Abstract
As the prevalence of obesity in Type 1 diabetes rises, the effects of emerging therapy options should be considered in the context of both weight and glycaemic control outcomes. Artificial pancreas device systems will ‘close the loop’ between blood glucose monitoring and automated insulin delivery and may transform day-to-day dietary management for people with Type 1 diabetes in multiple ways. In the present review, we draw directly from cognitive restraint theory to consider unintended impacts that closed-loop systems may have on ingestive behaviour and food intake. We provide a brief overview of dietary restraint theory and its relation to weight status in the general population, discuss the role of restraint in traditional Type 1 diabetes treatment, and lastly, use this restraint framework to discuss the possible behavioural implications and opportunities of closed-loop systems in the treatment of Type 1 diabetes. We hypothesize that adopting closed-loop systems will lift the diligence and restriction that characterizes Type 1 diabetes today, thus requiring a transition from a restrained eating behaviour to a non-restrained eating behaviour. Furthermore, we suggest this transition be leveraged as an opportunity to teach people lifelong eating behaviour to promote healthy weight status by incorporating education and cognitive reappraisal. Our aim was to use a transdisciplinary approach to highlight critical aspects of the emerging closed-loop technologies relating to eating behaviour and weight effects and to promote discussion of strategies to optimize long-term health in Type 1 diabetes via two key outcomes: glycaemic control and weight management.
Introduction
Historically, Type 1 diabetes was conceptualized as a ‘wasting disease’, and individuals presented with weight loss as a result of poor glucose utilization. Since the widespread adoption of intensified insulin therapy for the prevention of diabetic complications in 1993 [1,2], however, the same technologies that were meant to keep blood glucose as close to normal as possible have been shown also to promote weight gain [3]. Intensive insulin therapy and continuous subcutaneous insulin infusion improve metabolic control in Type 1 diabetes, but may also promote weight gain as a result of less dietary restriction and enhanced conversion of excess macronutrients to stored adipose tissue [4]. According to the SEARCH for Diabetes in Youth Study, the prevalence of obesity among young people with Type 1 diabetes in the USA now parallels that of the general population, with a slightly higher prevalence of overweight [5]; this trend is supported by additional registries [6,7]. Excess adiposity increases the risk of adverse cardiovascular disease events, compounding a risk that is already elevated in Type 1 diabetes [8]; thus, establishing weight control alongside glycaemic control as a priority of care in Type 1 diabetes is critical for the promotion of long-term health outcomes [9,10].
Considering the contemporary obesity trends, further development of technologies and clinical practice guidelines to promote glycaemic benefits should also minimize the risk of excessive weight gain. In the present paper, we focus on the critical gap that currently exists relating to the psychosocial implications and human factors associated with the emerging closed-loop insulin delivery systems that are poised to transform the care of people with Type 1 diabetes. We describe dietary restraint theory and restrained eating behaviour that is driven by cognitive processes rather than by physiological cues, apply this framework to understand eating behaviour in Type 1 diabetes, and suggest that transition to closed-loop systems may be leveraged as an opportunity to normalize lifelong eating behaviour and promote healthy weight status. We conclude with a call to action; more research that integrates measures of dietary intake and eating attitudes is critically needed to understand the behavioural implications of new technologies and optimize both the physiological and psychosocial aspects of Type 1 diabetes care in the future. In the absence of data for definitive hypothesis testing, we present a heuristic model for clinical practice as part of this perspective review.
Treatment of Type 1 diabetes: a changing landscape
As the prevalence of overweight and obesity in Type 1 diabetes is rising [5], the landscape of therapy options is rapidly changing as well. In particular, the family of closed-loop artificial pancreas devices systems (closed-loop systems) has shown great potential in people with Type 1 diabetes to achieve normal glucose levels by linking three technologies: a glucose-sensing component; an insulin delivery device; and an algorithm that calculates the amount of insulin needed in response to sensor glucose levels [11,12]. Closed-loop system development has progressed rapidly from inpatient clinical research trials to long-term, free-living studies [13]. Current closed-loop systems, also referred to as automated insulin delivery systems, incorporate various degrees of automation, including hybrid closed-loop systems such as the US Food and Drug Administration-approved 670G, which requires mealtime manual assist bolus, and the ‘fully-closed’ automated insulin delivery systems which are in development [14]. In parallel with the insulin-only closed-loop systems are the bihormonal closed-loop systems, which incorporate glucagon to prevent or treat insulin-induced hypoglycaemia [15]. Their use has been associated with improved glucose control, reduced hypoglycaemia and reduction in HbA1c levels, and although not a cure, these closed-loop systems have been praised as the most promising advance in the treatment of diabetes at this time [13].
Implications of the artificial pancreas system for body weight and eating behaviour
Uncertainty exists about what effect closed-loop systems will have on body weight and eating behaviour for people with Type 1 diabetes. First, it remains unforeseen how changes in insulin doses may drive changes in weight. In some studies in adolescents, total daily insulin delivery was similar when study participants used closed-loop systems vs conventional insulin pump therapy [16], although other studies have found up to 50% increases in mean total daily insulin doses associated with use of the closed-loop system in adults [12]. Other studies suggest that insulin delivery is higher overnight with closed-loop systems than with sensor-augmented pump therapy, which is probably a function of poor glucose control and insufficient insulin doses at baseline which are corrected with closed-loop automation [17].
Another hypothesis is that by facilitating insulin delivery, improving glycaemic control, and increasing fuel utilization, the closed-loop system may drive excessive weight gain, especially for those people with elevated HbA1c levels prior to initiation of closed-loop treatment who will undergo the most dramatic metabolic transition. In people who are ‘under-insulinized’ or who have poorly controlled blood glucose levels, improvements in glycaemic control and glucose utilization may have an effect similar to the weight gain reported in the 1993 Diabetes Control and Complication Trials in many intensively managed participants who had an average HbA1c decrease of 22 mmol/mol (2%) [9]; this excess weight gain was also associated with worsening of cardiovascular disease risk factors [18]. Similar trends in weight have also been reported in people who initiate insulin pump therapy and experience improved glycaemic control [19,20].
Finally, prolonged use of closed-loop systems may alter the glycaemic targets that are recommended to people with Type 1 diabetes, especially as evidence accumulates that lower target ranges are both feasible and safe. Over time, different clinical set points may affect weight status alongside other metabolic outcomes, and more research will be needed to characterize and optimize clinical targets within the context of closed-loop system use.
The behavioural implications of the imminent closed-loop systems, however, elicit another set of concerns with regard to weight change, where automated insulin delivery is poised to minimize hypoglycaemia and possibly remove dietary restraint, thereby necessitating a transition from the restrained eating practices that characterize the management of Type 1 diabetes to non-restrained eating tendencies. Currently, diligence and restriction are the cornerstone of the day-to-day management of Type 1 diabetes; people with Type 1 diabetes engage in strict day-to-day dietary management that ideally typically include measuring food, carbohydrate counting, correcting for or anticipating hypoglycaemia, and avoiding dietary exacerbation of unexpected episodes of hyperglycaemia [21,22]. By facilitating flexible insulin delivery through automation and by more broadly normalizing many day-to-day elements of living with Type 1 diabetes, the closed-loop systems may lift both qualitative and temporal food restrictions and liberate people with Type 1 diabetes from this mindset of food restraint. Even the ‘partially closed’ hybrid closed-loop systems, which require dietary assessment and meal announcements, may reduce much of the burden surrounding eating snacks and meals for people with Type 1 diabetes [12].
The population-wide effects of such enhanced dietary flexibility are yet to be seen. While clinical trials often control dietary intake tightly, preliminary evidence from free-living closed-loop clinical research studies raises concern about increased carbohydrate and caloric intake in people with Type 1 diabetes while on closed-loop systems compared with open-loop control groups [12]; however, such studies have compared carbohydrate intake in participants over a short time frame (< 1 week) [15], and longitudinal studies are needed to elucidate how the closed-loop systems will affect dietary intake and patient attitudes towards focal dietary components, such as carbohydrates. Anecdotal examples of short-term disinhibition of dietary boundaries, described as loss of control and binge eating, are commonly reported from week-long trials of closed-loop systems [23].
Considering the contemporary obesity trends, further development of clinical practice guidelines to promote the glycaemic benefits associated with the closed-loop systems should also minimize the risk of excessive adiposity. To this end, it is critical to understand eating behaviour in Type 1 diabetes, specifically as it predicts weight gain and develops a deeper understanding of the behavioural aspects that are implicit with adopting the closed-loop systems [9,10]. This work falls within the critical gap that currently exists relating to the psychosocial implications and human factors associated with uptake of the closed-loop systems, which will play a key role in future technology uptake, outcomes and user engagement [24]. Within this emerging field of study, there is even less research that specifically focuses on eating attitudes and behaviours, although this is a key patient-reported outcome [25,26]. In light of this gap, we propose that dietary restraint theory may offer insights into eating behaviour in people with Type 1 diabetes and illustrate the shift from high dietary restraint status to low dietary restraint status associated with uptake and use of the closed-loop systems. This framework may also guide future clinical recommendations surrounding the closed-loop systems and eating behaviours that promote optimized weight outcomes alongside glycaemic control.
Dietary restraint theory
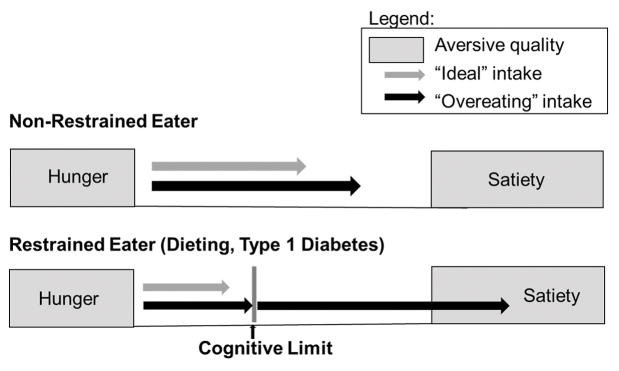
Cognitive dietary restraint is defined as the conscious attempt to limit and monitor food intake to achieve or maintain a desired weight and is associated with eating behaviour that is governed by cognitive processes rather than by physiological mechanisms such as hunger and satiety [27]. Dietary restraint theory distinguishes between restrained eaters and non-restrained eaters (Fig. 1) and posits that restrained eaters develop unusual eating patterns as a result of the stress inherent in chronic self-control, and that high levels of dietary restraint over time lead to dysregulated food intake, overeating, and ultimately, weight gain [28]. Whereas consumption in non-restrained eaters is driven primarily by the aversive qualities of hunger and satiety, food intake for restrained eaters is determined by the balance between a desire to eat and a wish to comply with cognitive dietary restrictions to promote weight loss.
FIGURE 1.
Schema of the two types of eaters outlined by cognitive restraint theory: the non-restrained eater and the restrained eater. In the non-restrained eater (top), eating is initiated by the aversive quality of hunger and is terminated by the aversive quality of hunger. Within the bracket of hunger and satiety, there is a zone of relative ‘biological indifference,’ where eating is driven by external and psychological factors. In the restrained eaters (bottom), such as individuals who are dieters or who have Type 1 diabetes, there are several key differences: 1) hunger and satiety signalling is disrupted where satiety thresholds are increased; 2) there is a cognitive dietary boundary (either self-imposed or medically imposed). When this diet boundary is exceeded by a ‘preload’ of food or another dietary disinhibition, restrained eaters continue to eat until they reach satiety (which is higher than that of a normal eater). When this boundary is removed (in hypoglycaemia for example, or using the flexible closed-loop systems), they may similarly overeat. Grey arrows represent typical ‘ideal’ food intake and black arrows represent typical food intake in episodes of ‘overeating.’
Although restrained eaters are sometimes successful in adhering to their diets, certain events, such as a dietary violation (i.e. eating something not recommended on the eating plan), alcohol consumption and emotional states, such as anxiety and depression, may interfere with their self-control, acting as disinhibitors and leading to overeating past the physiological boundary of satiety as well as excess food intake in satiety [29]. This pattern of overeating, disrupted satiety signalling, and eating in the context of satiety induced by transgression of the diet boundary is known as the ‘preload’ effect and has been demonstrated in laboratory studies [27,30]. Taken together, there is substantial evidence to support a causal relationship between dietary restraint and disordered eating patterns, specifically episodes of overeating in response to a preload or other dietary disruptor [31,32].
Dietary restraint theory in Type 1 diabetes
Currently, even with the most advanced and flexible treatment options, there are global elements of dietary restraint that heavily influence day-to-day life with Type 1 diabetes. In the past, conventional insulin therapy required people with Type 1 diabetes to match food intake to fixed insulin doses and people were encouraged to consume fixed meals to reduce mismatched intake [33,34]. Although more contemporary diabetes treatment paradigms permit some flexibility by allowing individuals to match insulin to food, people with diabetes are still tasked with counting carbohydrates and timing intake to insulin activity. As such, Type 1 diabetes remains a food culture of restraint in terms of the strict dietary regimen imposed by clinical recommendations, where the carbohydrate counting involved with day-to-day management of blood glucose drives imposed food preoccupation [35].
This universal, baseline element of cognitive dietary restraint in Type 1 diabetes invites the application of dietary restraint theory as a framework to describe eating. In Type 1 diabetes, the concept of an internally produced restrictive cognitive boundary is compounded by a medically imposed cognitive boundary of food intake. With this parallel cognitive boundary, the model shifts from restrained eaters, or individuals who maintain strict cognitive control over their eating behaviour in an attempt to maintain or decrease their body weight [27,29], to describe people with Type 1 diabetes who maintain strict cognitive control over their eating behaviour in an attempt to improve glycaemic control (Fig. 1). Despite the differences in source of restraint, the application of a dietary restraint theory framework is appropriate for this context; externally placed boundaries have been shown to mimic the effects of internally or self-imposed ones, notably in parent–child feeding studies [36,37].
Characteristics that are typically associated with high levels of dietary restraint have been independently implicated in reports of eating behaviour in Type 1 diabetes. Just as restrained eaters have a third, self-imposed dietary boundary that delineates acceptable food intake from intake that might drive weight gain [38], there is a relationship between consumption and virtue that prevails in the context of Type 1 diabetes, where control is often described as being ‘good’ and failure to comply with restriction is ‘bad’[39]. This creates a food culture akin to dieting, in which there is a disconnect between physiological and psychological eating cues. Second, just as restrained eaters are assumed to have lower hunger and satiety boundaries as a result of repeated dieting and overeating in the past [28], there is evidence that satiety may be disrupted by timed meals and insulin doses that are not based on hunger cues over time in people with Type 1 diabetes [40,41].
Although few studies have directly measured restraint in people with Type 1 diabetes, Martyn-Nemeth et al. [42] showed that, in a sample of 15 women (mean age 37 ± 13.5 years) with Type 1 diabetes, those with poorer glycaemic control had significantly higher levels of restrained eating and interpersonal distress [42], which suggests that high restraint may be associated with disordered eating habits, high diabetes distress, and worse glycaemic control.
Non-severe hypoglycaemia: dietary restraint theory in action
Restrained eating tendencies may be particularly challenged during an episode of non-severe hypoglycaemia. Increased hunger secondary to hypoglycaemia represents a major disruptor of physiological appetite signalling [43]. This hunger is thought to be a part of the cholinergic response, where transient declines in blood glucose stimulate counter-regulatory hormones such as cortisol, growth hormone and epinephrine and ghrelin, thereby increasing hunger and driving food consumption, particularly high-sugar and high-fat foods [44]. In addition, non-severe hypoglycaemic episodes represent temporary freedom from an otherwise pervasive condition of dietary restraint and are often accompanied with the associated disinhibited eating seen in restrained eaters. To this point, case studies have described people with diabetes who intentionally overdose insulin to enable the eating of sweets and/or other restricted high-carbohydrate food [45]. In a study in people with Type 1 diabetes aged 10–22 years, Schober et al. [45] found that 18% reported intentional overdosing of insulin, and half of these stated a desire for uncontrolled eating as the major motivation for this behaviour. Recently, the restraint theory framework was implicated in both the nature of and the high prevalence of disordered eating behaviour in Type 1 diabetes [35]; new models for disordered eating have identified hypoglycaemia as an important part of a cycle of chronic dietary restraint, spontaneous over-eating in the context of non-severe hypoglycaemia, guilt and compensatory restriction that is more similar to bulimia [46,47].
Automated insulin delivery systems and dietary restraint theory
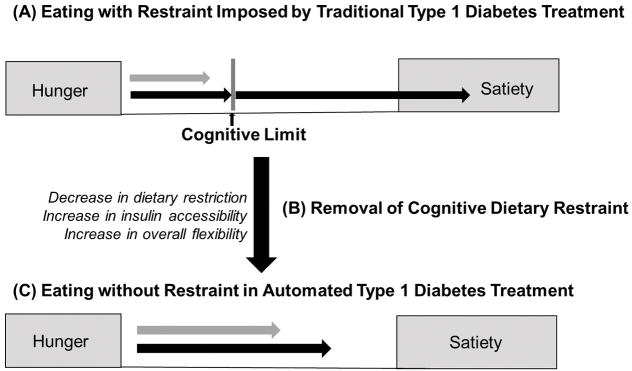
Looking ahead, the introduction of the closed-loop systems carries deep implications within the framework of dietary restraint theory, and if the model holds, the closed-loop systems will profoundly affect eating behaviour in many people with Type 1 diabetes. Restraint theory frames individuals with Type 1 diabetes using currently available therapy as eaters who operate in a pervasive culture of food restriction and carbohydrate preoccupation, with a medically imposed dietary boundary which explains much of the subclinical disordered eating behaviour previously described in Type 1 diabetes. Eating behaviour is further challenged regularly by disinhibition and impulsivity inherent in episodes of non-severe hypoglycaemia. Taken together, the partially and fully automated closed-loop systems are in place to minimize episodes of hypoglycaemia for people with Type 1 diabetes and possibly remove the medically imposed dietary boundaries, and they necessitate a fundamental cognitive transition and an associated behavioural transition from restrained eating to non-restrained eating tendencies (Fig. 2). This experience and effects of this shift in dietary restraint pose key, unanswered questions with regard to closed-loop system use.
FIGURE 2.
(a) Traditional Type 1 diabetes treatment: At diagnosis with Type 1 diabetes, there is transition into a state of cognitive dietary restraint that is compounded by introduction of nutritional guidelines, carbohydrate counting, eating in the absence of hunger, intense self-regulation, and dietary restraint. (b) Transition to be navigated with introduction of closed-loop systems: the flexibility lent by the closed-loop system will remove rigid eating patterns and lift the boundary of cognitive dietary restraint. We suggest this transition be leveraged as an opportunity to teach persons lifelong eating behaviour to promote healthy weight status by incorporating education and cognitive reappraisal. (c) In the absence of cognitive dietary restraint, eating behaviour should resemble non-restrained eaters, including increased flexibility, intuitive eating, and restored hunger and satiety signalling.
Possible unintended behavioural consequences of treatment with closed-loop systems
One of the most attractive parts of closed-loop systems for people with Type 1 diabetes, especially for young people, is the underlying promise that they will be able to ‘eat whatever they want’ for the first time since diagnosis. This misguided assumption that people can ‘eat whatever they want,’ instead of following healthy dietary advice, is also often heard from those with diabetes wanting to start insulin pumps and will probably be even more prevalent with closed-loop systems. This shift in treatment has been previously explored through structured education programmes for people with Type 1 diabetes such as the Dose Adjustment for Normal Eating (DAFNE) programme that is used widely throughout the UK [48–50]. The main purpose of the DAFNE course was for patients to learn skills and strategies to adjust insulin to match food intake, thereby increasing dietary flexibility and improving blood glucose control [51]; however, studies on the DAFNE programme have reported people with diabetes struggling to ‘break away’ from well-established routines, comfortable habits, and habitually reinforced protective behaviours and risk-avoiding strategies [51]. Such findings reinforce the need for training and support during treatment shifts that directly implicate large behavioural shifts.
In terms of restraint theory, the closed-loop systems may entirely remove the boundary condition through the dietary flexibility permitted by the algorithm and especially an eventual fully automated closed-loop system. Based on laboratory studies in restrainedoxforddiabetessymposium2017@mci-group.com eaters, it is reasonable to expect the disinhibited eating behaviour that results when the boundary is experimentally removed [52]. Disinhibited boundary behaviour may be different in the long term from in the short term. Examples of short-term disinhibition have been reported in week-long trials of the closed-loop systems, in which there is anecdotal evidence to describe increased carbohydrate intake at free-living meals [14]. Such disinhibited eating tendencies, if reinforced as habit and superimposed on the physiologically based potential for weight gain inherent in improved glycaemic control, threaten to increase body weight and cardiovascular disease risk factors [9,10,18]. This effect would probably be particularly severe in people with poor glycaemic control prior to initiating a closed-loop system; however, the lasting effect of the removal of the boundary, especially in terms of overeating, is more complex, but may prove to be beneficial in terms of long-term health outcomes. In the long-term, removal of the boundary may promote dietary flexibility, remove moral overtones and correct homeostatic signalling, by both minimizing hypoglycaemia and the forced consumption of calories in the absence of hunger as well as the ability to eat only when physiological hunger exists. People with HbA1c levels at goal who experience excessive hypoglycaemia may find the greatest benefit here; reducing the effect of consuming 15 g carbohydrate daily (60 kcal/day) to rescue hypoglycaemia, an unwanted yet necessary source of energy intake, translates to a potential loss of 6 pounds per year that will add up over decades. Notably, the increased dietary flexibility conferred through the DAFNE programme has not been associated with significant increases in weight as compared with the control group [48], and several studies have even reported modest weight loss over the first 2 years after the DAFNE programme [49,50], suggesting that improved flexibility does not inherently produce disinhibited eating among people with Type 1 diabetes.
Timely opportunities for weight management with closed-loop treatment
Dramatically increasing rates of closed-loop device development and future uptake are associated with psychosocial and behavioural implications which will necessarily shape patient attitudes, uptake and sustainability, and metabolic outcomes in individuals with Type 1 diabetes [25,26]. Just as researchers are beginning to consider and measure other psychosocial and behavioural implications of automated insulin delivery, the introduction of the closed-loop systems must be recognized as a necessary transition from dietary restraint to a lack thereof; this transition is fraught with potential pitfalls and may carry a great risk to create and reinforce lifelong patterns of disordered eating or related behaviours and cognitions that promote weight gain. As such, there exists a clear window in which it is critical to focus on behavioural and cognitive skills to prepare people with Type 1 diabetes to navigate this transition and equip them with the knowledge of how to eat in a healthy, intuitive and hunger-based way. Especially as individuals with Type 1 diabetes continue to move through the modern ‘obesogenic’ milieu that includes readily available energy-dense food and a sedentary lifestyle, this skillset is critical to avoid the prevalence of obesity further increasing in the Type 1 population and even surpassing that in the general population. We propose that education and cognitive reappraisal be integrated into the closed-loop transition protocol, which will help to reteach awareness of hunger and satiety cues, encourage people to eat on physiological cues instead of Type 1 diabetes treatment-imposed timelines, and correct ingrained aversions to foods that have been ‘forbidden.’ Various cognitive reappraisal strategies, such as training to internally highlight positive benefits of not performing a given behaviour or repeated acknowledgment of the negative aspects of performing that behaviour when facing a cue, have been shown to be effective in altering brain–behaviour relations for obesity treatment, smoking cessation as well as emotional regulation [53–55]. In addition, the bi-hormonal closed-loop systems may have behavioural implications distinct from the single-hormone system, particularly relating to dietary consumption requirements to correct hypoglycaemia. The glucagon rescue feature, meant to mitigate episodes of hypoglycaemia, may limit the introduction of disinhibition and associated binge eating, and thus may have additive benefits in promoting healthy weight status in people with Type 1 diabetes.
We propose a weight management intervention to be integrated into closed-loop transition that includes the following components:
Education on disordered eating, the components of a healthy diet, and resources for help, which will serve as skills for the obesogenic environment [56,57].
Cognitive reappraisal strategies to correct ingrained aversions to foods that have been ‘forbidden’, such as foods rich in carbohydrate [55].
Tools to recognize and eat based on physiological cues instead of treatment-imposed timelines, including awareness of hunger and satiety cue techniques in mindfulness and mindful eating [58,59].
Summary
To develop meaningful clinical practice guidelines to avoid weight gain and further increases in overweight and obesity in Type 1 diabetes, it is critical to consider eating behaviour in Type 1 diabetes, which may be fundamentally altered by the closed-loop systems. To this end, more research is critical, as is the integrating of measures of eating attitudes and behaviour into current efforts to study and optimize both the physiological and psychosocial aspects of closed-loop system uptake. We hypothesize that adopting the closed-loop systems will lift the diligence and restriction that characterizes Type 1 diabetes today, thus requiring a transition from restrained eating behaviour to non-restrained eating behaviour, but more data are critically needed to power a shift from heuristic models to hypothesis-testing to inform clinical practice. Recognizing and leveraging this transition as an opportunity for lifelong glycaemic control and weight management, we suggest nutrition and behavioural education to promote normalized eating behaviour and cognitive reappraisal as appropriate and mandatory techniques for people moving from traditional treatment to the closed-loop systems. Expanding protocols to guide the patient-oriented closed-loop system transition experience to include these cognitive and behavioural components will improve quality of life in the short term, by normalizing unique Type 1 diabetes eating behaviour aspects, as well as maximizing long-term population-level health by minimizing the known complications and risk of excessive adiposity in people with Type 1 diabetes.
What’s new?
We apply dietary restraint theory to characterize the behavioural implications of emerging closed-loop insulin delivery technology for use in Type 1 diabetes.
Restraint theory frames individuals with Type 1 diabetes who are using currently available therapy as eaters who operate in a pervasive culture of food restriction and carbohydrate preoccupation, with a medically imposed dietary boundary, which explains much of the subclinical disordered eating behaviour previously described in Type 1 diabetes.
We hypothesize that adopting closed-loop systems will lift the diligence and restriction that characterizes Type 1 diabetes today, thus requiring a transition from a restrained eating to a non-restrained eating behaviour.
We propose tools to support people with Type 1 diabetes in the transition from restrained eating tendencies to healthy, intuitive eating tendencies that may be necessitated by automated insulin treatment.
We propose heuristic models and a perspective review; no data exist for hypothesis-testing at this time.
Acknowledgments
Funding sources
A.R.K. is supported by funding from Flexible Lifestyles Empowering Change trial (FL3X, NIH 1UC4DK101132) and the University of North Carolina Renal Epidemiology Training Grant (NIH/NIDDK 5T32DK007750-16).
Footnotes
Competing interests
None declared.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pihoker C, Badaru A, Anderson A, Morgan T, Dolan L, Dabelea D, et al. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study. Diabetes Care. 2013;36:27–33. doi: 10.2337/dc12-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitri J, Hamdy O. Diabetes medications and body weight. Expert Opin Drug Saf. 2009;8:573–584. doi: 10.1517/14740330903081725. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JM, Liese AD, Liu L, Dabelea D, Anderson A, Imperatore G, et al. Weight-loss practices and weight-related issues among youth with type 1 or type 2 diabetes. Diabetes Care. 2008;31:2251–2257. doi: 10.2337/dc08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11:4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 7.DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, et al. Obesity in Youth with Type 1 Diabetes in Germany, Austria, and the United States. J Pediatr. 2015;167:627–32. e1–4. doi: 10.1016/j.jpeds.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 8.Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130:1532–1558. doi: 10.1161/CIR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 9.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37:2843–2863. doi: 10.2337/dc14-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, El-Khairi R, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204–1211. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313–325. doi: 10.1056/NEJMoa1314474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovatchev B, Tamborlane WV, Cefalu WT, Cobelli C. The Artificial Pancreas in 2016: A Digital Treatment Ecosystem for Diabetes. Diabetes Care. 2016;39:1123–1126. doi: 10.2337/dc16-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevitt S, Simpson S, Wood A. Artificial Pancreas Device Systems for the Closed-Loop Control of Type 1 Diabetes What Systems Are in Development? J Diabetes Sci Technol. 2016;10:714–723. doi: 10.1177/1932296815617968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blauw H, von Bon AC, Koops R, DeVries J. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18:671–677. doi: 10.1111/dom.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3:17–26. doi: 10.1016/S2213-8587(14)70226-8. [DOI] [PubMed] [Google Scholar]
- 17.Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824–833. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 18.Group DR. Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care. 1988;11:567–573. doi: 10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- 19.Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Adiposity and mortality in type 1 diabetes. Int J Obes (Lond) 2009;33:796–805. doi: 10.1038/ijo.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sands AL, Higgins LA, Mehta SN, Nansel TR, Lipsky LM, Laffel LM. Associations of youth and parent weight status with reported versus predicted daily energy intake and hemoglobin A1c in youth with type 1 diabetes mellitus. J Diabetes Sci Technol. 2013;7:263–270. doi: 10.1177/193229681300700131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litmanovitch E, Geva R, Rachmiel M. Short and long term neuro-behavioral alterations in type 1 diabetes mellitus pediatric population. World J Diabetes. 2015;6:259–270. doi: 10.4239/wjd.v6.i2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124:e1171–1179. doi: 10.1542/peds.2009-0207. [DOI] [PubMed] [Google Scholar]
- 23.Kovatchev B, Cheng P, Anderson SM, Pinsker JE, Boscari F, Buckingham BA, et al. Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther. 2017;19:18–24. doi: 10.1089/dia.2016.0333. [DOI] [PubMed] [Google Scholar]
- 24.Barnard KD, Hood KK, Weissberg-Benchell J, Aldred C, Oliver N, Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther. 2015;17:295–300. doi: 10.1089/dia.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naranjo D, Tanenbaum ML, Iturralde E, Hood KK. Diabetes Technology Uptake, Outcomes, Barriers, and the Intersection With Distress. J Diabetes Sci Technol. 2016;10:852–858. doi: 10.1177/1932296816650900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weissberg-Benchell J, Hood K, Laffel L, Heinemann L, Ball D, Kowalski A, et al. Toward Development of Psychosocial Measures for Automated Insulin Delivery. J Diabetes Sci Technol. 2016;10:799–801. doi: 10.1177/1932296815619637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman CP, Mack D. Restrained and unrestrained eating. J Pers. 1975;43:647–660. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 28.Tuschl RJ, Platte P, Laessle RG, Stichler W, Pirke KM. Energy expenditure and everyday eating behavior in healthy young women. Am J Clin Nutr. 1990;52:81–86. doi: 10.1093/ajcn/52.1.81. [DOI] [PubMed] [Google Scholar]
- 29.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84:66–72. [PubMed] [Google Scholar]
- 30.Williamson DA, Martin CK, York-Crowe E, Anton SD, Redman LM, Han H, et al. Measurement of dietary restraint: validity tests of four questionnaires. Appetite. 2007;48:183–192. doi: 10.1016/j.appet.2006.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wardle J, Beinart H. Binge eating: a theoretical review. Br J Clin Psychol. 1981;20(Pt 2):97–109. doi: 10.1111/j.2044-8260.1981.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 32.Polivy J, Herman CP. Dieting and binging. A causal analysis. Am Psychol. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 33.Lawton J, Rankin D, Cooke D, Clark M, Elliot J, Heller S, et al. Dose adjustment for normal eating: a qualitative longitudinal exploration of the food and eating practices of type 1 diabetes patients converted to flexible intensive insulin therapy in the UK. Diabetes Res Clin Pract. 2011;91:87–93. doi: 10.1016/j.diabres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Balfe M. Diets and discipline: the narratives of practice of university students with type 1 diabetes. Sociol Health Illn. 2007;29:136–153. doi: 10.1111/j.1467-9566.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 35.Peterson CM, Fischer S, Young-Hyman D. Topical review: a comprehensive risk model for disordered eating in youth with type 1 diabetes. J Pediatr Psychol. 2015;40:385–390. doi: 10.1093/jpepsy/jsu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell K, Andrianopoulos N, Hesketh K, Ball K, Crawford D, Brennan L, et al. Parental use of restrictive feeding practices and child BMI z-score. A 3-year prospective cohort study. Appetite. 2010;55:84–88. doi: 10.1016/j.appet.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Johannsen DL, Johannsen NM, Specker BL. Influence of parents’ eating behaviors and child feeding practices on children’s weight status. Obesity. 2006;14:431–439. doi: 10.1038/oby.2006.57. [DOI] [PubMed] [Google Scholar]
- 38.Herman CP, Polivy J. A boundary model for the regulation of eating. Res Publ Assoc Res Nerv Ment Dis. 1984;62:141–156. [PubMed] [Google Scholar]
- 39.Balfe M. Diets and discipline: the narratives of practice of university students with type 1 diabetes. Sociol Health Illn. 2007;29:136–153. doi: 10.1111/j.1467-9566.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 40.Dewan S, Gillett A, Mugarza JA, Dovey TM, Halford JC, Wilding JP. Effects of insulin-induced hypoglycaemia on energy intake and food choice at a subsequent test meal. Diabetes Metab Res Rev. 2004;20:405–410. doi: 10.1002/dmrr.471. [DOI] [PubMed] [Google Scholar]
- 41.Prodam F, Cadario F, Bellone S, Trovato L, Moia S, Pozzi E, et al. Obestatin levels are associated with C-peptide and antiinsulin antibodies at the onset, whereas unacylated and acylated ghrelin levels are not predictive of long-term metabolic control in children with type 1 diabetes. J Clin Endocrinol Metab. 2014;99:E599–607. doi: 10.1210/jc.2013-3294. [DOI] [PubMed] [Google Scholar]
- 42.Martyn-Nemeth P, Quinn L, Hacker E, Park H, Kujath AS. Diabetes distress may adversely affect the eating styles of women with type 1 diabetes. Acta Diabetol. 2014;51:683–686. doi: 10.1007/s00592-014-0575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 44.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121:4161–4169. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schober E, Wagner G, Berger G, Gerber D, Mengl M, Sonnenstatter S, et al. Prevalence of intentional under- and overdosing of insulin in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:627–631. doi: 10.1111/j.1399-5448.2011.00759.x. [DOI] [PubMed] [Google Scholar]
- 46.Pinhas-Hamiel O, Levy-Shraga Y. Eating disorders in adolescents with type 2 and type 1 diabetes. Curr Diab Rep. 2013;13:289–297. doi: 10.1007/s11892-012-0355-7. [DOI] [PubMed] [Google Scholar]
- 47.Verrotti A, Catino M, De Luca FA, Morgese G, Chiarelli F. Eating disorders in adolescents with type 1 diabetes mellitus. Acta Diabetol. 1999;36:21–25. doi: 10.1007/s005920050140. [DOI] [PubMed] [Google Scholar]
- 48.DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002;325:746. doi: 10.1136/bmj.325.7367.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunn D, Mansell P. Glycaemic control and weight 7 years after Dose Adjustment For Normal Eating (DAFNE) structured education in Type 1 diabetes. Diabet Med. 2012;29:807–812. doi: 10.1111/j.1464-5491.2011.03525.x. [DOI] [PubMed] [Google Scholar]
- 50.McIntyre HD, Knight BA, Harvey DM, Noud MN, Hagger VL, Gilshenan KS. Dose adjustment for normal eating (DAFNE)-an audit of outcomes in Australia. Med J Aust. 2010;192:637–640. doi: 10.5694/j.1326-5377.2010.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 51.Lawton J, Rankin D. How do structured education programmes work? An ethnographic investigation of the dose adjustment for normal eating (DAFNE) programme for type 1 diabetes patients in the UK. Soc Sci Med. 2010;71:486–493. doi: 10.1016/j.socscimed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Polivy J, Zeitlin SB, Herman CP, Beal AL. Food restriction and binge eating: a study of former prisoners of war. J Abnorm Psychol. 1994;103:409–411. doi: 10.1037//0021-843x.103.2.409. [DOI] [PubMed] [Google Scholar]
- 53.Hollmann M, Hellrung L, Pleger B, Schlögl H, Kabisch S, Stumvoll M, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes. 2012;36:648–655. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- 54.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yokum S, Stice E. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes. 2013;37:1565–1570. doi: 10.1038/ijo.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez-Campoy J, Jeor SS, Castorino K, Ebrahim A, Hurley D, Jovanovic L, et al. Clinical practice guidelines for healthy eating for the prevention and treatment of metabolic and endocrine diseases in adults: cosponsored by the American Association of Clinical Endocrinologists/the American College of Endocrinology and the Obesity Society. Endocr Pract. 2013;19(Suppl 3):1–82. doi: 10.4158/EP13155.GL. [DOI] [PubMed] [Google Scholar]
- 57.Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J Royal Soc Prom Health. 2006;126:262–267. doi: 10.1177/1466424006070487. [DOI] [PubMed] [Google Scholar]
- 58.Healy N, Joram E, Matvienko O, Woolf S, Knesting K. Impact of an intuitive eating education program on high school students’ eating attitudes. Health Educ. 2015;115:214–228. [Google Scholar]
- 59.Van Dyke N, Drinkwater EJ. Review article relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17:1757–1766. doi: 10.1017/S1368980013002139. [DOI] [PMC free article] [PubMed] [Google Scholar]