND), and the age × group interaction.
Results revealed that the interaction term was not a significant predictor of integrity in any region, p’s > 0.08, f2’s < 0.04. Thus, the effect of chronological age on white matter integrity did not significantly differ between groups in these regions. Select data for FA are presented in Figure 1.
3.2.3 Age-related integrity differences persist after controlling for cognitive performance
Separate multiple regression analyses between chronological age and each integrity metric (FA, MD, AD, RD) from the 18 white matter regions of interest were conducted again after controlling for MMSE, in addition to sex and education. Significant effects survived the false discovery rate (FDR) correction for 72 comparisons (FDR-adjusted p < 0.012). These data are presented in Table 3.
Table 3.
Age-Related Differences in White Matter Integrity in the Oldest-Old Controlling for Cognitive Status
| FA [β (p)] | MD [β (p)] | AD [β (p)] | RD [β (p)] | |
|---|---|---|---|---|
| Corpus Callosum | ||||
| Genu | −0.13 (0.204) | 0.15 (0.274) | 0.08 (0.469) | 0.13 (0.226) |
| Body | −0.18 (0.093) | 0.13 (0.232) | 0.08 (0.463) | 0.14 (0.186) |
| Splenium | −0.27 (0.010) | 0.29 (0.006) | 0.23 (0.033) | 0.29 (0.007) |
| Limbic | ||||
| Fornix body | −0.17 (0.104) | 0.22 (0.042) | 0.22 (0.041) | 0.22 (0.045) |
| Fornix cres | −0.29 (0.005) | 0.29 (0.006) | 0.27 (0.009) | 0.29 (0.005) |
| Cingulate gyrus | −0.25 (0.017) | 0.00 (0.978) | −0.17 (0.114) | 0.09 (0.404) |
| Hippocampal cingulum | −0.16 (0.128) | 0.15 (0.162) | 0.14 (0.204) | 0.16 (0.150) |
| Association | ||||
| External capsule | −0.30 (0.004) | 0.22 (0.037) | 0.16 (0.156) | 0.26 (0.015) |
| Superior longitudinal fasciculus | −0.19 (0.075) | 0.17 (0.121) | 0.12 (0.291) | 0.18 (0.095) |
| Inferior longitudinal fasciculus | −0.26 (0.013) | 0.19 (0.073) | 0.14 (0.200) | 0.21 (0.043) |
| Superior fronto-occipital fasciculus | −0.26 (0.017) | 0.15 (0.175) | 0.10 (0.369) | 0.17 (0.113) |
| Uncinate fasciculus | −0.24 (0.024) | −0.07 (0.503) | −0.13 (0.228) | −0.02 (0.861) |
| Projection/Thalamic | ||||
| Corona radiata | −0.21 (0.052) | 0.16 (0.128) | 0.14 (0.206) | 0.18 (0.103) |
| Corticospinal | −0.17 (0.095) | 0.10 (0.372) | 0.04 (0.729) | 0.13 (0.224) |
| Posterior thalamic radiation | −0.28 (0.011) | 0.25 (0.024) | 0.20 (0.074) | 0.27 (0.015) |
| Anterior limb of internal capsule | −0.22 (0.042) | 0.18 (0.101) | 0.14 (0.202) | 0.20 (0.072) |
| Posterior limb of internal capsule | −0.13 (0.225) | 0.30 (0.005) | 0.28 (0.009) | 0.28 (0.009) |
| Retrolenticular part of internal capsule | −0.08 (0.429) | 0.26 (0.017) | 0.25 (0.018) | 0.25 (0.022) |
Note. Significant (bolded, FDR-adjusted p < 0.012) and trending (italics, p < 0.05) relationships [β (p)] between chronological age and white matter integrity, controlling for sex, education, and MMSE, are presented separately for each integrity metric (FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; RD, radial diffusivity) and each white matter region. After controlling for MMSE, age-related differences in white matter integrity remained significant in the splenium of the corpus callosum, fornix cres, external capsule, posterior thalamic radiation, and posterior limb of the internal capsule.
Results revealed that, even after controlling for cognitive performance using MMSE, significant age-related declines in white matter integrity (decreased FA; increased MD, AD, RD) were observed in the splenium of the corpus callosum (FA, MD, RD), fornix cres (FA, MD, AD, RD), external capsule (FA), posterior thalamic radiation (FA), and posterior limb of internal capsule (MD, AD, RD). Age effects in the cingulate gyrus, inferior longitudinal fasciculus, superior fronto-occipital fasciculus, and retrolenticular part of the internal capsule no longer attained significance after controlling for MMSE.
3.2.4 White matter integrity does not differ with cognitive status
Finally, group differences in white matter integrity were assessed with a group (cognitively normal, CIND) × region (18 regions of interest) × integrity metric (FA, MD, AD, RD) ANCOVA that controlled for sex and education. Importantly, neither the main effect of group nor any interaction with group attained significance (p’s > 0.06).
This omnibus group × region × integrity metric ANCOVA was also re-analyzed using a Bayesian test with default prior scales in JASP (version 0.8.0.1; see Supplementary Table 2). Consistent with the previously reported non-significant main effect of group, a Bayes factor of 0.049 for the group model relative to the null model provided strong evidence in favor of there being no difference in white matter integrity between cognitively normal and CIND oldest-old.
4. Discussion
The current study examined age-related differences in white matter integrity in oldest-old adults without dementia and assessed whether these relationships differed between cognitively normal and cognitively impaired-not demented (CIND) subgroups. Results revealed significant age-related declines in white matter integrity in a handful of regions previously identified as showing the largest age effects in cognitively normal younger-old adults (e.g., fornix, external capsule). In the corpus callosum, however, relationships between age and white matter integrity were significantly weaker in the genu relative to the splenium. Observed age-related declines in white matter integrity also overlapped with regions previously identified as showing additional declines in younger-old adults with, or at risk for, dementia (e.g., fornix, cingulate gyrus, splenium of the corpus callosum). Importantly, relationships between age and white matter integrity in these regions were statistically similar in cognitively normal and CIND oldest-old, and remained significant after controlling for global cognition using MMSE performance, supporting the notion that the age effects observed here were not solely driven by cognitive dysfunction or increased dementia risk in this age group.
Previous DTI aging studies in cognitively normal younger-old adults have consistently reported brain-wide age-related declines in white matter integrity, with the largest age effects in the fornix, external capsule, and genu of the corpus callosum (Bennett et al., 2010; Burzynska et al., 2010). These regions overlap with the current findings of age-related declines in white matter integrity in the oldest-old. Specifically, increased age was associated with decreased integrity in half of the regions examined (9/18), including the fornix cres (decreased FA; increased MD, AD, RD) and external capsule (decreased FA; increased RD). In younger-old adults, normal age-related declines in white matter integrity are primarily attributed to demyelination and neuronal degeneration (Marner et al., 2003; Meier-Ruge et al., 1992; Peters, 2002; Tang et al., 1997). Age-related increases in radial (RD) and axial (AD) diffusivity, which were observed here for all regions that showed age effects for FA and MD, are often attributed to these neurobiological substrates, respectively (e.g., Kumar et al., 2013; cf., Wheeler-Kingshott and Cercignani, 2009). Yet, a definitive link between these microstructural alterations and regional age effects remains to be demonstrated. Measures of white matter integrity in oldest-old adults may be further influenced by a range of pathologies typically associated with various forms of dementia (e.g., neurofibrillary tangles, diffuse and neuritic plaques, microinfarcts, lacunes, white matter disease, cerebral amyloid angiopathy) that are also present in oldest-old adults without dementia (Balasubramanian et al., 2012; Bennett et al., 2006; Corrada et al., 2012; Kawas et al., 2015). Future neuroimaging studies can assess whether these dementia-related pathologies contribute to larger age differences in white matter integrity by directly comparing age effects in younger-old and oldest-old adults. As an important first step, here we demonstrated that oldest-old adults exhibit similar patterns of white matter aging as younger-old adults, especially in the fornix and external capsule, supporting the notion that white matter in these regions remains vulnerable to normal aging processes through the tenth decade of life.
In contrast to previous DTI aging studies in younger-old adults, oldest-old adults exhibited a different pattern of white matter aging in the corpus callosum. Specifically, relationships between age and white matter integrity did not attain significance in the genu of the corpus callosum for oldest-old adults in the current study. Instead, increased age was significantly related to decreased FA and increased MD and RD in the splenium of the corpus callosum, with the effect of age on splenium FA being significantly larger than the effect of age on genu FA. These results are not inconsistent with the notion of an anterior-posterior gradient of brain aging, where anterior white matter is thought to be more susceptible to brain aging (Head et al., 2004; Pfefferbaum et al., 2005). Indeed, the current data may indicate a temporal shift in the vulnerability of anterior and posterior corpus callosum, with the genu showing accelerated declines in younger-old adults that plateau in oldest-old adults, whereas the splenium remains relatively preserved in younger-old adults and begins to decline later in the aging process. This interpretation should be tested in future studies that compare regional differences in the aging of white matter integrity across the lifespan.
Before concluding that the age effects observed here are attributed to normal aging, we need to rule out the potential influence of preclinical dementia. Previous DTI studies have reported dementia-related declines in white matter integrity in the splenium of the corpus callosum, fornix, and cingulum in younger-old adults diagnosed with mild cognitive impairment and Alzheimer’s disease (Stebbins and Murphy, 2009). The same regions also show integrity declines in cognitively normal younger-old adults at increased risk for Alzheimer’s disease as determined by APOE4 carrier status, family history of Alzheimer’s disease, and neuroimaging assessment of amyloid burden (Gold et al., 2012; Rieckmann et al., 2016). Notably all three of these regions yielded significant age-related declines in white matter integrity in oldest-old adults. Importantly, however, moderation analyses revealed that the relationships between age and white matter integrity in these (or any) regions did not differ in cognitively normal and CIND oldest-old. Analyses controlling for MMSE also revealed that the age effects in these regions remained significant after controlling for global cognition. And group comparisons revealed no significant differences in white matter integrity between the oldest-old subgroups. Thus, the reported age effects do not appear to be attributed to cognitive dysfunction or underlying pathology associated with preclinical dementia in this advanced age group.
Despite constituting the fastest growing segment of our population (He and Muenchrath, 2011), the oldest-old have been significantly underrepresented in the scientific literature. This neuroimaging study provides initial evidence that age-related differences in white matter integrity in oldest-old adults without dementia are largely similar to those observed in younger-old adults, supporting the notion that white matter in the fornix, external capsule, and corpus callosum are particularly vulnerable to normal aging processes that extend from the sixth through tenth decades of life. Moreover, because similar patterns of age-related differences in white matter integrity were observed in cognitively normal and CIND oldest-old adults, the current data are consistent with the effects of normal aging, not preclinical dementia. As the first examination of age-related differences in white matter integrity in oldest-old adults, this study is strengthened by the large sample of well-characterized participants and by comparing age-matched groups of cognitively normal and CIND oldest-old within a narrow age range. The latter provides confidence that differences, if observed, are due to diagnosis and not age, which is sometimes difficult to control in studies of younger-old adults. Our interpretations, however, will benefit from future studies that directly compare younger-old and oldest-old adults and that include a subgroup of oldest-old adults with dementia.
Supplementary Material
Highlights.
White matter alterations in advanced age are largely unknown
94 oldest-old without dementia (>90 years) underwent diffusion tensor imaging
Integrity declined with age in corpus callosum, fornix, and external capsule
Age effects did not differ in cognitively normal versus impaired oldest-old
White matter remains vulnerable to normal aging through the tenth decade of life
Acknowledgments
This work was supported by the National Institutes of Health (NIH)/National Institute on Aging (NIA) grants: R01 AG021055 (Kawas and Corrada), K99/R00 AG047334 (Bennett), and P30 AG0101029 (DeCarli). We wish to thank Snigdha Karmarsu for assistance with data collection and Natalie Bryant for assistance with data analyses. We also wish to thank the staff and participants of the University of California, Irvine 90+ Study, the staff at Newport Diagnostic Center, and the staff at the Imaging of Dementia and Aging (IDeA) Laboratory at University of California, Davis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79:915–21. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neurosci. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–90. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–62. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29:1070–9. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li S-C, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–12. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res. 2012;9:709–17. doi: 10.2174/156720512801322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–43. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Bucjler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–41. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–5. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther. 2013;5:27. doi: 10.1186/alzrt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer’s disease: Preliminary findings and future directions. Biochim Biophys Acta Mol Basis Dis. 2012;1822:416–22. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Ger Psychiat. 2009;24:109–17. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Muenchrath MN. U.S. Census Bureau, American Community Survey Reports, ACS-17, 90+ in the United States: 2006–2008. Washington, DC: U.S. Government Printing Office; 2011. [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–23. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hsu JL, Van Hecke W, Bai CH, Lee CH, Tsai YF, Chiu HC, Jaw FS, Hsu CY, Leu JG, Chen WH. Microstructural white matter changes in normal aging: a diffusion tensor imaging study with higher-order polynomial regression models. Neuroimage. 2010;49:32–43. doi: 10.1016/j.neuroimage.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM. Alzheimer’s and dementia in the oldest-old: a century of challenges. Curr Alz Res. 2006;3:411. doi: 10.2174/156720506779025233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–42. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–27. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Harper RM. Brain axial and radial diffusivity changes with age and gender in healthy adults. Brain Res. 2013;1512:22–36. doi: 10.1016/j.brainres.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta Mol Basis Dis. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–35. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Matsusue E, Sugihara S, Fujii S, Ohama E, Kinoshita T, Ogawa T. White matter changes in elderly people: MR-pathologic correlations. Magn Res Med Sci. 2006;5:99–104. doi: 10.2463/mrms.5.99. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Ann NY Acad Sci. 1992;673:260–9. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychol Bull. 1992;111:172–5. [Google Scholar]
- Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. Neuroimage. 2010;52:1190–201. doi: 10.1016/j.neuroimage.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Peltz CB, Corrada MM, Berlau DJ, Kawas CH. Incidence of dementia in oldest-old with amnestic MCI and other cognitive impairments. Neurology. 2011;77:1906–12. doi: 10.1212/WNL.0b013e318238ee89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581–93. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. Brain aging: Models, methods, and mechanisms. Boca Raton: Taylor & Francis; 2007. The effect of normal aging on nerve fibers and neuroglia in the central nervous system In Riddle DR editor; pp. 97–125. [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–9. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–68. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–48. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Van Dijk KR, Sperling RA, Johnson KA, Buckner RL, Hedden T. Accelerated decline in white matter integrity in clinically normal individuals at risk for Alzheimer’s disease. Neurobiol Aging. 2016;42:177–88. doi: 10.1016/j.neurobiolaging.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Zhuang L, Braidy N, Wen W. Is Alzheimer’s a disease of the white matter. Curr Opin Psychiatry. 2013;26:244–51. doi: 10.1097/YCO.0b013e32835ed6e8. [DOI] [PubMed] [Google Scholar]
- Sala S, Agosta F, Pagani E, Copetti M, Comi G, Flippi M. Microstructural changes and atrophy in brain white matter tracts with aging. Neurobiol Aging. 2012;33:488–98. doi: 10.1016/j.neurobiolaging.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Salat DH. The declining infrastructure of the aging brain. Brain Connect. 2011;1:279–93. doi: 10.1089/brain.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Beh Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobeh Rev. 2006;30:749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging. 1997;18:609–15. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The modified mini-mental state examination (3MS) Can J Psychiatry. 1987;41:114–21. [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–68. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–60. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Yang Z, Slavin MJ, Sachdev PS. Dementia in the oldest old. Nat Rev Neurol. 2013;9:382–93. doi: 10.1038/nrneurol.2013.105. [DOI] [PubMed] [Google Scholar]
- Zhan X, Jickling GC, Ander BP, Liu D, Stamova B, Cox C, Jin LW, DeCarli C, Sharp FR. Myelin injury and degraded myelin vesicles in Alzheimer’s disease. Curr Alzheimer Res. 2014;11:232–8. doi: 10.2174/1567205011666140131120922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.