Abstract
In previous work, we developed a lightweight wearable hand exoskeleton (HandSOME) that improves range of motion and function in laboratory testing. In this pilot study, we added the ability to log movement data for extended periods and recruited 10 chronic stroke subjects to use the device during reach and grasp task practice at home for 1.5 hours/day, 5 days per week, for 4 weeks. Seven subjects completed the study, performing 448±651 hand movements per training day. After training, impairment was reduced (Fugl-Meyer Test; gain=4.9±4.1; p=.039) and function was improved (Action Research Arm Test; gain=3.3±2.6; p=.032). There was a significant correlation between gains in the Action Research Arm Test and the number of movements during training (r=0.90; p=.005). Proximal arm control also improved, as evidenced by a significant reduction in the reach path ratio (p=0.038). Five subjects responded well to the treatment, having gains of 6 points or more on the Fugl-Meyer or Action Research Arm Test, and achieving significant gains in digit extension (gain=19.8±10.2 degrees; p=0.024). However, all of the gains that were significant immediately after training were no longer significant at the 3 month follow-up. This treatment approach appears promising, but longer periods of home training may be needed to achieve sustainable gains.
Keywords: Exoskeleton, Hand, Neurorehabilitation, Stroke, Therapy
I. Introduction
There are 800,000 new strokes in the United States each year [1]. Movement deficits associated with stroke include a reduced range of motion (ROM) of the affected upper extremity and abnormal interjoint coordination. Individuals with stroke thus tend to have long lasting difficulty in performing activities of daily living (ADL) such as reaching, grasping and lifting objects. Rehabilitation technologies have the potential to promote motor recovery after stroke. Robotic therapies provide precise and repetitive movement training, and require less supervision from therapists [2]. A recent meta-analysis of 34 clinical trials found upper extremity robotic therapy improved ADL ability, function and strength when compared to other interventions, but the advantages of robotic therapy may not be large enough to be clinically relevant [3]. Robotic technologies that have been tested in clinical trials are mainly focused on recovery of the shoulder and elbow, and often involve practicing components of ADL without the ability to manipulate real objects [4]. However, the notion of task specificity demands that all limb segments involved in a task must be rehabilitated in a coordinated fashion [5]. While recent studies have challenged the importance of task specificity [6], [7], other studies have found that motor learning relies on sensory and biomechanical feedback loops during multi-joint movement [8]. To enable task specific training, devices are needed that allow practice of complex multi-DOF tasks involving use of the hand to grasp and manipulate objects, since hand function is crucial to a functional limb [9].
Several robots have been developed that assist movements of the hand isolated from the rest of the limb [10], [11], [12], [13], [14], [15], [16], [17], [18]. These devices require sitting in the clinic with the arm supported in a pre-defined posture and/or don’t allow interacting with objects. The potential to transfer gains to real life situations could be limited, given growing evidence of abnormal coupling of proximal and distal control in stroke patients, such that arm posture [19], activation level of proximal muscles [20], and level of arm support [21] can affect control of hand muscles. Examples of hand robots that can be used during ADL practice include the Hand-of-Hope, which is powered by five linear actuators and offers individual control of each digit [22]. The PneuGlove [23] is a pneumatically powered glove that contains air bladders that extend the fingers when inflated. Cybergrasp (Immersion Inc, San Jose, CA) uses cables routed through a linkage mounted to the back of the hand [24]. Extension force in each cable is controlled with five motors located remotely. The X-Glove is a portable device with 5 linear actuators that independently extend the digits [25]. These approaches are promising, with the ability to finely control assistance levels to each digit during task practice. However, these robots are complex, tethered and costly, which may limit integration with daily activities and transition to home-based therapy interventions.
In this study we performed a feasibility study on home use of Hand Spring Operated Movement Enhancer (HandSOME), which utilizes springs for finger extension assistance allowing for lightweight, inexpensive, and portable actuation that enables integration of the impaired hand into ADL practice. While some commercially available portable passive devices exist, including the SaeboFlex [26] and SaeboGlove (Saebo Inc., Charlotte NC), the spring assistance from these devices allows for limited finger ROM. In the case of the SaeboFlex, only large objects can be grasped because the applied torque increases rapidly as the fingers close, requiring high flexor forces when grasping small objects. Previously, we showed that HandSOME could improve ROM and functional grasp when worn by individuals with stroke [27]. While most stroke patients regain the ability to flex their fingers voluntarily, they have limited recovery of volitional extension. This pattern of recovery is due to involuntary activation of flexors, inability to activate the extensors and flexor hypertonia (increased resistance to passive extension) [28][29]. The path of the springs on HandSOME provide an extension torque that approximately matches the torque required to open the fingers passively, thereby compensating for flexor hypertonia and maximizing ROM [27]. HandSOME also couples thumb and finger movement through a linkage to ensure coordinated grasp and functional use of the hand.
The goal of this feasibility study was to determine the degree that subjects would comply with this home-based intervention, as measured by the number of movement repetitions performed. We identified the dropout rate and assessed the main challenges to compliance. Finally, we measured the magnitude of gains in function and ROM after home training with HandSOME, to determine if a larger scale controlled study was justified.
II. Methods
Ten subjects were enrolled into the study (Table 1). All subjects had a diagnosis of stroke more than six months prior to entry into the study, impaired ability to open the affected hand and difficulty performing reach and grasp tasks. Subjects were required to have trace ability to extend the wrist and fingers and full passive wrist ROM. All participants provided informed consent. The study protocol was approved by the Institutional Review Board of MedStar Health Research Institute.
Table 1.
Patient Characteristics
| Gender | Dominant side | Impaired side | Age (years) | Months Post | # of previous UE intervention studies | Baseline ARAT | Baseline FM | ||
|---|---|---|---|---|---|---|---|---|---|
| S01* | M | R | L | 65 | 44 | 0 | 21 | 25 | |
| S02 | M | L | R | 68 | 63 | 1 | 33 | 45 | |
| S03 | M | R | R | 55 | 35 | 0 | 28 | 42 | |
| S04 | M | R | L | 64 | 39 | 0 | 28 | 37 | |
| S05 | M | R | L | 36 | 59 | 1 | 14 | 28 | |
| S06* | F | R | L | 69 | 15 | 0 | 12 | 20 | |
| S07 | F | R | R | 42 | 26 | 0 | 9 | 37 | |
| S08 | M | R | R | 66 | 106 | 0 | 26 | 48 | |
| S09* | F | R | L | 54 | 30 | 0 | 14 | 42 | |
| S10 | M | R | L | 68 | 20 | 0 | 24 | 33 | |
Patients that withdrew from the study prior to completion.
A. HandSOME intervention
HandSOME (Fig.1) uses a four bar linkage to coordinate the movement of the finger metacarpophalangeal (MCP) joints and the thumb carpometacarpal (CMC) joint, ensuring normal kinematics during pinch-pad grasp. As the digits close, the spring assistance decreases, enabling a large ROM without fatiguing the subject when grasping objects. The magnitude of the torque assistance is adjustable by changing the number of elastic cords. We used two types of elastic cords: a thick polypropylene covered elastic cord (stiffness k = 297 N/m) and a thin elastic cord (k = 89 N/m). Both cord types were 5cm long at rest length and the therapist could customize the number and type of cords for each subject. Fig. 2 shows typical assistance profiles from the three most common spring configurations. Friction was less than 0.038 Nm [27].
Fig. 1.
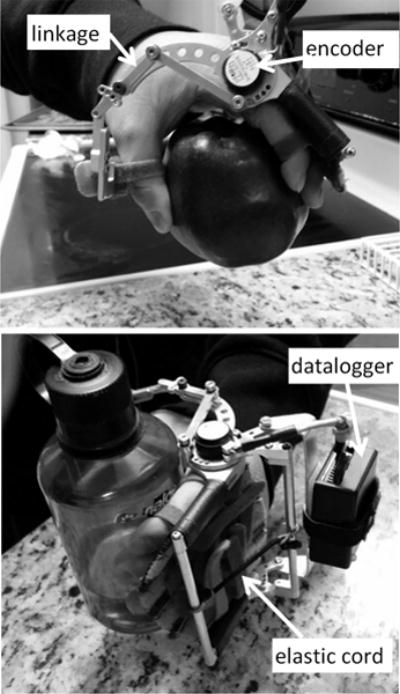
Hand Spring Operated Movement Enhancer (HandSOME). The encoder measures movement and a battery powered datalogger saves position data of the hand. The four bar linkage couples movement of the thumb and fingers. Elastic cords provide assistance torque to counter balance flexor hypertonia and assist weak extensor muscles. The assistance level can be changed by adjusting the number of cords. A fitting pad customizes the location of Velcro loops that hold the fingers in place.
Fig. 2.
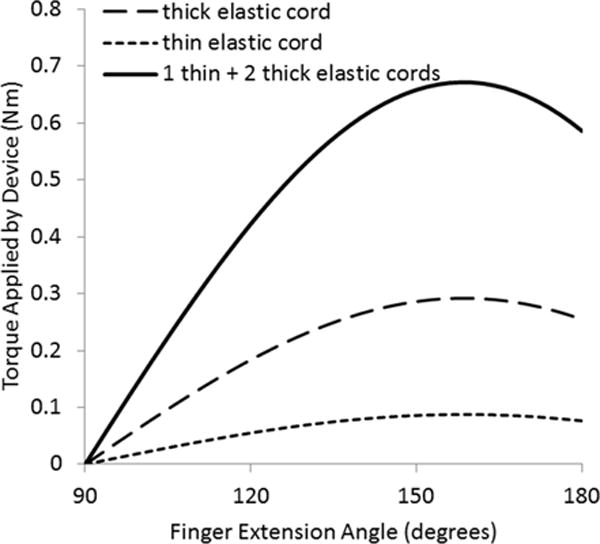
Typical assistance torque profiles used during training. Full flexion is 90 degrees and full extension is 180 degrees.
During the first visit to the clinic, the therapist fitted HandSOME to the subject’s hand and provided training on how to don the device independently. The subjects were asked to perform 90 minute therapy sessions at home, at least five times a week for four weeks. Each training session began with donning the HandSOME and focused on the object manipulation tasks prescribed by the physical therapist. The movement of the affected hand was measured by an encoder (E4 optical rotary encoder, US Digital, Vancouver, WA) at the center of the MCP joints and Arduino-based logging electronics that were integrated into HandSOME. Once a week, the subject returned to the clinic to download stored data and replace the battery integrated into the logging electronics. During this visit, the therapist would troubleshoot any problems the subject was having, adjust the tasks to be performed during the week and adjust the HandSOME spring configuration if needed. During all clinic visits, the number of practice repetitions was kept to a minimum so that any gains could be attributed to the home practice.
The therapist developed a list of tasks to perform during the home training based on subject ability and preferences. Subjects used objects at their home similar to the following: water bottle, pill bottle, pen, large object (3–4″ width), small object (1/2–3/4″ width), jar with lid. The bimanual tasks were to remove and then replace the pill bottle cap and jar lid. The other tasks were to pick up the object and place at another location. Tasks were graded in several ways based on therapist judgement. Targets started on the tabletop and progressed by moving further away from the body and more laterally. Once this was achieved, targets were progressed to different heights. If the subject had mastered all of these levels, objects were made heavier. Additionally, the therapist could ask the subject to perform the tasks standing (easier) or sitting (harder), or with a pronated (easier) or neutral forearm posture (harder).
B. Clinical outcome measures
All assessments were performed before and after the 4-week training intervention and again 3 months after the end of training. The Fugl-Meyer assessment of the upper extremity (FM) was used to assess motor impairments at the shoulder, elbow, wrist and fingers [30]. The FM evaluates reflexes, coordination patterns and the ability to perform several simple movements. The Action Research Arm Test (ARAT) was used to assess functional use of the upper extremities [31]. It is based on performance of 19 items that are divided into four subscales: Grasp, Grip, Pinch, and Gross movement. The Motor Activity Log (MAL) assessed use of the limb at home [32]. It is a structured interview during which respondents are asked to rate how they use their more-impaired arm for 28 ADL in the home. Activities include brushing teeth, buttoning a shirt or blouse, and eating with a fork or spoon. The Modified Ashworth Scale (MAS) was used to assess hypertonia at the fingers, wrist and elbow [33].
C. Biomechanical outcome measures
Subjects were seated in front of a table and performed 2 repetitions of 5 tasks. The tasks were: 1) full digit flexion/extension: straightening the fingers as much as possible from a closed fist position; 2) thumb opposition: touching the thumb to the tip of the 5th digit; 3) grasp a water bottle and bring to mouth to drink; 4) pick up a small nut and put it on the top of a shelf; 5) grip strength was quantified with a dynamometer (JAMAR 5030J1 Hand Dynamometer). Tasks 1 and 2 were used to measure the ROM in the thumb and fingers. Tasks 3 and 4 measured how well the arm and hand were coordinated during reach and grasp. Motion capture was performed with an electromagnetic motion capture system, the MiniBirds® (Ascension Technologies) controlled by the Motion Monitor® Software (Innovative Sports Technology). Electromagnetic markers were taped to the nail of the thumb, index, middle and ring fingers. Additional markers were placed on the back of the hand and at the wrist. The position and orientation of each marker were sampled at 120 Hz.
The thumb abduction angle and total extension angle of each digit, defined as the sum of the three extension joint angles within that digit, were calculated based on the Euler sequences recommended by the International Society of Biomechanics [34]. For Tasks 1 and 2, extension ROM of all 4 digits were averaged to provide a general measure of the ability to open the hand.
For the reach and grasp tasks (Tasks 3 and 4), the hand path ratio was calculated based on the wrist marker data. Hand path ratio is the length of the path of the wrist marker normalized to the length of the straight line that connects the start and stop points of the movement [35]. Smaller hand path ratios indicate more direct movements and less reliance on proximal compensation. For each trial, visual inspection was used to mark 3 time points: the start of movement, the time when the object was grasped and the time when the object was at its final location. The straight line path and actual path length taken between these 3 locations was calculated and used to form the hand path ratio.
D. Training Intensity
The total number of movements each subject performed during the training sessions was calculated from the HandSOME encoder data, which measured MCP flexion/extension. Velocity peaks greater than 5 deg/sec in amplitude and separated by more than 120ms were identified. A movement was defined by the time points before and after the peak where velocity dropped to below 10% of the peak velocity. The peak was ignored if velocity did not drop below 10% of peak velocity before the next peak. Movement amplitudes were calculated from these start and stop time points. Movements less than 4 degrees were not included.
E. Data analysis
The Shapiro Wilk test was performed on all data to test normality assumptions for statistical analysis. Paired t-tests were used to determine significant differences between the post-training and baseline time points, and between the follow-up and baseline time points. A Bonferroni correction was applied to account for multiple comparisons; all p values were multiplied by a factor of 2. The effect of training intensity on functional improvement was determined by calculating the correlation between ARAT score gains and number of movements performed during training.
III. Results
Seven subjects completed the protocol. Six of these subjects donned the device independently and one required help from a caregiver. Three subjects dropped out due to difficulty donning and doffing the device and lack of caregivers at home to assist. The 7 subjects who completed the study were generally positive about the treatment and several commented that they were trying to use their hand more after the 4 week training period. The number of movements (including both flexion and extension) varied considerably across the 7 subjects from a low of 43 per day to a high of 1873 per day (mean of 448±651 movements per day). One subject performed a total of 37460 movements, while the rest of the subjects performed less than 9300 movements. The total movement number distribution was normalized with a log transformation before statistical analysis (Shapiro-Wilk, p=0.55). The number of hours the device was used varied widely from 3 to 33 hours. There was a significant correlation between hours of training and movements performed (r=0.82, p=0.026), which supports the notion that subjects who performed a low number of movements did not comply with the 1.5 hours per day guideline. There was no evidence that compliance was affected by impairment level. The correlation between number of movements and impairment level (baseline FM) was not significant (r=0.52, p=0.23). Additionally, two of the dropouts had baseline FM scores below the mean, while the third had a baseline FM above the mean.
The training did not increase hypertonia in the fingers, wrist or elbow (Table 2). Average MAS scores were not increased at the post training or follow-up time points relative to baseline (p>0.6). There was a significant decrease in impairment at the post time point; FM scores increased by 4.9±4.1 points (p=0.039). There were also significant gains in function at the post time point; ARAT scores increased by 3.3±2.6 (p=0.032). There was a strong and significant correlation between the number of movements performed and gains in function, as measured by the ARAT (r=0.90, p=.005) (Fig. 3). Five subjects (#3, #4, #7, #8, #10) responded well to the intervention and had gains of 6 points or more on either the ARAT or the FM at the post time point, which meets or exceeds the Minimum Clinically Important Difference (MCID) for these clinical tests [36], [37]. However, gains in the FM and ARAT were no longer significant at the 3 month follow-up (Table 2). Gains in amount of functional limb use at home (MAL) were not significant at the post time point, but gains approached significance at the 3 month follow-up; MAL scores increased by 0.33±0.32 (p=0.07) at follow-up.
Table 2.
Mean (SD) Changes in Outcomes Measures
| Baseline | Change at Post | Change at Followup | |||
|---|---|---|---|---|---|
| FM (max=66) | 38.6 (6.9) | 4.9 (4.1) | p=0.039 | 4.0 (4.7) | p=0.133 |
| ARAT (max=57) | 23.1 (8.5) | 3.3 (2.6) | p=0.032 | 0.6 (3.3) | p=1.000 |
| MAL (max=5) | 1.3 (0.7) | 0.23 (0.28) | p=0.145 | 0.33 (0.32) | p=0.070 |
| MAS-elbow# | 1.1 (0.6) | 0.3 (0.7) | p=0.642 | 0.1 (0.4) | p=1.000 |
| MAS-wrist# | 0.9 (0.9) | −0.1 (0.5) | p=0.914 | 0.0 (0.6) | p=1.000 |
| MAS-fingers# | 1.4 (1.1) | −0.3 (0.8) | p=0.772 | 0.0 (0.5) | p=1.000 |
| finger extension (deg)* | 139.7 (46.0) | 6.5 (24.2) | p=1.000 | 1.2 (23.9) | p=1.000 |
| thumb abduction (deg) | 51.9 (17.7) | 8.1 (18.9) | p=0.598 | −0.5 (25.8) | p=1.000 |
| grip strength (lbs) | 24.7 (15.6) | 3.0 (4.0) | p=0.189 | 0.0 (9.6) | p=1.000 |
| reach path ratio# | 1.8 (0.3) | −0.4 (0.3) | p=0.038 | −0.4 (0.5) | p=0.340 |
full extension is 180 degrees
decreases indicate improvement
FM= Fugl-Meyer, ARAT= Action Research Arm Test, MAL=Motor Activity Log, MAS= Modified Ashworth Scale
Fig. 3.
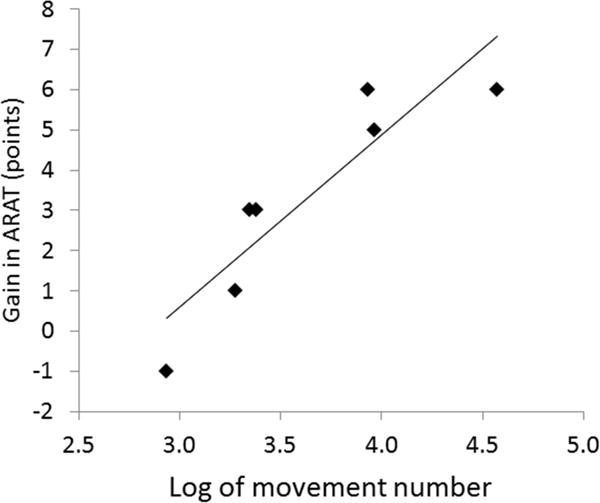
Correlation between log of number of movements and gains in the ARAT immediately after training (r=0.90, p=.005).
Biomechanical data were generally consistent with clinical outcomes. As a group, there were no significant changes in digit extension or thumb abduction throughout the study (Table 2). However, there was a large variance across subjects, and the 5 subjects who responded well to the treatment, as determined by clinical score gains greater than MCID, all had improved finger extension and thumb abduction (example data are shown in Figs. 4&5). This subgroup of 5 subjects achieved significant gains at the post-training time point in digit extension (mean gain=19.8±10.2 degrees, p=0.024). However, at the 3-month follow-up, gains were no longer significant in digit extension (mean gain=1.4±29.3 degrees, p=1.0).
Fig. 4.
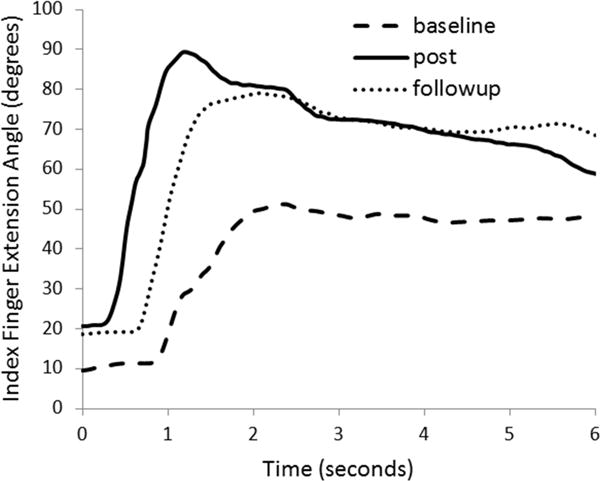
Data from subject 3 during Task 1 (range of motion test), showing gains in index finger extension post training. Gains were mostly retained at the followup time point. The ideal curve would peak at 180 degrees, full extension.
Fig. 5.
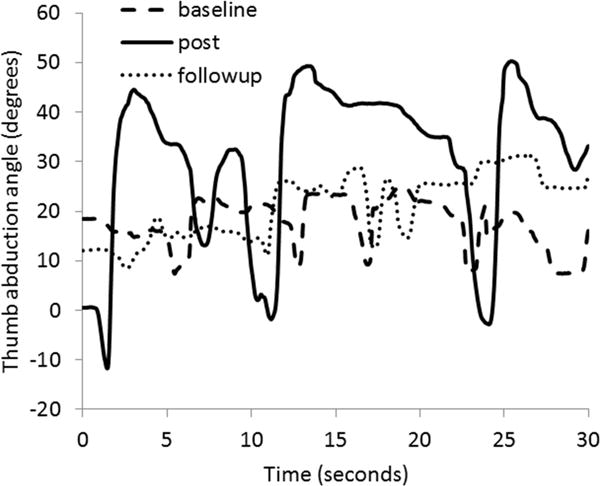
Data from subject 7 during Task 2 (thumb opposition task). Gains in thumb range of motion were apparent post training, but performance had returned to baseline levels by the follow-up time point. The ideal curve would range from 0 to 90 degrees of abduction.
Improvements in proximal arm control were evidenced by changes in the reach path ratio (Table 2). The ratio decreased significantly at the post-training time point (p=0.038), but changes were no longer significant at the 3-month follow up (p=0.340). Grip strength did not change significantly across the 3 time points.
IV. Discussion
In this convenience sample of 10 chronic stroke subjects, three individuals withdrew from the study due to difficulty donning the device. Of the remaining 7 individuals, 5 achieved clinically significant improvements in impairment (FM) and/or functional (ARAT) use of their affected limb. This result is promising considering that the training was done at home with inexpensive technology and without therapist supervision. The only treatment-related burden on the clinical staff was a weekly visit with the therapist to troubleshoot any problems and adjust the treatment regimen. This protocol could be easily integrated with the outpatient phase of usual care and could potentially improve the rate and level of recovery of individuals after stroke.
The ability to independently and easily don the device was critical for compliance. Three subjects dropped out predominantly due to difficulty donning the device and all subjects preferred trying to don the device themselves, despite having caregivers who could assist. Future work is needed to improve independent use for this population. Additionally, two individuals completed the study but did not have clinically significant gains after the intervention. At baseline these individuals were not substantially more impaired than other study subjects (Table 1). However, these individuals performed 2312 and 867 total movements during the intervention, which was well below the group mean of 8957 movements. Reduced compliance and engagement with the home-based intervention could have been a factor for these individuals. Additionally, the potential for gains may have already been exhausted in these 2 subjects; they were the only subjects in the group who participated in a prior treatment study that involved 24 hours of upper extremity therapy, 12 of which involved using the HandSOME in conjunction with an arm robot [38]. A larger sample size could provide additional information about the characteristics of the individuals most likely to benefit from the treatment.
Across all subjects, the average number of movements completed per day was 448, which is much higher than the number of movements performed during a conventional therapy session (32 functional and 54 ROM movements) [39]. However there was large variation across subjects in total movements performed, and a strong correlation was observed between ARAT score gains and the number of movements practiced. We chose to perform correlations with the ARAT because it tests functional reach and grasp tasks, which were the focus of the home training. However, this dose effect has not been consistently observed in studies of individuals with partial ability to open the hand (as were used in our study). A recent study carefully controlled the number of repetitions during massed practice therapy, and found no dosage effect in chronic stroke subjects who received 3200, 6400, 9600 or 10,808 repetitions of upper extremity tasks [40]. Additionally, a recent multisite study of 361 subacute stroke patients found no differences between groups who received 28.3 hours of intensive task oriented training, 26.7 hours of occupational therapy and a usual and customary care group that received 11.2 hours of occupational therapy [41]. While the number of movement repetitions were not reported in this study, it is likely the groups who received more time in therapy received much higher numbers of task repetitions. These studies support the notion that the effects of task specific training may plateau at a certain number of repetitions. While our small pilot study is not directly comparable to these larger controlled studies, the presence of subjects in our study who performed a very low number of movements may have contributed to the significant dosage effect we observed in our data.
Our study also showed the importance of the inclusion of long term follow-up assessments when examining clinical interventions. There was a striking decline in nearly all clinical and biomechanical measures between the post and follow-up time points. All of the significantly improved outcomes at the post time point were no longer significant at the follow-up time point (FM, ARAT, reach path ratio). The 5 subjects who responded well to the treatment and had significant gains in extension ROM immediately after treatment, returned to baseline levels 3 months later. The “threshold” hypothesis put forward by Schweighofer could explain this result [42]. They used a sophisticated computational model to predict that if motor training brings performance above a certain threshold, spontaneous arm use will be sufficient to drive further gains in performance and spontaneous use. However, performance gains that don’t reach the threshold for promoting spontaneous arm use will be in vain and any gains immediately after training will be lost at followup. One subject did perform a very large number of repetitions during training (1873 movements per training day). However, this subject also did not cross the threshold, as his clinical scores did not improve further at the 3-month followup. To promote spontaneous use of the affected limb, in future studies, subjects will be asked to wear the device as an orthosis to assist during real ADL in addition to the regimen prescribed by the therapist. We will also fabricate customized plastic versions of the HandSOME that will be given to subjects to use during the follow-up period, in the hopes that highly motivated subjects will continue using the device without any direct contact with therapists.
The learned nonuse hypothesis states that stroke patients do not spontaneously use the affected limb despite having adequate motor capacity because of a conditioned behavior to compensate with the other limb [43]. CI therapy has been designed to reverse learned nonuse and has been shown to improve both motor capacity and spontaneous arm use [44]. The learned nonuse phenomenon could explain the gains we observed immediately after treatment that were lost at followup, presumably because the subjects returned to their baseline levels of limb use during the followup period. The addition of the “transfer package”, used in CI therapy to promote spontaneous limb use, may have prevented the losses at followup. The role of learned nonuse could be tested by using subacute patients, who would be less affected by learned nonuse, or assessing the degree of nonuse in chronic subjects by comparing baseline clinical scores with those at hospital discharge.
HandSOME is similar conceptually to the Script Passive Orthosis (SPO), which provides individualized spring extension assistance at the wrist and each digit [45]. A home-training study with SPO reported gains in the FM similar in magnitude to what we observed [46], however a second controlled study found no differences between this experimental home treatment and a control group that received a standard home exercise regime [47]. Similar to our results, these 2 studies with SPO also observed a large variance across subjects in the amount of therapy performed and significant dosage effects. However, there are many differences between the SPO intervention and ours. The SPO intervention focused on joint ROM exercises, with the arm supported against gravity (SaeboMAS), and prompted by video games. Our intervention involved functional unimanual and bimanual tasks, such as reaching, grasping and manipulating real objects at the patient’s home, and allowed movement practice anywhere in the home, including while standing. This focus on functional tasks may explain the gains we observed in the ARAT. However, this required adequate proximal arm and grip strength to allow completion of tasks. These 2 protocols could be combined for maximum effect, with subjects initially performing ROM exercises with gravity support, and as strength improved, this could be followed by practice of functional tasks throughout the home.
There are also technical differences between the SPO and HandSOME. SPO allows individual control of the 5 digits, while HandSOME uses a 4-bar linkage to couple the digits together as one-DOF. This guarantees that objects can be grasped with a simple grasping pattern, even of coordination between joints is poor. At the wrist, HandSOME uses a standard soft wrist splint if needed, while the SPO provides spring assistance to wrist flexion/extension movement. SPO uses a leaf spring combined with an elastic cord to provide a fairly constant torque offset to the digits. In contrast, HandSOME provides a torque profile that decreases as the fingers flex, so that excessive grasp force is not needed to overcome the springs when grasping small objects. Further study is needed to determine if any of these differences are clinically relevant.
Although in many cases the alternative to independent home therapy is no therapy at all, the lack of a control group is a limitation of this study. It is not possible to determine if similar or even better results could have been achieved with a different home therapy program that did not include HandSOME. However, few functional tasks would have been possible without the use of HandSOME since subjects were selected who had major difficulty performing grasp and release tasks unassisted and use of the device greatly expanded the range of tasks that could be practiced at home.
Future work will focus on improving the usability of the device to increase compliance. We plan to use a single strap for all 4 fingers if subjects have difficulty using the multiple strap method. We also plan to reduce the overall bulkiness of the structure which may limit using the device as part of real ADL performance. Additionally, current work involves a more complicated high-DOF version of the HandSOME that allows for a larger range of movement patterns, including pointing, typing, key grip, power grasp and fine pinch [48]. We also observed that hand opening ability decreased when the arm was lifted against gravity, even when wearing HandSOME. We are developing a wearable passively powered exoskeleton to be used in conjunction with HandSOME that provides variable levels of gravity compensation for the shoulder [49]. This would reduce the effort required to complete tasks for subjects with proximal weakness, potentially improving compliance.
Overall the results from this study showed that individuals with stroke can achieve significant improvements after 4-weeks of independent home intervention with the HandSOME device. While these results are preliminary, and retention of gains remains to be demonstrated, these findings are promising due to the low cost of the intervention and the potentially straightforward integration of this intervention into outpatient therapy. Additional examination of the use of HandSOME for independent home therapy is recommended.
Acknowledgments
This work was supported by NIH R15 HD075166-01A1 and NIH R21 HD088783-01.
Biographies

Ji Chen (S’16) received the B.S. degree in mechanical engineering from University of Electronic Science and Technology of China, Chengdu, Sichuan Province, China, in 2003, the M.S. degree in mechanical engineering, Temple University, Philadelphia, PA, USA, in 2010, and Ph.D. degree in biomedical engineering, The Catholic University of America, Washington, DC, USA in 2017. He is currently a postdoc fellow in the Clinical Center at National Institute of Health, Bethesda, MD, USA. His research interests lie in the exoskeleton design and robotic rehabilitation for stroke patients and children with cerebral palsy. He was previously a research assistant at Center for Applied Biomechanics and Rehabilitation Research, MedStar National Rehabilitation Hospital, Washington, DC.

Diane Nichols has been a practicing physical therapist for more than 30 years. She focuses primarily on treating neurologically impaired individuals and her work spans all practice settings: acute care, outpatient, home care, long-term care and inpatient rehabilitation. Ms. Nichols works at the National Rehabilitation Hospital (NRH) in Washington, DC. She received her physical therapy degree from the University of Maryland at Baltimore and received the APTA Neurology Section’s 2009 Clinical Excellence Award.

Elizabeth B. Brokaw (M’10) received her B.S. from the University of Maryland, College Park MD in Biological Resources Engineering in 2008. She received the M.S. degree in Biomedical Engineering in 2009 and Ph.D. in 2012 from The Catholic University of America, Washington DC. Her research has focused on the development and clinical evaluation of a broad range of robotic and wearable technologies for rehabilitation and quantitative assessment of neurological conditions.

Peter S. Lum (M’09) received his B.S. from George Washington University in Mechanical Engineering in 1987. He received the M.S. degree in Applied Mechanics, from the California Institute of Technology, Pasadena CA in 1988. He received the PhD degree in Bioengineering from a joint program between the University of California at San Francisco and Berkley in 1993. He is currently a Professor in the Biomedical Engineering department at The Catholic University of America. He is also a Research Health Scientist at the Washington DC Veterans Affairs Medical Center and the Director of the Center for Applied Biomechanics and Rehabilitation Research (CABRR) at MedStar National Rehabilitation Hospital.
Contributor Information
Ji Chen, Biomedical Engineering Department, The Catholic University of America, Washington DC 20064. He was also with CABRR (Center for Applied Biomechanics and Rehabilitation Research), MedStar National Rehabilitation Hospital, Washington, DC 20010.
Diane Nichols, CABRR, MedStar National Rehabilitation Hospital, Washington, DC 20010.
Elizabeth B. Brokaw, Biomedical Engineering Department, The Catholic University of America, Washington DC 20064. She was also with CABRR, MedStar National Rehabilitation Hospital, Washington, DC 20010
Peter S. Lum, Biomedical Engineering Department, The Catholic University of America, Washington DC 20064. He is also with CABRR, MedStar National Rehabilitation Hospital, Washington, DC 20010.
References
- 1.Roger VL, et al. Executive summary: Heart disease and stroke statistics–2012 update: A report from the american heart association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Lum PS, Godfrey SB, Brokaw EB, Holley RJ, Nichols D. Robotic approaches for rehabilitation of hand function after stroke. Am J Phys Med Rehabil. 2012;91(11 Suppl 3):S242–54. doi: 10.1097/PHM.0b013e31826bcedb. [DOI] [PubMed] [Google Scholar]
- 3.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database of Systematic Reviews. 2015 Nov 7;11:CD006876. doi: 10.1002/14651858.CD006876.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maciejasz P, Eschweiler J, Gerlach-Hahn K, Jansen-Troy A, Leonhardt S. A survey on robotic devices for upper limb rehabilitation. J Neuroeng Rehabil. 2014 Jan 9;11:3. doi: 10.1186/1743-0003-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3(9):528–536. doi: 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milot MH, Spencer SJ, et al. A crossover pilot study evaluating the functional outcomes of two different types of robotic movement training in chronic stroke survivors using the arm exoskeleton BONES. J Neuroeng Rehabil. 2013 Dec 19;10:112. doi: 10.1186/1743-0003-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein J, Spencer SJ, Reinkensmeyer DJ. Breaking it down is better: haptic decomposition of complex movements aids in robot-assisted motor learning. IEEE Trans Neural Syst Rehabil Eng. 2012 May;20(3):268–75. doi: 10.1109/TNSRE.2012.2195202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon J. Movement Science: Foundations for Physical Therapy in Rehabilitation. Aspen Publishers Inc.; Rockville Md: 1987. Assumptions underlying physical therapy intervention: Theoretical and historical perspectives; p. 30. [Google Scholar]
- 9.Kwakkel G, Kollen B. Predicting improvement in the upper paretic limb after stroke: A longitudinal prospective study. Restor Neurol Neurosci. 2007;25(5–6):453–460. [PubMed] [Google Scholar]
- 10.Schabowsky CN, Godfrey SB, Holley RJ, Lum PS. Development and pilot testing of HEXORR: Hand EXOskeleton rehabilitation robot. J Neuroeng Rehabil. 2010 Jul 28;7:36. doi: 10.1186/1743-0003-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutner NG, Zhang R, Butler AJ, Wolf SL, Alberts JL. Quality-of-life change associated with robotic-assisted therapy to improve hand motor function in patients with subacute stroke: A randomized clinical trial. Phys Ther. 2010;90(4):493–504. doi: 10.2522/ptj.20090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouzit M, Popescu G, Burdea G, Boian R. The Rutgers Master II-New Design force-feedback glove. IEEE/ASME Trans Mechatronics. 2002;12(4):399–407. [Google Scholar]
- 13.Dovat L, Lambercy O, Gassert R, Maeder T, Milner T, Leong TC, Burdet E. HandCARE: A cable-actuated rehabilitation system to train hand function after stroke. IEEE Trans Neural Syst Rehabil Eng. 2008;16(6):582–591. doi: 10.1109/TNSRE.2008.2010347. [DOI] [PubMed] [Google Scholar]
- 14.Stein J, Bishop J, Gillen G, Helbok R. A pilot study of robotic-assisted exercise for hand weakness after stroke. IEEE Int Conf Rehabil Robot. 2011;2011:5975426. doi: 10.1109/ICORR.2011.5975426. [DOI] [PubMed] [Google Scholar]
- 15.Taheri H, Rowe JB, Gardner D, Chan V, Gray K, Bower C, Reinkensmeyer DJ, Wolbrecht ET. Design and preliminary evaluation of the FINGER rehabilitation robot: Controlling challenge and quantifying finger individuation during musical computer game play. J Neuroeng Rehabil. 2014 Feb 4;11:10. doi: 10.1186/1743-0003-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambercy O, Dovat L, Gassert R, Burdet E, Teo CL, Milner T. A haptic knob for rehabilitation of hand function. IEEE Trans Neural Syst Rehabil Eng. 2007;15(3):356–366. doi: 10.1109/TNSRE.2007.903913. [DOI] [PubMed] [Google Scholar]
- 17.Masia L, Krebs HI, Cappa P, Hogan N. Design and characterization of hand module for whole-arm rehabilitation following stroke. IEEE ASME Trans Mechatron. 2007;12(4):399–407. doi: 10.1109/TMECH.2007.901928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesse S, Kuhlmann H, Wilk J, Tomelleri C, Kirker SG. A new electromechanical trainer for sensorimotor rehabilitation of paralysed fingers: A case series in chronic and acute stroke patients. J Neuroeng Rehabil. 2008 Sep 4;5:21. doi: 10.1186/1743-0003-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann G, Schmit BD, Kahn JH, Kamper DG. Effect of sensory feedback from the proximal upper limb on voluntary isometric finger flexion and extension in hemiparetic stroke subjects. J Neurophysiol. 2011;106(5):2546–2556. doi: 10.1152/jn.00522.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller LC, Dewald JP. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012;123(6):1216–1225. doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo NJ, Rymer WZ, Kamper DG. Delays in grip initiation and termination in persons with stroke: Effects of arm support and active muscle stretch exercise. J Neurophysiol. 2009;101(6):3108–3115. doi: 10.1152/jn.91108.2008. [DOI] [PubMed] [Google Scholar]
- 22.Susanto EA, Tong RKY, Ho NSK. Hand exoskeleton robot for assessing hand and finger motor impairment after stroke. HKIE Transactions. 2015;22(2):78–87. [Google Scholar]
- 23.Connelly L, Jia Y, Toro ML, Stoykov ME, Kenyon RV, Kamper DG. A pneumatic glove and immersive virtual reality environment for hand rehabilitative training after stroke. IEEE Trans Neural Syst Rehabil Eng. 2010;18(5):551–559. doi: 10.1109/TNSRE.2010.2047588. [DOI] [PubMed] [Google Scholar]
- 24.Adamovich SV, Fluet GG, Mathai A, Qiu Q, Lewis J, Merians AS. Design of a complex virtual reality simulation to train finger motion for persons with hemiparesis: A proof of concept study. J Neuroeng Rehabil. 2009 Jul 17;6:28. doi: 10.1186/1743-0003-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer HC, Triandafilou KM, Thielbar KO, Ochoa JM, Lazzaro ED, Pacholski KA, Kamper DG. Use of a portable assistive glove to facilitate rehabilitation in stroke survivors with severe hand impairment. IEEE Trans Neural Syst Rehabil Eng. 2016;24(3):344–351. doi: 10.1109/TNSRE.2015.2513675. [DOI] [PubMed] [Google Scholar]
- 26.Farrell JF, Hoffman HB, Snyder JL, Giuliani CA, Bohannon RW. Orthotic aided training of the paretic upper limb in chronic stroke: Results of a phase 1 trial. Neurorehabilitation. 2007;22(2):99–103. [PubMed] [Google Scholar]
- 27.Brokaw EB, Black I, Holley RJ, Lum PS. Hand spring operated movement enhancer (HandSOME): A portable, passive hand exoskeleton for stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2011;19(4):391–399. doi: 10.1109/TNSRE.2011.2157705. [DOI] [PubMed] [Google Scholar]
- 28.Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve. 2003;28(3):309–318. doi: 10.1002/mus.10443. [DOI] [PubMed] [Google Scholar]
- 29.Kamper DG, Fischer HC, Cruz EG, Rymer WZ. Weakness is the primary contributor to finger impairment in chronic stroke. Arch Phys Med Rehabil. 2006;87(9):1262–1269. doi: 10.1016/j.apmr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 31.Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: Properties of the action research arm test. Arch Phys Med Rehabil. 2006;87(12):1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The motor activity log-28: Assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67(7):1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 33.Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 34.Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B, International Society of Biomechanics ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion–part II: Shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 35.Lang CE, Wagner JM, Bastian AJ, Hu Q, Edwards DF, Sahrmann SA, Dromerick AW. Deficits in grasp versus reach during acute hemiparesis. Exp Brain Res. 2005;166(1):126–136. doi: 10.1007/s00221-005-2350-6. [DOI] [PubMed] [Google Scholar]
- 36.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity fugl-meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 37.van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the action research arm test and the fugl-meyer assessment scale in chronic stroke patients. J Rehabil Med. 2001;33(3):110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- 38.Brokaw EB, Nichols D, Holley RJ, Lum PS. Robotic therapy provides a stimulus for upper limb motor recovery after stroke that is complementary to and distinct from conventional therapy. Neurorehabil Neural Repair. 2014;28(4):367–376. doi: 10.1177/1545968313510974. [DOI] [PubMed] [Google Scholar]
- 39.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson KT, Schindler-Ivens SM, Hornby TG, Ross SA, Scheets PL. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009 Oct;90(10):1692–8. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang CE, Strube MJ, Bland MD, Waddell KJ, Cherry-Allen KM, Nudo RJ, Dromerick AW, Birkenmeier RL. Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase II, single-blind, randomized, controlled trial. Ann Neurol. 2016;80(3):342–354. doi: 10.1002/ana.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, Cen SY, Azen SP, Interdisciplinary Comprehensive Arm Rehabilitation Evaluation (ICARE) Investigative Team Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: The ICARE randomized clinical trial. Jama. 2016;315(6):571–581. doi: 10.1001/jama.2016.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han CE, Arbib MA, Schweighofer N. Stroke Rehabilitation Reaches a Threshold. PLoS Comput Biol, vol. 2008;4(8):e1000133. doi: 10.1371/journal.pcbi.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006 Sep;42(3):241–56. [PubMed] [Google Scholar]
- 44.Wolf SL, Winstein CJ, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006 Nov 1;296(17):2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 45.Ates S, Lobo-Prat J, Lammertse P, van der Kooij H, Stienen AH. SCRIPT passive orthosis: design and technical evaluation of the wrist and hand orthosis for rehabilitation training at home. IEEE Int Conf Rehabil Robot. 2013 Jun;:6650401. doi: 10.1109/ICORR.2013.6650401. [DOI] [PubMed] [Google Scholar]
- 46.Nijenhuis SM, Prange GB, Amirabdollahian F, Sale P, Infarinato F, Nasr N, Mountain G, Hermens HJ, Stienen AH, Buurke JH, Rietman JS. Feasibility study into self-administered training at home using an arm and hand device with motivational gaming environment in chronic stroke. J Neuroeng Rehabil. 2015 Oct 9;12:89. doi: 10.1186/s12984-015-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nijenhuis SM, Prange-Lasonder GB, Stienen AH, Rietman JS, Buurke JH. Effects of training with a passive hand orthosis and games at home in chronic stroke: a pilot randomised controlled trial. Clin Rehabil. 2016 Feb 11; doi: 10.1177/0269215516629722. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Chen T, Lum PS. Hand rehabilitation after stroke using a wearable, high DOF, spring powered exoskeleton. Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Orlando, FL, USA. 2016. pp. 578–581. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Lum PS. Spring operated wearable enhancer for arm rehabilitation (SpringWear) after stroke. Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Orlando, FL, USA. 2016. pp. 4893–4896. [DOI] [PubMed] [Google Scholar]


