Abstract
Biomedical engineering and its associated disciplines play a pivotal role in improving our understanding and management of disease. Motivated by past accomplishments, such as the clinical implementation of coronary stents, pacemakers or recent developments in antibody therapies, disease management now enters a new era in which precision imaging and nanotechnology-enabled therapeutics are maturing to clinical translation. Preclinical molecular imaging increasingly focuses on specific components of the immune system that drive disease progression and complications, allowing the in vivo study of potential therapeutic targets. The first multicentre trials highlight the potential of clinical multimodality imaging for more efficient drug development. In this perspective, the role of integrating engineering, nanotechnology, molecular imaging and immunology to yield precision medicine is discussed.
This article is part of the themed issue ‘Challenges for chemistry in molecular imaging’.
Keywords: nanomedicine, molecular imaging, translational imaging, biomedical engineering
1. Introduction
Fundamental discoveries in the realm of disease biology [1–3] have identified inflammation-related processes as promising targets for more effective treatment [4]. However, the complexity of the immune system, and its role as a defence mechanism against infection, require the development of innovative tools to both identify and treat inflammatory diseases with high precision. In terms of scientific progress, the twenty-first century is characterized by exciting developments in biomedical engineering [5,6], ranging from cutting edge imaging techniques [7–9], novel devices to inventive nanomaterials [10,11]. Although biomedical engineering offers unique possibilities for inflammation diagnosis and treatment, cross-fertilization with immunology is vital to foster meaningful advances in disease management.
In this perspective, the role of integrating engineering, nanotechnology with molecular imaging to yield precision medicine is described. We will discuss how recent discoveries in immunology [1,3] can provide opportunities for diagnostic imaging [9], including translatable molecular imaging techniques [12]. We further highlight how biomedical engineering may produce precision therapeutics [13], with a focus on nanoparticle therapies [14].
2. The role of biomedical engineering
In modern medicine, medical procedures, agents and devices rely heavily on biomedical engineering [5,6]. Early devices included equipment for surgery, which could be as simple as specialized knives or syringes all the way to dedicated materials for stenting of obstructed arteries, as well as devices such as pacemakers or implantable defibrillators to help control abnormal heart rhythms [15]. Medical mechanics facilitated the development of new devices that physicians employ for interventional cardiology (stents, pulmonary artery catheters, intraaortic balloon pumps), electrophysiology (pacemakers, defibrillators, left atrial appendage occlusion devices), vascular surgery (polytetrafluoroethylene (PTFE) grafts, PTFE-stent grafts) and cardiac surgery (bioprosthetic and mechanical valves, destination ventricular assist devices). For the treatment of malignancies, biomedical engineering has yielded radiation therapy, a well-established and widely used treatment modality in which ionizing radiation is applied to kill cancer cells [16]. Recent advances in optical imaging and probe development have generated image-guided surgery methods, enabling surgeons to more accurately delineate tumours and metastases [17].
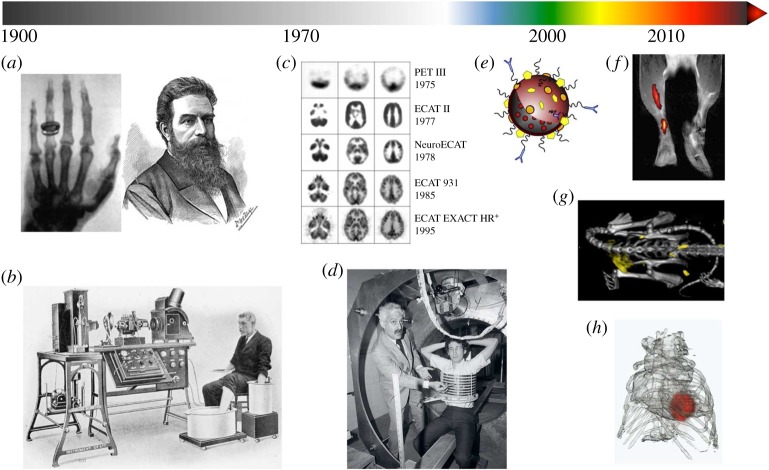
Medical physics contributed to the development of medical imaging methodologies, ranging from the first X-ray imaging, discovered by Wilhelm Röntgen in 1895 [18] (figure 1a), to the introduction of magnetic resonance imaging (MRI) in the 1970s and 1980s [22,23] (figure 1d). Advances in X-ray imaging and the development of mathematical reconstruction methods resulted in the establishment of X-ray computed tomography (CT), which allows the rapid acquisition of anatomical images with superb detail [24]. While CT excels in depicting hard tissues, such as calcifications in atherosclerotic plaques, MRI allows the visualization of soft tissues with exceptional contrast. The latter technique exploits water and fat protons' nuclear spin properties at a high magnetic field. Its rapid progression has heavily relied on sophisticated signal processing and the development of advanced hardware. Scintigraphy is a two-dimensional imaging technique that uses gamma cameras to detect emitted radiation from administered radioisotopes. This technique forms the basis of modern three-dimensional nuclear medical imaging such as positron emission tomography (PET, figure 1c) and single-photon emission computed tomography (SPECT) [25]. In addition to medical imaging, nuclear medicine not only contributed to radionuclide therapy, which is applied to selectively irradiate tumours, but can also be used to treat benign disorders such as thyrotoxicosis and arthritis. Intraoperative tumour-specific fluorescence imaging is an optical imaging technique that is currently being explored in the clinic [26].
Figure 1.
The evolution of diagnostic modalities: turning the light on in the twentieth century, adding colour in the twenty-first century. (a) Wilhelm Conrad Röntgen received the Nobel Prize in Physics in 1901 for discovering the fundamentals of X-ray imaging. (b) Willem Einthoven was awarded the Nobel Prize in Medicine for his work on developing electrocardiography (ECG) in 1924. (c) The progression of PET imaging quality (image credit The Crump Institute for Molecular Imaging). (d) Raymond Damadian subjects a volunteer to an NMR system. Paul C. Lauterbur and Peter Mansfield were awarded the Nobel Prize in Physiology or Medicine in 2003 for their work on developing methods to generate images from NMR signals, known as MRI. (e) Schematic of a multifunctional and target-specific nanoparticle. Nanoparticle imaging probes proved to be invaluable for the success of twenty-first century molecular imaging modalities. (f) 19F and 1H composite MR image of dendritic cell migration into the popliteal lymph node following a hind foot pad injection in a mouse (adapted with permission from Ahrens et al.[19]). (g) Tumour targeting of gold-labelled low-density lipoprotein visualized by spectral CT (adapted with permission from [20]). (h) FMT-CT reconstruction showing protease activity in the heart of a mouse with myocardial infarction (adapted with permission from Leuschner & Nahrendorf [21]).
Ultrasound is another widely used physical phenomenon with both diagnostic and therapeutic applications. As a diagnostic modality it can be used for different purposes, ranging from prenatal development monitoring to vessel wall thickness measurement in the coronary arteries of atherosclerosis patients using intravascular probes [27]. Therapeutic use of focused ultrasound includes lithotripsy of kidney stones [28].
Closely related to medical physics, the field of electrical engineering contributed electrocardiography [29] (figure 1b), or ECG for short, which is used to measure electrical irregularities disturbing the rhythm of the heart. In the development of electronics, hardware and computer components, electrical engineering has made crucial contributions. For example, the advances in radiofrequency coil design are integral to the success of MRI. The same applies to the electronics of any medical imaging device, which include transistors, diodes and integrated circuits.
Chemical and biochemical engineering are among the younger contributors to biomedical engineering. Their achievements include, but are not limited to, recombinant DNA technology, medicinal chemistry, analytical assays, radiochemistry, polymer and material chemistry, as well as nanochemistry. Whereas recombinant DNA technology has already had a tremendous impact on medicine, e.g. in human insulin production [30] and transgenic mouse models of disease, nanomedicine (figure 1e) only recently began to impact clinical care, which holds particularly true for diseases other than cancer [14].
3. The twenty-first century: nanomedicine and imaging cross paths
Novel biomedical engineering concepts began to sprout at the beginning of the twenty-first century. While biomedical physics dominated the twentieth century, biomedical engineering has defined medical innovation in the twenty-first century. In the early 2000s, molecular imaging techniques [7], in which chemically engineered probes are applied to identify molecular and cellular processes in vivo, found their way into preclinical research. MRI was revamped into a ‘molecular imaging’ technique that allowed identifying key processes related to inflammation, including the visualization of macrophages, the expression of adhesion molecules as well as markers of inflammation [31]. Fluorine (19F) MRI, a hotspot imaging technique [32], regained interest and found its application primarily in cell tracking [19,33] (figure 1f). At the same time, the advancement of multidetector CT enabled faster and higher resolution imaging of coronary arteries. The introduction of multicolour spectral CT [34] (figure 1g) and novel nanoparticles composed of iodine [35], gold [36] and other materials [37,38] even allowed molecular imaging of atherosclerotic plaque macrophages [39], despite CT's known reputation as an insensitive diagnostic technique. Optical imaging techniques (figure 1h) evolved, of which near infrared fluorescence (NIRF) imaging [40] proved to be useful for the simultaneous detection of multiple biomarkers in live mice. Catheter-based NIRF approaches then enabled imaging of inflammation in coronary-sized arteries [41–44]. Translationally, targeted fluorescent molecular reporters have recently been evaluated in cancer patients [26]. Remarkable advances in intravital microscopy now allow the study of single cell behaviour in haematopoietic tissues [45], in the blood and in tumours [46]. Biomedical engineering contributed substantially to overcome hurdles such as vigorous vascular motion with tissue stabilization and fast data acquisition and processing.
Advances in synthetic chemistry and nanochemistry propelled the above molecular imaging applications; yet, most of them have not advanced to the clinical arena. Inspired by the rapid growth of nanoparticle molecular imaging probes, several groups revitalized and extrapolated nanotherapeutic approaches to treating disease [14]. At the same time, the nanomedicine field has witnessed a dramatic scope increase, yielding nanomaterials that can be exploited for, e.g., drug [47] or nucleic acid [48,49] delivery. Unfortunately, the translation of such nanotechnologies is rare and associated with many hurdles. One example is BIND therapeutics, a next-generation nanotechnology biotech company that encapsulates chemotherapeutic drugs in targeted polymeric nanoparticles. Although their preclinical studies showed great promise, the company filed for bankruptcy in April 2016 [50]. Patient trials showed no benefits over treatment with the regular (non-nano-formulated) parent drug.
Among the many reasons for the observed discrepancy between preclinical success and failed clinical trials, two important parameters should be considered. First and foremost, are the nanoparticles optimally designed to retain the drug and efficiently accumulate in the tumour? Second, how do we properly screen for patients amenable for nanotherapy treatment? Both parameters are of paramount importance for successful nanodrug development and dictate nanotherapeutic efficacy. In a recently published study, we showed how advanced optical imaging can be integrated in a feedback loop to improve nanodrug design (figure 2a). Model drugs based on the fluorescent molecule Cy7 were derivatized to enhance compatibility and facilitate encapsulation in approximately 100 nm PEG-PLGA nanoparticles. It was found that, as a function of derivatization, the model drug was better retained in the nanoparticles in vivo, resulting in elevated effective drug doses in tumour tissue. Guidelines derived from these experiments were subsequently applied to the drug doxorubicin, a widely applied chemotherapeutic agent and the active ingredient of the first clinically approved nanodrug Doxil. We found that certain doxorubicin derivatives were better retained in nanoparticles, resulting in enhanced tumour accumulation and much-improved therapeutic efficacy.
Figure 2.
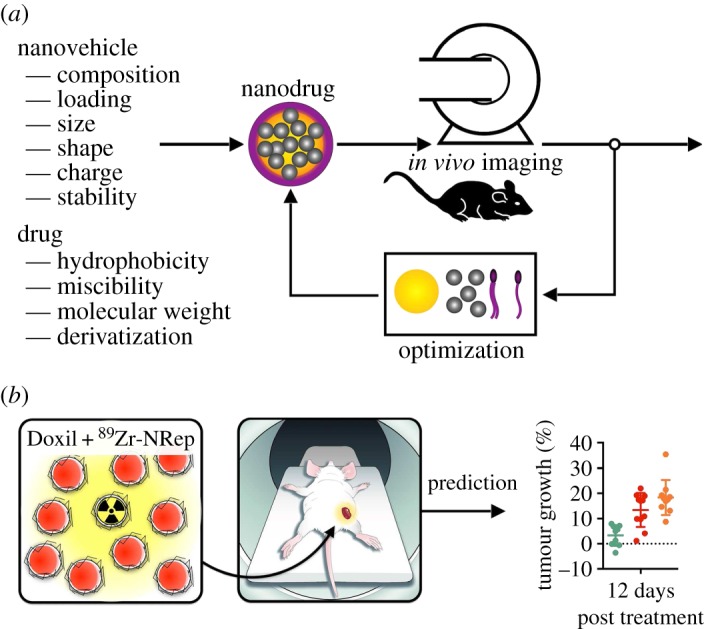
Imaging in nanodrug development and efficacy prediction. (a) Imaging can be integrated in nanodrug development feedback loop. (b) Companion diagnostics, such as the 89Zr-labelled liposome shown left, can serve as nanoreporters that exhibit a similar pharmacokinetic signature and target accumulation to a long circulating nanodrug.
Imaging can also play a pivotal role in the identification of subjects amenable to nanodrug treatment. The elegantly simple premise revolves around the question whether or not the nanodrug reaches and accumulates in the targeted site. This can be probed by PET imaging through labelling of nanodrugs with radioisotopes, as has been demonstrated for high-density lipoprotein nanoparticles in cardiovascular disease animal models [51] and patients [52]. Such radiolabelled nanoparticles can subsequently be employed as companion diagnostics that provide insight in nanodrug tumour accumulation. We showed that one and the same 89Zr-labelled liposomal companion diagnostic, referred to as nanoreporter, allowed accurate quantification of tumour accumulation of Doxil (figure 2b), nanoemulsions, PEG-PLGA nanoparticles and Abraxane [53], an albumin nanoparticle formulation of paclitaxel. An alternative approach, based on an MRI companion diagnostic, was also shown to be effective [54]. Both studies also demonstrated that nanodrug tumour accumulation was predictive of therapeutic outcome.
Finally, to evaluate nanodrugs' therapeutic effects, imaging can be employed to quantify tumour volume [8] or atherosclerotic plaque burden [9], but also metabolic activity [55] and the molecular signature of malignancies [8]. In preclinical studies, this facilitates studying nanodrugs in a non-invasive and longitudinal fashion, generating valuable feedback for further optimization [56]. Translational work, such as clinical trials, is also supported by the implementation of imaging endpoints [57]. Although this is common in anti-cancer treatment developments, this approach has recently also shown value in the dal-PLAQUE trial, in which the safety and efficacy of dalcetrapib on atherosclerotic disease was studied by multimodality imaging [58].
4. Outlook
As nanotechnologies and imaging techniques are continuously being developed, their integration occurs organically, but not necessarily optimally. Preclinically, theranostic approaches, for which, e.g. therapeutic agents with diagnostic features are developed [59], hold great promise. Unfortunately, clinical translation is not trivial. What do we do with a therapeutic agent's diagnostic features after establishing its specificity? Should two versions, with and without diagnostic features, of the same nanotherapeutic agent be developed and subjected to extensive evaluation and FDA approval, and at what costs? In view of the limited amount of nanodrugs that actually show benefits and received FDA approval, this scenario seems unrealistic. A blanket companion diagnostic, such as the PET nanoreporter technology discussed in this perspective, may represent an attractive alternative. This would require separate approval for the companion diagnostic, but once acquired it can be employed for most long circulating nanodrugs. Irrespective of the above, imaging's role in drug development, clinical trials and patient management will continue to grow. Specifically, for the development and monitoring of nanodrug treatments, integration of imaging has significant benefits. At the same time, nanoparticle contrast agents and tracers are continuously being developed, although their translation is falling behind in comparison to small molecule or peptide probes. A concentrated, strategic effort that does not focus on the development of yet another nanoparticle platform, with lots of whistles and bells, but on optimized implementation and translation of nanotechnologies is required. How can existing nanoparticle platforms be best used and translated; What is nanomedicine's reach beyond oncological applications; and How can imaging be employed to facilitate this?
Data accessibility
This article has no additional data.
Author contributions
All authors contributed to writing and critically evaluating the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by National Institute of Health grants R01 HL118440, R01 HL125703, R01 CA155432 and NWO Vidi (W.J.M.M.) as well as NIH R01 CA204441 (T.R.) and P30 CA008748.
References
- 1.Moore KJ, Tabas I. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355. ( 10.1016/j.cell.2011.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legein B, Temmerman L, Biessen EAL, Lutgens E. 2013. Inflammation and immune system interactions in atherosclerosis. Cell. Mol. Life Sci. 70, 3847–3869. ( 10.1007/s00018-013-1289-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swirski FK, Nahrendorf M. 2013. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166. ( 10.1126/science.1230719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabas I, Glass CK. 2013. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339, 166–172. ( 10.1126/science.1230720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nerem RM. Bioengineering in the 21st century. See https://smartech.gatech.edu/handle/1853/30214 .
- 6.Langer RA. 2009. conversation with Robert Langer: pioneering biomedical scientist and engineer. Interview by Paul S. Weiss ACS Nano 3, 756–761. ( 10.1021/nn900350p) [DOI] [PubMed] [Google Scholar]
- 7.Weissleder R, Mahmood U. 2001. Molecular imaging. Radiology 219, 316–333. ( 10.1148/radiology.219.2.r01ma19316) [DOI] [PubMed] [Google Scholar]
- 8.Weissleder R, Pittet MJ. 2008. Imaging in the era of molecular oncology. Nature 452, 580–589. ( 10.1038/nature06917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanz J, Fayad ZA. 2008. Imaging of atherosclerotic cardiovascular disease. Nature 451, 953–957. ( 10.1038/nature06803) [DOI] [PubMed] [Google Scholar]
- 10.Farokhzad OC, Langer R. 2009. Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20. ( 10.1021/nn900002m) [DOI] [PubMed] [Google Scholar]
- 11.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. 2007. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760. ( 10.1038/nnano.2007.387) [DOI] [PubMed] [Google Scholar]
- 12.Rudd JH, et al. 2010. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J. Am. Coll. Cardiol. 55, 2527–2535. ( 10.1016/j.jacc.2009.12.061) [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal A, Jaffer FA, Ntziachristos V. 2012. Intravascular multispectral optoacoustic tomography of atherosclerosis: prospects and challenges. Imaging Med. 4, 299–310. ( 10.2217/iim.12.20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobatto ME, Fuster V, Fayad ZA, Mulder WJM. 2011. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 10, 835–852. ( 10.1038/nrd3578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck H, Boden WE, Patibandla S, Kireyev D, Gupta V, Campagna F, Cain ME, Marine JE. 2010. 50th Anniversary of the first successful permanent pacemaker implantation in the United States: historical review and future directions. Am. J. Cardiol. 106, 810–818. ( 10.1016/j.amjcard.2010.04.043) [DOI] [PubMed] [Google Scholar]
- 16.Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, Richter C, Zips D, Bortfeld T. 2016. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 16, 234–249. ( 10.1038/nrc.2016.18) [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen FWB, Hardwick JCH, van Erkel AR. 2015. Luminescence-based imaging approaches in the field of interventional molecular imaging. Radiology 276, 12–29. ( 10.1148/radiol.2015132698) [DOI] [PubMed] [Google Scholar]
- 18.Hayter C. 1995. Making sense of shadows: Dr. James Third and the introduction of X-rays, 1896 to 1902. Can. Med. Assoc. J. 153, 1249–1256. [PMC free article] [PubMed] [Google Scholar]
- 19.Ahrens ET, Flores R, Xu H, Morel PA. 2005. In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 23, 983–987. ( 10.1038/nbt1121) [DOI] [PubMed] [Google Scholar]
- 20.Allijn, et al. 2013. Gold nanocrystal labeling allows low-density lipoprotein imaging from the subcellular to macroscopic level. ACS Nano. 7, 9761–9770. ( 10.1021/nn403258w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuschner F, Nahrendorf M. 2011. Molecular imaging of coronary atherosclerosis and myocardial infarction: considerations for the bench and perspectives for the clinic. Circ. Res. 108, 593–606. ( 10.1161/CIRCRESAHA.110.232678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damadian R. 1971. Tumor detection by nuclear magnetic resonance. Science 171, 1151–1153. ( 10.1126/science.171.3976.1151) [DOI] [PubMed] [Google Scholar]
- 23.Lauterbur PC. 1973. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 242, 190–191. ( 10.1038/242190a0) [DOI] [PubMed] [Google Scholar]
- 24.Goodman LR. 2010. The beatles, the Nobel Prize, and CT scanning of the chest. Radiol. Clin. N. Am. 48, 1–7. ( 10.1016/j.rcl.2009.09.008) [DOI] [PubMed] [Google Scholar]
- 25.Rahmim A, Zaidi H. 2008. PET versus SPECT: strengths, limitations and challenges. Nucl. Med. Commun. 29, 193–207. ( 10.1097/MNM.0b013e3282f3a515) [DOI] [PubMed] [Google Scholar]
- 26.van Dam GM, et al. 2011. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat. Med. 17, 1315–1319. ( 10.1038/nm.2472) [DOI] [PubMed] [Google Scholar]
- 27.Kastelein JJP, de Groot E. 2008. Ultrasound imaging techniques for the evaluation of cardiovascular therapies. Eur. Heart J. 29, 849–858. ( 10.1093/eurheartj/ehn070) [DOI] [PubMed] [Google Scholar]
- 28.Harper JD, et al. 2013. Focused ultrasound to expel calculi from the kidney: safety and efficacy of a clinical prototype device. J. Urol. 190, 1090–1095. ( 10.1016/j.juro.2013.03.120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivera-Ruiz M, Cajavilca C, Varon J. 2008. Einthoven's string galvanometer: the first electrocardiograph. Tex. Heart Inst. J. 35, 174–178. [PMC free article] [PubMed] [Google Scholar]
- 30.Keen H, Pickup JC, Bilous RW, Glynne A, Viberti GC, Jarrett RJ, Marsden R. 1980. Human insulin produced by recombinant DNA technology: safety and hypoglycaemic potency in healthy men. Lancet 2, 398–401. ( 10.1016/S0140-6736(80)90443-2) [DOI] [PubMed] [Google Scholar]
- 31.Choudhury RP, Fuster V, Fayad ZA. 2004. Molecular, cellular and functional imaging of atherothrombosis. Nat. Rev. Drug Discov. 3, 913–925. ( 10.1038/nrd1548) [DOI] [PubMed] [Google Scholar]
- 32.Bulte JWM. 2005. Hot spot MRI emerges from the background. Nat. Biotechnol. 23, 945–946. ( 10.1038/nbt0805-945) [DOI] [PubMed] [Google Scholar]
- 33.Partlow KC, et al. 2007. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 21, 1647–1654. ( 10.1096/fj.06-6505com) [DOI] [PubMed] [Google Scholar]
- 34.Roessl E, Proksa R. 2007. K-edge imaging in X-ray computed tomography using multi-bin photon counting detectors. Phys. Med. Biol. 52, 4679–4696. ( 10.1088/0031-9155/52/15/020) [DOI] [PubMed] [Google Scholar]
- 35.Hyafil F, et al. 2007. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 13, 636–641. ( 10.1038/nm1571) [DOI] [PubMed] [Google Scholar]
- 36.Mieszawska AJ, Mulder WJM, Fayad ZA, Cormode DP.. 2013. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 10, 831–847. ( 10.1021/mp3005885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan D, et al. 2010. Computed tomography in color: NanoK-enhanced spectral CT molecular imaging. Angew. Chem. Int. Ed Engl. 49, 9635–9639. ( 10.1002/anie.201005657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R. 2006. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat. Mater 5, 118–122. ( 10.1038/nmat1571) [DOI] [PubMed] [Google Scholar]
- 39.Cormode DP, et al. 2010. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology 256, 774–782. ( 10.1148/radiol.10092473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. 2005. Looking and listening to light: the evolution of whole-body photonic imaging. Nat. Biotechnol. 23, 313–320. ( 10.1038/nbt1074) [DOI] [PubMed] [Google Scholar]
- 41.Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, Ntziachristos V, Libby P, Weissleder R. 2008. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation 118, 1802–1809. ( 10.1161/CIRCULATIONAHA.108.785881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, Weissleder R, Libby P, Ntziachristos V. 2011. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J. Am. Coll. Cardiol. 57, 2516–2526. ( 10.1016/j.jacc.2011.02.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinegoni C, et al. 2011. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci. Transl. Med. 3, 84ra45 ( 10.1126/scitranslmed.3001577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo H, et al. 2011. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med. 17, 1680–1684. ( 10.1038/nm.2555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo Celso C, et al. 2009. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96. ( 10.1038/nature07434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, et al. 2013. Near-infrared fluorescence energy transfer imaging of nanoparticle accumulation and dissociation kinetics in tumor-bearing mice. ACS Nano 7, 10 362–10 370. ( 10.1021/nn404782p) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. 2008. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl Acad. Sci. USA 105, 17 356–17 361. ( 10.1073/pnas.0809154105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sager HB, et al. 2016. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 8, 342ra80 ( 10.1126/scitranslmed.aaf1435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kranz LM, et al. 2016. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401. ( 10.1038/nature18300) [DOI] [PubMed] [Google Scholar]
- 50.Ledford H. 2016. Bankruptcy filing worries developers of nanoparticle cancer drugs. Nature 533, 304–305. ( 10.1038/533304a) [DOI] [PubMed] [Google Scholar]
- 51.Pérez-Medina C, et al. 2016. In Vivo PET imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc. Imaging 9, 950–961. ( 10.1016/j.jcmg.2016.01.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng KH, et al. 2016. HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis 251, 381–388. ( 10.1016/j.atherosclerosis.2016.05.038) [DOI] [PubMed] [Google Scholar]
- 53.Pérez-Medina C, Abdel-Atti D, Tang J, Zhao Y, Fayad ZA, Lewis JS, Mulder WJM, Reiner T. 2016. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun. 7, 11838 ( 10.1038/ncomms11838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller MA, et al. 2015. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 7, 314ra183 ( 10.1126/scitranslmed.aac6522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiari N, Rudd JHF. 2011. FDG PET imaging and cardiovascular inflammation. Curr. Cardiol. Rep. 13, 43–48. ( 10.1007/s11886-010-0150-5) [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, et al. 2016. Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun. 7, 11221 ( 10.1038/ncomms11221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudin M, Weissleder R. 2003. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2, 123–131. ( 10.1038/nrd1007) [DOI] [PubMed] [Google Scholar]
- 58.Fayad ZA, et al. 2011. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 378, 1547–1559. ( 10.1016/S0140-6736(11)61383-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. 2011. Theranostic nanomedicine. Acc. Chem. Res. 44, 1029–1038. ( 10.1021/ar200019c) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.