Abstract
Sexual selection favours the expression of traits in one sex that attract members of the opposite sex for mating. The nature of sexually selected traits such as vocalization, colour and ornamentation, their fitness benefits as well as their costs have received ample attention in field and laboratory studies. However, sexually selected traits may not always be expressed: coloration and ornaments often follow a seasonal pattern and behaviours may be displayed only at specific times of the day. Despite the widely recognized differences in the daily and seasonal timing of traits and their consequences for reproductive success, the actions of sexual selection on the temporal organization of traits has received only scant attention. Drawing on selected examples from bird and mammal studies, here we summarize the current evidence for the daily and seasonal timing of traits. We highlight that molecular advances in chronobiology have opened exciting new opportunities for identifying the genetic targets that sexual selection may act on to shape the timing of trait expression. Furthermore, known genetic links between daily and seasonal timing mechanisms lead to the hypothesis that selection on one timescale may simultaneously also affect the other. We emphasize that studies on the timing of sexual displays of both males and females from wild populations will be invaluable for understanding the nature of sexual selection and its potential to act on differences within and between the sexes in timing. Molecular approaches will be important for pinpointing genetic components of biological rhythms that are targeted by sexual selection, and to clarify whether these represent core or peripheral components of endogenous clocks. Finally, we call for a renewed integration of the fields of evolution, behavioural ecology and chronobiology to tackle the exciting question of how sexual selection contributes to the evolution of biological clocks.
This article is part of the themed issue ‘Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals’.
Keywords: sexual selection, circadian rhythm, circannual rhythm, timing of reproduction, display behaviour
1. Introduction
Sexual selection occurs when individuals of either sex experience enhanced mating success based on their display of behaviours or ornaments [1]. With sexual selection defined as selection on traits that improve reproductive success [2], precise timing of ornaments and behaviour also becomes a key element. While much work has been devoted to study how sexual selection leads to sexual dimorphism in morphological or behavioural traits (i.e. big weapons, colourful ornaments, complex behaviour), less attention has been paid to physiological traits that determine when a trait will be expressed, even though variation in the timing of trait expression can also result in sexual selection.
After briefly summarizing the concepts in sexual selection that pertain to this framework, we follow with a short review of the role of the neurobiological and molecular regulation of circadian rhythms as well as its involvement in annual timing. Understanding the relationship between circadian and circannual mechanisms may provide insight into the pathways that potentially affect variation in timing simultaneously on both daily and seasonal scales. We then present selected studies especially from well-known avian taxa but also from mammal species as evidence for daily and annual timing of displays as potential sexually selected traits. As examples of traits in the daily and annual timescale domains, we present the timing of the dawn chorus, of reproductive readiness and of arrival at breeding grounds for birds, and the timing of hibernation termination and of daily activity times for squirrels (Sciuridae), one of the few mammalian taxa for which suitable data are available. Throughout this review, we aim to identify areas of future study to bolster evidence of timing being a sexually selected trait.
(a). Sexual selection and its application to timing processes
From its first formulation [3], sexual selection theory has been invoked to explain the existence of certain morphological or behavioural traits (secondary sexual characters) that can increase the mating success of an individual. In this review, we define sexual selection as among-individual variation in reproductive success and consider it to be one of three components that together constitute natural selection, the other two components being fecundity (fertility) and viability (survival) selection [4]. Sexual selection is a powerful evolutionary force based on social interactions and fostered by the existence of between-sex differences in mating potential and reproductive investment [5]. In the majority of species, it is the male that displays his quality, to attract mates (intersexual selection) or to compete with rivals in agonistic encounters (intrasexual selection), although the opposite pattern or mutual sexual selection also frequently occurs [1]. At an intraspecific level, the outcome of fights and choices depends on the expression of secondary sexual traits that can vary greatly among individuals. This variability represents the substrate for sexual selection [6–8]. The expression of secondary sexual traits in general is considered costly because these traits usually do not increase survival; instead, they require crucial resources to be converted into mating potential and reproductive success [9]. In this view, there exists a trade-off between the benefits of sexual selection and the costs paid through natural selection [10].
Throughout this review, we consider traits to be potentially under sexual selection if they increase the number of successful matings for an individual, thereby enhancing its reproductive success [2]. We focus primarily on traits that boost mate attraction (intersexual selection), but also consider competitive ability (intrasexual selection)—if it increases mating success—to play a role. Applied to the timing of daily or seasonal events, we expect that traits may be under sexual selection if they determine mating success (or proxies like number of mating partners), exhibit variation among members of one sex in timing and impose costs on their bearer. One prominent example is the dawn song of male songbirds (detailed below). If there exists variation among males in daily or seasonal singing times, males that sing earlier in the day or season obtain more fertilizations and early singing is more costly than late singing, sexual selection probably is at play. This contrasts with timing of traits that are well known to be under natural (fecundity or viability) selection, like the egg-laying dates of many songbirds or parturition times in mammals [11–18]. Here, ecological selection pressures like food availability or predation risk determine the number of offspring produced.
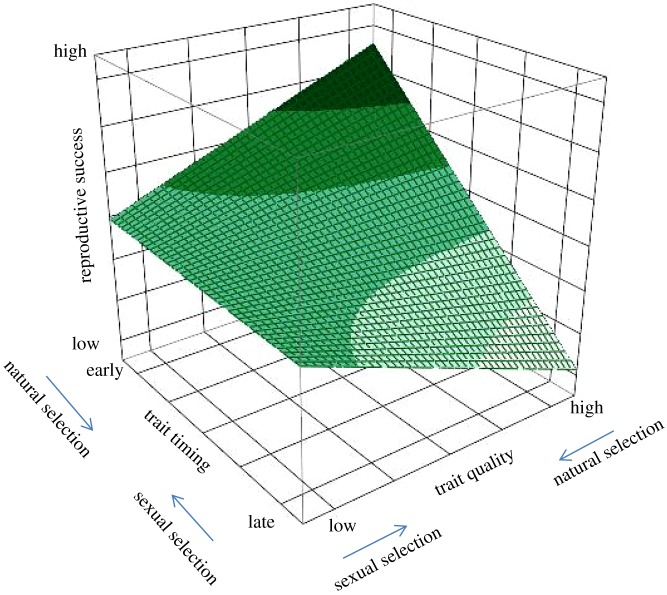
The assumption of costs for sexually selected traits also postulates the existence of interactions between sexual and fecundity/viability selection in the timing and the quality (e.g. size, colour and complexity) of a trait (figure 1). Staying with the example of song in male birds, sexual selection would be expected to favour an early expression of this trait because it would increase mating success while viability selection may act against the display of early song, perhaps because singing too early in the day or in the season is energetically costly or increases predation risk [19–21]. Likewise, a high quality of trait expression (for example, long song bouts or complex song) would be favoured by sexual selection, while fecundity/viability selection may reject the expression of the highest quality traits, perhaps because of associated costs. In this view, only individuals of the highest quality would be able to afford the costs of displaying song early and at high quality, and would gain maximal reproductive success [22] (figure 1). However, the interactions between trait timing and trait quality may not simply be additive but could be more complex. One reason for this complexity could be that trait quality primarily follows a Bateman's gradient (higher trait quality results in more matings, leading to higher reproductive success), while variation in trait timing can additionally result in variations in the operational sex ratio (for example, males with an early trait expression face fewer competitors and more potential mates) [2]. As a result, males that display a trait early may gain substantial mating success even if the quality of the trait they display is suboptimal, and males that display late may only be successful if their traits are of sufficient quality. It will be a rewarding challenge for future research to provide both a firm theoretical basis and empirical tests of such interactions. It should be noted that future work should incorporate both sexes equally in theoretical considerations, as there appears to exist a sex bias not only in empirical (see below), but also in theoretical work (e.g. [23,24]).
Figure 1.
Hypothetical surface profile representation of the timing of a trait and its interaction with the quality of its expression (e.g. size, colour and complexity). Examples for specific traits could be bird song, or ornaments in birds and mammals. Sexual selection would favour an early expression of this trait, while fecundity/viability selection may act against an early display. Likewise, sexual selection would promote a high quality of trait expression, while fecundity/viability selection would act against the expression of the highest quality traits. Consequently, only individuals of the highest quality would be able to sustain the costs of displaying this trait early and at high quality, but would gain maximal reproductive success. Specifics of surface profile depend on parametrization of the model, and this representation serves mainly illustrative and not quantitative purposes. For additional explanations, see text. (Online version in colour.)
2. Common molecular mechanisms of daily and seasonal timing as a substrate for sexual selection
Neural and neuroendocrine regulation of daily and seasonal timekeeping depends on photoneuroendocrine systems (PNES) with many conserved features in birds and mammals. Detailed reviews of the molecular and neuroanatomical features of the PNES, and a comparison of differences among vertebrate groups can be found elsewhere (e.g. [25]). Our brief overview primarily seeks to inform consideration of the likely genetic substrates for sexually selected timekeeping by outlining major components and genes involved in daily and seasonal clocks. An existing bias towards molecular studies on vertebrates being conducted on laboratory mammals (particularly rodent species) is reflected in this section.
Broadly conserved features of the PNES include opsin-based light-sensing pathways for daily and seasonal entrainment of clocks, circadian rhythm generation through transcriptional–translational feedback loop mechanisms running in hypothalamic pacemakers, and a key role for the pars tuberalis (PT) and hypothalamic tanycytes in seasonal neuroendocrine regulation. Important differences include varying complements of specific circadian clock genes (e.g. per1 in mammals, not birds), and differing emphasis on melatonin for circadian organization in birds as opposed to seasonal photoperiodic organization in mammals.
In birds, photic information reaches circadian timing systems through photoreceptors in the pineal gland and various opsin-expressing brain areas (figure 2a), with the eyes playing a species-specific role in circadian organization (that perhaps has more to do with melatonin secretion than actual photoreception) [26–30]. Avian photoperiodic control of seasonal reproduction involves primarily deep encephalic photoreceptors [29,31,32]. In both birds and mammals, the suprachiasmatic nucleus (SCN) contains a molecular circadian oscillator (figure 2a,b), which is entrained by light information and regulates nocturnal melatonin release by the pineal gland. The avian pineal gland also contains a self-sustained circadian oscillator that is intrinsically light-sensitive [33], which is not the case in mammals. In birds, the SCN, the eyes and the pineal gland jointly perform circadian pacemaker functions, regulating daily timing in physiology and behaviour (figure 2a; [30]). By contrast, in mammals the SCN is the dominant circadian pacemaker (figure 2b) and the photoperiod is exclusively sensed through the eye, where photoreceptors (notably containing melanopsin, OPN4) project to the SCN (figure 2b) [34].
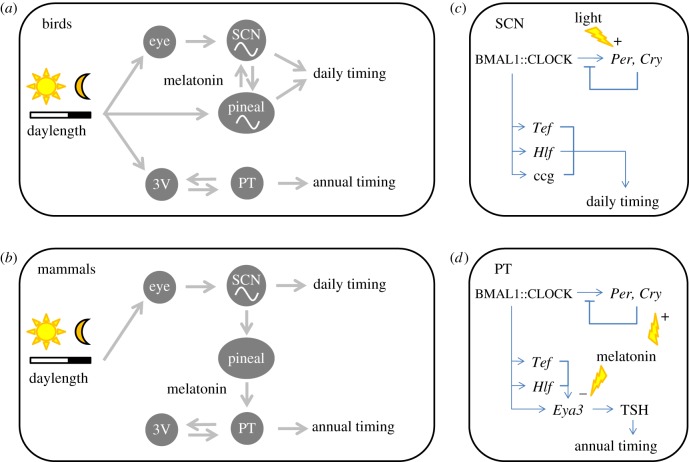
Figure 2.
Daily and annual timing share neurobiological and molecular pathways. In birds and mammals, the annual timing mechanism uses input from the circadian (daily) timing mechanism at the neurobiological and at the molecular level. In birds (a), light regulates the circadian system through photoreceptors in the pineal gland and various opsin-expressing brain areas, with the eyes playing a species-specific role in circadian organization. The avian pineal gland produces melatonin and contains a self-sustained circadian oscillator which, together with the suprachiasmatic nucleus (SCN), regulates daily timing in physiology and behaviour. In mammals (b), light input from the retina stimulates the SCN, which regulates daily timing in behaviour, physiology and melatonin production in the pineal gland. Melatonin receptors in the pars tuberalis (PT) of the pituitary regulate annual timing by thyroid-stimulating hormone (TSH) signalling to the tanycytes in the third ventricle (3V) wall, which in turn regulate thyroid hormone and gonadotropin-releasing hormone signalling, regulating gonadotropin secretion by the PT and subsequent annual timing of reproduction. Contrastingly, in birds (a), melanopsin-positive cerebrospinal fluid-contacting neurons in the 3V wall can directly signal photoperiodic information to the PT-tanycyte pathway, regulating annual timing of reproduction. At the molecular level, the core vertebrate circadian oscillator (c) consists of the BMAL1::CLOCK transcription factor inducing Per and Cry genes which, after dimerization, repress their own promotor activation by the BMAL1::CLOCK dimer. This oscillatory feedback mechanism causes rhythmical induction of BMAL1 which, after dimerizing with CLOCK, produces circadian regulation of output genes like Tef, Hlf and other clock-controlled genes regulating daily rhythms in cellular physiology and metabolism. Synaptic light input signalling to the SCN causes Per induction ((c) lightning symbol) and entrainment to the external light–dark cycle. In mammals, a similar circadian feedback network resides in the PT (d), but here melatonin induces Cry ((d) top lightning symbol), while BMAL1::CLOCK induces Tef and Hlf enhances BMAL1::CLOCK induction of Eya3. Under long winter nights, the induction of Eya3 is fully blocked by melatonin still present in the morning. When morning melatonin is absent during long summer days, EYA3 will cause TSH release, leading to tanycyte thyroid hormone production and, in long-day breeders ((d) bottom lightning symbol), to subsequent gonadotropin production by the pituitary, leading to seasonal gonadal development. ccg, clock-controlled gene. See www.genecards.org for full names of abbreviated genes. (Online version in colour.)
Within circadian pacemaker structures, as well as in target tissues, circadian rhythms at the cellular level are maintained by molecular oscillations in the so-called transcription translation feedback loops (TTFLs) [28]. In essence, TTFLs depend on transcriptional activators (e.g. CLOCK, BMAL1), which drive the expression of transcriptional repressors (e.g. CRY, PER), which generate negative feedback onto the activators. Entrainment of these feedback loops depends on light-dependent effects on the expression of negative elements, mediated through phosphorylation-dependent transcription factors such as CREB [35]. The dynamics of TTFLs depend heavily on post-translational effects such as phosphorylation and ubiquitination, which affect subcellular localization and stability of the core transcriptional regulators. The actions of these core transcriptional regulators on the so-called ‘clock controlled genes’ (including further transcription factors such as Tef, Hlf and Dbp; figure 2c,d) are responsible for overt circadian rhythms in cellular physiology (see www.genecards.org for abbreviations of gene names).
In both birds and mammals, the circadian timing system is also essential for measuring changes in day length, which then trigger seasonal neuroendocrine responses and synchronize circannual rhythms [29,36]. Especially in mammals, melatonin provides an important signal for the duration of darkness (and thus day length), forming a critical regulating input to the PT of the pituitary stalk, a crucial structure for circannual timing (figure 2b,d). The PT produces thyroid-stimulating hormone (TSH) under long summer days, through a mechanism thought to depend in mammals on melatonin-dependent control of TTFL oscillations and their impact on the expression of the clock-dependent transcriptional co-activator Eya3. PT Eya3 expression peaks some 12 h after dusk and appears to be directly suppressed by melatonin. This forms a ‘coincidence timer’ mechanism ensuring that Eya3 levels only rise when night length falls below a critical duration in spring [27]. Eya3 also enhances its own induction, thereby leading to positive feedback and full induction within a few days of long photoperiod [37]. In birds, photoperiodic control of PT production of TSH is also critical for the photoperiodic control of reproduction, and is also associated with strong Eya3 induction [38,39]. Here, however, melatonin does not relay the photoperiodic message, but instead photoreceptive, cerebrospinal fluid (CSF)-touching neurons containing OPN5, neuropsin or VAopsin project to the PT (figure 2b, illustrating this in mammals; [32,40–42]). Details of the activation pathway leading to TSH induction, including the putative role of Eya3 therein, remain to be established.
Downstream from TSH, induction of type II iodothyronine deiodinase (DIO2) expression in tanycytes lining the third ventricle wall is a conserved response to long photoperiod in birds and mammals. DIO2 converts thyroxine (T4) to the active form of thyroid hormone, triiodothyronin (T3). In long-day breeders, T3 stimulates the release of gonadotropins (i.e. follicle-stimulating hormone and luteinizing hormone) by the pituitary gland through interaction with hypothalamic gonadotropin-releasing hormone (GnRH)-producing neurons, resulting in gonadal development (for review, see [43]).
Overall, the selected regulatory networks summarized in this section offer a range of possible candidates through which sexual selection on daily or seasonal timing characteristics might operate. After all, selection requires heritable phenotypic variation (i.e. variation based on genetic mechanisms) to generate evolutionary change. Below we will discuss which components of these networks (central versus peripheral) may more likely be targets of sexual selection. We will also provide examples for specific clock genes/networks that are known or suspected to be involved in the timing of potentially sexually selected traits in the subsequent sections that discuss specific studies. It is also important to emphasize that other (non-PNES) physiological processes like endocrine signals are potent modifiers of daily and seasonal timekeeping, representing potential additional pathways and targets of selection (detailed further in the section below; see also [44]). For example, it has been shown in both mammals and birds that sex steroids (androgens, oestrogens) can affect circadian functioning, although phenotypic effects are species-specific. These sex steroids can cause both permanent (organizational) or temporary (activational) sex differences in daily timing [44,45]. Mechanistically, the actions of sex steroids are accomplished by binding to receptors that are located in the SCN itself, but also in pathways that provide input to and receive information from the SCN (at least in mammals, less is known in birds) [45].
3. Which PNES (or non-PNES) components may be under sexual selection?
When discussing potential effects of sexual selection on the functioning of biological clocks, one may also consider the clock components that may be targeted. Specifically, the question arises whether one would expect sexual selection to act on central or peripheral clock components (see also [44]). Selection on parts of the central clock (on core clock genes/networks and their expression in pacemaker structures; see molecular mechanisms above) may consequently permeate all clock tissues and exert pleiotropic effects on various traits – possibly at all times. Hence, selection on core clock components may be expected to lead to general (permanent, organizational) differences between the sexes, like, for example, a male-specific expression of certain traits like antlers, plumage ornaments or courtship displays or a female-specific expression of traits such as mate choice behaviour, cryptic coloration or maternal behaviour in uniparental species. Such pervasive sex differences may be more likely generated (or exaggerated) by the actions of fecundity (e.g. ability to perform courtship, establish a territory, produce offspring) or viability (e.g. avoid predation) selection, i.e. by the other two components of natural selection. However, the strength of any pleiotropic effects of central clock components will depend on the nature of their connections with other clock (or non-clock) components (for a related discussion concerning reproductive physiology, see [46,47]). For instance, in mammals the SCN plays a far more dominant role in both daily and seasonal processes than in birds (see molecular mechanisms above, figure 1), and thus selection on processes that affect the functioning of the SCN may have a larger impact on circadian phenotypes in mammals compared with birds.
By contrast, sexual selection would be expected to act on between-individual variation in one sex in the timing of signal expression (see sexual selection section above), which may be more likely generated by clock-controlled genes (ccgs; see molecular mechanisms above) that mediate the clock output and/or modulate gene functioning, perhaps even in a tissue-specific manner [48]. The latter may also involve tissue-specific DNA methylation [49], which can have sex-specific phenotypic effects (see below). Also, other systems like endocrine signals may be important (see also molecular mechanisms above), especially when the display of traits only happens at specific times of the year, like the dawn song of birds which is displayed during the reproductive season only ([44,50]; see also bird examples below).
(a). Indirect selection on timing mechanisms
To date, relatively little research has focused on the impact of sexual selection for timing on the functioning of the PNES, but there exist ample examples for the actions of fecundity/viability or artificial selection on PNES components (reviewed in [51–54]), of which a few select ones are summarized below. Here, we argue that a retrospective analysis of the genetic bases of ‘domestication selection’ in laboratory rodents and in commercial poultry breeds may be informative. In laboratory rodents, the regulation of reproduction by seasonal changes in day length via the PNES has been selected against, presumably because a weakened or non-functional PNES favours higher reproductive rates in colonies held on ambiguous 12 L : 12 D regimes, standard in rodent facilities [55,56]. For example, the mouse strain C57BL/6 J is severely compromised in its ability to produce melatonin, essential for suppressing reproduction in short days. The melatonin deficiency in the pineal glands of C57BL/6 J mice arises from mutations in melatonin-producing enzymes (HIOMT and AANAT), probably an inadvertent by-product of selection for breeding under laboratory conditions [57,58]. In layer breeds of poultry [59], domestication has had a strong selective effect on the TSH receptor gene [55,56], with domesticated species carrying a mutant allele that may be responsible for a reduced seasonality and consequently a greater readiness to breed under short days.
The existence of substantial within- and between-species differences in PNES mechanisms probably reflects the actions of fecundity or viability selection. A prominent example is the timing of reproduction in genus Peromyscus mice, which show considerable latitudinal variation within and between species in breeding times and responsiveness to short photoperiod [53,60]. Artificial directional selection experiments yielding lines of wild-derived Peromyscus mice with either strong or no responsiveness to short photoperiod (in inhibiting reproductive readiness [61]) support the hypothesis that fecundity or viability selection has shaped variation in the PNES. These selection-line Peromyscus mice showed clear differences in iodomelatonin binding in certain brain areas and in the number and location of GnRH neurons [62,63]. Furthermore, selection lines differed in the period of their free-running circadian rhythms (τ), although that appeared to be unrelated to their photoperiodic responses [64]. Fecundity or viability selection may also have affected the timing of expression of PNES genes in wild populations of a common Eurasian songbird, the great tit (Parus major). In a common garden experiment, great tit males from a Swedish (latitude 57°N) and a German (47°N) population differed in the timing of mRNA expression of Per2, DIO2, DIO3, GnRH and FSH-β following exposure to a single long day, which simulated an abrupt change from short to long days [65]. These differences in the timing of gene expression may be a result of adaptations to breeding at different latitudes, as Swedish great tits initiate reproduction a few weeks later and thus at longer day lengths than German birds [66].
Laboratory studies have also documented links between circadian clocks and reproductive-related behaviour, specifically between the speed of the circadian clock and behavioural traits and vice versa. For instance, experimental selection in mice for nest-building behaviour resulted in individuals that build bigger nests having a shorter circadian period length than individuals with hardly any nest building [67]. Likewise, selection in mice for aggression also yielded a circadian phenotype with shorter circadian periods for aggressive mice [68]. In humans, circadian chronotype has been linked to personality traits and even life-history strategies [69]. In this study, individuals self-characterized as morning-types (or larks) showed evidence for following a ‘slow’ life history in psychological and behavioural traits while evening-types (owls) were more likely to follow a ‘fast’ life history with opposing trait combinations. Because the circadian system is mechanistically tightly coupled to annual timing (see molecular mechanisms above), differences in daily timing may also be linked to differences in annual timing. Daily timing of display behaviour may thus convey information about the annual timing of the signaller. Indeed, a few phenotypic connections between circadian and annual timing systems have recently been discovered. For example, a phenotypic link between daily (first morning departure from the nest) and seasonal chronotypes (nest initiation dates) has recently been documented in females of two songbird species [70]. Additional specific examples will be discussed below.
4. Timing as a sexually selected trait in birds
(a). Daily timing
In several species of socially monogamous songbirds, the predawn period is a critical time for mating with partners outside of the social pair [71–75]. Much of the activity during this period before dawn is spent singing, participating in what is known as the dawn chorus [76,77]. The variation in the time at which an individual starts singing during this predawn period correlates with variation in extra-pair paternity; for example, male blue tits (Cyanistes caeruleus) that join the dawn chorus first are the most successful within the population at gaining extra-pair paternity [72,74,76]. Thus, the timing of the dawn song affects male mating success, and therefore appears to be a sexually selected signal.
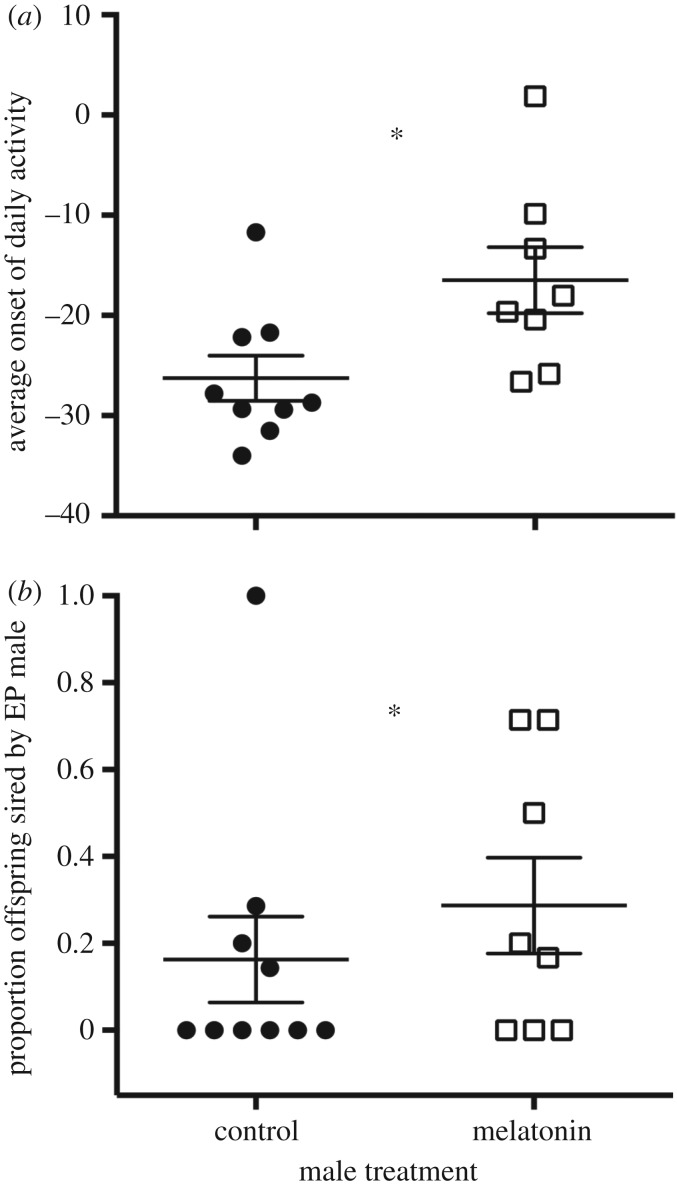
Recent studies suggest that components of circadian clocks may determine the timing of male activity onset, and thereby their initiation of the dawn song and subsequent mating success. Experimental disruption of the circadian rhythm of circulating melatonin levels delayed the onset of daily activity in wild male great tits compared with sham-treated individuals. Importantly, individuals with an experimentally delayed activity onset were more likely to be cuckolded (i.e. had a larger proportion of extra-pair nestlings in their nest), thus decreasing their genetic reproductive success (figure 3) [78]. These data suggest that females prefer to copulate with males that become active earlier in the day. This interpretation is corroborated by an earlier study on great tits brought into captivity as nestlings. When the free-running period length (τ) of their activity rhythms was recorded under constant dim light, individuals sired by an extra-pair father displayed a shorter τ than siblings sired by their social father [79]. This same study showed a relatively high heritability of circadian rhythms, suggesting that females may prefer to engage in extra-pair copulations with males that have a fast circadian rhythm.
Figure 3.
Onset of daily activity influences reproductive success. (a) Treatment of wild great tit males with a melatonin implant delayed their activity onset (data represent individual averages from 2 to 19 days of recording), and (b) melatonin-treated males also suffered a greater cuckoldry risk (higher proportion of extra-pair (EP) young in their nest). Data points represent mean values for individuals, and vertical lines indicate the mean ± s.e.m. for each treatment group. Adapted from [78].
Taken together, these studies in songbirds indicate that sexual selection probably is an important selective force that shapes circadian phenotypes. However, many questions still remain open. For example, while evidence is accumulating that the dawn song is aimed at attracting mates (i.e. intersexual selection), it could also function in male–male competition, implying a different kind of selection (i.e. intra-sexual selection, [72,75,80,81]). This distinction has implications for the receiver, because in intersexual selection it would be females that choose males based on their circadian phenotype, while in intrasexual selection the choosy sex would be other males. Both types of sexual selection require that early song signals an aspect of male quality that the receiver recognizes, but what aspect of quality is conveyed is presently unclear. Even more interesting, both types of selection require that the receiver be able to perceive the signal, i.e. is up and about equally early. Hence one critical prediction is that (certain) females and/or males also become active early during the period of the dawn chorus. However, there exists a researcher sex bias, and the timing of the behaviour of females is much less understood [82]. Results from the few studies that have been conducted on female blue and great tits thus far are puzzling. Females of both species do advance their activity onset and are active earlier on days that immediately precede the start of egg laying—but in general they tend to get up later than males [83,84]. Moreover, natural or manipulated female activity onset times do not correlate with extra-pair young in her brood [84]. Thus, how early male song may be selected for is still unclear. Interestingly, when female great tits were given a melatonin implant that was identical to the one that delayed the onset of activity in males [78], it did nothing to their daily activity onset but delayed their seasonal reproductive timing (clutch initiation dates [85]).
From the experimental evidence presented above, it is tempting to speculate that the circadian hormone melatonin is involved in mediating individual variation in activity onset and thus participation in the dawn chorus in male birds. The melatonin-induced delay in the onset of activity in male great tits described above may result from the implants swamping diel rhythms in endogenous melatonin, thus weakening circadian rhythms and altering chronotype, i.e. the time when individuals become active in the morning with respect to sunrise [78]. Such effects of continuous-release melatonin implants on the chronotype of entrained as well as periods of free-running circadian rhythms have indeed been demonstrated in songbirds [86–89]. Furthermore, it has recently been shown that zebra finches (Taeniopygia guttata) decrease nocturnally elevated melatonin levels roughly 2 h before lights on, with actual times of melatonin decreases differing among individuals [90]. It is therefore possible that natural variation exists among males in the timing of their early-morning melatonin decline, which in turn may influence their activity onset. Additionally, there could be interactions between the circadian system and sex steroids like testosterone, which is secreted at elevated levels at the start of the mating season and which may contribute to this variation in early morning melatonin decrease and/or activity onset. Testosterone can affect circadian properties in some avian species, leading to changes in τ, chronotypes and entrainment properties (see also discussion in [44,91]). However, the onset of the predawn crowing in roosters, which is under circadian control, is not influenced by testosterone administration [92].
Moreover, one critical link is still not fully established, which concerns the link between τ measured under constant conditions in the laboratory and the chronotype in nature. While studies in captivity on birds and humans convincingly show that a short period length is related to an onset of activity before lights-on [93,94], in captive great tits τ of individuals was not correlated with their chronotype [79]. Overall, we still have little information on the relationship between endogenous and overt rhythms in wild species. A recent study on Eurasian blackbirds (Turdus merula) has attempted to fill this gap [95]. Using automated radio telemetry, daily activity rhythms of urban and forest blackbirds were first recorded in the field, where urban birds showed a much earlier onset of dawn activity than the forest conspecifics. Blackbirds were then caught and their endogenous period length assessed in constant dim light in the laboratory. Urban blackbirds showed a shorter period length than the forest birds, and this correlated at the individual level with an earlier onset of activity in the field. Conversely, forest birds showed high variation in period length in the laboratory, but little or no variation in timing in the field, as they all precisely synchronized to dawn. As early dawn timing has been associated with higher extra-pair paternity gain in songbirds [72,74], an intriguing possibility is that urban life might select for early chronotypes and faster clocks. Again, altered daily patterns of melatonin may play a mechanistic role here. Indeed, when the same blackbirds were exposed to realistic levels of artificial light at night in captivity, simulating urban-like conditions, nocturnal melatonin levels dropped significantly, and especially in the early morning [96]. Such a drop was related at the individual level to the amount of activity that a bird showed in the morning, regardless of the origin of the animal (urban versus forest). This suggests that light at night, via changes in melatonin levels, can promote the emergence of early chronotypes, which could be favoured in urban environments [74,97]. This hypothesis requires further testing, but the availability of novel molecular tools might inspire further studies [98]. Indeed, it is now possible to infer the endogenous rhythm period of an animal using skin biopsies rather than having to maintain animals in captivity [99]. This could facilitate the collection of novel data to link chronotype and period length in natural populations.
Establishing a link between natural chronotype and τ in wild animals remains important because the circadian free-running period is not expressed under natural conditions and therefore cannot directly be subjected to sexual (nor fecundity/viability) selection. Circadian phase of entrainment (chronotype) is probably the phenotype that is selected for, but selection may also act on behavioural traits that may correlate with chronotype or other aspects of the circadian phenotype (see section ‘Indirect selection on timing mechanisms’).
(b). Annual timing as a sexually selected trait in birds
Many species of birds breed on a seasonal basis after which they regress their reproductive system and enter a non-breeding state [100]. Being able to breed requires a reactivation of the regressed reproductive system many weeks in advance of actual egg laying [100], and individuals that begin reproductive development later or more slowly than conspecifics will also display reproductive behaviours later and can be outcompeted by early individuals and/or selected against by potential mates [101,102]; but see also [103]. Sexual selection on the timing of reproductive development probably is stronger for males than for females [104], and may lead to earlier gonadal recrudescence in males compared with females [105,106]. This differential timing results from male reproductive success being strongly influenced by the ability to obtain a mate through between-individual variation in times of territory establishment and courtship display (i.e. by sexual selection) [104], while female reproductive success is predominantly determined by fecundity selection (i.e. her ability to lay eggs at the right time of the year). The circadian hormone melatonin plays a role in the seasonal expression of song in male songbirds, which is an important signal in sexual selection (see section on the dawn song above). Like in other vertebrates, photoperiod, i.e. the length of the daily light phase, determines the duration of nocturnal melatonin release in birds, thus providing an internal signal for the time of the year [30]. Melatonin receptors are present in various brain nuclei that are involved in song production [107–109] and melatonin contributes to regulating the photoperiodically induced timing of song [110]. Thus individual variation in the melatonin signal or its transduction at the receptor level could influence the time of the year when males begin to display song at the beginning of the reproductive season. Direct tests of this pathway in natural populations are still lacking.
What has been attempted, however, is to link circadian clock genes with broad-scale population-level variation in breeding times across latitudes. In some species including blue tits, there is evidence for latitudinal clines in Clock gene polymorphisms [111,112]. Furthermore, the Clock genotype shows a weak relationship with individual variation in breeding time in blue tits, though only in females (and not in males, [113]). In barn swallows (Hirundo rustica), Clock gene diversity as well as clock gene methylation is linked with individual variation in breeding time [48,114]. Understanding whether clock genes and/or their methylation causally underlie these relationships that can be sex-dependent, and are present in some species but not others [115], clearly requires further work. Furthermore, investigations of whether these core clock genes are amenable to sexual selection are also still lacking (see also [116]).
Migratory species that spend the winter away from their breeding habitats need to return to their breeding grounds before the reproductive season begins. While there undoubtedly exists fecundity selection on arrival times (simply because birds first need to arrive to be able to breed), sexual selection is also assumed to play a major role [117,118]. Sexual selection should promote earlier arrival times of males compared with females, through ‘rank advantage’ (male–male competition over high quality territories selects for the earliest arriving males) and/or ‘mate opportunity’ (early arriving polygynous males benefit from reduced sperm competition and increased mating opportunities [117,119–121]). Timing of migration has, at least in some species, been linked with polymorphisms both in Clock and in its paralogue Npas2 [122–125].
At the end of the breeding season, many seasonally breeding species replace their colourful breeding plumage with duller feathers (post-breeding or pre-basic moult). Individuals that re-achieve their breeding plumage through the subsequent prenuptial/alternate moult earlier in the breeding season should also be favoured by sexual selection [126], because brighter individuals are more successful in competitive interactions and mate choice, thereby benefiting from increased reproductive success (reviewed in [127]). The best evidence thus far for sexual selection on the timing of moult comes from studies on fairy wren species (Malurus spp.) [126]. Males of most fairy wrens display delayed plumage maturation, i.e. they moult into brighter plumage as they age. In superb fairy wrens (Malurus cyaneus) the earlier a male moults into breeding plumage, the more likely he is to increase his fitness by extra-pair paternity [128–130]. Indeed, a multi-year study found strong evidence for directional selection in promoting early moult in males [131].
Another excellent example for the importance of timing in sexual selection is the behavioural modification of the conspicuous male plumage in rock ptarmigan (Lagopus muta; [132]). While females moult into their cryptic breeding plumage around the time of snow melt, the males remain brilliantly white for at least three weeks longer. Their cryptic winter plumage thus not only serves as an attractive display during the mating season but also makes males highly vulnerable to predation from hawks, particularly gyrfalcons (Falco rusticolus). The dazzling male white plumage comes at a high cost and may thus form an ‘honest signal’ to available females and in male–male competition. As soon as the female begins egg incubation and can no longer be fertilized, the male starts soiling his plumage through mud- and dustbaths, thereby becoming cryptic before the two-to-three week long moult into summer plumage is achieved. Interestingly, polygamous and bachelor males remain white for longer, thus increasing their chances of extra-pair copulations. Should the female lose her clutch and become receptive again, the male immediately cleans his plumage to become conspicuous again [132]. This precise timing of male conspicuousness in relation to female fertility indicates that the delayed male spring moult as well as the timing of dirtying is under strong sexual selection.
Taken together, many lines of evidence suggest that sexual selection may shape the timing of avian seasonal processes like reproductive behaviour, migration and moult that are based on biological clocks. Complexity in understanding both selection pressures on and molecular mechanisms of annual timing is added by the fact that subsequent seasonal events can depend on each other and possibly constrain the action of sexual selection on individual seasonal components. For example, a change in the timing of one seasonal event like migration can have significant carry-over effects on subsequent events including reproduction and moult [133]. Such carry-over effects could result from trade-offs (individuals investing into reproduction may not be able to invest into moult at the same time) and/or from changes to aspects of the biological clock.
5. Timing as a sexually selected trait in mammals
Whether sexual selection exists among mammals on timing during the breeding season, either on daily or annual processes, is presently unclear. This, we propose, is at least in part due to the very few studies of biological timing and consequences for reproductive success in free-living mammals that are detailed enough to address this question (and the few existing ones were conducted primarily on squirrels (Sciuridae [116–120]). This may arise from the fact that field studies of daily and annual patterns of behaviour are logistically challenging, especially when a fine resolution of daily or seasonal patterns of individuals of both sexes along with estimates of individual reproductive success is required (though research on ecology and natural selection has clearly been done (e.g. [134–142])). Some of these hurdles can be overcome by employing small biologging devices which today allow collection of long-term and precise biological data from even quite small free-living mammals [143,144]. In addition, there are the associated difficulties in determining paternity of these same animals. Although there are published studies of paternity in free-living mammals (e.g. [145–149]), we are not aware of any studies of free-living mammals that detail both biological timing of males and females and individual reproductive fitness. The question remains as to whether mating displays by male mammals at specific times of the day or earlier or later in a season are reliable indicators of quality or if females preferentially choose mates based upon their circadian or circannual proclivities. In the field, selection of mates is driven in part by availability [150,151] as well as by pre- or post-copulatory choice related to perceived mate quality [152,153]. Thus, the potential for sexual selection exists, but requires further studies.
Despite a dearth of empirical evidence, we contend that, for some species, it is probable that daily or annual timing of mating is a sexually selected character trait in mammals. Below we discuss the potential for sexual selection on circadian and circannual timing in ground squirrels (Sciuridae), species that exhibit a relatively short gestational period and strong endogenously driven circannual rhythm of hibernation and reproduction [154–157].
(a). Do female ground squirrels prefer early-emerging males?
Seasonal timing of reproduction may be most critical to the arctic ground squirrel (Spermophilus parryii) owing to the environmental conditions of their high latitude distribution. Over winter they are exposed to extremely low temperatures during hibernation, with hibernacula temperatures decreasing to a minimum of −25°C. The brevity of the arctic summer necessitates mating to occur in the early spring when air temperatures are well below freezing, snow blankets the tundra and green-up is weeks away [158]. For males, high thermogenic costs of terminating hibernation at low ambient temperatures [159–161] and lack of available forage on the surface in spring [162] are offset by exogenous energy stores in the form of food cached in the previous summer and autumn [163]. In spring, male arctic ground squirrels draw from these food caches to fuel their approximately 30-day pre-emergent euthermic interval needed for reproductive development [164] and to recoup body mass lost over winter [158]. Because arctic ground squirrels are solitary hibernators, males are presented with the challenge of prognosticating when to end hibernation and initiate reproductive development relative to the timing of the end of hibernation of females. Ending heterothermy and initiating reproductive development too early in the season risks starvation after the food cache is consumed and before females emerge to the surface. Because androgens inhibit expression of torpor [165,166], re-entering hibernation once reproductive development has begun is not possible. Alternatively, ending heterothermy too late ensures that females are already impregnated during the approximately 10-day-long mating season [167]. On average, females are impregnated within about 2 days of ending heterothermy [168]; thus, reproductive success of male arctic ground squirrels depends upon ending heterothermy and initiating reproductive development at the right time relative to when females emerge from hibernation.
Although there is considerable variation in the timing of emergence of reproductively competent males between populations [168], within a population the emergence timing of reproductive males is highly synchronized and occurs within about 12 days with greater than 70% of reproductive males on the surface before the emergence of the first female [167]. This occurs despite the fact that animals are in solitary hibernation with no access to environmental cues for up to 270 days [169,170]. Males that successfully accumulate and defend a cache prior to hibernation can end heterothermy with sufficient time to increase body mass and become reproductively competent to compete for mating opportunities with other males. It is clear that timing is critical to reproductive success of arctic ground squirrels and it is possible that females select males based on their seasonal emergence times. However, many open questions remain, for example which cues females use to distinguish among males with different emergence times because females emerge much later.
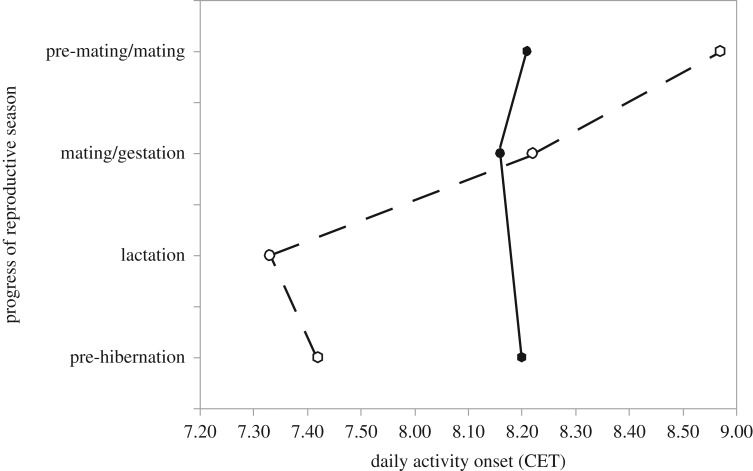
Within the daily cycle, is there evidence that females exhibit a preference for males at a specific time of the day? Upon emergence from hibernation, both male and female arctic ground squirrels initiate robust diurnal rhythms in body temperature and activity [163] and are active during the day [171]. Observation and quantification of courtship, mating and female choice in field studies have not been done, quite likely because copulations in free-living ground squirrels are rarely witnessed and are thought to most commonly occur underground [171]. The ground squirrel mating system is a scramble competition characterized by intense agonistic interaction among males [167,172]; females rarely refuse courtship advances by males but reproductive attempts are known to be interrupted by other competing males [149]. For ground squirrels, it appears that the challenge for males is finding receptive females and subsequent defence of that female from intruding males for a sufficient duration. Whether female mate choice occurs is not yet clear. Male European ground squirrels (Spermophilus citellus) are known to initiate activity earlier in the day ([173], figure 4) than do females, a characteristic that may serve to provide priority access to females ahead of later-sleeping counterparts. Future work in European ground squirrels may be fruitful to address possibilities of sexual selection on daily timing.
Figure 4.
Timing of daily activity onset (x-axis) in adult European ground squirrels at different seasonal stages (y-axis). Males: filled symbols and solid lines, females: open symbols and broken line. During the pre-mating and mating phases, males are active at earlier times than females, while the opposite pattern occurs during lactation and pre-hibernation. CET, Central European Time. Redrawn after data from Everts et al. [173].
Taken together, in some mammalian species it seems probable that circadian timing, circannual timing or both may be used as a sexually selected trait, but definitive proof is lacking. It is possible that the ideal study system for addressing mammalian sexual selection for circadian or circannual traits has yet to be found, but it also seems likely that vast existing datasets on sexual selection have not been fully exploited to address these issues. Another rewarding research area to explore further is that of potential indirect effects of sexual selection on seasonal timing in mammals. For instance, it has been argued that sexual selection has favoured sexual dimorphism in body size that can be rather pronounced in some mammal species (e.g. [174]). Size dimorphism in turn has allometric consequences, generating sex differences in morphology, physiology and life history including in metabolic rate and reproductive costs [174]. Hence, sexual size divergence can affect habitat use, and time and energy budgets (e.g. in marine mammals, [175,176]), possibly leading to differences in seasonal rhythms between males and females. Energy budgets and requirements can also affect daily foraging rhythms, the temporal niche used (day, night or crepuscular activity) and thus also circadian organization (reviewed in [44]). Indirect effects of sexual selection can therefore permeate an array of organismal traits, including seasonal and daily timing. However, the causes and mechanisms underlying sex differences in timing that are generated by such indirect effects of sexual selection still need to be clarified in natural populations (but see [44]).
6. Conclusion and future perspective
Sexual selection is a well-studied area in evolutionary biology, resulting in an ongoing flow of scientific papers mainly discussing the ornaments and behaviours involved. The timing of display behaviour, however, has received much less attention as a sexually selected trait in itself. In this review, we focused on two aspects of timing that may play a role as a sexually selected trait: daily timing and seasonal timing. Various cases have been described where both aspects of timing may be seen as sexually selected traits. The underlying neurobiological mechanisms of the annual timing of reproduction, moult and migration show that circadian clock genes play an essential role in both daily and annual timing. This opens the interesting possibility that daily timing of display behaviour may actually form a signal for the seasonal timing of the signalling animal. Future studies could therefore specifically test whether sexual selection may act upon the shared genes in seasonal and daily timing.
With the advent of technologies like biologging and tracking devices, we think that it is possible to address many of the open questions that we have outlined throughout this review in field populations—in birds, and especially also in mammals [141,144,177]. This will require long-term detailed behavioural studies to address how timing of display behaviour varies among individuals and how this variation relates to annual timing and reproductive success. Again, we note that such studies should be conducted on both sexes, as our brief review showed the existing studies to be biased towards males (for a similar bias in circadian studies of laboratory rodents, see [178]). On the other hand, many existing studies on sexual selection may have never considered the importance of timing of display behaviour as a trait in itself. It is possible that in addition to the importance of, say, antler size, feather coloration or complexity of bird song, it is also important when these traits are being used. This implies that studies on sexual selection may already have recorded timing of display behaviour, but never considered it as an important feature. Many data to test some of our hypotheses might therefore already exist. Moreover, in addition to studying the timing of display as an individual trait underlying sexual selection, we need to begin integrating issues of timing with trait quality. The quality of a trait undoubtedly matters in both intra- and intersexual selection and there may be important, but complex interactions with the timing of display (figure 1). Importantly, molecular advances now allow us to address questions regarding the specific genes and clock components that may be targeted by sexual selection [179]. Indeed, our review of select examples illustrates that there have been several attempts in recent years to take advantage of established molecular pathways in chronobiology to determine the genes that may underlie both daily and seasonal phenotypes. This field is ripe for detailed conceptual and empirical work on the timing of trait expression and the actions of sexual selection in birds, mammals and other taxa.
Acknowledgements
We thank Barbara Helm, Paul Heideman, Ellen Ketterson and three anonymous reviewers for their valuable comments that greatly improved the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to writing and editing the manuscript.
Competing interests
We declare we have no competing interests.
Funding
M.H. and S.C. thank the Max Planck Society for funding. R.A.H. was supported by the GELIFES ‘Adaptive Life’ programme, and D.D. by NWO, the Dutch Science Academy. T.G. was supported by NSF IOS-1257527 while writing this manuscript. Funding was provided to C.L.B. by the National Science Foundation (NSF-IOS nos 1602126 and 1558056).
References
- 1.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 2.Arnold SJ. 1994. Bateman's principles and the measurement of sexual selection in plants and animals. Am. Nat. 144, S126–S149. ( 10.1086/285656) [DOI] [Google Scholar]
- 3.Darwin C. 1871. Sexual selection and the descent of man. London, UK: Murray. [Google Scholar]
- 4.Arnold SJ, Wade MJ. 2013. On the measurement of natural and sexual selection. Evolution 38, 709–719. ( 10.1111/j.1558-5646.1984.tb00345.x) [DOI] [PubMed] [Google Scholar]
- 5.Kokko H, Klug H, Jennions MD. 2012. Unifying cornerstones of sexual selection: operational sex ratio, Bateman gradient and the scope for competitive investment. Ecol. Lett. 15, 1340–1351. ( 10.1111/j.1461-0248.2012.01859.x) [DOI] [PubMed] [Google Scholar]
- 6.Jones AG, Ratterman NL. 2009. Mate choice and sexual selection: what have we learned since Darwin? Proc. Natl Acad. Sci. USA 106, 10 001–10 008. ( 10.1073/pnas.0901129106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irschick DJ, Herrel A, Vanhooydonck B, Van Damme R. 2007. A functional approach to sexual selection. Funct. Ecol. 21, 621–626. ( 10.1111/j.1365-2435.2007.01281.x) [DOI] [Google Scholar]
- 8.Lailvaux SP, Irschick DJ. 2006. A functional perspective on sexual selection: insights and future prospects. Anim. Behav. 72, 263–273. ( 10.1016/j.anbehav.2006.02.003) [DOI] [Google Scholar]
- 9.Zahavi A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 10.Kokko H, Jennions MD, Brooks R. 2006. Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 37, 43–66. ( 10.1146/annurev.ecolsys.37.091305.110259) [DOI] [Google Scholar]
- 11.Caro SP, Schaper SV, Dawson A, Sharp PJ, Gienapp P, Visser ME. 2012. Is microevolution the only emergency exit in a warming world? Temperature influences egg laying but not its underlying mechanisms in great tits. Gen. Comp. Endocrinol. 190, 164–169. ( 10.1016/j.ygcen.2013.02.025) [DOI] [PubMed] [Google Scholar]
- 12.Thomas DW, Blondel J, Perret P, Lambrechts MM, Speakman JR. 2001. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600. ( 10.1126/science.1057487) [DOI] [PubMed] [Google Scholar]
- 13.Van Noordwijk AJ, McCleery RH, Perrins CM. 1995. Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64, 451–458. ( 10.2307/5648) [DOI] [Google Scholar]
- 14.Perrins CM. 1991. Tits and their caterpillar food supply. Ibis 133, 49–54. ( 10.1111/j.1474-919X.1991.tb07668.x) [DOI] [Google Scholar]
- 15.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 16.Post E, Forchhammer MC. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375. ( 10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Festa-Bianchet M. 1988. Birthdate and survival in bighorn lambs (Ovis canadensis). J. Zool. 214, 653–661. ( 10.1111/j.1469-7998.1988.tb03764.x) [DOI] [Google Scholar]
- 18.Loe LE, et al. 2005. Climate predictability and breeding phenology in red deer: timing and synchrony of rutting and calving in Norway and France. J. Anim. Ecol. 74, 579–588. ( 10.1111/j.1365-2656.2005.00987.x) [DOI] [Google Scholar]
- 19.Schmidt KA, Belinsky KL. 2013. Voices in the dark: predation risk by owls influences dusk singing in a diurnal passerine. Behav. Ecol. Sociobiol. 67, 1837–1843. ( 10.1007/s00265-013-1593-7) [DOI] [Google Scholar]
- 20.Hasselquist D, Bensch S. 2008. Daily energy expenditure of singing great reed warblers Acrocephalus arundinaceus. J. Avian Biol. 39, 384–388. ( 10.1111/j.0908-8857.2008.04427.x) [DOI] [Google Scholar]
- 21.McNamara JM, Mace RH, Houston AI. 1987. Optimal daily routines of singing and foraging in a bird singing to attract a mate. Behav. Ecol. Sociobiol. 20, 399–405. ( 10.1007/BF00302982) [DOI] [Google Scholar]
- 22.Gil D, Gahr M. 2002. The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141. ( 10.1016/S0169-5347(02)02410-2) [DOI] [Google Scholar]
- 23.Kokko H, Jennions M. 2003. It takes two to tango. Trends Ecol. Evol. 18, 103–104. ( 10.1016/S0169-5347(03)00009-0) [DOI] [Google Scholar]
- 24.Tang-Martínez Z. 2012. Repetition of Bateman challenges the paradigm. Proc. Natl Acad. Sci. USA 109, 11 476–11 477. ( 10.1073/pnas.1209394109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimura T. 2010. Neuroendocrine mechanism of seasonal reproduction in birds and mammals. Anim. Sci. J. 81, 403–410. ( 10.1111/j.1740-0929.2010.00777.x) [DOI] [PubMed] [Google Scholar]
- 26.Menaker M. 1968. Extraretinal light perception in the sparrow. I. Entrainment of the biological clock. Proc. Natl Acad. Sci. USA 59, 414–421. ( 10.1073/pnas.59.2.414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassone VM, Menaker M. 1984. Is the avian circadian system a neuroendocrine loop? J. Exp. Zool. 232, 539–549. ( 10.1002/jez.1402320321) [DOI] [PubMed] [Google Scholar]
- 28.Cassone VM. 2014. Avian circadian organization: a chorus of clocks. Front. Neuroendocrinol. 35, 76–88. ( 10.1016/j.yfrne.2013.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikegami K, Yoshimura T. 2012. Circadian clocks and the measurement of daylength in seasonal reproduction. Mol. Cell. Endocrinol. 349, 76–81. ( 10.1016/j.mce.2011.06.040) [DOI] [PubMed] [Google Scholar]
- 30.Brandstätter R. 2003. Encoding time of day and time of year by the avian circadian system. J. Neuroendocrinol. 15, 398–404. ( 10.1046/j.1365-2826.2003.01003.x) [DOI] [PubMed] [Google Scholar]
- 31.Menaker M, Keatts H. 1968. Extraretinal light perception in the sparrow, II. Photoperiodic stimulation of testis growth. Proc. Natl Acad. Sci. USA 60, 146–151. ( 10.1073/pnas.60.1.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menaker M, Roberts R, Elliott J, Underwood H. 1970. Extraretinal light perception in the sparrow. III. The eyes do not participate in photoperiodic photoreception. Proc. Natl Acad. Sci. USA 67, 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi JS, Menaker M. 1984. Multiple redundant circadian oscillators within the isolated avian pineal gland. J. Comp. Physiol. A 154, 435–440. ( 10.1007/BF00605243) [DOI] [Google Scholar]
- 34.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. ( 10.1126/science.1067262) [DOI] [PubMed] [Google Scholar]
- 35.Takahashi JS, Hong H-K, Ko CH, McDearmon EL. 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764–775. ( 10.1038/nrg2430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson A. 2002. Photoperiodic control of the annual cycle in birds and comparison with mammals. Ardea 90, 355–367. [Google Scholar]
- 37.Dardente H, Wyse CA, Birnie MJ, Dupré SM, Loudon ASI, Lincoln GA, Hazlerigg DG. 2010. A molecular switch for photoperiod responsiveness in mammals. Curr. Biol. 20, 2193–2198. ( 10.1016/j.cub.2010.10.048) [DOI] [PubMed] [Google Scholar]
- 38.Nakao N, et al. 2008. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452, 317–322. ( 10.1038/nature06738) [DOI] [PubMed] [Google Scholar]
- 39.Majumdar G, Yadav G, Rani S, Kumar V. 2014. A photoperiodic molecular response in migratory redheaded bunting exposed to a single long day. Gen. Comp. Endocrinol. 204, 104–113. ( 10.1016/j.ygcen.2014.04.013) [DOI] [PubMed] [Google Scholar]
- 40.Halford S, et al. 2009. VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 19, 1396–1402. ( 10.1016/j.cub.2009.06.066) [DOI] [PubMed] [Google Scholar]
- 41.Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Ebihara S, Kubo Y, Yoshimura T. 2010. A mammalian neural tissue opsin (opsin 5) is a deep brain photoreceptor in birds. Proc. Natl Acad. Sci. USA 107, 15 264–15 268. ( 10.1073/pnas.1006393107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakane Y, Shimmura T, Abe H, Yoshimura T. 2014. Intrinsic photosensitivity of a deep brain photoreceptor. Curr. Biol. 24, R596–R597. ( 10.1016/j.cub.2014.05.038) [DOI] [PubMed] [Google Scholar]
- 43.Hut RA, Dardente H, Riede SJ. 2014. Seasonal timing: how does a hibernator know when to stop hibernating? Curr. Biol. 24, R602–R605. ( 10.1016/j.cub.2014.05.061) [DOI] [PubMed] [Google Scholar]
- 44.van der Veen DR, Riede SJ, Heideman PD, Hau M, van der Vinne V, Hut RA. 2017. Flexible clock systems: adjusting the temporal programme. Phil. Trans. R. Soc. B 372, 20160254 ( 10.1098/rstb.2016.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey M, Silver R. 2014. Sex differences in circadian timing systems: implications for disease. Front. Neuroendocrinol. 35, 111–139. ( 10.1016/j.yfrne.2013.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adkins-Regan E. 2008. Do hormonal control systems produce evolutionary inertia? Phil. Trans. R. Soc. B 363, 1599–1609. ( 10.1098/rstb.2007.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ketterson ED, Atwell JW, McGlothlin JW. 2009. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr. Comp. Biol. 49, 365–379. ( 10.1093/icb/icp057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saino N, et al. 2017. Migration phenology and breeding success are predicted by methylation of a photoperiodic gene in the barn swallow. Sci. Rep. 7, 45412 ( 10.1038/srep45412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson TJ, Prendergast BJ. 2013. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc. Natl Acad. Sci. USA 110, 16 651–16 656. ( 10.1073/pnas.1310643110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heideman PD. 2004. Top-down approaches to the study of natural variation in complex physiological pathways using the white-footed mouse (Peromyscus leucopus) as a model. ILAR J. 45, 4–13. ( 10.1093/ilar.45.1.4) [DOI] [PubMed] [Google Scholar]
- 51.Bradshaw WE, Holzapfel CM. 2001. Phenotypic evolution and the genetic architecture underlying photoperiodic time measurement. J. Insect Physiol. 47, 809–820. ( 10.1016/S0022-1910(01)00054-3) [DOI] [Google Scholar]
- 52.Hut RA, Paolucci S, Dor R, Kyriacou CP, Daan S. 2013. Latitudinal clines: an evolutionary view on biological rhythms. Proc. R. Soc. B 280, 20130433 ( 10.1098/rspb.2013.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prendergast BJ, Kriegsfeld LJ, Nelson RJ. 2001. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q. Rev. Biol. 76, 293–325. ( 10.1086/393989) [DOI] [PubMed] [Google Scholar]
- 54.Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. 2010. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Phil. Trans. R. Soc. B 365, 3113–3127. ( 10.1098/rstb.2010.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubin C-J, et al. 2010. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464, 587–591. ( 10.1038/nature08832) [DOI] [PubMed] [Google Scholar]
- 56.Kijas JW, et al. 2012. Genome-wide analysis of the world's sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 10, e1001258 ( 10.1371/journal.pbio.1001258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebihara S, Marks T, Hudson DJ, Menaker M. 1986. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231, 491–493. ( 10.1126/science.3941912) [DOI] [PubMed] [Google Scholar]
- 58.Roseboom PH, Namboodiri MAA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. 1998. Natural melatonin ‘knockdown’ in C57BL/6 J mice: rare mechanism truncates serotonin N-acetyltransferase. Mol. Brain Res. 63, 189–197. ( 10.1016/S0169-328X(98)00273-3) [DOI] [PubMed] [Google Scholar]
- 59.Karlsson AC, Fallahshahroudi A, Johnsen H, Hagenblad J, Wright D, Andersson L, Jensen P. 2016. A domestication related mutation in the thyroid stimulating hormone receptor gene (TSHR) modulates photoperiodic response and reproduction in chickens. Gen. Comp. Endocrinol. 228, 69–78. ( 10.1016/j.ygcen.2016.02.010) [DOI] [PubMed] [Google Scholar]
- 60.Bronson FH. 1985. Mammalian reproductive biology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 61.Desjardins C, Bronson FH, Blank JL. 1986. Genetic selection for reproductive photoresponsiveness in deer mice. Nature 322, 172–173. ( 10.1038/322172a0) [DOI] [PubMed] [Google Scholar]
- 62.Kaugars KE, Rivers CI, Saha MS, Heideman PD. 2016. Genetic variation in total number and locations of GnRH neurons identified using in situ hybridization in a wild-source population. J. Exp. Zool. A 325, 106–115. ( 10.1002/jez.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heideman PD, Pittman JT. 2009. Microevolution of neuroendocrine mechanisms regulating reproductive timing in Peromyscus leucopus. Integr. Comp. Biol. 49, 550–562. ( 10.1093/icb/icp014) [DOI] [PubMed] [Google Scholar]
- 64.Majoy SB, Heideman PD. 2000. Tau differences between short-day responsive and short-day nonresponsive white-footed mice (Peromyscus leucopus) do not affect reproductive photoresponsiveness. J. Biol. Rhythms 15, 500–512. ( 10.1177/074873040001500607) [DOI] [PubMed] [Google Scholar]
- 65.Perfito N, Jeong SY, Silverin B, Calisi RM, Bentley GE, Hau M.. 2012. Anticipating spring: wild populations of great tits (Parus major) differ in expression of key genes for photoperiodic time measurement. PLoS ONE 7, e34997 ( 10.1371/journal.pone.0034997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silverin B, Massa R, Stokkan KA. 1993. Photoperiodic adaptation to breeding at different latitudes in great tits. Gen. Comp. Endocrinol. 90, 14–22. ( 10.1006/gcen.1993.1055) [DOI] [PubMed] [Google Scholar]
- 67.Bult A, Hiestand L, Van Der Zee EA, Lynch CB. 1993. Circadian rhythms differ between selected mouse lines: a model to study the role of vasopressin neurons in the suprachiasmatic nuclei. Brain Res. Bull. 32, 623–627. ( 10.1016/0361-9230(93)90164-7) [DOI] [PubMed] [Google Scholar]
- 68.Benus RF, Koolhaas JM, Van Oortmerssen GA. 1988. Aggression and adaptation to the light-dark cycle: role of intrinsic and extrinsic control. Physiol. Behav. 43, 131–137. ( 10.1016/0031-9384(88)90228-4) [DOI] [PubMed] [Google Scholar]
- 69.Ponzi D, Henry A, Kubicki K, Nickels N, Wilson MC, Maestripieri D. 2015. The slow and fast life histories of early birds and night owls: their future- or present-orientation accounts for their sexually monogamous or promiscuous tendencies. Evol. Hum. Behav. 36, 117–122. ( 10.1016/j.evolhumbehav.2014.09.008) [DOI] [Google Scholar]
- 70.Graham J, Cook N, Needham K, Hau M, Greives T. In press Early to rise, early to breed: a role for daily rhythms in seasonal reproduction. Behav. Ecol. ( 10.1093/beheco/arx088) [DOI] [Google Scholar]
- 71.Double M, Cockburn A. 2000. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. Lond. B 267, 465–470. ( 10.1098/rspb.2000.1023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poesel A, Kunc HP, Foerster K, Johnsen A, Kempenaers B. 2006. Early birds are sexy: male age, dawn song and extrapair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Anim. Behav. 72, 531–538. ( 10.1016/j.anbehav.2005.10.022) [DOI] [Google Scholar]
- 73.Sexton K, Murphy MT, Redmond LJ, Dolan AC. 2007. Dawn song of eastern kingbirds: intrapopulation variability and sociobiological correlates. Behaviour 144, 1273–1295. ( 10.1163/156853907781890922) [DOI] [Google Scholar]
- 74.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 75.Murphy MT, Sexton K, Dolan AC, Redmond LJ. 2008. Dawn song of the eastern kingbird: an honest signal of male quality? Anim. Behav. 75, 1075–1084. ( 10.1016/j.anbehav.2007.08.020) [DOI] [Google Scholar]
- 76.Krebs JR, Kacelnik A. 1983. The dawn chorus in the great tit (Parus major): proximate and ultimate causes. Behaviour 83, 287–308. ( 10.1163/156853983X00200) [DOI] [Google Scholar]
- 77.Mace R. 1987. The dawn chorus in the great tit Parus major is directly related to female fertility. Nature 330, 745–746. ( 10.1038/330745a0) [DOI] [Google Scholar]
- 78.Greives TJ, et al. 2015. Costs of sleeping in: circadian rhythms influence cuckoldry risk in a songbird. Funct. Ecol. 29, 1300–1307. ( 10.1111/1365-2435.12440) [DOI] [Google Scholar]
- 79.Helm B, Visser ME. 2010. Heritable circadian period length in a wild bird population. Proc. R. Soc. B 277, 3335–3342. ( 10.1098/rspb.2010.0871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amrhein V, Johannessen LE, Kristiansen L, Slagsvold T. 2008. Reproductive strategy and singing activity: blue tit and great tit compared. Behav. Ecol. Sociobiol. 62, 1633–1641. ( 10.1007/s00265-008-0592-6) [DOI] [Google Scholar]
- 81.Kunc HP, Amrhein V, Naguib M. 2005. Seasonal variation in dawn song characteristics in the common nightingale. Anim. Behav. 70, 1265–1271. ( 10.1016/j.anbehav.2005.02.010) [DOI] [Google Scholar]
- 82.Roth T, Sprau P, Schmidt R, Naguib M, Amrhein V. 2009. Sex-specific timing of mate searching and territory prospecting in the nightingale: nocturnal life of females. Proc. R. Soc. B 276, 2045–2050. ( 10.1098/rspb.2008.1726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halfwerk W, Bot S, Buikx J, van der Velde M, Komdeur J, ten Cate C, Slabbekoorn H. 2011. Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl Acad. Sci. USA 108, 14 549–14 554. ( 10.1073/pnas.1109091108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schlicht L, Valcu M, Loes P, Girg A, Kempenaers B. 2014. No relationship between female emergence time from the roosting place and extrapair paternity. Behav. Ecol. 25, 650–659. ( 10.1093/beheco/aru035) [DOI] [Google Scholar]
- 85.Greives TJ, Kingma SA, Beltrami G, Hau M. 2012. Melatonin delays clutch initiation in a wild songbird. Biol. Lett. 8, 330–332. ( 10.1098/rsbl.2011.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beldhuis HJA, Dittami JP, Gwinner E. 1988. Melatonin and the circadian rhythms of feeding and perch-hopping in the European starling (Sturnus vulgaris). J. Comp. Physiol. A 164, 7–14. ( 10.1007/BF00612712) [DOI] [Google Scholar]
- 87.Turek FW, McMillan JP, Menaker M. 1976. Melatonin: effects on the circadian locomotor rhythm of sparrows. Science 194, 1441–1443. ( 10.1126/science.1006311) [DOI] [PubMed] [Google Scholar]
- 88.Hau M, Gwinner E. 1994. Melatonin facilitates synchronization of sparrow circadian rhythms to light. J. Comp. Physiol. A 175, 343–347. ( 10.1007/BF00192993) [DOI] [Google Scholar]
- 89.Hau M, Gwinner E. 1995. Continuous melatonin administration accelerates resynchronization following phase shifts of a light-dark cycle. Physiol. Behav. 58, 89–95. ( 10.1016/0031-9384(95)00002-Z) [DOI] [PubMed] [Google Scholar]
- 90.Seltmann S, Trost L, Ter Maat A, Gahr M.. 2016. Natural melatonin fluctuation and its minimally invasive simulation in the zebra finch. PeerJ 4, e1939 ( 10.7717/peerj.1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turek F, Gwinner E.. 1982. Role of hormones in the circadian organization of vertebrates. In Vertebrate circadian systems. Proceedings in life sciences (eds Aschoff, J Daan S, Groos GA), pp. 173–182. Berlin, Germany: Springer. [Google Scholar]
- 92.Shimmura T, Yoshimura T. 2013. Circadian clock determines the timing of rooster crowing. Curr. Biol. 23, R231–R233. ( 10.1016/j.cub.2013.02.015) [DOI] [PubMed] [Google Scholar]
- 93.Aschoff J, Wever R. 1966. Circadian period and phase-angle difference in chaffinches (Fringilla coelebs L.). Comp. Biochem. Physiol. 18, 397–404. ( 10.1016/0010-406X(66)90197-6) [DOI] [PubMed] [Google Scholar]
- 94.Duffy JF, Rimmer DW, Czeisler CA. 2001. Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115, 895–899. ( 10.1037/0735-7044.115.4.895) [DOI] [PubMed] [Google Scholar]
- 95.Dominoni DM, Helm B, Lehmann M, Dowse HB, Partecke J. 2013. Clocks for the city: circadian differences between forest and city songbirds. Proc. R. Soc. B 280, 20130593 ( 10.1098/rspb.2013.0593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dominoni DM, Goymann W, Helm B, Partecke J.. 2013. Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): implications of city life for biological time-keeping of songbirds. Front. Zool. 10, 60 ( 10.1186/1742-9994-10-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dominoni DM. 2015. The effects of light pollution on biological rhythms of birds: an integrated, mechanistic perspective. J. Ornithol. 156, 409–418. ( 10.1007/s10336-015-1196-3) [DOI] [Google Scholar]
- 98.Dominoni D, Åkesson S, Klaassen R, Spoelstra K, Bulla M. 2017. Methods in field chronobiology. Phil. Trans. R. Soc. B 372, 20160247 ( 10.1098/rstb.2016.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown SA, et al. 2005. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 3, e338 ( 10.1371/journal.pbio.0030338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dawson A, King VM, Bentley GE, Ball GF. 2001. Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16, 365–380. ( 10.1177/074873001129002079) [DOI] [PubMed] [Google Scholar]
- 101.Daan S, Dijkstra C, Drent R, Meijer T. 1988. Food supply and the annual timing of avian reproduction In Acta XIX congressus internationalis ornithologici, volume I. 19th international ornithological congress, pp. 392–407. Ottawa: Ottawa University Press. [Google Scholar]
- 102.Verhulst S, Tinbergen JMJ. 1991. Experimental evidence for a causal relationship between timing and success of reproduction in the great tit Parus major. J. Anim. Ecol. 60, 269–282. ( 10.2307/5459) [DOI] [Google Scholar]
- 103.Schaper SV, Dawson A, Sharp PJ, Caro SP, Visser ME. 2012. Individual variation in avian reproductive physiology does not reliably predict variation in laying date. Gen. Comp. Endocrinol. 179, 53–62. ( 10.1016/j.ygcen.2012.07.021) [DOI] [PubMed] [Google Scholar]
- 104.Ball GF, Ketterson ED. 2008. Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Phil. Trans. R. Soc. B 363, 231–246. ( 10.1098/rstb.2007.2137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caro SP, Charmantier A, Lambrechts MM, Blondel J, Balthazart J, Williams TD. 2009. Local adaptation of timing of reproduction: females are in the driver's seat. Funct. Ecol. 23, 172–179. ( 10.1111/j.1365-2435.2008.01486.x) [DOI] [Google Scholar]
- 106.Partecke J, Van't Hof TJ, Gwinner E. 2005. Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J. Avian Biol. 36, 295–305. ( 10.1111/j.0908-8857.2005.03344.x) [DOI] [Google Scholar]
- 107.Bentley GE. 2001. Unraveling the enigma: the role of melatonin in seasonal processes in birds. Microsc. Res. Tech. 53, 63–71. ( 10.1002/jemt.1069) [DOI] [PubMed] [Google Scholar]
- 108.Jansen R, Metzdorf R, van der Roest M, Fusani L, ter Maat A, Gahr M. 2005. Melatonin affects the temporal organization of the song of the zebra finch. FASEB J. 19, 848–850. ( 10.1096/fj.04-2874fje) [DOI] [PubMed] [Google Scholar]
- 109.Cassone VM, Paulose JK, Whitfield-Rucker MG, Peters JL. 2009. Time's arrow flies like a bird: two paradoxes for avian circadian biology. Gen. Comp. Endocrinol. 163, 109–116. ( 10.1016/j.ygcen.2009.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang G, Harpole CE, Trivedi AK, Cassone VM. 2012. Circadian regulation of bird song, call, and locomotor behavior by pineal melatonin in the zebra finch. J. Biol. Rhythms 27, 145–155. ( 10.1177/0748730411435965) [DOI] [PubMed] [Google Scholar]
- 111.Johnsen A, et al. 2007. Avian clock gene polymorphism: evidence for a latitudinal cline in allele frequencies. Mol. Ecol. 16, 4867–4880. ( 10.1111/j.1365-294X.2007.03552.x) [DOI] [PubMed] [Google Scholar]
- 112.Liedvogel M, Cornwallis CK, Sheldon BC. 2012. Integrating candidate gene and quantitative genetic approaches to understand variation in timing of breeding in wild tit populations. J. Evol. Biol. 25, 813–823. ( 10.1111/j.1420-9101.2012.02480.x) [DOI] [PubMed] [Google Scholar]
- 113.Liedvogel M, Szulkin M, Knowles SCL, Wood MJ, Sheldon BC. 2009. Phenotypic correlates of Clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Mol. Ecol. 18, 2444–2456. ( 10.1111/j.1365-294X.2009.04204.x) [DOI] [PubMed] [Google Scholar]
- 114.Caprioli M, Ambrosini R, Boncoraglio G, Gatti E, Romano A, Romano M, Rubolini D, Gianfranceschi L, Saino N.. 2012. Clock gene variation is associated with breeding phenology and maybe under directional selection in the migratory barn swallow. PLoS ONE 7, e35140 ( 10.1371/journal.pone.0035140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dor R, Cooper CB, Lovette IJ, Massoni V, Bulit F, Liljesthrom M, Winkler DW. 2012. Clock gene variation in Tachycineta swallows. Ecol. Evol. 2, 95–105. ( 10.1002/ece3.73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Åkesson S, Ilieva M, Karagicheva J, Rakhimberdiev E, Tomotani B, Helm B. 2017. Timing avian long-distance migration: from internal clock mechanisms to global flights. Phil. Trans. R. Soc. B 372, 20160252 ( 10.1098/rstb.2016.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morbey YE, Ydenberg RC. 2001. Protandrous arrival timing to breeding areas: a review. Ecol. Lett. 4, 663–673. ( 10.1046/j.1461-0248.2001.00265.x) [DOI] [Google Scholar]
- 118.Kokko H, Gunnarsson TG, Morrell LJ, Gill JA. 2006. Why do female migratory birds arrive later than males? J. Anim. Ecol. 75, 1293–1303. ( 10.1111/j.1365-2656.2006.01151.x) [DOI] [PubMed] [Google Scholar]
- 119.Kokko H. 1999. Competition for early arrival in migratory birds. J. Anim. Ecol. 68, 940–950. ( 10.1046/j.1365-2656.1999.00343.x) [DOI] [Google Scholar]
- 120.Morbey YE, Coppack T, Pulido F. 2012. Adaptive hypotheses for protandry in arrival to breeding areas: a review of models and empirical tests. J. Ornithol. 153, 207–215. ( 10.1007/s10336-012-0854-y) [DOI] [Google Scholar]
- 121.Rainio K, Tøttrup AP, Lehikoinen E, Coppack T. 2007. Effects of climate change on the degree of protandry in migratory songbirds. Clim. Res. 35, 107–114. ( 10.3354/cr00717) [DOI] [Google Scholar]
- 122.Bazzi G, Pandolfini S, Gatti E, Gianfranceschi L, Cecere JGC, Pina FS, Saino N, Ubolini DR. 2016. Candidate genes have sex-specific effects on timing of spring migration and moult speed in a long-distance migratory bird. Curr. Zool. 365, 1–17. ( 10.1093/cz/zow103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bazzi G, et al. 2016. Clock gene polymorphism, migratory behaviour and geographic distribution: a comparative study of trans-Saharan migratory birds. Mol. Ecol. 25, 6077–6091. ( 10.1111/mec.13913) [DOI] [PubMed] [Google Scholar]
- 124.Saino N, et al. 2015. Polymorphism at the Clock gene predicts phenology of long-distance migration in birds. Mol. Ecol. 24, 1758–1773. ( 10.1111/mec.13159) [DOI] [PubMed] [Google Scholar]
- 125.Peterson MP, Abolins-Abols M, Atwell JW, Rice RJ, Milá B, Ketterson ED.. 2013. Variation in candidate genes CLOCK and ADCYAP1 does not consistently predict differences in migratory behavior in the songbird genus Junco. F1000Research 2, 115 ( 10.12688/f1000research.2-115.v1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peters A, Kingma SA, Delhey K. 2013. Seasonal male plumage as a multi-component sexual signal: insights and opportunities. Emu 113, 232–247. ( 10.1071/MU12083) [DOI] [Google Scholar]
- 127.Hill GE, McGraw KJ.. 2006. Bird coloration. Cambridge, MA: Harvard University Press. [Google Scholar]
- 128.Dunn PO, Cockburn A. 1999. Extrapair mate choice and honest signaling in cooperatively breeding superb fairy-wrens. Evolution 53, 938–946. ( 10.1111/j.1558-5646.1999.tb05387.x) [DOI] [PubMed] [Google Scholar]
- 129.Green DJ, Osmond HL, Double MC, Cockburn A. 2000. Display rate by male fairy-wrens (Malurus cyaneus) during the fertile period of females has little influence on extra-pair mate choice. Behav. Ecol. Sociobiol. 48, 438–446. ( 10.1007/s002650000258) [DOI] [Google Scholar]
- 130.Double MC, Cockburn A. 2003. Subordinate superb fairy-wrens (Malurus cyaneus) parasitize the reproductive success of attractive dominant males. Proc. R. Soc. Lond. B 270, 379–384. ( 10.1098/rspb.2002.2261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cockburn A, Osmond HL, Double MC. 2008. Swingin’ in the rain: condition dependence and sexual selection in a capricious world. Proc. R. Soc. B 275, 605–612. ( 10.1098/rspb.2007.0916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Montgomerie R, Lyon B, Holder K. 2001. Dirty ptarmigan: behavioral modification of conspicuous male plumage. Behav. Ecol. 12, 429–438. ( 10.1093/beheco/12.4.429) [DOI] [Google Scholar]
- 133.Marra PP, Hobson KA, Holmes RT. 1998. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282, 1884–1886. ( 10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 134.DeCoursey PJ, Walker JK, Smith SA.. 2000. A circadian pacemaker in free-living chipmunks: essential for survival? J. Comp. Physiol. A 186, 169–180. ( 10.1007/s003590050017) [DOI] [PubMed] [Google Scholar]
- 135.Ensing EP, Ciuti S, de Wijs FA, Lentferink DH, Ten Hoedt A, Boyce MS, Hut RA. 2014. GPS based daily activity patterns in European red deer and North American elk (Cervus elaphus): indication for a weak circadian clock in ungulates. PLoS ONE 9, e106997 ( 10.1371/journal.pone.0106997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kenagy GJ, Vásquez RA, Barnes BM, Bozinovic F. 2004. Microstructure of summer activity bouts of degus in a thermally heterogeneous habitat. J. Mammal. 85, 260–267. ( 10.1644/1545-1542(2004)085%3C0260:MOSABO%3E2.0.CO;2) [DOI] [Google Scholar]
- 137.van Oort BEH, Tyler NJC, Gerkema MP, Folkow L, Blix AS, Stokkan K-A. 2005. Circadian organization in reindeer. Nature 438, 1095–1096. ( 10.1038/4381095a) [DOI] [PubMed] [Google Scholar]
- 138.Daan S. et al. 2011. Lab mice in the field: unorthodox daily activity and effects of a dysfunctional circadian clock allele. J. Biol. Rhythms 26, 118–129. ( 10.1177/0748730410397645) [DOI] [PubMed] [Google Scholar]
- 139.Fernández-Duque E, de la Iglesia H, Erkert HG. 2010. Moonstruck primates: owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS ONE 5, 1–6. ( 10.1371/journal.pone.0012572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams CT, Barnes BM, Richter M, Buck CL. 2012. Hibernation and circadian rhythms of body temperature in free-living arctic ground squirrels. Physiol. Biochem. Zool. 85, 397–404. ( 10.1086/666509) [DOI] [PubMed] [Google Scholar]
- 141.Williams CT, et al. 2014. Light loggers reveal weather-driven changes in the daily activity patterns of arboreal and semifossorial rodents. J. Mammology 95, 1230–1239. ( 10.1644/14-MAMM-A-062) [DOI] [Google Scholar]
- 142.Tachinardi P, Valentinuzzi VS, Oda GA, Buck CL.. 2017. A test of the circadian thermo-energetics hypothesis in a subterranean rodent: a laboratory and field approach. Physiol. Biochem. Zool. 90, 546–552.. ( 10.1086/693003) [DOI] [PubMed] [Google Scholar]
- 143.Rutz C, Hays GC. et al. 2009. New frontiers in biologging science. Biol. Lett. 5, 289–292. ( 10.1098/rsbl.2009.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Williams CT, Barnes BM, Buck CL. 2016. Integrating physiology, behavior, and energetics: biologging in a free-living arctic hibernator. Comp. Biochem. Physiol. A 202, 53–62. ( 10.1016/j.cbpa.2016.04.020) [DOI] [PubMed] [Google Scholar]
- 145.Annavi G, Newman C, Dugdale HL, Buesching CD, Sin YW, Burke T, Macdonald DW. 2014. Neighbouring-group composition and within-group relatedness drive extra-group paternity rate in the European badger (Meles meles). J. Evol. Biol. 27, 2191–2203. ( 10.1111/jeb.12473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borkowska A, Borowski Z, Krysiuk K. 2009. Multiple paternity in free-living root voles (Microtus oeconomus). Behav. Processes 82, 211–213. ( 10.1016/j.beproc.2009.05.003) [DOI] [PubMed] [Google Scholar]
- 147.Nichols HJ, Cant MA, Sanderson JL. 2015. Adjustment of costly extra-group paternity according to inbreeding risk in a cooperative mammal. Behav. Ecol. 26, 1486–1494. ( 10.1093/beheco/arv095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raveh S, Heg D, Viblanc VA, Coltman DW, Gorrell JC, Dobson FS, Balmer A, Neuhaus P. 2011. Male reproductive tactics to increase paternity in the polygynandrous Columbian ground squirrel (Urocitellus columbianus). Behav. Ecol. Sociobiol. 65, 695–706. ( 10.1007/s00265-010-1071-4) [DOI] [Google Scholar]
- 149.Schwagmeyer PL. 1984. Multiple mating and intersexual selection in thirteen-lined ground squirrels. In Biology of ground-dwelling squirrels: annual cycles, behavioral ecology, and sociality (eds Murie JO, Michener GR), pp. 275–293. Lincoln, NE: University of Nebraska Press. [Google Scholar]
- 150.Eshel I. 1979. Sexual selection, population density, and availability of mates. Theor. Popul. Biol. 16, 301–314. ( 10.1016/0040-5809(79)90019-4) [DOI] [PubMed] [Google Scholar]
- 151.Clutton-Brock T, McAuliffe K. 2009. Female mate choice in mammals. Q. Rev. Biol. 84, 3–27. ( 10.1086/596461) [DOI] [PubMed] [Google Scholar]
- 152.Clutton-Brock TH, Huchard E.. 2013. Social competition and selection in males and females. Phil. Trans. R. Soc. B 368, 20130074 ( 10.1098/rstb.2013.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Orr TJ, Zuk M. 2014. Reproductive delays in mammals: an unexplored avenue for post-copulatory sexual selection. Biol. Rev. 89, 889–912. ( 10.1111/brv.12085) [DOI] [PubMed] [Google Scholar]
- 154.Prendergast BJ, Nelson RJ, Zucker I. 2010. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In Hormones, brain and behavior (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT), pp. 507–540. San Diego, CA: Academic Press; ( 10.1016/B978-008088783-8.00014-0) [DOI] [Google Scholar]
- 155.Pengelley ET, Fisher KC. 1963. The effect of temperature and photoperiod on the yearly hibernating behavior of captive golden-mantled ground squirrels (Citellus lateralis tescorum). Can. J. Zool. 41, 1103–1120. ( 10.1139/z63-087) [DOI] [Google Scholar]
- 156.Kenagy GJ. 1980. Interrelation of endogenous annual rhythms of reproduction and hibernation in the golden-mantled ground squirrel. J. Comp. Physiol. A 135, 333–339. ( 10.1007/BF00657649) [DOI] [Google Scholar]
- 157.Concannon P, Levac K, Rawson R, Tennant B, Bensadoun A. 2001. Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R951–R959. [DOI] [PubMed] [Google Scholar]
- 158.Buck CL, Barnes BM. 1999. Annual cycle of body composition and hibernation in free-living arctic ground squirrels. J. Mammol. 80, 430–442. ( 10.2307/1383291) [DOI] [Google Scholar]
- 159.Buck CL, Barnes BM. 2000. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R255–R262. [DOI] [PubMed] [Google Scholar]
- 160.Karpovich SA, Tøien Ø, Buck CL, Barnes BM. 2009. Energetics of arousal episodes in hibernating arctic ground squirrels. J. Comp. Physiol. B 179, 691–700. ( 10.1007/s00360-009-0350-8) [DOI] [PubMed] [Google Scholar]
- 161.Richter MM, Williams CT, Lee TN, Tøien Ø, Florant GL, Barnes BM, Buck CL. 2015. Thermogenic capacity at subzero temperatures: how low can a hibernator go? Physiol. Biochem. Zool. 88, 81–89. ( 10.1086/679591) [DOI] [PubMed] [Google Scholar]
- 162.Buck CL, Barnes BM. 1999. Temperatures of hibernacula and changes in body composition of arctic ground squirrels over winter. J. Mammal. 80, 1264–1276. ( 10.2307/1383177) [DOI] [Google Scholar]
- 163.Williams CT, Barnes BM, Buck CL. 2012. Daily body temperature rhythms persist under the midnight sun but are absent during hibernation in free-living arctic ground squirrels. Biol. Lett. 8, 31–34. ( 10.1098/rsbl.2011.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barnes BM. 1989. Freeze avoidance in a mammal: body temperatures below 0°C in an arctic hibernator. Science 244, 1593–1595. ( 10.1126/science.2740905) [DOI] [PubMed] [Google Scholar]
- 165.Dark J, Miller DR, Zucker I. 1996. Gonadectomy in the spring reinstates hibernation in male golden-mantled ground squirrels. Am. J. Physiol. 270, R1240–R1243. [DOI] [PubMed] [Google Scholar]
- 166.Lee TM, Pelz K, Licht P, Zucker I. 1990. Testosterone influences hibernation in golden-mantled ground squirrels. Am. J. Physiol. 259, R760–R767. [DOI] [PubMed] [Google Scholar]
- 167.Buck CL, Barnes BM. 2003. Androgen in free-living arctic ground squirrels: seasonal changes and influence of staged male-male aggressive encounters. Horm. Behav. 43, 318–326. ( 10.1016/S0018-506X(02)00050-8) [DOI] [PubMed] [Google Scholar]
- 168.Sheriff MJ, Kenagy GJ, Richter M, Lee T, Toien O, Kohl F, Buck CL, Barnes BM. 2011. Phenological variation in annual timing of hibernation and breeding in nearby populations of Arctic ground squirrels. Proc. R. Soc. B 278, 2369–2375. ( 10.1098/rspb.2010.2482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buck CL, Breton A, Kohl F, Toien O, Barnes BM. 2008. Overwinter body temperature patterns in free-living arctic squirrels (Spermophilus parryii). In Proc. 13th Int. hibernation symp. of hypometabolism in animals: hibernation, torpor, and cryobiology (eds Lovegrove B, McKechnie A), pp. 317–326. Pietermaritzburg: University of KwaZulu-Natal. [Google Scholar]
- 170.Williams CT, Sheriff MJ, Kohl F, Barnes BM, Buck CL. 2012. Interrelationships among timing of hibernation, reproduction, and warming soil in free-living female Arctic ground squirrels. In Living in a seasonal world (eds Ruf T, Bieber C, Arnold W, Millesi E), pp. 481–491. Berlin, Germany: Springer. [Google Scholar]
- 171.Murie JO, Michener GR. 1984. The biology of ground-dwelling squirrels: annual cycles, behavioral ecology, and sociality. Lincoln: University of Nebraska Press. [Google Scholar]
- 172.Edwards PD, Palme R, Boonstra R. 2016. Seasonal programming, not competition or testosterone, drives stress-axis changes in a partially-semelparous mammal. Horm. Behav. 85, 96–101. ( 10.1016/j.yhbeh.2016.08.007) [DOI] [PubMed] [Google Scholar]
- 173.Everts LG, Strijkstra AM, Hut RA, Hoffmann IE, Millesi E. 2004. Seasonal variation in daily activity patterns of free-ranging European ground squirrels (Spermophilus citellus). Chronobiol. Int. 21, 57–71. ( 10.1081/CBI-120027982) [DOI] [PubMed] [Google Scholar]
- 174.Isaac JL. 2005. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mamm. Rev. 35, 101–115. ( 10.1111/j.1365-2907.2005.00045.x) [DOI] [Google Scholar]
- 175.Breed GA, Bowen W, McMillan J, Leonard M. 2006. Sexual segregation of seasonal foraging habitats in a non-migratory marine mammal. Proc. R. Soc. B 273, 2319–2326. ( 10.1098/rspb.2006.3581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Beck CA, Bowen WD, Iverson SJ. 2003. Sex differences in the seasonal patterns of energy storage and expenditure in a phocid seal. J. Anim. Ecol. 72, 280–291. ( 10.1046/j.1365-2656.2003.00704.x) [DOI] [Google Scholar]
- 177.Williams CT, Wilsterman K, Zhang V, Moore J, Barnes BM, Buck CL.. 2016. The secret life of ground squirrels: accelerometry reveals sex-dependent plasticity in above-ground activity. R. Soc. open sci. 3, 160404 ( 10.1098/rsos.160404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS. 2013. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 154, 1501–1512. ( 10.1210/en.2012-1921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tauber E, Kyriacou CP. 2008. Genomic approaches for studying biological clocks. Funct. Ecol. 22, 19–29. ( 10.1111/j.1365-2435.2007.01343.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.