Abstract
Background and objective. Increasing physical activity (PA) is safe and beneficial in lung cancer (LC) patients. Advanced-stage LC patients are under-studied and have worse symptoms and quality of life (QoL). We evaluated the feasibility of monitoring step count in advanced LC as well as potential correlations between PA and QoL. Methods. This is a prospective, observational study of 39 consecutive patients with advanced-stage LC. Daily step count over 1 week (via Fitbit Zip), QoL, dyspnea, and depression scores were collected. Spearman rank testing was used to assess correlations. Correlation coefficients (ρ) >0.3 or <−0.3 (more and less correlated, respectively) were considered potentially clinically significant. Results. Most (83%) of the patients were interested in participating, and 67% of those enrolled were adherent with the device. Of those using the device (n = 30), the average daily step count was 4877 (range = 504-12 118) steps/d. Higher average daily step count correlated with higher QoL (ρ = 0.46), physical (ρ = 0.61), role (ρ = 0.48), and emotional functioning (ρ = 0.40) scores as well as lower depression (ρ = −0.40), dyspnea (ρ = −0.54), and pain (ρ = −0.37) scores. Conclusion. Remote PA monitoring (Fitbit Zip) is feasible in advanced-stage LC patients. Interest in participating in this PA study was high with comparable adherence to other PA studies. In those utilizing the device, higher step count correlates with higher QoL as well as lower dyspnea, pain, and depression scores. PA monitoring with wearable devices in advanced-stage LC deserves further study.
Keywords: exercise, non–small-cell lung cancer, physical activity, quality of life, small-cell lung cancer
Introduction
Despite increasing treatment options, lung cancer remains second in cancer frequency and first in cancer mortality.1 It is also one of the most burdensome cancers, with up to 90% of patients reporting symptoms during their course,2 and symptom intensity that is higher than that of most other cancers.3 Patients’ symptoms and activity levels worsen with time and treatment.4,5 Many clinicians associate symptom progression in chronic lung disease with the dyspnea spiral, wherein dyspnea develops, activity is avoided, functional capacity is lost, and dyspnea worsens.
Physical activity (PA) is a growing therapy for both patients with chronic lung disease and multiple cancer types (eg, lung, colon, breast, and prostate). Activity improves symptom burden, exercise tolerance, and quality of life (QoL).6-9 Because advanced-stage lung cancer patients have higher symptom burden and frequently concomitant lung disease, patients with more symptoms and activity limitations may have the most benefit. Though likely beneficial, studies increasing PA in lung cancer patients show low adherence in both early and advanced disease.10,11 Lung cancer patient and survivor surveys reveal preferences for activity guidance12 and that up to 80% of patients would prefer walking.13 Two recent randomized trials have shown that Fitbit-based interventions improved PA in postmenopausal women and overweight adults.14,15 Thus, digital accelerometers may motivate patients and help overcome the challenges of monitoring and maintaining PA in chronic disease.
Despite potential clinical benefit, data available regarding PA or the clinical utility of wearable accelerometers in patients with advanced-stage lung cancer are limited. We initiated this study to assess the feasibility of monitoring PA via daily step count in patients with advanced-stage lung cancer. Potential correlations between physical activity and symptoms, functional status, and QoL were also assessed. Some of the results of these studies have been previously reported in the form of an abstract.16
Methods
After institutional review board (IRB) approval (IRB for Human Research, Protocol 00028353), consecutive patients at the Medical University of South Carolina’s (MUSC’s) Thoracic Oncology clinic at the Hollings Cancer Center and Health East Cooper facility were approached. Inclusion criteria were pathological evidence of advanced-stage lung cancer, approval of the treating clinician, willingness to wear a Fitbit Zip (Fitbit Inc, San Francisco, CA), access to a smartphone, and willingness to download the Fitbit application to their smartphone. Advanced-stage lung cancer was defined as any stage small-cell lung cancer (SCLC) or stages III/IV non–small-cell lung cancer (NSCLC). Exclusion criteria were memory or communication impairment, the treating clinician’s request, physical inability to walk, or lack of access to a smartphone. The Fitbit Zip device is a popular, wearable accelerometer that clips to clothing and wirelessly synchronizes with smartphones. Fitbit Zip has been validated in COPD patients,17 and its accuracy is similar to other wearable pedometers.18 Fitbit technology has been utilized in 2 recent randomized controlled trials (RCTs) studying activity in postmenopausal women and overweight adults.14,15
This was a prospective, observational study that collected data on daily step count, QoL, functioning domains, depression, and dyspnea. After screening, patients were provided with a handout describing lung cancer symptoms, the dyspnea spiral, the study’s intent, the information to be collected, and contact information for the research team. After informed consent was obtained, 3 previously validated questionnaires were administered: European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire 30 (EORTC QLQ-C30), Patient Health Questionnaire 9 (PHQ-9), and modified Medical Research Council (MMRC) Dyspnea Scale.19-22 The questionnaires included a total of 40 items. The PHQ-9 and MMRC are single-domain questionnaires, with higher scores reflecting more depression or dyspnea, respectively. The EORTC QLQ-30 has multiple domains and assesses global QoL, physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning, and symptoms associated with cancer. Higher functional scores correspond to better functioning, whereas higher symptom scores correspond to worsened symptoms.
The Fitbit Zip was then provided to the patient. The Fitbit application was downloaded to the patient’s smartphone, and a brief tutorial of the device was undertaken. Participants were also given an addressed and postage-paid envelope for return of the device. Finally, patients were instructed to wear the device 24 h/d, to avoid exposing the device to water, to avoid changes to their daily routine, when to mail back the device, and that they might be contacted by the research team at a clinical appointment, by phone, or email. Step count data were collected for 7 consecutive days. Valid days were defined as having ≥200 steps/d collected by the device. Device adherence was defined as step counts collected for ≥5 days. Moy et al23,24 have used similar validity criteria in COPD, with >100 steps/d and 8 hours of wear time defining valid days, and averages calculated on weeks with ≥5 valid days. Wear time was not collected in this study and could not be used as a validity criterion.
Spearman rank correlation coefficients were calculated between average daily step counts, questionnaire domain scores, PHQ-9 scores, and symptom survey domains using SAS v9.4. With n = 30 individuals providing step counts, we were only powered to detect correlations as small as 0.5, assuming 2-sided hypothesis testing and an α level of .05. To not miss potentially clinically significant correlations with the small cohort, we also highlighted positive and negative correlations (more and less correlated) defined as >0.3 or <−0.3, respectively.
Results
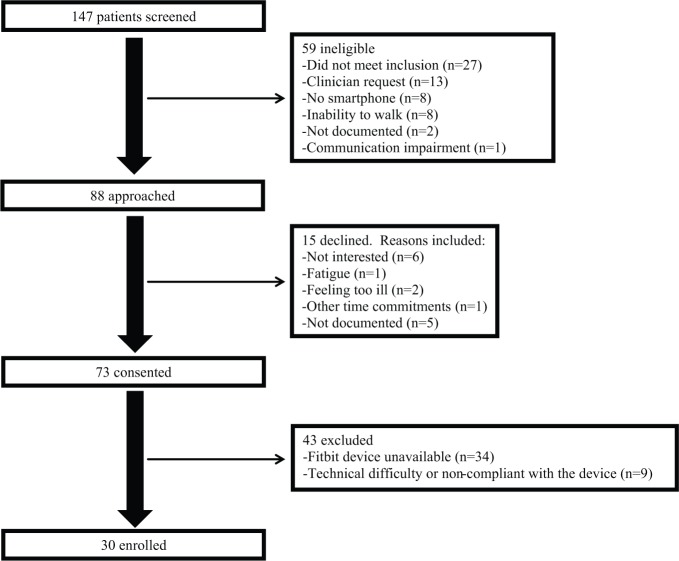
Between February 2014 and August 2015, a total of 88 patients met inclusion criteria and were approached (see Figure 1); 73/88 (83%) consented to participate. Because of consecutive enrollment, participants could be pretreatment, receiving treatment, or have received treatment in the past, Among nonparticipants (n = 15), reasons for declining included no interest (n = 6), fatigue (n = 1), feeling too ill (n = 2), or having other time commitments (n = 1). Five individuals did not provide reasons for not participating. Because of limited device availability, Fitbit accelerometers were unavailable for 34 patients. Fitbit devices were provided to 39 patients, but 9/39 (23%) did not utilize the device. Most patients (30/39, 77%) provided step counts, and 26/39 (67%) were adherent over the 7-day period.
Figure 1.
Inclusion flowchart.
For participants providing step count data (n = 30), 2/3 were male, and the mean age was 66 years (range = 51-80 years; see Table 1). The majority of patients had stage IV NSCLC (70%). Adenocarcinoma was the most common histology (77%). Smaller percentages had stage III NSCLC (20%) or SCLC (10%). The average daily step count (±SD) for the group was 4877 steps/d (±3055), with a wide interpatient range (504-12 118 steps/d).
Table 1.
Demographics.
| Age (years) | Range: 51-80; Mean: 66; SD: 7.75 |
|---|---|
| Gender | 20 (67%) male |
| Lung cancer histology | |
| Adenocarcinoma | 23 (77%) |
| Squamous cell carcinoma | 4 (13%) |
| Small-cell lung cancer (SCLC) | 3 (10%) |
| Stage | |
| IIIA | 4 (13%) |
| IIIB | 2 (7%) |
| IV | 21 (70%) |
| SCLC limited | 1 (3%) |
| SCLC extensive | 2 (7%) |
| Average daily step count (steps/d) | Range: 504-12 118; mean: 4877; SD: 3055 |
| Patients with ≥1 day with missing step counts | 8/30 (27%) |
| Total days with missing step counts | 20/210 (9.5%) |
| Patients with 1 or more days of ≥10 000 steps | 7/30 (23%) |
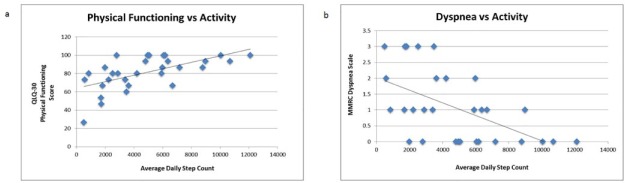
Higher PA (measured via average daily step count) was positively correlated with physical functioning, role functioning, emotional functioning, and global QoL scores (Table 2). Higher PA also correlated with lower dyspnea, depression, and pain scores. The highest correlation coefficients were noted between physical functioning (Spearman coefficient = 0.61) and MMRC Dyspnea Scale (Spearman coefficient −0.54). Linear relationships also were noted between average daily step count, physical functioning, and MMRC dyspnea scale (see Figure 2). Average step counts did not correlate with cognitive or social functioning, fatigue, nausea/vomiting, insomnia, appetite loss, constipation, diarrhea, or financial difficulties.
Table 2.
Spearman Correlation Coefficients Between Survey Domains and Average Daily Step Count.a
| Survey Domain | Spearman Correlation Coefficient |
|---|---|
| PHQ-9: Depression | −0.40 |
| EORTC QLQ-C30: Global health status/QoL | 0.46 |
| EORTC QLQ-C30: Physical functioning | 0.61 |
| EORTC QLQ-C30: Role functioning | 0.48 |
| EORTC QLQ-C30: Emotional functioning | 0.40 |
| EORTC QLQ-C30: Cognitive function | 0.12 |
| EORTC QLQ-C30: Social functioning | 0.20 |
| EORTC QLQ-C30: Fatigue | −0.12 |
| EORTC QLQ-C30: Nausea/Vomiting | −0.32 |
| EORTC QLQ-C30: Pain | −0.37 |
| EORTC QLQ-C30: Dyspnea | −0.44 |
| EORTC QLQ-C30: Sleep | 0.02 |
| EORTC QLQ-C30: Anorexia | −0.24 |
| EORTC QLQ-C30: Constipation | −0.17 |
| EORTC QLQ-C30: Diarrhea | 0.06 |
| EORTC QLQ-C30: Financial difficulty | −0.08 |
| MMRC: Dyspnea Scale: Description of breathlessness | −0.54 |
Abbreviations: PHQ, Patient Health Questionnaire-9; QoL, Quality of Life; EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Quesionnaire-30; MMRC, Modified Medical Research Council.
All bolded correlations are statistically significant (P < .05).
Figure 2.
A. European Organisation for the Research and Treatment of Cancer, Quality of Life Questionnaire (QLQ) 30 versus average daily step count. B. Modified Medical Research Council (MMRC) Dyspnea score versus average daily step count.
Discussion
This study in advanced-stage lung cancer has 3 main findings. First, monitoring step count with a wearable accelerometer in outpatients with advanced-stage lung cancer is feasible. Second, though advanced-stage lung cancer patients are often not considered candidates for PA, most patients in this cohort were interested in participating in low-impact PA and provided usable step counts. Third, in this cohort, higher step counts significantly correlate with higher QoL, physical functioning, role functioning, and emotional functioning as well as lower dyspnea, pain, and depression scores.
Research is accumulating that increased PA benefits cancer patients and survivors. The associations of higher PA with reduced cancer incidence,25 improved all-cause and cancer-specific mortality,26 and symptom improvement in advanced cancer27,28 underscore the role of exercise as an adjunctive therapy for lung cancer patients and survivors. Inactivity, by contrast, is a predictor of poor postoperative outcomes and worsened survival in lung cancer patients.6 Because the American College of Sports Medicine Roundtable recommends that cancer survivors avoid inactivity,29 clinicians should consider PA regimens in patients with lung cancer and survivors as a potential mechanism to reduce symptoms and improve QoL.
Though existing data are encouraging in that low-impact PA benefits advanced-stage lung cancer patients, there is no consensus on how to monitor PA, what intensity to recommend, or when to stop. Because patients with advanced-stage disease have the most frequent, intense, and refractory symptoms, this population may have the most opportunity for future interventions to improve QoL. But before recommendations can be made, feasibility of data collection needs to be established. Therefore, this study chose to evaluate the feasibility of remote step count data collection in patients with advanced-stage lung cancer.
The role of PA in advanced-stage lung cancer is unclear and understudied. In the recent review by Bade et al9 of PA in lung cancer, twice as many studies were available for lung cancer patients undergoing surgery compared with nonoperative patients. A common theme among patients and clinicians is that lung cancer patients are “too sick” to exercise. Our findings that patients with advanced-stage lung cancer are interested in activity monitoring and able to provide usable data supports further study into activity monitoring in the population. Interestingly, at least 2 prior studies suggest that sicker lung cancer patients may obtain more benefit from increasing their activity. Mujovic et al30 reported that pulmonary rehabilitation (PR) in preoperative NSCLC patients showed the greatest spirometric and 6-Minute Walk Distance improvements in those with the worst preoperative values. Similarly, Edvardsen et al31 studied 61 patients after undergoing lung cancer surgery and found that those with the worst preoperative oxygen uptake tended to have the most improvement. Thus, it is possible that patients with advanced-stage lung cancer may obtain more benefit from PA regimens than patients with early-stage disease, though maintaining activity adherence may be more challenging. Because Jensen et al32 showed that >90% of patients with advanced cancer can perform some type of exercise or physical therapy, clinicians should not exclude PA as a potential treatment for patients with advanced disease.
Five studies have addressed PA in patients with advanced-stage lung cancer. These studies have shown that increasing PA is feasible33 and improves mobility, fatigue, sleep, pain, strength, dyspnea, anxiety, depression, and QoL,27,28,34,35 though adherence may be <50%.33 Because most patients with lung disease can perform some type of exercise with variable compliance, the potential for digital accelerometers to remotely monitor low-impact PA is appealing. Other authors have implemented walking regimens with digital accelerometers and reported varying compliance. For example, Moy et al24 recently published a RCT of veterans with COPD who were provided pedometers and monitored for 1 year. The intervention group had valid step counts available for 76.7% of days. In contrast, in patients with stage I to IIIB NSCLC, Granger et al36 reported PA data for 56% of patients using an accelerometer. Thus, our results showing 77% of patients providing usable step count data and 67% device adherence are at least comparable to prior studies.
For several reasons, home step count monitoring may be a feasible alternative for patients with advanced disease to monitor and potentially increase their activity. A home-based, self-directed, low-intensity activity regimen may increase activity adherence in lung cancer patients. Though a prior Cochrane review suggests that center-based activity programs are superior to home-based programs in patients ≥50 years old,37 lung cancer patients may be more limited by their symptoms and benefit from a self-directed regimen. A recent study by Cadmus-Bertram et al14 found that a Fitbit-based intervention increased PA in postmenopausal women, suggesting that popular devices provide additional motivation that likely improve adherence to a home-based regimen. Second, cost is a prohibitive factor for some patients. Gym membership, PR fees, and the cost of traveling several times per week may be deterrents to regular activity. The low-cost Fitbit device that provides objective and trendable data (rather than self-reporting) is, therefore, appealing. Finally, using step counting to increase activity both prioritizes the patient’s schedule and increases patient autonomy. An interesting RCT by Brocki et al38 in 2014 showed no advantage to supervised versus unsupervised outpatient exercise sessions, and there was a trend toward improved outcomes in unsupervised sessions.
It is worth noting that the mechanisms of how PA improves outcomes in lung cancer are unclear. There are several studies showing evidence of altered immune response with increased PA or exercise training.39-41 Regarding symptomatic and spirometric benefit, it seems likely that increasing activity may minimize loss of function by interrupting the dyspnea spiral, which is usually debilitating and progressive. Because of the high prevalence of COPD in lung cancer patients,42 the mechanisms of improvement caused by activity could be optimized COPD management, a lung cancer-mediated mechanism, or both. More work is needed on this topic.
Because this study demonstrated feasibility and identified several clinically interesting correlations, further study is warranted. Though this study’s correlations showing improved QoL, less depression, and reduced symptoms with increased PA are intriguing (see Figure 2), causality cannot be determined. It is possible that patients who feel better at baseline are more likely to be physically active, rather than increased PA improving their QoL. The feasibility of implementing a step counting regimen in advanced-stage lung cancer patients might be followed by a randomized trial. Such studies would help determine if QoL improves with PA implementation; if adherence is affected by a home-based, low-impact regimen; and the causality between PA, symptom burden, and QoL.
This study has several strengths. First, home monitoring with Fitbit devices in advanced-stage lung cancer is unique in its approach. Second, our approach utilizes an approach that is patient centric. That is, whereas most regimens require patient travel, brief hospitalization, or an interruption in the patient’s life, home-based activity allows data gathering (and potential therapeutic measures) to proceed without interruption in day-to-day life. Third, home-based monitoring motivates and empowers patients to implement an activity regimen on their own terms and at low cost. The attractiveness to patients and ease of use are emphasized by high willingness to participate (83% of eligible patients).
There are several limitations to our study. First, our cohort is small, which could induce bias into our results. Second, this cohort is highly selected. That is, this study is in patients with advanced-stage lung cancer, attending clinic at MUSC, and motivated to participate in an activity study and may not be applicable to the general lung cancer population. Larger future studies would address both these issues. Third, causality cannot be proven by these correlations. Although it is tempting to assume that increased PA leads to improved symptoms, QoL, and depression, this data set can only show that higher step counts are positively correlated with QoL and negatively correlated with symptom and depression scores. Whereas increasing PA may improve outcomes, it is also possible that less-symptomatic patients are able to be more active. Confounding variables may include age, disease burden, preexisting lung disease, and preexisting activity levels. Finally, our patients were gathered via a convenience sample and were in all stages of their therapy (ie, at diagnosis, during chemoradiation, and posttreatment). Ideally, studies would focus enrollment at diagnosis or prior to cancer therapy because PA before lung cancer surgery has shown potentially more improvement than interventions after surgery.43,44
To our knowledge, this is the first study to show feasibility of home-based step count monitoring using a Fitbit as a measure of PA in patients with advanced-stage lung cancer. Prior studies to date have enrolled patients with all stages of lung cancer and patients with metastatic disease of multiple tissue types, utilized inpatient or PR regimens, or measured self-reported PA. This study focused enrollment on the sickest patients and an approach that may improve activity compliance. Given the baseline debility of patients with advanced-stage lung cancer, it is surprising that 83% of this cohort was interested in participating in an exercise study. The high interest of patients with advanced disease to participate in an activity study should challenge the conventional wisdom that patients with advanced disease should not be considered candidates for exercise therapies.
Conclusions
In summary, this is the first study we are aware of that shows feasibility of using a Fitbit accelerometer to measure step counts in advanced-stage lung cancer patients. Even in our small cohort, the high percentage of patient participation is encouraging and shows that PA monitoring is feasible. Finally, correlations showing high QoL, low depression, and low symptom scores in patients who are more active support further study. Future studies should assess feasibility and clinical response to a home-based exercise prescription. A larger, randomized trial would also better clarify the amount of improvement attributable to exercise and which patients may obtain the most benefit. Further research into the correlation between depression, symptoms, and QoL is needed because the treatment of depression may be an intervention that improves QoL and optimizes exercise compliance. At a time when we assess every advanced lung cancer patient for targeted cancer therapies of which a minority will be eligible, we should consider that “targeting” inactivity to improve symptoms could be recommended for all.
Footnotes
Authors’ Note: BCB, SBN, DDT, PJN, and GAS contributed to study design, data collection, interpretation, and writing of the manuscript. MCB, AU, and JBS contributed to data collection and writing of the manuscript. Critical manuscript revision was performed by BCB, GAS, and PJN. All authors approved this version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded, in part, by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program (NCATS Grant #UL1TR001450). This work was also supported by the South Carolina Clinical and Translational Research Institute with an academic home at the Medical University of South Carolina (NIH/NCATS Grant Number UL1TR000062). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1. The American Cancer Society. Cancer facts and figures 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed December 1, 2016.
- 2. Simone CB, II, Vapiwala N, Hampshire MK, Metz JM. Palliative care in the management of lung cancer: analgesic utilization and barriers to optimal pain management. J Opioid Manag. 2012;8:9-16. [DOI] [PubMed] [Google Scholar]
- 3. Mosher CE, Ott MA, Hanna N, Jalal SI, Champion VL. Coping with physical and psychological symptoms: a qualitative study of advanced lung cancer patients and their family caregivers. Support Care Cancer. 2015;23:2053-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Granger CL, McDonald CF, Irving L, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer. 2014;83:292-299. [DOI] [PubMed] [Google Scholar]
- 5. Koczywas M, Cristea M, Thomas J, et al. Interdisciplinary palliative care intervention in metastatic non-small-cell lung cancer. Clin Lung Cancer. 2013;14:736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rochester CL, Fairburn C, Crouch RH. Pulmonary rehabilitation for respiratory disorders other than chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:369-389. [DOI] [PubMed] [Google Scholar]
- 7. Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr Physiol. 2012;2:2775-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331-342. [DOI] [PubMed] [Google Scholar]
- 9. Bade BC, Thomas DD, Scott JB, Silvestri GA. Increasing physical activity and exercise in lung cancer: reviewing safety, benefits, and application. J Thorac Oncol. 2015;10:861-871. [DOI] [PubMed] [Google Scholar]
- 10. Granger CL, Chao C, McDonald CF, Berney S, Denehy L. Safety and feasibility of an exercise intervention for patients following lung resection: a pilot randomized controlled trial. Integr Cancer Ther. 2013;12:213-224. [DOI] [PubMed] [Google Scholar]
- 11. Quist M, Rorth M, Langer S, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. 2012;75:203-208. [DOI] [PubMed] [Google Scholar]
- 12. Cheville AL, Dose AM, Basford JR, Rhudy LM. Insights into the reluctance of patients with late-stage cancer to adopt exercise as a means to reduce their symptoms and improve their function. J Pain Symptom Manage. 2012;44:84-94. [DOI] [PubMed] [Google Scholar]
- 13. Leach HJ, Devonish JA, Bebb DG, Krenz KA, Culos-Reed SN. Exercise preferences, levels and quality of life in lung cancer survivors. Support Care Cancer. 2015;23:3239-3247. [DOI] [PubMed] [Google Scholar]
- 14. Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL. Randomized trial of a fitbit-based physical activity intervention for women. Am J Prev Med. 2015;49:414-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang JB, Cadmus-Bertram LA, Natarajan L, et al. Wearable sensor/device (Fitbit One) and SMS text-messaging prompts to increase physical activity in overweight and obese adults: a randomized controlled trial. Telemed J E Health. 2015;21:782-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bade BC, Nietert SB, Scott J, et al. Higher physical activity is correlated with better quality of life in lung cancer [abstract]. Am J Respir Crit Care Med. 2015;191:A5076. [Google Scholar]
- 17. Vooijs M, Alpay LL, Snoeck-Stroband JB, et al. Validity and usability of low-cost accelerometers for internet-based self-monitoring of physical activity in patients with chronic obstructive pulmonary disease. Interact J Med Res. 2014;3(4):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313:625-626. [DOI] [PubMed] [Google Scholar]
- 19. Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37:1345-1351. [DOI] [PubMed] [Google Scholar]
- 20. Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer. 2001;37:1331-1334. [DOI] [PubMed] [Google Scholar]
- 21. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farncombe M. Dyspnea: assessment and treatment. Support Care Cancer. 1997;5:94-99. [DOI] [PubMed] [Google Scholar]
- 23. Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest. 2015;148:128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moy ML, Martinez CH, Kadri R, et al. Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res. 2018;18(8):e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laukkanen JA, Pukkala E, Rauramaa R, Makikallio TH, Toriola AT, Kurl S. Cardiorespiratory fitness, lifestyle factors and cancer risk and mortality in Finnish men. Eur J Cancer. 2010;46:355-363. [DOI] [PubMed] [Google Scholar]
- 26. Arem H, Moore SC, Park Y, et al. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer. 2014;135:423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henke CC, Cabri J, Fricke L, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22:95-101. [DOI] [PubMed] [Google Scholar]
- 28. Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45:811-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426. [DOI] [PubMed] [Google Scholar]
- 30. Mujovic N, Subotic D, Marinkovic M, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10:68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edvardsen E, Skjonsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax. 2015;70:244-250. [DOI] [PubMed] [Google Scholar]
- 32. Jensen W, Bialy L, Ketels G, Baumann FT, Bokemeyer C, Oechsle K. Physical exercise and therapy in terminally ill cancer patients: a retrospective feasibility analysis. Support Care Cancer. 2014;22:1261-1268. [DOI] [PubMed] [Google Scholar]
- 33. Temel JS, Greer JA, Goldberg S, et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer. 2015;112:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jastrzebski D, Maksymiak M, Kostorz S, et al. Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy. Adv Exp Med Biol. 2015;861:57-64. [DOI] [PubMed] [Google Scholar]
- 36. Granger CL, Denehy L, McDonald CF, Irving L, Clark RA. Physical activity measured using global positioning system tracking in non-small cell lung cancer: an observational study. Integr Cancer Ther. 2014;13:482-492. [DOI] [PubMed] [Google Scholar]
- 37. Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005;(1):CD004017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brocki BC, Andreasen J, Nielsen LR, Nekrasas V, Gorst-Rasmussen A, Westerdahl E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery: a randomized controlled trial. Lung Cancer. 2014;83:102-108. [DOI] [PubMed] [Google Scholar]
- 39. Woods JA, Davis JM, Kohut ML, Ghaffar A, Mayer EP, Pate RR. Effects of exercise on the immune response to cancer. Med Sci Sports Exerc. 1994;26:1109-1115. [PubMed] [Google Scholar]
- 40. Wang R, Liu J, Chen P, Yu D. Regular tai chi exercise decreases the percentage of type 2 cytokine-producing cells in postsurgical non-small cell lung cancer survivors. Cancer Nurs. 2013;36:E27-E34. [DOI] [PubMed] [Google Scholar]
- 41. Karvinen KH, Esposito D, Raedeke TD, Vick J, Walker PR. Effect of an exercise training intervention with resistance bands on blood cell counts during chemotherapy for lung cancer: a pilot randomized controlled trial. Springerplus. 2014;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129:1305-1312. [DOI] [PubMed] [Google Scholar]
- 43. Rodriguez-Larrad A, Lascurain-Aguirrebena I, Abecia-Inchaurregui LC, Seco J. Perioperative physiotherapy in patients undergoing lung cancer resection. Interact Cardiovasc Thorac Surg. 2014;19:269-281. [DOI] [PubMed] [Google Scholar]
- 44. Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2011;72:139-153. [DOI] [PubMed] [Google Scholar]