Abstract
The CEST properties of EuDOTA-tetraamide complexes bearing pendant carboxylate and carboxyl ethyl esters were measured as a function of pH. The CEST signal from the Eu3+-bound water molecule decreased in intensity between pH 8.5 and 4.5 while the proton exchange rates (kex) increased over this same pH range. In comparison, the CEST signal in the corresponding carboxyl ester derivatives was nearly constant. Both observations are consistent with stepwise protonation of the four carboxylic acid groups over this same pH range. This indicates that negative charges on the carboxyl groups above pH 6 facilitate the formation of a strong hydrogen-bonding network in the coordination second sphere above the single Eu3+-bound water molecule, thereby decreasing prototropic exchange of protons on the bound water molecule with bulk water protons. The percentage of square antiprismatic versus twisted square antiprism coordination isomers also decreased as the appended carboxylic acid groups were positioned further away from the amide. The net effect of lowering the pH was an overall increase in kex and a quenching of the CEST signal.
This article is part of the themed issue ‘Challenges for chemistry in molecular imaging’.
Keywords: MRI contrast agent, Eu complex, pH sensor
1. Introduction
The paramagnetic chemical exchange saturation transfer (paraCEST) agents present an attractive alternative to gadolinium-based agents for molecular imaging applications. Agents of this class typically consist of a lanthanide ion chelated by a macrocyclic derivative of DOTA and the contrast generated originates from the slow-to-intermediate exchange of the highly shifted lanthanide-bound water molecule with bulk water [1,2]. Given the ability of lanthanide ions to induce shifts in the resonances of proximate ligand protons, the exchange of highly shifted labile protons of amides and alcohols has also proved to be an efficient way of generating CEST contrast [3–5]. Eu3+ complexes of a variety of DOTA-tetraamide ligands have been most widely studied as water exchange-based paraCEST agents. The reasons for this popularity reside in the fact that water exchange is found to be slowest in Eu3+ complexes in comparison to other lanthanide complexes [6]. A second factor is that the weak electron donating properties of tetraamide oxygen donor atoms compared with DOTA itself results in a three- to fourfold decrease in the rate of water exchange between an inner-sphere Eu3+-bound water molecule and bulk solvent, a condition highly favourable for CEST [7]. Furthermore, alteration of the DOTA-tetraamide structure has provided a way to design a variety of paraCEST agents responsive to physiological indicators such as pH [8,9], temperature [10,11], other metal ions [12–14], lactate [15,16] and enzyme activity [17,18]. Hence, the ability of these agents to provide functional information of this sort highlights their potential use as molecular imaging probes. The rate of proton exchange (kex) is one of the most important factors that determine CEST efficiency in these complexes [19], so most attempts to improve CEST sensitivity have been focused on designing paraCEST agents that exhibit slow proton exchange kinetics. The major factors influencing kex in Eu3+-based agents are the coordination geometry of the complex and the nature of the ligand side chains. It was previously reported that introduction of carboxyl groups or carboxyl ethyl esters on the amide substituents of EuDOTA-tetraamide complexes would substantially decrease the bound water proton exchange rate. This observation has been explained by the hydrogen-bonding network between carboxyl groups and surrounding second-sphere water molecules that restrain proton exchange with the single inner-sphere water molecule. In this study, we sought to obtain a better understanding of the factors that influence the proton exchange rate in EuDOTA-tetraamide complexes. While a vast majority of studies of such complexes have focused on varying the functional groups on the amide side chains to alter the water molecule exchange rates, this study focused on how a change in pH alters the rate of exchange of protons between inner- and outer-sphere water molecules and how this impacts CEST sensitivity.
2. Results and discussion
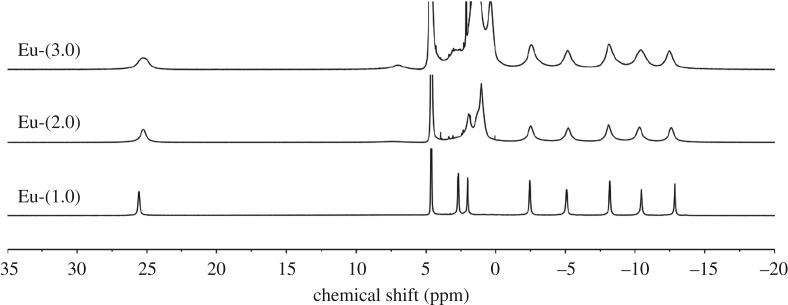
Given that the EuDOTA-tetraamide complexes studied here (scheme 1) have fourfold symmetry, the relative proportions of SAP and TSAP coordination isomers can be estimated by integrating the area of a single, highly shifted ethylenediamine proton in the macrocyclic backbone (usually referred to as the H4 proton). For Eu3+ complexes such as these, the H4 proton resonance is typically found near 24–28 ppm in the SAP isomer and near 5–10 ppm in the TSAP isomer. The high-resolution 1H NMR spectra of the three carboxylate complexes are shown in figure 1. It is evident that all three complexes exist mainly as SAP isomers. Closer examination of these 1H NMR spectra reveals that a second H4 resonance is also detectable in spectra of Eu-(2.0) and Eu-(3.0) near approximately 7 ppm, showing that a small amount of TSAP isomer is also present in the two complexes. The area of this resonance was found to increase as the length of the alkyl spacer between the amide group and the carboxyl group was increased.
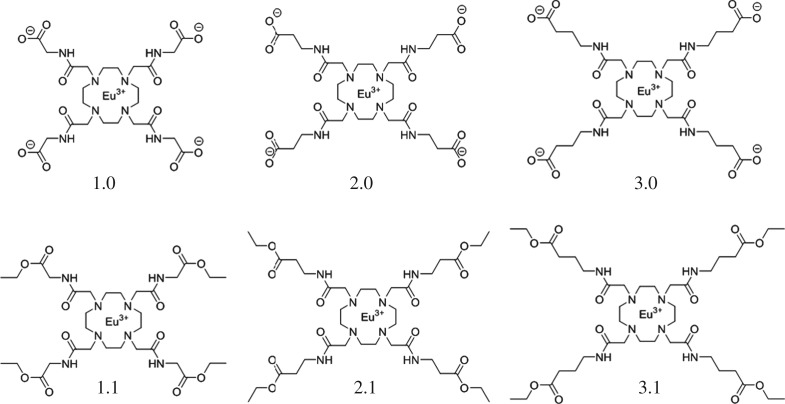
Scheme 1.
Structures of the complexes used in this study.
Figure 1.
1H NMR spectra of three Eu3+ complexes recorded at 400 MHz in D2O at pD 7.0.
Aime et al. [20] observed a similar trend with a few other Eu3+ complexes of DOTA-tetraamides wherein the SAP/TSAP ratio decreased in proportion to the number of alkyl groups on the amide. Thus, lengthening the alkyl spacer between the amide and carboxylic acid along the series, Eu-(1.0) → Eu-(2.0) → Eu-(3.0), seems to favour the TSAP isomer. It is also interesting that in those complexes where the TSAP isomer can be observed, the H4 proton resonances of both species are considerably broader. This indicates that the rate of interconversion between the SAP and TSAP isomers also increases as the length of the amide side chains was increased. This observation is useful in the design of paraCEST agents that preferentially exist as the SAP isomer in solution. The 1H NMR spectra of the three carboxylate derivatives showed no change in the relative proportions of SAP and TSAP isomers over the pH range 4–9 (figure 2).
Figure 2.
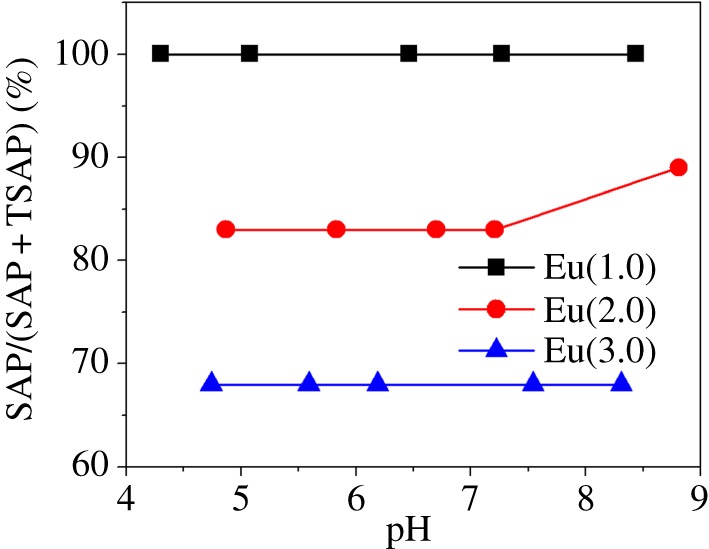
Plot of the proportion of SAP isomers as a function of pH.
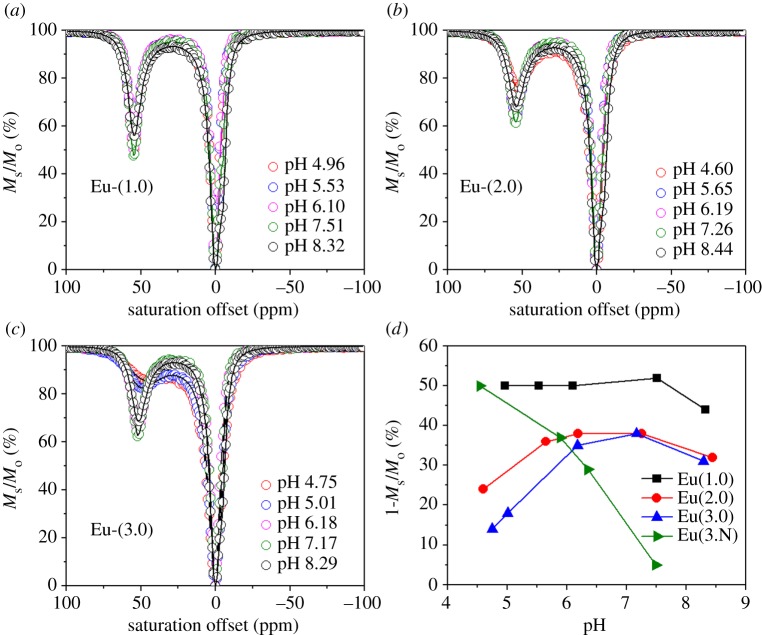
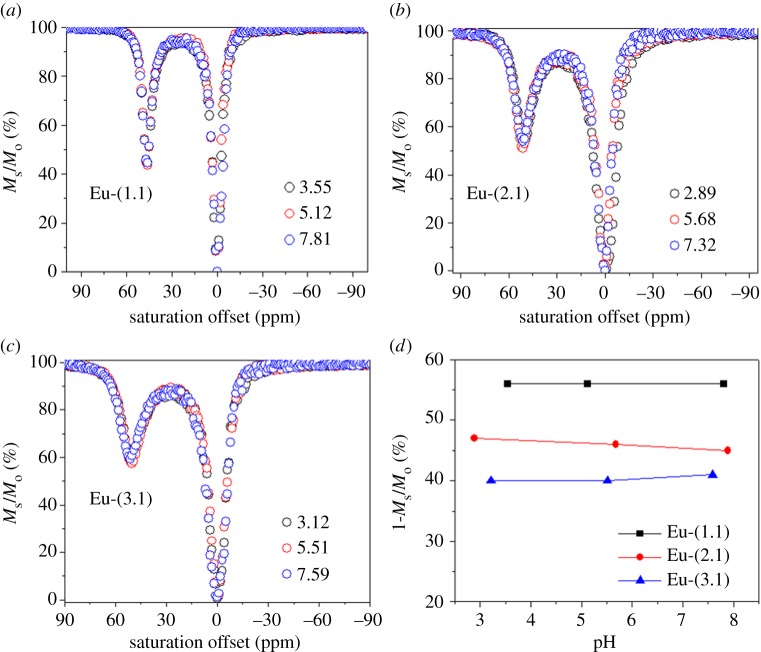
CEST spectra were also collected on these same three complexes as a function of pH. Several notable differences in these spectra were quite apparent (figure 3). First, the CEST intensity of the water molecule exchange peak near 50 ppm followed the same trend, Eu-(1.0) ≫ Eu-(2.0) > Eu-(3.0), at all pH values (see quantitative results in figure 3d). These differences were magnified under more acidic conditions. Since the CEST signal under observation here arises only from the SAP isomer present in solution (water exchange is too fast in the TSAP isomer), this partially reflects a decrease in population of the SAP isomer as the length of the alkyl group increases. The second notable difference relates to the pH dependency of the CEST intensities. Here, the intensity of the CEST signal of Eu-(1.0) remains constant over the pH range 4–7 and slightly decreases above pH 8. In comparison, Eu-(2.0) and Eu-(3.0) display considerably different pH responses. Between pH 7.2 and approximately 6, the CEST is relatively constant but drops off at both high pH and low pH. At the lower pH values, the CEST signal of Eu-(3.0) decreases in intensity more than that of Eu-(2.0). These differences approximately parallel the estimated pKa values of the extended carboxylate groups. The carboxylate groups in Eu-(1.0) would be expected to have pKa's similar to that of glycine, around 2–2.5, well below the range of pH values (4–7) examined here. The carboxylate groups in Eu-(2.0) should be similar to the pKa of the γ carboxyl group in aspartic acid, around 4.5. Consequently, the decrease in CEST intensity below pH approximately 5.5 is consistent with the initial stages of protonation of the carboxyl groups in this complex. Among these three complexes, the carboxyl groups in Eu-(3.0) are predicted to have the highest pKa values, consistent with a greater decrease in CEST signal that begins above pH 6 and drops more rapidly as the pH is lowered even further. The green line and data points in figure 3d show similar pH-dependent CEST data for a EuDOTA-tetraamide complex having three appended primary amine groups (labelled Eu-(3.N), an amine analogue of Eu-(3.0). More details of the amine series will be published elsewhere. However, it is interesting to note that in the case of the amine derivative, the pH dependence of CEST is opposite to those complexes described here. In Eu-(3.N), the CEST signal is small at pH 7.4, but increases as almost linearly to pH 4.5 as the amines become protonated. Hence, protonation of an appended carboxyl groups results in quenching of the CEST signal while protonation of appended amine groups results in significant enhancement of the CEST signal.
Figure 3.
CEST spectra of 20 mM Eu complexes (a), (b) and (c) as a function of pH. A pre-saturation pulse of 600 Hz of 3 s was applied at 25°C. The solid lines reflect the fit of the experimental data to Bloch theory [21]. (d) CEST intensity as a function as pH.
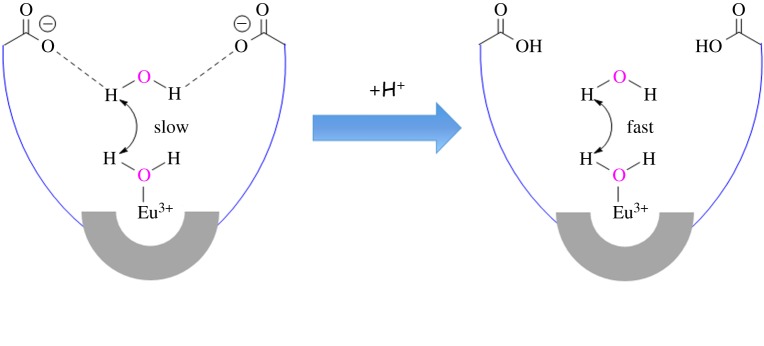
What is the origin of these interesting differences? As indicated in the introduction, the CEST intensity is highly dependent upon proton exchange rates in all complexes. To verify that these CEST changes are related to differences in proton exchange rates, each of the CEST spectra were fitted to Bloch theory for a two-site exchange model [21]. The resulting values are shown in figure 4. Not surprisingly, the trends shown in figure 4 for proton exchange rates largely parallel the CEST effects illustrated in figure 3d. The proton lifetimes (τm = 1/kex) show that protonation of a carboxyl group results in faster proton exchange and a parallel decrease in CEST intensity. This indicates that negatively charged carboxyl groups located near the Eu3+-bound water results in a decreased prototropic exchange of bulk water protons with the bound water protons. A carboxylate anion is more electron donating and can participate in more extensive hydrogen-bonding (H-bonding) networks with water molecules in the second coordination sphere. This hydrogen-bonding network serves to stabilize the Eu3+-bound water molecule, slowing-down or impeding proton exchange with bulk water and thereby enhancing the CEST intensity (figure 5) [22–26]. Above pH 8, a second factor, base-catalysed proton exchange, begins to play a role in the exchange kinetics and hence the CEST signal once again becomes quenched.
Figure 4.
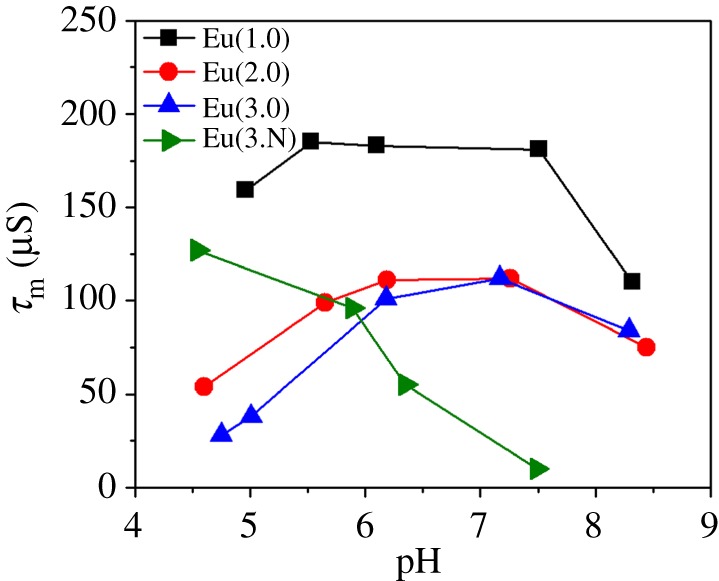
Plot of bound water proton residence lifetime (τm) as a function of pH at 25°C.
Figure 5.
Schematic of the prototropic effects described in this study. (Left) An ionized carboxyl group (COO–) forms a strong hydrogen-bonding network that slows prototropic exchange of all water protons. (Right) Protonated carboxyl groups form a weak hydrogen-bonding network thereby allowing rapid proton exchange between bulk water protons and the protons on the Eu3+-bound water molecule.
In additional support of these conclusions, we also prepared the corresponding ethyl ester derivatives of these same complexes. As shown in figure 6, the CEST signal of these complexes is not sensitive to changes in pH between 3 and 7.5. In this case, the ester-protected carboxyl groups cannot accept protons so remain pH insensitive over this entire pH range. At higher pH, these systems also show a decrease in pH due to base-catalysed exchange of protons.
Figure 6.
CEST spectra of 20 mM Eu complexes (a), (b) and (c) as a function of pH. A pre-saturation pulse of 600 Hz of 3 s was applied at 25°C. (d) CEST intensity as a function as pH.
3. Conclusions
In this study, the CEST properties of a series of EuDOTA-tetraamides bearing carboxylic acid, ester or amine substituents were studied as a function of pH. The results indicate that lengthening the alkyl spacer between the amide and the carboxylic acid functional group results in an increase in the population of TSAP isomer that parallels the increasing bulkiness of the amide pendant arms. It was shown that the pH dependence of CEST in these complexes is governed by the rate prototropic exchange between bulk solvent water protons and Eu3+-bound water protons. Protonation of appended carboxyl groups results in an increase in the rate of prototropic exchange and subsequent quenching of the CEST signal. The most favourable ionic forms for agents of this type are negatively charged carboxyl groups positioned above the central Eu3+-bound water molecule. The resulting hydrogen-bonding network stabilizes all protons in the immediate vicinity of the Eu-bound water molecule and this helps to maximize the CEST effect. The opposite is observed for complexes containing appended amine groups. In those complexes, protonation of the amine groups serves to stabilize the hydrogen-bonding network and increase CEST. Thus, any functional group that stabilizes protons in an inner-/outer-sphere network is advantageous for CEST enhancement. This work demonstrates that the development of paraCEST agents that are responsive to important biomarkers such as the pH can be optimized and tuned so that they can be used in a variety of possible medical applications. Finally, it is worth considering the potential applicability of this method for imaging pH in tumours, acidosis and kidney failure. Although the paraCEST agents mentioned in this work might still be limited by the inherent low sensitivity of the technique, this limitation can be easily overcome by delivering these agents in the form of nanoparticles or macromolecules (liposomes, dendrimers, polymers, viruses, lipoproteins, etc.). The delivery of these particles into the extracellular space can increase the sensitivity of the paraCEST agents by several orders of magnitude by accumulation and/or by simple tissue perfusion.
Supplementary Material
Data accessibility
All relevant data can be found in this paper and the electronic supplementary material.
Authors' contributions
L.Z., O.M.E. and A.D.S. carried out the experiments. L.Z., O.M.E., A.F.M. and P.Z. performed the data analysis. O.M.E., L.Z., A.F.M. and A.D.S. drafted the manuscript. All authors read and approved the manuscript’.
Competing interests
We declare we have no competing interest.
Funding
The authors acknowledge partial financial support for this work from the National Institutes of Health (CA-115531, EB-01598), Harold C. Simmons Cancer Center through an NCI Cancer Center Support Grant, 1P30 CA142543, and the Robert A. Welch Foundation (AT-584).
References
- 1.Zhang S, Wu K, Biewer MC, Sherry AD. 2001. 1H and 17O NMR detection of a lanthanide-bound water molecule at ambient temperatures in pure water as solvent. Inorg. Chem. 40, 4284–4290. ( 10.1021/ic0003877) [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Winter P, Wu K, Sherry AD. 2001. A novel europium(III)-based MRI contrast agent. J. Am. Chem. Soc. 123, 1517–1518. ( 10.1021/ja005820q) [DOI] [PubMed] [Google Scholar]
- 3.Woods M, Woessner DE, Zhao P, Pasha A, Yang M-Y, Huang C-H, Vasalitiy O, Morrow JR, Sherry AD. 2006. Europium(III) macrocyclic complexes with alcohol pendant groups as chemical exchange saturation transfer agents. J. Am. Chem. Soc. 128, 10 155–10 162. ( 10.1021/ja061498t) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aime S, Delli Castelli D, Terreno E. 2002. Novel pH-reporter MRI contrast agents. Angew. Chem. Int. Ed. 41, 4334–4336. ( 10.1002/1521-3773(20021115)41:22%3C4334::AID-ANIE4334%3E3.0.CO;2-1) [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Michaudet L, Burgess S, Sherry AD. 2002. The Amide protons of an ytterbium(III) dota tetraamide complex act as efficient antennae for transfer of magnetization to bulk water. Angew. Chem. Int. Ed. 41, 1919–1921. ( 10.1002/1521-3773(20020603)41:11%3C1919::AID-ANIE1919%3E3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Wu K, Sherry AD. 2002. Unusually sharp dependence of water exchange rate versus lanthanide ionic radii for a series of tetraamide complexes. J. Am. Chem. Soc. 124, 4226–4227. ( 10.1021/ja017133k) [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. 2010. Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem. Rev. 110, 2960–3018. ( 10.1021/cr900284a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aime S, Barge A, Delli Castelli D, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. 2002. Paramagnetic Lanthanide(III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications. Magn. Reson. Med. 47, 639–648. ( 10.1002/mrm.10106) [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Zhou Y, Ouari O, Woods M, Zhao P, Soesbe TC, Kiefer GE, Sherry AD. 2008. Polymeric PARACEST Agents for Enhancing MRI Contrast Sensitivity. J. Am. Chem. Soc. 130, 13 854–13 855. ( 10.1021/ja805775u) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li AX, Wojciechowski F, Suchy M, Jones CK, Hudson RHE, Menon RS, Bartha R. 2008. A sensitive PARACEST contrast agent for temperature MRI: Eu3+-DOTAM-glycine (Gly)-phenylalanine (Phe). Magn. Reson. Med. 59, 374–381. ( 10.1002/mrm.21482) [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Malloy CR, Sherry AD. 2005. MRI Thermometry Based on PARACEST Agents. J. Am. Chem. Soc. 127, 17 572–17 573. ( 10.1021/ja053799t) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esqueda AC, López JA, Andreu-de-Riquer G, Alvarado-Monzón JC, Ratnakar J, Lubag AJM, Sherry AD, De León-Rodríguez LM. 2009. A new gadolinium-based MRI Zinc sensor. J. Am. Chem. Soc. 131, 11 387–11 391. ( 10.1021/ja901875v) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotruvo JA Jr, Aron AT, Ramos-Torres KM, Chang CJ. 2015. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 44, 4400–4414. ( 10.1039/C4CS00346B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos-Torres KM, Kolemen S, Chang CJ. 2016. Thioether coordination chemistry for molecular imaging of copper in biological systems. Isr. J. Chem. 56, 724–737. ( 10.1002/ijch.201600023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, et al. 2017. Imaging extracellular lactate in vitro and in vivo using CEST MRI and a paramagnetic shift reagent. Chem - Eur J. 23, 1752–1756. ( 10.1002/chem.201604558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aime S, Delli Castelli D, Fedeli F, Terreno E. 2002. A Paramagnetic MRI-CEST agent responsive to lactate concentration. J. Am. Chem. Soc. 124, 9364–9365. ( 10.1021/ja0264044) [DOI] [PubMed] [Google Scholar]
- 17.Yoo B, Pagel MD. 2006. A PARACEST MRI contrast agent to detect enzyme activity. J. Am. Chem. Soc. 128, 14 032–14 033. ( 10.1021/ja063874f) [DOI] [PubMed] [Google Scholar]
- 18.Chauvin T, Durand P, Bernier M, Meudal H, Doan B-T, Noury F, Badet B, Beloeil J-C, Tóth E. 2008. Detection of enzymatic activity by PARACEST MRI: a general approach to target a large variety of enzymes. Angew. Chem. Int. Ed. 47, 4370–4372. ( 10.1002/anie.200800809) [DOI] [PubMed] [Google Scholar]
- 19.Sherry AD, Wu Y. 2013. The importance of water exchange rates in the design of responsive agents for MRI. Curr. Opin Chem. Biol. 17, 167–174. ( 10.1016/j.cbpa.2012.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aime S, Barge A, Bruce JI, Botta M, Howard JAK, Moloney JM, Parker D, de Sousa AS, Woods M. 1999. NMR, relaxometric, and structural studies of the hydration and exchange dynamics of cationic lanthanide complexes of macrocyclic tetraamide ligands. J. Am. Chem. Soc. 121, 5762–5771. ( 10.1021/ja990225d) [DOI] [Google Scholar]
- 21.Woessner DE, Zhang S, Merritt ME, Sherry AD. 2005. Numerical solution of the bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn. Reson. Med. 53, 790–799. ( 10.1002/mrm.20408) [DOI] [PubMed] [Google Scholar]
- 22.Liepinsh E, Otting G. 1996. Proton exchange rates from amino acid side chains— implications for image contrast. Magn. Reson. Med. 35, 30–42. ( 10.1002/mrm.1910350106) [DOI] [PubMed] [Google Scholar]
- 23.Aime S, Botta M, Fasano M, Terreno E. 1999. Prototropic and water-exchange processes in aqueous solutions of Gd(III) chelates. Acc. Chem. Res. 32, 941–949. ( 10.1021/ar970300u) [DOI] [Google Scholar]
- 24.Kálmán FK, Woods M, Caravan P, Jurek P, Spiller M, Tircsó G, Király R, Brücher E, Sherry AD. 2007. Potentiometric and relaxometric properties of a gadolinium-based MRI contrast agent for sensing tissue pH. Inorg. Chem. 46, 5260–5270. ( 10.1021/ic0702926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods M, Pasha A, Zhao P, Tircso G, Chowdhury S, Kiefer G, Woessner DE, Sherry AD. 2011. Investigations into whole water, prototropic and amide proton exchange in lanthanide(iii) DOTA-tetraamide chelates. Dalton Trans. 40, 6759–6764. ( 10.1039/c1dt10616c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delli Castelli D, Terreno E, Aime S. 2011. YbIII-HPDO3A: a dual ph- and temperature-responsive CEST agent. Angew. Chem. Int. Ed. 50, 1798–1800. ( 10.1002/anie.201007105) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data can be found in this paper and the electronic supplementary material.