Abstract
This study sought to evaluate whether myeloid-derived suppressor cells (MDSC) could be affected by chemotherapy and correlate with pathologic complete response (pCR) in breast cancer patients receiving neo-adjuvant chemotherapy. Peripheral blood levels of granulocytic (G-MDSC) and monocytic (M-MDSC) MDSC were measured by flow cytometry prior to cycle 1 and 2 of doxorubicin and cyclophosphamide and 1st and last administration of paclitaxel or paclitaxel/anti-HER2 therapy. Of 24 patients, 11, 6 and 7 patients were triple negative, HER2+ and hormone receptor+, respectively. 45.8% had pCR. Mean M-MDSC% were <1. Mean G-MDSC% and 95% confidence intervals were 0.88 (0.23–1.54), 5.07 (2.45–7.69), 9.32 (4.02–14.61) and 1.97 (0.53–3.41) at draws 1–4. The increase in G-MDSC by draw 3 was significant (p < 0.0001) in all breast cancer types. G-MDSC levels at the last draw were numerically lower in patients with pCR (1.15; 95% CI 0.14–2.16) versus patients with no pCR (2.71; 95% CI 0–5.47). There was no significant rise in G-MDSC from draw 1 to 3 in African American patients, and at draw 3 G-MDSC levels were significantly lower in African Americans versus Caucasians (p < 0.05). It was concluded that G-MDSC% increased during doxorubicin and cyclophosphamide therapy, but did not significantly differ between patients based on pathologic complete response.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2038-3) contains supplementary material, which is available to authorized users.
Keywords: Myeloid-derived suppressor cells, Breast cancer, Chemotherapy, Cytokines
Introduction
Over the past decade, it has become apparent that aberrant host immunity contributes to the growth and spread of cancer [2, 3]. Established cancers secrete factors that direct the recruitment and expansion of suppressive immune cells such as myeloid-derived suppressor cells, tumor-associated macrophages and T regulatory cells [4]. These immune suppressor cells normally function to prevent the development of harmful autoimmune reactions mediated by host lymphocytes, but in cancer patients, they become an important component of the tumor microenvironment and help cancer cells evade destruction [5]. The existence of multiple classes of immune suppressor cells, their presence in high numbers in most tumor types, and their requirement for tumor growth in animal models underscores their importance to tumor development. Consequently, suppressor immune cell levels may predict tumor burden, prognosis and response to therapy [6]. Furthermore, in the last decade, pre-clinical research demonstrated that certain chemotherapeutic agents such as platinum compounds, anti-metabolites and taxanes can modulate the levels and function of these suppressive immune cell subsets and by doing so can affect tumor growth indirectly [7–10]. Therefore, it is important to study the effects of chemotherapy on suppressive immune subsets in patients because these levels could impact and possibly predict the response to treatment.
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of early myeloid cells that accumulate in patients with cancer and are able to suppress immune function by multiple mechanisms. They produce cytokines such as TGF-β and IL-10 that can impair immune cell signaling. They also release arginase, which depletes l-arginine from the tumor microenvironment, crippling T cell function. MDSC produce reactive oxygen species (ROS) and nitric oxide, which can inhibit immune cell signal transduction [11–13]. In general, granulocytic MDSC (G-MDSC) generate ROS while monocytic MDSC (M-MDSC) express arginase and inducible nitric oxide synthase (iNOS), but do not produce high levels of ROS [14]. Human MDSC are characterized as being positive for CD33 and CD11b and negative for HLA-DR. G-MDSC also are CD15+, while M-MDSC are CD14+ [11, 14, 15].
In the current study, peripheral blood levels of MDSC and cytokines in a cohort of breast cancer patients receiving neo-adjuvant chemotherapy (NAC) were investigated [16, 17]. It was hypothesized that levels of MDSC in the peripheral blood mononuclear cell (PBMC) fraction would show an association with complete pathologic response (pCR) that might also be reflected in the levels of immune-derived cytokines.
Methods
Study design
A single arm, pilot study was conducted at the Ohio State University Comprehensive Cancer Center under an Institutional Review Board (IRB)-approved protocol (IRB protocol # 2010C0036) between May 2012 and March 2014 to measure levels of MDSC in women with operable breast cancer who received NAC. The primary objective was to evaluate the association between peripheral blood levels of MDSC and pathologic response following NAC. The secondary objective was to study the effect of NAC on the levels of circulating monocytic and granulocytic MDSC.
Patient population
Eligible patients included adult women (≥18 years old) with biopsy proven, non-metastatic breast cancer who, in the opinion of the treating physician, were suitable for NAC. Exclusion criteria were inoperable breast cancer or receipt of chemotherapy for breast cancer prior to study enrollment. All patients were required to sign an IRB-approved informed consent prior to enrollment.
Neo-adjuvant chemotherapy
Eligible study participants received intravenous (IV) NAC as determined by the treating physician (Supplementary Table 1). The majority of patients were treated with four cycles of doxorubicin at 60 mg/m2 and cyclophosphamide at 600 mg/m2 given every 2 weeks followed by 12 treatments with paclitaxel at 80 mg/m2 weekly (ddAC-wT) or four cycles of dose-dense paclitaxel at 175 mg/m2 given every 2 weeks (ddAC-ddT). Patients with HER2+ breast cancer also received trastuzumab at 2 mg/kg weekly (after a loading dose of 4 mg/kg on day 1, cycle 1) administered along with paclitaxel (ddAC-wTH). Trastuzumab was continued for 1 year. Following FDA approval of pertuzumab in combination with standard NAC in September 2013, pertuzumab was added to trastuzumab and paclitaxel at a dose of 420 mg every 3 weeks after a loading dose of 840 mg during cycle 1, day 1 (ddAC-wTHP). One patient received a regimen consisting of 12 weekly treatments with paclitaxel 80 mg/m2, lapatinib 750 mg daily and trastuzumab 2 mg/kg weekly after a loading dose of 4 mg/kg on day 1 of cycle 1 (THL) [18]. One additional patient was treated on a Cancer and Leukemia Group B (CALGB) clinical trial regimen. She received paclitaxel 80 mg/m2 weekly, bevacizumab 10 mg/kg every 2 weeks for 12 weeks followed by four cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks in combination with three cycles of bevacizumab 10 mg/kg every 2 weeks (CALGB 40603). Peg-filgrastim (pegylated granulocyte colony stimulating factor or G-CSF) at 6 mg was administered as a subcutaneous injection on day 2 of every cycle in patients receiving dose-dense anthracycline-based or taxane chemotherapy. Dose adjustments for toxicities were allowed per the treating physician’s discretion.
Sample collection and procurement
Peripheral blood was collected prior to cycle 1 and 2 of doxorubicin and cyclophosphamide and prior to the first and last dose of paclitaxel or paclitaxel/anti-HER2 therapy (in HER2+ patients). For other regimens, MDSC were measured prior to the 1st, 2nd and last chemotherapy treatment (Supplementary Fig. 1). PBMC were isolated from peripheral venous blood via density gradient centrifugation with Ficoll-Paque, (Amersham Pharmacia Biotech, Uppsala, Sweden) as previously described [19]. 1 × 106 PBMC were processed and analyzed by flow cytometry. The remainder of PBMC were cryopreserved for future analyses. Plasma samples were also collected and stored at −80 °C until analysis.
Assessment of clinical response
The response to NAC was assessed by the study pathologist (LS). Complete pathologic response was defined as the absence of residual invasive carcinoma in the breast or lymph nodes. Since patients with near complete response are expected to have a similar clinical outcome as those with complete response, an alternative method of assessing response was also used by calculating Residual Cancer Burden (RCB) class [20]. This was done by the use of online RCB index calculator developed by researchers at M.D. Anderson Cancer Center: (http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3). This calculator utilizes primary tumor bed area, cancer cellularity, percentage of in situ disease, number of positive lymph nodes, and size of the largest metastasis for determining the index. Patients are then categorized into the following RCB classes: 0—no residual disease, I—minimal residual disease, (RCB score 0–1.35), II—moderate residual disease (RCB score 1.35–3.27), III—extensive residual disease (RCB score of >3.28). Patients with RCB classes 0–I were deemed to have good response to chemotherapy (since outcomes of patients with RCB 0 and I do not differ), while patients with RCB classes II–III were considered not to have achieved an adequate pathologic response [20].
Flow cytometry for myeloid-derived suppressor cells
PBMC were analyzed for the presence of MDSC as previously described [21]. MDSC were defined as cells positive for CD33, CD11b and lacking HLA-DR with subsets expressing CD15 or CD14 representing granulocytic and monocytic MDSC, respectively (Fig. 1). Notably, the current method for phenotyping M-MDSC (CD33+/HLADR−/CD14+/CD11b+) compares favorably to methods employed by other investigators (e.g., CD14+/HLADRlow/−) with respect to the percentages of M-MDSC obtained [22]. Specific antibodies included CD15-FITC, CD33-APC, HLA-DR-PC7, CD11b-PE (all Beckman Coulter), and CD14-V450 (BD Biosciences). All samples were run on a BD LSR-II flow cytometer and data were analyzed with FlowJo software (Tree Star, Inc.).
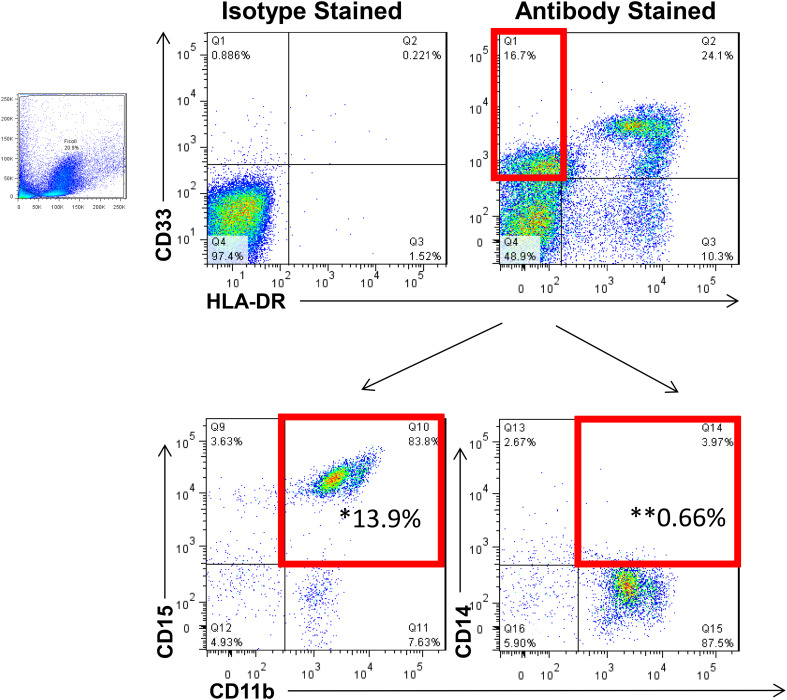
Fig. 1.
Representative flow cytometry staining for myeloid-derived suppressor cells. To quantify MDSC levels, flow cytometry was performed on cryopreserved PBMC from each patient. MDSC were identified as HLA-DR−, CD11b+, CD33+ cells with granulocytic and monocytic subsets expressing CD15 and CD14, respectively. *Granulocytic MDSC; **Monocytic MDSC. Data are representative of 24 patients analyzed at four time points each
T cell proliferation assay
Patient PBMC were depleted of CD33+ cells using CD33 Microbeads (Miltenyi). Total PBMC or PBMC depleted of CD33+ cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Life Technologies) and nonspecifically activated with anti-CD3/CD28 beads (Life Technologies). After 3 days, T cell proliferation was assessed by flow cytometry. APC anti-CD4 and anti-CD8 antibodies were used to identify T cell subsets (Biolegend).
Measurement of plasma cytokines
For quantitative detection of cytokines in patient plasma, a magnetic bead-based Bio-Plex Human Cytokine Panel 10-Plex Assay was used according to the manufacturer’s specifications (Bio-Rad Laboratories, Inc.). All samples were run in batches to minimize inter-assay variability, assayed in duplicate and quantitated using a standard curve.
Statistical analysis
The primary objective was to study an association between circulating MDSC levels and complete pathologic response defined as no residual invasive carcinoma in the breast or axilla. A sample size of 24 patients with operable breast cancer (expect six patients with pCR or RCB class 0–I and 18 patients without complete pathologic response or RCB class II or III) was determined to provide 90% power to detect at least an effect size of 1.5 standard deviation between responders and non-responder groups using a one-sided two sample t test with an alpha level of 0.05. Baseline levels of MDSC and changes in MDSC were compared between patients with or without pCR using a two-sample t test. Associations between MDSC levels and menopausal status, HR status, and tumor grade were explored using graphical analysis. Linear regression models were used to study associations between levels of MDSC, cytokine levels and pCR, including confounder variables, such as age, race, and menopausal status. Appropriate transformations of the data were employed to meet model assumptions and provide interpretable results.
Results
Patient characteristics
A total of 26 women with operable breast cancer were enrolled. Two patients did not complete all required blood draws and were therefore replaced in order to have all time points collected for 24 patients. Patient characteristics are summarized in Table 1. The median age of study patients was 48 years (range 32–69). The majority of patients were Caucasian (n = 19) and pre- or peri-menopausal (n = 15). Eleven patients were diagnosed with triple negative breast cancer (TNBC). Six patients had HER2+ breast cancer while seven had hormone receptor (HR)-positive and HER2-negative disease. Of seven patients with HR+, HER2-negative breast cancer, two had weak expression of HR defined as <10% staining for estrogen and progesterone receptors. Only one patient (4%) had clinical stage I disease (a 69-year-old female with high grade, triple negative breast cancer measuring 1.7 cm), while 20 (83%) and 3 (13%) study patients had stage II and III breast cancer, respectively. All 24 study patients had invasive ductal carcinoma as their tumor histology. These characteristics were felt to be representative of a typical patient population that is offered NAC at our institution [23].
Table 1.
Patient characteristics
| Characteristics | N (%) |
|---|---|
| All patients | 24 |
| Race | |
| White | 19 (79) |
| African American | 4 (17) |
| Hispanic | 1 (4) |
| Age (years) | |
| Median | 48 |
| Range | 32–69 |
| ECOGa performance status | |
| 0 | 20 (83) |
| 1 | 4 (17) |
| Menopausal status | |
| Pre/peri-menopausal | 15 (63) |
| Post-menopausal | 6 (25) |
| Unknown | 3 (13) |
| Tumor size (cm) | |
| Median | 2.8 |
| Range | 0.6–8.7 |
| Clinical N stage | |
| 0 | 12 (50) |
| 1 | 11 (46) |
| 2 | 1 (4) |
| Clinical stage | |
| I | 1 (4) |
| IIA | 12 (50) |
| IIB | 8 (33) |
| IIIA | 3 (13) |
| Grade | |
| 1 | 0 |
| 2 | 7 (29) |
| 3 | 17 (71) |
| Receptor status | |
| Hormone receptor (+) and HER-2/neu (−) | 7 (29) |
| Hormone receptor (+) and HER-2/neu (+) | 3 (13) |
| Hormone receptor (−) and HER-2/neu (+) | 3 (13) |
| Triple negative | 11 (46) |
aEastern Cooperative Oncology Group (ECOG)
Chemotherapy regimens
Supplementary Table 1 summarizes the chemotherapy regimens administered. Most patients received ddAC-wT regimen (n = 15, see Materials and Methods for regimen abbreviations). Two patients were treated with ddAC-ddT regimen. Of six patients with HER2 over-expressing breast cancer, three were treated with a ddAC-wTH regimen, one received ddAC-wTHP, and one was treated with a THL regimen. One patient with HER2+ breast cancer received four cycles of ddAC followed by THL chemotherapy. Finally, one patient with TNBC was co-enrolled in CALGB study 40603 as described in Materials and Methods [24].
Levels of circulating myeloid-derived suppressor cells
The mean percent G-MDSC and M-MDSC at baseline were 0.88 (95% CI 0.23–1.54) and 0.23 (95% CI 0.01–0.45), respectively. The mean frequency of G-MDSC increased significantly from draw 1 [0.88% (95% CI 0.23–1.54)] to draw 3 [9.32% (95% CI 4.02–14.61), p < 0.0001] (Fig. 2a) which correlates with administration of doxorubicin and cyclophosphamide along with peg-filgrastim in 22/24 patients. By the fourth draw, when the majority of patients were finishing 12 weeks of treatment with paclitaxel with or without trastuzumab, the mean percentage of G-MDSC had decreased towards the levels observed at draw 1 (1.97%). This decrease in G-MDSC between draws 3 and 4 was also statistically significant (p = 0.0006). Notably, one patient who was treated on CALGB 40603 trial did not have such an increase and instead her G-MDSC levels remained stable (0, 0.97, 0.45 and 0.37% at time points 1–4, respectively). It is worth pointing out that unlike other cases, doxorubicin and cyclophosphamide therapy (which was administered following four cycles of carboplatin, paclitaxel and bevacizumab) did not induce a rise in G-MDSC in this patient despite receiving G-CSF during anthracycline chemotherapy (possible explanations for this finding are given in the “Discussion”).
Fig. 2.
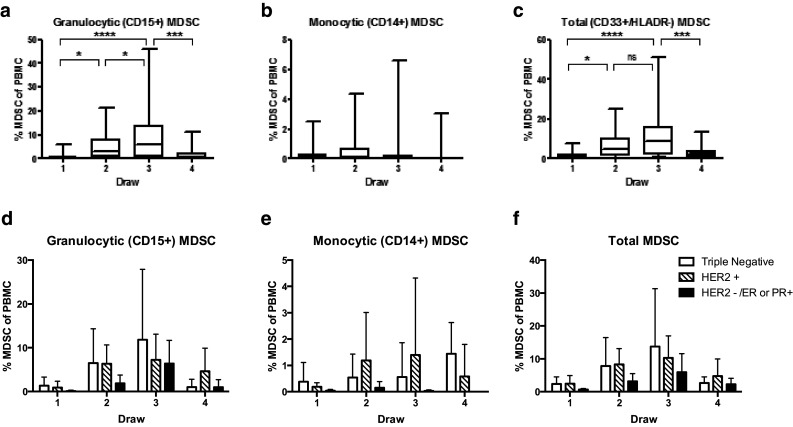
Levels of MDSC initially increase over the course of chemotherapy and do not vary by hormone receptor or HER2 expression. Circulating levels of granulocytic (HLADR−, CD33+, CD11b+, CD15+) or monocytic (HLADR−, CD33+, CD11b+, CD14+) MDSC were measured by flow cytometry prior to cycle 1 and 2 (draw 1, 2) of anthracycline and cycle 1 and 4 of taxane (draw 3, 4) for all 24 patients. Patient receptor status was determined and patients were grouped as either HER-2 positive, HER-2 negative and estrogen receptor (ER) or progesterone receptor (PR) positive, or triple negative. *p < 0.05, ***p < 0.001, ****p < 0.0001, ns not significant as determined by unpaired t test. a–c Box and whisker plots where center line indicates mean and whiskers span minimum to maximum values. d–f Data are shown as mean + SD
Monocytic MDSC did not display such a striking trend. Levels of M-MDSC were only slightly increased at draw two and three and then returned to baseline at draw four [mean and 95% CI was 0.23% (0.01–0.45), 0.56% (0.11–1.02), 0.61% (0–1.33) and 0.18% (0–0.45) for draws 1–4, respectively, Fig. 2b)]. The frequency of total MDSC (CD33+/HLA-DR−) largely mimicked the trend observed in the granulocytic subset [1.89% (1.04–2.74), 6.52 (3.63–9.41), 10.82 (5.21–16.43), 3.01 (1.72–4.31) between draws 1–4, respectively, Fig. 2c]. This was somewhat expected since G-MDSC comprised the majority of total MDSC. The rise in total MDSC between draw 1 and 3 (p = 0.0001) and decrease between draw 3 and 4 (p = 0.0009) were both statistically significant.
Levels of circulating MDSC in patient subsets
Figure 2d–f summarizes the levels of granulocytic and monocytic MDSC by HR and HER2/neu over-expression status. The levels of G-MDSC on average were greater in patients with TNBC at draws 1–3 compared to other breast cancer subtypes, but this difference was not significant. At draw 4, HER2+ patients had the highest levels of G-MDSC (mean 4.67 versus 1.03% in triple negative patients). Levels of G-MDSC at the end of NAC were lowest in patients with HR+, HER2-negative breast cancer (mean 0.98%). The HER2+ patients had the highest levels of M-MDSC at draws 2–4. While these trends were observed, overall, there were no significant differences between the breast cancer subsets and levels of G-MDSC or M-MDSC, possibly due to the sample size (n = 24 patients). These trends were not significantly affected by other factors such as menopausal status or tumor stage. However, at draw 3 (following four cycles of ddAC), the difference in G-MDSC between Caucasian and African American patients was statistically significant with African Americans having significantly less G-MDSC (p = 0.0315, Supplementary Fig. 2). In contrast to Caucasian patients, there was no significant rise in G-MDSC between draws 1 and 3 in African Americans patients with mean G-MDSC levels of 2.25% at draw 1 and 3.01% at draw 3 (p = 0.8751).
Myeloid-derived suppressor cells and response to chemotherapy
Following the completion of NAC and subsequent surgery, a pathological analysis was utilized to determine the pathologic response (pCR—defined as the absence of residual invasive carcinoma in the breast and lymph nodes) and the residual cancer burden (RCB) class in each patient (Table 2). The pCR rate in the study population was 45.8%. Five of 11 (45.5%) patients with TNBC, 3 of 6 (50%) patients with HER2+ breast cancer, 2 of 2 patients with weakly HR+ and HER2- breast cancer and 1 of 5 (20%) patients with strongly HR+ , HER2-negative breast cancer had a pCR. The rate of patients with RCB index 0-I (complete or near complete pCR) was 58.3%.
Table 2.
Clinical patient outcomes
| Residual cancer burden class | N (%) |
|---|---|
| All patients | 24 |
| RCB 0 | 10 (42) |
| RCB I | 4 (17) |
| RCB II | 8 (33) |
| RCB III | 2 (8) |
| Complete pathologic response (pCR) | 11 (46) |
| No complete pathologic response | 13 (54) |
Those patients who achieved a pCR or RCB index 0–I and those with no pCR (or RCB index II–III) were then grouped and their total, G-MDSC, and M-MDSC subsets were plotted by response (Fig. 3). The baseline MDSC percentages did not differ between response groups. However, at the last time point, G-MDSC percentages tended to be lower in patients with pCR than in patients with no pCR [1.15% (95% CI 0.14–2.16) vs. 2.71% (95% CI 0–5.47) respectively, Fig. 3a]. Similarly, in patients with RCB class 0–I, G-MDSC percentages at the end of chemotherapy were also lower compared to those with RCB class II–III [0.98% (95% CI 0.12–1.85) versus 3.25% (95% CI 0–6.53) respectively, Fig. 3d]. However, there was no statistically significant difference between RCB groups at any time point.
Fig. 3.
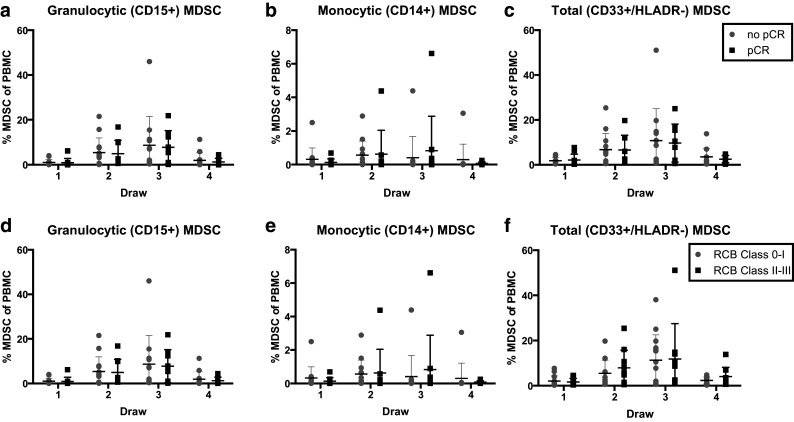
Levels of MDSC in study patients by pCR or RCB score. Circulating levels of total MDSC (HLADR−, CD33+) were measured by flow cytometry prior to cycle 1 and 2 of anthracycline and cycle 1 and 4 of taxane. Complete pathologic response (pCR) score was determined by a pathologist’s assessment of residual cancer invasive cancer in the breast and lymph nodes. Residual Cancer Burden (RCB) score was determined as follows: RCB 0—no residual disease, RCB I—minimal residual disease, (RCB score 0–1.35), RCB II—moderate residual disease (RCB score 1.35–3.27), RCB III—extensive residual disease (RCB score of >3.28). Patients with RCB classes 0–I were deemed as having complete pathologic response to chemotherapy, while patients with RCB classes II–III were considered not to have achieved good pathologic response. a–f Scatter plots for all MDSC measurements from the 24 enrolled patients with central horizontal line indicating mean levels, and error bars representing SD
We also observed one patient with striking differences in MDSC levels between time points 1 and 4 (6.17, 0.87, 0.11 and 0.02% in time points 1–4, respectively). The patient was a 39 year-old premenopausal female with grade 3 TNBC measuring 2.2 cm without lymph node involvement. The patient was treated with ddAC-wT regimen achieving pCR. Interestingly, this patient did not experience the increase in the levels of MDSC during anthracycline chemotherapy. There were three more patients who did not experience the rise in circulating G-MDSC during anthracycline chemotherapy and all had good response to NAC (two had minimal residual disease and RCB class I and one patient had complete response and an RCB class 0). Given these findings, we examined whether patients with lower G-MDSC levels at the end of anthracycline chemotherapy (draw 3) were more likely to have pCR. However, no statistically significant difference was observed.
Confirmation of immunosuppressive function of MDSC
To confirm that MDSC identified in patient samples using the indicated immunophenotype (HLA-DR−/CD11b+/CD33+) were functionally suppressive, their ability to inhibit T cell proliferation was measured (Supplementary Fig. 3). Total PBMC from study patients or PBMC depleted of CD33+ cells were labeled with CFSE, stimulated with anti-CD3/CD28 beads and cultured for 3 days. Proliferation of CD4+ and CD8+ T cells was subsequently measured by flow cytometry and proliferation of both CD4+ and CD8+ T cells was significantly increased following CD33+ cell depletion (and thus MDSC depletion) compared to proliferation of CD4+ and CD8+ T cells cultured in the presence of CD33+ cells.
Blood levels of cytokines
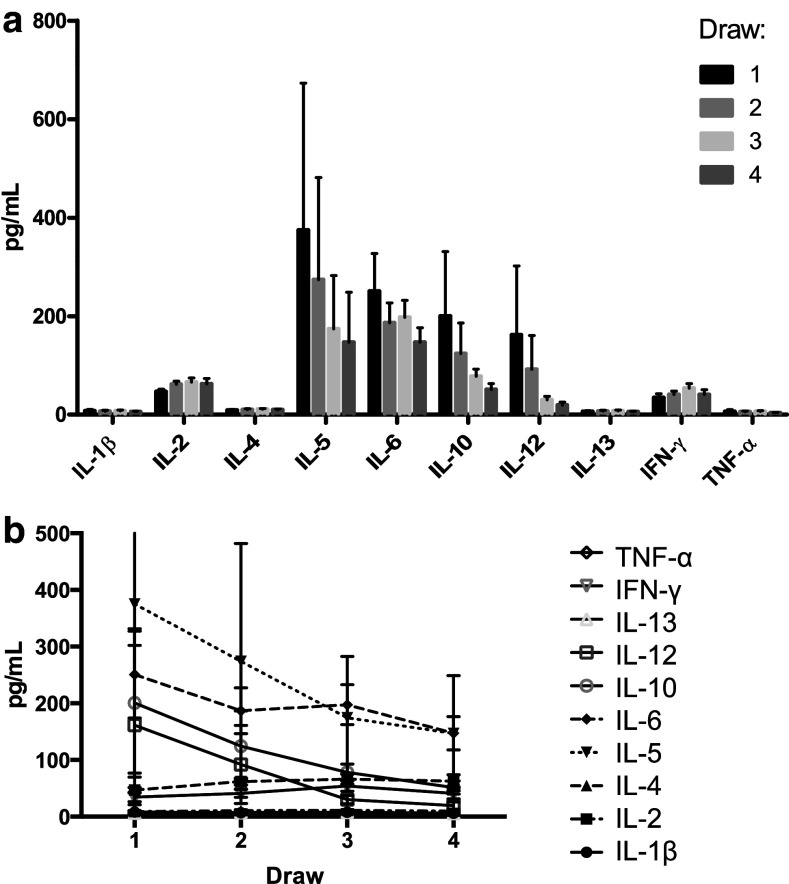
Levels of 10 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IFN-γ, and TNF-α) were measured in plasma samples collected at each draw from each patient and these data are summarized in Supplementary Table 2 and Fig. 4. Prior to the initiation of NAC, IL-5 levels were the highest overall in the total patient population with a subsequent gradual decrease at other time points (392.64 [95% CI 0–1074.59], 284.33 [95% CI 0–753.04], 176.8 [95% CI 0–425.97] and 169.06 pg/mL [95% CI 0–412.56] at time points 1–4, respectively, Fig. 4). The levels of IL-1β, IL-2, IL-4, IL-13 and IFN-γ all peaked at time point 3 and returned to near baseline levels by time point 4 (Supplementary Fig. 4), similar to the trends seen with G-MDSC. On the other hand, IL-10 levels decreased steadily in the course of NAC: 201.97 (95% CI 0–500.89), 122.26 (95% CI 0–260.83), 72.86 (95% CI 44.77–100.95) and 53.73 pg/mL (95% CI 28.87–78.59). Interestingly, IL-10 levels were stable overall for patients who had a pCR but gradually decreased in the course of NAC in patients without pCR [patients with pCR: 40.41 (20.65–60.17), 44.63, 64.13 (29.89–59.38), and 47.8 pg/mL (12.17–83.42); patients without pCR: 301.39 (0–803.46), 187.94 (0–455.35), 80.79 (30.87–130.71) and 58.68 pg/mL (18.93–98.43)]. A similar trend was seen with IL-12 [patients with pCR: 12.42 (6.49–18.35), 16.28 (12.19–20.37), 23.44 (13.67–33.22), and 21.13 pg/mL (0–44.66) and patients without pCR: 341.46 (0–1059.61), 164.6 (0–475.57), 34.41 (6.74–62.08), and 29.71 pg/mL (2.46–56.96)]. Cytokine levels did not significantly differ based on receptor status, pCR, or cancer stage.
Fig. 4.
Serum levels of IL-1β, IL-2, IL-4, IL-13 and IFN-γ vary over the course of chemotherapy. Serum levels of 10 cytokines were measured at each time point using a commercially available magnetic bead-based Bio-Plex Human Cytokine Panel 10-Plex Assay. Assay was performed in triplicate and bars represent the cytokine levels for 24 enrolled patients stratified by cytokine (a) or draw (b), displayed as mean ± SEM
Discussion
The goal of this study was to characterize the levels of circulating monocytic and granulocytic MDSC in patients with breast cancer treated with neo-adjuvant chemotherapy and correlate these levels with the clinical response. The G-MDSC subset was the predominant phenotype of circulating MDSC. G-MDSC levels increased sharply during doxorubicin and cyclophosphamide treatment but decreased during treatment with paclitaxel, reaching near baseline levels at the end of chemotherapy. Levels of M-MDSC were significantly lower, representing only about 1% or less of PBMC and did not differ significantly over time. Patients who did not achieve pCR had numerically higher levels of G-MDSC at the end of chemotherapy. The total number of patients in this pilot study and wide 95% confidence intervals could explain the reason why these findings did not reach statistical significance. The trends in G-MDSC levels were similar between patients with favorable vs. unfavorable response to chemotherapy irrespective of clinical stage, menopausal status, hormone receptor or HER2 expression. However, at draw 3 (following four cycles of ddAC), the difference in G-MDSC between Caucasian and African Americans patients was statistically significant with African Americans having significantly less G-MDSC (p = 0.0315). Racial differences in MDSC levels during cytotoxic chemotherapy have not been previously reported.
It is not entirely clear why most patients experienced elevation of G-MDSC during AC regimen followed by a drop during the taxane regimen. All patients treated with AC regimen also received granulocyte colony stimulating factor (G-CSF), which could be responsible for the increase in G-MDSC levels. However, G-MDSC levels dropped in two patients who also received G-CSF during the entire ddAC-ddT regimen, which argues against this possibility. Alternatively, the rise in G-MDSC could be due to a direct effect of the anthracycline regimen. The decrease in G-MDSC levels during administration of paclitaxel supports the hypothesis that there could be a direct effect of the AC regimen on rise in the levels of MDSC. Four patients in this study did not have the expected rise in G-MDSC levels during AC and these patients all achieved a complete or near complete response to chemotherapy. Therefore, it would be of interest to confirm in future studies whether flat levels of G-MDSC during the administration of an anthracycline regimen can predict a favorable outcome.
While previous studies have examined the impact of MDSC on immune function in murine models, only a few studies have tested how MDSC vary in patients with cancer and whether their levels are affected by cytotoxic chemotherapy. Several investigators reported that circulating MDSC correlate with tumor burden and could serve as a potential biomarker of prognosis and response to anti-neoplastic therapy [6, 25–29]. However, most of these studies were exploratory in nature. Gabitass and colleagues found a high correlation between peripheral blood MDSC levels and survival in 131 patients with pancreatic, gastric and esophageal cancer (p < 0.001). The strength of this study was the use of 54 age and sex matched volunteers as controls. In multivariate analysis, MDSC levels were found to be an independent prognostic factor for survival when adjustments were made for the effects of other prognostic factors such as disease type, subsequent treatment, stage, and performance status. The analysis found that a unit increase in MDSC percentage was associated with a 22% increase in the risk of death [hazard ratio of 1.22 (95% CI 1.06–1.41)] [29]. Similarly, a study by Cole and colleagues enrolled 25 patients treated for metastatic breast cancer and measured circulating MDSC levels using a method that relied on lineage-specific markers. Their study suggested that high levels of circulating MDSC correlated with poor survival with a level of accuracy that was similar to that of circulating tumor cells [28]. However, this study did not differentiate between monocytic and granulocytic populations of MDSC. Additionally, neither of these studies evaluated the effects of chemotherapy on the levels of MDSC in cancer patients.
Diaz-Montero and colleagues evaluated 17 patients with stage II–III breast cancer receiving adjuvant chemotherapy that included dose-dense doxorubicin plus cyclophosphamide followed by dose-dense paclitaxel [25]. Similar to the present results, this study found that levels of MDSC increased significantly during ddAC compared to baseline in breast cancer patients treated with adjuvant chemotherapy (2.2% MDSC at baseline and 11.7% after ddAC chemotherapy). The strength of Diaz-Montero’s study is the fact that all 17 patients received doxorubicin/cyclophosphamide and paclitaxel on a dose-dense schedule that requires G-CSF administration on day 2 of each chemotherapy cycle. This group was, therefore, able to confirm that MDSC elevation during the AC portion of chemotherapy could not be completely explained by G-CSF administration because G-CSF was also given during administration of paclitaxel when MDSC levels were lower [25]. However, this study used different immunophenotypic markers to identify circulating MDSC than the present study (Lin−/lo, HLA-DR−, CD11b+, CD33+). These markers cannot differentiate between monocytic and granulocytic populations of MDSC and in fact could exclude M-MDSC since CD14 is one of the lineage-specific (lin) markers used to exclude mature immune cells. In contrast, the present immunophenotypic panel allowed for the determination of levels of M-MDSC and G-MDSC. Therefore, it could be demonstrated that the effect of the AC regimen on circulating MDSC was largely due to changes in the granulocytic subset. Additionally, the Diaz-Montero study only evaluated tumor response to chemotherapy in a small cohort of six patients utilizing radiographic evidence of progression, while the present studied utilized pathologic complete response and Residual Cancer Burden calculator methods for evaluating tumor response to chemotherapy in all 24 study patients.
One patient in the present study who was treated with carboplatin, paclitaxel and bevacizumab had persistently low levels of G-MDSC and M-MDSC through the entire treatment and achieved a pCR. Unlike other cases in this study, doxorubicin and cyclophosphamide therapy (which was administered following four cycles of carboplatin, paclitaxel and bevacizumab) did not induce a rise in G-MDSC. Certain chemotherapeutic agents such as platinum or gemcitabine are known to deplete MDSC, and bevacizumab neutralizes the effects of VEGF, a known pro-MDSC factor [14]. We speculate that perhaps these effects may have resulted in the low levels of MDSC in this patient [10, 30]. Another possible explanation could be that reduction of tumor burden during the platinum regimen could have led to decreased MDSC generation and lower overall levels of circulating MDSC during the AC portion of chemotherapy in this patient. It is also noteworthy that there was no significant rise in G-MDSC from draw 1 to draw 3 in African American patients, and that at draw three G-MDSC levels were significantly lower in African American patients versus Caucasians. None of the four African American patients had a pCR. In addition, all four were classified as RCB class II. However, for the entire patient population, the association between G-MDSC at draw 3 and response was not statistically significant.
As an exploratory analysis, selected Th1 and Th2 cytokines in the peripheral blood of the study subjects were measured. It was found that a mixture of Th1 and Th2 cytokines peaked during chemotherapy but did not correlate with clinical response.
The present study does have several limitations. First, the study was not limited to a certain histopathologic type of breast cancer, patient characteristic or chemotherapy regimen. Second, the administration of growth factors could be a confounding factor. In addition, we utilized the Ficoll-Hypaque method to isolate MDSC. Some investigators have speculated that this method could lead to loss of G-MDSC and suggested that an analysis of whole blood rather than Ficoll-purified mononuclear cells might be necessary to fully monitor changes in human MDSC [31]. During the initial part of this study, we utilized the Ficoll-Hypaque method simultaneously with a whole blood RBC lysis technique and found very similar trends of M-MDSC and G-MDSC levels (Supplementary Fig. 5). Therefore, we were not concerned about G-MDSC loss.
In conclusion, we have observed that in women with operable breast cancer treated with neo-adjuvant chemotherapy, circulating G-MDSC increase during doxorubicin and cyclophosphamide chemotherapy and decrease sharply during paclitaxel. In patients with complete or near complete pathologic response following NAC, G-MDSC levels appear to be numerically lower compared to patients without pCR.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work has been supported by the National Institutes of Health Grants 2P01CA095426-11, T32 GM068412 (to M. Duggan), K12 CA 133250 as well as the Ohio State Center for Clinical and Translational Science Richard P. and Marie R. Bremer Medical Research Fund and William H. Davis Endowment for Basic Medical Research Pilot Grant (UL1TR000090).
Abbreviations
- CALGB
Cancer and Leukemia Group B
- G-CSF
Granulocyte colony stimulating factor
- G-MDSC
Granulocytic MDSC
- HR
Hormone receptor
- IRB
Institutional review board
- M-MDSC
Monocytic MDSC
- MDSC
Myeloid-derived suppressor cell(s)
- NAC
Neo-adjuvant chemotherapy
- PBMC
Peripheral blood mononuclear cell(s)
- pCR
Pathologic complete response
- RCB
Residual cancer burden
- TNBC
Triple negative breast cancer
Compliance with ethical standards
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Footnotes
Robert Wesolowski and Megan C. Duggan contributed equally to this paper.
References
- 1.Wesolowski R, Duggan M, Stiff A, et al. Abstract P4-09-18: Characterization of circulating myeloid derived suppressor cells and cytokines in patients undergoing neo-adjuvant chemotherapy for breast cancer. Cancer Res. 2016;76(4):P4-09-18. doi: 10.1158/1538-7445.SABCS15-P4-09-18. [DOI] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz J, Brooks TR, Duggan MC, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64:149–159. doi: 10.1007/s00262-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Feng Q-M, Wang Y, et al. The immunologic aspects in advanced ovarian cancer patients treated with paclitaxel and carboplatin chemotherapy. Cancer Immunol Immunother. 2010;59:279–291. doi: 10.1007/s00262-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 9.McDonnell AM, Nowak AK, Lake RA. Contribution of the immune system to the chemotherapeutic response. Semin Immunopathol. 2011;33:353–367. doi: 10.1007/s00281-011-0246-z. [DOI] [PubMed] [Google Scholar]
- 10.de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res. 2014;20:5384–5391. doi: 10.1158/1078-0432.CCR-14-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 12.Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Han Y, Guo Q, et al. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunt SK, Yang L, Sinha P, et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholl SM, Pierga JY, Asselain B, et al. Breast tumour response to primary chemotherapy predicts local and distant control as well as survival. Eur J Cancer. 1995;31A:1969–1975. doi: 10.1016/0959-8049(95)00454-8. [DOI] [PubMed] [Google Scholar]
- 17.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 18.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet (London, England) 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesinski GB, Kondadasula SV, Crespin T, et al. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy. J Natl Cancer Inst. 2004;96:1331–1342. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- 20.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 21.Mundy-Bosse BL, Young GS, Bauer T, et al. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4+ T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronte V, Brandau S, Chen S-H, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesolowski R, Budd GT. Neoadjuvant therapy for breast cancer: assessing treatment progress and managing poor responders. Curr Oncol Rep. 2009;11:37–44. doi: 10.1007/s11912-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 24.Sikov WM, Berry DA, Perou CM et al (2015) Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC ± carboplatin and/or bevacizumab in triple-negative breast cancer: outcomes from CALGB 40603 (Alliance). In: 2015 San Antonio Breast Cancer Symp. [Abstract]
- 25.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha P, Clements VK, Bunt SK, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 27.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 28.Cole S, Montero A, Garret-Mayer E, et al. Elevated circulating myeloid derived suppressor cells (MDSC) are associated with inferior overall survival (OS) and correlate with circulating tumor cells (CTC) in patients with metastatic breast cancer. Cancer Res. 2009;69:4135. doi: 10.1158/0008-5472.SABCS-09-4135. [DOI] [Google Scholar]
- 29.Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesolowski R, Markowitz J, Carson WE. Myeloid derived suppressor cells—a new therapeutic target in the treatment of cancer. J Immunother Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandruzzato S, Solito S, Falisi E, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.