Abstract
Gastrointestinal tract–secreted satiety hormones play a significant role in one of the largest health-care challenges for children and adults, obesity. Recent studies in mice identified a novel role for uroguanylin, the endogenous intestinal hormone that binds Guanylyl cyclase C (GUCY2C), in regulating satiety via a gut-brain signaling pathway. Mice bred without GUCY2C receptors over-ate and developed obesity. We hypothesized that intestinal uroguanylin expression in pediatric patients with obesity would be lower than patients without obesity, and we attempted to examine the difference with immunohistochemistry. Retrospective chart review of gastrointestinal endoscopic procedures at an academic children’s hospital identified patients with normal pathology findings on biopsy. Children aged 8–17 were included in the review; we analyzed biopsy samples from 20 matched pairs that differed only by body mass index (BMI)-for-age (average: 25%–75% vs. high: >95%). Biopsies of the duodenum, terminal ileum, ascending colon, and descending colon were subjected to immunohistochemistry for GUCY2C, uroguanylin, and the endogenous colonic hormone, guanylin. Intensity staining of all specimens was scored by a blinded pathologist. The overall staining intensity for females with high BMI-for-age was less for uroguanylin and guanylin as compared to average BMI-for-age females while GUCY2C staining was equal. Males did not exhibit different staining intensities for uroguanylin or guanylin. More matched female pairs had greater uroguanylin and guanylin staining in the average BMI-for-age cohort. The intestinal expression of uroguanylin, a key satiety hormone, appears to be diminished in female pediatric patients in the setting of obesity.
Keywords: gastrointestinal, pediatric, basic research, PediPath, metabolic, endocrine
Introduction
Obesity is a major problem in health care, particularly in children and adolescents.1–6 In pediatrics, children with obesity are increasingly burdened with adult-typical diseases.7,8 Research focusing on prevention and etiologies of obesity has intensified.9–15 Research on the development of satiety and appetite control, particularly in children, has included several gastrointestinal hormones.16–23 The control of appetite and satiety develops early in children and is one component of food intake self-regulation, a neuroendocrine-behavioral process important to controlling excess calorie consumption.24–28 Understanding the satiety signaling pathway in children, and how it is affected by excess calorie intake and obesity, will inform efforts to prevent disrupted food intake self-regulation and impact the development of obesity.
The novel satiety hormone, uroguanylin, is expressed in the gastrointestinal tract. It is one of two endogenous ligands for the brush border enzyme Guanylyl cyclase C (GUCY2C) with primary action in the intestine. Guanylin is the other endogenous ligand, predominantly acting in the large intestine (colon). Ligand binding leads to production of cyclic-3′ 5′-guanosine monophosphate (cGMP). Uroguanylin is the 16-amino acid active form of a peptide secreted as a 112-amino acid precursor and post-translationally modified to an 86-amino acid pro-hormone called pro-uroguanylin. Animal studies have shown the importance of GUCY2C and uroguanylin signaling for satiety and weight gain in obesity;16,17,19,21,29 specifically, Valentino et al. demonstrate that the GUCY2C receptor knock-out mouse has increased weight gain and appetite compared to wild-type mouse, an effect mediated by uroguanylin. Folgueira et al.16 demonstrate that uroguanylin levels in intestine and plasma are regulated by nutritional status in a leptin-dependent manner. In addition, adult human studies by Rodriguez et al.30 demonstrate that in the context of morbid obesity, plasma concentrations of uroguanylin are decreased relative to subject adiposity. These studies suggest that where uroguanylin–GUCY2C signaling is interrupted, obesity develops due to increased intake of excess calories.
There is a lack of information about GUCY2C and uroguanylin expression and regulation in children. We have recently conducted studies in adolescents to measure circulating levels of pro-uroguanylin in the plasma of female adolescents with obesity as compared to age-matched controls without obesity. The secretion of uroguanylin appears to be suppressed in the obesity state in adolescents, implying a direct effect of obesity on satiety regulation and appetite. In the present study, we set out to determine differences in expression of uroguanylin and GUCY2C in intestinal tissues of adolescents with and without obesity using immunohistochemistry (IHC). Pediatric intestinal biopsy tissue specimens, previously obtained during endoscopy for clinical evaluation, are the platform we used to test this hypothesis.
The present study’s objectives are (1) to review all elective outpatient upper/lower endoscopies performed at our institution during the last decade in order to select 20 age-, ethnicity-, and sex-matched pairs of patients, 1 per pair with and without obesity, who had normal biopsy tissue findings; (2) to prepare these biopsy tissues for immunohistochemical staining for uroguanylin, guanylin, and GUCY2C; and (3) to characterize expression by comparing intensity of staining as scored by a blinded pathologist.
We hypothesized that we would observe decreased staining for uroguanylin in children with obesity, indicative of a global downregulation of this novel satiety hormone during the pathophysiological changes that lead to the obesity state. We hypothesized that expression of guanylin would also be downregulated in obesity, confirming prior studies in the literature; and GUCY2C would be unchanged. We are the first to report findings on immunohistochemical staining of uroguanylin, GUCY2C, and its other ligand, guanylin, in human adolescent intestinal tissues.
Methods
Patient Selection/Chart Review/Tissue Collection
All protocols and procedures involving human subjects and review of protected health information were approved by the Nemours Institutional Review Board prior to the initiation of any part of the study. Using the electronic medical record, we generated a database of patients who underwent elective outpatient upper or lower endoscopy at the Nemours/Alfred I. duPont Hospital for Children between January 1, 2006 and September 30, 2014 at the same time (within 1 calendar day of the other procedure if not same day). Duplicate records were eliminated. Surgical pathology results were reviewed and classified as normal or abnormal by the study’s gastroenterologist (AA) and the study’s pathologist (DUC). Only patients with normal pathology results were organized into age-, sex-, and ethnicity-matched pairs. The pairs included 1 patient with a body mass index-(BMI)-for-age percentile between 25% and 75% at the time of the procedure and 1 patient with BMI-for-age percentile >95% at the time of the procedure. All BMI-for-age percentiles were based on Centers for Disease Control and Prevention growth charts, with the 25%–75% cohort representing average or typical size patients and >95% representing patients with obesity or morbid obesity. All patients selected for the study were coded in the database with a unique identifier that did not include any protected health information. The Department of Pathology was given the surgical pathology numbers for each selected patient’s specimens that were collected from the tissue bank, and tissue blocks were transferred to the Nemours Histology Core to undergo IHC.
Antibody Selection
Custom polyclonal (rabbit anti-human) antibodies for IHC application were created via collaboration with Thermo Fisher Scientific (Waltham, MA) using the following sequences as immunogens: for uroguanylin, amino acids 75–90, (DLQPVCASQEASSIFK); and for GUCY2C, amino acids 55–68, (EDAVNEGLEIVRGR). Sequences were selected based on design intended to increase the applicability for IHC. The antigen target maps to the C-terminal end of the pro-uroguanylin segment of the peptide, and it does not overlap with the active ligand sequence which has a high cysteine-cysteine–binding frequency. The GUCY2C target maps to the N-terminal ligand–binding extracellular domain on the surface epithelium. For the custom antibodies, briefly, pairs of New Zealand white rabbits were inoculated with 5 mg of each immunogen and subsequently boosted with 1 mg on a specified schedule (day 14, 28, and 42) and bled day 35, 56, and 58, concluding with a terminal bleed with ELISA evaluation at each step. Per Thermo Fisher Scientific, “serial 1:4 dilutions of serum samples (1:195, 1:781, 1:3,125, 1:12,500, 1:50,000, and 1:200,000) were tested by ELISA in duplicate. Titers were reported as the reciprocal of the dilution factor of the serum samples and were defined as the highest dilution at which the absorbance (405 nm) remained above the pre-bleed (pre-immune) control value from the same animal. Anti-antigen antibodies were detected by indirect ELISA with unconjugated antigens passively coated on plates, probed with anti-IgG-HRP conjugate, and detected with ABTS substrate.” For the uroguanylin antibodies, terminal bleed had a detection titer at 1:200,000 dilution for both rabbits; for the GUCY2C antibodies, titers of detection were 1:200,000 and 1:50,000 for each rabbit, and the 1:200,000 titer of detection rabbits were used for all experiments. The guanylin IHC was conducted with a commercially obtained polyclonal (rabbit anti-human) antibody (LS-B9726) from LifeSpan BioSciences (Seattle, WA) that was raised against amino acids 56–105 (FAPIPGEPVVPILCSNPNF PEELKPLCKEPNAQEILQRLEEIAEDPGTCEI), which map to the majority of the pro-guanylin (non-active guanylin) region of the peptide.
Immunohistochemistry
Formalin-fixed paraffin-embedded samples were obtained from the Nemours Department of Pathology. The samples were cut at 5 μm on a Leica RM2255 microtome (Leica, Buffalo Grove, IL) and floated onto Superfrost® Plus slides (Thermo Fisher Scientific, Fremont, CA). The sections were heat immobilized for 60 min at 60°C and stored at −20°C until ready to stain. Slides were equilibrated to room temperature. The slides were then placed on the Leica Bond RX Stainer (Leica, Buffalo Grove, IL) where they were dewaxed using Bond Dewax Solution (Leica, Buffalo Grove, IL) and then rinsed with Bond Wash Solution (Leica, Buffalo Grove, IL). Heat retrieval was done by using ER 1 (Leica, Buffalo Grove, IL) for all three antibodies. After which, the slides were stained using the Bond Polymer Refine Detection Kit (Leica, Buffalo Grove, IL). The slides were incubated in their prospective antibody, GUCY2C (Fisher Science Company, Pittsburgh, PA), Guanylin (LifeSpan BioSciences, Seattle, WA), and Uroguanylin (Fisher Science Company, Pittsburgh, PA), for 30 min, rinsed in Bond Wash Solution, followed by the Post Primary. The slides were rinsed in Bond Wash Solution and incubated in the Polymer. After additional rinses of Bond Wash, the slides were then developed in the kit’s DAB, rinsed, and then counter-stained with Hematoxylin from the kit. The slides were rinsed in deionized water, removed from the Bond RX, placed on the Sakura Tissue-Tek® Prisma™ Automated Stainer (Sakura, Torrance, CA), dehydrated, cleared, and then mounted in Permount® (Thermo Fisher Scientific, Fremont, CA).
The following antibody stains and controls were prepared for IHC on each patient sample: hematoxylin and eosin (H&E) staining in each of 4 anatomic locations (duodenum, terminal ileum, ascending colon, and descending colon); negative reagent control (omitting the primary antibody) IHC staining in 1 anatomic location (duodenum); positive control IHC staining using human small intestine for uroguanylin, human colon and intestine for GUCY2C, and human colon for guanylin; and IHC antibodies for uroguanylin, GUCY2C, and guanylin for patient specimens using appropriate anatomic locations (Figure 1).
Figure 1.
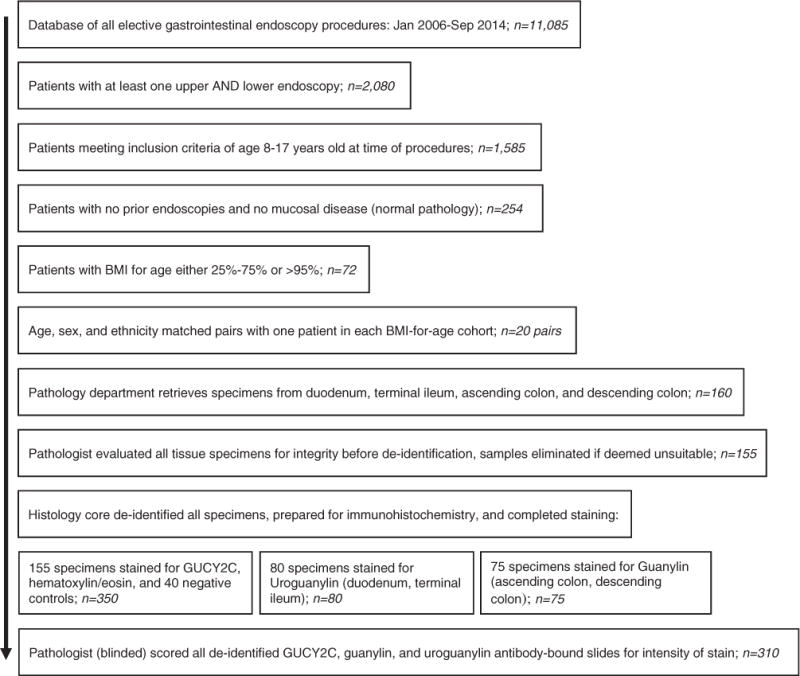
Patient selection and immunohistochemistry protocol. Electronic medical records from a 10-year period were examined for patients undergoing diagnostic endoscopy of the gastrointestinal tract. Patients with normal pathology meeting criteria for the study were selected and organized into matched pairs. Frozen biopsy specimens were subjected to immunohistochemistry using antibodies for guanylin, uroguanylin, and Guanylyl cyclase C (GUCY2C).
Pathologist Review and Scoring
The study pathologist, blinded to BMI-for-age cohort, age, sex, or ethnicity, scored all 310 slides and reviewed all H&E stains and negative reagent control and positive control slides for the study. For each patient, the pathologist reviewed H&E slides of the duodenum, terminal ileum, ascending colon, and descending colon; negative reagent control of the duodenum; uroguanylin stains (done on the duodenum and terminal ileum of all patients); guanylin stains (done on the ascending colon and descending colon of all patients); and GUCY2C stains (done on all four sites in every patient). Each immunohistochemically stained slide was scored using a three-tiered scoring system to evaluate intensity of the staining as follows: 1 indicates light diffuse staining, 2 indicates medium diffuse staining, and 3 indicates very intense diffuse staining. The staining was present in the epithelial cells and few stromal cells (most likely neuroendocrine cells). Staining was considered positive when present in the epithelial cells; it was mostly cytoplasmic and focally membranous. Scores were used to compare staining intensity for each of the three proteins between matched pairs, and overall between cohorts. Photographs of all slides were obtained using a Nikon Eclipse 80i microscope (Melville, NY).
Statistical Analysis
Descriptive statistics were used to report the patients whose tissues/specimens were reviewed and selected for immunohistochemical analysis. We conducted a univariate analysis of cohorts controlling for BMI-for-age percentiles to determine intensity staining pattern. A subsequent bivariate analysis, controlling for BMI-forage percentile between cohorts and sex within cohorts, compared the pathologist’s scoring intensity; significance was calculated using Student’s t test. To compare female pairs to female pairs in terms of intensity staining scores, we used 1-sided Fisher’s exact test.
Results
Patient Demographics/Chart Review/Tissue Collection
From January 2006 through September 2014, there were 11,085 upper or lower endoscopies performed at Nemours/AIDHC as elective outpatient procedures (Figure 1). Of this group, 2080 had at least 1 upper and lower endoscopy concomitantly. Our review of these charts determined that 1585 patients met the inclusion criteria of age 8–17 years at the time of the procedure. These patients’ charts were reviewed for pathology results from all biopsies. Two hundred fifty-four patients had no pathology on any biopsy specimen from their initial lower or upper endoscopy (16%). These records were sorted by BMI-for-age (either >95% or 25%–75%) and then sorted into age-, sex-, and ethnicity-matched pairs. From 72 patients with normal pathology who met criteria for age, we were able to identify 20 pairs matched by sex and ethnicity who had 1 patient in each pair with high BMI-for-age and 1 with average BMI-for-age. Demographics of the 2 cohorts are shown in Table 1. Matched cohort pairs are listed in Table 2. Five biopsy specimens out of 160 total specimens were not usable for the research project because of concern about sample integrity. The remaining 155 specimens were de-identified and prepared for IHC as noted in Methods. There were 155 specimens stained for GUCY2C, 80 stained for uroguanylin, and 75 stained for guanylin. All 5 of the specimens that were not usable were from the ascending or descending colon.
Table 1.
Cohort Demographics.
| Sex
|
%BMI-for-age
|
Ethnicity
|
Race
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Male | Female | 25–75 | >95 | Non-Hispanic | Hispanic | W | AA | O |
| 8–11 | 4 | 2 | 3 | 3 | 4 | 2 | 5 | 0 | 1 |
| 12–14 | 6 | 2 | 4 | 4 | 8 | 0 | 8 | 0 | 0 |
| 15–17 | 6 | 20 | 13 | 13 | 26 | 0 | 23 | 2 | 1 |
| Totals | 16 | 24 | 20 | 20 | 38 | 2 | 36 | 2 | 2 |
AA, African American; BMI: Body mass index; O, other; W, White. Demographics of patients whose tissue specimens were normal on review and met study criteria. Patients were predominantly female, older adolescents, non-Hispanic, and White.
Table 2.
Cohort Pairing for Immunohistochemistry.
| Pair | Sex | Age | Eth | Race | %BMI-for-age | Pair | Sex | Age | Eth | Race | %BMI-for-age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 8 | H | W | 25–75 | 11 | F | 15 | NH | W | 25–75 |
| 1 | M | 10 | H | W | >95 | 11 | F | 15 | NH | W | >95 |
| 2 | F | 9 | NH | W | 25–75 | 12 | F | 15 | NH | AA | 25–75 |
| 2 | F | 9 | NH | W | >95 | 12 | F | 15 | NH | AA | >95 |
| 3 | M | 11 | NH | O | 25–75 | 13 | F | 16 | NH | W | 25–75 |
| 3 | M | 11 | NH | W | >95 | 13 | F | 16 | NH | W | >95 |
| 4 | M | 13 | NH | W | 25–75 | 14 | F | 16 | NH | W | 25–75 |
| 4 | M | 13 | NH | W | >95 | 14 | F | 16 | NH | W | >95 |
| 5 | F | 14 | NH | W | 25–75 | 15 | M | 16 | NH | W | 25–75 |
| 5 | F | 14 | NH | W | >95 | 15 | M | 16 | NH | W | >95 |
| 6 | M | 14 | NH | W | 25–75 | 16 | F | 17 | NH | W | 25–75 |
| 6 | M | 14 | NH | W | >95 | 16 | F | 17 | NH | W | >95 |
| 7 | M | 14 | NH | W | 25–75 | 17 | M | 17 | NH | W | 25–75 |
| 7 | M | 14 | NH | W | >95 | 17 | M | 17 | NH | O | >95 |
| 8 | F | 15 | NH | W | 25–75 | 18 | F | 17 | NH | W | 25–75 |
| 8 | F | 15 | NH | W | >95 | 18 | F | 17 | NH | W | >95 |
| 9 | M | 15 | NH | W | 25–75 | 19 | F | 17 | NH | W | 25–75 |
| 9 | M | 15 | NH | W | >95 | 19 | F | 17 | NH | W | >95 |
| 10 | F | 15 | NH | W | 25–75 | 20 | F | 17 | NH | W | 25–75 |
| 10 | F | 15 | NH | W | >95 | 20 | F | 17 | NH | W | >95 |
AA: African American; BMI: Body mass index; Eth: Ethnicity; F: Female; H: Hispanic; M: Male; NH: Non-Hispanic; O: other; W, White.
Matched pairs were selected from 72 patients who met eligibility criteria. 40 patients are listed in 20 pairs by age, with each pair having an average BMI-for-age and high BMI-for-age patient. Due to few patients in the lower age range, pair 1 has different ages between cohorts.
Immunohistochemistry
We were successful in staining previously obtained, formalin-fixed, paraffin-embedded, small intestine, and colon biopsy specimens with antibodies for GUCY2C, guanylin, and uroguanylin using IHC. Slides representing staining intensities of 1, 2, and 3 are shown in Figure 2(a) to (c). Staining was predominantly seen in epithelial cells and was cytoplasmic. Figure 3 shows a representative pair of tissues from female (a, b) and male (c, d) patients, with (a, c) and without (b, d) obesity, stained for uroguanylin. We observed differences in staining within the female pair but not the male pair; this pattern was noted for uroguanylin staining in all pairs. Figure 4 is a similar representative pair of tissues from female (a, b) and male (c, d) patients, with (a, c) and without (b, d) obesity, stained for GUCY2C. We observed similar intensity of staining within each pair and in overall staining for GUCY2C in all pairs.
Figure 2.
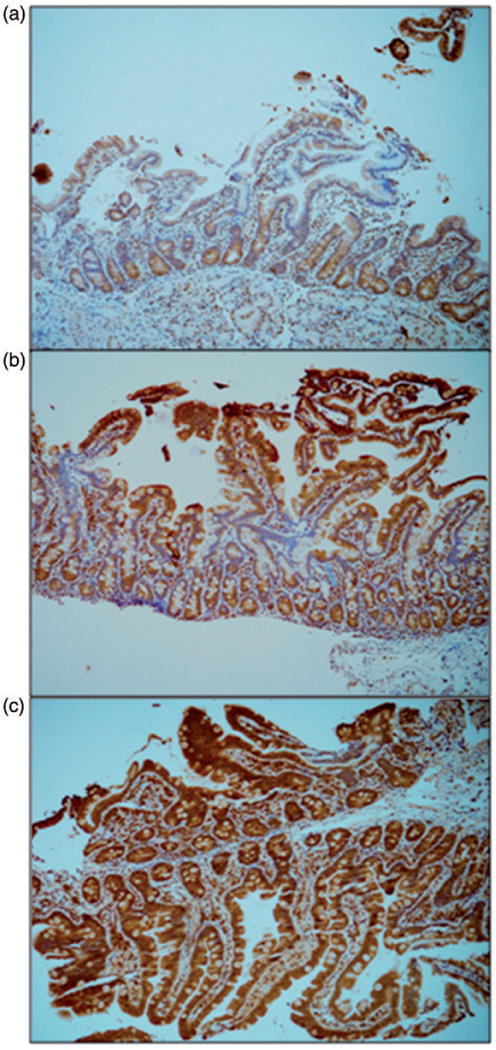
Immunohistochemical staining intensity scoring. Three different specimens of normal duodenum, each stained with immunohistochemistry antibody for Uroguanylin, were scored by the blinded pathologist. Base magnification is 10× with an ocular of 10×. These slides demonstrate the scoring of (a) “1,” (b) “2,” and (c) “3” based on the overall intensity of the brown pigment in the intestinal epithelium and lamina propria.
Figure 3.
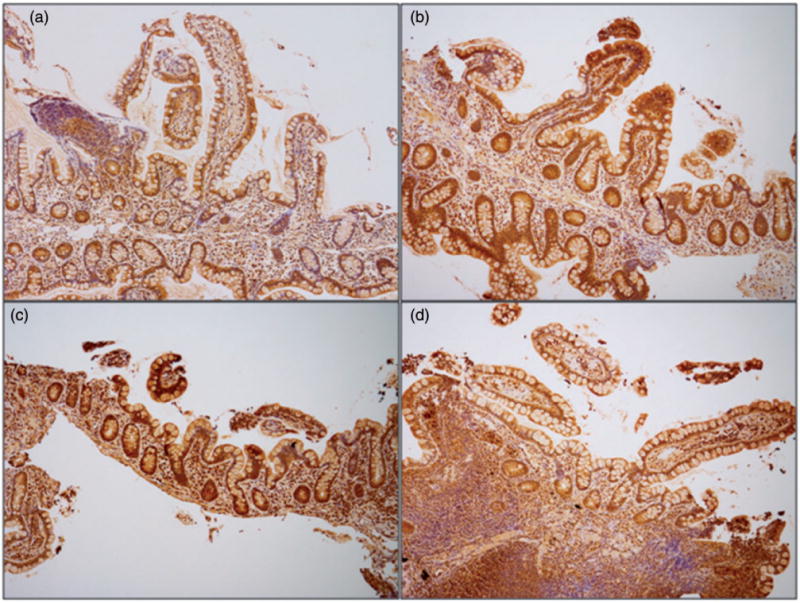
Lower intensity of staining for uroguanylin in terminal ileum biopsies from female patients with obesity. Terminal ileum sections were stained for uroguanylin via immunohistochemistry. Base magnification is 10× with an ocular of 10×. Terminal ileum section from representative female (a, b) and male (c, d) pairs of patients, with (a, c) and without (b, d) obesity; intensity score for (a), (c), and (d) of “1,” score for (b) of “2.”.
Figure 4.
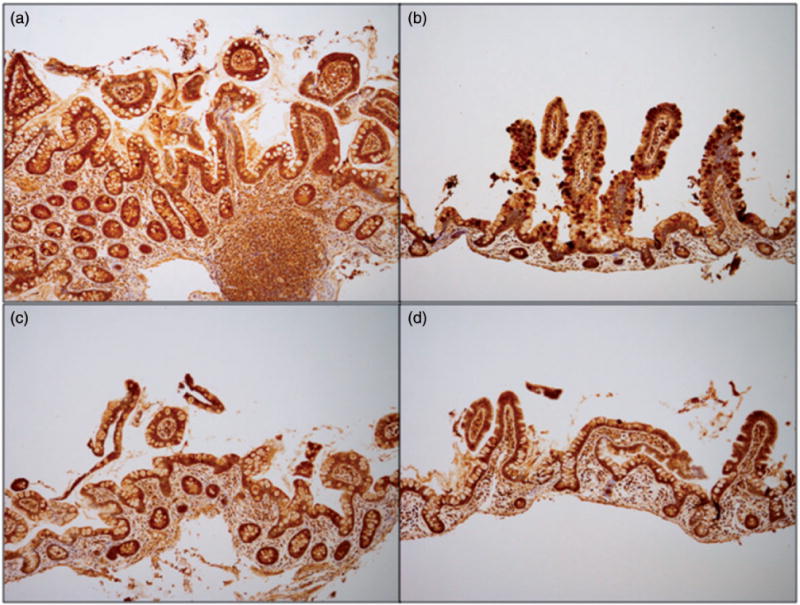
Equal intensity of staining for GUCY2C in terminal ileum biopsies from female and male patients with or without obesity. Terminal ileum sections were stained for Guanylyl cyclase C (GUCY2C) via immunohistochemistry. Base magnification is 10× with an ocular of 10×. Terminal ileum section from representative female (a, b) and male (c, d) pairs of patients, with (a, c) and without (b, d) obesity; intensity score for (a), (b) of “3,” score for (c), (d) of “2.”.
Overall Pathology Scoring—Uroguanylin, Guanylin, and Guanylyl Cyclase C
We observed that scoring for uroguanylin among pairs was as follows: 16/20 pairs had greater (11) or comparable (5) staining for uroguanylin in small intestinal tissues from patients without obesity, only 4/20 pairs had greater staining in patients with obesity. The overall scoring average for patients without obesity, from small intestinal tissues, for uroguanylin was 1.80 (standard deviation (S.D.) = 0.56, standard error (S.E.) = 0.09) versus 1.55 (S.D. = 0.55, S.E. = 0.09) = for patients with obesity, P < .03. For guanylin, the scoring among pairs was as follows: 16/20 pairs had greater or comparable staining for guanylin in large intestinal tissues from patients without obesity, only 4/20 pairs had greater staining in patients with obesity. The overall scoring average for patients without obesity, from large intestinal tissues, for guanylin was 2.26 (S.D. = 0.64, S.E. = 0.1) versus 1.89 (S.D. = 0.57, S.E. = 0.09) for patients with obesity, P < .01. GUCY2C staining in the small intestine and large intestine was 14/20 pairs with greater or comparable staining in patients without obesity, and 6/20 pairs with greater staining in patients with obesity in one or more specimens. The overall scoring average for patients without obesity, from both small and large intestinal tissues, for GUCY2C was 2.24 (S.D. = 0.59, S.E. = 0.09) versus 2.13 (S.D. = 0.52, S.E. = 0.08) for patients with obesity, P = 0.1.
Stratified Pathology Scoring—Uroguanylin
We further stratified our analysis of pairs and overall scoring by sex. Examining each age-, sex-, and ethnicity-matched pair, 9/12 female pairs (75%) had greater uroguanylin staining intensity in the average BMI-forage cohort; 2/12 had greater uroguanylin staining intensity in the high BMI-for-age cohort, and 1/12 had staining that was scored equally. Of the 8 male pairs, only 2 (25%) had greater staining intensity in the high BMI-for-age group. Looking at any small intestine location scoring of intensity of uroguanylin staining in females, the high BMI-for-age cohort had 13/24 scoring “1,” 10/24 scoring “2,” and 1/24 scoring “3.” The average BMI-for-age cohort had 5/24 scoring “1,” 16/24 scoring “2,” and 3/24 scoring “3.” These differences were statistically significant (P < .03). Summative scoring for uroguanylin staining intensity in all small intestine sites (duodenum and terminal ileum) was greater in the average BMI-for-age cohort overall (1.92, Table 3, P < .03) compared to the high BMI-for-age cohort (1.50); the difference was specific to female patients. Male patients had no difference (P = 1.0) in staining intensity between cohorts when all small intestine sites were analyzed.
Table 3.
Antibody Intensity Scoring.
| Sex | % BMI-for-age | Uroguanylin staining | Guanylin staining | GUCY2C staining (intestine) | GUCY2C staining (colon) | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | 25–75 | 1.92, 0.58 | P <.03 | 2.35, 0.71 | P <.01 | 2.21, 0.59 | P =.06 | 2.22, 0.71 | P =.24 |
| F | >95 | 1.50, 0.59 | 1.86, 0.48 | 1.96, 0.46 | 2.10, 0.48 | ||||
| M | 25–75 | 1.63, 0.50 | P = 1.0 | 2.13, 0.52 | P =.19 | 2.25, 0.58 | P = 1.0 | 2.33, 0.62 | P =.46 |
| M | >95 | 1.63, 0.50 | 1.94, 0.68 | 2.25, 0.58 | 2.31, 0.48 | ||||
BMI: Body mass index; F: Female; GUCY2C: Guanylyl cyclase C; M: Male.
Average intensity staining scores of specimens from patients by sex and BMI-for-age percentile (BMIFA) for each antibody tested. Values are expressed as mean intensity and standard deviation with P values representing Student’s t test.
Stratified Pathology Scoring—Guanylin
Examining each age-, sex-, and ethnicity-matched pair, 7/11 (63.6%) female pairs had greater guanylin staining intensity in the average BMI-for-age cohort (one pair had no available tissue for guanylin staining in the colon), 2/11 had staining intensity that was greater in the high BMI-for-age cohort, and 2/11 had staining that was scored equally between cohorts. Of the male pairs, 5/8 (62.5%) had greater staining intensity in the high BMI-for-age group. Looking at any colon location scoring of intensity of guanylin staining in females, the high BMI-for-age cohort had 4/21 scoring “1,” 16/21 scoring “2,” and 1/21 scoring “3.” The average BMI-for-age cohort of females had 3/23 scoring “1,” 9/23 scoring “2,” and 11/23 scoring “3” (3 specimens were not available for staining for guanylin in the high BMI-for-age cohort, 1 was not available for staining in the average BMI-for-age cohort). These differences were statistically significant (P < .003). Summative scoring for guanylin staining intensity in all large intestine sites (ascending colon and descending colon) was greater in the average BMI-for-age cohort overall (2.35, Table 3, P < .01) compared to the high BMI-for-age cohort (1.86); the difference was again more specific to female patients. Male patients had no difference in staining intensity (P = .19) between cohorts when all sites were analyzed overall.
Stratified Pathology Scoring—Guanylyl cyclase C
Examining each age-, sex-, and ethnicity-matched pair in the small intestine, 6/12 female pairs (50%) had greater staining intensity in the average BMI-for-age cohort; however, in 2 other pairs, the 2 cohorts had equal staining intensity. For males, 4/8 (50%) pairs had greater staining intensity in the average BMI-for-age cohort. Looking at overall (all locations) scoring of GUCY2C intensity staining in females, the high BMI-for-age cohort had 5/45 scoring 1, 34/45 scoring 2, and 6/45 scoring 3. The average BMI-for-age cohort of females had 4/47 scoring 1, 29/47 scoring 2, and 14/47 scoring 3 (3 specimens were not stained for GUCY2C in the high BMI-for-age cohort, 1 in the average BMI-for-age cohort). These differences were not statistically significant (P = .14). Summative scoring for GUCY2C staining intensity in all small and large intestine sites was comparable between both cohorts (Table 3). For female patients, the difference between cohorts for GUCY2C staining was not statistically significant both in the small intestine (P = .56) and in the large intestine (P = .24). For males, similar results were observed (Table 3, P = 1.0 for small intestine GUCY2C and P = .46 for large intestine GUCY2C).
Discussion
Obesity is a multifactorial disease and remains a heavy burden on the health of children and adolescents.2,5,6,12 Extensive study of the cause of obesity, of how obesity develops, and of the risk factors for obesity has generated many potential paths for further research and therapeutic intervention.1,4,31,32 Key among the interventions and research efforts are understanding the role of appetite in obesity’s development, and targeting early feeding and nutritional inputs for prevention of obesity.2,3,8,26,28,31,33–36 To prevent obesity, one strategy involves targeting satiety responsiveness early in childhood when the development of food intake self-regulation is unfolding, thus preventing excess consumption of calories. By understanding the mechanisms that drive satiety and what influences these mechanisms, we may be able to intervene before obesity can take hold.
Uroguanylin and GUCY2C are a novel gut hormone-receptor signaling pathway important in appetite/satiety and relevant to obesity.17,29,30,37,38 Uroguanylin, an endogenous intestinal peptide controlling fluid homeostasis by paracrine secretion into the small intestinal lumen, has been demonstrated in mice to function outside of the gastrointestinal tract via an endocrine pathway.21 Precursor (pro-) uroguanylin travels to the hypothalamus and binds GUCY2C, initiating a cGMP-controlled cascade of signaling that increases satiety in mice.19,21,39 In humans, some initial data on the uroguanylin satiety pathway exist.30 In this study, we aim to characterize intestinal expression of uroguanylin and guanylin and GUCY2C in the clinical setting of obesity. We use IHC and novel antibodies for uroguanylin and GUCY2C to identify staining in otherwise normal tissue from two groups of matched children/adolescents, one group with obesity and one without.
Review of a decade of records on diagnostic, outpatient endoscopic procedures involving the upper and lower gastrointestinal tract revealed that a small percentage of patients meet our inclusion criteria for normal histology (Figure 1) and fewer for criteria such as age and average or high BMI-for-age. We were able to identify at least 40 patients with normal pathology results and sort them into matched pairs, with 1 patient in each pair average BMI-for-age and 1 high BMI-for-age. We were successful in immunohistochemical staining of all available tissues from these patients using a commercial guanylin IHC antibody, and custom-made uroguanylin and GUCY2C IHC antibodies. Staining of all specimens demonstrated gradation of intensity for each of the three proteins.
We observe uroguanylin, shown in an animal model to be suppressed in obesity29 and in adult women with obesity to have decreased circulating pro-hormone concentrations,30 to have diminished staining intensity in high BMI-for-age females (Figure 3, Table 3) but not males. These data suggest a decrease in expression in the small intestine of uroguanylin that would reflect less pro-uroguanylin secretion into the circulation. The concomitant reduction would diminish uroguanylin’s pro-satiety effects. Other studies have examined gastrointestinal peptide expression in the setting of other pathology besides obesity;40–42 these studies were in adults or animal models. The setting of obesity has also been used to examine distribution of gastrointestinal peptides such as GLP-1 in adult tissues.43 To our knowledge, our study presents the first data on uroguanylin or GUCY2C staining in adolescent biopsy specimens with direct comparison between patients with and without obesity.
The inability to control or regulate food intake promotes the obesity state. It is not yet clearly demonstrated whether the development of obesity suppresses uroguanylin expression in humans or whether uroguanylin expression is suppressed by another mechanism, contributing to obesity. Animal studies imply that increased caloric intake leads to the obesity state (metabolic disruption/metabolic syndrome) by direct suppression of uroguanylin.16,17,29 Future studies may elucidate the directional mechanisms in humans. The data presented here support the concept of loss of uroguanylin signaling due to excess calories (less satiety) leads to obesity—a positive feedback loop.
Guanylin, previously shown to be suppressed in the obesity state in animal models,37 is observed to have diminished staining intensity in high BMI-for-age females (Table 3) but not males, suggesting a decrease of expression of guanylin in the colon in obesity. While guanylin is not known to travel extra-intestinally to regulate appetite, its decreased expression may be a marker for the effect of the obesity state on key regulatory peptides in the gut. Adult and animal studies have pointed to guanylin and GUCY2C’s role in colorectal cancer.37,44 GUCY2C staining intensity is observed to be fairly consistent in all specimens studied, both in the small intestine and the colon (Figure 4). In females, the small intestine GUCY2C staining scoring approaches statistical significance (P = .06) for a difference between cohorts with and without obesity (Table 3). But overall, GUCY2C expression appears to be stable in obesity as compared to average BMI-for-age specimens, both within pairs and between cohorts. The implication is that the effects of obesity center on the signaling peptides and not the downstream receptor, echoing observations in animal models,29,37,38 and recent studies in adults with obesity.30
Our study has limitations. Polyclonal antibody IHC is limited in its specificity; however, the staining appears robust and graded across our specimens. Moreover, notable differences exist between cohorts. Our single-blinded pathologist utilized a standard scoring system; however, we did not corroborate scoring with a second blinded observer. We would consider our technique semiquantitative and attempted to make qualitative assessments of the staining. Future alternative approaches could include qRT-PCR or quantitative RNA-Fluorescence in situ hybridization. Some of our pairs did not have all four tissue specimens available, and we were not able to examine any other biopsy specimens from other locations from patients in this study. While our uroguanylin and GUCY2C antibodies are custom designed and had adequate negative and positive controls, this is their first use in immunohistochemical assays without prior demonstration of affinity or avidity. Our data are not able to explain the sex difference in tissue intensity staining but agree with our prior work. Lastly, we were not able to find a substantial number of pairs of patients with normal biopsy specimens and who met our inclusion criteria that were of a more diverse racial or ethnic background. This limits the generalization of our observations.
Uroguanylin is emerging as a gut peptide of importance in the control of satiety and the development or maintenance of the obesity state/metabolic syndrome.19,39 Ongoing animal studies and some limited human studies have begun to elucidate the mechanisms that link uroguanylin satiety signaling to obesity and to diet effects in the gut. Uroguanylin analogs are considered possible targets for therapeutic intervention in adults with obesity.38 It was our hypothesis that uroguanylin is suppressed in high BMI-for-age individuals, as our prior data showed diminished secretion of its circulating precursor. We suspected that staining would mirror this pattern. We see decreased staining in the group of female patients who are high BMI-for-age for uroguanylin that implies a direct link between obesity and decreased satiety control through downregulation of uroguanylin expression.
The cellular mechanisms by which the obesity state and metabolic derangements cause decreased GUCY2C ligand expression and secretion have been hinted at in animal models29 but remain to be discovered in humans. For children, particularly, the development of appetite and satiety is often an early life event. Changes in diet, calorie intake, gastrointestinal microbiota, and environmental influences may all contribute to the regulation and coordination of satiety signals. Food intake self-regulation develops in the milieu of these signals, and in the backdrop of an individual’s genetic make-up. Future studies will focus on the uroguanylin-GUCY2C genotype differences between infants and children of different backgrounds and weights, as well as examine the gut-specific influences, such as nutrition and micro-flora, on the expression of satiety hormones such as uroguanylin.
Acknowledgments
The authors would like to thank Bobbie Boyce and Heather Hardy in the Nemours Histology Core for their expertise and hard work in preparing all of the patient slides and troubleshooting the immunohistochemistry; Scott Waldman for his scientific advice; and Dante Merlino and Adam Snook for discussions with troubleshooting the immunohistochemistry. They also thank Li Xie, who assisted with the statistical analyses for the manuscript. They also acknowledge the support of the Delaware CTR pilot investigator grant program, and particularly, Rob Akins, Julie Barthold, and Diane Abatemarco and invaluable institutional support from Erin Riegel, Denise Axsmith, Suzanne Purfield, Mary McElwain, and Linda Krawczuk.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: S. Binder-Macleod). The authors wish to acknowledge the Nemours Summer Undergraduate Research Scholars Program which supported one of the co-authors (LP).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Blake-Lamb TL, Locks LM, Perkins ME, et al. Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med. 2016;50:780–789. doi: 10.1016/j.amepre.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown CL, Halvorson EE, Cohen GM, et al. Addressing childhood obesity: opportunities for prevention. Pediatr Clin North Am. 2015;62:1241–1261. doi: 10.1016/j.pcl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels SR, Hassink SG. The role of the pediatrician in primary prevention of obesity. Pediatrics. 2015;136:e275–e292. doi: 10.1542/peds.2015-1558. [DOI] [PubMed] [Google Scholar]
- 4.Lumeng JC, Taveras EM, Birch L, et al. Prevention of obesity in infancy and early childhood: a National Institutes of Health Workshop. JAMA Pediatr. 2015;169:484–490. doi: 10.1001/jamapediatrics.2014.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spieker EA, Pyzocha N. Economic impact of obesity. Prim Care. 2016;43:83–95. doi: 10.1016/j.pop.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Ip EH, Leng X, Zhang Q, et al. Risk profiles of lipids, blood pressure, and anthropometric measures in childhood and adolescence: project heartbeat! BMC Obes. 2016;3:9. doi: 10.1186/s40608-016-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramanathan AS, Senguttuvan P, Prakash V, et al. Budding adult hypertensives with modifiable risk factors: “catch them young”. J Family Community Med. 2016;23:38–42. doi: 10.4103/2230-8229.172232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2015;74:227–234. doi: 10.1017/S0029665114001700. [DOI] [PubMed] [Google Scholar]
- 10.Connaughton RM, McMorrow AM, McGillicuddy FC, et al. Impact of anti-inflammatory nutrients on obesity-associated metabolic-inflammation from childhood through to adulthood. Proc Nutr Soc. 2016;3:1–10. doi: 10.1017/S0029665116000070. [DOI] [PubMed] [Google Scholar]
- 11.Huh SY, Rifas-Shiman SL, Taveras EM, et al. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics. 2011;127:e544–e551. doi: 10.1542/peds.2010-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister EJ, Dhurandhar NV, Keith SW, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr. 2009;49:868–913. doi: 10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal A, Lanigan J. Breastfeeding, early growth and later obesity. Obes Rev. 2007;8(Suppl 1):51–54. doi: 10.1111/j.1467-789X.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 14.Taveras EM, Rifas-Shiman SL, Belfort MB, et al. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voss JD, Atkinson RL, Dhurandhar NV. Role of adenoviruses in obesity. Rev Med Virol. 2015;25:379–387. doi: 10.1002/rmv.1852. [DOI] [PubMed] [Google Scholar]
- 16.Folgueira C, Sanchez-Rebordelo E, Barja-Fernandez S, et al. Uroguanylin levels in intestine and plasma are regulated by nutritional status in a leptin-dependent manner. Eur J Nutr. 2016;55:529–536. doi: 10.1007/s00394-015-0869-2. [DOI] [PubMed] [Google Scholar]
- 17.Folgueira C, Beiroa D, Callon A, et al. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes. 2016;65:421–432. doi: 10.2337/db15-0889. [DOI] [PubMed] [Google Scholar]
- 18.Horner K, Lee S. Appetite-related peptides in childhood and adolescence: role of ghrelin, PYY, and GLP-1. Appl Physiol Nutr Metab. 2015;40:1089–1099. doi: 10.1139/apnm-2015-0050. [DOI] [PubMed] [Google Scholar]
- 19.Fruhbeck G. Gastrointestinal hormones: uroguanylin-a new gut-derived weapon against obesity? Nat Rev Endocrinol. 2012;8:5–6. doi: 10.1038/nrendo.2011.206. [DOI] [PubMed] [Google Scholar]
- 20.Bascietto C, Giannini C, D’Adamo E, et al. Implications of gastrointestinal hormones in the pathogenesis of obesity in pre-pubertal children. J Pediatr Endocrinol Metab. 2012;25:255–260. doi: 10.1515/jpem-2011-0478. [DOI] [PubMed] [Google Scholar]
- 21.Valentino MA, Lin JE, Snook AE, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121:3578–3588. doi: 10.1172/JCI57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomenick JP, Melguizo MS, Mitchell SL, et al. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide YY secretion in prepubertal children. J Clin Endocrinol Metab. 2009;94:4463–4471. doi: 10.1210/jc.2009-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide YY, and appetite in normal weight and overweight children. Obesity (Silver Spring) 2008;16:547–552. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 24.Taut D, Baban A, Giese H, et al. Developmental trends in eating self-regulation and dietary intake in adolescents. Appl Psychol Health Well Being. 2015;7:4–21. doi: 10.1111/aphw.12035. [DOI] [PubMed] [Google Scholar]
- 25.Hughes SO, Power TG, O’Connor TM, et al. Executive functioning, emotion regulation, eating self-regulation, and weight status in low-income preschool children: how do they relate? Appetite. 2015;89:1–9. doi: 10.1016/j.appet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birch LL, Doub AE. Learning to eat: birth to age 2 y. Am J Clin Nutr. 2014;99:723s–728s. doi: 10.3945/ajcn.113.069047. [DOI] [PubMed] [Google Scholar]
- 27.Scaglioni S, Arrizza C, Vecchi F, et al. Determinants of children’s eating behavior. Am J Clin Nutr. 2011;94:2006s–2011s. doi: 10.3945/ajcn.110.001685. [DOI] [PubMed] [Google Scholar]
- 28.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–549. [PubMed] [Google Scholar]
- 29.Kim GW, Lin JE, Snook AE, et al. Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr Diabetes. 2016;6:e211. doi: 10.1038/nutd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez A, Gomez-Ambrosi J, Catalan V, et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int J Obes (Lond) 2016;40:1405–1415. doi: 10.1038/ijo.2016.66. [DOI] [PubMed] [Google Scholar]
- 31.Gortmaker SL, Taveras EM. Who becomes obese during childhood—clues to prevention. N Engl J Med. 2014;370:475–476. doi: 10.1056/NEJMe1315169. [DOI] [PubMed] [Google Scholar]
- 32.Woo Baidal JA, Locks LM, Cheng ER, et al. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;15:00752–00757. doi: 10.1016/j.amepre.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Brown CL, Skelton JA, Perrin EM, et al. Behaviors and motivations for weight loss in children and adolescents. Obesity (Silver Spring) 2016;24:446–452. doi: 10.1002/oby.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutelle KN, Peterson CB, Crosby RD, et al. Overeating phenotypes in overweight and obese children. Appetite. 2014;76:95–100. doi: 10.1016/j.appet.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewey KG. Nutrition, growth, and complementary feeding of the breastfed infant. Pediatr Clin North Am. 2001;48:87–104. doi: 10.1016/s0031-3955(05)70287-x. [DOI] [PubMed] [Google Scholar]
- 36.Faith MS, Carnell S, Kral TV. Genetics of food intake self-regulation in childhood: literature review and research opportunities. Hum Hered. 2013;75:80–89. doi: 10.1159/000353879. [DOI] [PubMed] [Google Scholar]
- 37.Lin JE, Colon-Gonzalez F, Blomain E, et al. Obesity-induced colorectal cancer is driven by caloric silencing of the Guanylin-GUCY2C paracrine signaling axis. Cancer Res. 2016;76:339–346. doi: 10.1158/0008-5472.CAN-15-1467-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blomain ES, Merlino DJ, Pattison AM, et al. GUCY2C hormone axis at the intersection of obesity and colorectal cancer. Mol Pharmacol. 2016;90:199–204. doi: 10.1124/mol.115.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeley RJ, Tschöp MH. Uroguanylin: how the gut got another satiety hormone. J Clin Invest. 2011;121:3384–3386. doi: 10.1172/JCI58297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran GW, Pennock J, McLaughlin JT. Enteroendocrine cells in terminal ileal Crohn’s disease. J Crohns Colitis. 2012;6:871–880. doi: 10.1016/j.crohns.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Rau TT, Sonst A, Rogler A, et al. Gastrin mediated down regulation of ghrelin and its pathophysiological role in atrophic gastritis. J Physiol Pharmacol. 2013;64:719–725. [PubMed] [Google Scholar]
- 42.Egerod KL, Engelstoft MS, Grunddal KV, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guedes TP, Martins S, Costa M, et al. Detailed characterization of incretin cell distribution along the human small intestine. Surg Obes Relat Dis. 2015;11:1323–1331. doi: 10.1016/j.soard.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Blomain ES, Waldman SA. Does obesity promote the development of colorectal cancer? Expert Rev Anticancer Ther. 2016;16:465–467. doi: 10.1586/14737140.2016.1162102. [DOI] [PMC free article] [PubMed] [Google Scholar]


