Abstract
Plasmodium vivax Duffy Binding Protein (PvDBP) is a promising vaccine candidate for P. vivax malaria. Recently, we reported the epitopes on PvDBP region II (PvDBP-II) for three inhibitory monoclonal antibodies (2D10, 2H2, and 2C6). In this communication, we describe the combination of native mass spectrometry and ion mobility (IM) with collision induced unfolding (CIU) to study the conformation and stabilities of three malarial antigen-antibody complexes. These complexes, when collisionally activated, undergo conformational changes that depend on the location of the epitope. CIU patterns for PvDBP-II in complex with antibody 2D10 and 2H2 are highly similar, indicating comparable binding topology and stability. A different CIU fingerprint is observed for PvDBP-II/2C6, indicating that 2C6 binds to PvDBP-II on an epitope different from 2D10 and 2H2. This work supports the use of CIU as a means of classifying antigen-antibody complexes by their epitope maps in a high throughput screening workflow.
Graphical Abstract
Introduction
Characterization of epitopes is important in the discovery and development of new therapeutics, vaccines, and diagnostics [1]. Owing to its sequencing capability, MS is one “read-out” for many functional epitope-mapping approaches [2, 3]. Moreover, MS-based footprinting (e.g., HDX-MS) have successfully bridged high and low-resolution structural epitope mapping methods by reliably localizing the binding site at the peptide level [4–8].
In parallel, native IM-MS is emerging for applications in the biophysical characterization of intact proteins [9–11]. Native MS introduces protein complexes up to the mega-Dalton range [12] into the gas phase in near-native states, while preserving non-covalent interactions. Ion mobility can be used to separate individual ions with similar m/z but different sizes and shapes. Drift times (DTs) provide information on conformational dynamics [13, 14], and folding/unfolding intermediates [15, 16]. Native MS and IM report on ligand-induced stability and conformational changes for protein-ligand and protein-protein interactions [17, 18].
CIU experiments can further characterize proteins by monitoring their unfolding. Following collisional activation, the drift times (DT) of activated ions are afford activation profiles as “fingerprint plots”. Such CIU experiments were first described for small monomeric protein ions [19], but they are now applied for stabilities and conformational changes even with ligand binding [20]. CIU can also report on the consequences of small-molecule attachments to large protein systems [21, 22], revealing cooperative binding mechanisms, overall stability of multi-protein systems [23], and antibody-isoform differentiation of disulfide bonding and glycosylation levels [24]. Software advances for CIU are allowing comparison between ions with subtle differences [25].
Although native MS, IM, and CIU have gained importance in biophysics, providing structural information for biomolecules, they have yet to see application in the field of antibody-antigen characterization. Herein, we differentiate antigen-antibody complexes with disparate binding topologies by using native IM-MS combined with CIU. We chose PvDBP, a leading vaccine candidate for preventing malaria caused by Plasmodium vivax. A conserved extracellular domain of this parasite cell-surface protein designated as region II (PvDBP-II) plays a key role in a critical step-wise interaction with Duffy antigen receptor for chemokines (DARC) on reticulocytes. This interaction is imperative for establishing successful invasion and subsequent host infection [26–28].
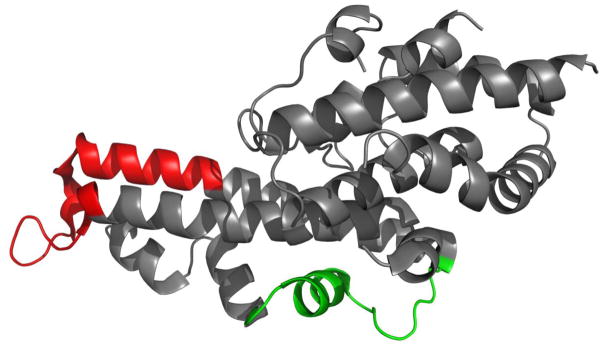
A panel of monoclonal antibodies against PvDBP was generated, and several inhibitory antibodies were described [29]. Epitopes for the inhibitory antibodies 2D10, 2H2, and 2C6 were structurally-defined by X-ray crystallography, small angle X-ray scattering (SAXS), site-directed mutagenesis, binding assays and MS-based HDX [29, 30]. All of these techniques show identical epitopes for two antibodies, 2D10 and 2H2, whereas antibody 2C6 binds to a different epitope (Figure 1) [30].
Figure 1.
Epitopes of 2D10 and 2H2 in red, 2C6 in green, mapped on PvDBP-II (PDB ID: 3RRC).
Experimental Section
PvDBP Region II and monoclonal antibodies were purified as described [29] and buffer exchanged into PBS. Stock antigen and antibodies solutions in PBS buffer were exchanged into 200 mM ammonium acetate solution (pH = 6.5–7). Before each experiment, the antigen and antibody were mixed and diluted to 5 μM and 6 μM, respectively. The sample solution was incubated at 25 ºC for 30 min before MS analysis.
Samples were analyzed using a quadrupole ion-mobility time-of-flight mass spectrometer (Synapt G2 HDMS, Waters, Milford, MA). Protein and complex ions were generated in a nESI source in the positive-ion mode. The source parameters were optimized to provide gentle ESI conditions. The traveling-wave IM separator was operated at a N2 pressure of ~1.5 mbar; the IMS wave velocity was 650 m/s; and the IMS wave height 40 V. The ToF-MS was operated over a m/z range of 100–15,000. For a CIU experiment, the trap-collision voltage applied to the ions in the traveling-wave-based ion trap situated prior to the IM separator was increased from 10 to 200 V in 10 V increments, and ion-mobility mass spectra were acquired at each increment.
Mass spectra were processed with Masslynx V4.1 software (Waters). Arrival time distributions at each collision voltage were extracted in the Drift Scope (Waters) and plotted as a 2D contour plot using Origin 2015 (OriginLab, Northampton, MA).
Results and Discussion
We introduced the antigen-antibody complexes into the gas phase by native MS, presumably preserving the non-covalent interactions (see supporting information for PvDBP-II and 2D10,). The relatively few and low charge states (from +23 to +28) strongly suggest that compact, near-native structures are maintained in the gas phase. Mass spectra clearly indicate that the binding stoichiometry between the antigen and antibodies is 1:1. We then selected the +26 ions for IM analysis because they are of high abundance and show low interference.
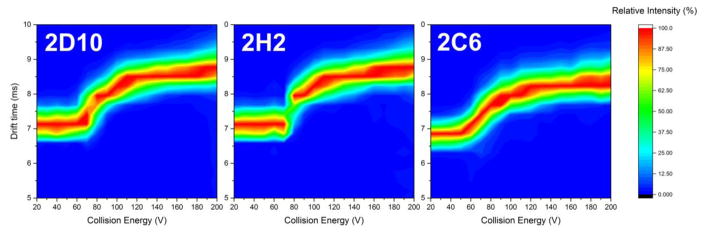
CIU fingerprints for complexes of PvDBP-II with antibodies 2D10, 2H2 and 2C6 were generated with collision voltage ranging from 20 to 200 V (Figure 1). Although charge-state selection can influence the CIU fingerprints, and high charges lead to some Coulombic unfolding prior to collisional activation [21, 31, 32], such effects do not compromise the comparison shown here because the same charge state was selected for all three complexes.
The unfolding pathways, illustrated by increasing drift times for the three complexes demonstrate a similar general trend over the entire voltage range. They all start from an initial compact state with short drift times. At higher accelerating voltages, the complexes undergo CIU and exhibit a gradual transition to a more elongated, unfolded state (increases in drift times). From that point on, the unfolded species dominate the CIU fingerprint, and only slight increases in drift times occur until the voltage reaches the maximum achievable.
A close examination reveals variations between complexes in the CIU fingerprints. At 100% relative intensity on each drift time spectra, the complexes with 2D10 and 2H2 exhibit identical compact state drift times (~7.1 ms) and unfolded state drift times (~8.5 ms). In contrast, the complex with 2C6 exhibits shorter drift times for both compact and unfolded states (~6.8 ms and 8.2 ms, respectively). This observation indicates that the orientationally averaged collision cross section (CCS) for PvDBP-II/2D10 and PvDBP-II/2H2 are comparable but significantly larger than that of DBP-II/2D6, indicating a different overall shape of the latter complex.
This observation is consistent with other evidence that PvDBP-II adopts an elongated structure with the epitopes for 2D10 and 2H2 located on one end of the molecule whereas the epitope for 2C6 is in the middle of the molecule [30] (Figure 1). By interacting with antibodies 2D10 and 2H2 at one end of the lengthy molecule, the complexes become more extended, whereas the interaction with 2C6 results in a more compact shape. Additional evidence is provided by small angle X-ray scattering (SAXS) that shows an elongated “boomerang-like” complex envelope for 2D10 and 2H2, but a more compact “T-shaped” envelope for 2C6 [30]. Although SAXS experiments were done with Fab fragments whereas CIU-IM was performed on full-length antibodies, the variation in complex shapes caused by different binding topology is consistent.
A clear stability shift was also observed. The complex of PvDBP-II/2C6 starts to unfold at collision energies corresponding to just under 60 V applied to the ions in the traveling-wave-based ion trap prior to IM, whereas the compact states of PvDBP-II/2D10 and PvDBP-II/2H2 persist over larger voltage ranges and do not unfold until the collision energies are 70 V, clearly requiring more activation voltage to undergo CIU. Although the complex with 2D10 shows a more gradual transition compared to that with 2H2, both can be considered highly comparable in contrast to the larger difference shown by the complex with 2C6. Thus, the CIU stability also supports the conclusion that 2C6 binds to a different epitope and brings less stabilization to the complex.
Encouragingly, HDX-MS reports that deuterium uptakes are increased significantly in many segments of DBP-II remote from the epitope upon binding to 2C6, indicating conformational changes that destabilize part of the molecule, whereas no such effect is detected for the antigen bound to either 2D10 or 2H2 [30]. Therefore, CIU and HDX results complement each other on the overall stabilities and different bindings of the complexes, and these insights are difficult to obtain via other epitope mapping methods.
In summary, we demonstrate for the first time that native IM-MS in combination with CIU can be used to distinguish complexes formed by one antigen (PvDBP-II) and various antibodies (2D10, 2H2 and 2C6) binding at different epitopes. Native MS allows complexes to be detected with sufficiently accurate mass to afford stoichiometric information. IM and CIU provide insights into the size, shape and stability of the complexes. CIU patterns for PvDBP-II bound to 2D10 and 2H2, and a different complex with 2C6 appear to classify the epitope for 2D10 and 2H2 vs. that of 2C6. This result is consistent with an extensive array of X-ray crystallography, SAXS, mutational mapping and HDX-MS. This outcome reinforces the concept that relevant solution protein-complex structures can be maintained in the gas phase.
CIU may become a high throughput screening for multi-pronged epitope-mapping. Facing a collection of antibody candidates for one antigen, we may be able to categorize antibody binding at different epitopes with high throughput and low sample consumption. Although this approach does not locate the epitope, it may in the future by top-down sequencing [33, 34].
Supplementary Material
Figure S1. Native MS of PvDBP-II and mAb 2D10, zoomed to show the antigen-antibody complex
Figure S2. Mass spectra of DBP-II and three mAbs before and after CIU (trap collision voltage at 10 V and 200 V respectively). The antigen signals are labeled with blue dots, antibody and antigen/antibody complexes with red and green dots respectively.
Figure S3. Drift time distribution of three complexes at 50 to 150 V of collision engergy.
Figure 2.
CIU fingerprints projected as contour plots for the +26 PvDBP-II in complex with antibodies 2D10, 2H2, & 2C6. Normalized intensities for the features are denoted by color-coding as per legend.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (NIGMS) of the NIH (Grant 8P41 GM103422 to MLG) and by NIH contract HHSN272201400018C to MLG and NHT.
References
- 1.Gershoni JM, Roitburd-Berman A, Siman-Tov DD, Freund NT, Weiss Y. Epitope mapping. BioDrugs. 2007;21:145–156. doi: 10.2165/00063030-200721030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opuni KF, Al-Majdoub M, Yefremova Y, El-Kased RF, Koy C, Glocker MO. Mass spectrometric epitope mapping. Mass spectrometry reviews. 2016 doi: 10.1002/mas.21516. [DOI] [PubMed] [Google Scholar]
- 3.Hager-Braun C, Tomer KB. Determination of protein-derived epitopes by mass spectrometry. Expert review of proteomics. 2005;2:745–756. doi: 10.1586/14789450.2.5.745. [DOI] [PubMed] [Google Scholar]
- 4.Guan X, Noble KA, Tao Y, Roux KH, Sathe SK, Young NL, Marshall AG. Epitope mapping of 7S cashew antigen in complex with antibody by solution-phase H/D exchange monitored by FT-ICR mass spectrometry. Journal of Mass Spectrometry. 2015;50:812–819. doi: 10.1002/jms.3589. [DOI] [PubMed] [Google Scholar]
- 5.Jensen PF, Jørgensen TJ, Koefoed K, Nygaard F, Sen JW. Affinity capture of biotinylated proteins at acidic conditions to facilitate hydrogen/deuterium exchange mass spectrometry analysis of multimeric protein complexes. Analytical chemistry. 2013;85:7052–7059. doi: 10.1021/ac303442y. [DOI] [PubMed] [Google Scholar]
- 6.Malito E, Faleri A, Surdo PL, Veggi D, Maruggi G, Grassi E, Cartocci E, Bertoldi I, Genovese A, Santini L. Defining a protective epitope on factor H binding protein, a key meningococcal virulence factor and vaccine antigen. Proceedings of the National Academy of Sciences. 2013;110:3304–3309. doi: 10.1073/pnas.1222845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei H, Mo J, Tao L, Russell RJ, Tymiak AA, Chen G, Iacob RE, Engen JR. Hydrogen/deuterium exchange mass spectrometry for probing higher order structure of protein therapeutics: methodology and applications. Drug discovery today. 2014;19:95–102. doi: 10.1016/j.drudis.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Willison LN, Tripathi P, Sathe SK, Roux KH, Emmett MR, Blakney GT, Zhang HM, Marshall AG. Epitope mapping of a 95 kDa antigen in complex with antibody by solution-phase amide backbone hydrogen/deuterium exchange monitored by Fourier transform ion cyclotron resonance mass spectrometry. Analytical chemistry. 2011;83:7129–7136. doi: 10.1021/ac201501z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laganowsky A, Reading E, Hopper JT, Robinson CV. Mass spectrometry of intact membrane protein complexes. Nature protocols. 2013;8:639–651. doi: 10.1038/nprot.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharon M, Robinson CV. The role of mass spectrometry in structure elucidation of dynamic protein complexes. Annu Rev Biochem. 2007;76:167–193. doi: 10.1146/annurev.biochem.76.061005.090816. [DOI] [PubMed] [Google Scholar]
- 11.Zhong Y, Hyung SJ, Ruotolo BT. Ion mobility–mass spectrometry for structural proteomics. Expert review of proteomics. 2012;9:47–58. doi: 10.1586/epr.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uetrecht C, Versluis C, Watts NR, Roos WH, Wuite GJL, Wingfield PT, Steven AC, Heck AJR. High-resolution mass spectrometry of viral assemblies: Molecular composition and stability of dimorphic hepatitis B virus capsids. Proceedings of the National Academy of Sciences. 2008;105:9216–9220. doi: 10.1073/pnas.0800406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH. Ion mobility–mass spectrometry. Journal of Mass Spectrometry. 2008;43:1–22. doi: 10.1002/jms.1383. [DOI] [PubMed] [Google Scholar]
- 14.McLean JA, Ruotolo BT, Gillig KJ, Russell DH. Ion mobility–mass spectrometry: a new paradigm for proteomics. International Journal of Mass Spectrometry. 2005;240:301–315. [Google Scholar]
- 15.Shi L, Holliday AE, Shi H, Zhu F, Ewing MA, Russell DH, Clemmer DE. Characterizing intermediates along the transition from polyproline I to polyproline II using ion mobility spectrometry-mass spectrometry. Journal of the American Chemical Society. 2014;136:12702–12711. doi: 10.1021/ja505899g. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Pierson NA, Valentine SJ, Clemmer DE. Conformation types of ubiquitin [M+ 8H] 8+ ions from water: methanol solutions: evidence for the N and A states in aqueous solution. The Journal of Physical Chemistry B. 2012;116:3344–3352. doi: 10.1021/jp210797x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyung SJ, Robinson CV, Ruotolo BT. Gas-phase unfolding and disassembly reveals stability differences in ligand-bound multiprotein complexes. Chemistry & biology. 2009;16:382–390. doi: 10.1016/j.chembiol.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Niu S, Ruotolo BT. Collisional unfolding of multiprotein complexes reveals cooperative stabilization upon ligand binding. Protein Science. 2015;24:1272–1281. doi: 10.1002/pro.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shelimov KB, Jarrold MF. Conformations, Unfolding, and Refolding of Apomyoglobin in Vacuum: An Activation Barrier for Gas-Phase Protein Folding. Journal of the American Chemical Society. 1997;119:2987–2994. [Google Scholar]
- 20.Hopper JTS, Oldham NJ. Collision Induced Unfolding of Protein Ions in the Gas Phase Studied by Ion Mobility-Mass Spectrometry: The Effect of Ligand Binding on Conformational Stability. Journal of the American Society for Mass Spectrometry. 2009;20:1851–1858. doi: 10.1016/j.jasms.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Rabuck JN, Hyung SJ, Ko KS, Fox CC, Soellner MB, Ruotolo BT. Activation state-selective kinase inhibitor assay based on ion mobility-mass spectrometry. Analytical chemistry. 2013;85:6995–7002. doi: 10.1021/ac4012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu S, Ruotolo BT. Collisional unfolding of multiprotein complexes reveals cooperative stabilization upon ligand binding. Protein Science : A Publication of the Protein Society. 2015;24:1272–1281. doi: 10.1002/pro.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Y, Han L, Buckner AC, Ruotolo BT. Collision Induced Unfolding of Intact Antibodies: Rapid Characterization of Disulfide Bonding Patterns, Glycosylation, and Structures. Analytical Chemistry. 2015;87:11509–11515. doi: 10.1021/acs.analchem.5b03291. [DOI] [PubMed] [Google Scholar]
- 25.Eschweiler JD, Rabuck-Gibbons JN, Tian Y, Ruotolo BT. CIUSuite: A Quantitative Analysis Package for Collision Induced Unfolding Measurements of Gas-Phase Protein Ions. Analytical Chemistry. 2015;87:11516–11522. doi: 10.1021/acs.analchem.5b03292. [DOI] [PubMed] [Google Scholar]
- 26.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Batchelor JD, Malpede BM, Omattage NS, DeKoster GT, Henzler-Wildman KA, Tolia NH. Red blood cell invasion by Plasmodium vivax: structural basis for DBP engagement of DARC. PLoS Pathog. 2014;10:e1003869. doi: 10.1371/journal.ppat.1003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batchelor JD, Zahm JA, Tolia NH. Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nature structural & molecular biology. 2011;18:908–914. doi: 10.1038/nsmb.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ntumngia FB, Schloegel J, Barnes SJ, McHenry AM, Singh S, King CL, Adams JH. Conserved and variant epitopes of Plasmodium vivax Duffy binding protein as targets of inhibitory monoclonal antibodies. Infection and immunity. 2012;80:1203–1208. doi: 10.1128/IAI.05924-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen E, Salinas ND, Huang Y, Ntumngia F, Plasencia MD, Gross ML, Adams JH, Tolia NH. Broadly neutralizing epitopes in the Plasmodium vivax vaccine candidate Duffy Binding Protein. Proceedings of the National Academy of Sciences. 2016;113:6277–6282. doi: 10.1073/pnas.1600488113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shelimov KB, Jarrold MF. Conformations, unfolding, and refolding of apomyoglobin in vacuum: An activation barrier for gas-phase protein folding. Journal of the American Chemical Society. 1997;119:2987–2994. [Google Scholar]
- 32.Clemmer DE, Jarrold MF. Ion mobility measurements and their applications to clusters and biomolecules. Journal of Mass Spectrometry. 1997;32:577–592. [Google Scholar]
- 33.Lu X, DeFelippis MR, Huang L. Linear epitope mapping by native mass spectrometry. Analytical biochemistry. 2009;395:100–107. doi: 10.1016/j.ab.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Wen J, Zhang H, Gross ML, Blankenship RE. Native electrospray mass spectrometry reveals the nature and stoichiometry of pigments in the FMO photosynthetic antenna protein. Biochemistry. 2011;50:3502–3511. doi: 10.1021/bi200239k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Native MS of PvDBP-II and mAb 2D10, zoomed to show the antigen-antibody complex
Figure S2. Mass spectra of DBP-II and three mAbs before and after CIU (trap collision voltage at 10 V and 200 V respectively). The antigen signals are labeled with blue dots, antibody and antigen/antibody complexes with red and green dots respectively.
Figure S3. Drift time distribution of three complexes at 50 to 150 V of collision engergy.