Abstract
Background
Irreversible electroporation (IRE) has emerged as a novel, safe ablative therapy for peri-vascular lesions. However, there remains a paucity of data on long-term outcomes.
Methods
We identified patients who underwent open IRE (1/2011 - 6/2015) for primary and secondary hepatic malignancies. Local ablation-zone recurrence (LR) was determined by cross-sectional imaging. Cumulative incidence (CumI) of LR was calculated and a competing risks regression assessed factors associated with LR.
Results
Forty patients had 77 lesions treated. The majority of lesions were of colorectal origin (74%). Median tumor size was 1.3cm (range 0.5 - 6). Most patients (86%) had prior systemic therapy and 29% received systemic therapy following IRE. With a median follow up of 25.7 months (range 4.5-58.8 months), 10 lesions in 9 patients recurred locally (CumI: 13.4%, 95% CI: 7.8-22.2%). Median estimated time to LR was not reached and no LR occurred after 19 months. Factors significantly associated with LR included ablation zone size (HR 1.58; 95% CI 1.12–2.23; p=0.0093) and body mass index (HR 1.21 95% CI 1.10–1.34; p=0.0001).
Conclusion
IRE LR rates were low after the treatment of well selected, small tumors. This technique is useful for lesions in anatomic locations precluding resection or thermal ablation.
Keywords: Irreversible electroporation, thermal ablation, hepatic metastases, hepatic malignancy, metastatic colorectal cancer, hepatocellular carcinoma, liver directed therapy
Introduction
Thermal ablative therapies have been increasingly used to treat primary and secondary hepatic malignancies. Radiofrequency (RFA) or microwave ablations (MWA) remain the most common of the thermal ablative modalities and are typically used in patients who are poor candidates for resection secondary to tumor location, comorbidities or previous hepatic resection (1, 2). Yet, proximity to portal pedicles, hepatic veins or bile ducts remains an impediment to these thermal ablative therapies (3, 4). Peri-vascular proximity not only allows for a heat sink effect, leading to a decrease in the efficacy of ablation, but also increases potential complications secondary to thermal injury to surrounding vascular or biliary structures (3, 4).
Recently, irreversible electroporation (IRE) has emerged as a novel, safe ablative therapy for peri-vascular hepatic lesions that otherwise cannot be resected or thermally ablated (5-9). Unlike thermal ablation which induces indiscriminate cellular necrosis within the ablation zone, IRE delivers a series of electrical pulses of millisecond duration that create irreversible pores in cell membranes therefore disrupting cellular homeostasis and ultimately leading to apoptosis (10). This modality is discriminate and only damages tumoral and parenchymal tissue while sparing hepatic veins, portal pedicles and/or bile ducts (11-13). One theory for this discriminate cell death postulates that the gap junctions within vasculature allow the electrical pulses to travel through the vessel walls rather than form nanopores (10). In doing so, these structures may be protected from IRE induced cell death. In essence, compared to thermal ablative technologies, the possible advantages of IRE include the lack of a heat-sink effect and the conservation of vasculature / biliary structures within the ablation zone. Therefore, IRE may overcome certain limitations of the current thermal ablative therapies.
Although the safety of this novel therapy has been evaluated, there remains a paucity of data on recurrence outcomes (9). Historically, local recurrence (LR) rates following thermal ablation have ranged from 3 – 30% (14). However, current reports on the effectiveness of IRE have predominantly included small sample sizes with short follow up intervals (15-19). Herein, we present the largest series to date assessing LR patterns following IRE for primary and secondary hepatic malignancies and discuss prognostic factors associated with LR.
Methods
We performed a retrospective review of a prospectively maintained database within the Memorial Sloan Kettering Cancer Center's (MSKCC) section of hepatopancreatobiliary surgery and identified patients who underwent open IRE alone or in combination with other surgical procedures between January 1, 2011 and June 30, 2015. Patients treated by a percutaneous approach were not included. All clinicopathologic data were obtained following approval from the Institutional Review Board. The NanoKnife (Angiodynamics) IRE device was used in all patients; of which the technical details have been previously reported by our group (9).
Patient Selection
All patients had primary or secondary hepatic malignancies and were staged preoperatively with contrast enhanced computed tomography (CT) imaging and/or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis. Select patients also had positron emission tomography (PET) obtained pre-operatively. All lesions precluded resection of thermal ablation due to their peri-vascular location (proximity to hepatic vein or portal pedicle). In the operating room, a laparotomy was performed and IRE was completed either alone or in combination with another procedure(s). One patient had a repeat IRE for a local recurrence and this lesion was excluded from analysis. At the time of operation, the size of the ablation zone was determined / measured by a NanoKnife algorithm using the distance between the active ends of the probes and the power settings. Size data was then entered into the operative report.
Patient Follow up
All patients were seen in follow-up after discharge from the hospital. Cross-sectional imaging was obtained at variable times, typically three times yearly for the first two years and then bi-annually if disease was stable during the 3-5 years following surgery. As previously reported, complications that developed during the peri-operative period were prospectively recorded into the MSKCC Surgical secondary-events program (SSE), which utilized a classification system similar to Clavien-Dindo (20).
Local recurrence was denoted by recurrence of tumor in an area of previously documented complete ablation as determined by a MSKCC radiologist on a post procedure scan. Specifically, we defined local recurrence as abnormal enhancement at the periphery, increasing size / parenchymal density of an ablation site on post-procedure contrast enhanced imaging or increased PET avidity at the ablation zone site.
Factors of Local Recurrence Assessed by Univariate Analysis
The following variables were assessed: age at surgery, body mass index (BMI), gender, alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, bilirubin, INR, concurrent procedures performed, proximity to a major bile duct, proximity to major vasculature, tumor size (continuous variable), tumor size (grouped: >1cm vs. ≤ 1cm), ablation zone size, tumor type (colorectal vs. all others) and the number of IRE per lesion.
Statistical Analysis
Patient, procedure and lesion level data were summarized with frequencies and percentages for categorical variables, and medians and ranges for continuous variables. Overall survival (OS) was defined as the time from initial IRE procedure to death. Patients alive at last follow up were censored. OS in colorectal cancer patients was estimated using Kaplan Meier (KM) methods and plots; 1 year, 2 year and 3-year KM estimates were provided. Time to LR was defined as the interval between IRE and LR on CT. Death was treated as a competing risk and lesions that did not recur by last follow up were censored. Cumulative incidence of LR on a lesion level was estimated along with 95% CI. The associations between preoperative and perioperative variables and LR were assessed using univariate competing risks regression with Fine and Grey methods modified by Zhou et al. to account for multiple lesions per patient (21). P-values less than 0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC).
Institutional Comparison of IRE to Thermal Ablation
We evaluated our IRE results in the context of a previously reported matched group of patients who underwent RFA or MWA at our institution (2). A formal statistical comparison was not possible between these groups because the tumors selected for IRE were, by definition, not amenable to other ablative modalities. Moreover, tumors included in the thermal ablative cohorts (MWA vs. RFA) did not include all lesions treated over the study period, but rather only those who were able to be matched to their counterpart.
Results
Cohort characteristics
Forty patients underwent 44 operations that included IRE ablation of 77 hepatic lesions, details of which are detailed in Table 1. The majority of IRE ablations (63/77, 82%) were performed in conjunction with another procedure. Median age at surgery was 53 years (range, 29.5 – 81 years) and the majority of patients were male (75%). Median body mass index (BMI) of the cohort was 27.5 (range, 17.3 – 39.7). The majority of patients (79.5%) received systemic therapy prior to IRE and 65.9% received systemic therapy following IRE. In the colorectal population, 39% received hepatic arterial infusion pump (HAIP) chemotherapy prior to IRE and 33% received HAIP chemotherapy following IRE. The majority of patients had normal pre-IRE transaminase levels (26/44 surgeries, 59%), albumin levels (median 4.2 g/dL, range 3.2 – 4.9), international normalized ratio levels (median 1, range 0.9 – 3.1), bilirubin levels (median 0.7 mg/dL, range 0.3 – 1.5) and platelet counts (median 181 per microliter, range 35 – 357).
Table 1.
Cohort characteristics of 40 patients encompassing 77 primary and secondary hepatic metastases treated with IRE. (key: HAIP – hepatic artery infusion pump, CEA – carcinoembryonic antigen, ALP- alkaline phosphatase, ALT- alanine aminotransferase, AST- aspartate aminotransferase, INR- international normalized ratio)
| Variable (N = 44 surgeries) | N | |
|---|---|---|
| Follow Up Time from IRE (Months) | Median (range) | 25.7 (4.5-58.8) |
| Age at Surgery (years) | Median (range) | 53.1 (29.5-81) |
| Body Mass Index | Median (range) | 27.5 (17.3-39.7) |
| CEA level (ng/ml) | Median (range) | 5.1 (1.2-1143.8) |
| Other Procedures Performed | No | 8 (18.2%) |
| Yes | 36 (81.8%) | |
| Pre-IRE Systemic Therapy | No | 9 (20.5%) |
| Yes | 35 (79.5%) | |
| Post-IRE Systemic Therapy | No | 15 (34.1%) |
| Yes | 29 (65.9%) | |
| Pre-IRE HAIP (colorectal; N = 33 surgeries) | No | 20 (61%) |
| Yes | 13 (39%) | |
| Post-IRE HAIP (colorectal; N = 33 surgeries) | No | 22 (67%) |
| Yes | 11 (33%) | |
Tumor and IRE Treatment Characteristics
The majority of lesions were of colorectal origin 57/77 (74%), however 7/77 (9%) were hepatocellular carcinoma, 3/77 (4%) leiomyosarcomas, 4/77 (5%) neuroendocrine tumors, 3/77 (4%) ampullary carcinoma, 2/77 (3%) uterine leiomyosarcomas and 1/77 (1%) cholangiocarcinoma. The median lesion size was 1.3 cm (range, 0.5-6 cm). Twenty four lesions (31%) were ≤ 5mm from the main right or left bile ducts. Lesion distance from a portal pedicle or hepatic vein was measured at the time of IRE and the distance recorded as <3mm (55/77, 71.4 %), 3 – 5mm (14/77, 18.2 %) or > 5mm (8/77, 10.4 %). Lesions were located within the following Couinaud segmental locations: segment 1 (12/77 16%), 2 (5/77 6 %), 3 (3/77 4 %), 4a (6/77 8 %), 4b (9/77 12 %), 5 (8/77 10%), 6 (8/77 10 %), 7 (6/77 8 %) and 8 (20/77 26 %). The median number of IRE treatments per lesion was 2 (range, 1 – 7) and this corresponded to a median ablation zone size of 3cm (range, 1.5 – 8cm). Note, the ablation zone size was reported by the treating surgeon at the time of operation. Clinicopathologic details are depicted in Table 2.
Table 2. Tumor and treatment characteristics of primary and secondary hepatic metastases treated with IRE (N=77).
| Variable (N=77) | N (%) | |
|---|---|---|
| Tumor Size (cm) | Median (range) | 1.3 (0.5-6) |
| Lesion Pathology | ||
| Colorectal | 57 (74) | |
| Hepatocellular | 7 (9) | |
| Leiomyosarcoma | 5 (6) | |
| Neuroendocrine | 4 (5) | |
| Ampullary | 3 (4) | |
| Cholangiocarcinoma | 1 (1) | |
| Distance to Major Vasculature | ||
| < 3mm | 55 (71.4) | |
| 3-5mm | 14 (18.2) | |
| 5-10mm | 2 (2.6) | |
| >10mm | 6 (7.8) | |
| Close to 1st degree bile duct | No | 53 (68.8) |
| Yes | 24 (31.2) | |
| IRE treatments per Lesion | Median (range) | 2 (1-7) |
| Ablation Zone Size (cm) | Median (range) | 3 (1.5-8) |
| IRE Combined with Other Procedures | 63 (82) | |
| Non-anatomic hepatectomy | 50 (64) | |
| Left lateral segmentectomy | 10 (13) | |
| Caudate lobectomy | 5 (6) | |
| Right lobectomy | 2 (3) | |
| Segmentectomy | 13 (17) | |
| Cholecystectomy | 8 (10) | |
| Microwave ablation | 32 (41) | |
| Radiofrequency ablation | 2 (3) | |
| Distal pancreatectomy | 3 (4) | |
| Partial gastrectomy | 1 (1) | |
| Placement of hepatic arterial infusion pump | 15 (19) | |
Complications
Post-operative complications within 30 days of the procedure occurred in 14/40 (35%) of patients and included cardiovascular (6/40, 15%), infection (4/40, 10%), paralytic ileus (4/40, 10%), pulmonary embolism (3/40, 8%), hematoma (2/40, 5%), hemorrhage (2/40, 5%) and pleural effusion (1/40, 3%), as depicted in Table 3. Complications specific to IRE (classified per patient) included intra-operative arrhythmia 1/40 (2.5%), post-operative hepatic vein perfusion defect 1/40 (2.5%) and post-operative hepatic vein thrombosis 1/40 (2.5%). In those patients who had IRE performed alone (14/77, 18%), 1 patient experienced a peri-hepatic hematoma (1/14, 7%).
Table 3.
Operative complications for the 40 patients treated and IRE specific complications as denoted per lesion, following IRE of primary and secondary hepatic metastases.
| Peri-Operative Complications | Number (%) |
|---|---|
| Overall complications (n = 40 patients) | 14 (35%) |
| Cardiovascular | 6 (15%) |
| Infectious | 4 (10%) |
| Paralytic ileus | 4 (10%) |
| Pulmonary embolism | 3 (8%) |
| Hematoma | 2 (5%) |
| Hemorrhage | 2 (5%) |
| Pleural effusion | 1 (3%) |
| IRE specific complications (n = 40 patients) | |
| Intra-operative cardiac arrhythmia | 1 (2.5%) |
| Post-operative hepatic vein perfusion defect | 1 (2.5%) |
| Post-operative hepatic vein thrombosis | 1 (2.5%) |
| Post-operative portal pedicle perfusion defect | 0 (0%) |
| Post-operative biliary stricture | 0 (0%) |
| Complications of IRE performed in absence of other procedures (n = 14 lesions) | |
| Peri-Hepatic Hematoma | 1 (7%) |
Local Recurrences
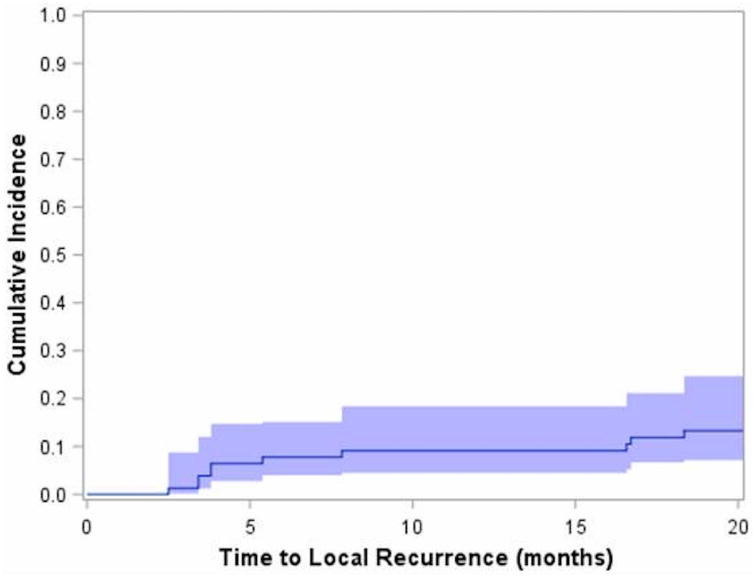
At a median follow up of 25.7 months (range, 4.5 – 58.8 months), 10 lesions recurred in 9 patients. The cumulative incidence for local recurrence (LR) was 13.4% (95% CI: 7.8%-22.2%). The median estimate of time to LR was not reached and no LR occurred after 19 months, Figure 1. Of the 67 lesions that did not recur, 53 had follow up time greater than 19 months.
Figure 1.
Cumulative incidence of local recurrence for all lesions. 10 lesions recurred in 9 patients with a cumulative incidence of 13.4% (95% CI: 7.8%-22.2%). Median time to local recurrence was not reached and no local recurrence occurred beyond 19 months.
Factors associated with local recurrence included 1) BMI (HR: 1.21, 95% CI: 1.10-1.34, p=0.0001), with each incremental unit of BMI (i.e 22 to 23) resulting in a 21% increase in the risk of local recurrence and 2) size of the ablation zone (HR: 1.58, 95% CI: 1.12-2.23, p=0.0093) with each incremental increase in cm resulting in a 58% increase in the risk of local recurrence. No other factors were significantly associated with local recurrence, though concurrent procedures (p=0.06), preoperative bilirubin levels (p=0.07) and age at surgery (p=0.08) approached significance, (Table 4). LR was not found to be associated with Couinaud segmental location, tumor size, distance to major vasculature, proximity to major bile ducts or the administration of systemic therapy.
Table 4.
Competing risks regression depicting factors associated with LR following IRE of primary and secondary hepatic metastases. (N=77)
| HR | [95% CI] | p-value | |
|---|---|---|---|
| Body Mass Index | 1.21 | [1.10 - 1.34] | .0001 |
| Ablation Zone Size (cm) | 1.58 | [1.12 - 2.23] | .0093 |
| Other Procedures Performed | 0.30 | [0.08 - 1.06] | 0.06 |
| Pre-IRE Bilirubin | 6.18 | [0.87 - 43.90] | 0.07 |
| Age at Surgery (years) | 0.97 | [0.93 -1.00] | 0.08 |
| Female Gender | 0.60 | [0.11 - 3.31] | 0.56 |
| Elevated Pre-IRE ALP | 0.66 | [0.17 - 2.62] | 0.55 |
| Elevated Pre-IRE ALT | 1.09 | [0.26 - 4.54] | 0.91 |
| High Pre-IRE AST | 1.08 | [0.25 - 4.73] | 0.91 |
| Pre-IRE Albumin | 0.82 | [0.20 - 3.42] | 0.79 |
| Pre-IRE INR | 1.74 | [0.47 - 6.37] | 0.40 |
| Close to Bile Duct | 1.46 | [0.43 - 4.98] | 0.54 |
| Close to Major Vasculature | 0.73 | [0.13 - 3.97] | 0.71 |
| Tumor Size (cm) | 1.35 | [0.88 - 2.07] | 0.17 |
| Tumor Size > 1cm | 2.15 | [0.61 - 7.64] | 0.23 |
| Colorectal Tumor Type | 0.28 | [0.04 - 1.95] | 0.20 |
| # Treatments per Lesion | 1.33 | [0.89 - 1.99] | 0.17 |
Institutional Comparison of IRE to Thermal Ablation
Our LR findings for IRE were similar to LR results in a previously reported matched group of patients who underwent RFA or MWA at our institution (2). The CumI of LR following IRE (13.4%, 95%CI: 7.8-22.2%) was lower than RFA (CumI LR: 21.4%, 14.5-29.1%), but higher than MWA (CumI LR: 6.8%, 2.9-13.1%). Of note, the clinicopathologic characteristics in these cohorts such as age, median tumor size, size of the ablation zone, performed in combination with other surgical procedures, use of pre-ablation chemotherapy and HAIP therapy were similar. Note however, a direct comparison cannot and should not be performed as IRE lesions precluded thermal ablation.
Discussion
This analysis of forty patients undergoing IRE of 77 hepatic metastases demonstrated a cumulative incidence of LR of 13.4%. The median estimate of time to LR was not reached and no LR occurred after 19 months. A secondary objective of this study was the assessment of prognostic factors of LR. As such, we found the size of the ablation zone and body mass index to be associated with LR. Further, IRE was implemented (within the liver remnant) during the first stage of a two stage hepatectomy in 5/40 (13%) patients. In other words, patients who would have otherwise been deemed unresectable were able to undergo a two-stage hepatectomy because of the IRE treatment. Overall, these findings suggest that IRE is a viable option for peri-vascular lesions which would otherwise preclude resection or thermal ablation.
As thermal ablative therapies have become more popular for the treatment of primary and secondary hepatic malignancies, their shortcomings have become more apparent. LR rates following thermal ablation have ranged from 3 – 30% for RFA at 12-49 months of follow up and 3 – 13% for MWA at 5-19 months of follow up (9, 14). Additionally, it is now well known that proximity to major vasculature is an impediment to thermal ablative therapies due to the heat sink effect, which can cause a drop in treatment efficacy below 50% (15, 22, 23). Additionally, thermal damage to surrounding structures, in particular vessels or bile ducts, substantially increases the risks of peri-operative complications. IRE on the other hand, may be able to overcome these limitations imposed by thermal ablation. Clinical studies have added weight to the above theory that IRE produces discriminate cell death, leaving vascular or biliary structures intact (7). In the COLDFIRE-1 trial, investigators prospectively IRE ablated ten peri-vascular hepatic malignancies which were then resected one hour later. In doing so, they demonstrated cell death of the ablated tumors within one hour after IRE however, large vascular and ductal structures within the ablation zone remained intact (7). IRE may ultimately allow for the treatment of liver tumors deemed unresectable or ineligible for other focal ablation techniques due to their peri-vascular location and should therefore be added to the armamentarium of providers caring for patients with primary or secondary liver cancers (24).
In agreement with our findings, others have also reported acceptable LR rates following IRE for hepatic malignancies (Table 5). In an analysis of 18 lesions treated with IRE, at a median follow up of 18 months, 72% of lesions were completely ablated (17). Moreover, the authors found a 93% success rate for lesions ≤ 3 cm with a local recurrence-free period of 18 ± 4 months (17). Similarly, Cannon and colleagues reviewed 44 hepatic lesions treated with IRE and found a local recurrence free survival (LRFS) of 60% at one year (16). However, in lesions < 3cm, the LRFS at one year increased to 98% (16). Following suit, Eisele et al reported on 13 hepatic lesions with a LR of 21% following IRE and again found lesion size to be the best predictor of LR (19). A possible reason for the higher recurrence with tumors larger than 3–4 cm is the number of probes required to treat these larger lesions (16). As instructed by the manufacturer, a 3 cm tumor is the upper limit which can be treated with a 3 probe array and the authors concluded that larger probe arrays cannot be placed with the precision necessary to treat larger tumors (16). On the contrary, Hosein et al reported on 29 patients in which 58 hepatic lesions were treated with IRE (18). At a median follow up of 11 months they found six cases of partial response (21%), seven cases of stable disease (25%), and five cases of progressive disease (18%) (18). However, unlike the previous studies presented, the sizes of the treated lesions were not associated with outcome differences (18). We postulate that a potential reason for a failure to show tumor size associated with LR relates to our median tumor size of 1.3cm. Specifically, only 4 lesions were over 3cm; therefore suggesting our database did not contain enough larger tumors to show an association with size and LR. On the other hand, the ablation zone size was measured by the surgeon at the time of surgery and may be a surrogate for size since we strived for a minimum of a 1cm ablation margin.
Table 5.
Local recurrence rates and factors associated with local recurrence following IRE of hepatic primary or secondary malignancies within the current literature (key: HCC – hepatocellular carcinoma; LRFS – local recurrence free survival; BMI – body mass index; CR – complete response, PR – partial response, PD – progression of disease, SD – stable disease)
| Median Follow Up | Lesions Treated | Local Recurrence | Factors Associated with Local Recurrence | |
|---|---|---|---|---|
| MSKCC IRE (n = 77) | 26 months | Colorectal (n = 58) HCC (n = 7) Other (n = 12) |
13.4% 95% CI: (7.8 - 22.2%) |
BMI (HR 1.21, 95% CI 1.10 – 1.34; p = 0.0001) Ablation zone size (HR 1.58; 95% CI 1.12 – 2.23; p = 0.0093) |
| Niessen et al15 (n = 48) | 6 months | Colorectal (n = 16) HCC (n = 22) Other (n = 10) |
29.2% < 5cm3 – 9.7% > 5cm3 – 65% |
BMI (p = 0.022) Lesion size > 5cm (p = 0.022) Tumor pathology (p = 0.023) |
| Cannon et al16 (n = 44) | Unknown | Colorectal (n = 20) HCC (n = 14) Other (n = 10) |
(LRFS) 60% at 1 year < 3cm – 98% at 1 year |
Lesion size > 4cm (HR 3.236, 95% CI: 0.585 – 17.891) |
| Cheung et al17 (n = 18) | 18 months | HCC (n = 18) | 28% | Lesion size > 3cm (p = 0.003) |
| Hosein et al18 (n = 58) | 11 months | Colorectal (n = 58) |
36% CR 21% PR 18% PD 25% SD |
N/A |
| Eisele et al19 (n = 13) | 6 months | Colorectal (n = 6) HCC (n = 5) Other (n = 2) |
21% | Lesion size (p < 0.001) |
Further, Niessen et al reported a LR of 29.2% following the treatment of 48 hepatic lesions (15). Similar to the previously presented studies, when the lesions were dichotomized by tumor density (surrogate for size), those < 5cm3 had a LR of 9.7% as compared to lesions > 5cm3 who saw a LR of 65% (15). As hypothesized following pre-clinical models, LR did not differ significantly between lesions that were and were not adjacent to large vessels or bile ducts (15). Interestingly and synonymous to our results, the authors found BMI to predict LR (15). We postulate that a fatty liver may cause a decrease in electrical conductivity. This conductivity differential may therefore contribute to a decrease in the efficacy of IRE; similar to the fact that electrical conductivity is higher within cirrhotic tissue (25). Further, ultrasound guided probe placement is more problematic in the obese patient due to hyperechoic ultrasound images secondary to their fatty liver.
One question that remains to be answered is how recurrence rates compare between IRE and thermal ablative therapies. Unfortunately, due to the fact that IRE is typically reserved for lesions which preclude thermal ablation, an unbiased, matched, direct comparison was not possible. However, we evaluated our IRE results in the context of patients who previously underwent RFA or MWA at our institution (data not shown) (2). In doing so, we found IRE to have similar LR rates to the thermal technologies. Though the study period and follow up time differed between cohorts and biases inherent in lesion selection are present, based on these LR's we suggest IRE may have an acceptable LR rate as compared to thermal ablative therapies.
With respect to safety, it should be mentioned that 35% of our patients experienced complications. However, we do not believe this represents an accurate percentage of IRE related complications since 82% of our patients had IRE performed in combination with another procedure. Yet, there were IRE specific complications including one hepatic venous perfusion defect and a left hepatic vein thrombosis. The hepatic venous perfusion defect was identified on CT imaging performed one week following IRE and was resolved by the following CT (6 months). No cross sectional images were obtained in the interim. The solitary case of hepatic vein thrombosis occurred in a patient who had previously undergone resection of segments 2 and 3 along with wedge resections of segments 4a and 4b for colorectal metastases. He then underwent IRE of a segment 8 lesion. At one month follow up, the left hepatic vein was found to be thrombosed. Unfortunately the patient succumbed to disease shortly after and no further cross sectional imaging was obtained. In brief, it is unclear whether this was caused by electrical effects of IRE, thermal effects of the IRE or tumor progression. Additionally, we had one patient experience a supraventricular arrhythmia despite EKG synchronization. This arrhythmia was triggered only when the device was actively administering IRE pulses, and the patient returned to a normal sinus rhythm when the IRE pulses stopped. A recent systemic review of the literature on IRE ablation found minor arrhythmias to occur in 2.2% of patients with cardiac gating and a 2.5% risk of hepatopancreatobiliary related complications such as portal vein thrombus, bile duct leakage or occlusion (24, 26). Moreover, Silk et al retrospectively assessed biliary complications after IRE ablation of twenty-two hepatic tumors in the immediate proximity of major bile ducts (27). They found no major biliary complications (27). Overall, as previously published, IRE remains a safe modality with peri-tumoral tumor complication rates.
Although we are filling a gap within the current literature, we acknowledge several limitations to our work. First, this was a retrospective analysis with a small sample size which included heterogenous pathology and systemic treatment. Second, the identification of LR was based off of non standardized cross-sectional imaging modalities at non-controlled intervals. This is a clear limitation. Moreover, the limited number of LR events restricted our ability to perform multivariate analyses or build risk models. Lastly, our use of hepatic arterial infusional pump therapy should be mentioned as a confounding factor; for as compared to other groups and historical controls, we utilized pump therapy in a higher percentage of patients. Therefore, we present our work as hypothesis generating, since concrete clinical conclusions cannot be drawn from our data.
Despite these limitations, the current study does identify important themes. In particular, it is currently the largest series with adequate follow up time to capture the majority of local recurrences (most reports have follow up periods of six months). To date, patients with central hepatic lesions unsuitable for resection or thermal ablation have limited to no curative options. IRE may therefore prove to add a survival benefit, specifically in those requiring two-stage hepatectomy. Additionally, outside of its benefit in two-stage hepatectomy, IRE may be useful in peri-vascularly located lesions precluding resection to clear all visible hepatic disease. A theoretical example would be a lesion on the left hepatic vein in a patient requiring an extended right hepatectomy.
Conclusion
The role of IRE within the context of expanding local and regional treatment options for hepatic malignancies is currently being defined. Due to the low peri-tumoral tissue toxicity, the lack of a heat sink effect and acceptable LR rates offered by IRE, it may be a beneficial ablative technology for peri-vascular hepatic malignancies which otherwise preclude resection or thermal ablation. IRE should therefore be included within multi-disciplinary discussions of the treatment of peri-vascular primary and secondary hepatic metastases. Moreover, IRE may allow otherwise unresectable patients the opportunity at resection or two-stage hepatectomy. Future studies assessing the most accurate imaging modality to identify recurrence and strategies that utilize IRE as an adjunct to systemic treatment may prove beneficial.
Synopsis.
Proximity to major vasculature or bile ducts remains an impediment to hepatic thermal ablative therapies. Irreversible electroporation (IRE) has emerged as a novel, safe ablative therapy for peri-vascular lesions; however, there remains a paucity of data on long-term outcomes. We present local recurrence rates and factors associated with local recurrence following IRE for hepatic malignancies.
Acknowledgments
This study was supported in part by NIH/NCI P30 CA008748 (Cancer Center Support Grant)
References
- 1.Leung U, Kuk D, D'Angelica MI, Kingham TP, Allen PJ, DeMatteo RP, et al. Long-term outcomes following microwave ablation for liver malignancies. The British journal of surgery. 2015;102(1):85–91. doi: 10.1002/bjs.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa-Gallego C, Fong Y, Gonen M, D'Angelica MI, Allen PJ, DeMatteo RP, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Annals of surgical oncology. 2014;21(13):4278–83. doi: 10.1245/s10434-014-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheffer HJ, Melenhorst MC, Echenique AM, Nielsen K, van Tilborg AA, van den Bos W, et al. Irreversible Electroporation for Colorectal Liver Metastases. Techniques in vascular and interventional radiology. 2015;18(3):159–69. doi: 10.1053/j.tvir.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Charpentier KP. Irreversible electroporation for the ablation of liver tumors: are we there yet? Archives of surgery. 2012;147(11):1053–61. doi: 10.1001/2013.jamasurg.100. [DOI] [PubMed] [Google Scholar]
- 5.Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut and liver. 2010;4(1):S99–S104. doi: 10.5009/gnl.2010.4.S1.S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AA, Vieveen JM, Bouwman AR, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. Journal of vascular and interventional radiology: JVIR. 2014;25(7):997–1011. doi: 10.1016/j.jvir.2014.01.028. quiz. [DOI] [PubMed] [Google Scholar]
- 7.Scheffer HJ, Nielsen K, van Tilborg AA, Vieveen JM, Bouwman RA, Kazemier G, et al. Ablation of colorectal liver metastases by irreversible electroporation: results of the COLDFIRE-I ablate-and-resect study. European radiology. 2014;24(10):2467–75. doi: 10.1007/s00330-014-3259-x. [DOI] [PubMed] [Google Scholar]
- 8.Scheffer HJ, Melenhorst MC, van Tilborg AA, Nielsen K, van Nieuwkerk KM, de Vries RA, et al. Percutaneous Irreversible Electroporation of a Large Centrally Located Hepatocellular Adenoma in a Woman with a Pregnancy Wish. Cardiovascular and interventional radiology. 2015;38(4):1031–5. doi: 10.1007/s00270-014-1041-8. [DOI] [PubMed] [Google Scholar]
- 9.Kingham TP, Karkar AM, D'Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. Journal of the American College of Surgeons. 2012;215(3):379–87. doi: 10.1016/j.jamcollsurg.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255(2):426–33. doi: 10.1148/radiol.10090337. [DOI] [PubMed] [Google Scholar]
- 11.Au JT, Wong J, Mittra A, Carpenter S, Haddad D, Carson J, et al. Irreversible electroporation is a surgical ablation technique that enhances gene transfer. Surgery. 2011;150(3):474–9. doi: 10.1016/j.surg.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Annals of biomedical engineering. 2005;33(2):223–31. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 13.Daniels C, Rubinsky B. Electrical field and temperature model of nonthermal irreversible electroporation in heterogeneous tissues. Journal of biomechanical engineering. 2009;131(7):071006. doi: 10.1115/1.3156808. [DOI] [PubMed] [Google Scholar]
- 14.Vogl TJ, Farshid P, Naguib NN, Darvishi A, Bazrafshan B, Mbalisike E, et al. Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiol Med. 2014;119(7):451–61. doi: 10.1007/s11547-014-0415-y. [DOI] [PubMed] [Google Scholar]
- 15.Niessen C, Igl J, Pregler B, Beyer L, Noeva E, Dollinger M, et al. Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: a prospective single-center study. Journal of vascular and interventional radiology: JVIR. 2015;26(5):694–702. doi: 10.1016/j.jvir.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC., 2nd Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. Journal of surgical oncology. 2013;107(5):544–9. doi: 10.1002/jso.23280. [DOI] [PubMed] [Google Scholar]
- 17.Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technology in cancer research & treatment. 2013;12(3):233–41. doi: 10.7785/tcrt.2012.500317. [DOI] [PubMed] [Google Scholar]
- 18.Hosein PJ, Echenique A, Loaiza-Bonilla A, Froud T, Barbery K, Rocha Lima CM, et al. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. Journal of vascular and interventional radiology: JVIR. 2014;25(8):1233–9 e2. doi: 10.1016/j.jvir.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Eisele RM, Chopra SS, Glanemann M, Gebauer B. Risk of local failure after ultrasound guided irreversible electroporation of malignant liver tumors. Interventional medicine & applied science. 2014;6(4):147–53. doi: 10.1556/IMAS.6.2014.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Fine J, Latouche A, Labopin M. Competing risks regression for clustered data. Biostatistics. 2012;13(3):371–83. doi: 10.1093/biostatistics/kxr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234(3):954–60. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 23.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Annals of surgery. 1998;227(4):559–65. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheffer HJ, Vroomen LG, Nielsen K, van Tilborg AA, Comans EF, van Kuijk C, et al. Colorectal liver metastatic disease: efficacy of irreversible electroporation--a single-arm phase II clinical trial (COLDFIRE-2 trial) BMC cancer. 2015;15:772. doi: 10.1186/s12885-015-1736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivorra A, Al-Sakere B, Rubinsky B, Mir LM. In vivo electrical conductivity measurements during and after tumor electroporation: conductivity changes reflect the treatment outcome. Phys Med Biol. 2009;54(19):5949–63. doi: 10.1088/0031-9155/54/19/019. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen K, Scheffer HJ, Vieveen JM, van Tilborg AA, Meijer S, van Kuijk C, et al. Anaesthetic management during open and percutaneous irreversible electroporation. British journal of anaesthesia. 2014;113(6):985–92. doi: 10.1093/bja/aeu256. [DOI] [PubMed] [Google Scholar]
- 27.Silk MT, Wimmer T, Lee KS, Srimathveeravalli G, Brown KT, Kingham PT, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. Journal of vascular and interventional radiology: JVIR. 2014;25(1):112–8. doi: 10.1016/j.jvir.2013.10.012. [DOI] [PubMed] [Google Scholar]