Abstract
Animals should time activities, such as foraging, migration and reproduction, as well as seasonal physiological adaptation, in a way that maximizes fitness. The fitness outcome of such activities depends largely on their interspecific interactions; the temporal overlap with other species determines when they should be active in order to maximize their encounters with food and to minimize their encounters with predators, competitors and parasites. To cope with the constantly changing, but predictable structure of the environment, organisms have evolved internal biological clocks, which are synchronized mainly by light, the most predictable and reliable environmental cue (but which can be masked by other variables), which enable them to anticipate and prepare for predicted changes in the timing of the species they interact with, on top of responding to them directly. Here, we review examples where the internal timing system is used to predict interspecific interactions, and how these interactions affect the internal timing system and activity patterns. We then ask how plastic these mechanisms are, how this plasticity differs between and within species and how this variability in plasticity affects interspecific interactions in a changing world, in which light, the major synchronizer of the biological clock, is no longer a reliable cue owing to the rapidly changing climate, the use of artificial light and urbanization.
This article is part of the themed issue ‘Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals’.
Keywords: temporal partitioning, biological rhythms, light pollution, global warming, urbanization
1. Introduction
Timing of specific activities has fitness consequences as being active in the same environment at different times, on the tidal, diel, lunar or seasonal scale, exposes animals to very different abiotic challenges and opportunities. These abiotic variables include not only the obvious differences between light and dark, but also differences in ambient temperatures, humidity, water level (tides) and more. These fluctuations result in a predictably rhythmic environment, which the organism has to exploit adaptively [1–3]. For example, diurnal desert mammals reduce activity during summer mid-day to avoid overheating and water loss [4], marine iguanas graze on algae in the intertidal zone during low tide [5], oystercatchers fly to tidal mudflats around the time of the expected ebb tide [6], arctic ground squirrels hibernate to avoid the freezing winter [7], birds migrate to other continents to avoid extreme conditions [8] and insects spend the unfavourable season in diapause [9]. The periodic abiotic changes in the environment, which affect the rhythmic behaviour of animals, result in a complex, rhythmic landscape of the biotic environment as well, and consequently affect interspecific interactions, which in turn may also influence the fitness consequences of timing of specific activities. Activities such as foraging, migration and reproduction, as well as seasonal physiological adaptation (e.g. thermogenesis capacity and changes in fur insulation and colour), should all be timed in a way that will maximize fitness not only in relation to the abiotic environment, but also in relation to the biotic environment, maximizing food (prey) availability, while minimizing predation risk, competition levels and parasite load.
To cope with the constantly changing, but predictable structure of the environment, organisms, from cyanobacteria to plants and animals, have evolved internal timing mechanisms termed biological clocks. These innate timing mechanisms enable the organism to anticipate and prepare for predicted changes in environmental conditions. The main synchronizer or Zeitgeber (time giver) of the internal timing system is light, on both the daily (timing of light and dark) and seasonal (day length) scales. Organisms are using this highly reliable cue for predicting the most probable timing of other changes in the environment, such as ambient temperature, food availability or interspecific interactions. Yet, other, non-photic Zeitgebers have also been described, including temperature [10] or social interactions (reviewed by [11,12]), which may interact with light conditions. The role of the internal timing system in determining activity patterns was extensively studied under laboratory conditions, but much less to under natural conditions [13].
While Zeitgebers are environmental variables that directly affect biological clocks, other environmental variables affect activity patterns without altering the clock, a process termed masking (see also [14]). The perceived daily and seasonal rhythms result from an integration between the innate, internal timing system and the influence of external variables. The direct or masking effects can be central in determining the species activity patterns. They allow the animal to respond to environmental conditions on the immediate and ecological time scales and are as important as Zeitgebers in determining activity patterns under natural conditions. For example, in many species, moonlight has a negative (lunophobic) or positive (lunophilic) masking effect, supressing and increasing activity levels during full moon nights, respectively (reviewed by [15]), extreme temperatures have negative masking (supressing activity) effect on activity levels, while comfortable temperatures have a positive effect, and increasing activity levels [16–20] and food availability can completely alter daily activity patterns [21]. Masking can also determine the activity pattern of species constantly: for example, golden spiny mice are diurnally active in their natural habitat all their life as a result of masking: individuals in the field have a diurnal activity pattern, and when they are transferred to a constant laboratory condition, they immediately start free-running with a nocturnal phase preference [17]. The relative level of the contributions of entrainment and masking in determining activity patterns may vary between species, environmental signals and processes, and in some cases, the same cue can mask a rhythm on the immediate scale and, at the same time, act as a synchronizer (e.g. the effect of light at night).
The mechanisms underlying the circadian system are well understood and were reviewed extensively (e.g. [22,23]). In short, in mammals, it is composed of a central pacemaker in the suprachiasmatic nuclei (SCN) and subsidiary clocks in nearly every body cell. The central clock is synchronized to geophysical time mainly by light perceived by intrinsically photosensitive retinal ganglion cells [23].
Seasonal timing mechanisms operate either as hourglass interval timers that need to be reset (more common in short-living species), or as self-sustaining circannual clocks (more common in long living species) [24]. The role of day length (photoperiod) in seasonal timing has been studied intensively (for review, see [24,25]). Photoperiod, the most accurate predictor of the annual phase, acts to grossly time seasonal changes by triggering, resetting and entraining internal interval timers and clocks, while other environmental cues such as food, ambient temperature and social cues adjust the rhythms continuously, either by synchronization (e.g. temperature, [10,26,27]) or by masking [24]. Photoperiod influences melatonin levels, which is produced and secreted only during the night, and therefore conveys photoperiod information to target tissues in the body. Evidence in sheep and European hamsters suggests that an autonomous circannual timing mechanism resides in thyrotrophs cells in the pars toberalis [28–32]. Like almost every cell in the body, these cells contain clock mechanisms, which interact with the melatonin signal to produce the molecular mechanism underlying seasonal timing (for review, see [25,33]).
In this paper, we will first review examples where the internal timing system is used to predict interspecific interactions, such as competition, predation, prey availability and parasitism (temporal partitioning, timing life history to prey/host availability and synchronization of phenology of different species to each other), and how these interactions affect the internal timing system and eventually activity patterns on the species or individual (chronotype) level.
The same features that make the internal timing system advantageous (relative rigidity) may turn disadvantageous when conditions change rapidly. In the second part of the review, we ask how plastic these mechanisms are, in terms of phenotypic plasticity and genetic adaptation, how this plasticity differs between and within species and how this variability in plasticity affects interspecific interactions in a changing world, in which light, the major synchronizer of the biological clock, is no longer a reliable cue owing to the rapidly changing climate, the use of artificial light and urbanization.
2. Use of the internal timing system to predict food availability, competition, predation risk and parasitism
Proving that an internal timing system is used to predict interspecific interactions such as competition, predation, prey availability and parasitism is extremely difficult. It demands following the behaviour or physiology of the species of interest using ‘natural experiments’, in which one of the interacting species no longer exists or changes its timing, or removing an interacting species under semi-natural conditions, while controlling for any proximate, external cues used as an indication of the interacting species' presence. Moreover, it could be argued that specific rhythmic patterns evolved in response to interspecific selective pressures, but activity patterns may have since become ‘fixed’ and are no longer amenable to manipulation. ‘Ghost of competition past’ is a term coined by Connell [34] to stress that interspecific competition, acting as an evolutionary force in the past, left its mark on the behaviour, distribution or morphology of species, even when there is no present-day competition between them. Later on, this term was also used for past predation [35] and even parasitism [36]. When such a phenomenon is a timed response, it may represent an innate, hard-wired, evolutionary response of the internal timing system. Such a phenomenon makes it even more difficult to prove that extant activity patterns are a consequence of interspecific interactions. Nevertheless, some experiments were able to demonstrate the role of innate, internal timing systems under natural or semi-natural conditions.
(a). Predation risk
Predation risk varies across temporal scales, including seasonal cycles [37], lunar cycles [15,38], diel cycles [39] and hourly variations [40–42]. On the temporal scale, animals can reduce risk of predation by temporal partitioning: (i) by reducing their activity when predators are active [43], (ii) by reducing their activity when predators find it easy to locate and capture prey [44–46] or (iii) by changing appearance in a way that makes it difficult for predators to detect and/or capture them [47].
There are many examples of changes in predation risk (including hunting) affecting daily activity patterns (reviewed by [2,3]). However, studies looking at the role of internal timing systems in these responses in nature are uncommon. Nevertheless, some examples exist, both in the laboratory and under natural conditions, and are described below.
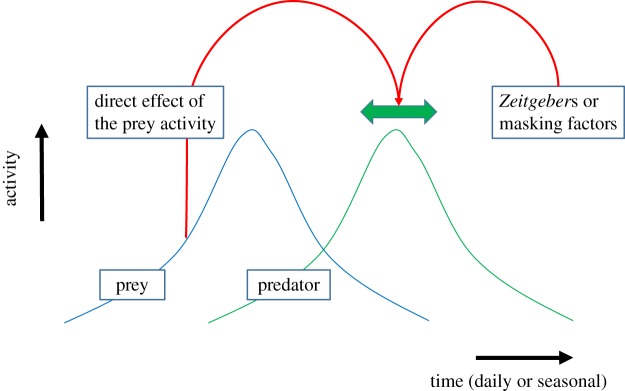
On the daily scale and under laboratory conditions, daily rhythms in anxiety-like behaviour measured using an elevated plus-maze were described in several nocturnal and diurnal rodent species [48]. In this experiment, nocturnal rodents had higher anxiety levels during the day, while in diurnal rodents an opposite rhythm or no rhythm at all was found. Melatonin, which is secreted during the night in both nocturnal and diurnal mammals, had an opposite effect in these nocturnal and diurnal species—it decreased anxiety levels in the nocturnal species and increased or had no effect on anxiety levels in the diurnal species [48]. Anxiety-like behaviour in rodents is expressed in a tendency to hide and reduce foraging and exploration behaviour [49–51]. Therefore, rhythms in anxiety are expected to influence activity patterns under natural conditions. Another study suggesting a daily rhythm in the response to the same predator cue was described in the green frog (Rana clamitans). Green toad tadpoles respond more strongly during, and for a longer time after, exposure to the same concentration of chemical cue of their predator, larval dragonflies (Anex spp.), during the day than during the night [52]. This suggests that the presence of a dragonfly larva is interpreted as an increase in predation risk during the day, when the larva is active and searching for food, and not during the night when it is inactive, and that the presence and timing of the predator can directly change (mask) activity pattern of the prey, but the magnitude of the response is clock-based (cf. figure 1). In this case, if for some reason the predator larva will change its activity pattern and become nocturnally active, and the tadpoles will not change their daily rhythm in response to its chemical cue, the cue may not be interpreted as an increased predation risk by the tadpoles, which will not respond adaptively.
Figure 1.
Predator activity patterns over the day or over the year may shift to earlier or later (thick green double-headed arrow) depending on environmental variables that affect the clock (Zeitgebers) or that alter activity patterns without altering the clock (masking factors) (thin red arrow on the right), but may also be affected directly by the activity pattern of their prey (thin red arrow on the left). A key question is whether the activity patterns of the prey directly affect the activity pattern of the predator (as a Zeitgeber or as a masking factor) as this determines the degree to which the two distributions can vary independently, for instance due to climate change or light pollution. Note that the same holds for the prey activity patterns, which may or may not be directly affected by the predator activity pattern. (Online version in colour.)
Under natural conditions, daily rhythms in vigilance, which is key for early detection of predators but involves costs in terms of other activities (e.g. foraging) [43], were described in several free-ranging angulates, including Sitka black-tailed deer (Odocoileus hemionus sitkensis) [35] and roe deer (Capreolus capreolus) [53]. Vigilance is expected to reduce in response to a decrease in perceived predation risk. Surprisingly, even in a predator-free island, Sitka black-tailed deer were much less vigilant during the night than during the day [35], which was interpreted by the authors as ‘ghost of competition past’. Such results suggest that an innate, internal timing system may be involved in anti-predator behaviours via daily rhythms in anxiety or vigilance.
In another study, which suggests that internally controlled, innate rhythms underlie ‘ghost of competition past’, radio-tagged Siberian polecats (Mustela eversmanii) and black-footed ferrets (Mustela nigripes) were released into the same ferret habitats in north America [54]. The ferrets tended to be nocturnal and most active after midnight, which is consistent with avoidance of increased predation risk by coyotes (Canis latrans) and other diurnal predators inhabiting this habitat, while Siberian polecats were not highly selective for any period of the day or night, i.e. far more active during the day, which is consistent with avoidance of nocturnal Asian red foxes (Vulpus vulpus) in their natural habitat [54]. In this case, activity patterns of ferrets and polecats did not result from a direct response to a predator presence, but appears to be a result of an expected rhythm in predation risk. Therefore, it appears to be controlled internally by an innate mechanism rather than a learned behaviour or a direct response to predation risk (masking); in accord, a similar activity pattern was documented in a wild population of ferrets [55] and in several generations of captive-bred ferrets that never encountered predators [56], which supports the presence of an innate response. The innate nature of the ferrets' activity pattern probably holds true also for the activity patterns of the polecats in the Biggins et al. study [54] and explains why the ferrets' activity pattern matched the expected predation risk rhythm in their natural habitat rather than the actual predation risk they experienced at the study site.
Experimental studies conducted on SCN-lesioned free-ranging squirrels (which become arrhythmic) support the role of internal timing system in anti-predator behaviour. A significantly higher proportion of SCN-lesioned eastern chipmunks (Tamias striatus) were killed by weasel predation during the first 80 days after animals were returned to their natural habitat compared with surgical control chipmunks or intact controls, and it was suggested that night-time restlessness of SCN-lesioned animals acted as a cue to the predator for locating its prey [57]. In antelope ground squirrels (Ammospermophilus leucurus), surface activity of SCN-lesioned animals at the food cache occurred both in daytime and at night, while controls were strongly day-active. Feral cat successfully killed 60% of the SCN-lesioned animals and 29% of the control animals in the enclosure [58].
Further support comes from a recent semi-natural, enclosures experiment, which used laboratory mice bearing a short circadian period mutation (tau mutation). These mutant mice are unable to entrain to a 24 h light–dark cycle in the laboratory. The study revealed a strong selection against the short-period mutants, which had both lower adult survival and recruitment compared with the wild type [59]. The tau mutants expressed significantly more activity during daytime, and the authors suggest that activity during the day may have led to a different predation risk between genotypes. Both nocturnal (great horned owls, Bubo virginianus) and diurnal (red-tailed hawks, Buteo jamaicensis) predators were video-recorded capturing mice during the experiment, but the extent of the ensuing mortality by these predators could not be quantified [59].
On the seasonal scale, examples showing the role of an internal biological clock in an anti-predator strategy include seasonal moult to a white or brown coat to match the presence or absence of snow in some species, including the snowshoe hare (Lepus americanus), Siberian hamster (Phodopus sungorus), long-tailed weasel (Mustela frenata) and stoat (Mustela erminea) [60], which is controlled by photoperiod. Background matching is a crypsis strategy that reduces the risk of detection by predators and should be timed to parallel the changes in snow cover [47,61]. If such a change occurred in response to the seasonal change in snow cover, it will expose animals to high risk of predation for the duration of fur colour change. By timing moult by photoperiod, animals are able to prepare for rather than respond to the changes in snow cover [47,60,61].
Predation can also act directly as a Zeitgeber of the SCN; although light is the most common Zeitgeber, other, non-photic cues, such as time-specific fear, can act as a non-photic entraining stimulus for the circadian system, timing foraging behaviour to a non-threatening part of the day [62]. A dark-phase-associated unsignalled threat (foot shock) caused rats to shift their foraging activity from the dark to the safer light phase, and this foraging pattern persisted even when both light cues and foot shocks were removed (free-running conditions). Moreover, fear entraining demanded an intact SCN and amygdala [62]. In this case, fear acted as a Zeitgeber, affecting activity pattern by influencing the internal circadian clock; therefore, the influence demanded an intact circadian clock and remained even after the Zeitgeber was removed. Nevertheless, time–place learning, the process in which animals link events with the spatial location and the time of day, which was demonstrated in many species and is influenced by timing manipulations with light or food, does not require functioning SCN and adrenal glands, as demonstrated in mice [63].
A longer term, hard-wired response to increased perceived predation risk is the response of rodents to moon-lit nights. The effect of moonlight as an indirect cue for predation risk for rodents has received considerable attention during the last decades (e.g. [64–67]). Rodents are preyed upon by owls and mammalian predators, whose predation efficiency increases during full moon nights (e.g. [46,65,66,68–70]); owl strike success rate is high [71], particularly under elevated illumination [46,65], and successful strikes are invariably lethal. As learning from experience is simply too costly, accurate perception of predation risk from owls and relevant behavioural responses may be predominantly genetically determined. Accordingly, many rodent species reduce activity or shift it to more sheltered habitats during moon-lit nights [38,46,64,72–76], in what appears to be a masking response to the increased light levels [77], which is genetically determined, because it is observed also under controlled laboratory conditions, in the absence of a predator, even in rodents who never encountered a predator during their lifetime (e.g. [78–82]).
Because anti-predator behaviour may determine the costly outcome of predator–prey interaction, it must form a strong selection pressure, and because anti-predator behaviour costs are relatively low, persistence over a long term, even when selection is relaxed, is expected to be adaptive. Timing anti-predator behaviour by the circadian system, to the expected rhythms in predation risk, should therefore be a possible underlying mechanism for such response. Such a mechanism will result in ‘ghost of predator past’ behaviour, which was documented in several field studies.
(b). Food availability
There is overwhelming evidence for cyclicity in food availability, at various temporal scales, across various taxa. Animals often respond to these food peaks, either by foraging more intensely (on hourly, daily, tidal or seasonal scales), by migrating to these sites (on seasonal time scales) or by reproducing during that period (on annual time scales). Animals can use an internal clock system to predict this variation (e.g. [83]) or use direct (food) or indirect cues (e.g. light) to prepare and respond to temporal food peaks. Here, we will briefly review responses to temporal variability in food at the scale of hours, tides, days and seasons. Since the prey itself perceives the predation risk posed by the predator and has its own rhythms, being active at different times exposes the prey and predator to different predators and prey worlds, respectively (e.g. [84,85]). Moreover, the prey can respond to the rhythms in predation risk (see above and figure 1). This results in a complex evolutionary interplay between predator and prey, food availability and predation risk.
A classic example of predator–prey interaction is the voles (Microtus arvalis) and kestrels (Falco tinnunculus) system (which is described by Helm et al. [14]). Voles are known to show peak activity at regular approximately 2 h intervals, owing to their need to feed that regularly; this is proximately regulated by an internal ‘ultradian clock system’ [86]. Kestrels, one of their main predators, anticipate this rhythmicity in food availability by adjusting their flight-hunting sessions to times of high perceived yield [87]. Ultradian rhythmicity is found in many small rodents [88], and it may be expected that the myriad of predators feeding on rodents, like the kestrel, are able to anticipate this temporal increase in food availability.
Many organisms living in tidal environments have tidal rhythms in their (foraging) activity, often in relation to tidal changes in food availability [89,90]. There is strong evidence, throughout various taxa, that this ‘circatidal’ behaviour is internally steered—however, the exact mechanism of this clock system remains unclear. For example, it has been experimentally verified in a venerid bivalve species, Austrovenus stutchburyi, that an internal clock for tidal food peaks does exist [91]. Under constant laboratory conditions, this animal opens its valves at expected times of high water—although this behaviour disappears after two tidal cycles if food is not presented during these times (free-running behaviour). Likewise, oystercatchers, Haematopus ostralegus, roosting inland behind a dyke, use some form of an internal clock to anticipate the exposure of mudflats on the other side of the dyke [6]. Tabulated high water times better predicted departure times from the roost than the actual wind-driven times of mudflat exposure. It is yet unclear what mechanism the birds use to generate tidal anticipation—at that time (1981), the paper listed five possible mechanisms, of which, as far we know, none has been refuted. Also, in a study on marine iguanas, the tide table predicted better the foraging activity than did the daily changes in the actual exposure of mudflats, again suggesting some kind of endogenous circatidal Zeitgeber [5].
Although diel rhythms in foraging activity are a very widespread phenomenon (e.g. [92,93]), there are not many studies actually showing that this is a response to daily fluctuation in food availabilities. One of the best-studied cases is the diel vertical migration of krill, which come to the water surface during the night, and the response by krill-feeding consumers [94]. Unlike this example of krill foragers responding to daily changes in availability, diel foraging patterns are often shaped by diel variations in food detectability (or variations in predation risk—see above), simply because food is just harder to see during the night [95]. At a proximate level, daily rhythmicity in foraging is not necessarily controlled by one internal central clock such as the SCN in mammals, but rather by a decentralized system of unknown oscillators [83]. For example, a recent study showed that mutant mice lacking known circadian clock functions are still able to anticipate a daily peak in food abundance [96].
Responses to food availability at the largest temporal scale of seasons are best known, with migration and reproduction as the best-studied phenomena. Especially, the problem of timing of reproduction in relation to food availability has occupied many research groups over many years, both from a proximate and from an ultimate angle. The latter was stimulated by the pioneering work of Lack [97,98]. In seasonal environments, reproduction is initiated in response to cues such as photoperiod [99] or temperature [100] as these cues predict the timing of the abundance of food. It is thus essential that such cues are predictive. In great tits (Parus major) for instance, the temperatures that correlate best with the year-to-year variation in breeding time are a predictor for the time at which caterpillars, the prey used to raise the chicks, are most abundant [101]. Recently, it has been shown, both within a species (great tit) and across taxa (birds, fish and insects), that there is an association between Clock genotype and latitude across populations, which matches latitudinal variation in breeding time (e.g. [102]), suggesting a clock-controlled timing of reproduction. However, in seasonal environments where the seasonal variation in food is not predicted by photoperiod or temperature, species may respond directly to changes in food abundance, e.g. zebra finches (Taeniopygia guttata) [103] or red crossbills (Loxia curvirostra) [104].
With respect to seasonal movements in response to changes in food availability, the best examples come from travelling herbivores which track and respond to changes in food quality and abundance across large scales [105], for example, migrant geese [106] or gazelles [107]. Changes in day length often in combination with unknown endogenous clocks control the timing of seasonal migrations [108].
(c). Competition
Temporal partitioning may facilitate coexistence between competitors through avoidance of direct confrontation (interference competition) or through the reduction of resource overlap (resource competition). For temporal partitioning to be a viable mechanism for reducing resource competition, one of the following conditions is needed:
1. The shared limiting resources differ between activity times.
2. The limiting resources are renewed within the time frame involved in the separation.
3. The spatial distribution of the perceived predation risk differs between activity times.
Interference competition and resource competition are not mutually exclusive, however; temporal partitioning may be generated by interference and yet act to reduce resource overlap, or vice versa. Many studies describe different diel activity patterns of potential competitors (reviewed in [2,3]). However, very few studies have tested the role of competition in this separation and even fewer have studied the role of biological clocks. Testing evolutionary-scale patterns is difficult, sometimes impossible, particularly for extinct taxa. However, resource overlap and its partitioning can be studied among temporally partitioned extant taxa, although this too has rarely been attempted.
A study of interference competition [109] suggesting that the temporal activity of prairie voles (Microtus ochrogaster) is internally controlled found that prairie voles, coexisting with their competitors—cotton rats (Sigmodon hispidus), are spatially segregated during a major portion of the cotton rat reproductive season, but co-occur during the rest of the year. During co-occurrence, prairie voles were primarily crepuscular and nocturnal, reducing overlap with cotton rats, but in the spatial segregation their activity was more evenly distributed between day and night. Following an accidental disappearance of cotton rats from a shared habitat, prairie voles continued to use nocturnal hours rather than daylight hours during times cotton rats would co-occur, and this was also the case in laboratory studies, where prairie voles were only sporadically active during daylight hours even in the absence of cotton rats during that period [109].
Competitive interactions were also shown to act as a synchronizer of the circadian clock and activity patterns in the laboratory: a single interaction of desert the hamster (Phodopus roborovskii) with its dominant competitor, the Mongolian gerbil (Meriones unguiculatus), resulted in a phase shift of the activity pattern, which is ecologically relevant because when occurring in nature it would shorten the interspecific contact period for the next days [110].
A well-studied model system for effect of competition on activity patterns occurs in the Judean rocky desert in Israel, where two ecologically similar congeneric species of spiny mice coexist (reviewed in [2,3]), displaying a unique temporal partitioning in activity patterns: the common spiny mouse, Acomys cahirinus, is nocturnal, as are most desert rodents, while the golden spiny mouse, Acomys russatus, is diurnally active [111]. Previous studies suggested that the golden spiny mouse was competitively excluded into diurnality by the common spiny mouse [76,112]. Under field experimental conditions, the removal of common spiny mice from the shared habitat enabled golden spiny mouse individuals to be active also during the night [112], although their activity remained largely diurnal [76]. Moreover, most golden spiny mice are nocturnal under controlled laboratory conditions (approx. 86%; [113]), and some individuals show spontaneous temporal shifts in activity between day and night and vice versa [113–115]. In disturbed areas near human settlements, where food availability is high, population densities increase, in particular those of common spiny mice. Therefore, it was suggested that food (as an energy and water source) may be limiting in this system under natural conditions [84]. In the field, both spiny mouse species overlap in their diet composition, with laboratory cafeteria experiments demonstrating a preference for arthropods [84]. In their natural habitat, more arthropods and greater arthropod biomass are available during the night at all seasons, suggesting that, in terms of resource availability, night should be the preferred activity time for spiny mice [85].
The golden spiny mouse's legacy as a nocturnal species is reflected in several morphological and physiological adaptations: a high capacity for non-shivering thermogenesis adaptive for cooler nights [116], a physiological and behavioural response to moon phase in spite of their diurnal activity [38], rod-based retinal structure suitable for night vision [117], its masking response to dark pulses [118] and an underlying internal circadian rhythm [17]. However, the species' overt activity pattern is diurnal and it has evolved dark skin pigmentation and a high concentration of ascorbic acid protecting skin and eyes from solar radiation [119]. Moreover, it shows no significant response to light pulses at night, which may reflect a mechanism enabling this species to occupy either a diurnal or a nocturnal niche in its natural habitat [77,118,120].
Since golden spiny mice remain mostly diurnal even in the absence of common spiny mice [17,76], it could be argued that diurnal activity in golden spiny mice is the ‘ghost of competition past’ [34]; diurnal activity has evolved in response to competition to the point where the species is more adapted to diurnal rather than nocturnal activity. Indeed, a biophysical model evaluating the metabolic costs of diurnal versus nocturnal activity of golden spiny mice predicted that energy expenditure during foraging is almost always lower during the day except during mid-day in summer in less sheltered microhabitats. Hence, the preferred temporal niche of this species is diurnal [121]. Thus, adaptation to diurnality may reflect the ‘ghost of competition past’.
(d). Parasitism
Parasites need to accurately time their activities in order to transmit to suitable hosts in which they can spread and develop. Likewise, hosts need to allocate resources for increasing their immune defences and avoid parasites via, for example, behavioural changes or movements.
Circadian rhythms of parasites have been accurately described according to four patterns: (i) rhythms depending on the synchronous cell division of the parasites according to a 24, 48 or 72 h pattern, for example, malaria parasites; (ii) synchronous discharge of infective forms from the host at some particular phase of day or night, for example, coccidial oocysts and pinworms; (iii) rhythms in which the same individuals migrate backwards and forwards in the body of the host according to a circadian pattern, for example, microfilariae and trypanosomes of frogs; and (iv) the migration of intestinal worms, such as Hymenolepis diminuta up and down the intestine [122].
Studies on different species of ticks provide examples of rhythmic detaching behaviour from the host resulting in enhanced parasite chances of survival and transmission. In the deer tick, Ixodes dammini, larvae and nymphs show similar synchronous diurnal timing of drop-off from their host, the white-footed mouse (Peromiscus leucopus) [123,124]. In a study where great tits (P. major) were used as host, two congeneric tick species, Ixodes arboricola and Ixodes ricinus, showed contrasting detachment strategies [125], but see [126].
Insect vector species play a dominant role in pathogen dynamics, in wild as well as in domesticated species and human-related diseases. In recent years, there has been a significant advance in the understanding of the molecular mechanisms underlying circadian rhythms in some of the prominent insect disease vectors (reviewed in [127]). Triatomine bugs (Hemiptera: Reduviidae: Triatominae), vectors of Chagas disease, feed during night by sucking blood and are inactive during day. Studies have shown that their peaks in activity, to look for host and to take shelter, are regulated by the circadian clock [128]. The sandfly Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) is the main vector of visceral leishmaniasis and in the wild is most active at dusk [129]. Also in this species, the molecular mechanisms regulating its circadian activity patterns have been described in laboratory studies [130].
The rhythms of hosts, parasites and the environment can impose temporal structure on epidemiological and evolutionary dynamics [131]. The extensive body of literature on mosquitos shows that these important vectors temporally regulate many aspects of their biological activities, such as biting time [132], resistance to insecticides [133], flight activity [134–137] and mating behaviour [137]. Moreover, recent genomic studies have also shown circadian rhythmic patterns in the gene expression in Anopheles gambiae and in Aedes aegypti [138,139]. Several of these genes were involved in metabolism, detoxification, vision, olfaction and immunity (including genes responsible for the melanization immune response, which encapsulates the Plasmodium parasites). The integration of these findings into genetic, molecular and population dynamics parasite–host interactions allows investigation of the role of circadian rhythms in malaria transmission in human populations [140].
Other experimental studies tested the consequences for the parasite being temporally mismatched to host circadian rhythms. Work by O'Donnell et al. [141] demonstrated how synchronization with host rhythms can influence in-host survival and between-host transmission potential. Mice were kept in two opposite light–dark regimes and infected with Plasmodium chabaudi. In the control treatment, the parasite rhythm matched the host rhythm, while in the focus treatment the parasite and host rhythm were mismatched. Results showed a reduction of 50% in both in-host replication and the production of transmission stages in the mismatched treatment when the mice were kept under phase-shifted conditions.
In host–parasite interactions, as well as in the other interactions discussed so far, circannual rhythms are not yet well understood and characterized as for circadian rhythms, although they are likely to play a major role in regulating phenological intraspecific interactions between host and parasites and thus strongly affecting fitness, especially for species with rigid phenological constrains. In the context of host–parasite interaction, ecological processes related to migration have been suggested to influence parasite transmission and prevalence as well as evolution of virulence [142]. In the monarch butterfly, Altizer et al. showed that prevalence of the protozoan parasite (Ophryocystis elektroscirrha) was different in three populations with different migratory incidence [143]. Further studies on this system have identified migratory culling (i.e. infected individuals are removed from the population during long-distance migration) as the mechanism via which parasite prevalence is reduced in populations with higher migration incidence [144].
3. Timing of interspecific interactions in a changing world
Two major anthropogenic rapid global changes, climate change and urbanization with the associated light pollution, may influence the finely tuned, orchestrated timing of interspecific interactions; both are altering the relative timing of species interactions and influencing which developmental stages interact with each other. Species within the same community may show variable plasticity or responses to climate change and light pollution, further contributing to the mistimed behaviours and desynchronization of species interactions. This may potentially have severe consequences for wild populations, ecosystem functioning and services (benefits to humans).
Animals can adapt to the changing environment by a genetic response to natural selection (microevolution) in either their mean trait value (mean character phenotype) or in their plasticity for the trait [145–148]. With the rapid pace at which environments change as a result of both light pollution and climate change, the more immediate solution is phenotypic plasticity. However, this is not always possible and is likely to be incomplete [149], which leads to directional selection on timing with potential consequences for population persistence (but see [150]).
(a). Global warming and timing of interspecific interactions
Environmental temperature plays an important role in timing of life-history events and activity patterns, including seasonal timing or phenology of many species. However, not all species respond to temperature at the same rate, which may result in mismatched interspecific interactions in response to climate change. For example, in response to global warming, not all organisms shift their phenology at the same rate. Especially in terrestrial species, it has been found that secondary consumers shift their phenology at only half the rate of primary producers, while the shift of primary consumers has an intermediate rate [151]. This will lead to a mismatched phenology between predators and their prey, plants and their pollinators, parasites and their hosts, and herbivores and their host plants.
Examples of such climate-change-induced phenological mismatches are now numerous. Caribou (Rangifer tarandus) in Greenland need to time the birth of their calves with the plant-growing season in order for their calves to have sufficient suitable food. Calving date advanced just 3.8 days between 1993 and 2006 (2.7 days per decade), whereas the phenology of onset of the plant-growing season advanced by 4.6 days (3.3 days per decade). Thus, caribou are getting increasingly mismatched with food availability, with consequences for reproduction: offspring production was strongly negatively correlated with trophic mismatch [152]. In a study on roe deer (C. capreolus), timing of the birth date did not shift over the 27-year study period [153] as can be expected for a species that initiates reproduction months earlier, and thus is unable to anticipate the timing of spring conditions. This failure to track environmental change resulted in a mismatch between vegetation flush and birth date, which has led to a decrease in survival of the offspring and thereby a reduction in roe deer fitness.
In birds, a well-known example is the differential shift in the laying date of great tits (P. major) and the date at which the biomass of their nestlings' food (caterpillar) peaks. The food peak shifts at about 0.7 d yr−1, whereas the lay date shifts at half that rate, leading to increased selection for earlier laying [154].
For insects, the phenological match between leaf-feeding moths and bud opening of their host plants has major fitness consequences [155]. As the phenologies of insects and plants are affected by temperatures of different periods of spring, and because climate change leads to differential warming during these different periods, there is ample opportunity for mismatching to occur. This has been shown for the winter moth (Opheroptera brumata), for which the egg hatching date has advanced more in the past 25 years than the bud burst date of the oak (Quercus robur) [156].
But not in all cases is the phenological mismatch causing reduced food availability or a decreased food quality. There can also be subtler effects such as the timing of seasonal camouflage and anti-predator behaviour. In the case of snowshoe hares described above, a decrease in snow cover persistence in recent decades has led to a mismatch between the presence of ground snow cover and seasonal colour moults. As there is almost no plasticity in moult phenology or behaviour during the autumn brown to white moult, which exposes the hares to high predation risk, future adaptation to climate change will require natural selection on moult phenology and behaviour [47].
In hibernating species such as the arctic ground squirrel, timing of hibernation is controlled by a biological clock, as evident by the fact that it free-runs under constant conditions [157]. Studies have found that the end of heterothermy in females is influenced by soil temperature and an endogenous circannual clock, but timing of male emergence from hibernation is influenced by the timing of female emergence. Males at two sites in Alaska end heterothermy on the same date in spring, but remain in their burrows while undergoing reproductive maturation. However, at one site, where snowmelt and female emergence occur relatively early, males emerge 8 days earlier than those at the other site, maintaining a 12-day period between male and female emergence found at each site, but reducing the pre-emergence euthermic period which is critical for reproductive maturation. This sensitivity in timing of male emergence to female emergence will need to be matched by phase shifts in the circannual clock and/or responsiveness to environmental factors that time the end of heterothermy, if synchrony in reproductive readiness between the sexes is to be preserved in a rapidly changing climate [158]. In a wild population of Columbian ground squirrels in Alberta, Canada, a significant delay (0.47 d yr−1, over a 20-year period) in the hibernation emergence date of adult females was found: females emerged later during years of lower spring temperature and delayed snowmelt. Although there has not been a significant annual trend in spring temperature, the date of snowmelt has become progressively later. The authors found that years of later emergence were associated with decreased individual fitness, which has resulted in a decline in population growth across the past two decades, probably as a result of reduced food availability (duration and/or amount) [159].
Pollination can also be affected by a phenological mismatch. An unusually warm spring in northern Japan led to substantial phenological advances in the flowering of several spring-ephemeral plants relative to their pollinating bees, resulting in dramatically decreased seed production of bee-pollinated species [160]. A more indirect effect of climate change on pollination is in a system of pseudo-copulation by male Andrena nigroaenea bees, which emerge slightly earlier than their females. During this interval, the males pseudo-copulate with flowers of the early spider-orchid (Ophrys sphegodesract), which gets pollinated this way. However, the flight date of the bees has advanced more due to higher temperatures than the flowering date of the orchid and with a warming of 2°C female bees will be out earlier than the orchid flowering date, leading to a reduction in the frequency of pseudo-copulation and thus pollination success rate [161].
Changing thermal regimes have also been shown to impact host–parasite interactions [162–164]. At community level, coexistence of species may be influenced via: (i) developmental time within the host becoming too short or too long in relation to the host developmental time itself; (ii) timing of host availability to the parasitoids shifting earlier or later in the season; as well as (iii) new competition arising due to phenological changes in temporal niches that were previously well differentiated [165,166]. In aquatic ecosystems, studies show increasing temperatures to influence life cycle dynamics via changes in temperature-induced parasite development [167,168]. Faster development in heteroxenous parasite (i.e. with more than one kind of host) has, in fact, been suggested to have implications for the number of life cycles that could be completed per year, and consequently the number of hosts that are likely to be exposed to the parasite [164]. Moreover, as global change is affecting migratory routes and dynamics, it is likely that these changes will also influence host–pathogen interactions and relative transmission. For example, the decrease in the number of migrating individuals in response to human-induced changes [169–172] has resulted in an increased infection risk [173].
Note, however, that although in all these examples in which climate change affects the seasonal timing we have no idea whether this is due to a shift in the underlying seasonal clock or whether these are masking effects. Circadian clocks are buffered against temperature variations [174]. However, at least in some cases, temperature interacts with photoperiod by changing the sensitivity of the seasonal clocks to photoperiod [10,27,175]. Therefore, temperature may affect seasonal timing via masking or by interacting with the seasonal clock. It is not clear whether there are other Zeitgebers for the circannual clock. However, it could well be that the degree of masking by temperature depends on the entrained clock [14]: this would explain the observed interaction between photoperiod and temperature in the effect on seasonal timing [176]. In any case, the different magnitude of the response, if any, of different species to climate change is expected to influence interspecific interactions.
(b). Light pollution and interspecific interactions
With the increasing use of artificial light, light is no more a predictable and reliable cue as it was during most of the evolutionary history of life on Earth. Light at night is prevalent, and its effects on organisms are accumulating at an increasing rate.
Many diurnal species have extended their foraging activity into the night in artificially illuminated areas. Waders, such as the common redshank (Tringa tetanus), forage at night in intertidal zones when these are illuminated by streetlights [177,178]. The birds in the area illuminated by lighting from a large petrochemical complex showed visually based foraging behaviour whereas birds in unlit areas used tactile foraging. Many prey species are more susceptible to predation under strong illumination [179]. Thus, the interaction between the different species, especially the visually foraging individuals in the ecological community, may be affected by artificial illumination.
Light at night can also disrupt the diel vertical migration of Daphnia retrocurva. Studies found that Daphnia had a smaller amplitude in their migration due to sky glow. This led to a change in the temporal overlap with their food, and this in turn reduced algal grazing which can potentially contribute to enhanced algal biomass in lakes near urban areas [180]. Light at night may also affect the pollination rate of plants pollinated by nocturnal insects, not only because these pollinators may suffer increased mortality rates due to their attraction to light [181], but also because these nocturnal insects alter their activity patterns over the 24 h cycle, which leads to lower pollination rates [182]. Something similar may occur in mosquitoes (A. gambiae), for which light was found to suppress flight, which will then affect the infection risks for their hosts [135].
A study of the effect of light pollution on a rocky desert community, focusing on two spiny mouse congeners, the nocturnal A. cahirinus (common spiny mouse) and the diurnal A. russatus (golden spiny mouse), found that A. cahirinus decreased activity and foraging with artificial lighting, restricting movement particularly in less-sheltered microhabitats, probably because of increased perceived predation risk. Because illumination restricted both activity time and space, intraspecific encounters of A. cahirinus over foraging patches increased during and following the illuminated hours. Light pollution had a negative influence by reducing overall activity and producing a relatively underexploited temporal niche, which may promote invasion of alien species that are less light sensitive, and by increasing intraspecific overlap in foraging A. cahirinus [120]. These shifts in activity patterns are likely due to masking rather than a phase shift in the circadian clock as despite the light at night there is still a very clear light signal (lux levels at night 1–10 lux but in daytime 10 000–100 000 lux). Moreover, a field experiment testing the effect of artificial light on activity in common spiny mice found a clear masking response [77].
Effects of light at night on seasonal timing are very rarely reported. In an experimental study on light at night, great tits bred earlier when their breeding site was in an illuminated area, but only did so in cold and late springs [183]. The effect of such shifts on predator–prey interaction is unclear. The date of bud burst in four species of deciduous trees in a UK dataset was correlated with light levels at night: bud burst occurred up to 7.5 days earlier in brighter areas [184]. It is unclear what are the effects of this shift on herbivore–tree interactions, but as timing of herbivore emergence and bud burst is under strong selection [155], there may be severe knock-on effects.
(c). Urbanization and interspecific interactions
A study of medium- and large-sized carnivores across a gradient of urbanization found that urbanization increased the opportunity for interspecific interactions between wild felids: in wildland habitats, bobcats (Lynx rufus) avoided using areas for short temporal periods after a puma (Puma concolor) visited an area. By contrast, bobcats did not appear to avoid areas that pumas lately visited in areas recently influenced by urbanization, and the overlap in circadian activity patterns between bobcats and pumas was higher in these areas [185]. The authors suggest that in urbanized areas, the animals are more active at night to avoid human disturbances during the day, leading to a greater temporal overlap.
4. Discussion
The temporal overlap, be it on a daily or a seasonal scale, of competitors, predators and their prey, parasites and their hosts or pollinators and their plants, directly determines the strength of their interspecific interaction. Key questions are whether the activity distributions of these interacting species are affected by the same environmental variables (either as a Zeitgeber or as a masking factor) and whether their sensitivity to these variables is the same or not. Given that the two interacting species are often very different (vertebrate versus invertebrate), it seems unlikely that this is the case. This will then lead to variation in the strength of interspecific interactions. However, a direct effect of the activity pattern of one species on the other (cf. figure 1) may dampen this variation.
As we have shown in this paper, interspecific interactions may form a strong selective force. In many cases, merely responding to the interacting species may be too costly (e.g. in the case of increased predation risk), or even impossible (e.g. timing of reproduction, which should be initiated well before peak time in food availability for the offspring). In these cases, animals are using other environmental cues, which can synchronize the biological clock used to predict the interspecific interaction (e.g. photoperiod in circannual rhythms) or mask the biological clock (e.g. the effect of ambient temperature on trees budding or the effect of increased light levels on activity in nocturnal animals). In a changing world, the association between these environmental variables and the interspecific interaction they cue, may dissociate and the cue become unreliable, affecting interspecific interactions. Moreover, because the mechanisms underlying the response and sensitivity of different species to climate change and light at night may differ, there is ample scope for the activity patterns of the interacting species to differentially shift on a seasonal, tidal or a diel scale. Whether this is likely depends on whether the activity pattern of one is a cue for the other. But when the environmental variables are merely predictors of the activity patterns of the interacting species, then environmental changes are likely to disrupt the predictive value of these cues and the activity patterns of the interacting species are likely to shift at different rates. Understanding the influences of such changes demands conducting controlled field experiments, in which the animals function in a complex ecological community, exposed to a complex cycling environment, in combination with laboratory experiments, looking at the mechanisms underlying these effects.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Hut R, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. 2012. In search of a temporal niche: environmental factors. Prog. Brain Res. 199, 281–304. ( 10.1016/B978-0-444-59427-3.00017-4) [DOI] [PubMed] [Google Scholar]
- 2.Kronfeld-Schor N, Dayan T. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 153–181. ( 10.1146/annurev.ecolsys.34.011802.132435) [DOI] [Google Scholar]
- 3.Kronfeld-Schor N, Dayan T. 2008. Activity patterns of rodents: the physiological ecology of biological rhythms. Biol. Rhythm Res. 39, 193–211. ( 10.1080/09291010701683268) [DOI] [Google Scholar]
- 4.Levy O, Dayan T, Porter WP, Kronfeld-Schor N, Mooij WM, Bronstein JL. 2016. Foraging activity pattern is shaped by water loss rates in a diurnal desert rodent. Am. Nat. 188, 205–218. ( 10.1086/687246) [DOI] [PubMed] [Google Scholar]
- 5.Wikelski M, Hau M. 1995. Is there an endogenous tidal foraging rhythm in marine iguanas? J. Biol. Rhythms 10, 335–350. ( 10.1177/074873049501000407) [DOI] [PubMed] [Google Scholar]
- 6.Daan S, Koene P. 1981. On the timing of foraging flights by oystercatchers, Haematopus ostralegus, on tidal mudflats. Neth. J. Sea Res. 15, 1–22. ( 10.1016/0077-7579(81)90002-8) [DOI] [Google Scholar]
- 7.Buck CL, Barnes BM. 1999. Annual cycle of body composition and hibernation in free-living arctic ground squirrels. J. Mammal. 80, 430–442. ( 10.2307/1383291) [DOI] [Google Scholar]
- 8.Cotton PA. 2003. Avian migration phenology and global climate change. Proc. Natl Acad. Sci. USA 100, 12 219–12 222. ( 10.1073/pnas.1930548100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauber MJ, Tauber CA, Masaki S. 1986. Seasonal adaptations of insects. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Steinlechner S, Stieglitz A, Ruf T, Heldmaier G, Reiter RJ. 1991. Integration of environmental signals by the pineal gland and its significance for seasonality in small mammals. In Role of melatonin and pineal peptides in neuroimmunomodulation. NATO ASI Series A Life Sciences, vol. 204 (eds Fraschini F, Reiter RJ), pp. 159–163. Boston, MA: Springer; ( 10.1007/978-1-4615-3756-4_16) [DOI] [Google Scholar]
- 11.Castillo-Ruiz A, Paul MJ, Schwartz WJ. 2012. In search of a temporal niche: social interactions. Prog. Brain Res. 199, 267–280. (doi:101016/B978-0-444-59427-300016-2) [DOI] [PubMed] [Google Scholar]
- 12.Mistlberger RE, Skene DJ. 2004. Social influences on mammalian circadian rhythms: animal and human studies. Biol. Rev. 79, 533–556. ( 10.1017/S1464793103006353) [DOI] [PubMed] [Google Scholar]
- 13.Kronfeld-Schor N, Bloch G, Schwartz WJ. 2013. Animal clocks: when science meets nature. Proc. R. Soc. B 280, 20131354 ( 10.1098/rspb.2013.1354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helm B, Visser ME, Schwartz W, Kronfeld-Schor N, Gerkema M, Piersma T, Bloch G. 2017. Two sides of a coin: ecological and chronobiological perspectives of timing in the wild. Phil. Trans. R. Soc. B 372, 20160246 ( 10.1098/rstb.2016.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronfeld-Schor N, Dominoni D, de la Iglesia H, Levy O, Herzog ED, Dayan T, Helfrich-Forster C. 2013. Chronobiology by moonlight. Proc. R. Soc. B 280, 20123088 ( 10.1098/rspb.2012.3088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daan S, et al. 2011. Lab mice in the field: unorthodox daily activity and effects of a dysfunctional circadian clock allele. J. Biol. Rhythms 26, 118–129. ( 10.1177/0748730410397645) [DOI] [PubMed] [Google Scholar]
- 17.Levy O, Dayan T, Kronfeld-Schor N. 2007. The relationship between the golden spiny mouse circadian system and its diurnal activity: an experimental field enclosures and laboratory study. Chronobiol. Int. 24, 599–613. ( 10.1080/07420520701534640) [DOI] [PubMed] [Google Scholar]
- 18.Lourens S, Nel JAJ. 1990. Winter activity of bat-eared foxes Otocyon megalotis on the Cape west-coast. S. Afr. J. Zool. 25, 124–132. ( 10.1080/02541858.1990.11448200) [DOI] [Google Scholar]
- 19.Rowsemitt CN, Petterborg LJ, Claypool LE, Hoppensteadt FC, Negus NC, Berger PJ. 1982. Photoperiodic induction of diurnal locomotor-activity in Microtus montanus, the montane vole. Can. J. Zool. 60, 2798–2803. ( 10.1139/z82-358) [DOI] [Google Scholar]
- 20.Whitford WG, Depree DJ, Hamilton P, Ettershank G. 1981. Foraging ecology of seed-harvesting ants, Pheidole spp. in a Chihuauan desert ecosystem. Am. Midl. Nat. 105, 159–167. ( 10.2307/2425021) [DOI] [Google Scholar]
- 21.van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA.. 2014. Cold and hunger induce diurnality in a nocturnal mammal. Proc. Natl Acad. Sci. USA 111, 15 256–15 260. ( 10.1073/pnas.1413135111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brancaccio M, Enoki R, Mazuski CN, Jones J, Evans JA, Azzi A. 2014. Network-mediated encoding of circadian time: the suprachiasmatic nucleus (SCN) from genes to neurons to circuits, and back. J. Neurosci. 34, 15 192–15 199. ( 10.1523/JNEUROSCI.3233-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dibner C, Schibler U, Albrecht U. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549. ( 10.1146/annurev-physiol-021909-135821) [DOI] [PubMed] [Google Scholar]
- 24.Paul MJ, Zucker I, Schwartz WJ. 2008. Tracking the seasons: the internal calendars of vertebrates. Phil. Trans. R. Soc. B 363, 341–361. ( 10.1098/rstb.2007.2143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helm B, Ben-Schlomo R, Sheriff M, Hut RA, Foster R, Barnes BM, Dominoni DM. 2013. Annual cycles and phenology: biological time-keeping meets environmental change. Proc. R. Soc. B 280, 20130016 ( 10.1098/rspb.2013.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caro SP, Schaper SV, Hut RA, Ball GF, Visser ME. 2013. The case of the missing mechanism: how does temperature influence seasonal timing in endotherms? PLoS Biol. 11, e1001517 ( 10.1371/journal.pbio.1001517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriegsfeld LJ, Ranalli NJ, Bober MA, Nelson RJ. 2000. Photoperiod and temperature interact to affect the GnRH neuronal system of male prairie voles (Microtus ochrogaster). J. Biol. Rhythms 15, 306–316. ( 10.1177/074873000129001413) [DOI] [PubMed] [Google Scholar]
- 28.Lincoln GA, Clarke IJ, Hut RA, Hazlerigg DG. 2006. Characterizing a mammalian circannual pacemaker. Science 314, 1941–1944. ( 10.1126/science.1132009) [DOI] [PubMed] [Google Scholar]
- 29.de Miera CS, Hanon EA, Dardente H, Birnie M, Simonneaux V, Lincoln GA, Hazlerigg DG. 2013. Circannual variation in thyroid hormone deiodinases in a short-day breeder. J. Neuroendocrinol. 25, 412–421. ( 10.1111/jne.12013) [DOI] [PubMed] [Google Scholar]
- 30.de Miera CS, Monecke S, Bartzen-Sprauer J, Laran-Chich M-P, Pevet P, Hazlerigg DG, Simonneaux V. 2014. A circannual clock drives expression of genes central for seasonal reproduction. Curr. Biol. 24, 1500–1506. ( 10.1016/j.cub.2014.05.024) [DOI] [PubMed] [Google Scholar]
- 31.Hut RA, Dardente H, Riede SJ. 2014. Seasonal timing: how does a hibernator know when to stop hibernating? Curr. Biol. 24, R602–R605. ( 10.1016/j.cub.2014.05.061) [DOI] [PubMed] [Google Scholar]
- 32.Wood SH, et al. 2015. Binary switching of calendar cells in the pituitary defines the phase of the circannual cycle in mammals. Curr. Biol. 25, 2651–2662. ( 10.1016/j.cub.2015.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dardente H. 2015. Circannual biology: the double life of the seasonal thyrotroph. Curr. Biol. 25, R988–R991. ( 10.1016/j.cub.2015.09.002) [DOI] [PubMed] [Google Scholar]
- 34.Connell JH. 1980. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35, 131–138. ( 10.2307/3544421) [DOI] [Google Scholar]
- 35.Le Saout S, Martin J-L, Blanchard P, Cebe N, Hewison AJM, Rames J-L, Chamaillé-Jammes S, Manser M. et al. 2015. Seeing a ghost? Vigilance and its drivers in a predator-free world. Ethology 121, 651–660. ( 10.1111/eth.12377) [DOI] [Google Scholar]
- 36.Mooring MS, Hart BL, Fitzpatrick TA, Reisig DD, Nishihira TT, Fraser IC, Benjamin JE. 2006. Grooming in desert bighorn sheep (Ovis canadensis mexicana) and the ghost of parasites past. Behav. Ecol. 17, 364–371. ( 10.1093/beheco/arj039) [DOI] [Google Scholar]
- 37.Creel S, Winnie JA Jr, Christianson D, Liley S. 2008. Time and space in general models of antipredator response: tests with wolves and elk. Anim. Behav. 76, 1139–1146. ( 10.1016/j.anbehav.2008.07.006) [DOI] [Google Scholar]
- 38.Gutman R, Dayan T, Levy O, Schubert I, Kronfeld-Schor N, Yamazaki S. 2011. The effect of the lunar cycle on fecal cortisol metabolite levels and foraging ecology of nocturnally and diurnally active spiny mice. PLoS ONE 6, e23446 ( 10.1371/journal.pone.0023446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones M, Dayan T. 2000. Foraging behavior and microhabitat use by spiny mice, Acomys cahirinus and A. russatus, in the presence of Blanford's fox (Vulpes cana) odor. J. Chem. Ecol. 26, 455–469. ( 10.1023/A:1005417707588) [DOI] [Google Scholar]
- 40.Jedrzejewska B, Jedrzejewski W. 1990. Antipredatory behaviour of bank voles and prey choice of weasels—enclosure experiments. Ann. Zool. Fenn. 27, 321–328. [Google Scholar]
- 41.Fenn MGP, Macdonald DW. 1995. Use of middens by red foxes—risk reverses rhythms of rats. J. Mammol. 76, 130–136. ( 10.2307/1382321) [DOI] [Google Scholar]
- 42.Hanski I, Henttonen H, Korpimaki E, Oksanen L, Turchin P. 2001. Small-rodent dynamics and predation. Ecology 82, 1505–1520. ( 10.1890/0012-9658(2001)082%5B1505:SRDAP%5D2.0.CO;2) [DOI] [Google Scholar]
- 43.Lima SL. 1998. Nonlethal effects in the ecology of predator-prey interactions. What are the ecological effects of anti-predator decision-making? BioScience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 44.Bouskila A. 1995. Interactions between predation risk and competition—a field-study of kangaroo rats and snakes. Ecology 76, 165–178. ( 10.2307/1940639) [DOI] [Google Scholar]
- 45.Campbell SR, Mackessy SP, Clarke JA. 2008. Microhabitat use by brown treesnakes (Boiga irregularis): effects of moonlight and prey. J. Herpetol. 42, 246–250. ( 10.1670/07-0681.1) [DOI] [Google Scholar]
- 46.Clarke JA. 1983. Moonlight's influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behav. Ecol. Sociobiol. 13, 205–209. ( 10.1007/BF00299924) [DOI] [Google Scholar]
- 47.Zimova M, Mills LS, Lukacs PM, Mitchell MS. 2014. Snowshoe hares display limited phenotypic plasticity to mismatch in seasonal camouflage. Proc. R. Soc. B 281, 20140029 ( 10.1098/rspb.2014.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bilu C, Kronfeld-Schor N. 2013. Effect of circadian phase and melatonin injection on anxiety-like behavior in nocturnal and diurnal rodents. Chronobiol. Int. 30, 828–836. ( 10.3109/07420528.2013.773439) [DOI] [PubMed] [Google Scholar]
- 49.Lister RG. 1987. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92, 180–185. ( 10.1007/BF00177912) [DOI] [PubMed] [Google Scholar]
- 50.Pellow S, Chopin P, File SE, Briley M. 1985. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167. ( 10.1016/0165-0270(85)90031-7) [DOI] [PubMed] [Google Scholar]
- 51.Pellow S, File SE. 1986. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 24, 525–529. ( 10.1016/0091-3057(86)90552-6) [DOI] [PubMed] [Google Scholar]
- 52.Fraker ME. 2008. The influence of the circadian rhythm of green frog (Rana clamitans) tadpoles on their antipredator behavior and the strength of the nonlethal effects of predators. Am. Nat. 171, 545–552. ( 10.1086/528961) [DOI] [PubMed] [Google Scholar]
- 53.Sönnichsen L, Bokje M, Marchal J, Hofer H, Jędrzejewska B, Kramer-Schadt S, Ortmann S, Janik V. 2013. Behavioural responses of European roe deer to temporal variation in predation risk. Ethology 119, 233–243. ( 10.1111/eth.12057) [DOI] [Google Scholar]
- 54.Biggins DE, Hanebury LR, Miller BJ, Powell RA. 2011. Black-footed ferrets and Siberian polecats as ecological surrogates and ecological equivalents. J. Mammol. 92, 710–720. ( 10.1644/10-MAMM-S-110.1) [DOI] [Google Scholar]
- 55.Biggins DE, Schroeder MH, Forrest SC, Richardson L. 1986. Activity of radio-tagged black-footed ferrets. Great Basin Nat. Mem. 8, 135–140. [Google Scholar]
- 56.Vargas A, Anderson SH. 1998. Black-footed ferret (Mustela nigripes) behavioral development: aboveground activity and juvenile play. J. Ethol. 16, 29–41. ( 10.1007/BF02896351) [DOI] [Google Scholar]
- 57.DeCoursey PJ, Walker JK, Smith SA. 2000. A circadian pacemaker in free-living chipmunks: essential for survival? J. Comp. Physiol. A 186, 169–180. ( 10.1007/s003590050017) [DOI] [PubMed] [Google Scholar]
- 58.DeCoursey PJ, Krulas JR, Mele G, Holley DC. 1997. Circadian performance of suprachiasmatic nuclei (SCN)-lesioned antelope ground squirrels in a desert enclosure. Physiol. Behav. 62, 1099–1108. ( 10.1016/S0031-9384(97)00263-1) [DOI] [PubMed] [Google Scholar]
- 59.Spoelstra K, Wikelski M, Daan S, Loudon AS, Hau M. 2016. Natural selection against a circadian clock gene mutation in mice. Proc. Natl Acad. Sci. USA 113, 686–691. ( 10.1073/pnas.1516442113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bissonnette TH, Bailey EE. 1944. Experimental modification and control of molts and changes in coat-color in weasels by controlled lighting. Ann. NY Acad. Sci. 45, 221–260. ( 10.1111/j.1749-6632.1944.tb47953.x) [DOI] [Google Scholar]
- 61.Kauffman AS, Cabrera A, Zucker I. 2001. Energy intake and fur in summer- and winter-acclimated Siberian hamsters (Phodopus sungorus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R519–R527. [DOI] [PubMed] [Google Scholar]
- 62.Pellman BA, Kim E, Reilly M, Kashima J, Motch O, de la Iglesia HO, Kim JJ. et al. 2015. Time-specific fear acts as a non-photic entraining stimulus of circadian rhythms in rats. Sci. Rep. 5, 14916 ( 10.1038/srep14916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mulder CK, Papantoniou C, Gerkema MP, Van Der Zee EA. 2014. Neither the SCN nor the adrenals are required for circadian time-place learning in mice. Chronobiol. Int. 31, 1075–1092. ( 10.3109/07420528.2014.944975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotler BP, Brown JS, Hasson O. 1991. Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72, 2249–2260. ( 10.2307/1941575) [DOI] [Google Scholar]
- 65.Longland WS, Price MV. 1991. Direct observations of owls and heteromyid rodents; can predation risk explain microhabitat use? Ecology 72, 2261–2273. ( 10.2307/1941576) [DOI] [Google Scholar]
- 66.Daly M, Behrends PR, Wilson MI, Jacobs LF. 1992. Behavioral modulation of predation risk—moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami. Anim. Behav. 44, 1–9. ( 10.1016/S0003-3472(05)80748-1) [DOI] [Google Scholar]
- 67.Price MV, Waser NM, Bass TA. 1984. Effects of moonlight on microhabitat use by desert rodents. J. Mammol. 65, 353–356. ( 10.2307/1381183) [DOI] [Google Scholar]
- 68.Theuerkauf J, Jedrzejewski W, Schmidt K, Okarma H, Ruczynski I, Sniezko S, Gula R. 2003. Daily patterns and duration of wolf activity in the Białowieża Forest, Poland. J. Mammol. 84, 243–253. ( 10.1644/1545-1542(2003)084%3C0243:DPADOW%3E2.0.CO;2) [DOI] [Google Scholar]
- 69.Griffin PC, Griffin SC, Waroquiers C, Mills LS. 2005. Mortality by moonlight: predation risk and the snowshoe hare. Behav. Ecol. 16, 938–944. ( 10.1093/beheco/ari074) [DOI] [Google Scholar]
- 70.Eggermann J, Gula R, Pirga B, Theuerkauf J, Tsunoda H, Brzezowska B, Rouys S, Radler S. et al. 2009. Daily and seasonal variation in wolf activity in the Bieszczady Mountains, SE Poland. Mamm. Biol. 74, 159–163. ( 10.1016/j.mambio.2008.05.010) [DOI] [Google Scholar]
- 71.Curio E. 1976. The ethology of predation. Berlin, Germany: Springer. [Google Scholar]
- 72.Jones M, Mandelik Y, Dayan T. 2001. Coexistence of temporally partitioned spiny mice: roles of habitat structure and foraging behavior. Ecology 82, 2164–2176. ( 10.1890/0012-9658(2001)082%5B2164:COTPSM%5D2.0.CO;2) [DOI] [Google Scholar]
- 73.Bowers MA. 1988. Seed removal experiments on desert rodents: the microhabitat by moonlight effect. J. Mammal. 69, 201–204. ( 10.2307/1381778) [DOI] [Google Scholar]
- 74.Vasquez RA. 1994. Assessment of predation risk via illumination level—facultative central place foraging in the cricetid rodent Phyllotis darwini. Behav. Ecol. Sociobiol. 34, 375–381. ( 10.1007/BF00197008) [DOI] [Google Scholar]
- 75.Topping MG, Millar JS, Goddard JA. 1999. The effects of moonlight on nocturnal activity in bushy-tailed wood rats (Neotoma cinerea). Can. J. Zool. 77, 480–485. ( 10.1139/z99-006) [DOI] [Google Scholar]
- 76.Gutman R, Dayan T. 2005. Temporal partitioning: an experiment with two species of spiny mice. Ecology 86, 164–173. ( 10.1890/03-0369) [DOI] [Google Scholar]
- 77.Rotics S, Dayan T, Levy O, Kronfeld-Schor N. 2011. Light masking in the field: an experiment with nocturnal and diurnal spiny mice under semi-natural field conditions. Chronobiol. Int. 28, 70–75. ( 10.3109/07420528.2010.525674) [DOI] [PubMed] [Google Scholar]
- 78.Kronfeld-Schor N, Visser ME, Salis L, van Gils JA. 2017. Chronobiology of interspecific interactions in a changing world. Phil. Trans. R. Soc. B 372, 20160248 ( 10.1098/rstb.2016.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eilam D, Dayan T, Ben-Eliyahu S, Schulman I, Shefer G, Hendrie CA. 1999. Differential behavioural and hormonal responses of voles and spiny mice to owl calls. Anim. Behav. 58, 1085–1093. ( 10.1006/anbe.1999.1224) [DOI] [PubMed] [Google Scholar]
- 80.Hendrie CA, Weiss SM, Eilam D. 1996. Exploration and predation models of anxiety: evidence from laboratory and wild species. Pharmacol. Biochem. Behav. 54, 13–20. ( 10.1016/0091-3057(95)02176-0) [DOI] [PubMed] [Google Scholar]
- 81.Hendrie CA, Weiss SM, Eilam D. 1998. Behavioral response of wild rodents to the calls of an owl: a comparative study. J. Zool. 254, 439–446. ( 10.1111/j.1469-7998.1998.tb00118.x) [DOI] [Google Scholar]
- 82.Mandelik Y, Dayan T, Eilam D. 2000. Foraging activity of Acomys cahirinus under different illumination levels: comparing giving-up densities to direct behavioral observations. Isr. J. Zool. 46, 167–168. [Google Scholar]
- 83.Mistlberger RE. 2011. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 104, 535–545. ( 10.1016/j.physbeh.2011.04.015) [DOI] [PubMed] [Google Scholar]
- 84.Kronfeld-Schor N, Dayan T. 1999. The dietary basis for temporal partitioning: food habits of coexisting Acomys species. Oecologia 121, 123–128. ( 10.1007/s004420050913) [DOI] [PubMed] [Google Scholar]
- 85.Vonshak M, Dayan T, Kronfeld-Schor N. 2009. Arthropods as a prey resource: patterns of diel, seasonal, and spatial availability. J. Arid Environ. 73, 458–462. ( 10.1016/j.jaridenv.2008.11.013) [DOI] [Google Scholar]
- 86.Gerkema MP, van der Leest F. 1991. Ongoing ultradian activity rhythms in the common vole, Microtus arvalis, during deprivations of food, water and rest. J. Comp. Physiol. A 168, 591–597. ( 10.1007/BF00215081) [DOI] [PubMed] [Google Scholar]
- 87.Rijnsdorp A, Daan S, Dijkstra C. 1981. Hunting in the kestrel, Falco tinnunculus, and the adaptive significance of daily habits. Oecologia 50, 391–406. ( 10.1007/BF00344982) [DOI] [PubMed] [Google Scholar]
- 88.Buttner D, Muschen U. 1981. Comparative study on the ultradian rhythm of small rodents. Dtsch. Tierarztl. Wochenschr. 88, 148. [Google Scholar]
- 89.Palmer JD. 1995. The biological rhythms and clocks of intertidal animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 90.Naylor E. (ed.) 2010. Chronobiology of marine organisms. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 91.Williams BG, Pilditch CA. 1997. The entrainment of persistent tidal rhythmicity in a filter-feeding bivalve using cycles of food availability. J. Biol. Rhythms 12, 173–181. ( 10.1177/074873049701200208) [DOI] [PubMed] [Google Scholar]
- 92.Reebs SG. 2002. Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 12, 349–371. ( 10.1023/A:1025371804611) [DOI] [Google Scholar]
- 93.McNeil R, Drapeau P, Gosscustard JD. 1992. The occurrence and adaptive significance of nocturnal habits in waterfowl. Biol. Rev. Camb. Philos. Soc. 67, 381–419. ( 10.1111/j.1469-185X.1992.tb01188.x) [DOI] [Google Scholar]
- 94.Blanchet MA, Biuw M, Hofmeyr GJG, de Bruyn PJN, Lydersen C, Kovacs KM. 2013. At-sea behaviour of three krill predators breeding at Bouvetøya—Antarctic fur seals, macaroni penguins and chinstrap penguins. Mar. Ecol. Prog. Ser. 477, 285–302. ( 10.3354/meps10110) [DOI] [Google Scholar]
- 95.Maccarone AD, Hamilton BL. 2014. Diurnal and nocturnal foraging activity by black-crowned night-herons (Nycticorax nycticorax) at an artificial weir. Waterbirds 37, 220–224. ( 10.1675/063.037.0211) [DOI] [Google Scholar]
- 96.Storch KF, Weitz CJ. 2009. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc. Natl Acad. Sci. USA 106, 6808–6813. ( 10.1073/pnas.0902063106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lack D. 1954. The natural regulation of animal numbers. Oxford, UK: Oxford University Press. [Google Scholar]
- 98.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen. [Google Scholar]
- 99.Gwinner E. 1989. Photoperiod as a modifying and limiting factor in the expression of avian circannual rhythms. J. Biol. Rhythms 4, 125–138. ( 10.1177/074873048900400210) [DOI] [PubMed] [Google Scholar]
- 100.Schaper SV, Dawson A, Sharp PJ, Gienapp P, Caro SP, Visser ME. 2012. Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. Am. Nat. 179, E55–E69. ( 10.1086/663675) [DOI] [PubMed] [Google Scholar]
- 101.Visser ME, Holleman LJ, Gienapp P. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172. ( 10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]
- 102.Liedvogel M, Szulkin M, Knowles SCL, Wood MJ, Sheldon BC. 2009. Phenotypic correlates of Clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Mol. Ecol. 18, 2444–2456. ( 10.1111/j.1365-294X.2009.04204.x) [DOI] [PubMed] [Google Scholar]
- 103.Zaan R. 1996. The zebra finch: synthesis of field and laboratory studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 104.Hahn TP. 1995. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the red crossbill, Loxia curvirostra (Aves: Carduelinae). J. Exp. Zool. 272, 213–226. ( 10.1002/jez.1402720306) [DOI] [Google Scholar]
- 105.Armstrong JB, Takimoto G, Schindler DE, Hayes MM, Kauffman MJ. 2016. Resource waves: phenological diversity enhances foraging opportunities for mobile consumers. Ecology 97, 1099–1112. ( 10.1890/15-0554.1) [DOI] [PubMed] [Google Scholar]
- 106.van Wijk RE, Kölzsch A, Kruckenberg H, Ebbinge BS, Müskens GJ, Nolet BA. 2012. Individually tracked geese follow peaks of temperature acceleration during spring migration. Oikos 121, 655–664. ( 10.1111/j.1600-0706.2011.20083.x) [DOI] [Google Scholar]
- 107.Fryxell JM, Wilmshurst JF, Sinclair AR. 2004. Predictive models of movement by Serengeti grazers. Ecology 85, 2429–2435. ( 10.1890/04-0147) [DOI] [Google Scholar]
- 108.Wikelski M, Martin LB, Scheuerlein A, Robinsoni MT, Robinson ND, Heim B, Hau M, Gwinner E. 2008. Avian circannual clocks: adaptive significance and possible involvement of energy turnover in their proximate control. Phil. Trans. R. Soc. B 363, 411–423. ( 10.1098/rstb.2007.2147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glass GE, Slade NA. 1980. The effect of Sigmodon hispidus on spatial and temporal activity of Microtus ochrogaster: evidence for competition. Ecology 61, 358–370. ( 10.2307/1935194) [DOI] [Google Scholar]
- 110.Scheibler E, Wollnik F. 2009. Interspecific contact affects phase response and activity in desert hamsters. Physiol. Behav. 98, 288–295. ( 10.1016/j.physbeh.2009.06.001) [DOI] [PubMed] [Google Scholar]
- 111.Shargal E, Kronfeld-Schor N, Dayan T. 2000. Population biology and spatial relationships of coexisting spiny mice (Acomys) in Israel. J. Mammal. 81, 1046–1052. ( 10.1644/1545-1542(2000)081%3C1046:PBASRO%3E2.0.CO;2) [DOI] [Google Scholar]
- 112.Shkolnik A. 1971. Diurnal activity in a small desert rodent. Int. J. Biometeorol. 15, 115–120. ( 10.1007/BF01803884) [DOI] [PubMed] [Google Scholar]
- 113.Cohen R, Kronfeld-Schor N. 2006. Individual variability and photic entrainment of circadian rhythms in golden spiny mice. Physiol. Behav. 87, 563–574. ( 10.1016/j.physbeh.2005.12.010) [DOI] [PubMed] [Google Scholar]
- 114.Cohen R, Smale L, Kronfeld-Schor N. 2009. Plasticity of circadian activity and body temperature rhythms in golden spiny mice. Chronobiol. Int. 26, 430–446. ( 10.1080/07420520902820939) [DOI] [PubMed] [Google Scholar]
- 115.Gutman R, Yosha D, Choshniak I, Kronfeld-Schor N. 2007. Two strategies for coping with food shortage in desert golden spiny mice. Physiol. Behav. 90, 95–102. ( 10.1016/j.physbeh.2006.08.033) [DOI] [PubMed] [Google Scholar]
- 116.Kronfeld-Schor N, Haim A, Dayan T, Zisapel N, Klingenspor M, Heldmaier G. 2000. Seasonal thermogenic acclimation of diurnally and nocturnally active desert spiny mice. Physiol. Biochem. Zool. 73, 37–44. ( 10.1086/316718) [DOI] [PubMed] [Google Scholar]
- 117.Kronfeld-Schor N, Dayan T, Jones ME, Kremer I, Mandelik Y, Wollberg M, Yassur Y, Gaton DD. et al. 2001. Retinal structure and foraging microhabitat use of the golden spiny mouse (Acomys russatus). J. Mammal. 82, 1016–1025. ( 10.1644/1545-1542(2001)082%3C1016:RSAFMU%3E2.0.CO;2) [DOI] [Google Scholar]
- 118.Cohen R, Smale L, Kronfeld-Schor N. 2010. Masking and temporal niche switches in spiny mice. J. Biol. Rhythms 25, 47–52. ( 10.1177/0748730409351672) [DOI] [PubMed] [Google Scholar]
- 119.Koskela TK, Reiss GR, Brubaker RF, Ellefson RD. 1989. Is the high concentration of ascorbic acid in the eye an adaptation to intense solar irradiation. Invest. Ophthalmol. Vis. Sci. 30, 2265–2267. [PubMed] [Google Scholar]
- 120.Rotics S, Dayan T, Kronfeld-Schor N. 2011. Effect of artificial night lighting on temporally partitioned spiny mice. J. Mammal. 92, 159–168. ( 10.1644/10-MAMM-A-112.1) [DOI] [Google Scholar]
- 121.Levy O, Dayan T, Kronfeld-Schor N, Porter WP. 2012. Biophysical modeling of the temporal niche: from first principles to the evolution of activity patterns. Am. Nat. 179, 794–804. ( 10.1086/665645) [DOI] [PubMed] [Google Scholar]
- 122.Hawking F. 1975. Circadian and other rhythms of parasites. Adv. Parasitol. 13, 123–182. ( 10.1016/S0065-308X(08)60320-6) [DOI] [PubMed] [Google Scholar]
- 123.Graf J-F, Mermod C, Aeschlimann A. 1978. Ecologie et éthologie d'Ixodes ricinus en Suisse (Ixododeia; Ixodidae). Sixième note: les rythmes de détachement chez Ixodes ricinus et leurs implications écologiques. [Ecology and ethology of Ixodes ricinus in Switzerland (Ixododeia, Ixodidae). Sixth note: the rates of detachment in Ixodes ricinus and their ecological implications]. Acarologia 20, 327–337 (in French with English abstract). [Google Scholar]
- 124.Mather TN, Spielman A. 1986. Diurnal detachment of immature deer ticks (Ixodes dammini) from nocturnal hosts. Am. J. Trop. Med. Hyg. 35, 182–186. ( 10.4269/ajtmh.1986.35.182) [DOI] [PubMed] [Google Scholar]
- 125.Heylen D, Matthysen E. 2010. Contrasting detachment strategies in two congeneric ticks (Ixodidae) parasitizing the same songbird. Parasitology 137, 661–667. ( 10.1017/S0031182009991582) [DOI] [PubMed] [Google Scholar]
- 126.Matuschka F-R, Richter D, Spielman A. 1991. Differential detachment from resting hosts of replete larval and nymphal Ixodes ticks. J. Parasitol. 77, 341–345. ( 10.2307/3283116) [DOI] [PubMed] [Google Scholar]
- 127.Meireles-Filho ACA, Kyriacou CP. 2013. Circadian rhythms in insect disease vectors. Mem. Inst. Oswaldo Cruz 108, 48–58. ( 10.1590/0074-0276130438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lorenzo MG, Lazzari CR. 1998. Activity pattern in relation to refuge exploitation and feeding in Triatoma infestans (Hemiptera: Reduviidae). Acta Trop. 70, 163–170. ( 10.1016/S0001-706X(98)00025-4) [DOI] [PubMed] [Google Scholar]
- 129.Feliciangeli M. 2004. Natural breeding places of phlebotomine sandflies. Med. Vet. Entomol. 18, 71–80. ( 10.1111/j.0269-283X.2004.0487.x) [DOI] [PubMed] [Google Scholar]
- 130.Meireles-Filho AC, da S. Rivas GB, Gesto JS, Machado RC, Britto C, de Souza NA, Peixoto AA. et al. 2006. The biological clock of an hematophagous insect: locomotor activity rhythms, circadian expression and downregulation after a blood meal. FEBS Lett. 580, 2–8. ( 10.1016/j.febslet.2005.11.031) [DOI] [PubMed] [Google Scholar]
- 131.Martinez-Bakker M, Helm B. 2015. The influence of biological rhythms on host–parasite interactions. Trends Ecol. Evol. 30, 314–326. ( 10.1016/j.tree.2015.03.012) [DOI] [PubMed] [Google Scholar]
- 132.Yohannes M, Boelee E. 2012. Early biting rhythm in the afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med. Vet. Entomol. 26, 103–105. ( 10.1111/j.1365-2915.2011.00955.x) [DOI] [PubMed] [Google Scholar]
- 133.Balmert NJ, Rund SS, Ghazi JP, Zhou P, Duffield GE. 2014. Time-of-day specific changes in metabolic detoxification and insecticide resistance in the malaria mosquito Anopheles gambiae. J. Insect. Physiol. 64, 30–39. ( 10.1016/j.jinsphys.2014.02.013) [DOI] [PubMed] [Google Scholar]
- 134.Jones M, Gubbins S, Cubbin C. 1974. Circadian flight activity in four sibling species of the Anopheles gambiae complex (Diptera, Culicidae). Bull. Entomol. Res. 64, 241–246. ( 10.1017/S0007485300031126) [DOI] [Google Scholar]
- 135.Jones M, Cubbin C, Marsh D. 1972. The circadian rhythm of flight activity of the mosquito Anopheles gambiae: the light-response rhythm. J. Exp. Biol. 57, 337–346. [Google Scholar]
- 136.Jones M, Gubbins S. 1978. Changes in the circadian flight activity of the mosquito Anopheles gambiae in relation to insemination, feeding and oviposition. Physiol. Entomol. 3, 213–220. ( 10.1111/j.1365-3032.1978.tb00151.x) [DOI] [Google Scholar]
- 137.Rund SS, Lee SJ, Bush BR, Duffield GE. 2012. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J. Insect. Physiol. 58, 1609–1619. ( 10.1016/j.jinsphys.2012.09.016) [DOI] [PubMed] [Google Scholar]
- 138.Rund SS, Hou TY, Ward SM, Collins FH, Duffield GE. 2011. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc. Natl Acad. Sci. USA 108, E421–E430. ( 10.1073/pnas.1100584108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leming MT, Rund SS, Behura SK, Duffield GE, O'Tousa JE. 2014. A database of circadian and diel rhythmic gene expression in the yellow fever mosquito Aedes aegypti. BMC Genomics 15, 1128 ( 10.1186/1471-2164-15-1128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rund SS, O'Donnell AJ, Gentile JE, Reece SE. 2016. Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 7, 14 ( 10.3390/insects7020014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O'Donnell AJ, Schneider P, McWatters HG, Reece SE. 2011. Fitness costs of disrupting circadian rhythms in malaria parasites. Proc. R. Soc. B 278, 2429–2436. ( 10.1098/rspb.2010.2457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331, 296–302. ( 10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 143.Altizer SM, Oberhauser KS, Brower LP. 2000. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol. Entomol. 25, 125–139. ( 10.1046/j.1365-2311.2000.00246.x) [DOI] [Google Scholar]
- 144.Bradley CA, Altizer S. 2005. Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol. Lett. 8, 290–300. ( 10.1111/j.1461-0248.2005.00722.x) [DOI] [Google Scholar]
- 145.Hoffmann AA, Sgro CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 146.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 147.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Asch M, Salis L, Holleman LJ, van Lith B, Visser ME. 2013. Evolutionary response of the egg hatching date of a herbivorous insect under climate change. Nat. Clim. Change 3, 244–248. ( 10.1038/nclimate1717) [DOI] [Google Scholar]
- 149.Gienapp P, Reed TE, Visser ME. 2014. Why climate change will invariably alter selection pressures on phenology. Proc. R. Soc. B. 281, 20141611 ( 10.1098/rspb.2014.1611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reed TE, Grotan V, Jenouvrier S, Saether BE, Visser ME. 2013. Population growth in a wild bird is buffered against phenological mismatch. Science 340, 488–491. ( 10.1126/science.1232870) [DOI] [PubMed] [Google Scholar]
- 151.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 152.Post E, Forchhammer MC. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2367–2373. ( 10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plard F, Gaillard J-M, Coulson T, Hewison AM, Delorme D, Warnant C, Bonenfant C, Mace GM. 2014. Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biol. 12, e1001828 ( 10.1371/journal.pbio.1001828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Visser M, Van Noordwijk A, Tinbergen J, Lessells C. 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870. ( 10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 155.van Asch M, Visser ME. 2007. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu. Rev. Entomol. 52, 37–55. ( 10.1146/annurev.ento.52.110405.091418) [DOI] [PubMed] [Google Scholar]
- 156.Visser ME, Holleman LJ. 2001. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. Lond. B 268, 289–294. ( 10.1098/rspb.2000.1363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Olson JM, Jinka TR, Larson LK, Danielson JJ, Moore JT, Carpluck J, Drew KL. 2013. Circannual rhythm in body temperature, torpor, and sensitivity to A(1) adenosine receptor agonist in Arctic ground squirrels. J. Biol. Rhythms 28, 201–207. ( 10.1177/0748730413490667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sheriff MJ, Richter MM, Buck CL, Barnes BM. 2013. Changing seasonality and phenological responses of free-living male arctic ground squirrels: the importance of sex. Phil. Trans. R. Soc. B 368, 20120480 ( 10.1098/rstb.2012.0480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lane JE, Kruuk LEB, Charmantier A, Murie JO, Dobson FS. 2012. Delayed phenology and reduced fitness associated with climate change in a wild hibernator. Nature 489, 554–557.. ( 10.1038/nature11335) [DOI] [PubMed] [Google Scholar]
- 160.Kudo G, Nishikawa Y, Kasagi T, Kosuge S. 2004. Does seed production of spring ephemerals decrease when spring comes early? Ecol. Res. 19, 255–259. ( 10.1111/j.1440-1703.2003.00630.x) [DOI] [Google Scholar]
- 161.Robbirt KM, Roberts DL, Hutchings MJ, Davy AJ. 2014. Potential disruption of pollination in a sexually deceptive orchid by climatic change. Curr. Biol. 24, 2845–2849. ( 10.1016/j.cub.2014.10.033) [DOI] [PubMed] [Google Scholar]
- 162.Marcogliese DJ. 2001. Implications of climate change for parasitism of animals in the aquatic environment. Can. J. Zool. 79, 1331–1352. ( 10.1139/z01-067) [DOI] [Google Scholar]
- 163.Lafferty KD. 2009. The ecology of climate change and infectious diseases. Ecology 90, 888–900. ( 10.1890/08-0079.1) [DOI] [PubMed] [Google Scholar]
- 164.Barber I, Berkhout BW, Ismail Z. 2016. Thermal change and the dynamics of multi-host parasite life cycles in aquatic ecosystems. Integr. Comp. Biol. 56, 561–572. ( 10.1093/icb/icw025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Le Lann C, Visser B, Mériaux M, Moiroux J, Van Baaren J, van Alphen JJ, Ellers J. 2014. Rising temperature reduces divergence in resource use strategies in coexisting parasitoid species. Oecologia 174, 967–977. ( 10.1007/s00442-013-2810-9) [DOI] [PubMed] [Google Scholar]
- 166.Sorribas J, Rodríguez R, Garcia-Mari F. 2010. Parasitoid competitive displacement and coexistence in citrus agroecosystems: linking species distribution with climate. Ecol. Appl. 20, 1101–1113. ( 10.1890/09-1662.1) [DOI] [PubMed] [Google Scholar]
- 167.Lv S, Zhou X-N, Zhang Y, Liu H-X, Zhu D, Yin W-G, Wang X-H, Jia T-W. 2006. The effect of temperature on the development of Angiostrongylus cantonensis (Chen 1935) in Pomacea canaliculata (Lamarck 1822). Parasitol. Res. 99, 583–587. ( 10.1007/s00436-006-0198-8) [DOI] [PubMed] [Google Scholar]
- 168.Tokeson JP, Holmes JC. 1982. The effects of temperature and oxygen on the development of Polymorphus marilis (Acanthocephala) in Gammarus lacustris (Amphipoda). J. Parasitol. 68, 112–119. ( 10.2307/3281332) [DOI] [Google Scholar]
- 169.Oberhauser K, Peterson AT. 2003. Modeling current and future potential wintering distributions of eastern North American monarch butterflies. Proc. Natl Acad. Sci. USA 100, 14 063–14 068. ( 10.1073/pnas.2331584100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brower LP, Kust DR, Rendon-Salinas E, Serrano EG, Kust KR, Miller J, Fernández del Rey C, Pape K. 2004. Catastrophic winter storm mortality of monarch butterflies in Mexico during January 2002. In The monarch butterfly: biology and conservation (eds Oberhauser K, Solensky MJ), pp. 151–166. Ithaca, NY: Cornell University Press. [Google Scholar]
- 171.Brower LP, Castilleja G, Peralta A, Lopez-Garcia J, Bojorquez-Tapia L, Diaz S, Melgarejo D, Missrie M. 2002. Quantitative changes in forest quality in a principal overwintering area of the monarch butterfly in Mexico, 1971–1999. Conserv. Biol. 16, 346–359. ( 10.1046/j.1523-1739.2002.00572.x) [DOI] [Google Scholar]
- 172.Brower LP, Malcolm SB. 1991. Animal migrations: endangered phenomena. Am. Zool. 31, 265–276. ( 10.1093/icb/31.1.265) [DOI] [Google Scholar]
- 173.Satterfield DA, Maerz JC, Altizer S. 2015. Loss of migratory behaviour increases infection risk for a butterfly host. Proc. R. Soc. B 282, 20141734 ( 10.1098/rspb.2014.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dunlap JC. 1996. Genetic and molecular analysis of circadian rhythms. Annu. Rev. Genet. 30, 579–601. ( 10.1146/annurev.genet.30.1.579) [DOI] [PubMed] [Google Scholar]
- 175.Larkin JE, Jones J, Zucker I. 2002. Temperature dependence of gonadal regression in Syrian hamsters exposed to short day lengths. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R744–RR52. ( 10.1152/ajpregu.00299.2001) [DOI] [PubMed] [Google Scholar]
- 176.Gienapp P, Hemerik L, Visser ME. 2005. A new statistical tool to predict phenology under climate change scenarios. Glob. Change Biol. 11, 600–606. ( 10.1111/j.1365-2486.2005.00925.x) [DOI] [Google Scholar]
- 177.Santos CD, Miranda AC, Granadeiro JP, Lourenço PM, Saraiva S, Palmeirim JM. 2010. Effects of artificial illumination on the nocturnal foraging of waders. Acta Oecol. 36, 166–172. ( 10.1016/j.actao.2009.11.008) [DOI] [Google Scholar]
- 178.Dwyer RG, Bearhop S, Campbell HA, Bryant DM. 2013. Shedding light on light: benefits of anthropogenic illumination to a nocturnally foraging shorebird. J. Anim. Ecol. 82, 478–485. (doi: 101111/1365-265612012) [DOI] [PubMed] [Google Scholar]
- 179.Mandelik Y, Jones M, Dayan T. 2003. Structurally complex habitat and sensory adaptations mediate the behavioural responses of a desert rodent to an indirect cue for increased predation risk. Evol. Ecol. Res. 5, 501–515. [Google Scholar]
- 180.Moore MV, Pierce SM, Walsh HM, Kvalvik SK, Lim JD. 2001. Urban light pollution alters the diel vertical migration of Daphnia. Int. Verein. Theor. Ang. Limnol. Verhand. 27, 779–782. [Google Scholar]
- 181.Macgregor CJ, Pocock MJ, Fox R, Evans DM. 2015. Pollination by nocturnal Lepidoptera, and the effects of light pollution: a review. Ecol. Entomol. 40, 187–198. ( 10.1111/een.12174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Knop E, Zoller L, Ryser R, Gerpe C, Hörler M, Fontaine C. 2017. Artificial light at night as a new threat to pollination. Nature 548, 206–209. ( 10.1038/nature23288) [DOI] [PubMed] [Google Scholar]
- 183.de Jong M, Ouyang JQ, Da Silva A, van Grunsven RH, Kempenaers B, Visser ME, Spoelstra K. 2015. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Phil. Trans. R. Soc. B 370, 20140128 ( 10.1098/rstb.2014.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.ffrench-Constant RH, Somers-Yeates R, Bennie J, Economou T, Hodgson D, Spalding A, McGregor PK. 2016. Light pollution is associated with earlier tree budburst across the United Kingdom. Proc. R. Soc. B 283, 20160813 ( 10.1098/rspb.2016.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lewis JS, Bailey LL, VandeWoude S, Crooks KR. 2015. Interspecific interactions between wild felids vary across scales and levels of urbanization. Ecol. Evol. 5, 5946–5961. ( 10.1002/ece3.1812) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.