Abstract
Objectives
Adrenergic receptor (ADR) genotypes have been associated with adverse outcomes in heart failure. Our objective was to evaluate the association of ADR genotypes with post-Norwood outcomes in infants with hypoplastic left heart syndrome (HLHS).
Methods
Infants with HLHS participating in the Pediatric Heart Network Single Ventricle Reconstruction Trial underwent genotyping for four single nucleotide polymorphisms in three ADR genes: ADRB1_231A/G, ADRB1_1165G/C, ADRB2_5318C/G and ADRA2A_2790C/T. The association of genotype with freedom from serious adverse events (SAE) (death, transplant, extra-corporeal membrane oxygenation, cardiopulmonary resuscitation, acute shunt failure, unplanned re-operations, or necrotizing enterocolitis) during 14 months follow-up was assessed using Cox regression and the association with post-Norwood complications was assessed using Poisson regression. Models were adjusted for clinical and surgical factors.
Results
The study included 351 eligible patients (62% male; 83% White). The mean age at Norwood procedure was 5.6±3.6 days. 152 patients had SAEs during 14-month follow-up including 84 deaths and 10 transplants. ADRA2A_2790CC genotype had lower SAE-free survival compared to CT/TT genotypes during follow-up (Log rank test, p=0.02) and this association was independent of clinical and surgical risk factors (Adjusted Cox regression. HR 1.54 [1.04, 2.30] p=0.033). Post-Norwood complication rate did not differ by genotype.
Conclusions
Infants with HLHS harboring ADR genotypes that are associated with higher catecholamine release or sensitivity had lower event-free survival after staged palliation. Excess catecholamine activation may adversely affect cardiovascular adaptation after the Norwood procedure. Future studies should explore if targeting adrenergic activation in those harboring risk genotypes can improve outcomes. (ClinicalTrials.gov number, NCT00115934)
Single ventricle lesions comprise over 7% of all congenital heart defects with hypoplastic left heart syndrome (HLHS) being the most common lesion.1, 2 In HLHS, a single right ventricle (RV) acts as a systemic ventricle while the left ventricle (LV) is hypoplastic or rudimentary. Patients with HLHS require multiple palliative surgeries to separate the pulmonary and systemic circulations with the first staged palliation i.e. the Norwood procedure performed in the newborn period. Maintenance of systemic cardiac output after the Norwood procedure requires a balance between the systemic and pulmonary circulations and adequate RV function. Abnormal systemic and pulmonary vascular resistance and ventricular dysfunction are major contributors to maladaptation to a single ventricle physiology. In one analysis, up to 40% of patients with staged palliation were noted to have some degree of heart failure.3
Single ventricle patients have fragile hemodynamics and are especially vulnerable after cardiac surgery. The post-operative physiological stress response involves a surge in endogenous catecholamine production which can have profound effects on cardiac function and vascular tone mediated by adrenergic receptors. Adrenergic receptors include presynaptic α2a and α2c receptors that inhibit norepinephrine release, cardiac β1 receptors that mediate chronotropic and inotropic response to norepinephrine, and β2 receptors that mediate vascular smooth muscle relaxation and response to β-blockers.4 Adrenergic upregulation is an important mechanism of cardiovascular adaptation during stress particularly in the infant where the circulation is highly catecholamine-dependent.5, 6 While acute upregulation is compensatory, chronic upregulation can have detrimental effects by increasing systemic and pulmonary vascular resistance, and increasing myocardial oxygen demands, resulting in adverse cardiac remodeling and ventricular dysfunction.4, 7–12 In children with dilated cardiomyopathy, α2 and β1 upregulation and β2 downregulation genotypes were associated with worse ventricular function, heart failure progression and acute hemodynamic decompensation.13 The impact of adrenergic receptor genotypes on adaptation to hemodynamic and surgical stress in infants with HLHS undergoing staged palliation is not known. We hypothesized that genetic modulation of receptor activity would alter catecholamine response and thereby influence post-operative clinical course. The purpose of our study was to evaluate if genetic variants that upregulate adrenergic receptor activity adversely impact post-Norwood outcomes in infants with HLHS enrolled in the single ventricle reconstruction (SVR) trial.
METHODS
Study population
This is an ancillary study that derives from the Pediatric Heart Network (PHN) SVR Trial, in which infants with HLHS (n=555) were enrolled from 15 North American centers between May 2005 and July 2008. The details of the study design, inclusion criteria, study assessments as well as trial results have been previously published.14, 15 In brief, inclusion criteria for the SVR trial included a diagnosis of HLHS or a related single morphologic RV anomaly, planned Norwood procedure, and absence of a genetic or medical condition that would affect transplant-free survival. Infants were randomized to receive a modified Blalock-Taussig shunt (MBTS) versus a right ventricle – pulmonary artery shunt (RVPAS) as part of their Norwood procedure and were followed for 14 months with serial assessment. The study was approved by the local institutional review boards and informed consent for study participation was obtained from the parents or legal guardians. Data collection included patient demographics and baseline characteristics, operative variables, hospitalization course, clinical outcomes, and echocardiographic assessment of RV ejection fraction (RVEF). All echocardiograms were reviewed centrally by an independent observer at the echocardiography core laboratory.14, 15
Genotyping
Buccal swab epithelial cells were collected at enrollment or during Norwood hospitalization using CytoSoft cytology brushes (Medical Packaging Corporation, Camarillo, CA) after obtaining informed consent. Methods have been reported previously.16 Genomic DNA was extracted using a PureGene kit (Gentra Systems, Inc, Minneapolis, Minn) according to the manufacturer’s protocol. Samples were retrieved from the PHN Biorepository for genotyping. Participants in the SVR trial who did not consent to future testing of their samples were not included in the ancillary study. The ancillary study was approved by the PHN Ancillary Study Review Committee and by the University of Michigan Institutional Review Board.
Adrenergic receptor single nucleotide polymorphisms (SNPs) were selected on the basis of previous association studies, functional effects, and population allele frequencies.13, 17,24 Genotyping (Sequenom SNP) was performed for four SNPs in three adrenergic receptor (ADR) genes: a A/G missense variant at position 231 in ADRB1 resulting in a serine to glycine substitution (rs1801252), a C/G missense variant at position 1165 in ADRB1 resulting in a glycine to arginine substitution (rs1801253), a C/G missense variant at position 5318 in ADRB2 resulting in a glutamine to glutamic acid substitution (rs1042714) and a C/T 3’ untranslated region (UTR) variant at position 2790 in ADR2A (rs553668). Patients carrying a single copy of the variant allele or SNP (also known as minor allele) at the genetic locus were defined as having a heterozygous genotype, those with two copies of the variant allele as having a homozygous genotype, and those without a variant allele i.e. carrying only the normal allele (also known as major allele) were defined as having a wild-type genotype.
Since variants in the renin-angiotensin-aldosterone signaling (RAAS) pathway genes have been previously associated with adverse ventricular remodeling in infants with single ventricle lesions, genotyping was also performed for four SNPs and one insertion-deletion variant in five RAAS genes. The following were considered RAAS-upregulation genotypes based on our previous work: AGT_CC, ACE_DD, AGTR1_CC, CYP11B2_CC, and CMA1_AA. The CYP11B2 and CMA1 variants were genotyped using Sequenom SNP genotyping and the AGTR1, AGT and the ACE insertion/deletion polymorphisms were genotyped using TaqMan primers and probes. For samples and SNPs which did not yield a valid result by Sequenom or TaqMan analysis, PCR amplification followed by Sanger sequencing was performed. The detailed genotyping methods, primers and probes for the Sequenom SNP analysis, the Taqman analysis and for the PCR amplification or Sanger sequencing are listed in Supplemental Methods and Supplemental Table 1.
Statistical Analysis
Allele and genotype frequencies were determined to calculate Hardy-Weinberg equilibrium. All demographic and clinical data were expressed as frequencies, mean with standard deviations, or medians with interquartile ranges (IQR). The primary outcome was a composite of any serious adverse event (SAE) during a 14-month follow-up after the Norwood procedure and included death, transplant, need for post-operative extra-corporeal membrane oxygenation (ECMO), postoperative cardiopulmonary resuscitation (CPR), acute shunt failure, unplanned re-operations or necrotizing enterocolitis.25, 26 Secondary outcomes included frequency of post-Norwood complications up to hospital discharge from stage 2 surgery, and RVEF during 14 months follow-up. Complications were defined as adverse events using the common terminology criteria for adverse events version 3.0 developed by the National Cancer Institute, NIH, but that did not meet criteria for SAEs.26 Since post-Norwood hospital and intensive care unit (ICU) length of stay were highly correlated with each other and with Norwood complication rate when analyzed using bootstrap Spearman rank correlation (data not shown), these outcomes were not analyzed separately.
ADR genotype associations were analyzed by comparing outcomes in homozygous or heterozygous SNP carriers versus wild-types using a dominant model. Freedom from the primary outcome was described using the Kaplan-Meier method with stratification by genotype. The log-rank test was used to assess between-stratum differences. Cox proportional hazard models adjusted for covariates of interest were used to test the association of the genotypes with the primary outcome. The proportional hazards assumption was verified using cumulative Martingale process plots. Associations were quantified by hazard ratios (HRs) and reported along with their 95% confidence intervals (CIs). P-values were calculated based on Wald’s statistics. Poisson regression adjusted for over-dispersion and for exposure time (number of days of hospitalization for Norwood procedure) was used to model the association of risk genotype with frequency of post-Norwood complications and results were presented as incidence rate ratios (IRR) with 95% confidence intervals. A mixed model was used to assess for association of genotype with RVEF during 14 months follow-up. All analyses were adjusted for gender, race, birth weight, gestational age, presence of aortic atresia or obstructed pulmonary venous return, presence of a genetic syndrome, age at Norwood procedure, total Norwood cardiopulmonary bypass time, deep hypothermic circulatory arrest time, type of shunt, and presence of a RAAS-upregulation genotype.27 The covariates selected were not found to exhibit significant collinearity. In regression models, when patients had missing data on the presence of identifiable syndromes, it was assumed that identifiable syndromes were not present. Observations with missing data in other variables were excluded from the models and this represented a very small percentage of the total cohort. All statistical analyses were performed using SAS v9.4.
RESULTS
Of 555 patients in the SVR trial, 436 provided DNA and 351 had complete genotype data (i.e. genotyping results for all 9 SNPs) and were included in the analysis (Figure 1). ADR genotype frequencies are shown in Table 1. All genotypes were in Hardy Weinberg equilibrium. Patient clinical characteristics and outcomes in the group with complete versus incomplete genotypes are shown in Table 2. The only significant difference was a higher incidence of death in the completely genotyped cohort compared to the incompletely genotyped cohort. Incomplete genotyping was the result of technical issues such as low quality or quantity of DNA. The results hereby focus on the 351 subjects with complete genotype data.
Figure 1.
A consort flow diagram showing the outcomes in the complete and incompletely genotyped groups from patients randomized in the single ventricle reconstruction (SVR) trial.
Table 1.
Adrenergic receptor variant frequencies
| Gene (SNP ID) |
Nucleotide substitution |
Amino acid substitution |
Function by genotype |
SNP carrier frequency |
HWE p value |
|---|---|---|---|---|---|
| ADRB1 (rs1801252) | 231 A/G (missense) | Ser49Gly | GG: Higher basal adenylate cyclase activity and lower isoproterenol sensitivity | 32% | 0.717 |
| ADRB1 (rs1801253) | 1165 C/G (missense) | Gly389Arg | CC: Increases β1 receptor sensitivity | 44% | 0.818 |
| ADRB2 (rs1042714) | 5318 C/G (missense) | Gln27Glu | GluGlu: Enhances response to β adrenergic receptor antagonist | 62% | 0.972 |
| ADRA2A (rs553668) | 2790 C/T (3' UTR) | CC: Increased norepinephrine release | 36% | 0.918 |
ADR, adrenergic receptor; HWE, Hardy Weinberg equilibrium; SNP, single nucleotide polymorphism
Table 2.
Patient characteristics and outcomes
| Characteristics | N | Complete genotyping |
N | Incomplete genotyping |
p† |
|---|---|---|---|---|---|
| Gender, Male (%) | 351 | 220 (62.7%) | 85 | 56 (65.9%) | 0.62 |
| Race, White (%) | 351 | 288 (82.1%) | 85 | 68 (80.0%) | 0.44 |
| Birth weight (kg) | 351 | 3.13 ± 0.53 | 85 | 3.13 ± 0.53 | 0.96 |
| Gestational age (weeks) | 344 | 38 ± 1 | 83 | 38 ± 2 | 0.38 |
| Identifiable syndrome (%) | 150 | 22 (14.7%) | 19 | 3 (15.8%) | 1.00 |
| Aortic atresia (%) | 351 | 215 (61.3%) | 85 | 50 (58.8%) | 0.71 |
| Obstructed PVR (%) | 351 | 10 (2.8%) | 85 | 1 (1.2%) | 0.70 |
| Age at Norwood (days) | 351 | 6 ± 4 | 85 | 6 ± 4 | 0.12 |
| Age at stage II palliation (days) | 276 | 162 ± 53 | 75 | 164 ± 77 | 0.78 |
| Total CPB time (mins) | 351 | 139 ± 51 | 85 | 140 ± 43 | 0.86 |
| DHCA time (mins) | 324 | 33 ± 21 | 76 | 31 ± 18 | 0.27 |
| MBTS (%) | 351 | 170 (48.4%) | 85 | 44 (51.8%) | 0.63 |
| RVPAS (%) | 351 | 183 (52.1%) | 85 | 43 (50.6%) | 0.81 |
|
| |||||
| Norwood course | |||||
|
| |||||
| Median hospital LOS (days) | 351 | 24 (15–42) | 85 | 22 (15–32) | 0.35 |
| Median ICU LOS (days) | 255 | 15 (9–33) | 52 | 13 (9–24) | 0.65 |
| Patients with complications (%) | 351 | 278 (79.2%) | 85 | 63 (74.1%) | 0.31 |
| Number of complications per patient | 351 | 3 ± 3 | 85 | 2 ± 3 | 0.25 |
| Pre-stage II RVEF (%) | 195 | 44 ± 9 | 56 | 43 ± 7 | 0.47 |
|
| |||||
| 14 months follow-up | |||||
|
| |||||
| Deaths (%) | 351 | 84 (23.9%) | 85 | 11 (12.9%) | 0.028 |
| Norwood hospitalization (%) | 84 | 36 (42.9%) | 11 | 4 (36.3%) | |
| After Norwood discharge (%) | 84 | 48 (57.1%) | 11 | 7 (63.6%) | |
| Transplants (%) | 351 | 10 (2.8%) | 85 | 2 (2.4%) | 1.00 |
| Norwood hospitalization (%) | 10 | 6 (60%) | 2 | 1 (50%) | |
| After Norwood discharge (%) | 10 | 4 (40%) | 2 | 1 (50%) | |
| Serious adverse events (%) | 351 | 152 (43.3%) | 85 | 29 (34.1%) | 0.14 |
| 14 months RVEF (%) | 168 | 43 ± 8 | 48 | 42 ± 7 | 0.52 |
p values were calculated using either Fisher exact test or Student t test.
PVR, pulmonary venous return; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; MBTS, modified Blalock-Taussig shunt; RVPAS, Right ventricle to pulmonary artery shunt; LOS, Length of stay; ICU, intensive care unit; IQR, interquartile range; RVEF, Right ventricular ejection fraction.
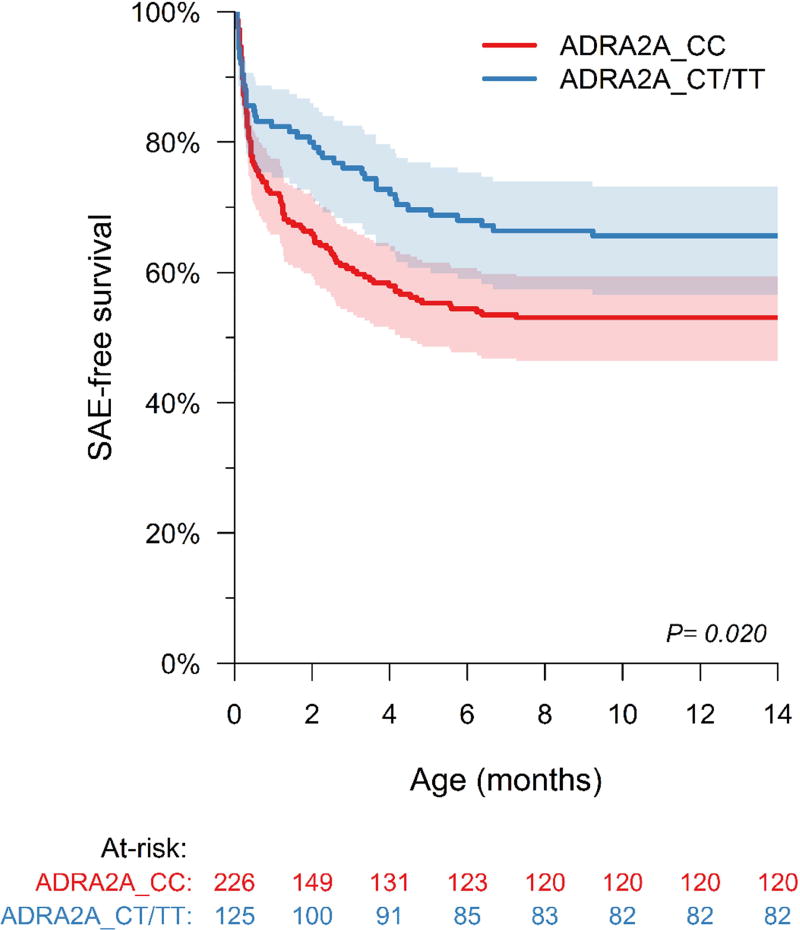
We evaluated the association of ADR genotypes with the primary outcome of composite SAEs. Sixty-four percent patients had the ADRA2A_2790CC genotype (wild-type). Kaplan-Meier analysis showed that patients with the CC genotype had lower SAE-free survival during 14 month follow-up compared to CT/TT genotypes (log rank test: p=0.02) (Figure 2). This association was independent of RAAS genotype, clinical and surgical risk factors (Adjusted Cox regression: HR 1.549 [1.04, 2.30] p=0.033). There was no synergistic adverse effect of multiple risk genotypes on outcomes.
Figure 2.
Kaplan Meier survival curve showing survival free from composite primary outcome stratified by ADRA2A_2790 genotype. The shaded bands represent 95% confidence intervals. Subjects with ADRA2A_2790CC genotype had lower survival free from serious adverse events (SAE) compared to subjects with CT/TT genotypes (p=0.02).
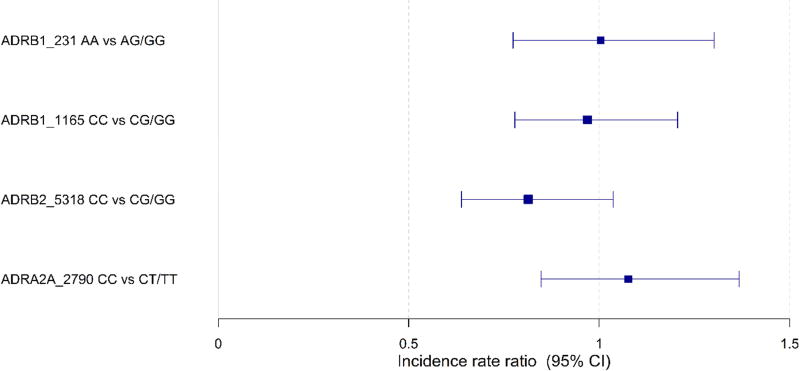
Tables 3 and 4 describe the incidence rate of Norwood complications by genotype groups. Overall, 79 percent patients had a post-Norwood complication with a median [95% CI] of 2 [1–4] complications per patient (Table 2). Complications by systems are shown in Table 5. The list of complications included for this trial have been previously published.26 Cardiac complications included arrhythmias, pericardial effusion, hypotension or hypertension, RV dysfunction, valvar insufficiency, and other. We evaluated the association of ADR genotypes with the rate of complications after the Norwood procedure using Poisson regression. All regression models were adjusted for demographic, clinical and surgical risk factors as well as for RAAS genotypes and the Poisson regression was adjusted for exposure time. There were no significant differences in incidence rates of Norwood complications by genotype (Figure 3). ADR genotypes were not associated with significant difference in RV function during 14 month follow-up (data not shown) as assessed by mixed effects models adjusted for clinical and surgical factors.
Table 3.
Frequency of SAEs during the first 14 months of follow-up and incidence rate of Norwood complications by ADR genotype.
| Gene (SNP ID) |
Proportion of subjects with at least one SAE in the first 14 months |
P-value | Incidence rate of Norwood complications (Number/subject/day of hospitalization) [95% CI] |
P-value | ||
|---|---|---|---|---|---|---|
| ADRB1 (rs1801252) | AA1 108/240 (45%) | GA/GG 44/111 (40%) | 0.36 | AA 0.09 [0.08, 0.10] | GA/GG 0.08 [0.07, 0.09] | 0.38 |
| ADRB1 (rs1801253) | CC1 85/195 (44%) | CG/GG 67/156 (43%) | 0.91 | CC 0.07 [0.06, 0.09] | CG/GG 0.09 [0.08, 0.1] | 0.07 |
| ADRB2 (rs1042714) | CC1 58/133 (43%) | CG/GG 94/218 (43%) | 1.00 | CC 0.09 [0.08, 0.09] | CG/GG 0.08 [0.07, 0.10] | 0.70 |
| ADRA2A (rs553668) | CC1 107/226 (47%) | CT/TT 45/125 (36.0%) | 0.04 | CC 0.08 [0.07, 0.10] | CT/TT 0.08 [0.07, 0.10] | 0.93 |
Major allele: AA, CC, CC, CC genotypes at the four loci represent wild-type genotypes; remainder are polymorphic genotypes i.e. heterozygotes or homozygotes for the SNP
ADR, adrenergic receptor; SAE, serious adverse event; CI, confidence interval
Table 4.
Comparison of occurrence of at least one SAE and incidence rate of Norwood complications during 14-month follow-up by ADR genotype
| Gene (SNP ID) |
Genotype | Odds ratio [95% CI] of SAE in first 14 months |
P- value† |
Incidence rate ratio [95% CI] of Norwood complications |
P- value†† |
|---|---|---|---|---|---|
| ADRB1 (rs1801252) | AA1 vs. GA/GG | 0.732 [0.215, 2.498] | 0.62 | 1.004 [0.774, 1.302] | 0.98 |
| ADRB1 (rs1801253) | CC1 vs. CG/GG | 0.564 [0.205, 1.555] | 0.27 | 0.969 [0.779, 1.206] | 0.78 |
| ADRB2 (rs1042714) | CC1 vs. CG/GG | 1.036 [0.346, 3.105] | 0.95 | 0.814 [0.638, 1.037] | 0.10 |
| ADRA2A (rs553668) | CC1 vs. CT/TT | 1.785 [1.040, 3.063] | 0.036 | 1.077 [0.875, 1.367] | 0.55 |
Logistic regression adjusted for covariates of interest. All subjects who did not experience an SAE were followed for at least 14 months.
Poisson regression adjusted for covariates of interest and exposure time.
Major allele: AA, CC, CC, CC genotypes at the four loci represent wild-type genotypes; remainder are polymorphic genotypes i.e. heterozygotes or homozygotes for the SNP
SAE, serious adverse event; CI, confidence interval
Table 5.
Frequency of post-Norwood complications by systems
| Post-Norwood complications by system | N=1025 |
|---|---|
| Cardiac | 239 (23.3%) |
| Gastro-intestinal | 100 (9.8%) |
| Hematologic | 84 (8.2%) |
| Infectious disease | 233 (22.7%) |
| Neurological | 41 (4.0%) |
| Renal | 35 (3.4%) |
| Respiratory | 249 (24.3%) |
| Vascular | 5 (0.5%) |
| Other | 39 (3.8%) |
Figure 3.
Forest plot of incidence rate ratios (IRRs) of Norwood complications by genotype. The IRR is the ratio of the expected number of complications per patient per day of Norwood hospitalization in each genotype group. There were no significant differences in the IRRs of Norwood complications by genotype.
DISCUSSION
In an era of precision medicine, there is a growing recognition of the importance of identifying not just clinical predictors but also genetic predictors of outcomes that can be used to individualize care and improve the safety and efficacy of medical and surgical interventions based on the unique genome and phenome of a patient. Previous studies have identified genetic variants that increase susceptibility to neurodevelopmental outcomes and adverse ventricular remodeling in single ventricle patients undergoing staged palliation as well as in patients with other types of congenital heart disease undergoing surgical repair.16, 28, 29, 30, 31 In this study, we evaluated the association of genetic variants that increase ADR signaling with post-Norwood outcomes in infants with HLHS. Using genetic material collected during the trial, we demonstrated that the ADR genotype is an important predictor of outcomes of stage 1 palliation in infants with HLHS with lower serious adverse event-free survival in patients harboring ADR risk genotypes. Although further study is required, these findings may have implications for individualizing peri-operative management in this cohort based on genotype.
We evaluated two SNPs in the ADRB1 gene and one in the ADRB2 gene. The ADRB1_389GG genotype has been previously associated with pediatric and adult heart failure and the ADRB1_231AA genotype is known to be associated with lower basal adenylate cyclase activity but increased sensitivity of the β1 receptor to norepinephrine with enhanced sympathetic blood pressure response.17, 18 Neither ADRB1 genotype nor the ADRB2 genotype were associated with post-operative complications or SAEs in our study.10
The ADR2A receptor is important in vascular tone as well as metabolic responses like insulin release from pancreatic cells and adipocyte metabolism in humans. Genetic variations lead to alterations in G-protein coupling and in agonist-promoted receptor phosphorylation and desensitization thereby modulating response to catecholamines.12 The ADRA2A_2790CC genotype (wild-type) has been reported to cause reduced feedback inhibition of norepinephrine and failure to inhibit sympathetic tone. While we did not measure sympathetic tone, we found that patients with the CC genotype had lower freedom from SAEs during 14 month follow-up compared to CT/TT genotypes. One explanation for this finding is that a milieu of high circulating catecholamines in patients with the CC genotype may have resulted in increased vasomotor tone and an adverse effect on systemic output and organ perfusion, thereby increasing the risk of post-Norwood SAEs. The event-free survival curves diverged relatively sharply after the early postoperative period, at around 15 days (Figure 1). This suggests that while early events were more likely secondary to surgical complications which were similar in both genotype groups, later events were more likely influenced by genetic differences in adrenergic system-mediated adaptation to the Norwood circulation as well as recovery from complications. Overall, our findings suggest that genotypes associated with increased adrenergic neurohormonal activation and responsiveness may have a detrimental effect in the post-Norwood circulation.
A limitation of our study is that only a small number of candidate SNPs were analyzed. The DNA in the trial was acquired via buccal swabs which required PCR amplification and did not yield sufficient quantity or quality of DNA for a more unsupervised approach using whole exome sequencing. Nonetheless, the SNPs were carefully selected based on prior associations with outcomes in other pediatric cardiac studies. Also, RV myocardial samples and detailed hemodynamic data were not collected during the Norwood procedure for the trial precluding assessment of tissue adrenergic receptor expression, and of potential influence of the genotypes on metabolic and hemodynamic function. Additionally, we were unable to evaluate the pharmacogenetic influences of the ADR genotypes with β-blocker response since less than 5% patients were receiving β-blocker therapy during follow-up. Also, the sample size of this study was reduced due to insufficient DNA or incomplete genotyping in the trial cohort. Nonetheless, the baseline characteristics of the genotyped and non-genotyped cohorts were similar excluding a survival bias in our study cohort.
In summary, ADR genotypes that enhance catecholamine levels and/or sensitivity increase the risk of serious adverse events in patients who survive beyond the first two weeks after the Norwood procedure. Further studies are needed to delineate the biological and hemodynamic effects of these genotypes and to validate these findings in additional cohorts. Pharmacologic modification of adrenergic receptors has been shown to positively influence outcome in other heart failure cohorts.21,22, 23 While there is no evidence to support empiric β-blocker use in HLHS patients, studies are needed to determine if pre-operative identification of these genetic subtypes can be used to target β-blockers and/or other forms of more aggressive systemic vasodilation post-Norwood to the subset with these risk genotypes.
Supplementary Material
Central Message.
Variations in adrenergic receptor genes influence postoperative outcomes in infants with hypoplastic left heart syndrome undergoing staged palliation.
Kaplan Meier plot: Lower serious adverse event-free survival with ADRA2A_7790CC genotype (log rank test p-value=0.020).
Perspective Statement.
Staged palliation for single ventricle lesions is associated with significant morbidity and mortality. Patients with adrenergic receptor genotypes associated with augmented catecholamine signaling had lower freedom from serious adverse events after Stage 1 palliation. Future studies should explore if targeting adrenergic activation in those harboring risk genotypes can improve outcomes.
Acknowledgments
See Appendix (Supplementary Material) for a complete list of the Pediatric Heart Network Investigators.
Sources of Funding:
Supported by grants HL068269, HL068270, HL068279, HL068281, HL068285, HL068288, HL068290, HL068292, HL109778, and HL085057 from the National Heart, Lung, and Blood Institute.
Glossary of Abbreviations
- ADR
Adrenergic receptor
- CI
confidence interval
- ECMO
Extra-corporeal membrane oxygenation
- HLHS
Hypoplastic left heart syndrome
- HR
Hazard ratio
- LV
left ventricle
- MBTS
modified Blalock- Taussig shunt
- OR
Odds ratio
- PHN
Pediatric Heart Network
- RAAS
Renin- angiotensin- aldosterone system
- RV
Right ventricle
- RVEF
right ventricular ejection fraction
- RVPAS
Right ventricle to pulmonary artery shunt
- SVR trial
Single Ventricle Reconstruction Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement:
The authors have no conflicts of interest.
Clinical Trial Registry Number: NCT00115934
Numbers and Dates of IRB Approvals*
Hospital for Sick Children, 1000006285, 02/11/2005
Children’s Hospital of Los Angeles, CCI-05-00038, 9/5/2008
Columbia University Medical Center, AAAB0870, 1/12/2005
Emory University, IRB00009510, 11/10/2005
Medical University of South Carolina, HR18084, 5/6/2008
Children’s Hospital of Wisconsin, CHW 05/29, 4/21/2005
University of Utah School of Medicine, 13354, 2/5/2005
Duke University Medical Center, Pro00004594, 4/5/2003
University of Michigan Health, 41816, 9/17/2010
Boston Children’s Hospital, 04-12-162, 12/13/2004
Children’s Hospital of Philadelphia, IRB 05-004136, 1/5/2004
SVR Biorepository at University of Michigan Health, HUM00031665, 9/3/2010
*These are original approval dates of trial participating sites, and of the SVR biorepository located at University of Michigan. All IRB approvals are active.
References
- 1.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary PW. Prevalence, clinical presentation and natural history of patients with single ventricle. Progress in Pediatric Cardiology. 2002;16:31–38. [Google Scholar]
- 3.Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189–1194. doi: 10.1161/hc1002.105182. [DOI] [PubMed] [Google Scholar]
- 4.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circulation Research. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hislop AA, Mak JCW, Kelly D, Reader JA, Barnes PJ, Haworth SG. Postnatal changes in β-adrenoceptors in the lung and the effect of hypoxia induced pulmonary hypertension of the newborn. British Journal of Pharmacology. 2002;135:1415–1424. doi: 10.1038/sj.bjp.0704597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitsett JA, Noguchi A, Moore JJ. Developmental aspects of alpha- and beta-adrenergic receptors. Semin Perinatol. 1982;6:125–141. [PubMed] [Google Scholar]
- 7.Brouri F, Hanoun N, Mediani O, Saurini F, Hamon M, Vanhoutte PM, et al. Blockade of β1- and desensitization of β2-adrenoceptors reduce isoprenaline-induced cardiac fibrosis. European Journal of Pharmacology. 2004;485:227–234. doi: 10.1016/j.ejphar.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proceedings of the National Academy of Sciences. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann DL, Kent RL, Parsons B, Cooper G. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 10.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, et al. Β2-adrenergic receptor redistribution in heart failure changes camp compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 11.Todd GL, Baroldi G, Pieper GM, Clayton FC, Eliot RS. Experimental catecholamine-induced myocardial necrosis: Morphology, quantification and regional distribution of acute contraction band lesions. Journal of Molecular and Cellular Cardiology. 1985;17:317–338. doi: 10.1016/s0022-2828(85)80132-2. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. Heterodimerization of α2a- and β1-adrenergic receptors. Journal of Biological Chemistry. 2003;278:10770–10777. doi: 10.1074/jbc.M207968200. [DOI] [PubMed] [Google Scholar]
- 13.Reddy S, Fung A, Manlhiot C, Selamet Tierney ES, Chung WK, Blume E, et al. Adrenergic receptor genotype influences heart failure severity and beta-blocker response in children with dilated cardiomyopathy. Pediatr Res. 2015;77:363–369. doi: 10.1038/pr.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the norwood procedure for single-ventricle lesions. New England Journal of Medicine. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, et al. Design and rationale of a randomized trial comparing the Blalock–Taussig and right ventricle–pulmonary artery shunts in the Norwood procedure. The Journal of Thoracic and Cardiovascular Surgery. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaynor JW, Kim DS, Arrington CB, Atz AM, Bellinger DC, Burt AA, et al. Validation of association of the apolipoprotein e ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. The Journal of Thoracic and Cardiovascular Surgery. 2014;148:2560–2568. doi: 10.1016/j.jtcvs.2014.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small KM, Wagoner LE, Levin AM, Kardia SLR, Liggett SB. Synergistic polymorphisms of β1- and α2c-adrenergic receptors and the risk of congestive heart failure. New England Journal of Medicine. 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 18.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a gprotein coupling domain of the human β1-adrenergic receptor. Journal of Biological Chemistry. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 19.Gilsbach R, Schneider J, Lother A, et al. Sympathetic α2-adrenoceptors prevent cardiac hypertrophy and fibrosis in mice at baseline but not after chronic pressure overload. Cardiovasc Res. 2010;86:432–444. doi: 10.1093/cvr/cvq014. [DOI] [PubMed] [Google Scholar]
- 20.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34:1057–1091. [PubMed] [Google Scholar]
- 21.Reddy S, Fung A, Manlhiot C, Tierney ES, Chung WK, Blume E, et al. Adrenergic receptor genotype influences heart failure severity and β-blocker response in children with dilated cardiomyopathy. Pediatric Research. 2015;77(2):363–369. doi: 10.1038/pr.2014.183. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truijen J, de Peuter OR, Kim Y, Bogaard B, Wouter EK, Kamphuisen PW, et al. Beta2-adrenergic receptor genotype influences the effect of nonselective vs. selective beta-blockade on baroreflex function in chronic heart failure. International Journal of Cardiology. 2011;153(2):230–232. doi: 10.1016/j.ijcard.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 23.de Groote P, Lamblin N, Helbecque N, Mouquet F, Mc Fadden E, Hermant X, et al. The impact of beta-adrenoreceptor gene polymorphisms on survival in patients with congestive heart failure. Eur J Heart Fail. 2005;7(6):966–973. doi: 10.1016/j.ejheart.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Sawczuk M, Maciehewska-Karlowska A, Cieszczyk P. A single nucleotide polymorphism rs553668 in the adra2a gene and the status of polish elite endurance athletes. Trends in Sport Sciences. 2013;1:30–35. [Google Scholar]
- 25.Ohye R, Schonbeck J, Eghtesady P, Laussen P, Pizarro C, et al. Cause, timing, and location of death in the Single Ventricle Reconstruction trial. Journal of thoracic and Cardiovascular Surgery. 2012;144(4):907–914. doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virzi L, Pemberton V, Ohye R, Tabbutt S, Lu M, et al. Reporting adverse events in a surgical trial for complex congenital heart disease: The Pediatric Heart Network experience. Journal of Thoracic and Cardiovascular Surgery. 2011;142:531–7. doi: 10.1016/j.jtcvs.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabbutt S, Ghanayem N, Ravishankar C, Sleeper L, Cooper D, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. Journal of Thoracic and Cardiovascular Surgery. 2012;144:882–995. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mital S, Chung WK, Colan SD, Sleeper LA, Manlhiot C, Arrington CB, et al. Renin-angiotensin-aldosterone genotype influences ventricular remodeling in infants with single ventricle. Circulation. 2011;123:2353–2362. doi: 10.1161/CIRCULATIONAHA.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman B, Auerbach S, Reddy S, Manlhiot C, Deng L, Prakash A, et al. Raas gene polymorphisms influence progression of pediatric hypertrophic cardiomyopathy. Hum Genet. 2007;122:515–523. doi: 10.1007/s00439-007-0429-9. [DOI] [PubMed] [Google Scholar]
- 30.Kim DS, Kim JH, Burt AA, Crosslin DR, Burnham N, McDonald-McGinn DM, et al. Patient genotypes impact survival after surgery for isolated congenital heart disease. The Annals of Thoracic Surgery. 2014;98:104–111. doi: 10.1016/j.athoracsur.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeewa A, Manickaraj AK, Mertens L, Manlhiot C, Kinnear C, Mondal T, et al. Genetic determinants of right-ventricular remodeling after Tetralogy of Fallot repair. Pediatr Res. 2012;72:407–413. doi: 10.1038/pr.2012.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.