Abstract
Background
The prognostic impact of central nervous system (CNS) involvement in children with acute myeloid leukemia (AML) has varied in past trials, and controversy exists over the degree of involvement requiring intensified CNS therapy. Two recent Children’s Oncology Group protocols, AAML03P1 and AAML0531, directed additional intrathecal (IT) therapy to patients with CNS2 (≤5 white blood cell [WBC] with blasts) or CNS3 (>5 WBC with blasts or CNS symptoms) disease at diagnosis.
Methods
We examined disease characteristics and outcomes of the 1,344 patients on these protocols, 949 with CNS1 (no blasts), 217 with CNS2, and 178 with CNS3, with the latter two receiving additional IT therapy.
Results
Young age (P = 0.003), hyperleukocytosis (P < 0.001), and the presence of inversion 16 (P < 0.001) were the only factors more prevalent in patients with CNS2 or CNS3 disease. Complete remission at the end of induction (EOI) 2 was achieved less often in patients with CNS involvement (P < 0.001). From diagnosis, event-free survival (EFS) for patients with CNS involvement was significantly worse (P < 0.001), whereas overall survival (OS) was not (P = 0.16). From the EOI1, there was a higher relapse rate (RR) and worse disease-free survival (DFS), but less impact on OS (CNS1:DFS 58.9%, RR 34.1%, OS 69.3%; CNS2:DFS 53.2%, RR 40.9%, OS 74.7%; CNS3:DFS 45.2%, RR 48.8%, OS 60.8%; P = 0.006, P < 0.001, P = 0.045, respectively). Multivariable analysis showed that independently CNS2 and CNS3 status adversely affected RR and DFS. Traumatic diagnostic lumbar puncture was not associated with worse outcome.
Conclusions
CNS leukemia confers greater relapse risk despite more aggressive locally directed therapy. Novel approaches need to be investigated in this group of patients.
Keywords: AML, CNS, therapy
1 INTRODUCTION
Pediatric patients with acute myeloid leukemia (AML) receive central nervous system (CNS) directed chemotherapy to minimize the risk of CNS relapse. Traditionally, pediatric patients with AML who have CNS disease at diagnosis (CNS3), defined as either >5 white blood cells (WBCs) with blasts in cytospin or with clinical or radiographic (chloroma) signs of CNS leukemia, receive more intensive CNS-directed therapy than those without any blasts or symptoms or signs (designated as CNS1). Those with <5 WBC but with blasts on cytospin are designated as CNS2 and in whom the use of extra CNS therapy for CNS2 patients varies among treatment protocols.1 Alternatively, adult protocols do not routinely administer intrathecal (IT) therapy based on the belief that medium- to high-dose cytarabine will prevent dissemination of leukemia into the CNS and eliminate any CNS disease present at diagnosis.2 In addition, the adult protocol standard is to perform a lumbar puncture (LP) at the time of diagnosis only if there are CNS symptoms present, and if absent, the LP is performed after a period of systemic therapy to avoid contamination of the cerebrospinal fluid (CSF) with circulating blasts.2–4 This differs from the practice in pediatric AML protocols in which LP is performed as part of the diagnostic workup with concomitant IT cytarabine given at this time, as well as during therapy.
One reason for this different approach is that the outcome of pediatric patients with AML with CNS disease at diagnosis has been reported to be worse than those without CNS disease in some studies, though similar or improved in others.5–7 A St. Jude study, retrospectively examined AML patients with CNS involvement treated on four consecutive treatment protocols.5 In these protocols, patients with CNS2 and CNS3 disease were treated with more intensive IT therapy than patients with CNS1 disease. They reported that patients with CNS3 disease had an improved 5-year event-free survival (EFS) than patients with CNS1 or CNS2 disease, but after adjusting for favorable genetic features with this approach there was no significant difference in EFS among the three groups. Another review of four Children’s Cancer Group (CCG) pediatric AML trials examined the risk of CNS disease at diagnosis on outcome.6 In these studies, patients with CNS2 disease, similar to those with CNS1 status, received no intensified IT therapy, which patients with CNS3 disease did receive. There was no significant outcome difference between CNS1, CNS2, and CNS3 patients. More recently, Children’s Oncology Group (COG) protocols AAML03P1 and AAML0531 treated patients with CNS2 disease similar to CNS3 patients with intensified IT therapy. The impact on outcome of this change compared to previous CCG studies is examined as is the impact overall of CNS disease at diagnosis.
2 METHODS
Treatment protocols COG AAML03P1 and AAML0531 have previously been reported.8,9 A chemotherapy backbone based on Medical Research Council (U.K.) trials was utilized and on AAML0531 patients were randomized to receive gemtuzumab ozogamicin (GO) in two cycles of chemotherapy, while the pilot protocol AAML03P1 gave GO to all patients during two cycles of chemotherapy. The age limits for AAML03P1 was ≥1 month and ≤21 years, and for AAML0531 was ≥1 month and ≤30 years. The treatment protocols included IT cytarabine at the beginning of each cycle of therapy except Capizzi II high-dose cytarabine therapy (fourth of five cycles) for patients without CNS involvement for a total of four cycles. Children required intensified CNS-directed therapy if they had any of the following: (i) any number of blasts on a cytospin prep in an a traumatic (<100 red blood cells [RBCs]) LP, (ii) blasts in a traumatic tap (≥100 RBCs) in which the WBC/RBC ratio in the CSF was twice than in peripheral blood, (iii) clinical signs of CNS leukemia (such as facial nerve palsy, brain/eye involvement, or hypothalamic syndrome), or (iv) radiographic evidence of an intracranial, intradural mass consistent with a chloroma. Patients with CNS2 or CNS3 disease received twice weekly IT cytarabine until the CSF was clear of blasts plus two additional treatments with a minimum of four IT treatments given and maximum of six during the first induction cycle. If the CSF did not clear of blasts, they were removed from protocol therapy. Patients who cleared the blasts went on to receive similar CNS-directed therapy in subsequent cycles to those without CNS involvement (i.e., IT cytarabine at the beginning of each cycle of chemotherapy except Capizzi II). Patients who developed a relapse at any site were removed from protocol.
Patient characteristics, disease characteristics, and clinical outcome for children who were CNS1, CNS2, or CNS3 at diagnosis were compared to each other as well as to the past CCG trial results. The impact of traumatic taps at diagnosis was also examined.
2.1 Statistical methods
Data were current on AAML03P1 and AAML0531 as of March 31, 2014. The significance of observed difference in proportions was tested using Pearson’s χ2 test or Fisher’s exact test when data were sparse. The Kruskal–Wallis test was used to test for differences in medians. The Kaplan–Meier method was used to estimate overall survival (OS), EFS, and disease-free survival (DFS). OS was defined as time from study entry to death.10 EFS was defined as the time from study entry until induction failure, relapse, or death. DFS was defined as the time from either the end of one or two induction courses (EOI1 or EOI2, respectively, where EOI is end of induction) of therapy for patients in complete remission (CR) until relapse or death. CR was defined as bone marrow (BM) aspirate containing regenerating normal hematopoietic cells and containing less than 5% blasts by morphology and without extramedullary disease. Low-risk cytogenetics were defined as the presence of inv(16)/t(16;16) or t(8;21). High-risk cytogenetics were defined as presence of monosomy 7, monosomy 5, or del5q, without inv(16)/t(16;16) or t(8;21). Standard risk was all other cytogenetics. Estimates of relapse risk (RR), treatment-related mortality (TRM), and cumulative incidence of isolated CNS relapse or any CNS relapse were obtained by methods that account for competing events.11 RR was defined as time from EOI1 or EOI2 for patients in CR to relapse in which deaths without a relapse were considered competing events. TRM was defined as time from EOI1 or EOI2 for patients in CR to death in which relapses were considered competing events. Isolated CNS relapse was defined as the first relapse and without an additional relapse in another site within 30 days. The cumulative incidence of isolated CNS relapse or any CNS relapse was estimated by considering all other relapses and first event deaths as competing events. Differences between groups of patients were tested using the log-rank test for OS, EFS, and DFS. Gray’s test was used to test significance for RR, TRM, and cumulative incidence of isolated CNS relapse or any CNS relapse. Cox proportional hazard models were used to estimate hazard ratios (HRs) for univariable and multivariable analyses of OS and DFS.12 Competing risk regression models were used to estimate HRs for analyses of RR and TRM.13 Children lost to follow-up were censored at their last known date of contact.
3 RESULTS
3.1 Patient characteristics
Among the 1,344 eligible patients enrolled on AAML03P1 and AAML0531 with available data for CNS status, 949 (71%) were CNS1, 217 (16%) were CNS2, and 178 (13%) were CNS3. Table 1 shows the characteristics of all patients. Patients with CNS2 status had a similar median age at diagnosis to CNS1 patients (10.5 vs. 9.7 years, P = 0.47), whereas CNS3 patients were younger (median 8.7 years, P = 0.05). In fact, 30% of those with CNS3 status were <1 year of age compared to 19% for CNS1 and 20.7% for CNS2 patients (P = 0.002). Median diagnostic WBC for patients was significantly different among the three CNS groups (16.4 vs. 54.7 vs. 43.9 for CNS1, CNS2, and CNS3, respectively, P < 0.001). Those with CNS2 or CNS3 had significantly elevated peripheral blast percentage with median blast percentages of 28%, 63%, and 43% for CNS1, CNS2, and CNS3, respectively (P < 0.001 for CNS1 vs. CNS2 and CNS1 vs. CNS3). Compared to CNS1 patients, those with CNS2 or CNS3 had a higher prevalence of inv(16)-associated AML (P < 0.001). No other recurring cytogenetic abnormalities were associated with the risk of CNS disease. Among molecular mutations, CEBPα was more prevalent in patients with CNS2 (9.6%) status than CNS1 (4.9%, P = 0.01) or CNS3 (4.6%, P = 0.08). In comparing cytogenetic and mutational risk groups at diagnosis, there was significantly more low-risk disease among CNS2 and CNS3 patients and significantly fewer standard-risk CNS2 patients compared to CNS1 and CNS3 patients. There was no difference in cytogenetic complexity, FLT3/ITD mutation, NPM1 mutation, WT1 mutation, BM minimal residual disease (MRD) at EOI1, or the receipt of GO among the three CNS groups.
TABLE 1.
Characteristics of patients in three CNS risk groups
| CNS1 | CNS2 | CNS3 | CNS1 vs. CNS2 vs CNS3 |
CNS1 vs. CNS2 |
CNS2 vs. CNS3 |
CNS1 vs. CNS3 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Characteristic | N | % | N | % | N | % | P | P | P | P |
| Total | 949 | 70.6% | 217 | 16.1% | 178 | 13.2% | ||||
|
| ||||||||||
| Study | ||||||||||
|
| ||||||||||
| AAML03P1 | 257 | 27.1% | 43 | 19.8% | 38 | 21.3% | 0.038 | 0.027 | 0.707 | 0.110 |
|
| ||||||||||
| AAML0531 | 692 | 72.9% | 174 | 80.2% | 140 | 78.7% | ||||
|
| ||||||||||
| Gender | ||||||||||
|
| ||||||||||
| Male | 482 | 50.8% | 111 | 51.2% | 92 | 51.7% | 0.975 | 0.923 | 0.916 | 0.827 |
|
| ||||||||||
| Female | 467 | 49.2% | 106 | 48.8% | 86 | 48.3% | ||||
|
| ||||||||||
| Age at diagnosis (year olds) | ||||||||||
|
| ||||||||||
| Median (range) | 9.7 | (0.003–29.8) | 10.5 | (0.15–23.9) | 8.7 | (0.02–20.9) | 0.096 | 0.470 | 0.050 | 0.054 |
|
| ||||||||||
| 0–1 | 180 | 19.0% | 45 | 20.7% | 55 | 30.9% | 0.002 | 0.551 | 0.021 | <0.001 |
|
| ||||||||||
| 1–2 | 51 | 5.4% | 10 | 4.6% | 10 | 5.6% | 0.881 | 0.648 | 0.649 | 0.895 |
|
| ||||||||||
| 3–10 | 296 | 31.2% | 57 | 26.3% | 37 | 20.8% | 0.012 | 0.154 | 0.203 | 0.005 |
|
| ||||||||||
| ≥11 | 422 | 44.5% | 105 | 48.4% | 76 | 42.7% | 0.475 | 0.295 | 0.259 | 0.662 |
|
| ||||||||||
| Cytogenetics | ||||||||||
|
| ||||||||||
| Normal | 228 | 25.0% | 39 | 19.3% | 28 | 16.4% | 0.020 | 0.088 | 0.462 | 0.015 |
|
| ||||||||||
| t(8;21) | 129 | 14.1% | 18 | 8.9% | 22 | 12.9% | 0.138 | 0.047 | 0.219 | 0.661 |
|
| ||||||||||
| inv(16) | 66 | 7.2% | 51 | 25.2% | 32 | 18.7% | <0.001 | <0.001 | 0.131 | <0.001 |
|
| ||||||||||
| t(9;11)/11q23 | 187 | 20.5% | 43 | 21.3% | 44 | 25.7% | 0.306 | 0.798 | 0.312 | 0.124 |
|
| ||||||||||
| t(6;9) | 20 | 2.2% | 3 | 1.5% | 1 | 0.6% | 0.330 | 0.784 | 0.628 | 0.230 |
|
| ||||||||||
| Monosomy 7 | 22 | 2.4% | 4 | 2.0% | 5 | 2.9% | 0.839 | 1.000 | 0.738 | 0.601 |
|
| ||||||||||
| del(7q) | 16 | 1.8% | 1 | 0.5% | 3 | 1.8% | 0.415 | 0.337 | 0.337 | 1.000 |
|
| ||||||||||
| Monosomy 5/del(5q) | 11 | 1.2% | 3 | 1.5% | 1 | 0.6% | 0.708 | 0.727 | 0.628 | 0.703 |
|
| ||||||||||
| +8 | 67 | 7.3% | 12 | 5.9% | 12 | 7.0% | 0.782 | 0.484 | 0.673 | 0.882 |
|
| ||||||||||
| Other | 167 | 18.3% | 28 | 13.9% | 23 | 13.5% | 0.134 | 0.134 | 0.908 | 0.127 |
|
| ||||||||||
| Unknown | 36 | 15 | 7 | |||||||
|
| ||||||||||
| FAB | ||||||||||
|
| ||||||||||
| M0 | 18 | 2.1% | 7 | 3.7% | 4 | 2.6% | 0.460 | 0.200 | 0.760 | 0.765 |
|
| ||||||||||
| M1 | 105 | 12.4% | 29 | 15.2% | 11 | 7.1% | 0.064 | 0.308 | 0.018 | 0.054 |
|
| ||||||||||
| M2 | 214 | 25.4% | 37 | 19.4% | 26 | 16.7% | 0.024 | 0.081 | 0.516 | 0.020 |
|
| ||||||||||
| M4 | 156 | 18.5% | 64 | 33.5% | 55 | 35.3% | <0.001 | <0.001 | 0.733 | <0.001 |
|
| ||||||||||
| M5 | 171 | 20.3% | 35 | 18.3% | 46 | 29.5% | 0.020 | 0.545 | 0.015 | 0.010 |
|
| ||||||||||
| M6 | 20 | 2.4% | 0 | 0.0% | 1 | 0.6% | 0.042 | 0.036 | 0.450 | 0.230 |
|
| ||||||||||
| M7 | 71 | 8.4% | 2 | 1.0% | 4 | 2.6% | <0.001 | <0.001 | 0.415 | 0.011 |
|
| ||||||||||
| Other | 89 | 10.5% | 17 | 8.9% | 9 | 5.8% | 0.166 | 0.499 | 0.270 | 0.065 |
|
| ||||||||||
| Unknown | 105 | 26 | 22 | |||||||
|
| ||||||||||
| Cytogenetic/molecular risk groups | ||||||||||
|
| ||||||||||
| Standard | 538 | 58.4% | 81 | 39.5% | 89 | 50.9% | <0.001 | <0.001 | 0.027 | 0.064 |
|
| ||||||||||
| Low | 277 | 30.1% | 94 | 45.9% | 65 | 37.1% | <0.001 | <0.001 | 0.086 | 0.064 |
|
| ||||||||||
| High | 106 | 11.5% | 30 | 14.6% | 21 | 12.0% | 0.462 | 0.214 | 0.453 | 0.853 |
|
| ||||||||||
| Unknown | 28 | 12 | 3 | |||||||
|
| ||||||||||
| WBC at study entry | ||||||||||
|
| ||||||||||
| <10,000 µL | 346 | 36.5% | 31 | 14.3% | 37 | 20.8% | <0.001 | <0.001 | 0.089 | <0.001 |
|
| ||||||||||
| 10,000–99,999 µL | 471 | 49.6% | 122 | 56.2% | 88 | 49.4% | 0.203 | 0.080 | 0.179 | 0.962 |
|
| ||||||||||
| ≥100,000 µL | 132 | 13.9% | 64 | 29.5% | 53 | 29.8% | <0.001 | <0.001 | 0.951 | <0.001 |
There were 53 of 178 (29.8%) patients classified as CNS3 who had either clinical signs of CNS leukemia (such as facial nerve palsy, hypothalamic syndrome, or brain/eye involvement) or radiographic evidence of an intracranial, intradural mass consistent with a chloroma. The remaining 125 patients who were CNS3 had either >5 WBC in the CSF with blasts present, or blasts in a traumatic tap in which the WBC/RBC ratio in the CSF was more than twice that in the peripheral blood.
The number of RBC in the diagnostic LP was significantly different among the three CNS groups (Supplementary Fig. 1). There were significantly more traumatic LPs (≥100 RBC) in CNS3 (52 [5.5%] CNS1, 12 [5.5%] CNS2, and 55 [30.9%] CNS3 [P < 0.001]). In addition, in patients with 6–100 RBC in the CSF, there was a significant difference between CNS groups with 150 (15.8%) CNS1, 64 (29.5%) CNS2, and 45 (25.3%) CNS3 (P < 0.001). The number of RBC was not significantly associated with the peripheral WBC count.
3.2 Patient outcomes
At the EOI 1,77% of CNS1 patients, 72.8% of CNS2 patients, and 69.9% of CNS3 patients were in CR (P = 0.091). At the EOI2, 89.6% of CNS1 patients, 85.2% of CNS2 patients, and 77.2% of CNS3 patients were in CR (P < 0.001; Table 2). Among patients with CNS2 and CNS3 disease, 9 of 216 (4.2%) and 16 of 172 (9.3%), respectively, were removed from protocol due to failure to clear their CSF or resolve their other CNS involvements by the EOI1 (P = 0.041). While persistent BM MRD at the EOI1 did not differ between CNS groups, there was a significant impact on the achievement of morphologic BM and extramedullary CR at the EOI (two cycles) with increasing remission failure as the degree of initial CNS involvement increased (P < 0.001, Table 2). The number of patients with refractory CNS disease or relapse in the CNS at EOI2 was 1 of 881 (0.1%) CNS1, 7 of 198 (3.5%) CNS2, and 2 of 147 (1.4%) CNS3.
TABLE 2.
Outcome of patients in three CNS risk groups
| CNS1 | CNS2 | CNS3 | CNS1 vs. CNS2 vs. CNS3 (three groups) |
CNS1 vs. CNS2 |
CNS2 vs. CNS3 |
CNS1 vs. CNS3 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Outcome | N | % ± 2SE% | N | % ± 2SE% | N | % ± 2SE% | P | P | P | P |
| CR at EOI1 | 712 | 77.0% | 155 | 72.8% | 121 | 69.9% | 0.091 | 0.194 | 0.541 | 0.047 |
|
| ||||||||||
| CR at EOI2 | 794 | 89.6% | 178 | 85.2% | 132 | 77.2% | <0.001 | 0.067 | 0.046 | <0.001 |
|
| ||||||||||
| Induction death | 22 | 2.3% | 2 | 0.9% | 9 | 5.1% | 0.027 | 0.288 | 0.027 | 0.075 |
|
| ||||||||||
| 5-year OS from study entry | 949 | 64.1% ± 3.3% | 217 | 69.0% ± 6.4% | 178 | 60.4% ± 7.6% | 0.161 | 0.209 | 0.069 | 0.220 |
|
| ||||||||||
| 5-year EFS from study entry | 949 | 51.9% ± 3.3% | 217 | 47.0% ± 6.9% | 178 | 39.1% ± 7.4% | <0.001 | 0.128 | 0.068 | <0.001 |
|
| ||||||||||
| 5-year TRM from study entry | 949 | 8.5% ± 1.8% | 217 | 6.2% ± 3.3% | 178 | 8.0% ± 4.1% | 0.505 | 0.242 | 0.436 | 0.878 |
|
| ||||||||||
| 5-year OS from EOI1 (CR patients only) | 712 | 69.3% ± 3.6% | 155 | 74.7% ± 7.2% | 121 | 60.8% ± 9.3% | 0.045 | 0.203 | 0.017 | 0.057 |
|
| ||||||||||
| 5-year DFS from EOI1 (CR patients only) | 712 | 58.9% ± 3.8% | 155 | 53.2% ± 8.2% | 121 | 45.2% ± 9.2% | 0.006 | 0.161 | 0.166 | 0.002 |
|
| ||||||||||
| 5-year RR from EOI1 (CR patients only) | 712 | 34.1% ± 3.6% | 155 | 40.9% ± 8.1% | 121 | 48.8% ± 9.3% | 0.001 | 0.076 | 0.152 | <0.001 |
|
| ||||||||||
| 5-year TRM from EOI1 (CR patients only) | 712 | 7.0% ± 1.9% | 155 | 6.0% ± 3.9% | 121 | 6.0% ± 4.4% | 0.825 | 0.611 | 0.986 | 0.671 |
|
| ||||||||||
| 5-year cumulative incidence of isolated BM relapse from EOI1 | 712 | 26.2% ± 3.3% | 155 | 25.3% ± 7.2% | 121 | 27.1% ± 8.2% | 0.874 | 0.789 | 0.617 | 0.696 |
|
| ||||||||||
| 5-year cumulative incidence of isolated CNS relapse from EOI1 | 712 | 0.6% ± 1.1% | 155 | 2.6% ± 2.6% | 121 | 5.8% ± 4.3% | <0.001 | 0.017 | 0.176 | <0.001 |
|
| ||||||||||
| 5-year cumulative incidence of concurrent CNS and BM relapse from EOI1 | 712 | 2.7% ± 1.3% | 155 | 8.5% ± 4.5% | 121 | 9.2% ± 5.3% | <0.001 | <0.001 | 0.811 | <0.001 |
|
| ||||||||||
| 5-year cumulative incidence of any CNS relapse from EOI1 | 712 | 3.9% ± 1.4% | 155 | 11.7% ± 5.2% | 121 | 17.7% ± 7.1% | <0.001 | <0.001 | 0.169 | <0.001 |
|
| ||||||||||
| 5-year cumulative incidence of any BM relapse from EOI1 | 712 | 30.2% ± 3.5% | 155 | 35.1% ± 7.9% | 121 | 37.8% ± 8.9% | 0.107 | 0.226 | 0.521 | 0.055 |
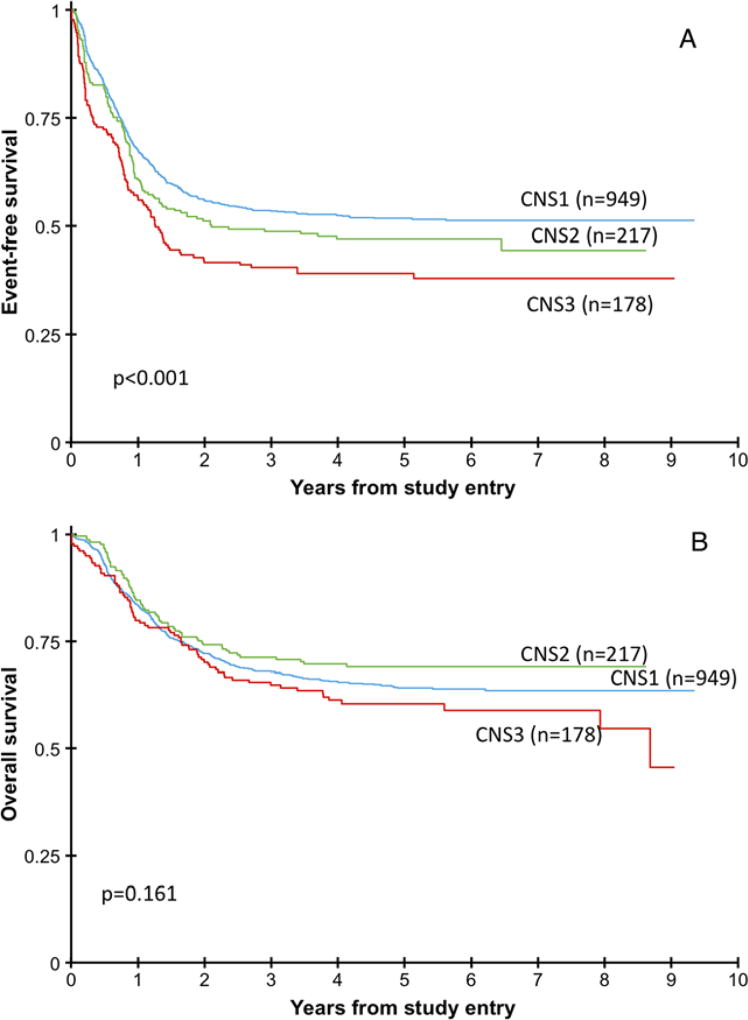
From study entry, there was no difference in OS, but there was a significant difference in EFS among the three groups (Fig. 1). There was significantly worse OS, DFS, and RR from EOI1 in CNS3 patients compared to the CNS1 and CNS2 patients. Interestingly, OS was significantly higher among CNS2 patients compared to CNS3 patients from the EOI1. Comparing to previous CCG studies, CNS2 patients on CCG2861/2891/2941/2961 had a 5-year OS of 59.1%,6 while on this study of COG03P1/0531 the CNS2 patients had a 5-year OS of 69% (P = 0.013).
FIGURE 1.
Event-free survival and overall survival for the three CNS groups (A) Event-free survival of patients in the three CNS groups showing significant difference among the three groups. (B) Overall survival of the patients in the three CNS groups showing no significant difference among the three groups
There was no difference in the incidence of isolated BM relapse from EOI1 in any of the three groups (Table 2). However, the 5-year cumulative incidence of isolated CNS relapse was significantly worse in CNS3 patients compared to CNS1 (5.8% vs. 0.6%, P < 0.001, and in CNS2 compared to CNS1 [2.6% vs. 0.6%, P = 0.017]). The 5-year cumulative incidence of concurrent CNS/BM relapse was significantly worse in CNS2 and CNS3 patients compared to CNS1 patients (8.5%, 9.2%, and 2.7%, respectively, P < 0.001). The 5-year cumulative incidence of any CNS relapse was significantly higher in CNS3 patients compared to CNS2 and CNS1 patients (17.7%, 11.7%, and 3.9%, respectively, P < 0.001). The 5-year cumulative incidence of any BM relapse was not significantly different among the three groups (30.2%, 35.1%, 37.8%, respectively, P = 0.11).
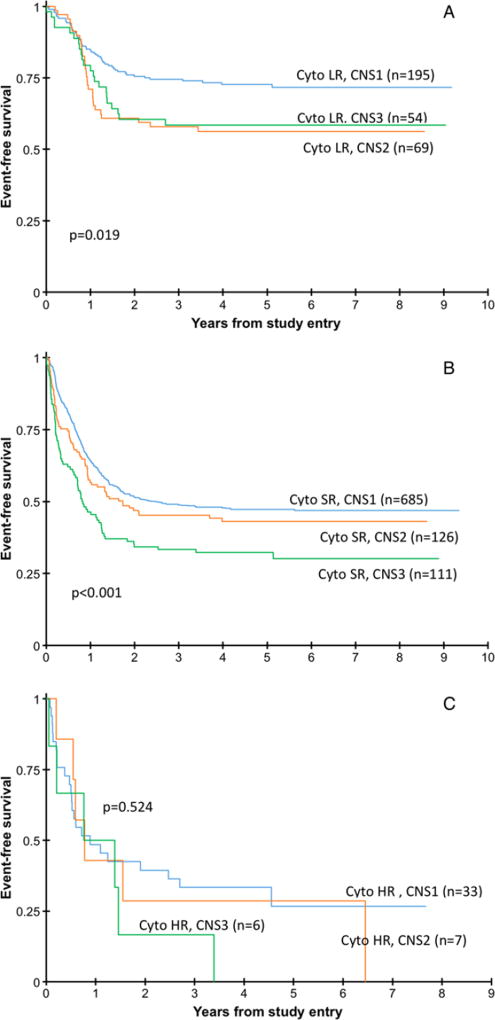
Given the higher prevalence of CNS2 and CNS3 disease in the favorable risk inv(16) AML cohort, we evaluated the clinical implications of CNS involvement (Supplementary Table S1). Outcome analyses for these low-risk inv(16) cytogenetic patients demonstrate no significant difference in 5-year EFS from study entry in CNS1 patients (69.0% ± 11.6%) compared to CNS2 (52.3% ± 14.2%) and CNS3 (61.4% ± 17.5%) patients, P = 0.187. Observing the other low-risk cytogenetic patients, those with t(8;21), the 5-year EFS from study entry was 74.6% in CNS1, 66.7% in CNS2, and 54.2% in CNS3 patients, P = 0.136. Combined, the EFS for all low-risk patients was worse in the CNS2 and CNS3 patients (P=0.019; Fig. 2A). The 5-yearOSfor the low-risk cytogenetic patients overall was not significantly different among the three groups (CNS1: 84.1%; CNS2: 80.2%; and CNS3: 81.0%). Also in these patients, the RR from EOI1 was significantly higher in the CNS2 (33.5% ± 12.7%) and CNS3 (38.6% ± 14.9%) patients compared to the CNS1 (20.4% ± 6.3%) patients, P = 0.032 and 0.009, respectively. Figure 3 demonstrates the EFS for the three cytogenetic groups.
FIGURE 2.
Event-free survival for the low-risk (LR), standard-risk (SR) and high-risk (HR) cytogenetic risk groups (A) Event-free survival for the low-risk cytogenetic patients in the three CNS groups showing a significant difference among the three groups. (B) Event-free survival for the standard-risk cytogenetic patients in the three CNS groups showing a significant difference among the three groups. (C) Event-free survival for the high-risk cytogenetic patients in the three CNS groups showing no significant difference among the three groups
When examining the other cytogenetic risk cohorts (Figs. 2B and 2C), the 5-year EFS for standard-risk cytogenetic patients was significantly worse for those with CNS3 status (47.2% for CNS1, 43.1% for CNS2, and 32.3% for CNS3, P < 0.001), while statistical differences were not achieved in the high-risk cytogenetic patients (26.7%, 28.6%, and 0%, respectively, P = 0.524) likely due to small numbers.
Multivariable Cox analyses and competing risk regression analyses examined the variables of CNS groups, age, race, cytogenetic risk group, WBC at diagnosis, MRD at EOI1, and treatment with GO. From study entry, CNS2 and CNS3 status independently adversely impacted EFS, but not OS (Table 3). From the EOI1, the OS and DFS for the CNS3 patients were significantly worse, due to a significantly higher RR, without a significant change in TRM. The multivariable competing risk regression analyses of isolated CNS relapse primary events showed that CNS3 patients had a significantly higher risk of isolated CNS relapse from EOI1 after adjusting for cytogenetic risk group (HR 7.82, P = 0.003; data not shown).
TABLE 3.
Multivariable analyses for patients in three CNS groups
| OS from study entry | EFS from study entry | |||||||
|
|
|
|||||||
| N | HR | 95% CI | P | HR | 95% CI | P | ||
|
| ||||||||
| CNS groups | ||||||||
|
| ||||||||
| CNS1 | 824 | 1 | 1 | |||||
|
| ||||||||
| CNS2 | 183 | 0.85 | 0.63–1.15 | 0.288 | 1.29 | 1.02–1.63 | 0.035 | |
|
| ||||||||
| CNS3 | 156 | 1.28 | 0.97–1.69 | 0.080 | 1.71 | 1.35–2.17 | <0.001 | |
|
| ||||||||
| Age | ||||||||
|
| ||||||||
| 3–10 years | 343 | 1 | 1 | |||||
|
| ||||||||
| 0–2 years | 295 | 1.25 | 0.94–1.65 | 0.130 | 1.11 | 0.87–1.41 | 0.422 | |
|
| ||||||||
| ≥11 years | 525 | 1.50 | 1.17–1.91 | 0.001 | 1.25 | 1.02–1.54 | 0.035 | |
|
| ||||||||
| Race | ||||||||
|
| ||||||||
| White | 934 | 1 | 1 | |||||
|
| ||||||||
| Nonwhite | 229 | 1.66 | 1.33–2.07 | <0.001 | 1.31 | 1.06–1.60 | 0.011 | |
|
| ||||||||
| Cytogenetic risk group | ||||||||
|
| ||||||||
| Standard | 851 | 1 | 1 | |||||
|
| ||||||||
| Low | 269 | 0.38 | 0.27–0.52 | <0.001 | 0.47 | 0.37–0.60 | <0.001 | |
|
| ||||||||
| High | 43 | 2.34 | 1.61–3.39 | <0.001 | 1.45 | 0.97–2.16 | 0.071 | |
|
| ||||||||
| WBC | ||||||||
|
| ||||||||
| <100K | 957 | 1 | 1 | |||||
|
| ||||||||
| ≥100K | 206 | 1.14 | 0.88–1.47 | 0.326 | 1.31 | 1.06–1.62 | 0.013 | |
|
| ||||||||
| Treatment | ||||||||
|
| ||||||||
| No GO | 440 | 1 | 1 | |||||
|
| ||||||||
| GO | 723 | 0.93 | 0.76–1.14 | 0.483 | 0.83 | 0.69–0.98 | 0.031 | |
|
| ||||||||
| End of induction I | ||||||||
|
|
||||||||
| RR from end induction I | TRM from end induction I | |||||||
|
|
|
|||||||
| N | HR | 95% CI | P | HR | 95% CI | P | ||
|
| ||||||||
| CNS groups | ||||||||
|
| ||||||||
| CNS1 | 470 | 1 | 1 | |||||
|
| ||||||||
| CNS2 | 109 | 1.37 | 0.99–1.89 | 0.060 | 1.56 | 0.70–3.47 | 0.279 | |
|
| ||||||||
| CNS3 | 89 | 1.83 | 1.27–2.61 | 0.001 | 1.34 | 0.51–3.56 | 0.551 | |
|
| ||||||||
| Age | ||||||||
|
| ||||||||
| 3–10 years | 196 | 1 | 1 | |||||
|
| ||||||||
| 0–2 years | 160 | 0.94 | 0.65–1.35 | 0.733 | 0.78 | 0.19–3.20 | 0.729 | |
|
| ||||||||
| ≥11 years | 312 | 0.91 | 0.68–1.22 | 0.518 | 3.92 | 1.52–10.1 | 0.005 | |
|
| ||||||||
| Race | ||||||||
|
| ||||||||
| White | 535 | 1 | 1 | |||||
|
| ||||||||
| Nonwhite | 133 | 1.23 | 0.91–1.66 | 0.172 | 2.04 | 1.02–4.06 | 0.043 | |
|
| ||||||||
| Cytogenetic risk group | ||||||||
|
| ||||||||
| Standard | 460 | 1 | 1 | |||||
|
| ||||||||
| Low | 191 | 0.57 | 0.42–0.78 | <0.001 | 1.12 | 0.54–2.33 | 0.764 | |
|
| ||||||||
| High | 17 | 1.21 | 0.64–2.29 | 0.557 | 2.14 | 0.58–7.96 | 0.256 | |
|
| ||||||||
| WBC | ||||||||
|
| ||||||||
| <100K | 545 | 1 | 1 | |||||
|
| ||||||||
| ≥100K | 123 | 1.43 | 1.05–1.95 | 0.024 | 0.27 | 0.07–1.10 | 0.067 | |
|
| ||||||||
| MRD at EOI1 | ||||||||
|
| ||||||||
| No | 525 | 1 | 1 | |||||
|
| ||||||||
| Yes | 143 | 1.45 | 1.09–1.94 | 0.010 | 1.63 | 0.79–3.37 | 0.188 | |
|
| ||||||||
| Treatment | ||||||||
|
| ||||||||
| No GO | 248 | 1 | 1 | |||||
|
| ||||||||
| GO | 420 | 0.65 | 0.50–0.84 | 0.001 | 2.19 | 1.00–4.79 | 0.050 | |
|
| ||||||||
| End of induction I | ||||||||
|
|
||||||||
| OS from end induction I | DFS from end induction I | |||||||
|
|
|
|||||||
| N | HR | 95% CI | P | HR | 95% CI | P | ||
|
| ||||||||
| CNS groups | ||||||||
|
| ||||||||
| CNS1 | 470 | 1 | 1 | |||||
|
| ||||||||
| CNS2 | 109 | 1.01 | 0.67–1.53 | 0.960 | 1.47 | 1.09–1.99 | 0.013 | |
|
| ||||||||
| CNS3 | 89 | 1.74 | 1.19–2.55 | 0.005 | 1.81 | 1.31–2.51 | <0.001 | |
|
| ||||||||
| Age | ||||||||
|
| ||||||||
| 3–10 years | 196 | 1 | 1 | |||||
|
| ||||||||
| 0–2 years | 160 | 1.00 | 0.67–1.51 | 0.984 | 0.96 | 0.69–1.34 | 0.809 | |
|
| ||||||||
| ≥11 years | 312 | 1.34 | 0.95–1.89 | 0.096 | 1.18 | 0.90–1.56 | 0.239 | |
|
| ||||||||
| Race | ||||||||
|
| ||||||||
| White | 535 | 1 | 1 | |||||
|
| ||||||||
| Nonwhite | 133 | 1.75 | 1.28–2.39 | <0.001 | 1.44 | 1.10–1.89 | 0.008 | |
|
| ||||||||
| Cytogenetic risk group | ||||||||
|
| ||||||||
| Standard | 460 | 1 | 1 | |||||
|
| ||||||||
| Low | 191 | 0.38 | 0.26–0.58 | <0.001 | 0.62 | 0.47–0.83 | 0.001 | |
|
| ||||||||
| High | 17 | 1.34 | 0.70–2.55 | 0.372 | 1.41 | 0.78–2.53 | 0.252 | |
|
| ||||||||
| WBC | ||||||||
|
| ||||||||
| <100K | 545 | 1 | 1 | |||||
|
| ||||||||
| ≥100K | 123 | 1.11 | 0.78–1.57 | 0.579 | 1.21 | 0.91–1.61 | 0.182 | |
|
| ||||||||
| MRD at EOI1 | ||||||||
|
| ||||||||
| No | 525 | 1 | 1 | |||||
|
| ||||||||
| Yes | 143 | 1.76 | 1.30–2.39 | <0.001 | 1.55 | 1.20–2.01 | <0.001 | |
|
| ||||||||
| Treatment | ||||||||
|
| ||||||||
| No GO | 248 | 1 | 1 | |||||
|
| ||||||||
| GO | 420 | 0.81 | 0.61–1.08 | 0.149 | 0.76 | 0.60–0.95 | 0.018 | |
The outcomes of all patients with traumatic (≥100 RBC) initial LP showed no significantly different OS (HR 1.14, P = 0.405) or EFS (HR 1.22, P = 0.132) from study entry, or RR (HR 1.06, P = 0.769) or TRM (HR 1.30, P = 0.510) compared to those with an a traumatic LP (Supplementary Table S2). Outcomes of CNS3 patients with or without a traumatic LP, showed no significant differences in OS, EFS, or RR (Supplementary Table S3) further illustrating the lack of adverse outcome resulting from a traumatic tap at diagnosis. In addition, the 53 patients with CNS3 disease based on, or also with, clinical signs of CNS leukemia or radiographic evidence of a chloroma had no significant difference in OS, EFS, DFS, or RR compared to the remaining CNS3 patients who had blasts in the CSF.
At the other end of the spectrum, in patients with no RBC in their initial CSF, OS was similar among the CNS1, CNS2, and CNS3 patients (62.6 ± 4.8%, 62.7 ± 11.6%, and 66.4 ± 17.5%, respectively, P = 0.974). These patients with no RBC in the CSF did have similarly worse differences in EFS, RR, and DFS between the three CNS groups but did not have the power to achieve statistical significance (CNS1 vs. CNS2 vs. CNS3 for EFS [0 RBC] vs. [all pts]—[52.1 ± 4.9% vs. 47.1 ± 12.0% vs. 43.6 ± 18.2%] vs. [51.9 ± 3.3% vs. 47.0 ± 6.9% vs. 39.1 ± 7.4%], for DFS [58.3 ± 5.5% vs. 55.0 ± 14.3% vs. 50.0 ± 21.5%) vs. (58.9 ± 3.8% vs. 53.2 ± 8.2% vs. 45.2 ± 9.2%), and RR (34.8 ± 5.4% vs. 38.8 ± 14.1% vs. 45.4 ± 22.2%) vs. (34.1 ± 3.6% vs. 40.9 ± 8.1% vs. 48.8 ± 9.3%]).
4 DISCUSSION
This analysis of children with AML from two consecutive national COG Phase III trials with identical, contemporary backbones, and IT therapies shows that increasing degrees of CNS involvement adversely impacts EFS and DFS primarily by increasing relapse risk. This is despite additional IT chemotherapy doses for both CNS2 and CNS3 patients. Patients with CNS3 status had the worst DFS and EFS and highest RR of the three CNS groups with CNS2 status imparting an intermediate adversity though statistically nonsignificant. Even when adjusting for age, race, WBC, cytogenetic risk group, or use of GO, their EFS from study entry, and OS and DFS from the EOI1 were worse than the patients with CNS1 status, although the OS from study entry was not significantly different. Worse DFS was solely due to a higher RR, as there was no difference in TRM. This demonstrates that the additional dosing of single-agent IT therapy is not adequate to fully control the negative effect of CNS3 or CNS2 status using this chemotherapy regimen. Among the three cytogenetic risk groups, CNS3 status had its greatest adverse impact on EFS in low- and intermediate-risk patients, whereas CNS2 status primarily impacted low-risk cytogenetic patients.
Patients with CNS2 disease had an intermediate outcome that was not statistically significant between CNS1 and CNS3 patients, primarily driven by an increase in CNS relapse, despite their receipt of more IT therapy. Their 5-year OS from study entry was significantly better than on the previous CCG studies in which they did not receive intensified IT therapy.6,14–17 However, similar to the overall comparison of contemporary outcomes to the previous CCG studies, the CNS1 patients had a significantly better OS from study entry compared to the past studies (64.1% vs. 49.9%, P < 0.001). This suggests that the change in systemic therapy is likely the reason for the improved survival more so than the addition of extra IT therapies for CNS2 patients.
Examining other potentially confounding variables, we found that CNS2 patients had a higher incidence of several favorable risk factors compared to CNS1 including inv(16), CEBPα, and overall low-risk disease. These CNS2 patients had worse EFS compared to CNS1 patients, yet OS was better in the CNS2 patients overall, likely a result of the known higher salvage rate for low-risk patients who relapse. This association of CNS disease with low-risk cytogenetics has also been seen in previous studies.5,18 Additionally, we show the favorable CEBPα mutation to be associated with the presence of CNS disease, whereas a previous study failed to find this in pediatric AML19 possibly due to fewer patients compared to our current study.
Traumatic LPs, traditionally defined as those with ≥100 RBC/µL, were examined to determine if this had an impact on outcome. In examining the number of RBC in the diagnostic LP, there were significantly more CNS3 patients with≥100 RBC in the CSF than in the other 2 CNS groups (P < 0.001), raising the concern a traumatic tap could introduce leukemic cells into the CSF and worsen the outcome. In examining the outcome of these patients using univariable and multivariable Cox analysis, those who had a traumatic initial LP had no difference in EFS, OS, or RR. Examining only the CNS3 patients, there was no difference in outcome among those with traumatic versus a traumatic tap (data not shown). Examining patients with no RBC in their initial CSF, there was again little change in outcome differences between the three CNS groups though now due to small numbers these differences were no longer significant (data not shown). In adult AML protocols, a diagnostic LP is not performed in order to prevent contamination of the CSF with blasts unless there are signs of CNS disease present.2–4 Our data suggest that traumatic taps in patients who are treated similar to our protocols do not confer a higher risk of treatment failure.
In a previous analysis of children with CNS disease treated on CCG protocols, the CNS groups did not differ significantly in OS from study entry or from EOI, EFS from study entry, or DFS from EOI after adjusting for age, WBC at diagnosis, race, and cytogenetic risk group.6 A St. Jude review, in 2003, of CNS disease found that patients with CNS3 disease had a significantly higher EFS compared to CNS1 and CNS2 patients. Although this difference persisted after adjusting for treatment protocol and for age at diagnosis, it was lost after adjusting for cytogenetic features, in a subset restricted to 290 patients.5 Our current study is similar to the St. Jude analysis in which patients with CNS2 and CNS3 disease received more intensive IT therapy. However, in the St. Jude study approximately half of the patients with CNS2 and CNS3 disease also received cranial irradiation that was not used in our study nor is it used or recommended any longer in most contemporary therapies due to its long-term toxicity.20 In the legacy CCG protocols that utilized intensive timing induction,14–17 patients with CNS2 disease did not receive intensified IT therapy but instead, and similar to the CNS1 patients, did receive a total of 8–10 IT injections over the course of the protocol. This doubling what the CNS1 patients received on the recent two COG protocols, as does the differing methods of backbone intensification, confounds our direct comparisons of intensified IT therapy. A recent expert panel review of AML therapy in children and adolescents addressed CNS-directed therapy.21 This review recommended chemotherapeutic CNS prophylaxis for all patients regardless of the presence of CNS disease, but did not specify timing or optimal number of IT therapies. St. Jude investigators in a 2008 review of CNS involvement by leukemia reported their own experience showing that the use of single-agent cytarabine was less effective than triple therapy with cytarabine, methotrexate, and hydrocortisone in preventing CNS relapse.22 This may be an effective approach to investigate for those with CNS2 or CNS3 status in future trials.
Overall, this study shows that current intensified IT therapy for patients with CNS2 or CNS3 disease in the setting of current-day intensive systemic therapy is not yet optimal. This study gives support to further investigating the therapy for these patients with CNS disease to improve their relapse-free survival, as current methods of intensifying IT therapy are not adequate. While overall marrow and CNS RR failed to be statistically worse among the CNS2 patients compared to CNS1 patients, this study does show that patients with CNS2 status have a worse CNS relapse risk than CNS1 patients and similar to CNS3 patients’ CNS relapse risk. Therefore, improved methods of therapy are still needed for this cohort of patients presenting with leukemic involvement of the CNS.
Supplementary Material
Acknowledgments
The research reported in this publication was supported by the Children’s Oncology Group, National Cancer Institute of the National Institutes of Health under award numbers U10CA180886, U10CA180899, U10CA098543, U10CA098413, and St. Baldrick’s Foundation.
Abbreviations
- AML
acutemyeloid leukemia
- BM
bone marrow
- CCG
Children’s Cancer Group
- CNS
central nervous system
- COG
Children’s Oncology Group
- CR
complete remission
- CSF
cerebrospinal fluid
- DFS
disease-free survival
- EFS
event-free survival
- EOI
end of induction
- GO
gemtuzumab ozogamicin
- HR
hazard ratio
- IT
intrathecal
- LP
lumbar puncture
- MRD
minimal residual disease
- OS
overall survival
- RBC
red blood cell
- RR
relapse rate
- TRM
treatment-related mortality
- WBC
white blood cell
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Johnston DL. A review of central nervous system leukaemia in paediatric acutemyeloid leukaemia. J Hematol Malig. 2011;1:1–5. [Google Scholar]
- 2.Rozovski U, Ohanian M, Ravandi F, et al. Incidence of and risk factors for involvement of the central nervous system in acute myeloid leukemia. Leuk Lymphoma. 2015;56:1392–1397. doi: 10.3109/10428194.2014.953148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng CL, Li CC, Hou HA, et al. Risk factors and clinical outcomes of acute myeloid leukaemia with central nervous system involvement in adults. BMC Cancer. 2015;15:344. doi: 10.1186/s12885-015-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 5.Abbott BL, Rubnitz JE, Tong X, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution’s experience. Leukemia. 2003;17:2090– 2096. doi: 10.1038/sj.leu.2403131. [DOI] [PubMed] [Google Scholar]
- 6.Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG. The presence of central nervous system disease at diagnosis in pediatric acutemyeloid leukemia does not affect survival: a Children’s Oncology Group study. Pediatr Blood Cancer. 2010;55:414–420. doi: 10.1002/pbc.22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusenbery KE, Howells WB, Arthur DC, et al. Extramedullary leukemia in children with newly diagnosed acute myeloid leukemia. J Pediatr Hematol Oncol. 2003;25:760–768. doi: 10.1097/00043426-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Kalbfleisch JDPR. The Statistical Analysis of Failure Time Data. Hoboken, NJ: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 12.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J R Stat Soc. 1999;94:496–509. [Google Scholar]
- 14.Woods WG, Kobrinsky N, Buckley J, et al. Intensively timed induction therapy followed by autologous or allogeneic bone marrow transplantation for children with acute myeloid leukemia or myelodysplastic syndrome: a Children’s Cancer Group pilot Study. J Clin Oncol. 1993;11:1448–1457. doi: 10.1200/JCO.1993.11.8.1448. [DOI] [PubMed] [Google Scholar]
- 15.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acutemyeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Lange BJ, Dinndorf P, Smith FO, et al. Pilot study of idarubicin-based intensive-timing induction therapy for children with previously untreated acute myeloid leukemia: Children’s Cancer Group study 2941. J Clin Oncol. 2004;22:50–156. doi: 10.1200/JCO.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children’s Oncology Group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the Children’sOncologyGroup. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shihadeh F, Reed V, Faderl S, et al. Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer. 2012;118:112–117. doi: 10.1002/cncr.26253. [DOI] [PubMed] [Google Scholar]
- 19.Ho PA, Alonzo TA, Gerbing RB, et al. Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): a report from the Children’s Oncology Group. Blood. 2009;113:6558–6566. doi: 10.1182/blood-2008-10-184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creutzig U, Ritter J, Zimmermann M, Schellong G. Does cranial irradiation reduce the risk for bone marrow relapse in acute myelogenous leukemia? Unexpected results of the Childhood Acute Myelogenous Leukemia Study BFM-87. J Clin Oncol. 1993;11:279–286. doi: 10.1200/JCO.1993.11.2.279. [DOI] [PubMed] [Google Scholar]
- 21.Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120:3187–3205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 22.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.