Abstract
Protein modification by O-linked beta-N-acetylglucosamine (O-GlcNAc) is emerging as an important factor in the pathogenesis of sporadic Alzheimer’s disease, however detailed molecular characterization of this important protein posttranslational modification at proteome level has been highly challenging, due to its low stoichiometry and labile nature. Herein we report the most comprehensive, quantitative proteomics analysis for protein O-GlcNAcylation in post-mortem human brain tissues with and without Alzheimer’s disease using isobaric tandem mass tags labeling, chemoenzymatic photocleavage enrichment and liquid chromatography coupled to mass spectrometry. A total of 1,850 O-GlcNAc peptides covering 1,094 O-GlcNAcylation sites were identified from 530 proteins in the human brain. One hundred and thirty one O-GlcNAc peptides covering 81 proteins were altered in Alzheimer’s brain as compared to controls (q <0.05). Moreover, alteration of O-GlcNAc peptide abundance could be attributed more to O-GlcNAcylation level than to protein level changes. The altered O-GlcNAcylated proteins belong to several structural and functional categories, including synaptic proteins, cytoskeleton proteins and memory-associated proteins. These findings suggest that dysregulation of O-GlcNAcylation of multiple brain proteins may be involved in the development of sporadic Alzheimer’s disease.
Keywords: O-GlcNAcylation, Alzheimer’s disease, Isobaric labeling, Mass spectrometry
Introduction
O-GlcNAcylation is an important protein modification in which Ser/Thr residues are modified with a single β-N-acetylglucosamine [1]. O-GlcNAcylation is generally considered as a post-translational modification (PTM), but it may also occur co-translationally [2]. In living organisms, O-GlcNAc is dynamically regulated by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). O-GlcNAc has been found to modify many proteins and may play important roles in regulating diverse cellular processes such as metabolism [3], signalling [4], transcription [5] and protein quality control [2].
Recently, the possible link between O-GlcNAc and Alzheimer’s disease (AD) has attracted attention [6,7]. Most cases of AD (~99%) are sporadic and may be caused by multiple etiological factors including epigenetic, environmental and metabolic factors. Metabolic factors may play the most significant roles in the development of this disease [8]. One of the earliest and most obvious brain metabolic changes is decreased brain glucose uptake and metabolism [9]. As a known molecular sensor of intracellular metabolism and stress, O-GlcNAcylation may mediate several pathways in disrupted brain metabolism. Thus, comprehensive understanding of brain protein O-GlcNAcylation and its dysregulation in AD may contribute to a better understanding of the molecular mechanisms leading to its sporadic development.
However, issues such as the low levels and labile nature of O-GlcNAcylation have posed significant analytical challenges for the direct proteomic characterization of O-GlcNAc peptides using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS); the ability to quantify O-GlcNAc in large scale is also largely absent. Advances recently made in the proteomics field are increasingly enabling high-fidelity MS characterization of O-GlcNAc peptides [10] using techniques such as lectin affinity chromatography [11] and chemoenzymatic photocleavage (CEPC) [12] methods for enrichment, electron transfer dissociation (ETD) for site determination [13], and stable isotope labeling methods for precise quantification [14–16]. In this report, we developed an integrated proteomic pipeline consisting of isobaric tandem mass tag (TMT) labeling [17], high-pH reversed-phase LC (bRPLC) fractionation and concatenation [18], CEPC enrichment [12,19] and LC-MS/MS for comprehensive, quantitative and large-scale analysis of protein O-GlcNAc. Application of this proteomic pipeline to characterize O-GlcNAcylation in post-mortem human brains from individuals with or without AD led to the identification of a total of 1,850 O-GlcNAc peptides from 530 proteins (with 1,094 confidently assigned O-GlcNAc sites), among which 131 O-GlcNAc peptides altered significantly in AD.
Materials and Methods
Brain tissue homogenization and digestion
Autopsied post-mortem frozen brain tissue samples from 10 pathologically confirmed AD cases and 10 matched controls with no neurological pathologies or symptoms (Table S1) were obtained from the Banner Sun Health Research Institute Brain and Body Donation Program (Sun City, AZ). The use of the frozen human brain tissue was in accordance with the National Institutes of Health guidelines and was approved by the Institutional Review Boards of New York State Institute for Basic Research and the Pacific Northwest National Laboratory. All AD cases were in Braak stage V or VI. AD cases and controls were matched in terms of age of death, post-mortem interval and gender. The grey matter (~0.1 g) of the mid frontal gyrus of each sample was homogenized in 600 μl lysis buffer (8 M urea, 60 mM NH4HCO3, 1x HALT phosphatase inhibitor cocktail (Pierce), 20 μM PUGNAc and 10 mM dithiothreitol). After homogenization, proteins were reduced at 37 °C for 1 h and alkylated at room temperature (RT) for 30 min in the dark (25 mM iodoacetamide). The sample was then diluted one fold with 50 mM NH4HCO3, 2 mM CaCl2, and digested twice with trypsin (1:50 enzyme-to-substrate ratio), first for 3 h at 37 °C, then overnight at RT. The sample was acidified with 10% trifluoroacetic acid and centrifuged at 14,000 g for 20 min before being desalted by C18 solid phase extraction and dried. Peptide concentration was determined with BCA assay (Pierce).
TMT labeling and fractionation
A pooled reference was first created with equal contribution from each sample. Each individual sample containing ~600 μg of peptides was dried and dissolved in 180 μL of 250 mM sodium borate, pH 8.6, before 5 mg of TMT reagent in 430 μL acetonitrile was added. The TMT-6 labeling scheme is shown in Table S2. The reaction was allowed to carry on at RT for 90 min before termination with 5% hydroxylamine. Samples with different TMT labeling in the same TMT-6 experiment were combined, acidified and desalted on C18 solid phase extraction column, followed by bRPLC fractionation and concatenation [18]. Mobile phases were: A) 10 mM triethylammonium bicarbonate (TEAB), pH 7.5 and B) 10 mM TEAB, 90% acetonitrile, pH 7.5. Sample separation was carried out at a flow rate of 0.5 mL/min using the following gradient (minute: B%): 0:0, 35:0, 41:10, 127:30, 137:42.5, 142:55, 147:100, 148:0 and 178:0. Fractions were collected from 48 to 164 min with equal intervals of 1.2 min and the fractions were concatenated into 6 final fractions.
Enrichment of O-GlcNAc peptide
Enrichment of O-GlcNAc peptide from the fractions followed the CEPC method [12,19] with minor modifications. After removing a small aliquot (~10 μg) for direct LC-MS/MS analysis to provide global protein quantification and as a means for loading normalization, the fractionated peptides (~300 μg) were dried in vacuum and re-suspended in 1.1 mL of 20 mM HEPES, pH 7.9, and 80 μL of 100 mM MnCl2, 75 μL of 0.5 mM UDP-GalNAz, 62 μL of GalT1 and 2.5 μL of PNGase F (New England Biolabs) were added (N-glycans were removed by PNGase F to improve specificity of the O-GlcNAc enrichment). The mixtures were incubated at 4 °C for ~20 h and then at RT for 3 h. After this glycotransfer step, the peptides were reacted with 1 mM biotin-PEG-PC-alkyne (Ambergen) in 20 mM HEPES, pH 7.9 using 0.8 mM CuSO4, 4 mM tris(3-hydroxypropyltriazolylmethyl)amine, 20 mM sodium ascorbate, 20 mM aminoguanidine as the catalyst for copper catalyzed alkyne-azide cycloaddition [20]. Extra reagents were removed by C18 and strong cation exchange solid phase extractions. The peptides were neutralized with PBS and incubated with NeutrAvidin agarose resin (Pierce) for 2 h at RT, and washed thoroughly with 2 M Urea in PBS (6x), 4 M Urea in 50 mM NH4HCO3 (6x), 2 M NaCl (6x), water (1x) and 70% methanol (5x). The O-GlcNAc peptides were released from the resin by 365 nm UV radiation for 25 min. One fourth of the enriched sample was injected for LC-MS/MS analysis.
LC-MS/MS analysis
Samples were injected and analyzed using a Waters nanoAquity UPLC system coupled online to a LTQ Orbitrap Velos MS (Thermo Scientific). Mobile phases are: A) 0.1% formic acid in water and B) 0.1% formic acid in acetonitrile. Samples were separated on a 75 μm-i.d. and 70 cm-long column packed with 3μm C18 particles (Phenomenex) using gradient 1 (minute: B%): 0:1, 6:5, 60:12, 225:30, 290:45, 300:95, 310:95 and 315:1 (Experiment 1 and 2; Table S2) or gradient 2: 0:1, 4:8, 45:12, 160:35, 208:60, 215:75, 220:95 and 222:1 (Experiment 3 and 4; Table S2); both at a flow rate of 300 nl/min. Full MS spectra (400–2,000 m/z) were acquired with a resolution of 60,000. Top-10 most intensive precursor ions were selected for MS/MS using alternating ETD and higher energy collision dissociation (HCD), both with an isolation window of 2 Da. 100-ms activation time and supplemental activation were used in ETD, and the product ions were detected in an ion trap. 32% normalized collision energy was used in HCD fragmentation, and the product ions were detected in the Orbitrap with a resolution of 7,500.
MS/MS identification
As previously reported [19], the MS raw data were searched against a UniProt protein database (version 2015_04) using MS-GF+ (v9881) [21]. The searching parameters are: precursor ion mass tolerance (±10 ppm), partial tryptic specificity, dynamic oxidation of Met (15.9949 Da), static alkylation on Cys (57.0215 Da), static TMT6 labeling on N-terminal and Lys (229.1629 Da), and dynamic O-GlcNAcylation on Ser and Thr (502.2023 Da). The search results from HCD and ETD spectra were first filtered separately to obtain a false identification rate of <1% at peptide level. The identification of an O-GlcNAc peptide was then confirmed/filtered by the presence of the diagnostic 300.13 m/z ion in subsequent corresponding HCD spectra [19]. An Ascore >13 was required for confident O-GlcNAc site assignment (p <0.05) [22].
Quantitative and statistical analysis
The intensities of all six TMT reporter ions were extracted using MASIC software [23]. The reporter ion intensities from different scans and/or different fractions corresponding to the same O-GlcNAc peptide or protein (in the case of global proteome analysis) were summed. Relative peptide/protein abundance was then calculated as the ratio of sample abundance to abundance of the pooled reference (in the 126 channel) using the summed reporter ion intensities (i.e., sample/reference) and log2 transformed to obtain final relative expression values. This allows comparison of relative protein abundances across the entire sample set with minimum variation from each TMT-6 experiment. The strategy of using a common “internal” reference for merging different multiplexed experiments in isobaric labeling analysis has been demonstrated recently as a highly effective and reliable way for large-scale quantitative proteomics analysis [24,25].
To correct for potential difference in sample loading in the same TMT6 experiment, the median log2 relative abundance of the peptides present in each of the original labeled but not enriched samples were computed and re-centered, and the normalization factors obtained therein were applied to corresponding O-GlcNAc data. Moderated t-test was performed using the limma package [26]. The null hypothesis was that there is no difference between the AD and control groups. To correct for multiplicity of hypothesis testing, individual O-GlcNAc or protein values were adjusted by converting to q-values using corresponding R package [27].
Gene function classification and pathway analysis of the O-GlcNAc proteins in AD were performed using DAVID (https://david.ncifcrf.gov/) [28]. Motif analysis was carried out using pLogo (https://plogo.uconn.edu/) [29].
Western blotting
Tissue lysates containing 200 μg of protein were subjected to reduction (10 mM dithiothreitol) and alkylation (90 mM iodoacetamide) before precipitation by the methanol/chloroform method and re-constituted in 40 μL of 1% SDS, 20 mM HEPES, pH 7.9. The sample was then subjected to GalNAz labeling with Click-iT labeling kit (ThermoFisher C33368) according to the manufacturer’s instructions. The azide-labeled proteins were linked with a 5 kDa PEG molecule via copper free click chemistry as reported [30]. After the reactions, the proteins were precipitated and re-constituted for SDS-PAGE, transferred to PVDF membrane, and detected with rabbit antibodies for QK1 or SYNPO (Pirece PA5-30563 and PA5-21062) using the Fast Western Kits (Pierce) with β-tubulin antibody as loading control.
Data deposition
The LC-MS/MS raw data, database searching results and TMT reporter ion intensity information have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org dataset PXD004510).
Results
Integrated quantitative proteomics for O-GlcNAc modification
An overview of our new quantitative proteomic pipeline for comprehensive O-GlcNAc analysis is shown in Figure 1A. It has several attractive features: (1) TMT labeling allows for precise quantification and sample multiplexing hence much increased analysis throughput, and can be applied to tissues, cells, or biofluids; (2) the “common” reference strategy [24,25] allows for robust O-GlcNAc quantification in a large sample cohort; (3) the CEPC method (Figure 1B) for enriching O-GlcNAc peptides is robust and highly specific and sensitive; and (4) every precursor is analyzed by both ETD and HCD for peptide identification and quantitation and O-GlcNAc site determination. A pair of alternating ETD/HCD spectra are shown in Figure 1C,D.
Figure 1.
Quantitative proteomic pipeline for O-GlcNAc analysis. (A) Pipeline overview. Peptides were labeled individually with TMT-6 reagents before being equally mixed and separated into six fractions, followed by enrichment of O-GlcNAc peptides and LC-MSMS analysis. (B) Chemical principle of the CEPC enrichment method for O-GlcNAcylated peptides. First, the terminal GlcNAc motif (O-GlcNAc and GlcNAc on N-glycans) was labeled with an azide modified monosaccharide (GalNAz) using the Click-iT O-GlcNAc Enzymatic Labeling System. At the same time, N-glycans were removed by enzyme PNGase F to improve specificity. Second, the O-GlcNAc peptides were linked with a cleavable biotin reagent through click chemistry between the azide group and the alkyne group. Third, O-GlcNAc peptides were enriched by avidin chromatography and released by ultraviolet radiation after washing. The ETD spectra (C) and HCD (D) spectra respectively of an example O-GlcNAc peptide (sequence: GPQVSgSALNLDTSK, g indicates GlcNAc) after TMT labeling and CEPC enrichment reactions. The insert in (D) shows the TMT reporter ions region. The oxonium ion with m/z value of 300.13 was the fragment of the part shown in gray in the final product in (B).
Mapping of O-GlcNAc in human brain
A total of 1,850 unique peptides (Table S3) from 530 O-GlcNAc proteins (Table S4) were identified. Gene ontology (GO) classification results for these O-GlcNAc proteins are shown in Figure S1. These results supported the involvement of O-GlcNAc in various cellular functions in the brain, including cytoskeleton organization, axonogenesis, cell adhesion and regeneration. KEGG pathway analysis indicated the involvement of O-GlcNAc in extracellular matrix (ECM)-receptor interaction, focal adhesion, lysosome, adherens junction, long-term potentiation and the MAPK signaling pathway. Interestingly, nine secreted proteins related to ECM (TNXB, TNC, COL6A2, COL6A3, LAMA5, LAMC1, LAMB2, HSP2, and LAMA) as well as some membrane proteins including NOTCH were also found to be O-GlcNAcylated. O-GlcNAcylation on these proteins may be the result of the action of the epidermal growth factor (EGF) domain specific O-GlcNAc transferase (EOGT), which is located in the endoplasmic reticulum and is responsible for O-GlcNAcylation of extracellular proteins [31].
A total of 1,094 O-GlcNAcylation sites (Table S5) were unambiguously determined (≥95% confidence). More than 85% of the O-GlcNAc sites were identified from ETD (data not shown), while HCD provided additional O-GlcNAc peptide identifications as well as important information on the oxonium ion (additional evidence of O-GlcNAcylation) and TMT reporter ion intensities (quantitation). Eighty six sites were in the O-GlcNAc database from the Phosphosite website (http://www.phosphosite.org) [32], which covered 411 previously reported O-GlcNAcylation sites from human proteins. Many of the O-GlcNAc sites have reciprocal (20.3%) or proximal (within ±10 aa, 54.3%) relationships with phosphorylation sites (Table S5). Moreover, most of (98.9%) O-GlcNAc-modified proteins identified in the present study are known phosphoproteins (Table S4). In our previous study, significantly fewer O-GlcNAc sites found in the healthy mouse brain appeared to have reciprocal (4.6%) or proximal (within ±10 aa, 21.4%) relationships with phosphorylation sites [19].
Interestingly, there is virtually no difference in the motifs of O-GlcNAc modification between human (Figure 2) and mouse (Figure S2). Moreover, the motifs of Ser O-GlcNAcylation and Thr O-GlcNAcylation are surprisingly similar (Figures 2 and S2). This brain O-GlcNAcylation motif feature is consistent to that predicted using the O-GlcNAc prediction programs YinOYang 1.2 server and OGlcNAcScan [33], and predictions based on 410 experimentally verified O-GlcNAcylation sites extracted from dbOGAP, OGlycBase and UniProtKB [34]. Thus, the O-GlcNAcylation motifs appear to be fairly conserved crossing various species and in various organs.
Figure 2.

Sequence motif analysis of protein O-GlcNAcylation in human using sites determined in this study. The images were generated in pLogo. The red horizontal lines suggest a threshold of p = 0.05. Upper panel, the T motif; lower panel, the S motif. FG, foreground; BG, background.
Quantification of O-GlcNAcylation
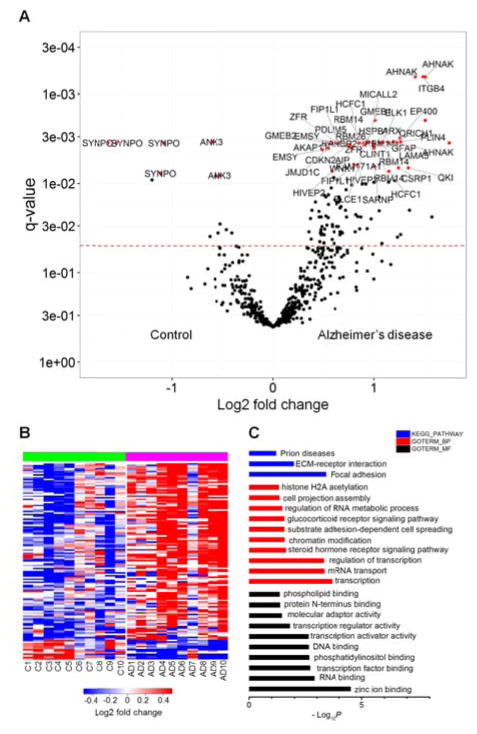
A total of 623 peptides with quantitative information and also >50% occurrence in the samples were further considered for downstream analyses (Table S6). 131 of the 623 O-GlcNAc peptides showed significant change of abundance in AD (q <0.05; Table S6 and Figure 3A). The changes of these O-GlcNAc peptides across the individual samples are shown as a heatmap in Figure 3B. GO analysis of the proteins carrying these peptides showed that these proteins are involved in zinc ion binding, mRNA transport, transcription, chromatin modification and cell projection organization, as well as the focal adhesion, ECM-receptor interaction and prion diseases pathways (Figure 3C). Interestingly, among these 131 O-GlcNAc peptides, only 12 peptides from three proteins (SYNPO, ANK3 and SH3RF1) decreased in AD while the majority, 119 peptides, increased in the disease.
Figure 3.
The O-GlcNAc abundance in human brain was quantified and compared between Alzheimer’s disease and control cases. (A) Volcano plot of the O-GlcNAc peptides with >50% occurrence in all samples. Dotted line indicates the significance cutoff of q <0.05; Label: the protein (gene name) that carries the O-GlcNAc modifiication/peptide; log2 fold change = log2 (AD/C). (B) Heatmap of the O-GlcNAc peptides with >50% occurrence in samples and q value <0.05. Value = log2(Sample channel/Reference channel). (C) GO (DAVID) analysis of O-GlcNAc proteins showing significant changes in Alzheimer’s disease: molecular function, biological process and KEGG pathways.
We briefly analyzed an aliquot of the TMT-6-labeled, unenriched peptide samples for correcting loading differences in the TMT channels for the enriched O-GlcNAc samples. This also allows us to initially examine if the changes in O-GlcNAc abundance are affected by the changes in protein expression. We quantified a total of 1,635 proteins, and the results are provided in Table S7. Among these quantified proteins, only 27 proteins (1.65%) showed significant changes (q <0.05) in AD (Figures S3 and S4), a much lower frequency of changes than that observed in the O-GlcNAcylated peptides (>20% differed significantly). Moreover, statistical tests of the global peptide quantitation results with (Table S7) and without (Table S9) protein roll-up showed that the proportion of differentially abundant global peptides appeared to be even lower (0.35%) than proteins (see Table S10), confirming the significantly higher level of change in O-GlcNAcylation in these AD samples.
The expression of O-GlcNAc cycling enzymes (OGT and OGA, both quantified in our study) may be an important contributor to the differences in O-GlcNAc abundance. However, we did not find significant changes in the abundances of these enzymes in brains with Alzheimer’s (Figure S5; q =0.60 and q =0.224 for OGT and for OGA, respectively). These global proteome results suggest that most of the changes in O-GlcNAc abundance were due to changes at the O-GlcNAc modification level instead of protein abundance.
The net changes in O-GlcNAcylation (i.e., the change of O-GlcNAcylation on top of the protein expression) were calculated for 306 O-GlcNAc peptides where the protein expression data were also available (Table S8). For these O-GlcNAc net changes, a heatmap is shown in Figure S6, and a volcano plot is shown in Figure S7. Twelve peptides showed increase and twelve peptides showed decrease in O-GlcNAc net changes (q <0.05, Table 1).
Table 1.
O-GlcNAcylation sites with significant net change from control to Alzheimer’s disease (q <0.05). S# or T# denotes the O-GlcNAcylation sites in the peptide sequences.
| Protein | Peptide | log2(AD/C) | q value |
|---|---|---|---|
| AHNAK | VS#GPDLDLNLK | 1.203 | 0.013 |
| AHNAK | LQVT#M*PGIK | 1.126 | 0.016 |
| ITGB4 | VLSTS#STLTR | 1.069 | 0.013 |
| AHNAK | GPQVS#SALNLDTSK | 1.067 | 0.013 |
| AHNAK | LQVT#MPGIK | 1.044 | 0.014 |
| CSRP1 | GFGFGQGAGALVHS#E | 1.013 | 0.026 |
| NFASC | YVAFNGT#K | 0.910 | 0.044 |
| RBM14 | AQPSAS#LGVGYR | 0.879 | 0.044 |
| RBM14 | AQPSVS#LGAAYR | 0.853 | 0.049 |
| WNK1 | EGPVLAT#S#SGAGVFK | 0.782 | 0.043 |
| PLIN3 | TLTAAAVS#GAQPILSK | 0.709 | 0.049 |
| HNRNPU | SSGPTSLFAVT#VAPPGAR | 0.650 | 0.050 |
| ANK3 | SGFTSLSSS#SSNTPSASPLK | −0.530 | 0.043 |
| ANK3 | APAVT#EATPLK | −0.568 | 0.049 |
| ANK3 | STLGASTT#SSVK | −0.692 | 0.016 |
| ANK3 | VFSTT#TAM*PFSPLR | −0.753 | 0.014 |
| ANK3 | VFSTT#TAMPFSPLR | −0.761 | 0.013 |
| ANK3 | VFST#TTAM*PFSPLR | −0.769 | 0.042 |
| ANK3 | TSPVTT#AGSLLER | −0.773 | 0.021 |
| SYNPO | TPAT#TTSTFSR | −0.792 | 0.043 |
| ANK3 | STLGAST#TSSVK | −0.803 | 0.013 |
| ANK3 | TSPVT#TAGSLLER | −0.841 | 0.013 |
| SYNPO | VSTPATT#TSTFSR | −1.257 | 0.014 |
| SYNPO | TPATT#TSTFSR | −1.343 | 0.019 |
Initial validation of protein O-GlcNAcylation level
Western blotting was performed to initially verify the MS quantification data for two proteins, SYNPO (Synaptopodin) and QKI (Protein quaking). The O-GlcNAc was first labelled with GalNAz using Click-iT kit, then a PEG tag (5 kDa) [35] was linked to the azide through copper free click chemistry [30]. Anti-SYNPO and anti-QKI antibodies were used to visualize the proteins in the non-glycosylated and glycosylated forms (Figure 4). O-GlcNAcylation of SYNPO decreased in the Alzheimer’s samples while QKI increased in these samples compared with control samples. These data are in concordance with the MS quantification results.
Figure 4.
Verification of O-GlcNAc quantification with Western blotting analysis using lysate of the human brain tissue. (A) PEG labeling of O-GlcNAcylated proteins. First, the O-GlcNAc motif was labeled with GalNAz by GalT1 and UDP-GalNAz while the N-glycans were removed by PNGaseF. Second, the azide group was linked to a PEG tag with 5 K Da through copper free click chemistry. The proteins were precipitated and separated on SDS page and detected by antibody against synaptopodin (SYNPO) (B) or protein quaking (QKI) (C) through Western blotting. β-tubulin was used as loading control.
Discussion
AD has two types, early onset familial AD and late onset sporadic type. Most (~99%) cases are sporadic and appear to be caused by multiple etiological factors through several pathogenetic mechanisms [36]. Our findings of the alterations of multiple O-GlcNAcylated proteins in the brains of sporadic Alzheimer’s are consistent with the complex nature of the disease. These altered O-GlcNAcylated proteins cover various functional categories, with more proteins involved in mRNA transport and transcriptional and posttranscriptional regulation of gene expression. A pivotal role of epigenetic dysregulation has gained much attention recently in the development of sporadic AD, which could result from or through the altered O-GlcNAcylation of these proteins [37,38].
There have been several studies on the overall O-GlcNAcylation level in AD using antibody-based approaches, however with conflicting results (downregulated [39,40] and upregulated [41,42]). Note that no PUGNAc was used to preserve O-GlcNAc in these studies. With PUGNAc used in the present study, our MS quantification data showed more O-GlcNAc peptides increased rather than decreased in abundance in brains with AD. The MS-based methods also provided a powerful tool for site-specific quantification of O-GlcNAcylation (both total and “net” changes in abundance) of hundreds of brain proteins simultaneously (623 O-GlcNAc peptides covering 284 proteins in this study).
To determine whether the changes in O-GlcNAc peptide abundance are the result of the alteration of native protein expression or OGT/OGA function/activity, two approaches were taken. First, the same TMT-6-labeled samples without enrichment for O-GlcNAc peptides were analyzed and compared to the O-GlcNAc results. The remarkable differences noted between the O-GlcNAc and global proteome analyses (>20% vs. <2% changed) suggest the selective alterations of either protein O-GlcNAcylation levels or the expression levels of O-GlcNAcylated proteins. Second, we analyzed the net change in O-GlcNAcylation of 306 O-GlcNAc peptides where their protein expression data were also available. We found significant increase and decrease in net change of O-GlcNAc of 12 and 12 peptides, respectively. Therefore, the O-GlcNAc cycling enzymes have functioned differently on these sites/peptides than other sites in AD brain. The imbalance of O-GlcNAc cycling on these sites may be due to the changes of the structure of the proteins that carry these O-GlcNAc sites, or differential neuronal cell loss and/or glial response in AD.
Among the proteins carrying altered O-GlcNAcylation in Alzheimer’s, ankyrin-3 (ANK3, also called ankyrin-G), synaptopodin (SYNPO) and A-kinase anchor protein 11 (AKAP11) are of particular interest. ANK3 is an adaptor protein expressed in neurons and epithelial cells and mediates the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. This linkage is required to maintain the integrity of the plasma membrane and to anchor specific transmembrane proteins in the plasma membrane. The pivotal role of the disrupted integrity of the brain plasma membrane in AD brain has been recognized [43]. Altered trafficking of amyloid beta (Aβ) peptide and other critical proteins in the membrane is thought to be crucial to the development of AD [44]. A previous gene linkage study found significant association between sporadic Alzheimer’s and the single nucleotide polymorphism of ANK3 [45]. Mis-localization of ANK3 and its role in the disruption of axon polarity have been recently reported [46]. Our findings of the marked decrease in ANK3 O-GlcNAcylation in AD brains may open a new avenue for further investigation of its role in the molecular pathogenesis of AD, such as, the influence of O-GlcNAc of ANK3 in the membrane integrity and axon polarity. Synaptopodin is a postsynaptic protein important for synaptic plasticity [47]. Our finding of the marked decrease in synaptopodin O-GlcNAcylation in AD brains suggests its role in the synaptic dysfunction and degeneration that occur in AD. AKAP11 has one O-GlcNAc site (S719) that showed significant increase in abundance in AD. Change of O-GlcNAc on this site is worthy of attention because AKAP11 is related with important kinases in AD. AKAP11 was reported to target protein kinase-A, which may inhibit glycogen synthase kinase 3α, a kinase regulating the production of Aβ [48]. AKAP11 also binds to glycogen synthase kinase 3β [49], which is responsible for the phosphorylation of tau protein [50]. Thus, the function of this site is of great interest for further research and highlights the importance of the crosstalk between O-GlcNAc and phosphorylation in the pathology of AD.
It has been reported that O-GlcNAc modifies several important brain proteins, such as tau [39,40,51,52] and Aβ [53–55], which have been implied in the pathogenesis of AD. We also detected O-GlcNAc-modified tau peptides (Table S3), but too many missing values occurred (i.e., only detected in <50% of the samples) in the present study. Thus, tau O-GlcNAcylation could not be quantified reliably. O-GlcNAcylated peptides from Aβ were not detectable in the present study, probably due to the very low level of O-GlcNAcylation of Aβ comparing to other proteins, e.g., bassoon. Nevertheless, the regulation of O-GlcNAcylation on these proteins may still be important for the pathogenesis of AD, as reported by previous studies.
In conclusion, the present study is the most comprehensive quantitative proteomic study for brain O-GlcNAcylation to date, reporting O-GlcNAc modifications on over one thousand sites from human brain tissue samples. The use of multiplexed isobaric labeling with the pooled common reference strategy enabled us to compare the levels of hundreds of O-GlcNAc peptides in 20 individual samples quickly and reliably and to discover changes of more than a hundred O-GlcNAc peptides in AD. The number of samples analyzed in this study is still relatively small and hence some of the findings can be underpowered; it is also possible that not all of these changes were due to AD. However, the initial identification of altered O-GlcNAc peptides/proteins, such as AKAP11, opens new avenues for future investigations of the molecular mechanisms of AD. Moreover, the quantitative proteomics analytical pipeline developed and implemented in the current study can be further expanded to accommodate the need for higher throughput (e.g., using TMT-10) as well as integrated proteome, phosphoproteome and/or O-GlcNAc proteome analysis (e.g., via sample splitting or sequential enrichment). The increased number of samples to be included in the comparison and the integrated proteome/phosphoproteome/O-GlcNAc-ome analysis will help prioritize some of these findings for further verification, and shed light on the crosstalk between protein phosphorylation and O-GlcNAcylation in AD pathology.
Supplementary Material
Motif analysis of protein O-GlcNAcylation in mouse using sites determined in our previous work
GO (DAVID) analysis of O-GlcNAc proteins identified from all the samples.
Heatmap for human brain proteins that showed significant difference in O-GlcNAc abundance (q <0.05) between Alzheimer’s and control brains
Volcano plot for protein abundance between AD human brain and control brains
The abundance of proteins OGT and OGA in human brains with or without Alzheimer’s disease
The net changes of O-GlcNAcylation of human brain proteins, as shown in heatmap of proteins with q <0.05
The net changes of O-GlcNAcylation of human brain proteins are shown in Volcano plot
Human brain tissues used in this study
TMT-6 labeling of the human brain samples
Dataset of O-GlcNAc peptides
Dataset of O-GlcNAc proteins
Dataset of O-GlcNAc sites
Quantitative O-GlcNAc peptides
Table S7. Quantitative O-GlcNAc proteins
Table S8. Quantitative O-GlcNAc net changes
Table S9. Quantitative global peptides
Table S10. Differential abundance of O-GlcNAc, global protein and global peptide differences
Acknowledgments
We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona, for the provision of autopsied frozen human brain tissue. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026, National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610, Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium), and the Michael J. Fox Foundation for Parkinson’s Research. Portions of this work were supported by the New York State Office for People with Developmental Disabilities and by NIH grants U24-CA-160019 from the National Cancer Institute Clinical Proteomic Tumor Analysis Consortium (CPTAC) and NIGMS Biomedical Technology Research Resource P41GM103493, and an Interagency Agreement from the United States Department of Defense through the Henry M. Jackson Foundation under MIPR2DO89M2058. The experimental work described herein was performed in the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by United States Depart of Energy (DOE) Office of Science Biological and Environmental Research and located at Pacific Northwest National Laboratory, which is operated by Battelle Memorial Institute for the DOE under Contract DE-AC05-76RL0 1830.
Footnotes
Author Contributions: T.L., W.-J.Q, F.Y., and C.-X.G. designed research; S.W. performed protein digestion, TMT labeling and O-GlcNAc enrichment; M.A.G. performed peptide fractionation; A.K.S. acquired the LC-MS/MS data; V.A.P., S.W., T.L., and M.E.M analyzed data; S.W., T.L., K.D.R, R.D.S., W.-J.Q., and C.-X.G. wrote the manuscript.
Conflict of interest: C.-X.G. serves on the Scientific Advisory Board of Alectos Therapeutics. Other authors declare no competing financial interests.
References
*Cited only in supplementary material
- 1.Torres C-R, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 2.Abeshouse A, Ahn J, Akbani R, et al. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan H-B, Singh JP, Li M-D, et al. Cracking the O-GlcNAc code in metabolism. Trends Endocrinol Metab. 2013;24:301–309. doi: 10.1016/j.tem.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11:678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Shan X, Yuzwa SA, et al. The emerging link between O-GlcNAc and Alzheimer disease. J Biol Chem. 2014;289:34472–34481. doi: 10.1074/jbc.R114.601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong CX, Liu F, Iqbal K. O-GlcNAcylation: A regulator of tau pathology and neurodegeneration. Alzheimers Dement. 2016;10:1078–1089. doi: 10.1016/j.jalz.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Demetrius LA, Driver J. Alzheimer’s as a metabolic disease. Biogerontology. 2013;14:641–649. doi: 10.1007/s10522-013-9479-7. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer S. Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: an update. Exp Gerontol. 2000;35:1363–1372. doi: 10.1016/s0531-5565(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Yang F, Camp DG, et al. Proteomic approaches for site-specific O-GlcNAcylation analysis. Bioanalysis. 2014;6:2571–2580. doi: 10.4155/bio.14.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinidad JC, Barkan DT, Gulledge BF, et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteomics. 2012;11:215–229. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Udeshi ND, O’Malley M, et al. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2010;9:153–160. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalkley RJ, Thalhammer A, Schoepfer R, et al. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc Natl Acad Sci USA. 2009;106:8894–8899. doi: 10.1073/pnas.0900288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khidekel N, Ficarro SB, Clark PM, et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol. 2007;3:339–348. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- 15.Skorobogatko YV, Deuso J, Adolf-Bergfoyle J, et al. Human Alzheimer’s disease synaptic O-GlcNAc site mapping and iTRAQ expression proteomics with ion trap mass spectrometry. Amino Acids. 2011;40:765–779. doi: 10.1007/s00726-010-0645-9. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Udeshi ND, Slawson C, et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal. 2010;3:ra2. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson A, Schäfer J, Kuhn K, et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75:1895–1904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Yang F, Gritsenko MA, et al. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics. 2011;11:2019–2026. doi: 10.1002/pmic.201000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfaro JF, Gong C-X, Monroe ME, et al. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci USA. 2012;109:7280–7285. doi: 10.1073/pnas.1200425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong V, Presolski SI, Ma C, et al. Analysis and optimization of copper-catalyzed azide–alkyne cycloaddition for bioconjugation. Angew Chem Int Ed Engl. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Pevzner PA. MS-GF+ makes progress towards a universal database search tool for proteomics. Nat Commun. 2014;5:5277. doi: 10.1038/ncomms6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beausoleil SA, Villén J, Gerber SA, et al. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 23.Monroe ME, Shaw JL, Daly DS, et al. MASIC: a software program for fast quantitation and flexible visualization of chromatographic profiles from detected LC-MS(/MS) features. Comput Biol Chem. 2008;32:215–217. doi: 10.1016/j.compbiolchem.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Liu T, Zhang Z, et al. Integrated oroteogenomic characterization of human high-grade serous ovarian cancer. Cell. 2016;166:755–765. doi: 10.1016/j.cell.2016.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertins P, Mani DR, Ruggles KV, et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature. 2016;534:55–62. doi: 10.1038/nature18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.O’Shea JP, Chou MF, Quader SA, et al. pLogo: a probabilistic approach to visualizing sequence motifs. Nat Methods. 2013;10:1211–1212. doi: 10.1038/nmeth.2646. [DOI] [PubMed] [Google Scholar]
- 30.Teo CF, Wells L. Monitoring protein O-linked β-N-acetylglucosamine status via metabolic labeling and copper-free click chemistry. Anal Biochem. 2014;464:70–72. doi: 10.1016/j.ab.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaidani Y, Nomura T, Matsuura A, et al. O-linked-N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nat Commun. 2011;2:583. doi: 10.1038/ncomms1591. [DOI] [PubMed] [Google Scholar]
- 32.Hornbeck PV, Zhang B, Murray B, et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–D520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jochmann R, Holz P, Sticht H, et al. Validation of the reliability of computational O-GlcNAc prediction. Biochim Biophys Acta. 2014;1844:416–421. doi: 10.1016/j.bbapap.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Kao HJ, Huang CH, Bretana N, et al. A two-layered machine learning method to identify protein O-GlcNAcylation sites with O-GlcNAc transferase substrate motifs. BMC Bioinformatics. 2015;16(Suppl 18):S10. doi: 10.1186/1471-2105-16-S18-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rexach JE, Rogers CJ, Yu S-H, et al. Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags. Nat Chem Biol. 2010;6:645–651. doi: 10.1038/nchembio.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Mut JV, Graff J. Epigenetic Alterations in Alzheimer’s Disease. Front Behav Neurosci. 2015;9:347. doi: 10.3389/fnbeh.2015.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lardenoije R, Iatrou A, Kenis G, et al. The epigenetics of aging and neurodegeneration. Prog Neurobiol. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F, Iqbal K, Grundke-Iqbal I, et al. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Shi J, Tanimukai H, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132:1820–1832. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Förster S, Welleford AS, Triplett JC, et al. Increased O-GlcNAc levels correlate with decreased O-GlcNAcase levels in Alzheimer disease brain. Biochim Biophys Acta. 2014;1842:1333–1339. doi: 10.1016/j.bbadis.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffith LS, Schmitz B. O-linked N-acetylglucosamine is upregulated in Alzheimer brains. Biochem Biophys Res Commun. 1995;213:424–431. doi: 10.1006/bbrc.1995.2149. [DOI] [PubMed] [Google Scholar]
- 43.Walter J, van Echten-Deckert G. Cross-talk of membrane lipids and Alzheimer-related proteins. Mol Neurodegener. 2013;8:34. doi: 10.1186/1750-1326-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang BL. Neuronal protein trafficking associated with Alzheimer disease: from APP and BACE1 to glutamate receptors. Cell Adh Migr. 2009;3:118–128. doi: 10.4161/cam.3.1.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan AR, Hamilton G, Turic D, et al. Association analysis of 528 intra-genic SNPs in a region of chromosome 10 linked to late onset Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147b:727–731. doi: 10.1002/ajmg.b.30670. [DOI] [PubMed] [Google Scholar]
- 46.Tsushima H, Emanuele M, Polenghi A, et al. HDAC6 and RhoA are novel players in Abeta-driven disruption of neuronal polarity. Nat Commun. 2015;6:7781. doi: 10.1038/ncomms8781. [DOI] [PubMed] [Google Scholar]
- 47.Zhang XL, Poschel B, Faul C, et al. Essential role for synaptopodin in dendritic spine plasticity of the developing hippocampus. J Neurosci. 2013;33:12510–12518. doi: 10.1523/JNEUROSCI.2983-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phiel CJ, Wilson CA, Lee VM-Y, et al. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 49.Whiting JL, Nygren PJ, Tunquist BJ, et al. Protein kinase A opposes the phosphorylation-dependent recruitment of glycogen synthase kinase 3β to A-kinase anchoring protein 220. J Biol Chem. 2015;290:19445–19457. doi: 10.1074/jbc.M115.654822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner U, Utton M, Gallo J-M, et al. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci. 1996;109:1537–1543. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- 51.Arnold CS, Johnson GV, Cole RN, et al. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271:28741–28744. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 52.Yuzwa SA, Yadav AK, Skorobogatko Y, et al. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids. 2011;40:857–868. doi: 10.1007/s00726-010-0705-1. [DOI] [PubMed] [Google Scholar]
- 53.Griffith LS, Mathes M, Schmitz B. Beta-amyloid precursor protein is modified with O-linked N-acetylglucosamine. J Neurosci Res. 1995;41:270–278. doi: 10.1002/jnr.490410214. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen KT, Iverfeldt K. O-GlcNAcylation increases non-amyloidogenic processing of the amyloid-beta precursor protein (APP) Biochem Biophys Res Commun. 2011;404:882–886. doi: 10.1016/j.bbrc.2010.12.080. [DOI] [PubMed] [Google Scholar]
- 55.Chun YS, Park Y, Oh HG, et al. O-GlcNAcylation promotes non-amyloidogenic processing of amyloid-beta protein precursor via inhibition of endocytosis from the plasma membrane. J Alzheimers Dis. 2015;44:261–275. doi: 10.3233/JAD-140096. [DOI] [PubMed] [Google Scholar]
- 56.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD. Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 57.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Motif analysis of protein O-GlcNAcylation in mouse using sites determined in our previous work
GO (DAVID) analysis of O-GlcNAc proteins identified from all the samples.
Heatmap for human brain proteins that showed significant difference in O-GlcNAc abundance (q <0.05) between Alzheimer’s and control brains
Volcano plot for protein abundance between AD human brain and control brains
The abundance of proteins OGT and OGA in human brains with or without Alzheimer’s disease
The net changes of O-GlcNAcylation of human brain proteins, as shown in heatmap of proteins with q <0.05
The net changes of O-GlcNAcylation of human brain proteins are shown in Volcano plot
Human brain tissues used in this study
TMT-6 labeling of the human brain samples
Dataset of O-GlcNAc peptides
Dataset of O-GlcNAc proteins
Dataset of O-GlcNAc sites
Quantitative O-GlcNAc peptides
Table S7. Quantitative O-GlcNAc proteins
Table S8. Quantitative O-GlcNAc net changes
Table S9. Quantitative global peptides
Table S10. Differential abundance of O-GlcNAc, global protein and global peptide differences