Abstract
In multiple sclerosis (MS), there is a growing interest in inhibiting the pro-inflammatory effects of granulocyte-macrophage colony-stimulating factor (GM-CSF). We sought to evaluate the therapeutic potential and underlying mechanisms of GM-CSF receptor alpha (Rα) blockade in animal models of MS. We show that GM-CSF signaling inhibition at peak of chronic experimental autoimmune encephalomyelitis (EAE) results in amelioration of disease progression. Similarly, GM-CSF Rα blockade in relapsing-remitting (RR)-EAE model prevented disease relapses and inhibited T cell responses specific for both the inducing and spread myelin peptides, while reducing activation of mDCs and inflammatory monocytes. In situ immunostaining of lesions from human secondary progressive MS (SPMS), but not primary progressive MS patients shows extensive recruitment of GM-CSF Rα+ myeloid cells. Collectively, this study reveals a pivotal role of GM-CSF in disease relapses and the benefit of GM-CSF Rα blockade as a potential novel therapeutic approach for treatment of RRMS and SPMS.
Keywords: GM-CSF, Multiple sclerosis, MS, EAE, Dendritic cells, Inflammatory monocytes
1. Introduction
Multiple sclerosis (MS) is an immune-mediated disorder of the central nervous system (CNS) characterized by multifocal areas of leukocyte infiltration, demyelination and axonal damage. The immune response in MS is believed to be mediated by autoreactive T lymphocytes that recognize myelin peptides. MS and its animal model, experimental autoimmune encephalomyelitis (EAE), are driven by both TH1 [producing interferon (IFN)-γ, interleukin (IL)-2 and tumor necrosis factor (TNF)], and TH17 [producing IL-17, IL-21, IL-22 and Granulocyte-macrophage colony-stimulating factor (GM-CSF)] subsets. Surprisingly, IFN-γ, IL-12, IL-17A, IL-17F, IL-21 and IL-22 have all been shown to be dispensable for the development of EAE [1,2], however the pathogenicity of TH17 cells has been linked with production of GM-CSF [3–6]. GM-CSF is a hematopoietic growth factor produced by a number of hematopoietic and non-hematopoietic cells including activated CD4+ T cells, monocytes/macrophages, B cells, NK cells, endothelial cells and epithelial cells. GM-CSF has a wide array of functions, notably inducing the survival and activation of myeloid cells, differentiation of dendritic cells (DCs), the polarization of macrophages towards an M1 profile, enhancing antigen presentation, inducing complement- and antibody-mediated phagocytosis, and mobilizing monocytes and other myeloid populations from bone marrow to blood [7–9].
The GM-CSF receptor (GM-CSF R) is a heterodimer comprised of a specific low-affinity α chain (CD116; GM-CSF Rα) and a common β chain (CD131; GM-CSF Rβ) that is shared by IL-3 and IL-5 [10]. The GM-CSF R is expressed in multipotent myeloid progenitor cells and continues to be expressed throughout myeloid development on monocytes, DCs, macrophages and neutrophils [11,12]. It is not expressed by lymphocytes [11].
In EAE, GM-CSF is necessary for disease expression as GM-CSF KOs are resistant to disease induction [13]. Disease can be rescued by the administration of recombinant GM-CSF. Adoptive transfer using cytokine-deficient mice showed that wild-type, IL-17A−/−, and IFN-γ−/− T cells induced transfer EAE with similar kinetics. By contrast, GM-CSF−/− T cells were incapable of inducing EAE and invading the CNS [14]. Significantly, a recent study demonstrated that peripheral myeloid cells, but not microglia, are the key GM-CSF responder cells [14] corresponding with earlier observations that GM-CSF administration stimulated egress of CD11b+Ly6Chi monocytes into the circulation [15]. These data were recently confirmed using conditional gene targeting in which the beta chain of the GM-CSF receptor (Csf2rb) was deleted in specific subpopulations throughout the myeloid lineages [4]. It was found that deletion of Csf2rb in CCR2+Ly6Chi monocytes phenocopied the EAE resistance seen in complete Csf2rb-deficient mice.
Approximately 85% of MS patients are initially diagnosed with the relapsing-remitting form of the disease (RRMS) and approximately half of these patients go on to develop secondary progressive MS (SPMS), which is characterized by steady clinical deterioration, independent of relapses. Primary progressive MS (PPMS) affects only 10—15% of the MS population and is associated with a rapid unremitting disease progression. Despite the importance of GM-CSF in MS and EAE, and the growing interest in therapeutic potential of blocking this cytokine, the pathologic role of GM-CSF in relapses and in the chronic phases of CNS autoimmunity remains unknown, as does the exact effect of GM-CSF on myeloid cells. In the current study, we investigated the effects of specifically blocking GM-CSF signaling using a monoclonal antibody (mAb) directed again the GM-CSF Rα chain (CAM-3003) in both chronic (C-EAE) and relapsing-remitting (RR-EAE) models of MS and the underlying mechanisms of disease amelioration. Since GM-CSF is produced locally in the CNS [16–18], targeting the receptor allows us to address the consequence of GM-CSF signaling blockade on infiltrating myeloid cell populations in response to the local production of GM-CSF. CAM-3003 has been well characterized and previously shown to reduce disease severity and the number of synovial macrophages in a collagen-induced arthritis model [19].
2. Materials and methods
2.1. Mice
Female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Female SJL/J mice were obtained from Harlan Laboratories (Bethesda, MD). All mice were housed under specific pathogen-free conditions in the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)—approved Center for Comparative Medicine SPF facility at Northwestern University.
2.2. Active EAE
Eight to ten week-old female C57BL/6 mice were used to induce EAE by active immunization. Mice were injected subcutaneously with 200 µg of MOG35–55 peptide (Genemed Synthesis, San Francisco, CA) emulsified in complete Freund's adjuvant (CFA) containing 200 µg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) distributed over three sites on the flank. On day 0 and 2 after immunization, 200 ng pertussis toxin (List Biological Laboratories, Campbell, CA) were administered intraperitoneally (i.p.). Mice were treated with anti-GM-CSF Rα antibody (CAM3003; 3 mg/kg, 10 mg/kg or 30 mg/kg), anti-GM-CSF blocking antibody (clone 22E9; 10 mg/kg or 30 mg/kg) or isotype control (3 mg/kg, 10 mg/kg or 30 mg/kg) at the peak of disease (n = 8 mice per group). The injections were done i.p. three times a week, until the completion of the study.
For RR-EAE disease induction, eight to ten week-old female SJL/J mice were injected with CFA emulsion containing 50 µg PLP139–151. Mice were treated with anti-GM-CSF Rα antibody or isotype control (10 mg/kg) either at the time of disease induction or at the peak of disease. The injections were done i.p. three times a week, until the completion of the study. Two independent experiments were performed for mice treated at time of disease induction, both experiments with 10 mice per group. Three independent experiments were performed for mice treated at peak of disease, one experiment with 10 mice per group, and two experiments with 8 mice per group.
Clinical signs of EAE were assessed daily according to the following scores: 0, no clinical sign of disease; 1, limp tail; 2, hind limp weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; 5, hind and fore limb paralysis. Data are reported as the mean daily clinical score.
2.3. Ex vivo recall responses and LPS activation of splenocytes
Spleens were harvested from mice at the peak of disease relapse, counted, and cultured in 96-well microtiter plates at a density of 106 cells/well in a total volume of 200 µl of R10 media (RPMI with 10% (vol/vol) fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin). Cells were cultured at 37 °C in the presence of OVA323–339 (purity: 97.29%), PLP139–151 (purity: 97.78%), PLP178–191 (purity: 95.12%) and MBP84–97 (purity: 96.34%) (Genemed Synthesis; 20 µg/ml) for 72 h. Proliferation was evaluated after staining for Ki-67 (eBioscience) nuclear antigen. Live-dead discrimination was performed using LIVE/DEAD fixable staining reagents (Life Technologies) and intracellular staining for Ki-67 was done using Foxp3/Transcription Factor Staining Buffer Set (eBiosciences). The frequency of Ki-67 positive cells was assessed on gated live CD3+ CD4+ cells. For cytokine quantification, media samples were measured by multiplex cytokine assays (Millipore) for IFN-γ, IL-17, GM-CSF and TNF-α production according to manufacturer's instructions.
Day 34 post-immunization splenocytes (106 cells/well) were also activated for 24 h in presence of LPS (10 ng/ml) from E. coli serotype 0111:B4 (Sigma) in 200 µl of R10 media. IL-1β, IL-6, IL-12p70, IL-23 and TNF-α cytokines were measured by multiplex cytokine assays (Millipore) following manufacturer's instructions.
2.4. Isolation of CNS leukocytes
CNS-immune cells were isolated by Percoll gradient centrifugation from homogenized combined brain and spinal cords as previously described [20]. The numbers of cell subpopulations in the CNS were determined by multiplying the percentage of lineage marker—positive cells by the total number of mononuclear cells isolated from the CNS.
2.5. Flow cytometry analysis
Fc receptors were blocked using anti-mouse CD16/32 (0.25 µg; eBioscience). Cells were then stained for 30 min at 4 °C using the specified antibodies (GM-CSF Rα from R&D Systems; all the others from BD Biosciences or eBioscience). To detect T cell cytokine expression, cells were activated for 18 h with 1 mg/ml ionomycin and 20 ng/ml phorbol 12-myristate 13-acetate 40 (PMA) in the presence of 2 mg/ml brefeldin A (Sigma) for the last 6 h of co-culture. To detect antigen-presenting cell cytokine expression, cells were activated for 18 h with 100 ng/ml LPS from E. coli serotype 0111:B4 (Sigma) in the presence of 2 mg/ml brefeldin A (Sigma) for the last 6 h of co-culture. Cells were stained for surface markers and a fixation and permeabilization kit (eBioscience) was used. Cells were acquired on a BD Canto II and analyzed using BD FACSDiva version 6.1 software.
2.6. Bone marrow monocyte isolation and chemokine receptors expression
Bone marrow cells (BM) were isolated by flushing femurs and tibias of mice. Cells were passed through a 70 µm cell strainer and treated with erythrocyte lysing solution. Monocytes were purified using magnetic cell sorting (Miltenyi Biotec; Auburn, CA) according to the manufacturer's instructions. CD11b+Ly6Chi monocytes purity was >85% as assessed by flow cytometry.
To evaluate the expression of chemokine receptors on BM-monocytes, cells were plated at a density of 106 cells per well in a 96 well plate in R10 media. Cells were pre-treated with the anti-GM-CSF Rα antibody or the isotype control (100 µg/ml) for 2 h. After washing the media, cells were either left untreated or activated in presence of GM-CSF (10 ng/ml) for 18 h. Analysis of the chemokine receptors expression was then performed by flow cytometry.
2.7. Immunostaining of human and mouse CNS material
Luxol Fast Blue (LFB) and hematoxylin and eosin (H&E) staining were performed on mouse spinal cord specimens as previously described [21]. Sections were scores by an investigator 'blinded' to the identity of the treatment group. The following scale was used for histology score: 0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = marked; 5 = severe.
Chromogenic immunohistochemistry of GM-CSF Rα and CD68 in formalin-fixed paraffin embedded (FFPE) sections were performed on non-neurological disease controls (6 donors total, 5 donors shown), secondary progressive MS patients (8 donors total, 5 donors shown) and primary progressive MS patients (6 donors total, 5 donors shown). Sections showing acute demyelinating lesions and active perivascular mononuclear cell infiltration were selected, and compared to adjacent normal regions and to non-neurological disease controls. The causes of death from non-neurological disease controls were colorectal cancer and myocardial infarction. The SPMS patients were 3 females and 2 males, aged of 41, 43, 66, 81 and 83 years old, the PPMS patients were also 3 females and 2 males, aged of 45, 50, 68, 71 and 77 years old, while the controls were 2 females and 3 males, aged of 59, 66, 75 and 87 (2×) years old. Paraffin-embedded blocks were cut into 4 µm thick sections. Dako PT Link and Target Retrieval Solution were used for deparaffinization and antigen retrieval of the sections. Staining was performed employing the Dako EnVision Flex kit and using the mouse monoclonal antibody specific for GM-CSF Rα (IgG1, Santa Cruz, final concentration 8 µg/ml) or CD68 (IgG1, Abcam, diluted 1 In 2) for 30 min at 4 °C.
Quantification was performed by manually counting the number of positive staining. The data is presented as the proportion number of positive cells/number of total cells in each section.
For GM-CSF Rα and CD11c co-immunostaining, frozen CNS material from MS patients (n = 3) was obtained after autopsy. Time of death to snap freezing of the blocks varied from 1 to 2.5 h. These blocks are distinct from the paraffin-embedded material used. Spinal cord material from EAE animals was collected following rapid intra-cardiac phosphate-buffered saline (PBS) perfusion and snap-frozen in dry ice. Human and murine tissues were cryosectioned (7 µm thick), mounted on superfrost slides (Thermo Scientific), fixed in −20 °C acetone for 10 min and hydrated in PBS. Endogenous biotin was blocked with the Avidin/Biotin blocking kit (Invitrogen) when required. Non-specific immunoglobulin binding was blocked with serum for 30 min. Sections were then incubated for 1 h with the primary antibody diluted in serum. Slides were washed 7 times for 3 min with PBS Tween 20 0.05% (v/v) after each incubation. This was followed by 1 h incubation with the secondary antibody. All incubations were done at room temperature. Corresponding isotypes were used as controls for the immunostains. Sections were then mounted with Gelvatol containing either TOPRO-3 (for human sections; Invitrogen) or DAPI (4′,6-diamidino-2-phenylindole; for mouse sections; Molecular Probes) for nuclear staining. Fluorescence acquisition was done on a Leica SP5 confocal microscope for human samples, and on a Nikon A1R Confocal microscope for mouse samples.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad PRISM 6.0 (GraphPad software). Data are presented as the mean ± the standard error of the mean (SEM). The chemokine receptors analysis was performed by nonparametric Wilcoxon matched-pairs test. All the other analyses including EAE scores were analyzed by nonparametric Mann-Whitney test. Only p values < 0.05 were considered significant.
3. Results
3.1. Anti-GM-CSF Rα treatment ameliorates chronic EAE
C57BL/6 mice undergoing MOG35–55-induced C-EAE were treated i.p. 3 times a week with 3 different doses of anti-GM-CSF Rα antibody (3,10 and 30 mg/kg) or isotype control antibody starting at peak of disease (day 14). Blockade of the GM-CSF Rα at 10 and 30 mg/kg resulted in a sustained and significant reduction in disease clinical score, as compared to animals receiving the isotype control (Fig. 1A; n = 8 mice per group, ***p < 0.001). The treatment was associated with reduced immune cell infiltration as determined by H&E staining and demyelination as determined by Luxol fast blue staining (Fig. 1B). In particular, the total numbers of peripherally-derived CD45hi CNS infiltrating cells, as well as the numbers of CD45hi CD4+ IFN-γ+ cells, and CD45hi CD4+ IL-17+ cells were markedly reduced in CAM-3003-treated animals, as compared to IgG-treated controls (Fig. 1C; n = 5 mice analyzed per group, *p < 0.05). These data are in accordance with previous reports showing the potential use of GM-CSF targeting in C-EAE [6,13,22]. We next compared the efficiency of anti-GM-CSF (clone 22E9) vs. anti-GM-CSF Rα (CAM-3003) blocking antibodies in treating C-EAE. Targeting GM-CSF Rα showed equivalent efficiency in reducing EAE severity as blockade of the cytokine itself (Fig. S1; n = 8 mice per group). Together these data demonstrate the critical role of GM-CSF in the chronic phase of the disease and provide arguments for a potential therapeutic role for targeting GM-CSF Rα in MS patients.
Fig. 1. Therapeutic treatment with anti-GM-CSF Rα ameliorates progression of C-EAE clinical scores and reduces CNS cell infiltration.
Active C-EAE was induced in C57BL/6 animals by immunization with MOG35–55. (A) anti-GM-CSF Rα-treated mice at 10 mg/kg (center panel) and 30 mg/kg (right panel) show a significant reduction in clinical scores as compared to isotype control animals and PBS mice (n = 8 mice per group; ***p < 0.001 by nonparametric Mann-Whitney test); Mice receiving no treatment (No Rx) are also represented. (B) Frozen spinal cord specimens (8 µm sections) from EAE mice were stained for hematoxylin and eosin (H&E; upper panels) and luxol fast blue (LFB; lower panels). Photomicrographs shown are representative of >10 sections performed on five separate animals. (C) Number of total cells, CD45hiCD11b−CD3+CD4+IL-17+ T cells, and CD45hiCD11b−CD3+CD4+IFN-γ+ T cells in the CNS of EAE mice treated with control Ig (squares) vs. anti-GM-CSF Rα-treated mice (10 mg/kg; circles) at day 34 post-immunization (n = 5 mice per group; *, p < 0.05 by unpaired Student's t-test). Data shown are representative of 3 independent experiments.
3.2. Prophylactic and therapeutic treatment of RR-EAE
We next tested the ability of anti-GM-CSF Rα to block EAE development in PLP139–151-induced RR-EAE in SJL/J mice. Anti-GM-CSF Rα administered 3 times a week starting from day 0 totally inhibited development of RR-EAE (Fig. S2A; n = 10 mice per group, ***p < 0.001) highlighting the importance of GM-CSF in the initiation stages of disease. Prophylactic treatment with anti-GM-CSF Rα resulted in a significant reduction of the numbers of CNS-infiltrating inflammatory myeloid cells, T cells and DCs, as well as encephalitogenic T cells producing IFN-γ, IL-17, GM-CSF and TNF (Fig. S2B; n = 6 mice per group, **p < 0.01).
We next examined the therapeutic capacity of anti-GM-CSF Rα Ab for treating ongoing RR-EAE. Anti-GM-CSF Rα or isotype control (10 mg/kg) were injected i.p. into mice 3 times a week starting at peak of acute disease (day 16 post-immunization). GM-CSF Rα blockade resulted in a significant reduction of the relapse severity, as compared to animals receiving isotype control antibody (Fig. 2A; n = 10 mice per group, ***p < 0.001). Anti-GM-CSF Rα treatment decreased the numbers of CD45hi B220− CD11b+ infiltrating myeloid cells, CD45hi B220− CD11b+ CD11c+ mDCs, CD45hi B220−CD11b+ CD11c− Ly6Chi Ly6G− inflammatory monocytes, and CD45hi CD3+ CD4+ T lymphocytes in the CNS of EAE mice sacrificed 32 days post-immunization (Fig. 2B; n = 10 mice per group, *p < 0.05, **p < 0.01) corresponding to the peak of disease relapse. Mice treated with anti-GM-CSF Rα Ab had significantly reduced numbers of CD4+ lymphocytes producing IL-17, IFN-γ, GM-CSF and TNF (Fig. 2C; n = 10 mice per group, **p < 0.01). Thus this shows for the first time the crucial role of GM-CSF in relapse severity in the RR-EAE model of MS, and the potential therapeutic benefit for targeting GM-CSF.
Fig. 2. Therapeutic treatment with anti-GM-CSF Rα ameliorates disease relapses in RR-EAE and reduces CNS cell infiltration.
EAE was induced in SJL/J animals by active immunization with PLP139–151. Anti-GM-CSF Rα blocking Ab or isotype control (10 mg/kg) were injected i.p. 3 times a week, starting at day 16 until day 34 post-induction. (A) Anti-GM-CSF Rα-treated mice (close circles) show a significant reduction in clinical scores as compared to isotype control Ig-treated animals (open circles; n = 10 mice per group; ***p < 0.001 by nonparametric Mann-Whitney test). Data shown are representative of 3 independent experiments. (B) Pool data of 3 experiments of the number of live CD45hiB220−CD11b+ infiltrating myeloid cells, CD45hiB220−CD11b+Ly6G−CD11c+ mDCs, CD45hiB220−CD11b+Ly6G−Ly6Chi inflammatory monocytes, CD45hiCD11b−CD3+CD4+ T cells, and (C) IL-17, IFN-γ, GM-CSF and TNF-producing CD4+ T cells in the CNS of EAE mice treated with control Ig (squares) vs. anti-GM-CSF Rα (circles) at day 32 post-immunization represented as % of controls for each cell subpopulation (n = 10 mice per group; *p < 0.05, **p < 0.01 by nonparametric Mann-Whitney test).
In addition, we evaluated the cellular content of the spleens from therapeutic treated mice. While the lymphocytes population remained unchanged, we observed a decrease in numbers of myeloid cells and in particular the inflammatory monocytes sub-population (Fig. S3; n = 10 mice per group, *p < 0.05, **p < 0.01).
We also assessed if therapeutic anti-GM-CSF Rα treatment decreased myelin peptide-specific T cell responses. Spleens harvested at day 34 from mice given anti-GM-CSF Rα at day 16 exhibited a decrease recall responses to the disease-inducing PLP139–151 peptide as well as to PLP178–191 and MBP84–97 spread epitopes as determined by proliferation and by IFN-γ, IL-17, GM-CSF and TNF cytokine secretion (Fig. 3A and B; n = 9 mice per group, *p < 0.05, **p < 0.01, ***p < 0.001). These results demonstrate that anti-GM-CSF Rα is able to inhibit effector cytokine production by both previously and newly activated T cells, and therefore limits epitope spreading.
Fig. 3. Anti-GM-CSF Rα treatment inhibits myelin-specific T cell responses.
Following therapeutic treatment on SJL/J mice, spleens were harvested at day 34 post-immunization and recall responses of total splenocytes (106 cells/well) to PLP139–151, PLP178–191, MBP84–97 and OVA323–339 (20 µg/ml) were measured after 72 h of culture. (A) Proliferation, as assessed by % of Ki-67+ staining from live CD4+ T cells (n = 9 mice per group, *p < 0.05, **p < 0.01 by nonparametric Mann-Whitney test), and (B) cytokines production in response to myelin peptides were reduced in spleens of anti-GM-CSF Rα-treated mice (10 mg/kg; white squares) in comparison to isotype control Ig-treated mice (grey circles) (n = 9 mice per group; *p < 0.05, **p < 0.01, ***p < 0.001 by nonparametric Mann-Whitney test).
3.3. CNS-infiltrating inflammatory monocytes and mDCs highly express GM-CSF Rα in both EAE and MS
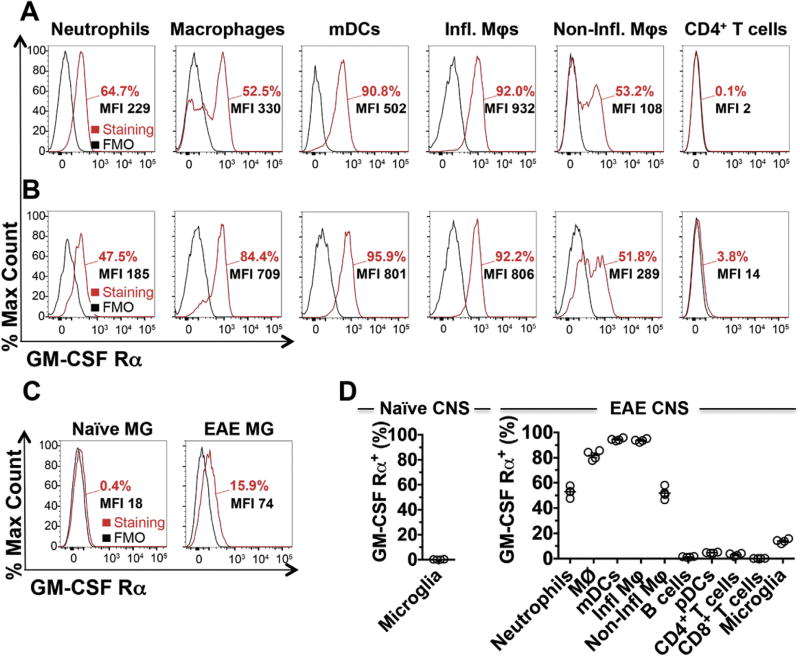
In an initial attempt to understand the mechanism of action of anti-GM-CSF Rα, we first measured the expression level of GM-CSF Rα on different immune populations. We initially validated our flow cytometry approach by measuring the expression level of GM-CSF Rα on bone marrow (BM) monocytes, which are highly responsive to GM-CSF and are thus expected to express significant levels of the receptor. As anticipated, the vast majority (97.3%) of BM monocytes expressed high level of GM-CSF Rα (Fig. S4A). In naïve spleen, receptor expression is limited to myeloid cells (Figs. S4B and S4C). B220− CD11b+ CD11c+ Ly6G− mDCs (98.6%) and B220− CD11b+ CD11c− Ly6G− Ly6Chi inflammatory monocytes (87.9%) express the highest levels. At peak of acute EAE (d15 post-immunization), splenic expression of GM-CSF Rα is still restricted to myeloid cells (Fig. 4A and Fig. S4C). In the CNS at peak of disease, we observed high expression of the GM-CSF Rα on infiltrating CD45hi B220− CD11b+ CD11c+ Ly6G− mDCs (95.9%), on CD45hi B220− CD11b+ CD11c− Ly6G− Ly6Chi inflammatory monocytes (92.2%) and on CD45hi B220− CD11b+ CD11c− Ly6G− macrophages (84.4%) (Fig. 4B and D). CD4+ T cells in the CNS of EAE mice as well as other lymphoid cells tested express very limited levels of the GM-CSF Rα. In the naïve CNS, microglia expressed negligible levels of the receptor (Fig. 4C and D). However, in CNS of EAE mice, we observed a small upregulation of GM-CSF Rα expression by microglia (Fig. 4C). Despite this upregulation, the expression levels of the alpha chain of the receptor by microglia is still significantly less in percentage and MFI when compared to other myeloid cells in the CNS, thus confirming recent studies showing that deletion of the non-specific β chain of the GM-CSF receptor (GM-CSF Rβ) on CXC3CR1+ cells does not modify C-EAE in C57BL/6 mice [4]. Analysis of spinal cord sections from naïve and EAE mice confirmed the upregulation of the GM-CSF Rα chain on CD11b+ myeloid cells at peak of the disease (Fig. 5A).
Fig. 4. GM-CSF Rα is highly expressed on mDCs and inflammatory monocytes.
(A) FACS analyses of GM-CSF Rα expression on splenocytes from EAE mice at peak of disease (14—16 days post-immunization) demonstrate high levels on B220−CD11b+CD11c+Ly6G− mDCs and B220−CD11b+CD11c−Ly6ChiLy6G− inflammatory monocytes. Expression on B220−CD11b+Ly6ChiLy6G+ neutrophils, B220−CD11b+CD11c−Ly6G− macrophages, B220−CD11b+CD11c−Ly6CloLy6G− non-inflammatory monocytes and CD11b−CD3+CD4+ T cells was also assessed. The Δ median fluorescence intensity (MFI; MFI of the staining minus the MFI of the fluorescence minus one (FMO)), is also depicted in each graph. FMO is represented in black histograms and GM-CSF Rα immunostaining is shown in red. (B) GM-CSF Rα expression on CNS infiltrating cells (CD45hi) from EAE mice at peak of disease. (C) CD45loCD11b+Ly6Clo microglia from naïve mice (left panel) and EAE mice at peak of disease (right panel) were examined to evaluate GM-CSF Rα expression. (D) Compilation of GM-CSF Rα expression by flow cytometry on neutrophils, macrophages (MØ), mDCs, inflammatory monocytes (inf φ), non-inflammatory monocytes (Non-inf φ), CD11b−B220+CD11c− B cells, CD11b−B220+CD11c+ pDCs, CD11b−CD3+CD4+ T cells, CD11b−CD3+CD8+ T cells and microglia from the CNS of naïve mice (n = 6) and EAE mice at peak of disease (n = 4). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5. GM-CSF Rα is upregulated in the CNS of EAE mice and in SPMS, but not PPMS lesions.
(A) Spinal cord sections from naïve mice (left panels) and EAE mice (right panels) immunostained for CD11b (red, upper panels), GM-CSF Rα (green, center panels), and DAPI (blue) at the peak of the disease. Scale bars, 30 µm. Photomicrographs are representative of immunostaining performed on 3 sections for each mouse of n = 4 mice per group for a total of 24 sections. (B) Chromogenic immunohistochemistry of GM-CSF Rα in control brains (left panels), lesions site of SPMS brains (2nd from the left panels), adjacent to lesions site of SPMS brains (3rd from the left panels), lesions site of PPMS brains (2nd from the right panels) and adjacent to lesions site to PPMS brains (right panels) (n = 5 donors). All scale bars: 200 µm. (C) Immunofluorescent staining and confocal microscopic analysis of active SPMS lesions expressing GM-CSF Rα (CD116; green) and CD11c (red). Co-localization is shown in lower panels, along with TO-PRO-3 (blue). Scale bar, 30 µm. Photomicrographs shown are representative of immunostaining performed on 12 active plaque areas obtained from the CNS material of 3 SPMS patients.
While numerous studies have shown an important and non-redundant role for GM-CSF in EAE, the role of this cytokine in MS is much less well established. We therefore evaluated the expression level of the receptor on sections from control non-inflamed human brain tissue and from MS lesions in comparison to normal and EAE mouse brains. Matching our observations in mice (Fig. 5A - Left Column), GM-CSF Rα was barely detectable in control brain samples (Fig. 5B - Column 1 and Fig. S5B). However, in EAE brain (Fig. 5A — Right Column) and SPMS lesions (Fig. 5B - Column 2), GM-CSF Rα expression was greatly upregulated. GM-CSF Rα expression was similar to control brain sections in tissues adjacent to lesions (Fig. 5B - Column 3 and Fig. S5B). GM-CSF Rα staining in SPMS patients closely matches the CD68 staining pattern found in the same patients (Fig. S5 - Column 2 and Fig. S5C). CD68 staining, similar to GM-CSF Rα expression, was considerably reduced in sections adjacent to lesions (Fig. S5 - Column 3). Strikingly, GM-CSF Rα expression was not found in lesions or adjacent tissue in PPMS patients (Fig. 5B - Columns 4&5 and Fig. S5B), even though upregulation of CD68 staining was observed in PPMS lesions (Fig. S5 - Columns 4 and Fig. S5C). Double immunostaining analyses of active SPMS lesions (n = 12 active lesions from 3 MS patients) revealed that GM-CSF Rα was expressed by CD11c+ myeloid cells (Fig. 5C). Collectively, the staining data confirm an enrichment of GM-CSF Rα+ myeloid APCs in the inflamed CNS, specifically in the relapsing remitting form and the chronic phase of the disease, and validate the potential therapeutic use in targeting this molecule. Importantly, lesions in PPMS patients are fundamentally different than lesions from SPMS patients in that very little expression of GM-CSF Rα+ cells is observed.
3.4. Reduced costimulatory molecule and inflammatory cytokines expression on mDCs and inflammatory monocytes following anti-GM-CSF Rα treatment
In an effort to determine the mechanism(s) by which anti-GM-CSF Rα blockade regulated EAE severity, we analyzed the myeloid cell populations in the spleen of therapeutically treated mice, at day 34 post-immunization. We observed a significant downregulation by percentage of positive cells and by median fluorescence intensity (MFI) of CD80, CD86, CD40 and MHC II expression on splenic mDCs (Fig. 6A - Left and Middle Panels; n = 10 mice per group, *p < 0.05, **p < 0.01, ***p < 0.001). Percentages of cells expressing these costimulatory molecules and the MFI of MHC II were not affected on other splenic subpopulations tested (Fig. 6B, Figs. S6A and S6B, and data not shown). We also analyzed cytokine profiles, and observed a significant reduction in IL-6 expression by mDCs (Fig. 6A–Right panel; n = 5 mice per group, *p < 0.05). In general, inflammatory monocytes were the more potent producers of cytokines when compared to other myeloid cell populations and the analysis of cytokine expression by inflammatory monocytes revealed a significant reduction in IL-1β, IL-6, IL-12p40, IL-23p19, and TNF-α expression (Fig. 6B and Fig. S6C; n = 5 mice per group, *p < 0.05, **p < 0.01) in response to GM-CSF receptor inhibition. Macrophages also showed a significant reduction of IL-6, IL-23p19 and TNF-α expression in mice treated with anti-GM-CSF Rα (Fig. S6A; n = 5 mice per group, *p < 0.05). Other populations such as pDCs and B cells do not exhibit any differences in expression of cytokines (data not shown). Confirming the reduction in cytokine production by isolated myeloid cells, we found a significant reduction of IL-1β, IL-6 and TNF-α production in LPS-activated splenocytes from mice treated with anti-GM-CSF Rα (Fig. 6C; n = 5 mice per group, **p < 0.01). IL-23 and IL-12p70 were below detection for most of the samples, and thus could not be analyzed with confidence.
Fig. 6. Anti-GM-CSF Rα treatment reduces myeloid cell activation as well as expression of chemokine receptors.
At day 34 post-immunization, spleens from therapeutically treated SJL/J mice were harvested, and immune cells were isolated. Cells were gated on (A) mDCs (B220−CD11b+CD11c+Ly6G−), and (B) inflammatory monocytes (B220−CD11b+CD11c−Ly6ChiLy6G−). Expression levels of CD80, CD86, CD40, MHC II, IL-1β, IL-6, IL-12p40, IL-23p19, TGF-β and TNF-α were assessed by flow cytometry (percentage and MFI) on anti-GM-CSF Rα-treated mice (10 mg/kg; grey circles) and isotype controls-treated mice (white squares) (n = 10 mice per group for the surface staining and n = 5 mice per group for the cytokines staining; *p < 0.05, **p < 0.01; ***p < 0.001 by nonparametric Mann-Whitney test). (C) 24 h post LPS activation, splenocytes from anti-GM-CSF Rα mice show a significant decrease in IL-1β, IL-6 and TNF-α production when compared to isotype controls mice (n = 5 mice per group; **p < 0.01, by nonparametric Mann-Whitney test). (D) Bone Marrow monocytes (CD11b+ Ly6C+) were isolated and exposed to GM-CSF (10 ng/ml) in presence of anti-GM-CSF Rα (100 µg/ml; black triangles) or the isotype control (white squares). After 24 h of activation in presence of GM-CSF, CXCR2 and CCR6 were significant upregulated. Pre-treatment of the cells with anti-GM-CSF Rα blocked these upregulations and the expressions were back to the control level, the anti-GM-CSF Rα alone (grey circles) (n = 6 BM-monocytes preparation; *p < 0.05 by nonparametric Wilcoxon matched-pairs test).
3.5. Anti-GM-CSF Rα treatment reduced chemokine receptors expression
CCL2 (also known as MCP-1) as well as CCL7 (also known as MCP-3) are chemokines that bind CCR2 and mediate monocyte recruitment [23]. Isolated BM-monocytes showed no difference in their expression of CCR2 when cultured in presence of anti-GM-CSF Rα (Fig. 6D). However, CXCR2 and CCR6 are both decreased in presence of anti-GM-CSF Rα (Fig. 6D, n = 6 preparation of BM-monocytes, *p < 0.05). CXCR2 may promote chemotaxis when bound to its atypical ligand macrophage migration inhibitory factor (MIF). MIF binds to CD74 and CXCR2 on monocytes and macrophages, leading to CXCR2 signaling and integrin-dependent chemotaxis of monocytes [24,25]. Finally, transfer experiments have shown that CCR6 is necessary for the migration of inflammatory monocytes into cutaneous tissues after immunization [26]. These results demonstrate that in addition of reducing activation of myeloid cells, anti-GM-CSF Rα can affect cell migration by reducing chemokine receptor expression.
4. Discussion
The goal of the current study was to evaluate the therapeutic potential and underlying mechanisms of GM-CSF receptor blockade in animal models of MS. GM-CSF Rα blockade at peak of C-EAE disease results in amelioration of disease progression, significantly reducing clinical symptoms, and inhibiting both innate and adaptive CNS immune responses. Similarly, in the SJL/J PLP139–151-induced RR-EAE model, anti-GM-CSF Rα blockade of GM-CSF signaling totally protects mice from disease induction and, more importantly, therapeutic treatment during disease remission blocked expression of disease relapse accompanied by a reduction of T cells specific for both the inducing and spread myelin peptides. Collectively, our results emphasize the important role played by GM-CSF in EAE, and the potential benefit of blocking this cytokine as a novel therapeutic approach for the treatment of MS.
Given their central role in the regulation of immune responses, cytokines and their receptors are clearly appealing targets for therapeutic intervention [27]. Such targeting strategies have proven successful in diverse autoimmune diseases in the past years. For example, in rheumatoid arthritis and Crohn's disease, TNF-α and IL-6 receptor targeted drugs are now approved. Blockade of IL-1β is also approved in the treatment of rheumatoid arthritis. In psoriasis, antibody blockade of IL-12/23p40 has been shown to be efficient in reducing disease severity. There are numerous ways to block cytokines. The most established are monoclonal antibodies, soluble receptors or receptor—Fc fusion molecules, as well as cytokine antagonists.
None of these drugs have proven useful for treatment of MS. In fact, TNF-α blockade exacerbated disease [28]. IL-12/23p40 was thought to be an excellent target in MS, due to fact that IL-12/23p40 is involved in the formation of IL-12 and IL-23, which are crucial for induction of autoreactive Th1 and Th17 lymphocytes, respectively. However, clinical trials failed to show any therapeutic benefit of neutralizing IL-12/23p40 in MS patients [29]. It has been suggested that the inability of the drug to cross the blood-brain barrier (BBB) as well as the late time frame of treatment initiation may explain this failure [30]. At this time, only Daclizumab (anti-IL-2Rα) has been approved for the treatment of RRMS [31]. A potential confounder is that most of the cytokine-targeted therapies in MS are directed towards reducing T cell activity and/or function. The recent success of rituximab (anti-CD20) indicates an important benefit in targeting other cell populations, even in later stage of the disease [32,33].
APCs are involved in multiple phases of neuropathology in MS and EAE, thus making them important cells to study and manipulate therapeutically. It was shown that in vivo pharmacologic or chemical depletion of peripheral blood monocytes restricts demyelination as well as accumulation of effector lymphocytes into the CNS, leading to a significant reduction of EAE clinical signs in mice and rats [34–36]. Moreover, Greter et al. have shown that bone marrow-derived CD11c+ DCs present antigen to myelin-reactive T lymphocytes in vivo and promote CNS T cell invasiveness and inflammation [37]. In previous studies, our group as well as others has shown that mDCs are responsible for initiating epitope spreading of myelin antigen responses within the CNS, activating naive CD4+ T cells and inducing the differentiation of TH17 cells in EAE [16,38,39].
In this study, we show that blockade of GM-CSF Rα alters activation of mDCs and inflammatory monocytes and leads to a significant decrease of disease severity in RR-EAE and C-EAE. Our data thus builds upon the recent report of Croxford et al. showing that only deletion of Csf2rb in CCR2+ Ly6Chi monocytes leads to inhibition of disease induction in chronic EAE [4]. In addition, anti-GM-CSF Rα decreased CXCR2 and CCR6 expression, receptors crucial for cells to respond to the chemokines CXCL1 and CXCL8 (ligands of CXCR2), as well as CCL20 (ligand of CCR6), and thus to traffic to inflamed sites. Specifically, it was shown that blocking CXCR2 enhances recovery and remyelination in spinal cords of C-EAE mice. The reduction in demyelinated areas in anti-CXCR2 treated EAE animals correlated with a reduction in inflammatory cell infiltration, most notably macrophages [40]. Interestingly another study demonstrated that classical EAE, characterized by predominant spinal cord inflammation, occurred in the combined absence of IFN-β and IL-17 signaling, but was dependent on GM-CSF and CXCR2 [41]. Moreover, since CXCR2 is crucial for neutrophil trafficking, it is possible that blockade of GM-CSF Rα, expressed in high proportion by neutrophils, resulted in a decreased migration of these cells in the CNS. However, we observed no difference between control-treated mice and anti-GM-CSF Rα-treated mice at the time-points examined. Regarding CCR6, our data are in agreement with a previous study showing a significant increase of CCR6 expression on human monocytes following GM-CSF activation [42]. It has been demonstrated on numerous occasions that CCR6 plays a prominent role in the recruitment of innate immune cells to the sites of epithelial inflammation [43]. Furthermore, since GM-CSF is able to act as a chemotactic agent [17,44,45], and was found to be involved in recruiting myeloid cells into the CNS critical for EAE development [3], it is highly likely that in addition to reduce myeloid cell activation, anti-GM-CSF Rα reduced cell migratory capacity to the CNS, leading to a milder disease course.
It was recently reported that mouse spleen serves as a reservoir for monocytes that can be mobilized in response to inflammatory signals [46]. Ly6chiCD11b+CD11c+ monocyte-derived dendritic cells (Mo-DCs) are a prominent component of CNS-infiltrates cells in EAE [14,15]. Mo-DCs are believed to arise from inflammatory monocytes following their migration into inflamed tissue [47]. Intriguingly, we observed a significant decrease in inflammatory monocytes in the spleen following anti-GM-CSF Rα treatment. At this moment, we are unable to demonstrate whether this decrease is a cause or a consequence of the reduced inflammation observed in animals treated with blocking antibody. Indeed, a reduced number of monocytes in the spleen can be explained by a reduced capacity of the cells to migrate from the bone marrow to the spleen, a reduced necessity of the immune system to produce more monocytes and/or monocytes returned to the bone marrow as a consequence of a more “controlled” inflammation in anti-GM-CSF Rα treated animals.
Although, there was a previous report showing human microglial responses to GM-CSF ex vivo [48], surprisingly, we found minimal steady state expression of GM-CSF Rα on these cells, although at the peak of acute EAE, we did observe a marginal increase of expression of GM-CSF Rα on microglia (CD45loCD11b+Ly6Clo). It is conceivable that microglia upregulate their expression of CD45 once activated, and are thus included in our gating of infiltrating macrophages (CD45hiB220−CD11b+CD11c−Ly6G−). These observations parallel the findings in humans, in which the receptor is expressed by a small subset of activated microglia in the healthy brain, but is upregulated in MS lesions [17]. Nonetheless, our analysis of human and mouse in situ CNS immunostaining, as well as mouse ex vivo flow cytometry staining clearly show that mDCs and inflammatory monocytes express the highest levels of GM-CSF Rα. Interestingly, we observed an increase in GM-CSF Rα expression on macrophages in the CNS of EAE mice compared to macrophages in the spleen of the same mice. This increase is likely due to the pro-inflammatory microenvironment in the CNS inducing expression of the receptor. Moreover, migrating inflammatory monocytes have the ability to differentiate into mDCs and macrophages [15,16]. It is thus possible that part of the macrophage population in the CNS originates from inflammatory monocytes, which express very high levels of the receptor. Nonetheless the effects of GM-CSF Rα blockade on splenic macrophages are modest in comparison to the effects on mDCs and inflammatory monocytes. These results suggest that most of the immune modulatory effects of GM-CSF Rα mAb are achieved on these two cell populations.
Other CNS resident cells express the GM-CSF receptor [49,50] and since the blood-brain barrier is disrupted in EAE/MS, it is thus possible for the anti-GM-CSF Rα antibody to access the CNS, and to exert some of its effects on the CNS resident cells. For example, GM-CSF has been shown to be neuroprotective [51], and induce proliferation of neural progenitor cells in vitro [52]. In addition, GM-CSF directly inhibits oligodendrocytes progenitor cells proliferation and maturation (unpublished results). Also it has been reported that GM-CSF stimulates in vitro proliferation of simian astrocytes in primary cultures potentially leading to astrocytosis [53].
Intriguingly, we observed very little GM-CSF Rα expression in PPMS lesions, which may suggest that the neuroinflammatory effector mechanisms driving PPMS differ from those in RRMS-SPMS. However, it has been proposed that lesion development in PPMS patients develops over a much longer time period than in RRMS/SPMS patients [54,55]. Therefore these in situ data possibly reflect the different timing in lesion formation, with GM-CSF Rα+ cells present early in lesion formation, but less frequent later on, explaining the reduced presence of cells expressing the receptor. These observations reveal an interesting trend, but additional experimentation will be required to assess the functional significance of this finding and how it may be used to develop therapeutic approaches for PPMS.
4.1. Conclusions
Collectively our results demonstrate a crucial role of GM-CSF in RR-EAE and C-EAE and provide a novel therapeutic approach to specifically restrict the action of this cytokine. Blockade of the GM-CSF Rα leads to significantly lessened disease severity, reduction in mDC activation, and reduced pro-inflammatory cytokine production by inflammatory monocytes. Additionally, anti-GM-CSF Rα alters the expression of chemokine receptors, and because GM-CSF can also act as a chemoattractant, antibody treatment may impede cell migration. Altogether, these effects on APCs resulted in significantly reduced activation of myelin peptide-specific TH1 and TH17 cells. Lately, interest for impeding GM-CSF action in MS has been recently raised by the results of a Phase Ib clinical trial employing antibody blockade of GM-CSF in patients with relapsing-remitting or secondary-progressive MS showing the drug to be safe and well tolerated [56]. This study enhances our knowledge of GM-CSF functions in EAE, and points to the potential benefits of GM-CSF Rα blockade as a novel therapeutic target for treatment of RRMS and SPMS.
Supplementary Material
Acknowledgments
We would like to acknowledge the important contribution of the patients who have donated autopsy material for our study. This work was supported by a grant from MedImmune (SDM) and NIH Grant NS-026543 (SDM). II was supported by a National MS Society Postdoctoral Fellowship FG 2065-A-1. We thank members of the Miller lab for their insightful comments.
Conflict of interest
This study was supported by funding from MedImmune which is developing mavrilimumab, a human GM-CSF receptor antibody, for therapeutic use in autoimmune disease.
Footnotes
Author contributions
TD, CJ and MAS provided the a-GM-CSF Rα and the isotype control. II performed the experiments related to RR-EAE. He was helped by DX, JR, ZH and SB. TD performed the experiments related to c-EAE, as well as the chromogenic immunohistochemistry staining and was helped by DPM and JC. CJ and MAS provided the chromogenic immunohistochemistry on human brain sections. HK performed the double immunostaining on human samples, and was helped by CP. II, TD, CJ, AP, MAS and SDM provided intellectual input throughout the project. The paper was written by II and SDM, with review from TD, CJ and MAS.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2017.06.005.
References
- 1.Rodgers JM, Miller SD. Cytokine control of inflammation and repair in the pathology of multiple sclerosis. Yale J. Biol. Med. 2012;85:447–468. [PMC free article] [PubMed] [Google Scholar]
- 2.Herndler-Brandstetter D, Flavell RA. Producing GM-CSF: a unique T helper subset? Cell Res. 2014;24:1379–1380. doi: 10.1038/cr.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 4.Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Croxford AL, Spath S, Becher B. GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol. 2015;36:651–662. doi: 10.1016/j.it.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 6.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The ence-phalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte-macrophage colony-stimulating factor. Crit. Rev. Immunol. 2005;25:405–428. doi: 10.1615/critrevimmunol.v25.i5.50. [DOI] [PubMed] [Google Scholar]
- 8.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan Y, Xu Y, Lew AM. The regulation of the development and function of dendritic cell subsets by GM-CSF: more than a hematopoietic growth factor. Mol. Immunol. 2012;52:30–37. doi: 10.1016/j.molimm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, et al. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol. Rev. 2012;250:277–302. doi: 10.1111/j.1600-065X.2012.01164.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosas M, Gordon S, Taylor PR. Characterisation of the expression and function of the GM-CSF receptor alpha-chain in mice. Eur. J. Immunol. 2007;37:2518–2528. doi: 10.1002/eji.200636892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testa U, Fossati C, Samoggia P, Masciulli R, Mariani G, Hassan HJ, et al. Expression of growth factor receptors in unilineage differentiation culture of purified hematopoietic progenitors. Blood. 1996;88:3391–3406. [PubMed] [Google Scholar]
- 13.McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J. Exp. Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codarri L, Greter M, Becher B. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol. 2013;34:114–119. doi: 10.1016/j.it.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 15.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ifergan I, Kebir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, et al. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain J. Neurol. 2008;131:785–799. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- 17.Vogel DY, Kooij G, Heijnen PD, Breur M, Peferoen LA, van der Valk P, et al. GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur. J. Immunol. 2015;45:1808–1819. doi: 10.1002/eji.201444960. [DOI] [PubMed] [Google Scholar]
- 18.Choi SS, Lee HJ, Lim I, Satoh J, Kim SU. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;9:e92325. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greven DE, Cohen ES, Gerlag DM, Campbell J, Woods J, Davis N, et al. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:1924–1930. doi: 10.1136/annrheumdis-2014-205234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terry RL, Ifergan I, Miller SD. Experimental autoimmune encephalomyelitis in mice. Methods Mol. Biol. 2016;1304:145–160. doi: 10.1007/7651_2014_88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, et al. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke MA, Segal BM. IL-23 modulated myelin-specific T cells induce EAE via an IFNgamma driven, IL-17 independent pathway. Brain Behav. Immun. 2011;25:932–937. doi: 10.1016/j.bbi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 25.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 26.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 2010;9:703–718. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- 28.TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The lenercept multiple sclerosis study group and the University of British Columbia MS/MRI analysis group. Neurology. 1999;53:457–465. [PubMed] [Google Scholar]
- 29.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 30.Longbrake EE, Racke MK. Why did IL-12/IL-23 antibody therapy fail in multiple sclerosis? Expert Rev. Neurother. 2009;9:319–321. doi: 10.1586/14737175.9.3.319. [DOI] [PubMed] [Google Scholar]
- 31.Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A, et al. Daclizumab HYP versus interferon Beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2015;373:1418–1428. doi: 10.1056/NEJMoa1501481. [DOI] [PubMed] [Google Scholar]
- 32.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 33.Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009;66:460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 34.Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vredent C, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 2014;6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J. Exp. Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J. Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- 37.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 38.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 39.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 40.Kerstetter AE, Padovani-Claudio DA, Bai L, Miller RH. Inhibition of CXCR2 signaling promotes recovery in models of multiple sclerosis. Exp. Neurol. 2009;220:44–56. doi: 10.1016/j.expneurol.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroenke MA, Chensue SW, Segal BM. EAE mediated by a non-IFN-gamma/non-IL-17 pathway. Eur. J. Immunol. 2010;40:2340–2348. doi: 10.1002/eji.201040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dabritz J, Weinhage T, Varga G, Wirth T, Walscheid K, Brockhausen A, et al. Reprogramming of monocytes by GM-CSF contributes to regulatory immune functions during intestinal inflammation. J. Immunol. 2015;194:2424–2438. doi: 10.4049/jimmunol.1401482. [DOI] [PubMed] [Google Scholar]
- 43.Ito T, Carson WFt, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp. Cell Res. 2011;317:613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez-Cambronero J, Horn J, Paul CC, Baumann MA. Granulocyte-macrophage colony-stimulating factor is a chemoattractant cytokine for human neutrophils: involvement of the ribosomal p70 S6 kinase signaling pathway. J. Immunol. 2003;171:6846–6855. doi: 10.4049/jimmunol.171.12.6846. [DOI] [PubMed] [Google Scholar]
- 45.Khajah M, Millen B, Cara DC, Waterhouse C, McCafferty DM. Granulocyte-macrophage colony-stimulating factor (GM-CSF): a chemoattractive agent for murine leukocytes in vivo. J. Leukoc. Biol. 2011;89:945–953. doi: 10.1189/jlb.0809546. [DOI] [PubMed] [Google Scholar]
- 46.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee SC, Liu W, Brosnan CF, Dickson DW. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12:309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- 49.Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci. Lett. 1993;160:131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- 50.Dame JB, Christensen RD, Juul SE. The distribution of granulocyte-macrophage colony-stimulating factor and its receptor in the developing human fetus. Pediatr. Res. 1999;46:358–366. doi: 10.1203/00006450-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Schabitz WR, Kruger C, Pitzer C, Weber D, Laage R, Gassler N, et al. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF) J. Cereb. Blood Flow. Metab. 2008;28:29–43. doi: 10.1038/sj.jcbfm.9600496. [DOI] [PubMed] [Google Scholar]
- 52.Kim JK, Choi BH, Park HC, Park SR, Kim YS, Yoon SH, et al. Effects of GM-CSF on the neural progenitor cells. Neuroreport. 2004;15:2161–2165. doi: 10.1097/00001756-200410050-00003. [DOI] [PubMed] [Google Scholar]
- 53.Guillemin G, Boussin FD, Le Grand R, Croitoru J, Coffigny H, Dormont D. Granulocyte macrophage colony stimulating factor stimulates in vitro proliferation of astrocytes derived from simian mature brains. Glia. 1996;16:71–80. doi: 10.1002/(SICI)1098-1136(199601)16:1<71::AID-GLIA8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 54.Kuhlmann T. Relapsing-remitting and primary progressive MS have the same cause(s)—the neuropathologist's view: 2. Mult. Scler Houndmills, Basing-stoke, Engl. 2013;19:268–269. doi: 10.1177/1352458513476563. [DOI] [PubMed] [Google Scholar]
- 55.Ingle GT, Stevenson VL, Miller DH, Thompson AJ. Primary progressive multiple sclerosis: a 5-year clinical and MR study. Brain J. Neurol. 2003;126:2528–2536. doi: 10.1093/brain/awg261. [DOI] [PubMed] [Google Scholar]
- 56.Constantinescu CS, Asher A, Fryze W, Kozubski W, Wagner F, Aram J, et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurology(R) Neuroimmunol. neuroinflammation. 2015;2:e117. doi: 10.1212/NXI.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.