Abstract
Most processes within organisms, and most interactions between organisms and their environment, have distinct time profiles. The temporal coordination of such processes is crucial across levels of biological organization, but disciplines differ widely in their approaches to study timing. Such differences are accentuated between ecologists, who are centrally concerned with a holistic view of an organism in relation to its external environment, and chronobiologists, who emphasize internal timekeeping within an organism and the mechanisms of its adjustment to the environment. We argue that ecological and chronobiological perspectives are complementary, and that studies at the intersection will enable both fields to jointly overcome obstacles that currently hinder progress. However, to achieve this integration, we first have to cross some conceptual barriers, clarifying prohibitively inaccessible terminologies. We critically assess main assumptions and concepts in either field, as well as their common interests. Both approaches intersect in their need to understand the extent and regulation of temporal plasticity, and in the concept of ‘chronotype’, i.e. the characteristic temporal properties of individuals which are the targets of natural and sexual selection. We then highlight promising developments, point out open questions, acknowledge difficulties and propose directions for further integration of ecological and chronobiological perspectives through Wild Clock research.
This article is part of the themed issue ‘Wild Clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals’.
Keywords: chronotype, phenotypic plasticity, time programme, reaction norm, circannual, circadian
1. Introduction
Since antiquity, it has been appreciated that wild organisms exhibit predictable periodic behaviours in concert with the regular alternation of day and night, the seasons, the tides, and the waxing and waning of the moon. Studied early on were the daily leaf movements of heliotropic plants; in 1729, the French astronomer de Mairan reported that these rhythms persisted when the plants were locked away from the light [1]. The importance of this discovery was realized by Charles and Francis Darwin, who suggested how rhythmic leaf movements might serve a protective function [2]. Persistence of biological rhythms even when organisms are sheltered from experiencing the earth's geophysical cycles was subsequently confirmed across the plant and animal kingdoms [3]. In addition to the best-studied diel time scale (‘circadian’ clocks, with a period length of about: (‘circa’) 1 day (‘dian’)), similar rhythms were shown on circannual, circatidal and circalunar scales [4–6]. Nonetheless, only in the latter part of the twentieth century, when experiments including a space mission proved that no hidden earthly cues are needed to drive biological rhythms, was endogenous rhythm-generation fully accepted [7–9].
In the real world, unconstrained by artificial conditions like those chosen by de Mairan, in animal facilities or during space missions, organisms perceive rhythmic information from the external environment. Their biological clocks effectively provide timing programmes [10,11] to use such information in highly specific ways, so that, for example, twilight prompts different processes in the morning compared to evening. Biological clocks integrate endogenous timekeeping with environmental information to generate internal representations of time, so that at any given moment, the organism will be in a particular temporal state which we here call ‘internal clock time’ (e.g. morning activation, or gonadal reproductive activation; cf. [12,13] for specific, formal definitions of internal time).
Keeping track of time internally offers major advantages compared with solely responding to the immediate external environment [14,15]. In their interactions with the environment, organisms benefit from internal clocks for anticipating conditions that are remote in time and space (e.g. [16]); for tracking time in environments where temporal information is unavailable or misleading (e.g. in caves or hibernacula); and for reference time-consulting to correctly use environmental information (e.g. the changing position of the sun in navigation; [17]). Internally, organisms benefit by maintaining time-structuring of different physiological processes, that should, or must not, occur at the same time (temporal compartmentalization; for in-depth reviews, see [14,15]).
Although these advantages are intuitively clear, they are difficult to demonstrate. This is because in studies of organisms, their periodic behaviour or physiology (e.g. waking up, breeding) is directly recorded, whereas the internal clock time that orchestrates these activities is not readily observed. For many of us, internal clock time becomes acutely evident when it is misaligned with external time (i.e. time measured conventionally as clock time at a given site; [12]), such as by jet lag, when bright morning sunshine subjectively feels like deep midnight and we find it difficult to start our day [18]. Experiences like these underline the importance of internal clock time for how, or whether, biological processes occur. For example, internal clock time determines whether high temperatures in winter trigger flowering or breeding in wild organisms [19], how the immune system responds to a pathogen attack or a flu shot [20] or how efficiently a food source can be exploited [21]. Chronobiology, as a field, is centred around such studies of biological clocks. Efforts are abound to characterize or infer the internal clock times that orchestrate organisms' lives (e.g. for epidemiological approaches, see [18]; for physiological characterization, see [22].)
Consequently, biological clocks are recognized for their importance in most fields of the life sciences and medicine, and in many sectors of public life and industry [23,24]. Surprisingly, despite this broad interest, fundamental questions about the evolutionary biology and functional importance of biological clocks in natural ecosystems have been largely neglected [25]. Ecology, as a field, holds the expertise that is needed to close this major gap in chronobiology. Ecology has a fundamental interest in the timing of events and in the functional significance of interactions between organisms and their environment. Yet in turn, without an understanding of underlying biological clocks, ecology cannot fully address these themes. Thus, ecology and chronobiology complement each other and can greatly benefit from shared research [26]. This first requires efforts to understand, and appreciate, each other's perspectives.
2. ‘Wild Clocks’: time's many components affect organisms in nature
The two thriving fields of chronobiology and ecology, which jointly approach ‘Wild Clocks’ in this theme issue, are connected by their interest in timing, but integration of their respective research is often difficult. These difficulties are routed in differences in the main interest of the fields. The content of today's core research in chronobiology is the systematic study of the mechanisms that organisms use to time body processes relative to geophysical cycles [14], whereas ecologists classically describe, compare and functionally analyse rhythms in natural environments [27]. In simple terms, the key interest of chronobiologists is in ‘how do organisms time biological processes’, and that of ecologists is in ‘why do they do it’. These different interests affect concepts and practical approaches, beginning with identifying and describing the ‘time’ that is relevant for an organism's behaviour and physiology [28].
At first sight, the definition of time for the purpose of biological studies would seem straight-forward. We commonly use calendars and clocks to measure local time, and the passage of time, relative to geophysical processes (i.e. the Earth's rotation and orbit, the rotation of the Moon around the Earth). Geophysical processes are associated with a host of further abiotic cycles, primarily in the duration and intensity of light exposure, and as a consequence also with cycles in temperature, wind patterns, humidity or precipitation (figure 1). Related to the abiotic cycles are biotic cycles of the environment, for example, annual changes in vegetation cover, which imply fluctuating food availability, predator detection or predation and parasite pressure. Measuring biotic cycles is now instituted in multiple ecological and citizen-science projects that record phenology (i.e. the timing of recurrent seasonal processes [29]).
Figure 1.
Schematic of the factors that affect an organism's manifest timing. The central, orange circle represents an organism, containing its biological timekeeping system shown in blue. Components of external abiotic cycles (shown in grey) and biotic cycles (shown in green) are perceived by an organism's sensory system. External information is interpreted based on an individual's internal clock time (e.g. whether warm winter temperatures should induce breeding), but at the same time, external time components can also modify internal clock time. Jointly, external time components and internal clock time influence individual timing outcomes, which on average can be used to characterize an individual's chronotype. The organism's behaviour and physiology can, in turn, feed back to affect rhythms of conspecifics (social time) or interspecifics (ecological time).
Consideration of abiotic and biotic cycles can be revealing on a macro-ecological scale, but identifying the relevant external time for a particular organism is often much more complex because abiotic and biotic time affect organisms in highly specific ways [28]. Organisms can substantially alter the ways they experience abiotic cycles, for example, by modifying their micro-environment (e.g. by retreating to shelters or hibernacula, or building nests) or by undertaking migrations [16,19]. High specificity of biotic effects is well established. For example, for Rhagoletis fruit flies and associated parasitoid wasps [30], a centrally important component of time is the fruiting state of the host plants in which Rhagoletis larvae develop. Rhagoletis flies have diversified to reproduce on specific host plants which fruit at different times of year, and the parasitoid wasps have correspondingly diversified [30]. Thus, for these flies and wasps, specific fruiting phenologies, not calendar date or macro-ecological phenology, constitute correct time.
Such specificity is associated with the above-introduced, additional component of time that is relevant for an organism's behaviour and physiology: Internal timekeeping (figure 1) can anticipate external abiotic and biotic cycles and regulate an organism's response to its environment. The flies and wasps discussed above will be internally prepared to match the phenology of their respective hosts through their biological clocks. On a given date, differently specialized flies and wasps will differ in the timing of their annual cycles, and each individual's specific internal clock time (figures 1 and 2) will affect its ability to exploit their hosts. Importantly, biological clocks do not simply predict the correct time for a given activity, but equip organisms with mechanisms to adjust timing in response to abiotic and biotic time components that vary between years at a given location and date [31,32]. Effects of internal clock time on organisms' use of opportunities apply to many species, for example, migratory birds, whose internal clock time influences spring return dates and hence their use of breeding opportunities [16], or to bees maximizing their foraging reward by precisely timing visits to flowers [17]. The mechanisms by which biological clocks enable organisms to respond correctly to the external environment are a core interest of the field of chronobiology.
Figure 2.
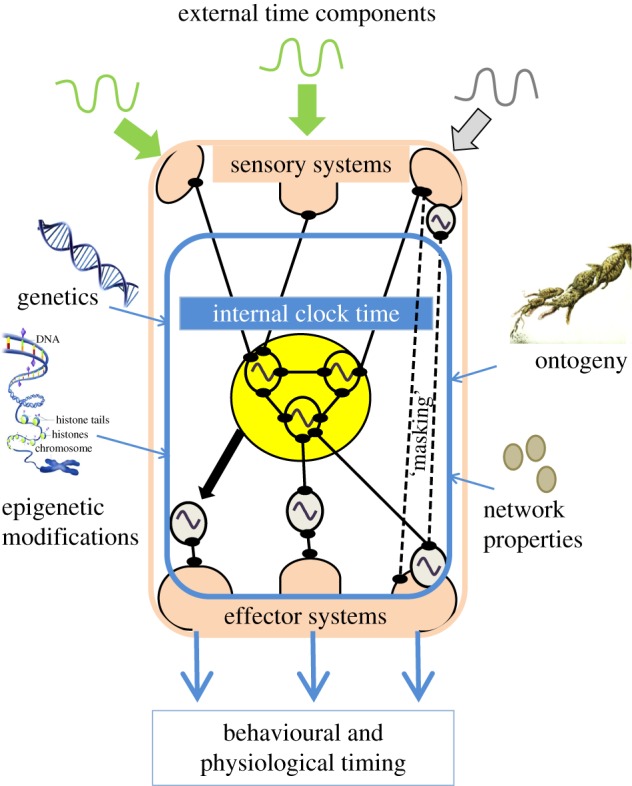
Plasticity in the clock system. Multiple environmental factors may act on sensory systems that may or may not contain peripheral clocks (indicated by sine waves). The sensory systems are connected to, and can entrain internal clock time of the central clock (yellow central shape, showing multiple central oscillators with a sine wave). The central clock entrains peripheral oscillators and acts on effector systems via neuronal (circle-ended black lines) or other (e.g. humoral, thermal, black arrow) pathways. Environmental signals that are perceived by the sensory system can also act directly on effector systems (e.g. masking) (circle-ended dashed lines). Effector systems generate rhythms in organismal behaviour and physiology. Individual differences in the biological timekeeping system that integrates this information can arise in multiple ways, for example, through genetic variation, epigenetic variation, ontogeny and network properties.
This overview suggests that to properly understand an organism's manifest timing, several components of ‘time’ must be looked at simultaneously, of which some are external (environmental factors) and others internal (figures 1 and 2). However, the fields of chronobiology and ecology give different weight to these components and correspondingly, differ in their views of an organism's environment. The field of chronobiology acknowledges that interactions with external time are fundamental for the functioning of internal biological clocks, but emphasizes that they are not needed to sustain rhythmicity. It identifies effects of components of time that act to modify internal clock time through entrainment of the clock (synchronization of internal clock time by environmental cues or ‘zeitgebers’ [25]).
Zeitgebers are mainly photic (i.e. aspects of the light environment) and have been prioritized in chronobiological studies. Zeitgeber effects are distinguished from effects of abiotic and biotic time that directly modify the expression of an organism's behavioural and physiological rhythms (such direct effects are classically termed ‘masking’ by chronobiologists, because they mask the true state of the internal clock [25,33]). In contrast with the persistent effects of entrainment, in masking temporal adjustments to an organism's observed timing immediately disappear when the external factor is no longer present (also see [31]). Notably, however, a single environmental factor can act as both zeitgeber and to mask a behaviour (e.g. light).
This chronobiological view contrasts with ecological views that prioritize, or are limited to, the ways in which the external context of an organism orchestrates its functioning. Ecological perspectives traditionally do not consider the importance of internal clock time, nor the twofold effects of environment (entrainment and masking). As we will discuss in this issue, ecologists increasingly appreciate that they cannot fully understand, and even less so predict, responses of organisms to the biotic and abiotic environment without considering the physiological systems that orchestrate these responses. In turn, in chronobiology there is a growing concern about knowledge gaps arising from predominantly laboratory-based research. Recent studies have highlighted discrepancies between rhythms in the laboratory, compared with the field where organisms are exposed to a wide variety of abiotic and biotic influences [31]. Thus, chronobiologists increasingly conclude that pure consideration of internal clocks and photic entrainment, although perfected in laboratory settings, has insufficient explanatory power in the real world.
An example of the importance of time in ecology, and of complementary chronobiological insights in how it is achieved, is the two-way interaction between kestrels Falco tinnunculus and common voles Microtus arvalis [34,35]. In this predator–prey system, the two species display rhythms of hunting and above-ground foraging, respectively, on a daily as well as ultradian (i.e. shorter than 24 h) time scale. Both kestrels and voles use identified, internal timing systems which directly affect the ecological processes involved in their predator–prey relationship, defining opportunities and restrictions. Voles are active on the surface only a few times per day even in winter, and otherwise hide in burrows. The voles' activity bouts repeat rhythmically over the day, driven by a rigid ultradian internal clock, which is functionally related to, but not caused by optimal food processing [36]. Likely, ultradian synchronization in the voles plays a crucial role at the family level as they save energy by huddling [37] and employ warning against predators [35]. Kestrels, in turn, appear to adjust their flight-hunting to times of expected high yields, using a daily hunting routine in which they incorporate ultradian hunting success of the preceding day [34]. Circadian time-place learning, highlighted in this issue for bees [17], may enable kestrels to optimize their hunting behaviour. This example demonstrates the interactions of biological clocks with abiotic and biotic components of time, underlining the relevance of precise timing mechanisms [32].
Given the complementary perspectives of ecology and chronobiology of the same biological processes, it seems evident that greater progress can be made when both perspectives are integrated, e.g. by the use of well-defined, shared concepts and terminology. This is what we endeavour below. We believe that this will help both fields to answer questions which each one alone cannot well explain, and to truly understand biological rhythms as ‘Wild Clocks’ that have evolved in the complexity of natural environments. In the following, we will first review in greater detail how timing is investigated in each field. We then propose that despite their differences, the two fields intersect in two concepts that are centrally important for both, and that both fields strive to balance against each other. These key concepts are plasticity of timing on the one hand, and consistent individual chronotypes [25] on the other.
3. Timing from chronobiological and ecological perspectives
(a). Chronobiology
The field of chronobiology has established that circa-rhythmicity across kingdoms in biology shares the following characteristics, which we here detail for circadian rhythms: it is endogenously generated, with a circa-24 h period that does not match any known geophysical oscillation (hence, called ‘free-running’; table 1a; [25]); and innate, persistent in organisms and across generations maintained in constant environmental conditions. The realization that such a mechanism could be used for the actual measurement of time led to the conceptualization of the existence of an internal clock: a ‘self-winding, self-regulating’ [38] continuously consulted timepiece with an accuracy and precision great enough for use as a time standard. Critical requirements for such a clock are a certain rigidity—that it runs faithfully despite varying external (e.g. ambient temperature, humidity) and internal (e.g. hunger, hormonal and arousal state) conditions. Importantly, despite the rigidity, this clock also needs to maintain a measure of plasticity—that it can be adjusted to the environment in many ways.
Table 1.
Phenotypic plasticity in timekeeping. The table conceptualizes for diurnal species forms of plasticity of timing and of clock-dependent plasticity from chronobiological and ecological perspectives.
 |
The metaphor of an internal ‘clock’—accurate and precise but resettable—focused theoretical and experimental attention in chronobiology on how such a biological system was built, leading to a wave of mathematical, neurobiological and molecular genetic advances in our understanding of circadian timekeeping over the last 50 years [25]. Prominent in these analyses has been the study of organisms in artificially controlled constant environmental conditions (i.e. constant darkness or dim light, unvarying temperature, food and water ad libitum). These experiments were conducted in order to measure the clock's intrinsic free-running circadian period, and to determine the component molecular and cellular ‘gears’ that enable its rigid sustained oscillation. Efforts have also been directed to investigating input pathways that link the clock to the sensory systems that perceive entraining environmental signals, in particular light. While determination of free-running period in constant conditions is critical for tracking the clock's motion, under real-life conditions rhythms are entrained to external time, so that internal clock time can be defined as the phase angle of a rhythm relative to an external phase reference ([25]; henceforth referred to as ‘phase’; e.g. phase of activity onset relative to sunrise, measured in minutes or in degrees). Clearly, there are countless rhythms within an organism at any time (e.g. activity, body temperature, metabolite levels or gene expression [22]) which could be used to derive ‘phase’ for characterizing internal clock time (see discussion in §4). For simplicity, phase is usually assessed from a rhythm that is relatively easy to measure repeatedly in an individual and thought to capture multiple traits, in particular locomotor activity [39] and body temperature. Importantly, it is the phase of the clock (i.e. internal clock time) that determines the state of an individual and its responses to the environment. Phase is thus critical for understanding the adaptive function of organismal rhythms. Free-running period length and phase of entrainment are systematically related to each other (see §4), such that faster clocks (shorter period length) tend to lead to earlier phase (more positive phase angles) [14].
Under free-running conditions, chronobiologists observed an often striking individual consistency in the timing of an organism's biological rhythms [14]. Recordings of individual differences in free-running period lengths paved the way to identifying the inheritance of the properties of the clock, especially the role of particular genes for features of circadian rhythms (e.g. different free-running period lengths). Based on this, a principal cellular clock mechanism was identified, by which a set of intracellular ‘clock’ genes functions within autoregulatory feedback loops, with proteins rhythmically suppressing the transcription of their own mRNAs [14]. Establishing links between genotype and phenotype, and identifying an underlying cellular clock mechanism, has been a paradigmatic contribution of chronobiology to science (the ‘first revolution’ in chronobiology [40,41]).
Subsequent research has discovered many further genes involved in clock regulation and entrainment, but has also continuously added layers of complexity. We now know that several interacting cellular feedback loops contribute to timing, and that many additional molecular mechanisms partake. The emerging view posits an intricate multi-level system regulated by processes involving epigenetics, transcription–translation feedback loops and post-transcriptional and post-translational modifications within cells, cellular interactions among oscillators and non-oscillator cells [25], and cross-talks between tissues in different parts of the body [42–44]. In the case of circadian organization, we know now that a multiplicity of clocks oscillate throughout body organs and tissues, expressing defined but permutable phase relationships to each other and to the environmental day–night cycle (figure 2; [31]).
Importantly, therefore, an organism can be thought of as consisting of many clocks (millions in complex multi-cellular organisms; [45]) that are co-ordinated within the body in various ways (the ‘second revolution’ in chronobiology; [40]). This complex system is thought to adaptively orchestrate the daily temporal organization of organismal physiology and behaviour. More recently, the field of chronobiology has refocused its interest on entrained clocks in the laboratory and particularly in the real world (the ‘third revolution’ in chronobiology; [40]). This research has uncovered often substantial differences between individuals. It also found far greater plasticity of circadian clocks than expected from controlled laboratory settings, and has highlighted the responsiveness to a host of environmental factors [31,46]. Nonetheless, similarly as for free-running period length, individuals often showed high consistency in their temporal alignment (i.e. phase), captured by the term chronotype [25]. In today's chronobiology, chronotype is under intense study using the molecular tools mentioned above, as well as large-scale epidemiological methods. As we will summarize below, the emerging interests in plasticity and chronotype are paralleled in ecology.
The focus of this description so far has been on circadian rhythms, but biological rhythms on other time-scales are also discussed in this issue [6,16,30,47]. Knowledge of clock mechanisms are most elaborated for circadian rhythms, but similar principles appear to apply for other ‘circa-rhythms’ (circannual, circatidal and circalunar clocks) [4,5,48]. These rhythms interact with each other to various extents. Interactions with circadian clocks are partly understood for annual cycles of mammals, but debated in other taxa, for example, insects [30]. The mammalian ‘clock’ can serve to measure changing photoperiod (i.e. the daylight fraction of the 24 h day), as a seasonal ‘calendar’. However, at least in the suprachiasmatic nucleus (SCN) of the hypothalamus, the site of the pre-eminent photo-entrainable circadian pacemaker of mammals, the mechanisms for encoding day/night and day length appear to be distinct. While the former employs the autoregulatory transcription–translation feedback loop, for the latter, intercellular coupling within the SCN neuronal network is reconfigured [49] (figure 2). Our understanding of interactions of circadian rhythms with rhythms on other time-scales is still in its infancy, but recent years have seen substantial progress [50–52]. In addition to circa-rhythms, which approximate geophysical time-scales, biological processes are organized by further internal rhythms with no counterparts in the environment [26]. These range from ultradian time-scales, exemplified above for voles, to multi-year rhythms, for example, in cicada [53]. Although conceptually distinct, these rhythms can interact with circa-rhythms in ways that are poorly understood [54].
(b). Ecology
Getting the timing right provides legions of interesting adaptive problems that organisms have to solve [26]. Animals live in a world where the abundance of resources and the incidence of threats fluctuate on daily, seasonal and potentially further temporal scales. In addressing these problems, the field of ecology has focused on the timing of individuals relative to the external environment, and on its consequences at intraspecific and interspecific levels [32,55]. The importance of cyclic repeatable variability in ecological conditions has been realized for a long time, and has been highlighted by phenology studies that reported species-specific patterns, which sometimes were stunningly precise between years [29]. On a daily time-scale, naturalists, including Linné, have described similarly species-specific, and often precise, temporal patterns, for example, in the timing of opening and closing of flowers [17]. Explicit consideration of rhythms in ecology was fuelled by developments of nascent chronobiology, in particular, by ecology-minded chronobiologists like Pat DeCoursey, Eberhard Gwinner and Serge Daan [4,14,26]. Aspects of timing have been integrated into a number of key concepts, of which two, the concepts of phenotypic plasticity and repeatable phenotypes (i.e. chronotypes), will be highlighted below in §4.
Another integrated ecological concept, termed the ‘temporal niche’, refers to temporal segregation of resource use among potentially competing species or individuals sharing the same habitat [56]. The temporal niche concept has been commonly applied to seasonal and annual cycles in which it can explain the differentiation of flowering and fruiting time among sympatric plant species sharing similar pollinators or seed dispersers [57]. However, temporal niches are also found at finer time-scales such as daily cycles. For example, in this issue, Bloch et al. [17] review the interactions between the clocks of bees and flowers and show that both plants and pollinators can reduce competition by segregating the local times of flowering or foraging activity, respectively. Temporal resource segregation can play an important role in increasing the diversity of communities by allowing for the coexistence of species that otherwise would be in strong competition with one another. Likewise, even within species, individuals or subsets of the population, for example, the sexes, can occupy different temporal niches (e.g. [58]).
Finally, a further key concept, life-history theory, aims to explain the diversity of patterns and timing of an organism's life cycle in evolutionary terms. It assumes that because time and resources are restricted, the expression and timing of various activities cannot all be optimized independently and are instead traded off against each other to maximize fitness [59,60] (see also [47]). Timing, like other traits, has fitness consequences that are captured in fitness curves. Theoretical ecologists have generalized life-history theory approaches to identify optimal timing for activities, including complete annual and daily routines [61]. In such models, time is traded off against other assets, such as energy balance and predator avoidance [26,62,63]. For example, for many avian species fitness curves for reproductive timing decline over the season, implying that early breeders have a higher relative fitness than late breeders. Subsequent research aimed to identify whether fitness declined because of factors associated with date, or whether late breeders were low quality birds which had intrinsically low fitness (the date versus quality hypothesis [64]). To distinguish between these hypotheses, animals need to be experimentally manipulated to shift their timing so that fitness consequences can be measured, which can be challenging in the wild.
Applying these concepts, the ecological literature has abundantly classified and analysed timing in a functional context, with major focus on diel and annual rhythms (e.g. [27,65–68]). Comparative studies have discussed transitions between nocturnality and diurnality, and have suggested that mammals have undergone a ‘nocturnal bottleneck’ during evolution to escape predation [69,70]. Macro-ecologists have recently identified large-scale patterns in daily timing of mammals across the globe [68,71]. The proportion of nocturnal species is highest in arid regions and lowest at extremely high latitudes, while crepuscularity (activity during dawn and dusk) is correlated with longer twilight durations. Cathemerality (activity that is spread across day and night) is also more common in cold habitats and under long hours of daylight and twilight in the northern Holarctic region [68,72]. Cathemeral activity, organized in ultradian rhythmicity, is widespread in herbivore mammals, from small voles to horses [71,73]. Furthermore, animals from various taxa can show rhythmic behaviour that does not align with the 24 h day [26]. For example, a recent, large-scale comparison among waders (order Charadriiformes) showed highly variable rhythms of incubation shifts, with period lengths between subsequent parental nest attendance ranging from 6 to 43 h. As in mammals, the prevalence of 24 h rhythms declined with increasing latitude [74,75].
The broad-scale patterns of variation are generally consolidated by detailed studies of populations or individuals. These studies underlined both considerable evolutionary lability of chronobiological traits [76] but also rigid cycles where benefits of rhythmicity are not evident. For example, on the one hand, nearly continuous or ultradian activity has been shown in increasing numbers of species [72]. On the other hand, an increasing number of species is reported to maintain rhythms in seemingly largely arrhythmic environments, for example, caves [77], the deep sea [78] or continuous polar light or darkness [75,79,80]. For some species, such apparent contradictions arise from individual plasticity (see §4). For example, honeybees are known for their highly precise clocks during the forager stage, but at other stages they may show prolonged intervals of activity with no circadian rhythms [72]. In the absence of circadian behaviour, some pacemakers nevertheless continue to tick in the brains of the bees, even under the tightly regulated physical environment of the hive. This observation underlines a high degree of plasticity in the circadian system [81–83].
So far, functional explanations of the often striking rhythmic patterns of animals remain mostly speculative, opening intriguing research questions for ecologists and chronobiologists alike [32]. In some cases, environmental drivers of interspecific diversity of patterns have been identified (reviewed by [56,84]). Food availability can diversify rhythms when limited resources replenish within the timeframe in which different species partition their access to them (e.g. tidally shifting grain availability in sand dunes [62]), or when different resources are available at different times (daily shifts in arthropod availability [85,86]). In desert habitats, in turn, heat dissipation probably plays a role in the ‘siesta’ which interrupts activity patterns in many species (e.g. rodents, lions, oryx [87,88]). Energetic constraints also have direct consequences for the daily timing of activity [31] and for reproductive timing and output [47,89]. These and other natural selection pressures, for example, predation, are detailed in §4. A poorly studied form of possible selection on timing, sexual selection, is discussed in the contribution by [55]. Some studies indicate direct reproductive benefits of specific behavioural timings. For example, recent research on an Arctic wader species reported that males that courted mates most persistently around the clock had the highest reproductive success [90].
Thanks to breathtaking developments in animal-tracking technologies, ecological studies of timing are surging, in particular, for birds and mammals [74]. Tracking of individuals can detail the timing of specific behaviours (e.g. nocturnal roosting of swifts exclusively during the breeding season [91]) and physiology (e.g. sleep [92]). Extensions include tracking of individuals across the lifespan or in the context of conspecific interactions. One group whose timing has been studied in particular detail are migratory birds, which exploit annual resource peaks across the globe [16]. Very much in parallel to findings in chronobiology, ecological studies on these and other groups have demonstrated various degrees of plasticity in timing, for example, in response to weather or between seasons. Ecological studies of migratory birds also echo the findings on chronotypes described above for chronobiology (figure 3). Studies of repeated journeys of individuals in some species have revealed between-individual variation in combination with high individual consistency [16,93–96]. Similar findings have been reported for daily behaviours of animals, where chronotype has become an increasingly popular measure [55,97–101].
Figure 3.
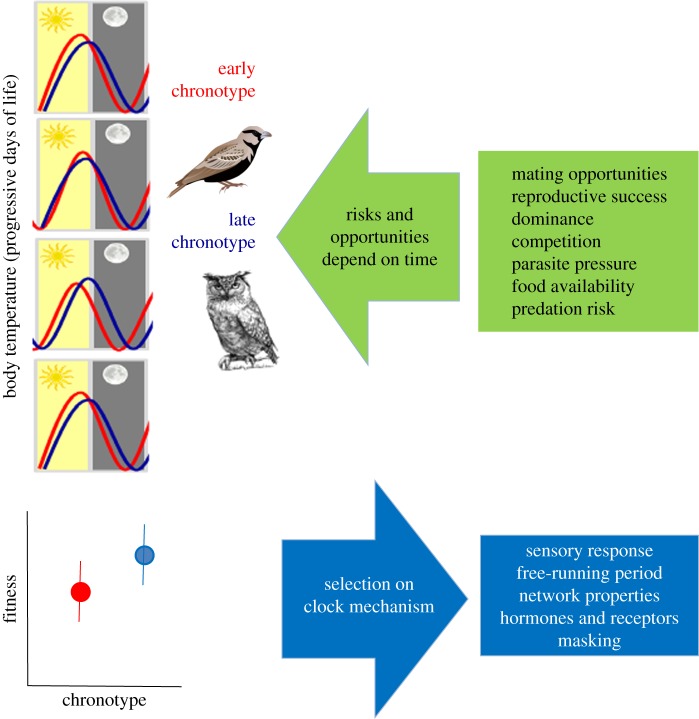
Chronotype as subject to selection. Most individuals will show some day-to-day variation in timing of physiology or behaviour, but often the relative timing compared with others in the population is quite consistent. Consistent differences in timing map to different chronotypes (red: early chronotype; blue: late chronotype). Because many environmental opportunities as well as risks to an animal (green arrows) depend on time, different chronotypes will have different fitness under different environmental conditions. When selection is directional this will lead to modification of the mechanisms that determine chronotype (blue arrows), and over time, to shifts in the distribution of chronotypes in the population (i.e. microevolution of chronotype).
4. Converging key concepts of both fields: plasticity and chronotype
(a). Plasticity
From a perspective of chronobiology, the circadian system of animals provides a fascinating example of a system that, paradoxically, shows both rigidity and plasticity. Historically, there has been great interest in the rigidity of the system. Animals indeed can keep a precise circadian period over many weeks and even months. The consistency and rigidity of the system is important to precisely organize animal behaviour and physiology, and is critical for measuring day length (photoperiod) that underlies many annual and circannual rhythms. As indicated above, recent years have seen also a spate of studies that highlight substantial plasticity, as evident for example from discrepancies between rhythms in the laboratory and in the field [31]. Whereas laboratory studies in constant conditions essentially neutralize the need for, or triggers of, plasticity, in the constantly changing real-world substantial plasticity is probably the rule.
How all the ecologically relevant information summarized in figure 1 is integrated to plastically adjust an organism's rhythms to its environment is unknown, but studies in ecological context force the old clock imagery to be reframed as a malleable temporal programme [10,11]. The transforming of abiotic and biotic time to behavioural and physiological timing (figure 1) may involve multiple clocks and their variable coupling functions (figure 2). Chronobiology describes various types of plasticity, which we exemplify for circadian rhythms in table 1. To some authors, a biological rhythm in a behaviour or physiological process in itself is seen as ‘plasticity’ (‘endogenous’ plasticity [102]; table 1a). For example, an animal regulates its body temperature rhythmically across the 24 h day, adjusting its set point in response to internal cues that are provided by its biological clock. Because most processes in the body are under regulation of the clock, an organism's plastic response to the external environment also fluctuates (table 1b; [102]). Thereby, an organism may respond to one and the same environmental challenge or opportunity in completely different ways at different times of day. An example is the immune system, whose arms exert action that depends on internal clock time [103]. Table 1b shows daily fluctuations in the inflammatory response of animals to experimental infection, which in laboratory mice were decisive for their survival prospects. Other examples are responses to olfactory cues. Thus, the male moth antenna responds differentially to a similar dose of female sex attractants delivered at different times of day [104], and tadpoles respond differently when exposed during the day or night to the same concentration of chemical cues of their predator [105].
The most widespread notion of plasticity of biological clocks is seen in their own entrainment to potent environmental cues (zeitgebers) (table 1c). The response of biological clocks to zeitgebers is an interactive process because the type of response depends on internal clock time. For example, in a diurnal animal, exposure to light just before its early-morning phase will advance its activity rhythm, whereas exposure to identical light after its late evening phase will delay the rhythm. The variation in the clock's response to the zeitgeber is captured in ‘phase-response curves’, which are similar in shape for diurnally- and nocturnally active animals. By systematically applying light pulses at different phases of an organism's free-running rhythm, researchers have established the clock's rhythm in photic entrainment (table 1c; after [14]). This feature can mostly account for synchronization of the clock's oscillation to the environmental day–night cycle through resetting the speed and/or phase of the clock. Differences between individuals in free-running period affect the ways the clock is reset to match the 24 h day: the speed of the clock is increased and its phase advanced if an animal's free-running period is longer than 24 h; conversely, its speed is decreased and the phase delayed if an animal's free-running period is shorter than 24 h. These forms of plasticity are generally reversible but in some cases entrainment to a given zeitgeber can lead to lasting effects (called after-effects), even when the zeitgeber is again modified. For example, mice that experienced long or short days subsequently showed longer or shorter circadian periods, respectively, in locomotor activity when monitored under constant conditions. The most persistent plasticity are early-life effects of light exposure, which appear to be irreversible (photoperiodic imprinting [106]). In recent years, chronobiologists have come to also appreciate non-photic zeitgebers, such as food, temperature or conspecifics. Recent studies emphasized the richness of zeitgebers that entrain animal clocks in a more complex and natural context, but also show that non-photic time givers may override photic entrainment [107–109].
Distinct from plasticity through changes in internal clock time, the expression of behavioural and physiological rhythms can also be directly modified by abiotic and biotic factors (masking; table 1d; see [25,31,33]). For example, studies of the circadian body temperature rhythm in humans revealed that masking factors such as light at night, activity levels, postural changes, meal times and sleep, may account for roughly half of the rhythm's amplitude. In table 1d, we show an example from human recordings of activity and sleep (see also [92]). Many humans live out their natural chronotype during the weekend, but on work days, timing is masked by a (cultural) time table. In our example, a late chronotype accommodated an early-starting job during the work week, but on the weekend immediately reverted to late onset and end of activity (table 1d). As a consequence of cultural time tables, many humans incur a sleep deficit over the work week termed ‘social jet lag’ [24]. Classical examples of masking have focused on responses of animals to light. For example, a nocturnal animal may repress its nocturnal activity under artificial light at night or under full moon light [110] without changing its internal clock time [111,112]. Thus, light has dual effects: entrainment and masking; and overt (i.e. measurable) rhythms, such as locomotor activity, are the sum of both.
Binary distinction between plasticity through the clock (entrainment) versus plasticity outside the clock (masking) falls short of capturing the ability of animals to adjust their rhythms to the environment. Under natural conditions, animals are predominantly entrained to the 24 h day, but may nonetheless modify their circadian system and suites of traits under its regulation (table 1e). An example is the rapid shift of bumblebees and honeybees between precisely timed diurnal activity and activity around the clock, depending on whether they forage or nurse a brood. This plasticity is regulated by contact with the brood (reviewed in [72,113]). Gene expression studies have shown that shifts to brood care are associated with attenuation in the cyclic expression of whole brain mRNA levels of clock genes such as Period, Cryptochrome-m, Cycle and Clockwork Orange, but not in the abundance of PERIOD protein in pacemaker neurons, suggesting complex socially modulated reorganization of the circadian system of social bees [81,83,114]. Shifts also occur seasonally, for example, to nocturnality of diurnal birds during the migration season, which can be triggered endogenously by circannual rhythms. They are characterized by distinct changes in the circadian system, for example, in free-running period length [16,72,115].
Plasticity of the circadian system can be profoundly complex because it may involve the response to multiple external and internal factors (figures 1 and 2). For example, circadian rhythms are commonly influenced by photoperiod and temperature and therefore naturally change with season [116]. Many species are largely diurnal when it is cold (e.g. winter), but show more crepuscular or nocturnal activity when it is warm. For Drosophila, which is the best-studied species showing this pattern, some of the underlying molecular mechanisms have been elucidated (e.g. [117,118]; reviewed in [119]). In mammals, one driver of seasonal switches between diurnal winter activity and nocturnal summer activity are changes in energy balance [120,121]. This switch, and the associated circadian thermo-energetic hypothesis, are explained in greater detail in [31]. Activity at daytime can offer ways, even for a nocturnal mouse, to evade lower night temperatures or lower food availability in its burrow, although this advantage could be counter-balanced by increased predation risk [85]. More generally, interactions with other species (e.g. food or predators [27,32]) and with conspecifics [122,123] can be powerful modulators of biological rhythms, as explained above for social insects (table 1e). In addition to a more general plasticity in circadian rhythms that is associated with maternal behaviour or physiology [124], social effects have been observed in many other contexts. For example, Drosophila flies that are placed with conspecifics change their locomotor activity rhythms compared with the pattern they show in solitude, and these changes depend on whether flies are grouped with a male or a female partner [125].
The examples above show that understanding plasticity is at the intersection of the fields of chronobiology and ecology. From a chronobiological perspective, the circadian system displays many layers of plasticity, enabling it to adjust the temporal organization of organisms to an ever changing environment. Such plasticity is enhanced by its mechanistic complexity, whose many parts and multiple oscillators offer numerous ways of adjusting internal clock time, and thereby making the system less rigid. Therefore, complexity could be the key to addressing the apparent paradox of ‘How can a biological system be rigid and conserved, but at the same time plastic’? Resolving this paradox requires us to understand how the system works—what are the gears, how do they work together and how do they respond to ecologically relevant factors, such that the timing system as a whole generates plasticity.
For the field of ecology, whose central theme is the interaction of species with their environment, investigating plasticity is a fundamental and well conceptualized approach [76]. Key interests in plasticity concern the environmental factors that animals respond to, the form of their responses, and the implications of such responses for fitness. Rhythms in behaviour and physiology display plasticity in several forms, of which some, but not all, can be translated into the conceptual framework of ecology (table 1). As described above, many species show behavioural and physiological rhythms even under constant conditions (table 1a). These rhythms do not fit the conceptual framework of responses to the environment and are not directly translatable to ecological concepts of phenotypic plasticity (they may be conceptualized as ‘endogenous’ plasticity; table 1a; [102]). In this context, ecologists can greatly benefit from chronobiological insights, especially if rhythms affect an organism's response to its environment. An example is the time-dependent response to pathogens or to olfactory cues outlined above (table 1b).
By contrast, ecological concepts do take hold when the timing can be explicitly related to changes in environmental factors. These include not only highly predictable geophysical cycles like photoperiod, but also a host of further rhythms in the abiotic and biotic environment which to different degrees differ from day to day, or from year to year (figure 1). The timing of sunrise and sunset depends on geophysical processes, but is further modified by environmental conditions, for example, between-day differences in cloud cover, which may affect the time when an animal becomes active. Likewise, timing of snow melt differs between years, and in turn modifies the annual onset of the growing season. Because the match of behavioural and physiological rhythms with abiotic and biotic time is important for fitness, animals may modify their rhythms from day to day, or year to year: species that breed in High Arctic areas need to adjust the seasonal timing of migration and breeding to the between-year variation in snow melt to avoid increased mortality risks or failed reproduction (e.g. [126]).
Individuals are thus predicted to show plasticity in their response to the timing of environmental variables that impact their fitness, the so-called selective agents (also sometimes called ultimate factors [65]). Ecologists term the environmental variable that affects the phenotype a cue (also sometimes called a proximate factor [65]), and the phenotype is said to be phenotypically plastic [127]. Ecologists then plot the phenotype against the cue—usually using a linear regression rather than a higher order relationship—to derive what is termed a reaction norm [25]. The reaction norm (if linear) has two characteristics: the slope, which is the sensitivity of the phenotype to the cue, and the elevation, which is the value of the phenotype in the mean environment (table 1b–e). It is important to realize that the environmental variable the animals respond to (the cue) and the environmental variable that affects their fitness (the selective agent) do not need to be the same. In fact, very often they are different as the phenotype is shaped at a different time than when the phenotype is under selection [29]: in the case of the Arctic-breeding birds the selective agent is the date of snow melt [128], but the cues used to shape their phenotype, their arrival date, are likely to be different. The cue can be a geophysical predictor, such as increasing day length in the winter quarters or at a stop-over site, or another abiotic (e.g. temperature at the staging areas) or biotic factor (e.g. observation of other migratory individuals; figure 1).
Effects of these cues can depend on the phase of an animal's annual cycle (cf. table 1c): for example, in autumn, migration of North-temperate migrants is cued by shortening days, whereas in spring it is cued by lengthening days [10,129]. The cues often interact, so that, for example, the responsiveness to temperature may increase with increasing day length [130,131]. It is, however, essential that these cues are predictive for the selective agents, thus the temperature at the staging area has to be correlated with the date of snow melt in the breeding area [132]. In this case, the predictiveness hinges on spatial autocorrelation between environmental variables, but in case of resident species, for instance, it hinges on temporal autocorrelation: rainfall may predict the timing of the abundance of grass seeds, and hence Zebra finches (Taeniopygia guttata) may use rainfall as a cue for breeding [133]. In some cases, the selective agent is also the cue, such as the presence of cones on coniferous trees, which attract nomadic crossbills (Loxia curvirostra) to settle and breed in a given area [134]. Clearly, because cues need to be predictive for the selective agents, species (or even populations of the same species) will use very different cues or prioritize similar cues differently. These examples of annual timing are paralleled by those of daily timing. For example, cave-dwelling nocturnal mammals may ‘light sample’ near the entrance of their den, using relative light levels as a cue to time their evening emergence [14]. Light levels then are used as zeitgebers, used by the animals to synchronize their emergence to times without risk from diurnal predators [135].
Annual changes in day length (photoperiod) and regular daily changes in light intensity (determined by solar angle) are the most common cues for annual and daily timing, but they have a particular role. Because they are dictated by geophysical cycles, there is no between-cycle variation in their temporal patterns. This makes them particularly useful for providing organisms with the correct time coordinates for their specific situation, such as time of birth or local time if they have moved. However, at a given location, such changes obviously cannot be used as a predictor for between-day or between-year environmental variation. Sometimes it is stated that photoperiod opens and closes an annual ‘time window’ in which other (so-called supplementary or fine-tuning) cues play a role. We suggest that extending this view by ecological concepts enables a more dynamic perspective, where the role of photoperiod is conceptualized in its interaction with other cues. In this conceptualization, the slope of the reaction norm to a cue varies with photoperiod (time of year, time of day; table 1c). It may even be flat in parts of the year, when animals do not respond to certain cues [29,129].
From an ecological perspective, it matters to which extent timing can be additionally modified, for example, by masking (table 1d; e.g. conspecific cues overriding clock regulation), or by additional, built-in response mechanisms (table 1e; e.g. energy-dependent modification of reproductive timing; [47]). Species that rely exclusively on photoperiod and have no additional plasticity are at particular risk to suffer from consequences of day- or year-specific variation. For example, in roe deer (Capreolus capreolus), the reproductive phenotype is shaped during the preceding autumn rut, but selection occurs during the following spring when the mother lactates, which should be the time when there is plenty of young grass. If the timing of the upcoming spring is not accurately predicted by autumnal photoperiod, this species can suffer severe losses of fitness [136,137].
Thus, in the context of phenotypic plasticity, the integration of chronobiological concepts into ecology is well on its way. The move from viewing biological timekeeping as merely a constraint for optimal timing, towards integrating photoperiod and biological clocks as adaptive programmes, is an exciting new direction. Yet because ecologists are only partly familiar with chronobiological concepts, they rarely pose more refined questions. For example, whether photoperiod directly, or in interaction with a circannual clock, alters the sensitivity to cues is important for predicting the response of avian migrants to novel light conditions when they change their winter ranges [129]. Ecologists can also not distinguish between situations when an animal performs an activity against its clock (table 1d), as a consequence of masking, from those when an animal shifts its clock (resulting from entrainment; table 1c), although this difference can greatly affect an animal's state and fitness (table 1b).
Ecologists can greatly advance chronobiological theory by their interest in what causes individual variation in the timing reaction norm to environmental cues. The variation in elevation can be seen as variation in chronotype, independent of plasticity. But also the slope can be seen as a trait: some individuals are more ‘sensitive’ to a range of cues (figure 1) than others, and thus, differ in plasticity. For chronobiologists, this view fits well with conceptualizing clocks as programmes, inspiring re-evaluation of clock plasticity beyond entrainment. If selection is seen to also act on plasticity, masking (table 1d) and various forms of clock plasticity (table 1e; [72]) are potentially adaptive features, rather than undesirable noise. For example, when an animal experiences predation risk, it is adaptive to escape even at times when the biological clock promotes sleep time. Variation in both the slope and the elevation of the reaction norm can be due to many mechanisms that affect rhythm generation or responses to the environment (summarized in figure 2). The multi-level complexity of the circadian system provides many opportunities for evolution to shape reaction norms, such that a population may get less or more sensitive to a cue [138].
(b). Chronotype
Complementary to plasticity, the fields of chronobiology and ecology have observed high consistency in the individual timing of diverse organisms, spanning many animal taxa, herbaceous mountain plants and tropical rainforest trees [93,100,101,139–142]. To designate consistent phenotypes, both fields use the term chronotype [25] as an attribute of an individual [99]. Chronotypes are classified as ‘early’ or ‘late’ by relating a defined phase point of a measured biological rhythm (e.g. sleep onset, peak of locomotory activity, lowest body temperature) to an external phase reference point, for example, midnight or sunrise [13]. Chronotype can refer to rhythms of diverse processes, such as locomotion, body temperature, hormone or metabolite levels, gene expression, cognitive function, eating or sleeping (figure 3; [22]). Individuals are considered ‘early’ or ‘late’ not in absolute terms, but relative to conspecifics measured under similar conditions. For example, if several individuals are measured repeatedly under various environmental conditions (such as weather), they may continue to show consistent chronotypes (i.e. remaining relatively early or late members of the population). In practice, environmental effects can often be accounted for, for example, by identifying work days for the exemplary human chronotype discussed above (table 1d; [13]), or by accounting for year of study when analysing reproductive phenology [143] or avian activity and sleep timing [101,142]. Moreover, whenever possible, chronotype should reflect an individual's characteristic phase, rather than singular timing events. Establishing consistency requires repeated measurements, and underlying mechanisms and evolutionary implications can only be inferred if the behaviour is reasonably stable. Beyond these shared features, chronobiology and ecology address the concept of chronotype from different backgrounds.
From a chronobiology perspective, applying the concept of chronotype is challenging: any process could theoretically be used to define an individual's chronotype because most biological processes are controlled by biological clocks. Originally, the term chronotype had covered this broad range of processes. It was described by Charles Ehret [144] as the ‘temporal phenotype’ of an organism, a 24-h map of the phases of the peaks of various rhythms. Obviously, some of these rhythms are independent of one another, while others are not. Translating such a 24-h map into an individual's phase of entrainment requires careful deliberation, both in the marker rhythm(s) chosen and in the phase reference point (e.g. rhythm onset, offset, or a measure of midpoint [39]). Over the last decades, chronotype has held a prominent position in research on humans. It has been widely popularized by online questionnaires that collect data from the public [13]. For example, the Munich questionnaire by pioneering researcher Till Roenneberg on timing of mid-sleep during weekends and work days at present has nearly 300 000 entries (T. Roenneberg, personal communication 2017), so that the distribution of chronotypes can be well characterized. Extreme human chronotypes are commonly referred to as ‘larks’—morning people (those who wake up early and are most alert in the first part of the day) and ‘owls’—evening people (those who are most alert in the late evening hours and prefer to go to bed late) [145,146]. This descriptor of chronotype, used in epidemiological and association studies, has revealed a wealth of chronobiological information, including evidence for its partly genetic determination and its links to circadian mechanisms [147,148]. Studies of animal behaviour have adopted this approach and have likewise identified associations between clock genes and chronotypes [99,142]. These studies take account of factors that obscure the links between chronotype and internal clock time. In the case of humans, imposed work schedules are seen as unnatural, and, hence, chronotype is calculated from mid-sleep on weekends [13]. In the case of wild animals, factors such as nocturnal light exposure or time of year are factored into analyses (e.g. [141,142]).
From an ecological perspective, the concept of ‘chronotype’, with its dual focus on individual consistency and inter-individual differences, fits seamlessly with important key concepts (figure 3). As described above, analysis of individual variation is fundamental in ecology, and chronotype can be studied in the framework of reaction norm approaches. Individual consistency of chronotype depends on both the elevation of the reaction norm and the plasticity of its slope. Variation in elevation describes chronotype, but if different individuals (I) have different sensitivity to the environment (E) then their reaction norms will cross (I × E interaction). Hence, there is no consistency: individual A has a higher trait value than individual B in environment E1 while it has a lower trait value in environment E2.
For evolutionary analysis, it is important to establish the consistency (measured as repeatability) of phenotypes because it indicates an upper limit to the heritability, and, hence, evolvability of traits [149]. Repeatability quantifies the variation of expression of a trait within an individual relative to variation between individuals (e.g. [150]). It can be estimated as the proportion of variance within individuals relative to the overall variance measured within a population (ranging from 1 = fully repeatable to 0 = not repeatable; but see [95] for caveats). Traits that show high repeatability, and whose repeatability is to a large extent genetic or epigenetic, are labile to evolutionary changes to the mechanisms that determine their expression. Accordingly, chronotype is labile to selection on its underlying mechanisms to the extent that it fulfils the requirements of inter-individual variation, individual repeatability, and genetic and epigenetic inheritance [151]. If so, shifts can occur in the distribution of chronotypes in the population (i.e. microevolution of chronotype) provided that chronotypes differ in fitness. Such fitness differences could arise from timing-dependent selection pressures identified above, for example via energy requirements, foraging and mating opportunities, or predation risks (figure 3). Consequently, ecologists are keen to quantify chronotype and its repeatability for individuals [93,100,101,139–142]. Under strong directional environmental pressure, entire local populations can modify chronotype, as for example observed in marine midges exposed to different tidal regimes [50]. As explained above, a promising future development of studies of chronotype would also measure individual differences in plasticity (slope of the reaction norm), which depending on conditions can confer selective costs or benefits (e.g. [138,143]).
Appealing as the concept of chronotype is, ecologists, too, perceive challenges. As for chronobiologists, the choice of descriptors of chronotype requires their consideration and can depend on the study context [39]. For example, in birds, differences in inter-individual variation and in associated pay-offs suggest that timing of the onset of an activity (e.g. wake-up time) can be more relevant for fitness than the timing of its offset (e.g. return to roost) [55,152], and the timing of one activity may be more important than that of others. A recent study of waders found that different aspects of their annual cycle were varying in ways suggestive of process-specific chronotypes, making it difficult to define the most appropriate descriptor [16,153]. Furthermore, plasticity of chronotype to environmental factors, as described above, can complicate analyses, especially if repeated measurements are not feasible (e.g. timing of reproduction in short-lived species). In these cases, pedigreed data and sophisticated statistical models might help to estimate the inherited component that underlies an individual's temporal behaviour [143]. Overall, however, ecological studies using chronotype have great prospects to increase our understanding of the functional role of biological clocks.
Thus, chronobiology and ecology show exciting convergence in their research interests: chronobiologists are now looking at distributions of chronotypes in the real world, while ecologists have become interested in the mechanisms underlying distinct chronotypes and in the ways selection may have acted on them. To highlight and boost the potential of this convergence, below we summarize main recent advances of chronotype research.
Chronobiological research strives to understand the mechanisms that shape chronotype, viewed as being the consistent, observable, synthetic timing outcome of a biological rhythm in response to ‘abiotic’ and ‘biotic’ inputs (i.e. consistent phase, [13,25]; figures 1 and 2). Thus, research aims to identify internal clock time and its manifestation through clock entrainment, masking and programmed plasticity. In recent years, there has been an explosion of interest regarding the relationship between chronotype and human health and well-being, in particular with respect to misalignment of societal time with an individual's biological clock [145]. Effects of sleep deprivation and social jet lag on mental and physical disorders are under active investigation [24].
As explained above, one important contributing component of chronotype is an organism's free-running period length. Free-running periods are distributed around a species-specific mean in animals [154–156] including humans (e.g. [145,157,158]. It has been suggested that people with longer free-running circadian periods have later phases of behaviour under normal day–night conditions, and people with shorter periods have earlier phases [145,159]. A correlation between free-running period and chronotype was also described in intra- and interspecific studies of animals, including birds [141,160], insects [161,162] and mice [163,164], although not all studies were confirmatory [156]. Further clock-governed aspects of chronotype arise from an individual's specific responsiveness for example to light (e.g. based on features of the light input pathway and neuronal networks) and from age-related changes.
Studies of chronotypes of free-living and captive wild animals are at the intersection between chronobiology and ecology [97,98,141,165,166]. In some studies, measures of associated fitness consequences have provided hints to the benefits of a particular chronotype or underlying circadian trait [55]. For example, in passerine great tits (Parus major), free-running period is variable and highly heritable [156]. This variation was associated with extra-pair paternity (EP): EP young found in broods with long period lengths had significantly shorter period lengths than their half siblings. Assuming that period lengths of offspring partly reflect those of their fathers, the study suggested that females chose males with fast clocks (i.e. short period length) for EP matings, in particular if their social mate had a slow clock. Such studies link directly to ecological studies, which for several avian species have demonstrated territorial and reproductive benefits of early activity, including high EP success (reviewed by [55]). EP matings are thought to be constrained by spatial limitations, but temporal niches, like early-morning hours, may provide opportunities to increase reproductive success for socially monogamous birds such as great tits. Similarly, Dominoni et al. [141] reported particularly early, repeatable chronotypes of an urban population of European Blackbird, coupled with shorter circadian period length than in a forest population. It is possible that this difference reflects adaptations to the blackbirds' respective temporal environments, which, for example, differ in nocturnal light levels.
Chronotype may also affect the utilization of specific resources such as food. For example, bees with an early onset of morning activity can arrive first to early opening flowers and exploit their reward, including non-replenished pollen which can give them an important competitive advantage when pollen resources are limited [17]. Functional correlates of individually consistent chronotypes were also identified in a study of foraging and torpor in desert golden spiny mice (Acomys russatus). Sequence of arrival at a foraging patch was not random; some individuals tended to arrive early, while others tended to arrive late. The study found strong relationships between the sequence of arrival at the patch (used as a measure of chronotype), amount of food foraged and time spent torpid [97,98]. Individuals that arrived early to the foraging patch gained consistently greater energy returns, and over time, spent much less time torpid than late arriving individuals.
In summary, the convergent interests of ecology and chronobiology indicate rich ground for fruitful interactions. Stimulated by their common wish to determine mechanisms and implications of chronotype, researchers are already taking up methodologies from each other's fields.
5. Outlook: Wild Clock research across levels of biological organization
Within the field of chronobiology, there is a rapid increase in our understanding of the physiology and molecular biology of the clock, thanks largely to the importance of biological rhythms in biomedical research. Its historical identification of specific gene-phenotype relationships has put chronobiology in a leading position within systems approaches in biology, and these advances still continue. Thus, chronobiology shares the excitement of frontline molecular methods development, but also the challenges of the discovery of ever greater layers of complexity. At the same time, breathtaking developments of animal-tracking technologies are flooding the field of ecology with spatio-temporal data that are yet to be fully explored. These data range from lifelong, large-scale activity patterns to quantify migrations, to high-resolution EEGs to infer daily patterns of sleep. For Wild Clock research, which is positioned at the intersection of these fields, their combined advances open visionary opportunities. Here we give an overview of the perceived potential of this research, while in-depth discussion is provided in the individual contributions to this theme issue.
(a). Organisms and environment: mechanistic perspectives for integrative research
From its mechanistic perspective, chronobiological research investigates the ways organisms integrate different environmental influences (figure 1) and information on internal state (figure 2), which are important concerns for ecologists. Chronobiology has been selective in the aspects of the environments under study, but once chosen, examines their influence on the circadian system across levels of biological organization, from sensory input pathways to the integrated organismic responses, and from molecular processes in cells to physiological processes in tissue and the organism as a whole.
Extensive molecular research within chronobiology has identified many regulatory factors that are well positioned to integrate the environmental influences that are important in natural ecological contexts. These involve epigenetic modifications, chromatin landscape, miRNA, transcription factors, and many other proteins and non-coding RNAs [44]. Thus, one promising platform for collaborative research is a molecular ecology approach focusing on the processes that are involved in integrating environmental influences on the clock. This approach requires ecologists to include into their research portfolio molecular analyses, while researchers with a molecular chronobiology background may need to strengthen their basis in ecology and evolution.
The sensory pathways of environmental input to the clock are of main interest to chronobiology, with a focus on light as the main zeitgeber for the central clock. The sensory systems and molecules that sense light and convey this information to the circadian clock are quite well understood in model organisms [3,14], but much will be gained by studying species with different life histories (e.g. polar species that experience continuous light, or cavity and subterranean dwelling species that stay mostly in the dark; e.g. [77,123]). Recently, chronobiological studies have also made progress in detailed understanding of effects of temperature, studied in the brain clock network of Drosophila [119]. For example, temperature may affect alternative splicing of ‘clock genes’, and sub-populations of the Dorsal Neurons in the fly's brain circadian network are specifically responsive to temperature cycles. Thus, light and temperature information can be integrated by the molecular machinery in clock cells and at the system level by coupling light and temperature responsive cells.
Food availability, a key theme in ecology, might be an extremely important zeitgeber. Animals show food anticipatory behaviour that is characterized by increased locomotor activity well before the predicted feeding time, and is associated with many physiological and molecular changes in various tissues [167,168]. Increasing attention to peripheral tissue clocks, for example, in the liver has provided compelling mechanistic evidence for linking clocks to metabolism [169,170]. In laboratory rodents, timed food availability entrains circadian rhythms in locomotor activity by affecting clocks outside the SCN. However, it is not yet clear how the different clocks and light and food availability inputs are integrated to adjust overall locomotion and feeding behaviour [31]. This line of research opens an exciting venue for eco-chronobiological research.
On a molecular level, links are emerging between biological rhythms and metabolic sensors. Studies of how nutrition and the cell metabolic state influence the molecular clockwork indicate a role for epigenetic mechanisms, which modify DNA and its associated proteins (e.g. histones), and thereby modify when and where gene transcription is initiated. One possible link between the clock and epigenetic processes is that clock proteins such as CLOCK in mammals function as chromatin modifiers [170,171]. Metabolic states and epigenetic modifications are important not only in peripheral clocks, such as the liver, but also for the regulation of the central brain clock. Therefore, epigenetic processes can potentially integrate the influence of light and other internal and external time components (figure 1). For example, recent research has highlighted the importance of epigenetic modifications in SCN cells [171]. DNA methylation was implicated in regulating the relatively long term ‘after-effect’ of day length on the free-running period of mice via methylation-dependent changes in the expression of genes, including clock genes, in the SCN [172], with possible implications for seasonal modifications of clock responses. DNA methylation is also involved in photoperiodism in both mammals and insects [173,174]. The compelling evidence that the clock machinery is regulated by epigenetic mechanisms, which in turn may be coupled to ecologically relevant environmental factors, offers exciting opportunities for Wild Clock research.
Another approach by which chronobiology and ecology have been integrated is the growing trend to incorporate experimental conditions that are ecologically meaningful in chronobiological research. For example, metabolic challenge by cold and hunger induces diurnality in laboratory mice that are typically nocturnal [121], and nocturnality in migratory birds that are typically diurnal [175]. There is also evidence suggesting that the quality of food available at a certain time of day can entrain the clock [176,177]. These observations, and evidence that drug abuse affects circadian rhythms, suggest that reward pathways entrain the circadian clock [171,178]. The idea of links between the reward pathway and the circadian clock is promising from an ecological perspective because it may provide mechanisms by which other rewarding interactions, such as maternal care and mating, might entrain the clock. Conversely, effects of dangerous or stressful events on the clock may be equally important for wild organisms. Laboratory studies have indeed established that stressors and diseases affect circadian rhythms in animals (e.g. [179–181]), including effects of predator odor and social defeat [182,183]. For humans, it has been suggested that circadian disruption imposed by the chronic stress of modern societies underlies the increase in pathologies such as anxiety, depression, sleep disorders, metabolic diseases and various forms of cancer [24,171,184]. Similar effects could be present in animals exposed to human stressors. Detailed, mechanistic insights may reveal how the circadian system could be kept from showing the disorders currently seen in human societies.
However, a mechanistic understanding of how biological rhythms are influenced by the environment will face many additional hurdles. For example, the various clocks in different tissues may respond differentially to the same environmental change [31,185]. Furthermore, effects of the environment on biological rhythms typically depend on time of year, due to both differences in seasonal activities (e.g., breeding) and to annual changes in physiology [4,23]. Thus, to fully understand effects of environmental changes may require characterizing molecular responses in several relevant clocks, along with insights on how the various clocks are integrated to create an adaptive organismal response.
(b). Clocks in a changing world: ecological research highlights a need for integrative research
There is a pressing need for comprehensive insights of the interplay between environment and clocks because our world is rapidly changing. Two of the main changes, global climate change and urbanization, directly affect timing, and their effects are exacerbated by further anthropogenic changes, in particular agricultural intensification, that can disrupt environmental rhythms [186].
Circadian timing is affected by a key component of urbanization: artificial light at night. While evidence is now consolidating that light at night has major implications for humans, such as disrupted sleep and increasing metabolic syndrome [24], studies of wild species have heralded problems as early as during the 1930s [187]. Initial concerns for wild species had focused on altered reproductive rhythms as a consequence of photoperiodic effects of artificial light at night, and these concerns are now robustly supported across taxa, for example, in birds, mammals, fish and plants [188–191]. Recent ecological examples include modified sleep and circadian rhythms in light-exposed, wild birds [141,192] and light-dose-dependent temporal activity patterns in captive birds [193]. These behavioural changes are astonishing because the wild animals exposed to light at night still get a clear signal from the natural photoperiod to which they can entrain, as there is a 3–4 order of magnitude difference between artificial light levels at night (typically 1–10 lx) and day time (10 000–100 000 lx). The consequences of shifted activity patterns for wild animals are still not clear. Animals' sleep may be affected, and there may be physiological costs of living under artificial light conditions which may be due to circadian disruption [189,192,194]. Indication for this comes, for example, from modified secretion of the circadian hormone melatonin in animals exposed to low-level artificial light at night [191,195]. A potential measure that can be taken to reduce the impact of light at night, other than simply reducing the amount of light, is to modify the spectrum of the light. For example, blue tits (Cyanistes caeruleus) exposed to green light at night shifted their activity patterns less than those exposed to white light [194]. Lunar cycles are also affected by artificial light at night. Moonlight confers information that is being used by diverse species as a cue and possibly a zeitgeber, influencing activity patterns and timing of reproduction, but effects of light at night on lunar cycles, so far, are poorly understood [46].
On an annual time-scale, climate change has clearly disruptive effects on seasonal biology [23]. Many species have shifted their phenology in the past decades. Parmesan [196] estimated for a set of 203 Northern Hemisphere species across taxa that the shift in spring phenology was 2.8 days per decade. Because little is known about the circannual clock under natural conditions, we have no idea whether these shifts involved internal clock time. The shift in phenology can largely be attributed to the increased temperature as in all taxonomic groups there is clear evidence that the phenology of a majority of species is correlated with some metric of temperature [197]. There is, however, substantial taxonomic variation in this rate: phenology in amphibians shifted twice as fast as that of trees, birds and butterflies, and almost eight times as fast as in herbs, grasses and shrubs. When grouped into primary producers, primary consumers and secondary consumers, the former two shifted their phenology much faster than the latter (especially in terrestrial environments [197]). This may lead to phenological mismatches between predators and their prey, and herbivores and their plants, with consequences for natural selection on circannual rhythms [198] and population viability [67,199]. Such disruptions of seasonal biology, on an ecosystem level, can have detrimental effects for individual species, but also for human health, animal health and ecosystem services [23]. As an example of species-specific effects, migratory species that utilize seasonal resources at sequential sites over the course of the year now have to cope with strongly diverging changes in phenology at the various sites. They will have to adjust multiple components of their annual cycles, putting multiple selection pressures on the integrated chronotypes [153]. On the level of species interactions, a potential powder barrel are changes in host-parasite relationships, if for example parasites and vectors can extend their annual temporal niches and expand into regions that no longer show prohibitive seasonal changes [23,103].
Various other anthropogenic changes are also likely to affect biological timekeeping, either on their own or by reinforcing effects of climate change and light pollution. For example, agricultural intensification is affecting the abundance of the plants, insects and birds of what were formerly speciose agricultural landscapes (e.g. [200–202]). This loss of biodiversity, intense changes in soil and water management, and surges in the application of agrochemicals and manipulated crops, may well have affected the daily and seasonal timing of key parts of the food chains. For example, highly synchronous seasonal flowering pulses of crops could affect the phenology of pollinators, and an increasing number of widely used agrochemicals that target the nervous systems of insects could possibly affect their clock systems [203,204]. Changes to timekeeping of plants and animals of agricultural landscapes have gone largely undescribed, with arguably the best information being available for birds (e.g. [186]).
Finding solutions to such major challenges will be facilitated by a fundamental understanding of the mechanistic and ecological processes that determine the responses of wild organisms to a changing world. Wild Clock research can make a paradigmatic contribution.
(c). Prospects of a Wild Clock approach
Despite the ubiquity of biological clocks, chronobiologists today have a poor understanding of the functions and evolution of internal timekeeping, and ecologists largely treat timekeeping as a black box, foregoing opportunities to understand and predict the behaviour of free-living organisms. Hence, combining concepts and field methods from ecology with concepts and laboratory-derived insights from chronobiology, might alter long-held assumptions in both fields.
For chronobiologists, the scope for a Wild Clock approach has been highlighted by evidence that clock regulation in real-world contexts tends to be more complex, and may differ, from expectations based on laboratory studies [109,121,205]. Hence, an important import from ecology is an appreciation of the complexity of the environmental signals that affect biological clocks. Research in chronobiology has focused on circadian rhythms assuming a central circadian pacemaker that is entrained by environmental zeitgebers and affects downstream processes in physiology and behaviour by means of output pathways. In practice, however, most of the research has focused on light–dark cycles as the input and on locomotor activity as an output. A wave of new discoveries points to the limitation of this approach. As described above, it is now clear that the ‘core’ or ‘central’ pacemaker is actually composed of heterogeneous groups of networked cells that interact in complex ways to organize time. Cells in this central clock network and in many other tissues respond differentially to zeitgebers, which in turn are not limited to light [42,108,206] (figure 2). In order to understand basic questions in chronobiology, such as why there are so many oscillators and how they interact to organize the organism's internal clock time, chronobiologists need to know the many environmental cues that are important to their model organism and the ways they influence the circadian system (figure 1).
A deeper understanding of their organisms' ecology gives chronobiologists also access to an adaptive framework that can facilitate the generation of new hypotheses and predictions for their functional relevance and evolutionary change, for example, by integrating reaction norm approaches with chronobiological views of dynamic timing programmes. The increasing appreciation of ecology by chronobiologists stems from acknowledging the complexity of the circadian system, but also from realizing that richer ecological contexts produce unexpected results. For these and other advances, chronobiologists who study Wild Clocks can benefit from the surge of data that are collected by powerful animal-tracking technology, and from technical developments that allow more integrative ways to capture chronotype, for example, by remote collection of data on body temperature [74,207,208].
For ecologists, engaging with a Wild Clock approach means opening the black box, rather than limiting their interest to environmental inputs and organismal outputs. Use of chronobiological concepts can improve predictability in ecological studies. For example, knowing whether an activity rhythm is generated by a light-entrained clock or in direct response to light helps to predict future activity patterns after changing illumination, for example, in urban areas. More generally, knowledge of the ways different zeitgebers such as light, temperature, social interactions and food availability affect animal behaviour can help predict the outcomes of the above-mentioned situations in which zeitgebers are modified by changes such as global warming or light at night. This requires an understanding of reaction norms of timing, of selection pressures that act on them, and of genetic variation of reaction norms that determines their scope for evolutionary modification.
While ecologists are well on their way to establish such knowledge for chronotypes of daily and annual activities, establishing the link to internal clocks is still very challenging. Here, the most promising future avenues involve the use of molecular tools inspired by chronobiology, for example, automated reading of clock gene expression in animal fibroblasts that are cultured in the laboratory [74,209]. Another example are candidate genes for timing, identified by chronobiologists, whose sequences, expression patterns or epigenetic modification can be compared in wild animals that differ in timing (e.g. [16,210]). With these methods, animals in the wild can be characterized for their clock, and chronotype, plasticity and fitness can be assessed, so that the long-standing question of the adaptive value of Wild Clocks can ultimately be resolved.
That chronobiology and ecology are already benefitting from cross-fertilization is evident from inspiring findings at the intersection of the two disciplines. Ecologists are repeatedly pointed to clock pathways when they compare genomes and transcriptomes, for example, between urban and rural birds, or migratory and non-migratory states (e.g. [16,211,212]). These findings emphasize the importance of biological clocks in the real world. Chronobiologists, in turn, have been able to disentangle, and experimentally reconstruct, the neuronal networks that govern the distinct activity patterns of Drosophila species living at high latitudes [213], thereby demonstrating how much can be learned from the diversity of patterns and mechanisms in the natural world.
Acknowledgments
We gratefully acknowledge the organizers and funders of the Wild Clocks meeting on Texel, The Netherlands, in March 2015. We also thank Helen Eaton for her patient and insightful support of this theme issue, and appreciate the very helpful advice of the anonymous referees.
Authors' contributions
All co-authors contributed to the concept and content of the review. B.H. drafted the main manuscript with input from the co-authors, who all also provided comments on the text. All the authors gave final approval for publication.
Competing interests
All authors declare that they have no competing interests.
Funding
We received no funding for this study.
References
- 1.de Mairan JJO. 1729. Observation botanique. In Histoire de l'academie Royale des sciences, p. 35 Paris. [Google Scholar]
- 2.Darwin CR, Darwin F. 1880. The power of movement in plants. London, UK: John Murray. [Google Scholar]
- 3.Foster RG, Kreitzman L. 2005. Rhythms of life: the biological clocks that control the daily lives of every living thing. New Haven, CT: Yale University Press. [Google Scholar]
- 4.Gwinner E. 1986. Circannual rhythms, p. 154 Berlin, Germany: Springer. [Google Scholar]
- 5.Naylor E. 2010. Chronobiology of marine organisms. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Bulla M, Oudman T, Bijleveld AI, Piersma T, Kyriacou CP. 2017. Marine biorhythms: bridging chronobiology and ecology. Phil. Trans. R. Soc. B 372, 20160253 ( 10.1098/rstb.2016.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulzman FM, Ellman D, Fuller CA, Moore-Ede MC, Wassmer G. 1984. Neurospora circadian rhythms in space: a reexamination of the endogenous-exogenous question. Science 225, 232–234. ( 10.1126/science.11540800) [DOI] [PubMed] [Google Scholar]
- 8.Schwartz WJ, Daan S. 2017. Origins: a brief account of the ancestry of circadian biology. In Biological timekeeping: clocks, rhythms and behaviour (ed. Kumar V.), pp. 3–22. New Delhi, India: Springer. [Google Scholar]
- 9.Daan S. 2010. A history of chronobiological concepts. In The circadian clock (ed. Albrecht U.), pp. 1–35. Berlin, Germany: Springer. [Google Scholar]
- 10.Gwinner E. 1996. Circadian and circannual programmes in avian migration. J. Exp. Biol. 199, 39–48. [DOI] [PubMed] [Google Scholar]
- 11.Pittendrigh CS. 1993. Temporal organization: reflections of a Darwinian clock-watcher. Ann. Rev. Physiol. 55, 17–54. ( 10.1146/annurev.ph.55.030193.000313) [DOI] [PubMed] [Google Scholar]
- 12.Daan S, Merrow M, Roenneberg T. 2002. External time–internal time. J. Biol. Rhythms 17, 107–109. ( 10.1177/074873002129002375) [DOI] [PubMed] [Google Scholar]
- 13.Roenneberg T. 2015. Having trouble typing? what on earth is chronotype? J. Biol. Rhythms 30, 487–491. ( 10.1177/0748730415603835) [DOI] [PubMed] [Google Scholar]
- 14.Dunlap JC, Loros JJ, Decoursey PJ. 2004. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 15.Yerushalmi S, Green RM. 2009. Evidence for the adaptive significance of circadian rhythms. Ecol. Lett. 12, 970–981. ( 10.1111/j.1461-0248.2009.01343.x) [DOI] [PubMed] [Google Scholar]
- 16.Åkesson S, Ilieva M, Karagicheva J, Rakhimberdiev E, Tomotani B, Helm B. 2017. Timing avian long-distance migration: from internal clock mechanisms to global flights. Phil. Trans. R. Soc. B 372, 20160252 ( 10.1098/rstb.2016.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloch G, Bar-Shai N, Cytter Y, Green R. 2017. Time is honey: circadian clocks of bees and flowers and how their interactions may influence ecological communities. Phil. Trans. R. Soc. B 372, 20160256 ( 10.1098/rstb.2016.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. 2007. Epidemiology of the human circadian clock. Sleep Med. Rev. 11, 429–438. ( 10.1016/j.smrv.2007.07.005) [DOI] [PubMed] [Google Scholar]
- 19.Foster RG, Kreitzman L. 2009. Seasons of life: the biological rhythms that enable living things to thrive and survive. New Haven, CT: Yale University Press. [Google Scholar]
- 20.Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC. 2016. Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34, 2679–2685. ( 10.1016/j.vaccine.2016.04.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18 664–18 669. ( 10.1073/pnas.1008734107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasukawa T, et al. 2012. Human blood metabolite timetable indicates internal body time. Proc. Natl Acad. Sci. USA 109, 15 036–15 041. ( 10.1073/pnas.1207768109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson TJ, et al. 2015. Disrupted seasonal biology impacts health, food security and ecosystems. Proc. R. Soc. Lond. B 282, 20151453 ( 10.1098/rspb.2015.1453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roenneberg T, Merrow M. 2016. The circadian clock and human health. Curr. Biol. 26, R432–R443. ( 10.1016/j.cub.2016.04.011) [DOI] [PubMed] [Google Scholar]
- 25.Schwartz WJ, Helm B, Gerkema MP. 2017. Wild clocks: preface and glossary. Phil. Trans. R. Soc. B 372, 20170211 ( 10.1098/rstb.2017.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daan S, Aschoff J. 1982. Circadian contributions to survival. In Vertebrate circadian systems: structure and physiology (eds Aschoff J, Daan S, Groos GA), pp. 305–321. Berlin, Germany: Springer. [Google Scholar]
- 27.Halle S, Stenseth NC. 2000. Activity patterns in small mammals. Berlin, Germany: Springer. [Google Scholar]
- 28.Helm B, Shavit A. 2017. Dissecting and reconstructing time and space for replicable biological research. In Stepping in the same river twice: replication in biological research (eds Ellison AM, Shavit A), pp. 233–249. New Haven, CT: Yale University Press. [Google Scholar]
- 29.Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. 2010. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Phil. Trans. R. Soc. B 365, 3113–3127. ( 10.1098/rstb.2010.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denlinger DL, Hahn DA, Merlin C, Holzapfel CM, Bradshaw WE. 2017. Keeping time without a spine: what can the insect clock teach us about seasonal adaptation? Phil. Trans. R. Soc. B 372, 20160257 ( 10.1098/rstb.2016.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Veen DR, Riede SJ, Heideman PD, Hau M, van der Vinne V, Hut RA. 2017. Flexible clock systems: adjusting the temporal programme. Phil. Trans. R. Soc. B 372, 20160254 ( 10.1098/rstb.2016.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kronfeld-Schor N, Visser ME, Salis L, van Gils JA. 2017. Chronobiology of interspecific interactions in a changing world. Phil. Trans. R. Soc. B 372, 20160248 ( 10.1098/rstb.2016.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mrosovsky N. 1999. Masking: history, definitions, and measurement. Chronobiol. Int. 16, 415–429. [DOI] [PubMed] [Google Scholar]
- 34.Rijnsdorp A, Daan S, Dijkstra C. 1981. Hunting in the kestrel, Falco tinnunculus, and the adaptive significance of daily habits. Oecologia 50, 391–406. ( 10.1007/bf00344982) [DOI] [PubMed] [Google Scholar]
- 35.Gerkema MP, Verhulst S. 1990. Warning against an unseen predator: an experimental study in the common vole, Microtus arvalis. J. Anim. Behav. 10, 1169–1178. ( 10.1016/S0003-3472(05)80183-6) [DOI] [Google Scholar]
- 36.Gerkema MP, Leest DF. 1991. Ultradian rhythms in the common vole Microtus arvalis during short deprivations of food, water and rest. J. Comp. Physiol. A 168, 591–597. ( 10.1007/BF00215081) [DOI] [PubMed] [Google Scholar]
- 37.Gerkema MP. 2002. Ultradian rhythms. In Biological rhythms (ed. Kumar V.), pp. 207–215. New Delhi, India: Narosa. [Google Scholar]
- 38.Johnson MS. 1939. Effect of continuous light on periodic spontaneous activity of white-footed mice (Peromyscus). J. Exp. Zool. 82, 315–328. ( 10.1002/jez.1400820209) [DOI] [Google Scholar]
- 39.Daan S, Aschoff J. 1975. Circadian rhythms of locomotor activity in captive birds and mammals: their variations with season and latitude. Oecologia 18, 269–316. ( 10.1007/BF00345851) [DOI] [PubMed] [Google Scholar]
- 40.Menaker M. 2006. Circadian organization in the real world. Proc. Natl Acad. Sci. USA 103, 3015–3016. ( 10.1073/pnas.0600360103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi JS, Shimomura K, Kumar V. 2008. Searching for genes underlying behavior: lessons from circadian rhythms. Science 322, 909–912. ( 10.1126/science.1158822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeman GM, Webb AB, An S, Herzog ED. 2008. For whom the bells toll: networked circadian clocks. Sleep Biol. Rhythms 6, 67–75. ( 10.1111/j.1479-8425.2008.00344.x) [DOI] [Google Scholar]
- 43.Mehra A, Baker CL, Loros JJ, Dunlap JC. 2009. Post-translational modifications in circadian rhythms. Trends Biochem. Sci. 34, 483–490. ( 10.1016/j.tibs.2009.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Partch CL, Green CB, Takahashi JS. 2014. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 24, 90–99. ( 10.1016/j.tcb.2013.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herzog ED. 2007. Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 8, 790–802. ( 10.1038/nrn2215) [DOI] [PubMed] [Google Scholar]
- 46.Kronfeld-Schor N, Dominoni D, de la Iglesia H, Levy O, Herzog ED, Dayan T, Helfrich-Forster C. 2013. Chronobiology by moonlight. Proc. R. Soc. B 280, 20123088 ( 10.1098/rspb.2012.3088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams CT, Klaassen M, Barnes BM, Buck CL, Arnold W, Giroud S, Vetter SG, Ruf T. 2017. Seasonal reproductive tactics: annual timing and the capital-to-income breeder continuum. Phil. Trans. R. Soc. B 372, 20160250 ( 10.1098/rstb.2016.0250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tessmar-Raible K, Raible F, Arboleda E. 2011. Another place, another timer: marine species and the rhythms of life. BioEssays 33, 165–172. ( 10.1002/bies.201000096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. 2007. Seasonal encoding by the circadian pacemaker of the SCN. Curr. Biol. 17, 468–473. ( 10.1016/j.cub.2007.01.048) [DOI] [PubMed] [Google Scholar]
- 50.Kaiser TS, et al. 2016. The genomic basis of circadian and circalunar timing adaptations in a midge. Nature 540, 69–73. ( 10.1038/nature20151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zantke J, Ishikawa-Fujiwara T, Arboleda E, Lohs C, Schipany K, Hallay N, Straw AD, Todo T, Tessmar-Raible K. 2013. Circadian and circalunar clock interactions in a marine annelid. Cell Rep. 5, 99–113. ( 10.1016/j.celrep.2013.08.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, et al. 2013. Dissociation of circadian and circatidal timekeeping in the marine Crustacean Eurydice pulchra. Curr. Biol. 23, 1863–1873. ( 10.1016/j.cub.2013.08.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sota T, Yamamoto S, Cooley JR, Hill KBR, Simon C, Yoshimura J. 2013. Independent divergence of 13- and 17-y life cycles among three periodical cicada lineages. Proc. Natl Acad. Sci. USA 110, 6919–6924. ( 10.1073/pnas.1220060110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Veen DR, Gerkema MP. 2017. Unmasking ultradian rhythms in gene expression. FASEB J. 31, 743–750. ( 10.1096/fj.201600872R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hau M, Dominoni D, Casagrande S, Buck CL, Wagner G, Hazlerigg D, Greives T, Hut RA. 2017. Timing as a sexually selected trait: the right mate at the right moment. Phil. Trans. R. Soc. B 372, 20160249 ( 10.1098/rstb.2016.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kronfeld-Schor N, Dayan T. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 151–181. ( 10.1146/annurev.ecolsys.34.011802.132435) [DOI] [Google Scholar]
- 57.Kelly CK, Bowler MG, Fox GA. 2013. Temporal dynamics and ecological process. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 58.Huffeldt NP, Merkel FR. 2016. Sex-specific, inverted rhythms of breeding-site attendance in an Arctic seabird. Biol. Lett. 12, 20160289 ( 10.1098/rsbl.2016.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 60.Cuthill IC, Houston AI. 1997. Managing time and energy. In Behavioral ecology. An evolutionary approach (eds Krebs JR, Davies NB), pp. 97–121, 4th edn Oxford, UK: Blackwell Science. [Google Scholar]
- 61.Houston A, McNamara J. 1999. Models of adaptive behaviour. An approach based on state. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 62.Ziv Y, Abramsky Z, Kotler BP, Subach A. 1993. Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 66, 237–246. ( 10.2307/3544810) [DOI] [Google Scholar]
- 63.Alerstam T. 2011. Optimal bird migration revisited. J. Ornithol. 152, 5–23. ( 10.1007/s10336-011-0694-1) [DOI] [Google Scholar]
- 64.Verhulst S, Nilsson J-A. 2008. The timing of birds' breeding seasons: a review of experiments that manipulated timing of breeding. Phil. Trans. R. Soc. Lond. B 363, 399–410. ( 10.1098/rstb.2007.2146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker JR. 1938. The evolution of breeding seasons. In Evolution: essays on aspects of evolutionary biology (ed. de Beer GR.), pp. 161–177. London, UK: Oxford University Press. [Google Scholar]
- 66.Martin GR. 1990. Birds at night. London, UK: Poyser. [Google Scholar]
- 67.Reed TE, Grøtan V, Jenouvrier S, Sæther B-E, Visser ME. 2013. Population growth in a wild bird is buffered against phenological mismatch. Science 340, 488–491. ( 10.1126/science.1232870) [DOI] [PubMed] [Google Scholar]
- 68.Bennie JJ, Duffy JP, Inger R, Gaston KJ. 2014. Biogeography of time partitioning in mammals. Proc. Natl Acad. Sci. USA 111, 13 727–13 732. ( 10.1073/pnas.1216063110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angielczyk KD, Schmitz L. 2014. Nocturnality in synapsids predates the origin of mammals by over 100 million years. Proc. R. Soc. B 281, 20141642 ( 10.1098/rspb.2014.1642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerkema MP, Davies WI, Foster RG, Menaker M, Hut RA. 2013. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc R. Soc. B 280, 20130508 ( 10.1098/rspb.2013.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roll U, Dayan T, Kronfeld-Schor N. 2006. On the role of phylogeny in determining activity patterns of rodents. Evol. Ecol. 20, 479–490. ( 10.1007/s10682-006-0015-y) [DOI] [Google Scholar]
- 72.Bloch G, Barnes BM, Gerkema MP, Helm B. 2013. Animal activity around the clock with no overt circadian rhythms: patterns, mechanisms and adaptive value. Proc. R. Soc. B 280, 20130019 ( 10.1098/rspb.2013.0019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin AM, et al. 2010. Circadian regulation of locomotor activity and skeletal muscle gene expression in the horse. J. Appl. Physiol. 109, 1328–1336. ( 10.1152/japplphysiol.01327.2009) [DOI] [PubMed] [Google Scholar]
- 74.Dominoni D, Åkesson S, Klaassen R, Spoelstra K, Bulla M. 2017. Methods in field chronobiology. Phil. Trans. R. Soc. B 372, 20160247 ( 10.1098/rstb.2016.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bulla M, et al. 2016. Unexpected diversity in socially synchronized rhythms of shorebirds. Nature 540, 109–113. ( 10.1038/nature20563) [DOI] [PubMed] [Google Scholar]
- 76.Piersma T, Van Gils J. 2011. The flexible phenotype. A body-centered integration of ecology, physiology, and behaviour. Oxford, UK: Oxford University Press. [Google Scholar]
- 77.Beale AD, Whitmore D, Moran D. 2016. Life in a dark biosphere: a review of circadian physiology in ‘arrhythmic’ environments. J. Comparat. Physiol. B 186, 947 ( 10.1007/s00360-016-1000-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersen DM, Keafer BA. 1987. An endogenous annual clock in the toxic marine dinoflagellate Gonyaulax tamarensis. Nature 325, 616–617. ( 10.1038/325616a0) [DOI] [PubMed] [Google Scholar]
- 79.Ashley NT, Ubuka T, Schwabl I, Goymann W, Salli BM, Bentley GE, Buck CL. 2014. Revealing a circadian clock in captive arctic-breeding songbirds, lapland longspurs (Calcarius lapponicus), under constant illumination. J. Biol. Rhythms. 29, 456–469. ( 10.1177/0748730414552323) [DOI] [PubMed] [Google Scholar]
- 80.Tran D, Sow M, Camus L, Ciret P, Berge J, Massabuau J-C. 2016. In the darkness of the polar night, scallops keep on a steady rhythm. Sci. Rep. 6, 32435 ( 10.1038/srep32435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shemesh Y, Eban-Rothschild A, Cohen M, Bloch G. 2010. Molecular dynamics and social regulation of context-dependent plasticity in the circadian clockwork of the honey bee. J. Neurosci. 30, 12 517–12 525. ( 10.1523/jneurosci.1490-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez-Zas SL, Southey BR, Shemesh Y, Rubin EB, Cohen M, Robinson GE, Bloch G. 2012. Microarray analysis of natural socially regulated plasticity in circadian rhythms of honey bees. J. Biol. Rhythms 27, 12–24. ( 10.1177/0748730411431404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuchikawa T, et al. 2017. Neuronal circadian clock protein oscillations are similar in behaviourally rhythmic forager honeybees and in arrhythmic nurses. R. Soc. open biol. 7,170047 ( 10.1098/rsob.170047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kronfeld-Schor N, Dayan T. 2008. Activity patterns of rodents: the physiological ecology of biological rhythms. Biol. Rhythm Res. 39, 193–211. ( 10.1080/09291010701683268) [DOI] [Google Scholar]
- 85.Kronfeld-Schor N, Dayan T. 1999. The dietary basis for temporal partitioning: food habits of coexisting Acomys species. Oecologia 121, 123–128. ( 10.1007/s004420050913) [DOI] [PubMed] [Google Scholar]
- 86.Vonshak M, Dayan T, Kronfeld-Schor N. 2009. Arthropods as a prey resource: patterns of diel, seasonal, and spatial availability. J. Arid. Environ. 73, 458–462. ( 10.1016/j.jaridenv.2008.11.013) [DOI] [Google Scholar]
- 87.Levy O, Dayan T, Porter WP, Kronfeld-Schor N. 2016. Foraging activity pattern is shaped by water loss rates in a diurnal desert rodent. Am. Nat. 188, 205–218. ( 10.1086/687246) [DOI] [PubMed] [Google Scholar]
- 88.Davimes JG, Alagaili AN, Gravett N, Bertelsen MF, Mohammed OB, Ismail K, Bennett NC, Manger PR. 2016. Arabian Oryx (Oryx leucoryx) respond to increased ambient temperatures with a seasonal shift in the timing of their daily inactivity patterns. J. Biol. Rhythms 31, 365–374. ( 10.1177/0748730416645729) [DOI] [PubMed] [Google Scholar]
- 89.Simons AM. 2011. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc. R. Soc. B 278, 1601–1609. ( 10.1098/rspb.2011.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lesku JA, Rattenborg NC, Valcu M, Vyssotski AL, Kuhn S, Kuemmeth F, Heidrich W, Kempenaers B. 2012. Adaptive sleep loss in polygynous pectoral sandpipers. Science 337, 1654–1658. ( 10.1126/science.1220939) [DOI] [PubMed] [Google Scholar]
- 91.Hedenström A, Norevik G, Warfvinge K, Andersson A, Bäckman J, Åkesson S. 2016. Annual 10-month aerial life phase in the common swift Apus apus. Curr. Biol. 26, 3066–3070. ( 10.1016/j.cub.2016.09.014) [DOI] [PubMed] [Google Scholar]
- 92.Rattenborg NC, de la Iglesia HO, Kempenaers B, Lesku JA, Meerlo P, Scriba MF. 2017. Sleep research goes wild: new methods and approaches to investigate the ecology, evolution and functions of sleep. Phil. Trans. R. Soc. B 372, 20160251 ( 10.1098/rstb.2016.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Battley PF. 2006. Consistent annual schedules in a migratory shorebird. Biol. Lett. 2, 517–520. ( 10.1098/rsbl.2006.0535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lourenço PM, Kentie R, Schroeder J, Groen NM, Hooijmeijer JCEW, Piersma T. 2011. Repeatable timing of northward departure, arrival and breeding in Black-tailed Godwits Limosa l. limosa, but no domino effects. J. Ornithol. 152, 1023–1032. ( 10.1007/s10336-011-0692-3) [DOI] [Google Scholar]
- 95.Conklin JR, Battley PF, Potter MA. 2013. Absolute consistency: individual versus population variation in annual-cycle schedules of a long-distance migrant bird. PLoS ONE 8, e54535 ( 10.1371/journal.pone.0054535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Senner NR, Verhoeven MA, Abad-Gomez JM, Gutierrez JS, Hooijmeijer JC, Kentie R, Masero JA, Tibbitts TL, Piersma T. 2015. When Siberia came to the Netherlands: the response of continental black-tailed godwits to a rare spring weather event. J. Anim. Ecol. 84, 1164–1176. ( 10.1111/1365-2656.12381) [DOI] [PubMed] [Google Scholar]
- 97.Levy O, Dayan T, Rotics S, Kronfeld-Schor N. 2012. Foraging sequence, energy intake and torpor: an individual-based field study of energy balancing in desert golden spiny mice. Ecol. Lett. 15, 1240–1248. ( 10.1111/j.1461-0248.2012.01845.x) [DOI] [PubMed] [Google Scholar]
- 98.Levy O, Dayan T, Kronfeld-Schor N, Porter WP. 2012. Biophysical modeling of the temporal niche: from first principles to the evolution of activity patterns. Am. Nat. 179, 794–804. ( 10.1086/665645) [DOI] [PubMed] [Google Scholar]
- 99.Randler C. 2014. Sleep, sleep timing and chronotype in animal behaviour. Anim. Behav. 94, 161–166. ( 10.1016/j.anbehav.2014.05.001) [DOI] [Google Scholar]
- 100.Alós J, Martorell-Barceló M, Campos-Candela A. 2017. Repeatability of circadian behavioural variation revealed in free-ranging marine fish. R. Soc. open sci. 4, 160791 ( 10.1098/rsos.160791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Graham JL, Cook NJ, Needham KB, Hau M, Greives TJ. 2017. Early to rise, early to breed: a role for daily rhythms in seasonal reproduction. Behav. Ecol. 28, 1266–1271. ( 10.1093/beheco/arx088) [DOI] [Google Scholar]
- 102.Stamps JA. 2016. Individual differences in behavioural plasticities. Biol. Rev. Camb. Philos. Soc. 91, 534–567. ( 10.1111/brv.12186) [DOI] [PubMed] [Google Scholar]
- 103.Martinez-Bakker M, Helm B. 2015. The influence of biological rhythms on host-parasite interactions. Trends Ecol. Evol. 30, 314–326. ( 10.1016/j.tree.2015.03.012) [DOI] [PubMed] [Google Scholar]
- 104.Merlin C, Lucas P, Rochat D, Francois MC, Maibeche-Coisne M, Jacquin-Joly E. 2007. An antennal circadian clock and circadian rhythms in peripheral pheromone reception in the moth Spodoptera littoralis. J. Biol. Rhythms 22, 502–514. ( 10.1177/0748730407307737) [DOI] [PubMed] [Google Scholar]
- 105.Fraker ME. 2008. The influence of the circadian rhythm of green frog (Rana clamitans) tadpoles on their antipredator behavior and the strength of the nonlethal effects of predators. Am. Nat. 171, 545–552. ( 10.1086/528961) [DOI] [PubMed] [Google Scholar]
- 106.Brooks E, Canal MM. 2013. Development of circadian rhythms: Role of postnatal light environment. Neurosci. Biobehav. Rev. 37, 551–560. ( 10.1016/j.neubiorev.2013.02.012) [DOI] [PubMed] [Google Scholar]
- 107.Simoni A, Wolfgang W, Topping MP, Kavlie RG, Stanewsky R, Albert JT. 2014. A mechanosensory pathway to the Drosophila circadian clock. Science 343, 525–528. ( 10.1126/science.1245710) [DOI] [PubMed] [Google Scholar]
- 108.Chen C, Buhl E, Xu M, Croset V, Rees JS, Lilley KS, Benton R, Hodge JJ, Stanewsky R. 2015. Drosophila ionotropic receptor 25a mediates circadian clock resetting by temperature. Nature 527, 516–520. ( 10.1038/nature16148) [DOI] [PubMed] [Google Scholar]
- 109.Fuchikawa T, Eban-Rothschild A, Nagari M, Shemesh Y, Bloch G. 2016. Potent social synchronization can override photic entrainment of circadian rhythms. Nat. Commun. 7, 11662 ( 10.1038/ncomms11662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gutman R, Dayan T, Levy O, Schubert I, Kronfeld-Schor N. 2011. The effect of the lunar cycle on fecal cortisol metabolite levels and foraging ecology of nocturnally and diurnally active spiny mice. PLoS ONE 6, e23446 ( 10.1371/journal.pone.0023446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rotics S, Dayan T, Levy O, Kronfeld-Schor N. 2011. Light masking in the field: an experiment with nocturnal and diurnal spiny mice under semi-natural field conditions. Chronobiol. Int. 28, 70–75. ( 10.3109/07420528.2010.525674) [DOI] [PubMed] [Google Scholar]
- 112.Rotics S, Dayan T, Kronfeld-Schor N. 2011. Effect of artificial night lighting on temporally partitioned spiny mice. J. Mammal. 92, 159–168. ( 10.1644/10-MAMM-A-112.1) [DOI] [Google Scholar]
- 113.Eban-Rothschild A, Bloch G. 2012. Circadian rhythms and sleep in honey bees. In Honeybee neurobiology and behavior (eds Galizia CG, Eisenhardt D, Giurfa M), pp. 31–45. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 114.Shemesh Y, Cohen M, Bloch G. 2007. Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 21, 2304–2311. [DOI] [PubMed] [Google Scholar]
- 115.Bartell PA, Gwinner E. 2005. A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J. Biol. Rhythms 20, 538–549. ( 10.1177/0748730405281826) [DOI] [PubMed] [Google Scholar]
- 116.Erkinaro E. 1969. Free-running circadian rhythm in Wood Mouse (Apodemus flavicollis Melch.) under natural light-dark-cycle. Experientia 25, 649. [DOI] [PubMed] [Google Scholar]
- 117.Tomioka K, Sakamoto M, Harui Y, Matsumoto N, Matsumoto A. 1998. Light and temperature cooperate to regulate the circadian locomotor rhythm of wild type and period mutants of Drosophila melanogaster. J. Insect. Physiol. 44, 587–596. ( 10.1016/S0022-1910(98)00046-8) [DOI] [PubMed] [Google Scholar]
- 118.Majercak J, Sidote D, Hardin PE, Edery I. 1999. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron 24, 219–230. [DOI] [PubMed] [Google Scholar]
- 119.Maguire SE, Sehgal A. 2015. Heating and cooling the Drosophila melanogaster clock. Curr. Opin. Insect Sci. 7, 71–75. ( 10.1016/j.cois.2014.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hut RA, Kronfeld-Schor N, Van der Vinne V, De la Iglesia H. 2012. In search of a temporal niche: environmental factors. In Neurobiology of circadian timing (eds Foster RG, Kalsbeek A, Merrow M, Roenneberg T). Amsterdam, Netherlands: Elsevier. [Google Scholar]
- 121.van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA. 2014. Cold and hunger induce diurnality in a nocturnal mammal. Proc. Natl Acad. Sci. USA 111, 15 256–15 260. ( 10.1073/pnas.1413135111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Castillo-Ruiz A, Paul MJ, Schwartz WJ. 2012. In search of a temporal niche: social interactions. Prog. Brain Res. 199, 267–280. ( 10.1016/b978-0-444-59427-3.00016-2) [DOI] [PubMed] [Google Scholar]
- 123.Bloch G, Herzog ED, Levine JD, Schwartz WJ. 2013. Socially synchronized circadian oscillators. Proc. R. Soc. B 280, 20130035 ( 10.1098/rspb.2013.0035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eban-Rothschild A, Belluci S, Bloch G. 2011. Maternity-related plasticity in circadian rhythms of bumble-bee queens. Proc. R. Soc. B 278, 3510–3516. ( 10.1098/rspb.2011.0579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujii S, Krishnan P, Hardin P, Amrein H. 2007. Nocturnal male sex drive in Drosophila. Curr. Biol. 17, 244–251. ( 10.1016/j.cub.2006.11.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Drent R, Both C, Green M, Madsen J, Piersma T. 2003. Pay-offs and penalties of competing migratory schedules. Oikos 103, 274–292. ( 10.1034/j.1600-0706.2003.12274.x) [DOI] [Google Scholar]
- 127.Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155. ( 10.1016/S0065-2660(08)60048-6) [DOI] [Google Scholar]
- 128.Lameris TK, Jochems F, van der Graaf AJ, Andersson M, Limpens J, Nolet BA. 2017. Forage plants of an Arctic-nesting herbivore show larger warming response in breeding than wintering grounds, potentially disrupting migration phenology. Ecol. Evol. 7, 2652–2660. ( 10.1002/ece3.2859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Helm B, Schwabl I, Gwinner E. 2009. Circannual basis of geographically distinct bird schedules. J. Exp. Biol. 212, 1259–1269. ( 10.1242/jeb.025411) [DOI] [PubMed] [Google Scholar]
- 130.Gienapp P, Hemerik L, Visser ME. 2005. A new statistical tool to predict phenology under climate change scenarios. Glob. Change Biol. 11, 600–606. [Google Scholar]
- 131.Bauer S, Gienapp P, Madsen J. 2008. The relevance of environmental conditions for departure decision changes en route in migrating geese. Ecology 89, 1953–1960. ( 10.1890/07-1101.1) [DOI] [PubMed] [Google Scholar]
- 132.Winkler DW, et al. 2014. Cues, strategies, and outcomes: how migrating vertebrates track environmental change. Mov. Ecol. 2, 10 ( 10.1186/2051-3933-2-10) [DOI] [Google Scholar]
- 133.Zann R, Morton S, Jones K, Burley N. 1995. The timing of breeding by zebra finches in relation to rainfall in Central Australia. Emu 95, 208–222. ( 10.1071/MU9950208) [DOI] [Google Scholar]
- 134.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press. [Google Scholar]
- 135.DeCoursey PJ. 1986. Light-sampling behavior in photoentrainment of a rodent circadian rhythm. J. Comp. Physiol. A 159, 161–169. ( 10.1007/BF00612299) [DOI] [PubMed] [Google Scholar]
- 136.Plard F, Gaillard J-M, Coulson T, Hewison AM, Delorme D, Warnant C, Bonenfant C. 2014. Mismatch between birth date and vegetation phenology slows the demography of Roe Deer. PLoS Biol. 12, e1001828 ( 10.1371/journal.pbio.1001828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plard F, Gaillard J-M, Bonenfant C, Hewison AM, Delorme D, Cargnelutti B, Kjellander P, Nilsen EB, Coulson T. 2013. Parturition date for a given female is highly repeatable within five roe deer populations. Biol. Lett. 9, 20120841 ( 10.1098/rsbl.2012.0841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Asch M, Salis L, Holleman LJ. M., van Lith B, Visser ME. 2013. Evolutionary response of the egg hatching date of a herbivorous insect under climate change. Nat. Clim. Change 3, 244–248. ( 10.1038/nclimate1717) [DOI] [Google Scholar]
- 139.Dainou K, Laurenty E, Mahy G, Hardy OJ, Brostaux Y, Tagg N, Doucet JL. 2012. Phenological patterns in a natural population of a tropical timber tree species, Milicia excelsa (Moraceae): Evidence of isolation by time and its interaction with feeding strategies of dispersers. Am. J. Bot. 99, 1453–1463. ( 10.3732/ajb.1200147) [DOI] [PubMed] [Google Scholar]
- 140.Salmela MJ, Greenham K, Lou P, McClung CR, Ewers BE, Weinig C. 2016. Variation in circadian rhythms is maintained among and within populations in Boechera stricta. Plant Cell Environ. 39, 1293–1303. ( 10.1111/pce.12670) [DOI] [PubMed] [Google Scholar]
- 141.Dominoni DM, Helm B, Lehmann M, Dowse HB, Partecke J. 2013. Clocks for the city: circadian differences between forest and city songbirds. Proc. R. Soc. B 280, 20130593 ( 10.1098/rspb.2013.0593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stuber EF, Baumgartner C, Dingemanse NJ, Kempenaers B, Mueller JC. 2016. Genetic correlates of individual differences in sleep behavior of free-living great tits (Parus major). G3 6, 599–607. ( 10.1534/g3.115.024216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nussey DH, Postma E, Gienapp P, Visser ME. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306. ( 10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 144.Ehret CF. 1974. The sense of time: evidence for its molecular basis in the eukaryotic gene-action system. Adv. Biol. Med. Phys. 15, 47–77. ( 10.1016/B978-0-12-005215-8.50009-7) [DOI] [PubMed] [Google Scholar]
- 145.Roenneberg T, Wirz-Justice A, Merrow M. 2003. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. J. Biol. Rhythms 18, 80–90. ( 10.1177/0748730402239679) [DOI] [PubMed] [Google Scholar]
- 146.Jones SE, et al. 2016. Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 12, e1006125 ( 10.1371/journal.pgen.1006125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A, Herzel H, Kramer A. 2008. Molecular insights into human daily behavior. Proc. Natl Acad. Sci. USA 105, 1602–1607. ( 10.1073/pnas.0707772105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Allebrandt KV, et al. 2010. CLOCK gene variants associate with sleep duration in two independent populations. Biol. Psychiatry 67, 1040–1047. ( 10.1016/j.biopsych.2009.12.026) [DOI] [PubMed] [Google Scholar]
- 149.Roff DA. 1997. Evolutionary quantitative genetics. New York, NY: Chapman & Hall. [Google Scholar]
- 150.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 151.Jablonka E, Lamb MJ. 2014. Evolution in four dimensions: genetic, epigenetic, behavioral, and symbolic variation in the history of life. Boston, MA: MIT Press. [Google Scholar]
- 152.Kacelnik A, Krebs JR. 1982. The dawn chorus in the Great tit (Parus major): proximate and ultimate causes. Behaviour 83, 287–309. ( 10.1163/156853983X00200) [DOI] [Google Scholar]
- 153.Karagicheva J, Rakhimberdiev E, Dekinga A, Brugge M, Koolhaas A, ten Horn J, Piersma T. 2016. Seasonal time keeping in a long-distance migrating shorebird. J. Biol. Rhythms 31, 509–521. ( 10.1177/0748730416655929) [DOI] [PubMed] [Google Scholar]
- 154.Pittendrigh CS, Daan S. 1976. A functional analysis of circadian pacemakers in nocturnal rodents I. the stability and lability of spontaneous frequency. J. Comp. Physiol. 106, 223–252. ( 10.1007/BF01417856) [DOI] [Google Scholar]
- 155.Lee TM, Labyak SE. 1997. Free-running rhythms and light-and dark-pulse phase response curves for diurnal Octodon degus (Rodentia). Am. J. Physiol. Regulatory Integr. Comparat. Physiol. 273, R278–R286. [DOI] [PubMed] [Google Scholar]
- 156.Helm B, Visser ME. 2010. Heritable circadian period length in a wild bird population. Proc. R. Soc. B 277, 3335–3342. ( 10.1098/rspb.2010.0871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dijk DJ, Lockley SW. 2002. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. 92, 852–862. ( 10.1152/japplphysiol.00924.2001) [DOI] [PubMed] [Google Scholar]
- 158.Roenneberg T, Daan S, Merrow M. 2003. The art of entrainment. J. Biol. Rhythms 18, 183–194. ( 10.1177/0748730403018003001) [DOI] [PubMed] [Google Scholar]
- 159.Duffy JF, Rimmer DW, Czeisler CA. 2001. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115, 895–899. ( 10.1037/0735-7044.115.4.895) [DOI] [PubMed] [Google Scholar]
- 160.Aschoff J, Wever R. 1962. Über Phasenbeziehungen zwischen biologischer Tagesperiodik und Zeitgeberperiodik. Zeitschrift für vergleichende Physiologie 46, 115–128. ( 10.1007/BF00341546) [DOI] [Google Scholar]
- 161.Fleury F, Allemand R, Vavre F, Fouillet P, Bouletreau M. 2000. Adaptive significance of a circadian clock: temporal segregation of activities reduces intrinsic competitive inferiority in Drosophila parasitoids. Proc. R. Soc. Lond. B 267, 1005–1010. ( 10.1098/rspb.2000.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nikhil KL, Abhilash L, Sharma VK. 2016. Molecular correlates of circadian clocks in fruit fly Drosophila melanogaster populations exhibiting early and late emergence chronotypes. J. Biol. Rhythms 31, 125–141. ( 10.1177/0748730415627933) [DOI] [PubMed] [Google Scholar]
- 163.Pfeffer M, Wicht H, von Gall C, Korf H.-W. 2015. Owls and Larks in mice. Front. Neurol. 6, 101 ( 10.3389/fneur.2015.00101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wicht H, Korf HW, Ackermann H, Ekhart D, Fischer C, Pfeffer M. 2014. Chronotypes and rhythm stability in mice. Chronobiol. Int. 31, 27–36. ( 10.3109/07420528.2013.820739) [DOI] [PubMed] [Google Scholar]
- 165.Spoelstra K, Wikelski M, Daan S, Loudon AS, Hau M. 2016. Natural selection against a circadian clock gene mutation in mice. Proc. Natl Acad. Sci. USA 113, 686–691. ( 10.1073/pnas.1516442113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Refinetti R, Wassmer T, Basu P, Cherukalady R, Pandey VK, Singaravel M, Giannetto C, Piccione G. 2016. Variability of behavioral chronotypes of 16 mammalian species under controlled conditions. Physiol. Behav. 161, 53–59. ( 10.1016/j.physbeh.2016.04.019) [DOI] [PubMed] [Google Scholar]
- 167.Antle MC, Silver R. 2009. Neural basis of timing and anticipatory behaviors. Eur. J. Neurosci. 30, 1643–1649. ( 10.1111/j.1460-9568.2009.06959.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mistlberger RE. 1994. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 18, 171–195. ( 10.1016/0149-7634(94)90023-X) [DOI] [PubMed] [Google Scholar]
- 169.Lu C, Thompson CB. 2012. Metabolic regulation of epigenetics. Cell Metab. 16, 9–17. ( 10.1016/j.cmet.2012.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Masri S, Sassone-Corsi P. 2013. The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat. Rev. Neurosci. 14, 69–75. ( 10.1038/nrn3393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Orozco-Solis R, Sassone-Corsi P. 2014. Epigenetic control and the circadian clock: linking metabolism to neuronal responses. Neuroscience 264, 76–87. ( 10.1016/j.neuroscience.2014.01.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Azzi A, Dallmann R, Casserly A, Rehrauer H, Patrignani A, Maier B, Kramer A, Brown SA. 2014. Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat. Neurosci. 17, 377–382. ( 10.1038/nn.3651) [DOI] [PubMed] [Google Scholar]
- 173.Stevenson TJ, Prendergast BJ. 2013. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc. Natl Acad. Sci. USA 110, 16 651–16 656. ( 10.1073/pnas.1310643110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pegoraro M, Bafna A, Davies NJ, Shuker DM, Tauber E. 2016. DNA methylation changes induced by long and short photoperiods in Nasonia. Genome Res. 26, 203–210. ( 10.1101/gr.196204.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gwinner E, Schwabl H, Schwabl-Benzinger I. 1988. Effects of food-deprivation on migratory restlessness and diurnal activity in the garden warbler Sylvia borin. Oecologia 77, 321–326. ( 10.1007/BF00378037) [DOI] [PubMed] [Google Scholar]
- 176.Challet E, Mendoza J. 2010. Metabolic and reward feeding synchronises the rhythmic brain. Cell Tissue Res. 341, 1–11. ( 10.1007/s00441-010-1001-9) [DOI] [PubMed] [Google Scholar]
- 177.Mendoza J, Angeles-Castellanos M, Escobar C. 2005. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur. J. Neurosci. 22, 2855–2862. ( 10.1111/j.1460-9568.2005.04461.x) [DOI] [PubMed] [Google Scholar]
- 178.Falcon E, McClung CA. 2009. A role for the circadian genes in drug addiction. Neuropharmacology 56(Suppl. 1), 91–96. ( 10.1016/j.neuropharm.2008.06.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dunn JD, Arimura A, Scheving LE. 1972. Effect of stress on circadian periodicity in serum LH and prolactin concentration. Endocrinology 90, 29–33. ( 10.1210/endo-90-1-29) [DOI] [PubMed] [Google Scholar]
- 180.Tornatzky W, Miczek KA. 1993. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol. Behav. 53, 983–993. ( 10.1016/0031-9384(93)90278-N) [DOI] [PubMed] [Google Scholar]
- 181.Yocum GD, Žďárek J, Joplin KH, Lee RE, Smith DC, Manter KD, Denlinger DL. 1994. Alteration of the eclosion rhythm and eclosion behavior in the flesh fly, Sarcophaga crassipalpis, by low and high temperature stress. J. Insect. Physiol. 40, 13–21. ( 10.1016/0022-1910(94)90107-4) [DOI] [Google Scholar]
- 182.Funk D, Amir S. 2000. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 866, 262–267. ( 10.1016/S0006-8993(00)02249-6) [DOI] [PubMed] [Google Scholar]
- 183.Pellman BA, Kim E, Reilly M, Kashima J, Motch O, de la Iglesia HO, Kim JJ. 2015. Time-specific fear acts as a non-photic entraining stimulus of circadian rhythms in rats. Sci. Rep. 5, 14916 ( 10.1038/srep14916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Archer SN, Oster H. 2015. How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J. Sleep Res. 24, 476–493. ( 10.1111/jsr.12307) [DOI] [PubMed] [Google Scholar]
- 185.Yamazaki S, et al. 2000. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685. ( 10.1126/science.288.5466.682) [DOI] [PubMed] [Google Scholar]
- 186.Schroeder J, Piersma T, Groen NM, Hooijmeijer JC, Kentie R, Lourenço PM, Schekkerman H, Both C. 2012. Reproductive timing and investment in relation to spring warming and advancing agricultural schedules. J. Ornithol. 153, 327–336. ( 10.1007/s10336-011-0747-5) [DOI] [Google Scholar]
- 187.Rowan W. 1938. London starlings and seasonal reproduction in birds. Proc. Zool. Soc. Lond. A 108, 51–77. ( 10.1111/j.1469-7998.1938.tb00021.x) [DOI] [Google Scholar]
- 188.Dominoni D, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 ( 10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Robert KA, Lesku JA, Partecke J, Chambers B. 2015. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc. R. Soc. B 282, 20151745 ( 10.1098/rspb.2015.1745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bennie J, Davies TW, Cruse D, Gaston KJ. 2016. Ecological effects of artificial light at night on wild plants. J. Ecol. 104, 611–620. ( 10.1111/1365-2745.12551) [DOI] [Google Scholar]
- 191.Bruning A, Holker F, Franke S, Kleiner W, Kloas W. 2016. Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Sci. Total Environ. 543, 214–222. ( 10.1016/j.scitotenv.2015.11.023) [DOI] [PubMed] [Google Scholar]
- 192.Raap T, Pinxten R, Eens M. 2015. Light pollution disrupts sleep in free-living animals. Sci. Rep. 5, 13557 ( 10.1038/srep13557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.de Jong M, Jeninga L, Ouyang JQ, van Oers K, Spoelstra K, Visser ME. 2016. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 155, 172–179. ( 10.1016/j.physbeh.2015.12.012) [DOI] [PubMed] [Google Scholar]
- 194.Ouyang JQ, de Jong M, van Grunsven RHA, Matson KD, Haussmann MF, Meerlo P, Visser ME, Spoelstra K. In press. Restless roosts: light pollution affects behavior, sleep, and physiology in a free-living songbird. Glob. Change Biol. ( 10.1111/gcb.13756) [DOI] [PubMed] [Google Scholar]
- 195.Dominoni D, Goymann W, Helm B, Partecke J. 2013. Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): implications of city life for biological time-keeping of songbirds. Front. Zool. 10, 60 ( 10.1186/1742-9994-10-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872. ( 10.1111/j.1365-2486.2007.01404.x) [DOI] [Google Scholar]
- 197.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 198.Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM. 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870. ( 10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 199.Both C, Bouwhuis S, Lessells CM, Visser ME. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83. ( 10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 200.Benton TG, Bryant DM, Cole L, Crick HQP. 2002. Linking agricultural practice to insect and bird populations: a historical study over three decades. J. Appl. Ecol. 39, 673–687. ( 10.1046/j.1365-2664.2002.00745.x) [DOI] [Google Scholar]
- 201.Newton I. 2004. The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146, 579–600. ( 10.1111/j.1474-919X.2004.00375.x) [DOI] [Google Scholar]
- 202.Hallmann CA, Foppen RPB, van Turnhout CAM, de Kroon H, Jongejans E. 2014. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511, 341–343. ( 10.1038/nature13531) [DOI] [PubMed] [Google Scholar]
- 203.Goulson D. 2013. REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. ( 10.1111/1365-2664.12111) [DOI] [Google Scholar]
- 204.Chagnon M, Kreutzweiser D, Mitchell EAD, Morrissey CA, Noome DA, Van der Sluijs JP. 2015. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 22, 119–134. ( 10.1007/s11356-014-3277-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP. 2012. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484, 371–375. ( 10.1038/nature10991) [DOI] [PubMed] [Google Scholar]
- 206.Hermann-Luibl C, Helfrich-Förster C. 2015. Clock network in Drosophila. Curr. Opin. Insect Sci. 7, 65–70. ( 10.1016/j.cois.2014.11.003) [DOI] [PubMed] [Google Scholar]
- 207.Nord A, Lehmann M, MacLeod R, McCafferty DJ, Nager RG, Nilsson J-Å, Helm B. 2016. Evaluation of two methods for minimally invasive peripheral body temperature measurement in birds. J. Avian Biol. 47, 417–427. ( 10.1111/jav.00845) [DOI] [Google Scholar]
- 208.McCafferty D, Gallon S, Nord A. 2015. Challenges of measuring body temperatures of free-ranging birds and mammals. Anim. Biotelem. 3, 33 ( 10.1186/s40317-015-0075-2) [DOI] [Google Scholar]
- 209.Lu W, Meng QJ, Tyler NJ, Stokkan KA, Loudon AS. 2010. A circadian clock is not required in an arctic mammal. Curr. Biol. 20, 533–537. ( 10.1016/j.cub.2010.01.042) [DOI] [PubMed] [Google Scholar]
- 210.Saino N, et al. 2017. Migration phenology and breeding success are predicted by methylation of a photoperiodic gene in the barn swallow. Sci. Rep. 7, 45412 ( 10.1038/srep45412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Boss J, et al. 2016. Gene expression in the brain of a migratory songbird during breeding and migration. Mov. Ecol. 4, 4 ( 10.1186/s40462-016-0069-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Watson H, Videvall E, Andersson MN, Isaksson C. 2017. Transcriptome analysis of a wild bird reveals physiological responses to the urban environment. Sci. Rep. 7, 44180 ( 10.1038/srep44180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Menegazzi P, Dalla Benetta E, Beauchamp M, Schlichting M, Steffan-Dewenter I, Helfrich-Förster C. 2017. Adaptation of circadian neuronal network to photoperiod in high-latitude European drosophilids. Curr. Biol. 27, 833–839. ( 10.1016/j.cub.2017.01.036) [DOI] [PubMed] [Google Scholar]