Abstract
T-cell receptor (TCR)-pMHC affinity has been generally accepted to be the most important factor dictating antigen recognition in gene-modified T-cells. As such, there is great interest in optimizing TCR-based immunotherapies by enhancing TCR affinity to augment the therapeutic benefit of TCR gene-modified T-cells in cancer patients. However, recent clinical trials using affinity-enhanced TCRs in adoptive cell transfer (ACT) have observed unintended and serious adverse events, including death, attributed to unpredicted off-tumor or off-target cross-reactivity. It is critical to re-evaluate the importance of other biophysical, structural, or cellular factors that drive the reactivity of TCR gene-modified T-cells. Using a model for altered antigen recognition, we determined how TCR–pMHC affinity influenced the reactivity of hepatitis C virus (HCV) TCR gene-modified T-cells against a panel of naturally occurring HCV peptides and HCV-expressing tumor targets. The impact of other factors, such as TCR–pMHC stabilization and signaling contributions by the CD8 co-receptor, as well as antigen and TCR density were also evaluated. We found that changes in TCR–pMHC affinity did not always predict or dictate IFNγ release or degranulation by TCR gene-modified T-cells, suggesting that less emphasis might need to be placed on TCR–pMHC affinity as a means of predicting or augmenting the therapeutic potential of TCR gene-modified T-cells used in ACT. A more complete understanding of antigen recognition by gene-modified T-cells and a more rational approach to improve the design and implementation of novel TCR-based immunotherapies is necessary to enhance efficacy and maximize safety in patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2032-9) contains supplementary material, which is available to authorized users.
Keywords: T-cell, T-cell receptor (TCR), Gene-modified T-cells, Adoptive cell therapy, Affinity, Altered peptide ligands
Introduction
Adoptive cell transfer (ACT) of T-cell receptor (TCR) gene-modified T-cells has had mixed, but promising clinical results as a cancer immunotherapy [1]. To enhance the efficacy/safety of TCR gene-modified T-cells, the field should continually refine the fundamental understanding of antigen recognition, which is generally thought to be dictated by the affinity and specificity of the interaction between TCR and peptide-major histocompatibility complex proteins (pMHC) [2]. We and others have predicted that T-cells engineered with high-affinity TCRs would create better effectors than those engineered with lower affinity TCRs by enhancing their sensitivity [3–5] and generating a novel population of MHC class-I-restricted CD4+ T-cells [5–7]. However, identifying naturally occurring, high-affinity, tumor-reactive TCRs is challenging as thymic selection generally excludes high-affinity TCRs against self-antigens [8]. Efforts to enhance TCR binding to pMHC have relied on yeast display, phage display, and computational design [9–11], which many believe will translate into improved antigen recognition [12–14].
Despite the emphasis on using high-affinity TCRs, we and others have suggested that enhancing TCR–pMHC affinity may not necessarily engineer a better T-cell [15–17]. For example, a TCR transgenic mouse model showed that TCRs with the same affinity possess distinctly different activation requirements [15]. Additionally, sister T-cell clones or progeny of individually isolated clones displayed a range of avidities for antigen [16, 17]. In the clinic, high-affinity TCR-engineered T-cells induced serious on- and off-target adverse events including inflammatory colitis [18], neurological toxicity [19], and death by cardiogenic shock [20, 21]. Collectively, these studies provide evidence that TCR–pMHC affinity may not necessarily dictate the quality of the biological response and caution against the use of high-affinity TCRs in ACT. A refined understanding of the fundamentals governing antigen recognition by TCR gene-modified T-cells may help lay the groundwork for developing safer and more effective strategies.
Recent studies by our group provide a model to address the importance of TCR–pMHC affinity on T-cell function. We reported on an HLA-A2-restricted hepatitis C virus (HCV)-reactive TCR (HCV1406 TCR) that was not only therapeutic in an HCV-associated hepatocellular carcinoma model [22], but was also cross-reactive against naturally occurring variants of the antigenic/mutagenic HCV epitope NS3:1406-1415 [23]. Structural modeling of the TCR–pMHC interface helped justify altered recognition, but did not identify biophysical/cellular mechanism(s) responsible for altered T-cell function. Thus, this TCR and set of altered pMHC ligands provide a framework for examining which parameters beyond affinity influence antigen recognition by TCR gene-modified T-cells.
Surprisingly, we found that changes in TCR–pMHC affinity did not necessarily correlate with altered ligand recognition. Rather, signaling contributions by the CD8 co-receptor as well as changes in both TCR and ligand densities played critical roles, sometimes independent of TCR–pMHC affinity. Together, these data challenge the hypotheses that TCR–pMHC affinity is the driving factor behind antigen recognition or that enhancing TCR affinity will augment function of TCR gene-modified T-cells. These data also encourage TCR-transduced T-cells to be individually evaluated for their reliance on CD8, ability to recognize low and high levels of antigen, and the level of transgene expression required to maximize efficacy and safety of these novel immunotherapies.
Materials and methods
Cell lines
293GP, PG13, T2, HepG2, and Jurkat cell lines were obtained from the American Type Culture Collection (Rockford, MD, USA) and maintained as previously described [22, 23]. Generation of CD8+ Jurkat cell lines was previously described using a modified SAMEN retroviral vector containing either full length human CD8 α and β (CD8αβ) or chains lacking the intracellular Lck-binding domains (CD8α′β′), separated by an internal SRα promoter [24]. HCV+ HepG2 cell lines were generated using pMFG retroviral vectors containing wildtype (WT) and mutant HCV NS3:1406-1415 minigenes linked to eGFP by a P2A linker, previously described [22].
T-cells
Apheresis products of normal donors were purchased from Key Biologics (Memphis, TN, USA). Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO, USA) density gradient centrifugation was used to isolate PBMCs. PBMCs were stimulated with 50 ng/mL anti-CD3 mAb (Miltenyi Biotec, Bergisch Gladbach, Germany) for 3 days in AIM-V medium (Life Technologies, Carlsbad, CA, USA) supplemented with 5% heat-inactivated pooled human AB serum (hAB; Valley Biomedical, Inc., Winchester, VA, USA), 300 IU/mL recombinant human IL-2 (Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA), and 100 ng/mL recombinant human IL-15 (Biological Resources Branch, National Cancer Institute, Bethesda, MD, USA).
Retroviral transduction
Retroviral supernatants were prepared using a stable, high-titer retroviral producer cell line PG13 expressing HCV1406 TCR in a modified SAMEN retroviral vector containing the TCR α chain linked to β chain and a truncated CD34 molecule by a P2A or T2A self-cleaving sequence, respectively. Generation of producer cell lines, collection of retrovirus, and TCR-transduction by spinoculation have been described elsewhere [22, 23]. Cultures were enriched for high/uniform transgene expression by positive selection using anti-CD34 mAb-coated immunomagnetic beads (Miltenyi Biotec) [22, 25]. T-cells were also sorted into CD4+ or CD8+ fractions by immunomagnetic selection.
Peptides
Rationale for and generation of naturally occurring mutant HCV epitopes was previously described [23]. Peptides used as stimulators in functional assays were obtained at 95% purity from Synthetic Biomolecules (San Diego, CA, USA).
Cytokine-release assay
Targets (T2 or HepG2 cells) were pulsed with peptide (concentrations ranging 10–0.0001 µg/mL) for 2 h and co-cultured in 1:1 ratio with effectors (TCR-transduced T-cells or Jurkat cells) in 96-well U-bottom tissue-culture plates [22, 23]. Ten ng/mL phorbol 12-myristate 13-acetate (Sigma-Aldrich, St. Louis, MO) was added to Jurkat cell co-cultures to enhance sensitivity of stimulation [23]. Co-cultures were incubated at 37 °C for 18 h, supernatants harvested, and the amount of IFNγ or IL-2 released by 1x105 T-cells or Jurkat cells, respectively, was measured by ELISA (R&D Systems, Minneapolis, MN). We define “reactive” effectors as producing >200 pg/mL cytokine and at least twice above background (tyrosinase:368–376 stimulation). When comparing variant ligand to WT, “reactive” ligands must also exceed 5% of WT-stimulated cytokine magnitude.
Degranulation CD107a assay
3 × 105 target and effector cells were co-cultured in a 1:1 ratio in a 96-well U-bottom tissue-culture plate with 5.0 ng/mL brefeldin-A, 2.0 nM monensin, and 250 ng anti-CD107a-Brilliant Violet 510 mAb (Biolegend, San Diego, CA). Co-cultures were incubated at 37 °C for 5 h. Cells were stained for surface markers (anti-CD3-APC/Cy7, anti-CD4-PE/Cy7, anti-CD8-PerCP/Cy5.5, anti-CD34-PE mAbs; Biolegend). Data were acquired using an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA, USA).
Intracellular cytokine detection
3 × 105 of target and effector cells were co-cultured in a 1:1 ratio in a 96-well U-bottom tissue-culture plate with 5.0 ng/mL brefeldin-A and 2.0 nM monensin (Biolegend). Co-cultures were incubated at 37 °C for 5 h. Cells were stained for surface markers above, fixed, permeabilized, and counterstained for intracellular IFNγ (anti-IFNγ-Brilliant Violet 421 mAb) according to manufacturer’s protocols (Biolegend). Data were acquired using an LSRFortessa flow cytometer.
Protein
For binding studies, recombinant HLA-A*0201 heavy chain and β-2 microglobulin were expressed as inclusion bodies in Escherichia coli [26]. MHC folding and assembly from inclusion bodies was performed according to standard procedures [27]. Protein was purified using ion-exchange followed by size-exclusion chromatography.
Binding studies
TCR–pMHC binding affinity between HCV1406 TCR and WT or mutant HCV NS3:1406-1415/HLA*0201 was measured via surface plasmon resonance (SPR) using a Biacore 3000 instrument (GE Healthcare) in 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20 (pH 7.4) as previously described [28, 29]. TCR was covalently coupled to a CM5 sensor chip via standard amine coupling. Equilibrium experiments were performed at 25 °C, injecting 70 µL of pMHC complex (concentrations ranging from 0.5 µM to 200 µM) at a flow of 5 µL/min. Responses at equilibrium were determined by averaging signal over the final 10 s of the injection and subtracting responses from identical injections over a mock surface. Injections were repeated three times. Measurements of binding kinetics used a flow rate of 100 µL/min and injected pMHC concentrations up to 100 µM. Dissociation rates were calculated using single exponential fits to baseline subtracted dissociation phases. Association rates were calculated using dissociation rates and independently measured K D values. Data analysis was performed with Biaevaluation 4 using a 1:1 binding model.
Statistical analysis
Comparisons of mean cytokine release or CD107a expression were performed using two-sample t-tests with unequal variances. Dose response relationships between peptide concentration and cytokine release were estimated using four parameter logistic models in the R library ‘drc.’ Half maximal effective concentration (EC50) estimates were extracted from fitted model results.
Results
TCR–pMHC affinity does not necessarily dictate antigen recognition
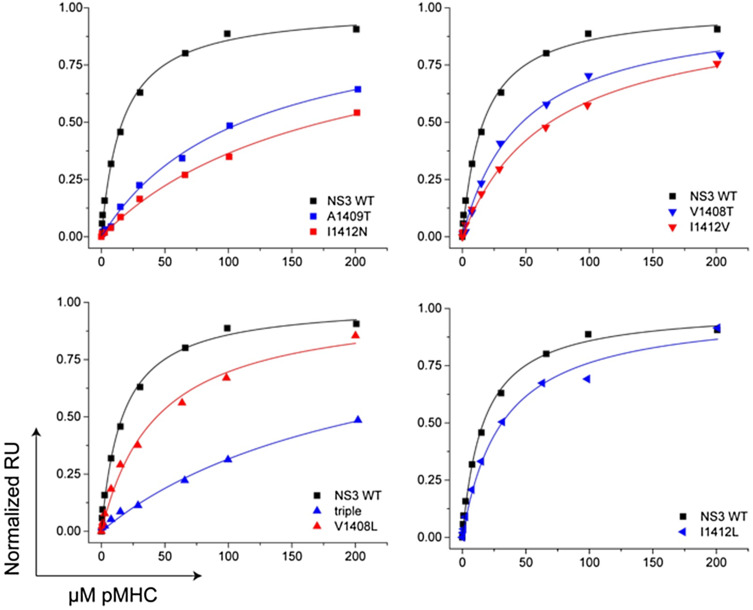
We previously reported HCV1406 TCR gene-modified T-cells recognized wildtype (WT) HCV NS3:1406–1415 peptide-loaded targets and multiple naturally occurring mutant variants [23]. Many investigators believe that the most critical feature of a productive TCR–pMHC interaction is the affinity [2], which may be the determining factor for functional recognition. Our antigen recognition model enables us to examine how TCR–pMHC interactions with varying affinities impact function of TCR gene-modified T-cells. Using surface plasmon resonance (SPR), we measured the K D and ΔG values for the HCV1406 TCR binding a panel of NS3:1406–1415 variants bound to HLA-A2. (Fig. 1; Table 1). We also measured binding kinetics, as these have been the emphasis of previous studies [30, 31].
Fig. 1.
Surface plasmon resonance measurements of the affinity of the HCV1406 TCR for variant NS3 peptides presented by HLA-A2. Binding was measured to TCR-coated sensor surfaces by injecting increasing concentrations of pMHC. Data are shown in separate panels to facilitate comparisons; the wildtype data set (black line and black symbols) are replicated in each panel for clarity. For all measurements, data are representative of at least three separate experiments. For all mutants, data were fit using global analysis with the sensor surface as a shared parameter
Table 1.
Naturally occurring HCV NS3:1406-1415 variant peptides and their pMHC binding affinities to HCV1406 TCR
| Epitope | Sequence | K D (µM)a | ΔG (kcal/mol)b |
|---|---|---|---|
| WT | KLVALGINAV | 16.8 ± 0.3 | −6.51 ± 0.02 |
| I1412Lc | KLVALGLNAV | 32.4 ± 0.7 | −6.12 ± 0.02 |
| V1408Tc | KLTALGINAV | 45.9 ± 0.6 | −5.92 ± 0.01 |
| V1408Lc | KLLALGINAV | 60.1 ± 5.2 | −5.76 ± 0.09 |
| I1412Vc | KLVALGVNAV | 63.4 ± 3.0 | −5.73 ± 0.05 |
| A1409Td | KLVTLGINAV | 119.7 ± 9.2 | −5.35 ± 0.08 |
| I1412Nd | KLVALGNNAV | 168.0 ± 17.6 | −5.15 ± 0.11 |
| V1408S/A1409G/I1412Ld | KLSGLGLNAV | 169.3 ± 23.4 | −5.15 ± 0.13 |
| V1408S/A1409S/I1412L/A1414Sd | KLSSLGLNSV | NBD | NBD |
NBD no binding detected
aAverage K D of three independent experiments ± standard error
bΔG = RT·ln(K D)
c“Moderate affinity” interaction
d“Lower affinity” interactions
Although HCV1406 TCR performs functionally as a high-affinity TCR exhibiting CD8-independent target recognition [22, 32], the affinity of HCV1406 TCR–WT pMHC interaction, while strong (K D = 17 μM), falls short of naturally occurring, high-affinity TCRs (<10 µM) [33]. TCR–pMHC interactions between variants I1412L (32 μM), V1408T (46 μM), V1408L (60 μM), and I1412V (63 μM) exhibited affinities within a twofold range, which we categorized as “moderate affinity” compared to WT. TCR–pMHC interactions containing variants A1409T (120 μM), I1412N (168 μM), and 8S/9G/12L (169 μM) were approximately one log-fold weaker than WT, a range we called “low affinity.” Although the TCR-8S/9S/12L/14S–MHC interaction could not be measured by SPR, it is reasonable to predict that it falls into the “low affinity” range based on similar amino acid substitutions and subsequent functional studies. Taken together, we have established three ranges of TCR–pMHC affinities for comparing recognition of naturally occurring, related ligands by TCR-engineered T-cells. TCR dissociation rates clustered around the WT value of 0.09 s−1, with only a single variant possessing a dissociation rate more than twofold different than WT (Supplementary Table S1).
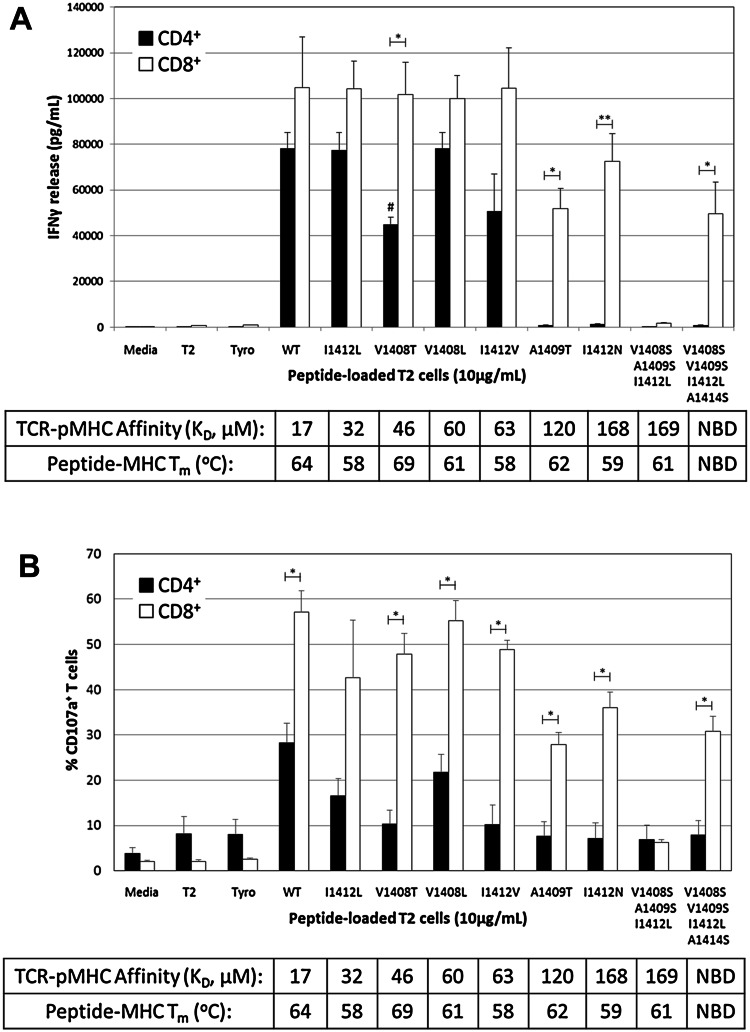
Using a Jurkat system, we have previously shown that recognition of some but not all mutant HCV NS3:1406-1415 peptides required the CD8 co-receptor [23]. To complement these findings, we evaluated whether changes in TCR–pMHC affinity correlated with CD8-dependence in primary T-cells. Interestingly, CD8+ and CD4+ T-cell patterns of IFNγ secretion (Fig. 2a) and CD107a expression (marker for degranulation; Fig. 2b) were not always directly related to TCR–pMHC affinity. CD8+ TCR-transduced T-cells secreted IFNγ and degranulated against all HCV peptide-loaded T2 cells except for mutant 8S/9S/12L. Despite the broad range of affinities among altered ligands, IFNγ release by CD8+ T-cells was not significantly different from WT, and only A1409T and 8S/9S/12L/14S stimulated significantly fewer CD107a+ CD8+ T-cells. Surprisingly, variant 8S/9S/12L exhibited nearly identical TCR–pMHC affinity as mutant I1412N (169 versus 168 μM) but did not stimulate IFNγ secretion or degranulation, suggesting that the affinity of the TCR–pMHC interaction does not solely dictate antigen recognition. It may be notable that variant 8S/9S/12L showed faster dissociation kinetics than I1412N (k off = 0.20 s−1 for 8S/9S/12L vs. 0.09 s−1 for I412N; Supplementary Table S1); however, this apparent correlation did not persist in other experiments.
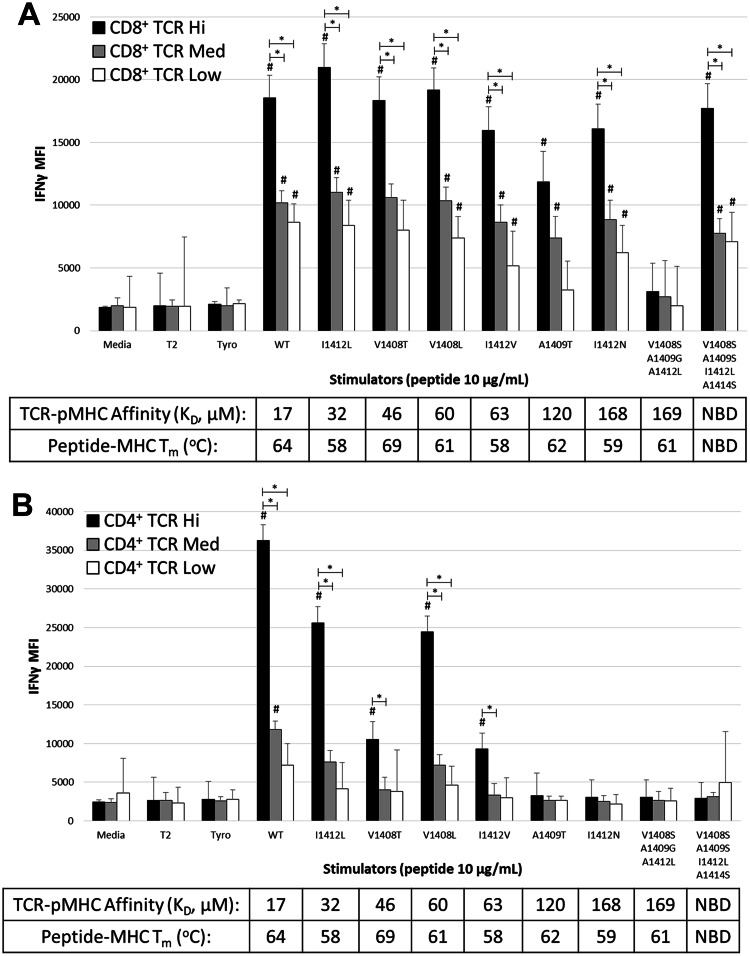
Fig. 2.
Recognition of altered TCR–pMHC interactions is not entirely explained by changes in TCR–pMHC affinity. PBL from a normal donor were transduced with the HCV1406 TCR retroviral vector. TCR-transduced cells were enriched for CD34 expression and separated into CD4+ (black bars) and CD8+ (white bars) populations using immunomagnetic beads. T2 cells were pulsed with 10 µg/mL of WT or mutant HCV NS3:1406–1415 peptides or tyrosinase:368-376 as a control and co-culture with T-cells for 18 h. a IFNγ secretion was measured by ELISA. Mean and standard deviation of triplicate measurements of a single experiment are shown. b Degranulation of T-cells was measured by surface CD107a expression. Mean and standard deviation of three experiments for a single donor are shown. Cultures are considered “antigen-reactive” if they secrete at least 200 pg/mL IFNγ, twice above background and at least >5% of WT-stimulated cytokine release. Significant differences in IFNγ release between CD4+ and CD8+ T-cells are shown, *p < 0.05, **p < 0.01. V1408T-stimulated IFNγ release by CD4+ T-cells is significantly lower than WT, I1412L, or V1408L stimulations, # p < 0.05. SPR-measured affinities of TCR–pMHC interactions are shown, and thermal stability of peptide-MHC interactions is shown. NBD, no binding detected. Data are representative of three independent experiments with three PBL donors
Peptide reactivity by TCR-transduced CD4+ T-cells clustered more predictably, recognizing WT and all “moderate affinity” ligands, but not “low affinity” ligands suggesting that HCV1406 TCR’s threshold for CD8 dependence is between 63 and 120 μM. However, significant differences in IFNγ release were seen between ligands despite similar affinities. For example, V1408T (45 μM) stimulated significantly less IFNγ secretion compared to WT (17 μM), I1412L (32 μM), and V1408L (60 μM) (p < 0.05). I1412V (63 μM) also reproducibly stimulated a less robust response, but was not significantly different in the experiment shown here. Degranulation against I1412V and V1408T was also blunted compared to WT, I1412L, and V1408L but these comparisons did not reach statistical significance. These functional differences were also not easily attributable to differences in TCR–pMHC binding kinetics. The clearest example is V1408L and I1412V, which, despite significant functional differences, had nearly identical binding kinetics and affinities (Table 1 and Supplementary Table S1).
Thermal stability (T m values) of the peptide-MHC interactions (which we have previously reported [23]) was all within a range typically considered to reflect high-affinity peptide binding and failed to reconcile diminished (or absent) antigen recognition. Specifically, the T m of 8S/9S/12L-HLA-A2 (61 °C) was actually slightly higher than that of I1412N-HLA-A2 (59 °C), suggesting that decreased peptide-MHC stability was not the cause of the inability to recognize 8S/9S/12L despite exhibiting the same TCR–pMHC affinity as I1412N. Similarly, V1408T had a slightly higher T m (69 °C) than both WT (64 °C) and V1408L (61 °C) but stimulated significantly lower IFNγ release by CD4+ T-cells.
Altogether, these data suggest that TCR–pMHC affinity is not necessarily a singular defining characteristic dictating antigen recognition. With that in mind, we explored how other biological parameters influence the recognition of altered pMHC ligands.
CD8-dependent antigen recognition relies on Lck-binding
Although we observed a relationship between CD8-dependence and TCR–pMHC affinity, it was uncertain how CD8 improved recognition of altered ligands. The CD8 co-receptor is known to stabilize the TCR–pMHC complex, enhancing relative affinity [34], which might explain differences in recognition patterns between CD4+ and CD8+ T-cells. CD8 also facilitates TCR signaling by binding Lck, recruiting it to the TCR/CD3 complex [35, 36]. Some suggest that antigen sensitivity is more dependent on signaling capacity than affinity-enhancement by CD8 [24], and that antigen-engaged TCRs scan multiple co-receptors to find one coupled to Lck to initiate signaling [37].
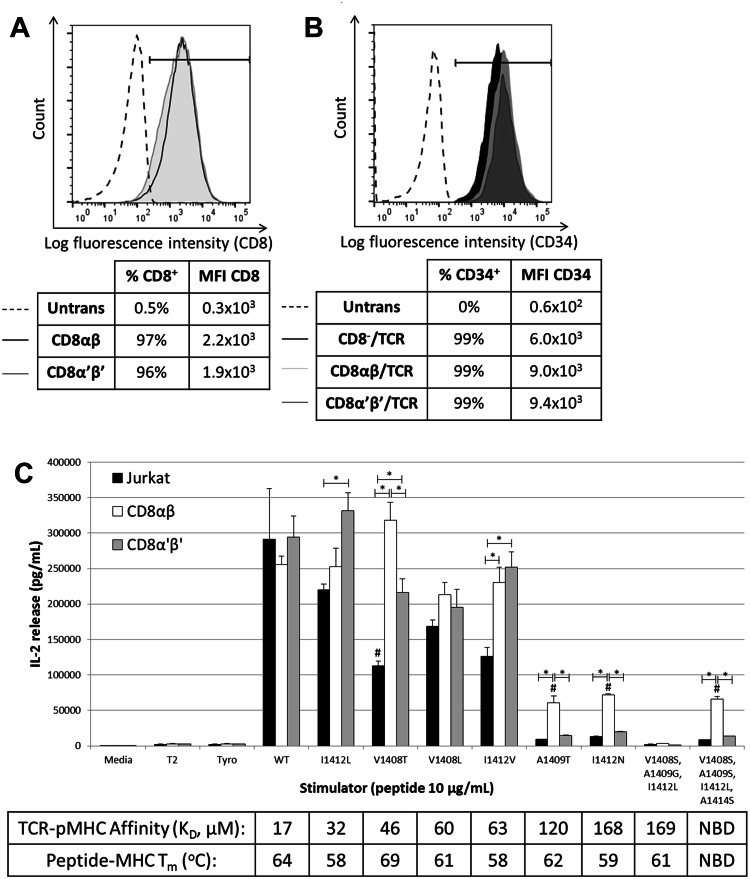
We determined the relative importance of affinity-enhancement versus signaling augmentation by CD8 on the recognition of altered ligands by engineering HCV1406 TCR-expressing CD8− Jurkat cells to express full length CD8αβ, or a truncated form (CD8α′β′) lacking the intracellular Lck-binding domain (Fig. 3a, b). All three groups (CD8−, CD8αβ, and CD8α′β′ Jurkat cells) secreted similar amounts of IL-2 against WT peptide (Fig. 3c). “Moderate affinity” CD8-independent ligands also stimulated robust IL-2 release from all groups. As predicted, CD8− Jurkat cells were non-reactive against “low affinity” ligands, whereas CD8αβ rescued the reactivity against all except 8S/9G/12L. Interestingly, Jurkat cells expressing truncated CD8α′β′ were minimally or non-reactive against “low affinity” variants. Even dose-dependent titrations of variant pMHC ligands showed that EC50 values for CD8α′β′ Jurkat cells were consistently below CD8αβ and/or similar to CD8− Jurkat cells (Supplementary Figure S1). These data suggest that TCR–pMHC stabilization by CD8 is alone not sufficient for efficient antigen recognition and that intracellular Lck-binding domains of CD8 can be critical for recognition of altered pMHC ligands, further supporting our hypothesis that TCR–pMHC affinity is not necessarily the critical determinant for antigen recognition.
Fig. 3.
CD8 co-receptor signaling components are required for reactivity against CD8-dependent ligands. a Jurkat cells were transduced to express either full length CD8αβ or truncated CD8α′β′ lacking the intracellular Lck-binding domain. Transduced cells were sorted for high and uniform expression of CD8. b Each group was transduced with a retrovirus encoding the HCV1406 TCR and immunomagnetically enriched for CD34 expression. c T2 cells were loaded with WT or variant HCV NS3:1406–1415 peptides or tyrosinase:368–376 peptide as a negative control and were co-cultured with TCR-transduced Jurkat cells for 18 h. IL-2 secretion by Jurkat (black bars) CD8αβ Jurkat (white bars) or CD8α′β′ (gray bars) was measured by ELISA. Mean and standard deviation of triplicate measurements are shown. Significant differences in IL-2 release between effectors are shown, *p < 0.05. Significantly less IL-2 release compared to WT stimulated is denoted with #, p < 0.05. Cultures are considered “antigen-reactive” if they secrete at least 200 pg/mL IL-2, twice above background and at least >5% of WT-stimulated cytokine release. Affinities of TCR–pMHC interactions are shown. NBD no binding detected. Data are representative of three independent experiments
Antigen density influences recognition of altered pMHC ligands
Clinical reports of on- or off-target effects by TCR-transduced T-cells have been attributed to recognition of low levels of antigen by high-affinity TCRs [18–21, 38]. Evaluating reactivity against peptide-loaded T2 cells is also not representative of antigen density on virally infected cells or tumor. Therefore, we established high, intermediate, and low antigen presentation system to evaluate how changes in antigen density impact the recognition of altered pMHC ligands.
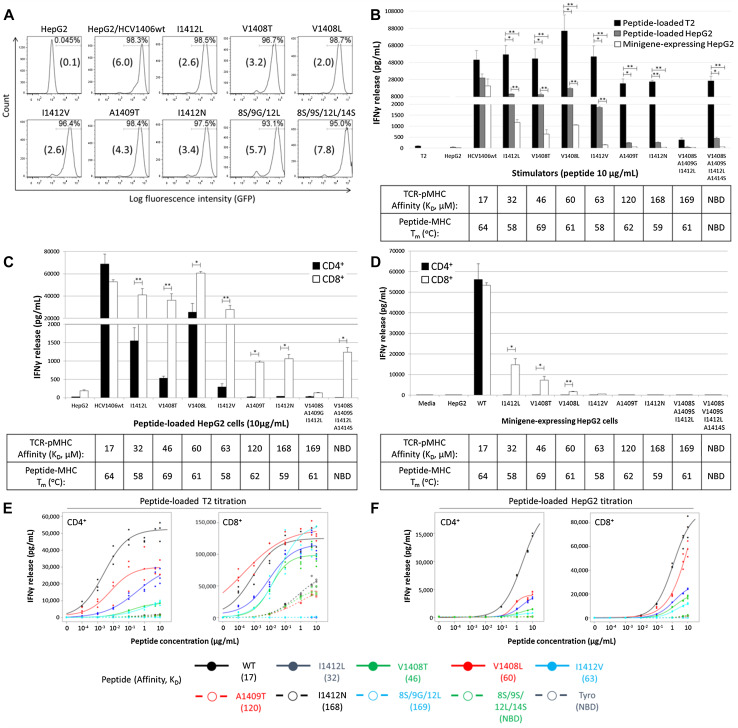
We used peptide-loaded TAP− T2 cells for our high antigen density system because exogenously loaded peptides can saturate their empty MHC molecules. We used peptide-loaded TAP+ HepG2 hepatocellular carcinoma cells for our intermediate level antigen density system. Exogenously loaded antigen has to compete with peptide already bound to MHC and is presented at a lower density than T2 cells. We developed a panel of HepG2 cells expressing endogenously processed HCV antigen (WT and variant HCV NS3:1406-1415 epitope minigenes) for our low peptide density system (Fig. 4a). Minigene-derived HCV peptides must compete with endogenously processed peptides for available MHC-I, thus presenting at a lower density than peptide-loaded cells. Coupled with peptide titrations, this panel of APC enabled us to determine how antigen density requirements related to TCR–pMHC affinity.
Fig. 4.
HCV1406 TCR recognition of altered ligands is substantially diminished with lower levels of HCV antigen. a HepG2 cells were transduced with retroviral vectors encoding HCV NS3:1406–1415 variants as minigenes fused to GFP by a T2A linker. Cells were sorted for high and uniform expression of GFP. Mean fluorescence intensity (×104) is shown in parentheses for each cell line. b PBL from a normal donor were transduced with the HCV1406 TCR retroviral vector and enriched for CD34 expression using anti-CD34 immunomagnetic beads. TCR-transduced T-cells were co-cultured for 18 h with WT or mutant HCV NS3:1406–1415 peptide-loaded T2 cells (black bars), peptide-loaded HepG2 cells (gray bars) or minigene-expressing HepG2 cells (white bars). c, d HCV1406 TCR-transduced PBL were immunomagnetically isolated into CD4+ (black bars) or CD8+ (white bars) populations and co-cultured with, c HepG2 cells loaded with WT or variant HCV NS3:1406–1415 peptides or d HepG2 cells engineered to express HCV NS3:1406–1415 minigenes. IFNγ secretion after 18 h co-culture was measured by ELISA. Mean and standard deviation of triplicate measurements are shown. IFNγ secretion was measured by ELISA. Mean and standard deviation of triplicate measurements are shown. Significant differences in IFNγ release between CD4+ and CD8+ T-cells are shown, *p < 0.05, **p < 0.01. Peptide titrations (10–0.0001 μg/mL) using peptide-loaded e T2 cells or f HepG2 cells as stimulators were also performed with CD4+ (left panels) and CD8+ (right panels) TCR-transduced T-cells. Dose-dependent relationships between peptide concentration and IFNγ release were estimated using four parameter logistic models in R library ‘drc.’ EC50 estimates were extracted from the fitted model, listed in Supplemental Table S2. Cultures are considered “antigen-reactive” if they secrete at least 200 pg/mL IFNγ, twice above background and at least >5% of WT-stimulated cytokine release. Affinities of TCR–pMHC and peptide–MHC interactions are shown. SPR-measured affinities of TCR–pMHC interactions are shown, and thermal stability of peptide-MHC interactions is shown. NBD no binding detected. These data are representative of three independent experiments
Figure 4b illustrates differences in cytokine release by bulk cultures of TCR-transduced T-cells stimulated by peptide-loaded T2 cells (high antigen density), peptide-loaded HepG2 cells (intermediate density), and HCV minigene-expressing HepG2 cells (low density). As expected, the overall magnitude of cytokine release follows the density of antigen presented. Recognition of WT antigen was not significantly impacted by antigen densities. However, significantly less IFNγ was secreted when stimulated with APC containing intermediate or low densities of altered ligands (p < 0.05). Despite similar TCR–pMHC affinity to V1408L, APC containing I1412V were not recognized at low density. Additionally, APC containing “low affinity” ligands were not recognized at intermediate or low densities. These results suggest that recognition of altered ligands with low TCR–pMHC affinities can be overcome at high levels of antigen. However, even at lower densities, recognition is not always dependent on TCR–pMHC affinity.
To evaluate how CD8 impacts recognition of altered ligands at lower densities, we stimulated purified CD4+ or CD8+ HCV1406 TCR-transduced T-cells with intermediate density (Fig. 4c) or low density (Fig. 4d) APC. We found that CD4+ T-cells were extremely sensitive to changes in antigen density, but there was no distinct relationship between reactivity and affinity. For example, different amounts of IFNγ were secreted by CD4+ T-cells stimulated with “moderate affinity” ligands despite similar affinities. CD8 facilitated strong recognition across WT and “moderate affinity” ligands, but “low affinity” variants induced significantly less IFNγ secretion (p < 0.001). CD4+ and CD8+ TCR-transduced T-cells secreted similar levels of IFNγ against HepG2 cells expressing WT minigene at low density (Fig. 4d). However, recognition of “moderate affinity” ligands at low density required the presence of CD8, and cytokine release was significantly lower than WT. Peptide titration curves using T2 cells (Fig. 4e) and HepG2 cells (Fig. 4f) and respective estimated EC50 values (Supplementary Table S2) also suggest that sensitivity to variant epitopes is not necessarily consistent with changes (or similarities) in TCR–pMHC affinity, supporting earlier Jurkat data (Supplementary Figure S1). Together, these data suggest that even at lower antigen densities, recognition of altered ligands is not solely driven by TCR–pMHC affinity.
TCR transgene levels influence IFNγ production independent of TCR–pMHC affinity
Given that antigen density influences recognition of altered ligands, it is logical to predict that TCR density may also play an important role. Our TCR retroviral vector contains a truncated CD34 cassette [25]. We demonstrated that increased CD34 relates to increased tetramer binding [22, 25], suggesting that CD34 is a good surrogate marker for TCR expression. This also allows us to evaluate TCR transgene expression without staining the TCR with an antibody or tetramer, which can influence the outcome of functional assays [39, 40]. Based on CD34 expression, we determined the mean fluorescence intensity (MFI) of intracellular IFNγ and surface CD107a (degranulation) of CD4+ or CD8+ T-cells expressing high, medium, or low levels of HCV1406 TCR (Supplementary Figure S2).
TCR density had a significant impact on CD8+ T-cell function with the amount of IFNγ produced being directly related to TCR expression (Fig. 5a). While TCR density was related to antigen recognition, changes in TCR–pMHC affinity generally had no impact on the intensity of IFNγ at a given TCR density. We also found that TCR density influenced antigen recognition by CD4+ T-cells with significant IFNγ production being generally restricted to high TCR-expressing CD4+ T-cells. (Figure 5b). However, TCR–pMHC affinity also had an impact on the amount of IFNγ produced. High-affinity WT ligand stimulated significantly greater IFNγ than “moderate affinity” ligands, and “low affinity” ligands did not stimulate IFNγ production by CD4+ T-cells even at high TCR density. However, the impact of TCR–pMHC affinity on CD4+ T-cells was not absolute since there was considerable variation in IFNγ intensity when stimulated with ligands of similar “moderate” affinities. A similar relationship between TCR density and CD107a expression against altered pMHC ligands was also observed (Supplementary Figure S2c-d). Collectively, these data indicate that recognition of altered pMHC ligands can be influenced by the density of introduced TCR. While TCR expression and TCR–pMHC affinity both appear to be critical for antigen recognition by CD4+ T-cells, only TCR expression appears to be important for antigen recognition by CD8+ T-cells. These results further support our assertion that TCR–pMHC affinity is not the most critical requirement for antigen recognition by T-cells.
Fig. 5.
The requirement for TCR expression to recognize altered pMHC ligands is different for CD8+ and CD4+ T-cells. PBL from a normal donor were transduced to express HCV1406 TCR and enriched for TCR-transduced cells using anti-CD34 immunomagnetic beads. T-cells were co-cultured with peptide-loaded T2 cells for 5 h and evaluated for IFNγ production by intracellular cytokine staining. Mean fluorescence intensity (MFI) of IFNγ production in TCR high (black), medium (gray), and low (white) expression populations is shown for a CD8+ and b CD4+ TCR-transduced T-cells. Significant differences in MFI of IFNγ between TCR expression levels are shown, *p < 0.05. Effector groups that display significantly greater IFNγ staining intensity compared to background tyrosinase stimulation is also denoted #p < 0.05. SPR-measured affinities of TCR–pMHC interactions are shown, and thermal stability of peptide-MHC interactions is shown. NBD no binding detected. These data are representative of three independent experiments with three donors
Discussion
Efforts to augment the efficacy of TCR gene-modified T-cells have focused on TCRs with enhanced affinity. However, increasing evidence suggests that TCR–pMHC affinity may not always influence the quality of effector responses [15–17, 24]. Moreover, high-affinity TCRs used in ACT have caused serious clinical adverse events [18–21]. Therefore, it is critical to evaluate the importance of affinity on antigen recognition by TCR gene-modified T-cells to help guide improvements in cancer immunotherapy.
We have developed a model to address what factors, including TCR–pMHC affinity, CD8 co-receptor, antigen density, and TCR density influence the recognition of altered pMHC ligands by a single TCR. K D values for TCR–pMHC interactions segregated ligands into ranges of affinity we defined as “high,” “moderate,” and “low” affinities. Consistent with the generalization of how affinity influences antigen recognition, WT and “moderate affinity” ligands were CD8-independent, and “low affinity” variants were CD8-dependent. However, similar “moderate affinity” ligands induced varying levels of cytokine release and degranulation in the absence of CD8. Additionally, of two ligands with identical affinities, only one was recognized by CD8+ T-cells and Jurkat cells at high and low antigen densities. This disconnect between TCR–pMHC affinity and T-cell reactivity contradicts general expectations and supports our assertion that TCR–pMHC affinity is not a singular determinant of T-cell function.
One potential explanation for these inconsistencies is that conventional affinity measurements by SPR may not accurately reflect the physiologic TCR–pMHC interaction. SPR is performed in three-dimensional (3D) space with soluble TCRs/pMHCs; however, physiologically the TCR and pMHC are anchored on two-dimensional (2D) membranes of opposing cells [41]. Thus, 3D measurements by SPR may not account for regulations imposed by the complex T-cell membrane environment, including reduced spatial degrees of freedom and co-receptors/co-stimulatory molecules [42]. Various groups have developed methods to establish binding partners anchored onto 2D surfaces to overcome limitations of SPR [43, 44]. Early studies have suggested that 2D measurements may better correlate with T-cell responses [43, 45] and measure TCR–pMHC-CD8 tri-molecular interactions [46], which may explain functional contributions by CD8. As 2D techniques become better established, they may provide a more predictive/correlative marker for antigen recognition and evaluating therapeutic TCRs.
Another set of parameters influencing antigen recognition are TCR binding kinetics. Kinetic studies suggest that slower TCR off-rates and longer dwell times may be required to complete intracellular signaling cascades and subsequent T-cell activation [30, 31]. Others believe that shorter dissociation rates and/or higher association rates are necessary for rapid exchange of TCR–pMHC interactions, known as serial triggering [47, 48]. Some studies have suggested that elements of both models may be relevant and not mutually exclusive [49]. In our study, TCR binding kinetics were unable to clearly rationalize different functional outcomes.
Differences in peptide-MHC binding can also influence antigen recognition and an immune response, but to what degree is unclear [50, 51]. Thermal denaturation of HCV peptide-HLA-A2 complexes indicated native and altered peptides bound MHC with high-affinity, but with slight variances [23]. These variances, however, were unable to explain altered antigen recognition patterns in light of small/large changes in TCR–pMHC affinity. It is possible that subtle differences in peptide sequence might alter the natural processing/presentation of antigen, not detectable by conventional assays. Further studies are necessary to improve the evaluation of peptide-MHC binding interactions and their relationship to T-cell function. Overall, specific biophysical requirements for T-cell activation and importance of each parameter may depend on individual TCR-antigen interactions.
TCR–pMHC affinity can also be enhanced by CD8 stabilizing the TCR–pMHC interaction, augmenting T-cell sensitivity [34]. However, we have previously shown that Lck-recruitment to the TCR/CD3 complex by CD8 may also be essential for T-cell function [24]. The relative importance of TCR–pMHC stabilization versus signaling by CD8 has been debated [24, 37, 52–54]. In this study, CD8-dependence relied on the ability to bind Lck, suggesting that merely stabilizing TCR–pMHC may not permit/enhance T-cell function, but that augmenting TCR/CD3 signaling can play a critical role. This further suggests that TCR–pMHC affinity is not necessarily the most influential parameter driving T-cell function. In light of these observations, it may be worth considering how modifying other accessory and/or co-stimulatory molecules influences antigen recognition by TCR-engineered T-cells.
Examining the ability of TCR gene-modified T-cells to recognize altered pMHC ligands at different densities is also important because clinical use of high-affinity TCR gene-modified T-cells induced severe adverse events by recognizing low-level cognate and/or related antigen [18–21, 38]. In our model, the ability to recognize altered ligands was markedly reduced with decreasing levels of antigen, and TCR–pMHC affinity became a better predictor for recognition and CD8 requirement. But we know that CD8-dependence is not solely based on affinity-enhancement. Additionally, differences in cytokine secretion at lower levels of antigen were not always reconciled by changes in TCR–pMHC affinity, reinforcing the need to differentiate requirements of cognate/altered antigen recognition when evaluating the efficacy/safety of TCR-engineered T-cells.
TCR density also influenced recognition of altered ligands, sometimes independent of TCR–pMHC affinity. While even low levels of TCR transgene expression in CD8+ T-cells facilitated IFNγ production, CD4+ T-cells were more reliant on high levels of TCR expression. Additionally, the requirement for high TCR expression was not consistent among altered ligands with similar affinities. The impact that TCR expression levels have on antigen recognition is important to highlight because investigators are modifying TCR sequences and/or gene-delivery vectors to promote transgene expression and limit mispairing with the endogenous TCR, both impacting relative surface density [55–58]. It has not yet been considered how these approaches might impact TCR cross-reactivity. Our data suggest that TCR density plays an important role in recognition of altered ligands. Therefore, the use of such pairing-enhanced TCRs/vectors has the potential for related antigen recognition affecting efficacy and safety. Thus, consequences of pairing-enhanced TCRs’ increased surface density should be further evaluated when designing TCRs for ACT.
It is important to consider that as with affinity, both TCR and antigen density are integral components in determining the number of receptors engaged. Thus, changing the amount of TCR/pMHC will surely impact T-cell function. However, the inconsistencies between the magnitude of cytokine release and/or the requirements for recognition given known affinities suggest that the population of engaged receptor is not solely dictating T-cell functional responses. Observing architectural changes in TCR–pMHC complexes may provide an answer to this dichotomy, with recent findings illustrating how altered TCR binding geometries can impact T-cell function [45, 59].
While these observations are an important first step in re-evaluating the role of TCR–pMHC affinity on antigen recognition, they describe the behavior of a single TCR. TCRs with other specificities may exhibit different recognition requirements. However, ongoing studies evaluating other well-characterized TCRs including HCV1073 [23, 60], TIL 1383I [5, 24], and DMF5 [61] suggest that our observations are not unique to HCV1406. Further in vivo modeling will help evaluate the role of TCR–pMHC affinity in a complex tumor microenvironment and may enhance the overall understanding of what factors govern T-cell responses.
In summary, we utilized our HCV1406 TCR model to address how a single TCR recognizes multiple related epitopes and how TCR–pMHC affinity influences functional responses. Our results suggest that less emphasis be placed on TCR–pMHC affinity as a means to improve the therapeutic potential of TCR gene-modified T-cells. Instead, we should also evaluate candidate TCRs for their CD8 requirements (structural and signaling), functional avidity, and amount of TCR expression required to stimulate antigen recognition (summarized in Supplementary Figure S3). Additionally, how these factors impact the ability to recognize altered, physiologically relevant targets is critical to evaluate their efficacy and safety. By evaluating the roles of multiple factors in antigen recognition and refining the way in which we evaluate TCR–pMHC binding interactions, we can more appropriately advance the design/implementation of TCR-based immunotherapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- EC50
Half maximal effective concentration
- MFI
Median fluorescence intensity
- pMHC
Peptide-major histocompatibility complex
- SPR
Surface plasmon resonance
- WT
Wildtype
Compliance with ethical standards
Funding
The authors would like to acknowledge funding provided by the National Cancer Institute: P01 CA154779 (Nishimura), R01 CA102280 (Nishimura), R01 CA104947 (Nishimura), R01 CA90873 (Nishimura), R21 CA153789 (Nishimura), F30 CA180731 (Spear), and the National Institute of General Medical Sciences: R35 GM118166 (Baker).
Ethical standards
All recombinant DNA and retroviral transduction work was done under approved Loyola University Chicago or University of Notre Dame Institutional Biosafety Committee protocols. Human materials used were established tumor cell lines or derived from apheresis products purchased from commercial sources. Therefore, these studies are not considered Human Subjects Research and did not require Institutional Review Board approval.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Spear TT, Nagato K, Nishimura MI. Strategies to genetically engineer T cells for cancer immunotherapy. Cancer Immunol Immunother. 2016;65(6):631–649. doi: 10.1007/s00262-016-1842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian S, Maile R, Collins EJ, Frelinger JA. CD8 + T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179(5):2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 3.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163(1):507–513. [PubMed] [Google Scholar]
- 4.Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96(17):9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roszkowski JJ, Lyons GE, Kast WM, Yee C, Van Besien K, Nishimura MI. Simultaneous generation of CD8 + and CD4 + melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65(4):1570–1576. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- 6.Chhabra A, Yang L, Wang P, Comin-Anduix B, Das R, Chakraborty NG, Ray S, Mehrotra S, Yang H, Hardee CL, Hollis R, Dorsky DI, Koya R, Kohn DB, Ribas A, Economou JS, Baltimore D, Mukherji B. CD4 + CD25 − T cells transduced to express MHC class I-restricted epitope-specific TCR synthesize Th1 cytokines and exhibit MHC class I-restricted cytolytic effector function in a human melanoma model. J Immunol. 2008;181(2):1063–1070. doi: 10.4049/jimmunol.181.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray S, Chhabra A, Chakraborty NG, Hegde U, Dorsky DI, Chodon T, von Euw E, Comin-Anduix B, Koya RC, Ribas A, Economou JS, Rosenberg SA, Mukherji B. MHC-I-restricted melanoma antigen specific TCR-engineered human CD4 + T cells exhibit multifunctional effector and helper responses, in vitro. Clin Immunol. 2010;136(3):338–347. doi: 10.1016/j.clim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisen HN, Sykulev Y, Tsomides TJ. Antigen-specific T-cell receptors and their reactions with complexes formed by peptides with major histocompatibility complex proteins. Adv Protein Chem. 1996;49:1–56. doi: 10.1016/S0065-3233(08)60487-8. [DOI] [PubMed] [Google Scholar]
- 9.Dunn SM, Rizkallah PJ, Baston E, Mahon T, Cameron B, Moysey R, Gao F, Sami M, Boulter J, Li Y, Jakobsen BK. Directed evolution of human T cell receptor CDR2 residues by phage display dramatically enhances affinity for cognate peptide-MHC without increasing apparent cross-reactivity. Protein Sci. 2006;15(4):710–721. doi: 10.1110/ps.051936406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holler PD, Holman PO, Shusta EV, O’Herrin S, Wittrup KD, Kranz DM. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc Natl Acad Sci USA. 2000;97(10):5387–5392. doi: 10.1073/pnas.080078297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23(3):349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 12.Chlewicki LK, Holler PD, Monti BC, Clutter MR, Kranz DM. High-affinity, peptide-specific T cell receptors can be generated by mutations in CDR1, CDR2 or CDR3. J Mol Biol. 2005;346(1):223–239. doi: 10.1016/j.jmb.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 13.Malecek K, Grigoryan A, Zhong S, Gu WJ, Johnson LA, Rosenberg SA, Cardozo T, Krogsgaard M. Specific increase in potency via structure-based design of a TCR. J Immunol. 2014;193(5):2587–2599. doi: 10.4049/jimmunol.1302344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malecek K, Zhong S, McGary K, Yu C, Huang K, Johnson LA, Rosenberg SA, Krogsgaard M. Engineering improved T cell receptors using an alanine-scan guided T cell display selection system. J Immunol Methods. 2013;392(1–2):1–11. doi: 10.1016/j.jim.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8alphabeta versus CD8alphaalpha expression. J Immunol. 2001;167(5):2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 16.Kroger CJ, Alexander-Miller MA. Cutting edge: CD8 + T cell clones possess the potential to differentiate into both high- and low-avidity effector cells. J Immunol. 2007;179(2):748–751. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura MI, Roszkowski JJ, Moore TV, Brasic N, Mckee MD, Clay TM (2005) Antigen recognition and T-cell biology. In: Khleif SN (ed) Tumor immunology and cancer vaccines. Springer US, Boston, pp 37–59. doi:10.1007/0-387-27545-2_2 [DOI] [PubMed]
- 18.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK (2013) Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5 (197):197ra103. doi:10.1126/scitranslmed.3006034 [DOI] [PMC free article] [PubMed]
- 21.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spear TT, Callender GG, Roszkowski JJ, Moxley KM, Simms PE, Foley KC, Murray DC, Scurti GM, Li M, Thomas JT, Langerman A, Garrett-Mayer E, Zhang Y, Nishimura MI. TCR gene-modified T cells can efficiently treat established hepatitis C-associated hepatocellular carcinoma tumors. Cancer Immunol Immunother. 2016;65(3):293–304. doi: 10.1007/s00262-016-1800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear TT, Riley TP, Lyons GE, Callender GG, Roszkowski JJ, Wang Y, Simms PE, Scurti GM, Foley KC, Murray DC, Hellman LM, McMahan RH, Iwashima M, Garrett-Mayer E, Rosen HR, Baker BM, Nishimura MI. Hepatitis C virus-cross-reactive TCR gene-modified T cells: a model for immunotherapy against diseases with genomic instability. J Leukoc Biol. 2016;100(3):545–557. doi: 10.1189/jlb.2A1215-561R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons GE, Moore T, Brasic N, Li M, Roszkowski JJ, Nishimura MI. Influence of human CD8 on antigen recognition by T-cell receptor-transduced cells. Cancer Res. 2006;66(23):11455–11461. doi: 10.1158/0008-5472.CAN-06-2379. [DOI] [PubMed] [Google Scholar]
- 25.Norell H, Zhang Y, McCracken J, Martins da Palma T, Lesher A, Liu Y, Roszkowski JJ, Temple A, Callender GG, Clay T, Orentas R, Guevara-Patino J, Nishimura MI. CD34-based enrichment of genetically engineered human T cells for clinical use results in dramatically enhanced tumor targeting. Cancer Immunol Immunother. 2010;59(6):851–862. doi: 10.1007/s00262-009-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89(8):3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce BG, Hellman LM, Hossain M, Singh NK, Vander Kooi CW, Weng Z, Baker BM. Computational design of the affinity and specificity of a therapeutic T cell receptor. PLoS Comput Biol. 2014;10(2):e1003478. doi: 10.1371/journal.pcbi.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis-Harrison RL, Armstrong KM, Baker BM. Two different T cell receptors use different thermodynamic strategies to recognize the same peptide/MHC ligand. J Mol Biol. 2005;346(2):533–550. doi: 10.1016/j.jmb.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 29.Piepenbrink KH, Gloor BE, Armstrong KM, Baker BM. Methods for quantifying T cell receptor binding affinities and thermodynamics. Methods Enzymol. 2009;466:359–381. doi: 10.1016/S0076-6879(09)66015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92(11):5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinowitz JD, Beeson C, Lyons DS, Davis MM, McConnell HM. Kinetic discrimination in T-cell activation. Proc Natl Acad Sci USA. 1996;93(4):1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callender GG, Rosen HR, Roszkowski JJ, Lyons GE, Li M, Moore T, Brasic N, McKee MD, Nishimura MI. Identification of a hepatitis C virus-reactive T cell receptor that does not require CD8 for target cell recognition. Hepatology. 2006;43(5):973–981. doi: 10.1002/hep.21157. [DOI] [PubMed] [Google Scholar]
- 33.Kammertoens T, Blankenstein T. It’s the peptide-MHC affinity, stupid. Cancer Cell. 2013;23(4):429–431. doi: 10.1016/j.ccr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Wooldridge L, van den Berg HA, Glick M, Gostick E, Laugel B, Hutchinson SL, Milicic A, Brenchley JM, Douek DC, Price DA, Sewell AK. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem. 2005;280(30):27491–27501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60(5):755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 36.Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci USA. 1989;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stepanek O, Prabhakar AS, Osswald C, King CG, Bulek A, Naeher D, Beaufils-Hugot M, Abanto ML, Galati V, Hausmann B, Lang R, Cole DK, Huseby ES, Sewell AK, Chakraborty AK, Palmer E. Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance. Cell. 2014;159(2):333–345. doi: 10.1016/j.cell.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cebecauer M, Guillaume P, Hozak P, Mark S, Everett H, Schneider P, Luescher IF. Soluble MHC-peptide complexes induce rapid death of CD8 + CTL. J Immunol. 2005;174(11):6809–6819. doi: 10.4049/jimmunol.174.11.6809. [DOI] [PubMed] [Google Scholar]
- 40.Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 2009;126(2):147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dustin ML, Bromley SK, Davis MM, Zhu C. Identification of self through two-dimensional chemistry and synapses. Annu Rev Cell Dev Biol. 2001;17:133–157. doi: 10.1146/annurev.cellbio.17.1.133. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475(7357):510–513. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464(7290):932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu B, Chen W, Evavold BD, Zhu C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. 2014;157(2):357–368. doi: 10.1016/j.cell.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams JJ, Narayanan S, Liu B, Birnbaum ME, Kruse AC, Bowerman NA, Chen W, Levin AM, Connolly JM, Zhu C, Kranz DM, Garcia KC. T cell receptor signaling is limited by docking geometry to peptide-major histocompatibility complex. Immunity. 2011;35(5):681–693. doi: 10.1016/j.immuni.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang N, Huang J, Edwards LJ, Liu B, Zhang Y, Beal CD, Evavold BD, Zhu C. Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination. Immunity. 2011;34(1):13–23. doi: 10.1016/j.immuni.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalergis AM, Boucheron N, Doucey MA, Palmieri E, Goyarts EC, Vegh Z, Luescher IF, Nathenson SG. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2(3):229–234. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 48.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375(6527):148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 49.Schmid DA, Irving MB, Posevitz V, Hebeisen M, Posevitz-Fejfar A, Sarria JC, Gomez-Eerland R, Thome M, Schumacher TN, Romero P, Speiser DE, Zoete V, Michielin O, Rufer N. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol. 2010;184(9):4936–4946. doi: 10.4049/jimmunol.1000173. [DOI] [PubMed] [Google Scholar]
- 50.Chervin AS, Stone JD, Holler PD, Bai A, Chen J, Eisen HN, Kranz DM. The impact of TCR-binding properties and antigen presentation format on T cell responsiveness. J Immunol. 2009;183(2):1166–1178. doi: 10.4049/jimmunol.0900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell. 2013;23(4):516–526. doi: 10.1016/j.ccr.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore TV, Lyons GE, Brasic N, Roszkowski JJ, Voelkl S, Mackensen A, Kast WM, Le Poole IC, Nishimura MI. Relationship between CD8-dependent antigen recognition, T cell functional avidity, and tumor cell recognition. Cancer Immunol Immunother. 2009;58(5):719–728. doi: 10.1007/s00262-008-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Loenen MM, Hagedoorn RS, de Boer R, Falkenburg JH, Heemskerk MH. Extracellular domains of CD8alpha and CD8ss subunits are sufficient for HLA class I restricted helper functions of TCR-engineered CD4(+) T cells. PLoS ONE. 2013;8(5):e65212. doi: 10.1371/journal.pone.0065212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voelkl S, Moore TV, Rehli M, Nishimura MI, Mackensen A, Fischer K. Characterization of MHC class-I restricted TCRalphabeta + CD4- CD8- double negative T cells recognizing the gp100 antigen from a melanoma patient after gp100 vaccination. Cancer Immunol Immunother. 2009;58(5):709–718. doi: 10.1007/s00262-008-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang HC, Bao Z, Yao Y, Tse AG, Goyarts EC, Madsen M, Kawasaki E, Brauer PP, Sacchettini JC, Nathenson SG, et al. A general method for facilitating heterodimeric pairing between two proteins: application to expression of alpha and beta T-cell receptor extracellular segments. Proc Natl Acad Sci USA. 1994;91(24):11408–11412. doi: 10.1073/pnas.91.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66(17):8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109(6):2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyerhuber P, Conrad H, Starck L, Leisegang M, Busch DH, Uckert W, Bernhard H. Targeting the epidermal growth factor receptor (HER) family by T cell receptor gene-modified T lymphocytes. J Mol Med (Berl) 2010;88(11):1113–1121. doi: 10.1007/s00109-010-0660-z. [DOI] [PubMed] [Google Scholar]
- 59.Gras S, Chadderton J, Del Campo CM, Farenc C, Wiede F, Josephs TM, Sng XY, Mirams M, Watson KA, Tiganis T, Quinn KM, Rossjohn J, La Gruta NL. Reversed T cell receptor docking on a major histocompatibility class I complex limits involvement in the immune response. Immunity. 2016;45(4):749–760. doi: 10.1016/j.immuni.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Liu Y, Moxley KM, Golden-Mason L, Hughes MG, Liu T, Heemskerk MH, Rosen HR, Nishimura MI. Transduction of human T cells with a novel T-cell receptor confers anti-HCV reactivity. PLoS Pathog. 2010;6(7):e1001018. doi: 10.1371/journal.ppat.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177(9):6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.