Abstract
The i.v. lipid emulsion (LIP) is a source of oxidants, which may stimulate inflammation. Coadministration of parenteral multivitamins (MVP) with LIP prevents lipid peroxidation in light-exposed total parenteral nutrition (TPN). We hypothesized that this modality of TPN administration affects systemic inflammation, which may be modulated by exposure to oxygen. Premature infants were allocated to three TPN regimens: control regimen — MVP coadministered with amino acid/dextrose exposed to ambient light, LIP provided separately (n = 9) — LIP+MVP light exposed (LE): MVP coadministered with light-exposed LIP (n = 9) — LIP+MVP light protected (LP): MVP coadministered with light-protected LIP (n = 8). In LE and LP, amino acid/dextrose was provided separately. On reaching full TPN, infants were sampled for IL-6 and IL-8 in plasma and the redox potential of glutathione in whole blood (E, mV). Data were compared (ANOVA) in infants exposed to low (<0.25) versus high (≥ 0.25) FiO2. Patients (mean ± SD: birth weight 797 ± 172 g; GA 26 ± 1 wk) had similar clinical characteristics in TPN groups. Cytokine levels correlated positively (p < 0.01) with FiO2 and E. High FiO2 stimulated an increase (p < 0.01) in cytokines in control regimen, whereas these markers remained unaffected by oxygen in the LE and LP groups. The choice of a TPN admixture may have important consequences on the systemic inflammatory response triggered by an oxidant stress.
Supplemental oxygen, assisted ventilation, and total parenteral nutrition (TPN) are therapies that expose premature infants to significant oxidant and inflammatory stress (1–4). As these infants are particularly deficient in antioxidant and anti-inflammatory defenses, such therapies may also have potentially harmful long-term effects on their developing lungs and brain (5).
Synergistic deleterious effects of inflammation and oxidant stress in preterm infants can occur through a number of potential mechanisms. Exposure to oxygen leads to the accumulation of reactive oxygen species (ROS), which results in the generation of hydrogen and lipid peroxides. When antioxidant defenses are overwhelmed, this reaction self-propagates, and the peroxides generated can damage cells and tissues (6). In preterm infants, even brief periods of supplemental oxygen at birth may produce sufficient oxidant stress to cause sustained lung disease (7). Also, oxidized lipids and hydrogen peroxides have a direct modulatory effect on inflammatory pathways, including Toll-like receptors (8), and the transcriptional activator nuclear factor (NF)-kB (9). Such a direct activation of inflammatory pathways by oxygen byproducts can potentiate inflammatory responses and promote further endothelial injury and pulmonary capillary leakage, resulting in systemic generalization of the inflammation with potentially harmful consequences on the developing preterm brain (10). Hydrogen peroxides are markedly increased in brain tissues of animals exposed to hyperoxia (11). Oxidant stress and systemic inflammation each have independent and synergistic deleterious apoptotic effects on brain microglial cells and immature oligodendrocyte (12–14). Notably, prolonged supplemental oxygen exposure and systemic inflammation as measured by plasma levels of IL-6 and IL-8 have both been strongly associated with adverse long-term pulmonary and neurodevelopmental outcomes in preterm infants (15–19).
Quality of nutrition early in life has documented beneficial effects on health outcomes later in life (20). Premature infants often require i.v. nutritional support (TPN) until optimal gut maturity is achieved to allow full enteral feeding. Light exposure of TPN induces the loss of antioxidant vitamins (21) and the generation of oxidant products of photooxidation such as hydrogen peroxide (22), lipid peroxides (23), aldehydes (24), and ascorbylperoxide (25). The generation of these products of oxidation can be decreased by protection from light (23). It has been recently shown in vitro that multivitamin coadministration with the lipid moiety of TPN prevents lipid peroxidation and antioxidant vitamin loss in the infusate (21). Based on the previously documented antioxidant effect of light protection and coadministration of multivitamins with lipids, which limits the principal sources of peroxides in infants receiving TPN, we hypothesized that coadministration of i.v. multivitamins with lipids would reduce the proinflammatory effect of supplemental oxygen in the early days of life.
METHODS
Subject recruitment and intervention
Infants born at 28 wk of gestation or lower admitted to the Children’s & Women’s Health Centre of British Columbia neonatal intensive care unit between 2006 and 2009 were prospectively recruited as part of a study evaluating the antioxidant response to different TPN regimens. Written informed consent was obtained. Infants were allocated by chance in pharmacy to one of three different TPN regimens initiated in the first 24 h of age and continued for at least 10 d:
Infants in the control regimen (AA) group received parenteral multivitamin (MVP) mixed with the amino acids/dextrose solution (AA+MVP) exposed to ambient light (clear syringes and tubing), and the lipid emulsion (LIP) was administered separately.
Infants in the LIP+MVP light-exposed (LE) group received parenteral multivitamin mixed within the LIP (LIP+MVP) exposed to ambient light (clear syringes and tubing), and the amino acid/dextrose solution was provided separately.
Infants in the LIP+MVP light-protected (LP) group received parenteral multivitamin mixed within the LIP (LIP+MVP) protected from light (using amber syringes and tubing (26), and the light-exposed amino acid/dextrose solution was provided separately.
In all three groups, nutrient intake was provided according to the institutional TPN protocol (27), so that all infants in the study would have a similar nutrient intake. Inclusion criteria: premature infants with birth weight <1000 g and GA <28 wk who required TPN. To control for variability in the nutrient intake, subjects were excluded if enteral energy intake exceeded the parenteral energy intake at any given time during the study. To minimize blood letting, every other infant recruited in the trial was considered for plasma cytokine analyses. Two samples for plasma cytokine and whole blood redox potential analyses were collected 72 h apart, between days 7 and 10 of life. IL-6 and IL-8 were specifically included in the analysis as they most strongly correlated with outcomes in previous studies (18). The study protocol was approved by the Clinical Research Ethics Board of the University of British Columbia.
Definitions
Chorioamnionitis was defined histologically as a maternal stage 2 or greater with involvement of fetal membranes, according to criteria previously established by Redline (28). The Score for Neonatal Acute Physiology (SNAP-II) was measured within 12 h of age (29). The fractional inspired oxygen (FiO2) was recorded on the day of blood sampling and categorized into a low (FiO2 < 0.25) or high (FiO2 ≥0.25) exposure to oxygen. A cutoff of 25% FiO2 was chosen to better discriminate between the clinical statuses of infants with mild versus more significant lung disease. Necrotizing enterocolitis (NEC) was defined according to the attending neonatologist if the infant presented signs of acute gastrointestinal deterioration with radiologic pneumatosis, free or portal air, or signs of fixed bowel dilatation with bowel wall thickening. Culture-proven sepsis was defined by either a positive blood or cerebrospinal fluid (CSF) culture. Because we were interested in identifying a confounding effect of NEC or sepsis on the levels of oxidant stress or inflammatory cytokines, only diagnoses occurring during the 10-d study period were considered in the data analysis.
Laboratory analyses
Within 4 min of sampling, aliquots of whole blood (EDTA tube) were homogenized in freshly prepared metaphosphoric acid (5% wt/vol). The plasma fraction was separated by centrifugation within minutes of blood sampling (EDTA tube) and stored at −80°C until batch analysis. Concentrations of IL-6, IL-8, IL-1β, IL-10, IL-12, and TNF-α were measured in duplicate using a cytometric-bead array multiplex assay (BD Bioscience, San Diego, CA) and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). Interduplicate variations were within 10% and 6% for the two detectable cytokines, IL-6 and IL-8, respectively. Reduced glutathione (GSH) and oxidized glutathione (GSSG) in centrifuged whole blood (5000×g) were determined at 200 nm after separation by capillary electrophoresis (30). The redox potential of glutathione [GSH/(GSSG+GSH), E, mv], a measure of overall oxidant/antioxidant status, was calculated from the Nernst equation (31).
Statistical analysis
Cytokine data (mean ± SD) were compared by factorial ANOVA 3 × 2 × 2, mode of MVP admixture {[LE versus LP (effect of light)] versus AA} × effect of oxygen (low versus high) × duration of TPN (sample 1 versus sample two). When indicated, data were transformed by natural log to satisfy homoscedasticity tested by Barlett’s χ2. The orthogonality of the comparisons was reached using the harmonic mean of the sample size in each group. Correlations between oxygen exposure and cytokine levels were determined using a Spearman’s rank test. Correlations between redox potential and cytokines were determined by Pearson’s correlation coefficient. Statistical analyses were performed using SPSS 11.0 for windows (SPSS Inc., Chicago, IL). The level of significance was set at p < 0.05.
RESULTS
Clinical characteristics of infants
Twenty-six preterm infants had samples drawn for determination of the redox potential and cytokines on postnatal days 7 and 10. Clinical characteristics, include anthropometric parameters and factors that contribute to generating systemic inflammation or oxidant stress, such as the presence of chorioamnionitis, blood-culture proven infections or NEC, severity of illness score (SNAP-2), are presented in Table 1. These variables, as well as nutrient intakes on seventh day of life, did not differ between the three study groups. However, there was an overall increase in total energy intake (i.v. + p.o.,) between seventh day of life and 10 (72 ± 19 versus 83 ± 21 kcal/kg/d, p < 0.001) that is accounted for by the expected advancement in enteral feeds.
Table 1.
Clinical characteristics of infants
| Variable | AA (n = 9) | LE (n = 9) | LP (n = 8) |
|---|---|---|---|
| GA, mean ± SD (wk) | 26 ± 1 | 26 ± 1 | 26 ± 0 |
| Birth weight, mean ± SD (g) | 751 ± 211 | 763 ± 163 | 749 ± 118 |
| Gender, n (%) male | 5 (63) | 6 (60) | 3 (38) |
| Chorioamnionitis, n (%) | 3 (38) | 2 (20) | 2 (25) |
| Apgar score at 5 min, mean ± SD | 7 ± 2 | 6 ± 2 | 7 ± 2 |
| SNAP score, median (IQ range) | 9 (14–23) | 6 (16–32) | 14 (19–33) |
| Culture-proven sepsis or NEC, n (%) | 1 (13) | 1 (10) | 2 (25) |
| Glucose i.v. intake on day 7, mean ± SD (g/kg/d) | 10.3 ± 2.0 | 8.4 ± 2.5 | 10.0 ± 2.2 |
| Protein i.v. intake on day 7, mean ± SD (g/kg/d) | 2.9 ± 0.7 | 2.5 ± 0.8 | 2.8 ± 1.1 |
| Lipid i.v. intake on day 7, mean ± SD (g/kg/d) | 2.1 ± 0.8 | 2.6 ± 0.8 | 2.7 ± 0.8 |
| Multivitamin i.v. intake on day 7, mean ± SD (mL/kg/d) | 1.2 ± 0.2 | 1.1 ± 0.3 | 1.1 ± 0.4 |
| Enteral intake on day 7, mean ± SD (mL/kg/d) | 14 ± 17 | 20 ± 22 | 13 ± 19 |
| Days of supplemental oxygen, median (IQ range) | 48 (25–84) | 79 (37–92) | 76 (71–95) |
| Fractional inspired oxygen, day 7 median (75th centile) | 0.23 (0.30) | 0.23 (0.25) | 0.24 (0.26) |
| Fractional inspired oxygen, day 10 median (75th centile) | 0.26 (0.53) | 0.26 (0.30) | 0.28 (0.36) |
Effect of exposure to oxygen on systemic inflammation
There was a positive correlation between the amount of oxygen infants were exposed to (FiO2) and plasma cytokine levels (r2 = 0.28 for IL-6 and r2 = 0.31 for IL-8, n = 50; p < 0.001). Overall, cytokine levels also correlated positively with E (r2 = 0.20 for IL-6; r2 = 0.20 for IL-8, n = 50; p < 0.002); when analyzing samples 1 and 2 separately, this correlation reached statistical significance only for sample 2 (r2 = 0.40 for IL 6, n = 24, p < 0.001 and r2 = 0.28 for IL 8, n = 24, p < 0.01). For IL-1β, IL-10, IL-12, and TNF-α, measurements were low and even below the level of detection in the majority of infants and therefore not shown.
Effect of TPN regimen on oxygen-associated systemic inflammation and oxidant stress
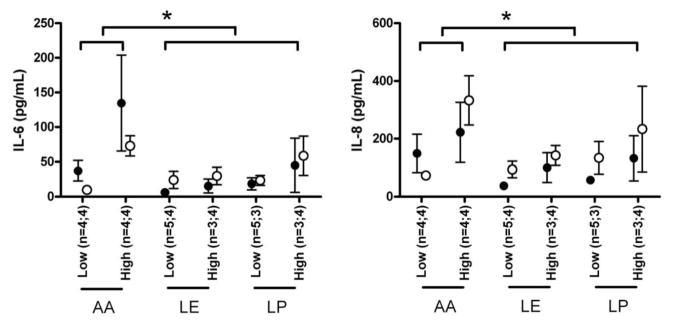
The analysis of variance showed an overall significant effect of oxygen on both IL-6 (F1,34 = 7.33, p < 0.01) and IL-8 plasma concentrations (F1,34 = 9.92, p < 0.01). Administration of MVPs with lipids seemed to protect against the oxygen-associated inflammatory response as a high FiO2 induced significantly lower IL-6 (F1,34 = 5.11, p < 0.05) and IL-8 (F1,34 = 4.48, p < 0.05) cytokine responses in the LE and LP regimen, compared with the AA regimen (Fig. 1). Within our sample size, there was no additional detectable effect of light protection on the cytokine responses as no differences were found between LE and LP regimens (F1,34 <1.6). Furthermore, no significant effect of duration of TPN [sample 1 versus sample 2, (F1,34 <4.0)] was detected.
Figure 1.
Plasma levels of IL-6 and IL-8 in preterm infants receiving TPN. Preterm infants were allocated to receive one of the following TPN regimens: AA, multivitamin (MVP) +amino acid/dextrose solution with lipid (LIP) provided separately (n = 9); LE, MVP administered with LIP exposed to light with amino acid/dextrose solutions provided separately (n = 9); LP, MVP administered with LIP protected from light with amino acid/dextrose solutions provided separately (n = 8). IL-6 (left panel) and IL-8 (right panel) were measured from plasma sampled on 7th day of life (● sample 1) and 10th day of life (○ sample 2). Data (mean ±SD) were compared by ANOVA between infants (n = sample 1; sample 2) exposed to low (<0.25) vs high (≥ 0.25) FiO2; *p < 0.05.
DISCUSSION
This study reports the effect of different modalities of coadministration of parenteral amino acid, lipid, and MVP solutions on the proinflammatory responses detected on exposure to supplemental oxygen. Infants in this study represent a subgroup of extremely low birth weight (ELBW) premature infants enrolled in a larger study designed to compare modalities of MVP admixture with TPN on antioxidant vitamin bioavailability and on the redox status of ELBW premature infants. In previous studies, we demonstrated that administering the parenteral LIP with multivitamins (whether light exposed or not) protected premature infants against the oxidant stress induced by oxygen supplementation (FiO2 ≥25%), independently of antioxidant vitamin levels (Chessex P, unpublished data). A cutoff of 25% FiO2 was chosen to better discriminate the clinical status of infants. In addition, oxidant stress has been reported with even relatively mild changes in supplemental oxygen (32). Furthermore, the inflammatory response to FiO2 >50% does not change substantially (33). We also confirmed that ELBW premature infants exhibited biologic markers of oxidant stress (redox potential of glutathione, dityrosine, and iso-PG-F2α) that were significantly more oxidized than in adults (34), generally indicating a greater vulnerability to ROS-mediated tissue injury.
Similarly, in this subgroup, we demonstrate that the mode of administration of TPN modulates the inflammatory response induced by oxygen (Fig. 1). Indeed the AA in which MVP was mixed with the amino acid plus dextrose solution resulted in a greater systemic inflammatory response, as detected by levels of IL-6 and IL-8 in plasma. Both exposure to supplemental oxygen and TPN regimens influence markers of inflammation and oxidation. Modulation of the proinflammatory effect of oxygen by TPN can potentially be explained by a reduction in the generation of lipid and hydrogen peroxide, which likely impact on the activity of proinflammatory intracellular signaling events (e.g. NF-kB translocation to the nucleus) (9,35). This model is supported by recent data showing that ROS, such as hydrogen peroxide, cause the scavenger protein thioredoxin (TRX) to dissociate from its thioredoxin-interacting partner (TXNIP), which can then directly interact with the inflammasome component NLRP3 to cause production of an inflammatory responses (36). The infusion of TPN solutions containing peroxides, which are relatively stable and permeable oxidants, would also induce a traditional “inside-out” stressor in the vessels, directly affecting the integrity of the endothelium (37). It has recently been proposed that stimulation of cytokines can also induce an “outside-in” paradigm that produces in turn a large amount of H2O2 in the vessel wall, potentiating an inflammatory response and causing initiation of necrosis, hypertrophy, and angiogenesis (35). This may account for some of the mechanisms (38) by which both inflammation and TPN are involved in the later development of bronchopulmonary dysplasia (18,39). The presence of positive linear correlations between the redox potential and the systemic cytokine response supports a similar physiologic link between these two stressors in preterm infants.
Limitations of this study include the small sample size and the short duration of observation. However, the effect was reproducible at two time points (samples 1 and 2) even if both samples were not systematically distributed in the same high or low oxygen groups. The ANOVA showed no statistically significant effect related to samples. Although the clinical characteristics of infants in the three groups are not statistically different, a logistic regression model to exclude other confounders was not performed because of the limited sample size. The clinical complications of inflammation could have extended well beyond the treatment period of our protocol, which was limited to the first 2 wk of life.
In conclusion, supplemental oxygen exposure is associated with a systemic inflammatory response, detected by measuring plasma levels of IL-6 and IL-8: two significant markers of adverse long-term pulmonary and neurodevelopmental outcomes in preterm infants. Delivering light-exposed lipids separate from amino acids may enhance the proinflammatory effect of oxygen. Furthermore, we provide compelling evidence that the proinflammatory response generated by supplemental oxygen exposure is attenuated in infants exposed to lipids mixed with the multivitamin preparation. Therefore, the different modalities of coadministration of TPN components may have important protective effects against potentially deleterious inflammation. These results need to be confirmed on a larger sample size incorporating relevant clinical outcomes.
Acknowledgments
Supported by a Grant MOP 53270 from the Canadian Institutes of Health Research.
We thank Indira Genowati and Mihoko Whalen for experimental assistance with the cytokine measurements and Jas Aulack for technical assistance.
Abbreviations
- AA
control regimen
- AA+MVP
parenteral multivitamin preparation mixed within the amino acid dextrose solution
- E
redox potential expressed in mV
- LIP+MVP
parenteral multivitamin preparation mixed within the lipid emulsion
- LE
LIP+MVP light exposed
- LP
LIP+MVP light protected
- MVP
multivitamin preparation
- NEC
necrotizing enterocolitis
- ROS
reactive oxygen species
- TPN
total parenteral nutrition
References
- 1.Bohrer B, Silveira RC, Neto EC, Procianoy RS. Mechanical ventilation of newborns infant changes in plasma pro- and anti-inflammatory cytokines. J Pediatr. 2010;156:16–19. doi: 10.1016/j.jpeds.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Laborie S, Lavoie JC, Chessex P. Increased urinary peroxides in newborn infants receiving parenteral nutrition exposed to light. J Pediatr. 2000;136:628–632. doi: 10.1067/mpd.2000.105131. [DOI] [PubMed] [Google Scholar]
- 3.Fujinaga H, Baker CD, Ryan SL, Markham NE, Seedorf GJ, Balasubramanian V, Abman SH. Hyperoxia disrupts vascular endothelial growth factor-nitric oxide signaling and decreases growth of endothelial colony-forming cells from preterm infants. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1160–L1169. doi: 10.1152/ajplung.00234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozycki HJ, Comber PG, Huff TF. Cytokines and oxygen radicals after hyperoxia in preterm and term alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1222–L1228. doi: 10.1152/ajplung.00230.2001. [DOI] [PubMed] [Google Scholar]
- 5.Yeung MY. Influence of early postnatal nutritional management on oxidative stress and antioxidant defence in extreme prematurity. Acta Paediatr. 2006;95:153–163. doi: 10.1080/08035250500301133. [DOI] [PubMed] [Google Scholar]
- 6.Warner BB, Wispe JR. Free radical-mediated diseases in pediatrics. Semin Perinatol. 1992;16:47–57. [PubMed] [Google Scholar]
- 7.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, Roberts LJ, 2nd, Arduini A, Escobar JJ, Sastre J, Asensi MA. Preterm resuscitation with low oxygen causes less oxidative Stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:439–449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 8.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira-Marques V, Cyrne L, Marinho HS, Antunes F. A quantitative study of NF-kappaB activation by H2O2: relevance in inflammation and synergy with TNF-alpha. J Immunol. 2007;178:3893–3902. doi: 10.4049/jimmunol.178.6.3893. [DOI] [PubMed] [Google Scholar]
- 10.Volpe JJ. Postnatal sepsis, necrotizing enterocolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr. 2008;153:160–163. doi: 10.1016/j.jpeds.2008.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusa T, Beckman JS, Crapo JD, Freeman BA. Hyperoxia increases H2O2 production by brain in vivo. J Appl Physiol. 1987;63:353–358. doi: 10.1152/jappl.1987.63.1.353. [DOI] [PubMed] [Google Scholar]
- 12.Gerstner B, Buhrer C, Rheinlander C, Polley O, Schuller A, Berns M, Obladen M, Felderhoff-Mueser U. Maturation-dependent oligodendrocyte apoptosis caused by hyperoxia. J Neurosci Res. 2006;84:306–315. doi: 10.1002/jnr.20880. [DOI] [PubMed] [Google Scholar]
- 13.Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thebaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2009;65:430–436. doi: 10.1203/PDR.0b013e31819984ce. [DOI] [PubMed] [Google Scholar]
- 14.Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL, Felderhoff-Mueser U, Volpe JJ, Rosenberg PA. Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci. 2008;28:1236–1245. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 17.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr. 2009;154:39–43. e3. doi: 10.1016/j.jpeds.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 20.Lucas A, Morley R, Cole TJ. Randomized trial of early diet in preterm babies and later intelligence quotient. BMJ. 1998;317:1481–1487. doi: 10.1136/bmj.317.7171.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvers KM, Sluis KB, Darlow BA, McGill F, Stocker R, Winterbourn CC. Limiting light-induced lipid peroxidation and vitamin loss in infant parenteral nutrition by adding multivitamin preparations to Intralipid. Acta Paediatr. 2001;90:242–249. doi: 10.1111/j.1651-2227.2001.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 22.Lavoie JC, Bélanger S, Spalinger M, Chessex P. Admixture of a multivitamin preparation to parenteral nutrition: the major contributor to in vitro generation of peroxides. Pediatrics. 1997;99:E6. doi: 10.1542/peds.99.3.e6. [DOI] [PubMed] [Google Scholar]
- 23.Neuzil J, Darlow BA, Inder TE, Sluis KB, Winterbourn CC, Stocker R. Oxidation of parenteral lipid emulsion by ambient and phototherapy lights: potential toxicity of routine parenteral feeding. J Pediatr. 1995;126:785–790. doi: 10.1016/s0022-3476(95)70412-4. [DOI] [PubMed] [Google Scholar]
- 24.Helbock HJ, Motchnik PA, Ames BN. Toxic hydroperoxides in intravenous lipid emulsions used in preterm infants. Pediatrics. 1993;91:83–87. [PubMed] [Google Scholar]
- 25.Knafo L, Chessex P, Rouleau T, Lavoie JC. Association between hydrogen peroxide-dependent byproducts of ascorbic acid and increased hepatic acetyl-CoA carboxylase activity. Clin Chem. 2005;51:1462–1471. doi: 10.1373/clinchem.2005.050427. [DOI] [PubMed] [Google Scholar]
- 26.Laborie S, Lavoie JC, Pineault M, Chessex P. Protecting solutions of parenteral nutrition from peroxidation. JPEN J Parenter Enteral Nutr. 1999;23:104–108. doi: 10.1177/0148607199023002104. [DOI] [PubMed] [Google Scholar]
- 27.Khashu M, Harrison A, Lalari V, Gow A, Lavoie JC, Chessex P. Photoprotection of parenteral nutrition enhances advancement of minimal enteral nutrition in preterm infants. Semin Perinatol. 2006;30:138–145. doi: 10.1053/j.semperi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 29.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 30.Lavoie JC, Rouleau T, Tsopmo A, Friel J, Chessex P. Influence of lung oxidant and antioxidant status on alveolarization: role of light-exposed total parenteral nutrition. Free Radic Biol Med. 2008;45:572–577. doi: 10.1016/j.freeradbiomed.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Schafer FQ, Buetner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith CV, Hansen TN, Martin NE, McMicken HW, Elliot SJ. Oxidant stress response in premature infants during exposure to hyperoxia. Pediatr Res. 1993;34:360–365. doi: 10.1203/00006450-199309000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Harling AE, Beresford MW, Vince GS, Yoxall CW. Does the use of 50% oxygen at birth in preterm infants reduce lung injury? Arch Dis Child Fetal Neonatal Ed. 2005;90:F401–F405. doi: 10.1136/adc.2004.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belik J, Gonzalez-Lius GE, Perez-Vizcaino F, Villamor E. Isoprostanes in fetal and neonatal health and diseases. Free Radic Biol Med. 2010;48:177–188. doi: 10.1016/j.freeradbiomed.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 37.Lavoie JC, Chessex P. Gender-related response to a tert-butyl hydroperoxide-induced oxidation in human neonatal tissue. Free Radic Biol Med. 1994;16:307–313. doi: 10.1016/0891-5849(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 38.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Bassiouny MR, Almarsafawy H, Abdel-Hady H, Nasef N, Hammad TA, Aly H. A randomized controlled trial on parenteral nutrition, oxidative stress, and chronic lung disease in preterm infants. J Pediatr Gastroenterol Nutr. 2009;48:363–369. doi: 10.1097/mpg.0b013e31818c8623. [DOI] [PubMed] [Google Scholar]