Abstract
Migratory birds regularly perform impressive long-distance flights, which are timed relative to the anticipated environmental resources at destination areas that can be several thousand kilometres away. Timely migration requires diverse strategies and adaptations that involve an intricate interplay between internal clock mechanisms and environmental conditions across the annual cycle. Here we review what challenges birds face during long migrations to keep track of time as they exploit geographically distant resources that may vary in availability and predictability, and summarize the clock mechanisms that enable them to succeed. We examine the following challenges: departing in time for spring and autumn migration, in anticipation of future environmental conditions; using clocks on the move, for example for orientation, navigation and stopover; strategies of adhering to, or adjusting, the time programme while fitting their activities into an annual cycle; and keeping pace with a world of rapidly changing environments. We then elaborate these themes by case studies representing long-distance migrating birds with different annual movement patterns and associated adaptations of their circannual programmes. We discuss the current knowledge on how endogenous migration programmes interact with external information across the annual cycle, how components of annual cycle programmes encode topography and range expansions, and how fitness may be affected when mismatches between timing and environmental conditions occur. Lastly, we outline open questions and propose future research directions.
This article is part of the themed issue ‘Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals’.
Keywords: clock, circannual programmes, orientation, migration strategies, photoperiod, environment
1. Introduction
The migration of birds has been a perpetual source of inspiration, from ancient observations to frontline biological research. The striking, repetitive temporal and spatial patterns of avian migrants have played a particularly important role in the emergence of the field of biological rhythms. Observations had strongly suggested that birds timed migration not only by immediate responses to the environment but also by using internal timekeeping mechanisms [1,2]. Many migratory species are remarkably punctual when they return to their breeding grounds, despite inter-annual differences in local conditions [3]. Equally, birds have admirable navigational abilities, using among other cues the sun for orientation, whose direction from earth (i.e. its azimuth) changes with time of day. Furthermore, many species perform migration-related behaviours at appropriate times of the year, even if kept in captivity without exposure to environmental change [4–7]. Investigation of these behaviours enabled major breakthroughs for discovering endogenous (i.e. self-sustained) biological rhythms. The discoveries of the time-compensated sun compass [8], and of internal triggers for the spring return of long-distance migrants [9], pin-pointed circadian and circannual rhythms (i.e. rhythms with period lengths of roughly 1 day and 1 year, respectively). Importantly, although these clock studies were mostly carried out in captivity, they investigated biological rhythms in an explicitly ecological context, and thus established their functional significance.
The captive environment had the advantage of allowing long-term studies of otherwise elusive migratory species, as well as experimental manipulation of important factors, for example by providing controlled levels of food, temperature and day length [7,10]. If kept in individual cages, many nocturnally migrating species show migratory restlessness (or German: ‘Zugunruhe’) at the times when wild conspecifics migrate. The birds hop and fly at night, and if kept in circular cages they often show seasonally appropriate directional preference [8,11,12]. These observations prompted Kramer [8] to propose that the integration of an inherited sense of time with an inherited sense of direction could function as a ‘clock and compass’ mechanism, which could guide inexperienced birds on their first journey before learning about the environment en route. Since then, captive studies of a wide range of migratory species and systematic experimentation of the captive environment have identified detailed time programmes and their interactions with selected environmental factors [4,10,13–15]. Many of the sophisticated adaptations of migratory programmes can best be studied in individually migrating songbird species, especially in hand-raised individuals during their first journey, when young birds are still naive.
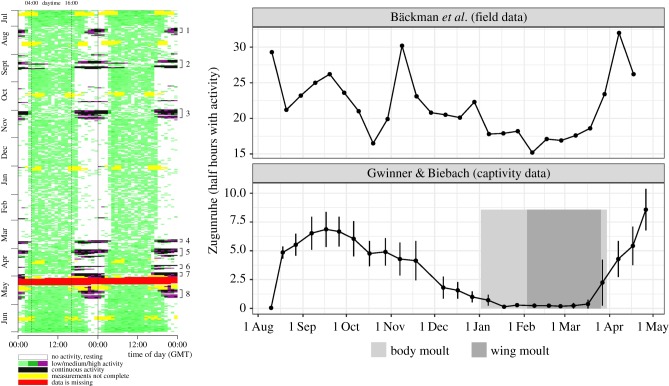
Studies of freely moving birds have in turn provided a much more comprehensive account of the environmental context of migratory timing. However, they have long been mostly limited to recording arrival and departure times at target and stop-over sites, and to some experimental manipulation, for example of locally available food [7]. Over the last decades, technological advances [16] have rapidly opened opportunities to follow entire migratory journeys of individuals, sometimes over several years. These studies generally support the involvement of endogenous biological time programmes, for example by identifying population-specific departure patterns of long-distance migrants from identical sites [17–19], and in some cases greater fidelity for timing than for non-breeding site or migration route [3,19]. Detailed spatio-temporal information from free-flying birds, combined with advances in the study of captive birds, provide an excellent basis for a synthesis between ecological expertise of the environment and chronobiological expertise of the regulation of bird migration. Recently, first data have become available that allow comparisons of year-round activities of wild and captive conspecifics [20] (figure 1). This opens intriguing possibilities to compare the timing of migration studied with such different methods, and shows the complementary information that can be generated by combining these approaches.
Figure 1.
Activity profiles of red-backed shrikes (Lanius collurio) in the wild and in captivity. Left: movement and nocturnal migratory flight of a single, free-flying Danish shrike monitored from July 2014 until July 2015 by Bäckman et al. [20]. Right: comparison of nocturnal activity of the same free-flying shrike (top) with mean activity of 10 hand-raised conspecifics recorded in captivity (bottom) [21]. Shrikes originated from Gribskov, Denmark (top, 56°N) and Lemsjoeholm, South Finland (bottom, 60°N), respectively. On the left, each horizontal line represents accelerometer data from 2 consecutive days, where the second day is repeated as the first day on the next line. Colours encode mean activity level for each hour ranging from no activity (white) to continuous flight (black) with intermediary levels in colour. On the right, data are approximately monthly average numbers ±s.e. of half-hour periods between 18.00 and 06.00 h when birds were active during dark hours, plotted against time of year. Captive birds were kept under constant photoperiodic conditions (LD 12 : 12 h) [21]. For captive birds, vertical shadings indicate the timing of prenuptial (prealternate) moult (light grey: body moult, embedded in it, in dark grey: wing moult).
One converging theme from ecological and chronobiological studies of migration is the diversity of patterns and mechanisms. This diversity is based on several independent evolutionary origins of long-distance migration in animals [22–24]. In birds that breed at northern latitudes, the diversity of routes and distances was promoted by range expansions occurring after the last glaciation and continues to evolve at a fast pace, but rapid change of migration is not limited to these systems [7,25–27]. Here, we exploit some of the diversity of avian migration to review how migratory birds master the multiple timing challenges they face throughout the annual cycle because efficient transport over long distances needs to be well prepared [28]. To tackle the challenges, natural selection acting on the migration phenotype of individual birds is expected to lead to the evolution of fine-tuned interactions between genetically controlled timing mechanisms and the environments the birds experience. Physiological and morphological limitations to the performance of birds, and inter-annual variation and progressive change in global environments, require further adjustments and often involve trade-offs between physiological capacities, and between multiple ecological costs and rewards. Below, we will highlight the timing challenges we perceive along the journey of long-distance migrating bird species and will summarize the knowledge of the ways biological rhythms are used to tackle them. We will then use case studies to explain migratory timing and movement adaptations in birds in greater detail.
2. Challenges
(a). Departing in time, in anticipation of future environmental conditions
A central question in migration research is how migrants know when to leave areas they use during the non-breeding season to return in time to breed. Over short distances, local changes in seasonal conditions may be good predictors for conditions in the breeding areas, but over longer distances more general, reliable cues are needed. For some species, photoperiodism (i.e. responses to the predictable annual change in the length of the light fraction of the day) can function as a seasonal calendar and a trigger for homeward migration. However, for Northern-Hemisphere long-distance migrants that migrate to, or cross, the equator, photoperiodism cannot sufficiently explain a timely return [1]. Day length is nearly constant at the equator and decreases after the December solstice in the Southern Hemisphere so that activating effects of increasing day length are ruled out. Following speculations about internal annual clocks [1,2], Gwinner [9,10] showed that, instead, birds can use circannual programming to time homeward departure. Studying captive Phylloscopus warblers in Africa and Germany, he found that they started spring migratory restlessness (Zugunruhe) at identical times. Gwinner was then the first to show that Zugunruhe occurred at approximately the right time in the year even under constant, simulated equatorial photoperiods (12 h light (L) alternating with 12 h of darkness (D), referred to as LD 12 : 12 h). Zugunruhe, and other events in the avian life-cycle, re-occurred for many years under such constant conditions, displaying rhythms with period lengths of approximately 1 year [7,10].
However, as detailed below in case study 1, under constant conditions, these rhythms start to drift relative to the calendar year (i.e. they are ‘free-running’), indicating the need for synchronization by environmental cues. This synchronization is achieved by responsiveness of circannual programmes to synchronizing cues (so-called Zeitgebers; mainly photoperiod), which can adjust the speed and phase (i.e. the life-cycle stage) of the birds' annual cycle. Although circannual programming is thereby highly responsive to the environment, the encoded responses differ across the annual cycle [10,29]. A prominent example are so-called refractory periods, i.e. times of year when particular responses to the environment are attenuated (e.g. inactivated reproductive competence on the winter grounds [30,31]). Such programming is specific to species and even populations [4,29], allowing birds that carry out less extreme migrations to show greater direct responses to day length and other environmental cues [4,32]. It is important to note that while circannual rhythms and photoperiodism can initiate seasonal processes, for example, a readiness to migrate, moult or breed, an individual's behaviour is usually fine-tuned by a range of environmental factors [33].
Departure from the breeding grounds in autumn often also involves anticipation of future environmental conditions, as many long-distance migrants leave the breeding grounds before conditions get harsh [1,7,34]. Like spring departure, autumn departure of long-distance migrants is encoded in circannual programmes, but in autumn, many temperate species can also use day length on the breeding grounds as a reliable cue. Most species breed as the day lengths increase and initiate post-breeding activities when the days shorten. Autumn activities, including moult, Zugunruhe and migration-related physiology, are finely regulated by photoperiod [7,35], and further modified by environmental factors (e.g. ambient temperature) as well as by preceding breeding activities [32,36].
The daily timing of departure has also been investigated by chronobiological and ecological approaches, which showed that internal clocks can trigger the start of migratory restlessness [37]. Apparently, migratory birds use two separate daily clocks (i.e. two oscillators), of which one times the daytime activity and the other the nocturnal restlessness (Zugunruhe). The clock that times Zugunruhe is highly responsive to nutritional state and ticks more slowly than the daytime clock [38]. Although captivity findings generally corresponded well with field data, recent studies also revealed differences: whereas in captive migrants, Zugunruhe usually sets in shortly after sunset [39], free-living migrants adjusted the timing of departure depending on environmental factors and their body condition [40–43]. Furthermore, whereas in captivity, autumn Zugunruhe usually builds up by a gradual activity shift into the night, a recent study on free-living short-distance migrants revealed that the birds left instantaneously during their first restless night [44].
(b). Using clocks en route, to move through time and space
Once a bird has commenced its migratory journey, timekeeping plays an important role for navigation and for pacing the progress of migration. Answers to the intriguing question how birds navigate to their target areas implicate circadian clocks in several ways. For daytime movements, they can use the azimuth of the sun for orientation, provided they closely kept track of time of day [8]. Because the azimuth changes over the day, birds must factor in time to estimate the time-of-day specific angle of the sun, to say, cardinal South. Numerous experiments involving simulated positions of the sun, and time-shifting of the birds' circadian clock, proved that the birds indeed interpreted the azimuth relative to their subjective perception of time of day and adjusted their flight direction accordingly [45,46]. For migratory birds that travel along east-west axes, this implies that they must quickly reset their circadian clocks to solar time at their current location. If their clocks were still on the time of the areas they travelled from (i.e. showing jet lag), the consequent navigational errors could be substantial. However, there are also advantages to using non-adjusted clocks during continuous flights. At high latitudes, an uncompensated sun compass will lead birds along efficient flight routes that approximate great circles. Birds could set a course at sunset or sunrise and follow it relative to the position of the sun as they cross longitudes using their time-compensated sun compass without updating it for local time during flight [47,48]. To which extent the sun compass is indeed used by birds during migration flights across the globe has however been questioned. Alternatively, birds could use their geomagnetic compass (i.e. inclination compass [49]), keeping track of the apparent inclination angle during long continuous flights [50]. Thus, their internal clock may not be connected to the compass used during migration flight, but during stop-over a reset clock would benefit local use and help to time the next flight departure [51]. It is likely that different compass systems are prioritized differently depending on environmental circumstances. For example, the sun compass route may explain migration trajectories starting at high latitudes, but in contrast to the magnetoclinic route, it fails to explain routes starting nearer to the equator [52]. A magnetic compass based on magnetoclinic routes [50], however, seems to function across a larger span of latitudes including starting points nearer to the equator [52].
Whatever the navigational mechanisms may be, the directional courses of birds need to be adjusted at the appropriate times of year. Some migratory species, in captivity, adjusted the preferred direction of their Zugunruhe movements, shifting from southward in autumn to northward in spring, and from southwest in early autumn to south in late winter [10]. Cross-breeding studies in captivity [53], and geolocator studies of wild birds, suggest that even detailed directional changes may be encoded in the migration programme [54]. In the wild, these programmes are further adjusted to environmental conditions, in ways that differ greatly between species. For example, tracking data have shown that some species may reach their destinations along complex routes, which are shaped by local topography and for which the navigation mechanism and the potentially underlying programming still remain to be revealed [55–57]. Other species show much more stereotyped and simple routes across substantial ranges [58]. Navigation and timing mechanisms will be discussed further in case study 2.
Timekeeping is also important for pacing the progress of migration. Once birds are on the move, the overall speed of migration, and consequently arrival time, is determined during both, in-flight periods and periods of stop-over. Birds on migration need to recover fat reserves for around 7 days at stopover compensating for the energy loss spent at 1 day of migration flight [59]. This highlights the need to time both flight segments and stopover periods during migration in relation to the internal physiological state as well as to environmental conditions, barriers and quality of stopover sites. Studies in the field and captivity have shown that nocturnal activity (onset of Zugunruhe and of nocturnal departure, respectively), and hence the progress of migration, are finely adjusted depending on the nutritional state [60,61]. At stop-over and arrival sites, speedy re-entrainment of the circadian clock and suppression of night activity are thought to help birds to explore the new locations with full benefits of a functional circadian system. Migratory birds may have special adaptations that enable such rapid adjustments: a systemic signal of the birds' circadian clock, the hormone melatonin, is usually high at night. However, nightly melatonin is downregulated as part of the migration programme, implying a weaker clock that accordingly is easier to shift [62]. Melatonin is then temporarily increased during stop-over, when birds encounter rich sources for foraging and fat accumulation, and remain at a site [63]. Similarly, the response of nocturnal activity to modified food availability also changes as part of the migration programme [60], indicating that annual and daily time programmes interact to enable successful migration. Several of these sophisticated adaptations will be discussed in case study 2.
(c). Fitting it all in an annual cycle, allowing for flexibility but staying on time
Long distance migrants have by definition complex annual cycles because migration, in addition to moulting and breeding, involves a suite of morphological and physiological preparations [64]. These life-cycle stages can be independently adjusted (see case study 1) according to need and in response to timing cues. Importantly, the effects of cues vary depending on the annual-cycle stage because to be relevant they must predict conditions in the environment that will ultimately affect fitness [65]. For example, for some annual stages photoperiod will convey important information, whereas others may be more directly responsive to food availability or ambient temperature [33]. Thus, the annual cycle can get delayed or accelerated in various ways, but at some point, birds will need to re-synchronise their activities if they are to follow the timely migration programme.
It is, therefore, a tricky timing challenge to find the right balance between flexibly adjusting migration to the current environment on the one hand, and keeping track of seasonally appropriate behaviour on the other [33,66]. Flexibility to the environment offers many benefits, for example avoiding unfavourable conditions or taking advantage of suitable conditions at unexpected times of day or year, as increasingly observed under climate change (see below). However, from an annual-cycle perspective, it bears the risk of mistiming at subsequent destinations, for example, delayed breeding site arrival resulting from extended stopover during spring migration [67], or, conversely after rushed stopover, premature return to areas that might still be in winter conditions [68]. To fit their activities in the annual cycle, birds usually correct for their shifts in timing, but intermediately they may show ‘carry-over’ effects between life-cycle stages [69]. Birds compensate for shifts either by acceleration or deceleration of activities (adjusting the speed of their clock; for example faster moult), or by modifying their activities (adjusting the phase of the clock; for example, arresting moult in autumn and resuming in spring, or overlapping moult with breeding) [70]. When, and how, birds restore appropriate seasonal timing is of interest from a chronobiological perspective because it can give clues to the mechanisms that underlie seasonal timing. Species differ greatly in how they balance flexibility and consistency across their annual cycle. Recent studies have highlighted remarkable individual consistency of migratory timing in extremely punctual species, for example, bar-tailed godwits Limosa lapponica and collared flycatchers Ficedula albicollis [17,19]. In contrast to their individual consistency, conspecifics differed widely among each other in departure timing at the exact same wintering areas, further indicating robust individual- or population-specific patterns. When individuals spread across non-breeding habitat of different quality, environmental effects can also contribute to differential migration patterns [71]. On the other end of a spectrum from consistent to flexible, species such as large seabirds may greatly adapt the migration programmes, for example depending on whether or not they breed in a given year [72] (see also case study 3).
(d). Adjusting the time programme, to keep pace with global change
A species' position along a consistency-to-flexibility gradient is thought to be highly relevant for its ability to cope with global change. Recent climate change has caused dramatic increases in temperatures across the globe, with large ecological and evolutionary consequences for organisms [73], including migratory birds [74]. The reported effects of climate change on migratory birds vary from altered patterns of migration, with shortened distances [75] or shifts to residency [76], to changes in morphology [77]. One of the most evident effects of climate change, however, is a change in phenology (i.e. timing of events [34,74]). For example, the timing of breeding has advanced in many bird species, including migratory ones [78]. Likewise, migration has advanced in some species and these changes were attributed to climate change [74,79]. Temperature variation, or its effects, can thus also be a relevant cue for the timing of migration. However, because the increase in global temperatures caused by climate change is not accompanied by changes in photoperiodic cues, and because temperature changes are not equal in space and time, the birds' responses to climate change are also not necessarily uniform. This is evidenced by the inconsistent patterns of advancements in the timing of migration reported in some but not all cases [79–81]. At least in some species, it is well possible that the scheduling of migration depends more strongly on the animal's endogenous rhythms and photoperiodism [19,82,83] than on temperature cues. If birds solely rely on photoperiodic cues and/or endogenous rhythms to initiate their migration, they may be too inflexible to accommodate phenology changes, for example during their subsequent arrival at the breeding grounds, unless they adjust the speed of migration to conditions en route [84,85] or shorten their migration distances [75]. Such adjustments could compensate for inflexible departure timing as long as advancements of phenology at the breeding grounds are not too great. In addition to mismatches with the environment, climate change could also cause mismatches within the annual cycle, increasing or decreasing the time available for particular life cycle stages or leading to their overlap [86] if certain annual cycle stages are more affected by temperature cues than others [80].
Potential mismatches under climate change have been studied in some bird species for which long-term datasets are available. The European pied flycatcher (Ficedula hypoleuca) readily breeds in nest-boxes, making this species especially suitable for long-term monitoring and a popular study system for the effects of climate change on birds. In a Dutch population of pied flycatchers, advancements in the timing of breeding were not accompanied by a similar change in arrival dates. Thus, in this population, the interval between arrival and breeding became progressively shorter, and further advancement of laying dates would be constrained by the lack of advancement in arrival dates [80]. Curiously, this pattern can be very different in other populations; for example, in a Finnish population of pied flycatchers, the observed pattern was the opposite: while arrival dates advanced, the timing of breeding did not, causing an increase in the time between arrival and breeding [87,88]. Still, both cases suggest that timing of migration and breeding do not respond similarly to climate change.
It is complicated to assess the fitness consequences of changes in phenology due to the complexity of avian annual cycles [89]. For example, conditions on the wintering grounds or during migration could be even more important for fitness consequences or population decline than those experienced during breeding [90]. Thus, the whole annual cycle may need to be taken into account [91]. However, some examples of detrimental effects exist. For instance, shifts in time of breeding and/or migration have been reported to be insufficient for some species to track concomitant changes in food availability [76,89,92]. Effects of such temporal mismatches are assumed to be particularly detrimental to populations when associated with the deterioration of their wintering and breeding habitats [77,93,94].
3. Case studies
(a). CASE STUDY 1: circannual programming in waders
Key questions: Are life-cycle stages independently modified within the circannual programme? Does the rigidity of the endogenous programme differ for the timing of different life-cycle stages? Could such variation enable flexibility within annual cycles?
Birds migrating long distances have intense annual schedules (figure 2c), during which some of the stages require rigid timing mechanisms, while others are best scheduled conditionally, as a function of carry-over, current environmental conditions and individual state [7,66,95,96]. Several studies report a seasonal trend in the degree of flexibility in timing: spring (pre-breeding) stages of the annual cycle are more punctual than those later in the year [32,83,97,98]. Correspondingly, synchronicity between individuals also changes across the annual cycle and declines after the pre-breeding stage [98]. The high punctuality and synchronicity in spring can be explained by a restricted time period suitable for reproduction at the breeding grounds [98]. After breeding, these constraints are relaxed, and differences owing to individual circumstances will have accumulated (timing of nest initiation, breeding success etc.), leading to greater flexibility in scheduling.
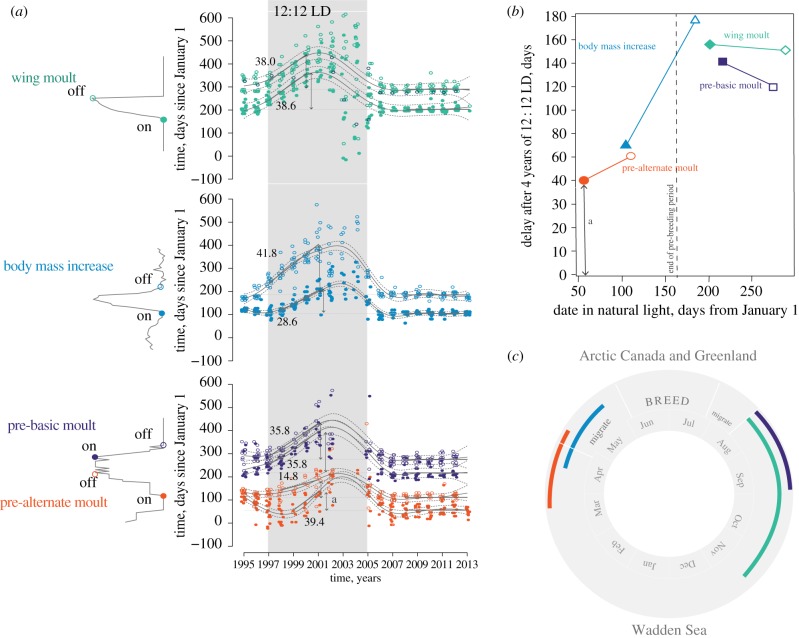
Figure 2.
Timing of life-cycle stages in captive red knots under natural (wintering site) and constant (LD 12 : 12 h) photoperiodic conditions. (a) Timing of wing moult, spring body mass increase, and pre-alternate and pre-basic plumage moult in red knots across their stay in captivity. The period of treatment with 12 : 12 LD is shown in grey shade. Onsets (closed dots) and offsets (open dots) of each stage for each individual red knot are plotted together with predicted mean values (curved solid grey lines) and 95% confidence intervals (curved dotted lines). Horizontal solid black lines represent predicted means of onsets and offsets in natural photoperiod during 2008–2013 and in LD 12 : 12 h during 1997–2001. The numbers next to the lines stand for the predicted mean values of the rate (days/year) of linear drift in timing. Two-sided vertical arrows represent cumulative delay after 4 years in LD 12 : 12 h. One of the arrows (marked with ‘a’) is projected on panel (b). (b) Annual-cycle overview of the free-running timing of life-cycle stages of knots maintained under unvarying LD 12 : 12 h conditions. Cumulative delays in onsets (closed symbols) and offsets (open symbols) of spring body mass increase (triangles), pre-alternate (circles), pre-basic (squares) and wing-moult (diamonds) are plotted against median dates (days since January 1). Delays in the phases of the same life-cycle stage are connected with lines. (c) Annual cycle of the islandica Red Knot. Adapted from Karagicheva et al. [14]. Colours represent the life-cycle stages, as in (a) and (b).
The need for punctuality is especially high for long-jump migrants, such as Arctic- and subarctic-breeding shorebirds (i.e. waders) [99,100], which can cover vast distances of up to 12 000 km by non-stop flights [101]. These long endurance flights require physiological preparations, such as storing adequate fuel reserves and undergoing prealternate (i.e. prenuptial) plumage moult (e.g. [102,103]). Preparations should begin several weeks before the birds start migration [104]. At their non-breeding grounds, long-distance migrants have no direct cues of upcoming local phenological conditions at their remote destinations. Thus, the birds will predominantly rely on circannual programming, which works in concert with photoperiodism, or, in some non-breeding ranges, on photoperiodism [30,105]. These mechanisms are thought to have been fine-tuned over evolutionary time by trial and error of ancestral generations. As the birds approach the breeding grounds, environmental cues will become progressively more informative predictors of the phenological conditions at their destinations. Consistent with this seasonal decline in requirements for rigid timing, different external cues are used to regulate the different life-cycle stages. Non-photic environmental cues, which provide more direct information on the phenological process, may take over later in the season and enable more flexible timing, e.g. of arrival at the breeding grounds [3,31,94,105,106]. Mechanistically, the seasonal variation in punctuality should be reflected by a modular endogenous timekeeping system, which enables independent temporal adjustment of life-cycle stages within the annual cycle [29,107,108]. Such a regulatory system would fit well with current mechanistic ideas of tissue-specific circannual timing mechanisms [109]. Testing of these ideas requires observing the output of endogenous timekeeping separately from the action of cue-response systems, by placing animals in temporally uninformative environments for longer than a year [10].
Some such studies have shown that in the absence of temporal cues the free-running cycles of different life-cycle stages can become dissociated (i.e. that they will show a time-shift relative to each other), suggesting that they may be differentially regulated [10,14,110]. Twenty-year-long observations of the repeated seasonal changes in body mass, plumage state and primary moult in captive red knots Calidris canutus islandica revealed that even under unvarying conditions (constant temperature and photoperiod 12 : 12 LD) circannual cycles of spring life-cycle stages (pre-alternate moult and spring body mass gain) continued robustly for a few years, similar to the rhythms of free-living conspecifics [14]. In contrast, the post-breeding life-cycle stages of red knots drifted more swiftly toward later dates (i.e. free-ran with period lengths that far more exceeded 365 days; see also [110]; figure 2a). Due to the differences in the starting time and circannual period lengths, the cumulative delay after 4 years under constant conditions varied between life-cycle stages, and between their onsets and offsets (figure 2b).
Such differences in rigidity between spring and autumn life-cycle stages fit well with the seasonal life of red knots. The red knot is a High Arctic breeding shorebird, whose islandica subspecies breeds in Northern Canada and Greenland and spends the nonbreeding season along the Wadden Sea coast, migrating between the sites over a distance of about 5000 km [104,111] (figure 2c). The islandica red knots depart from the Wadden Sea in the first week of May, stop to fuel-up in Iceland, and arrive at the breeding grounds in early June. They prepare for migration and breeding long in advance by starting to moult into breeding plumage and to gain body mass in late March [104]. At this time, as described above the knots can by no means anticipate what phenological conditions they will find at the breeding sites thousands of kilometres away, and more than two months in the future, and thus adhere to punctual, internal timekeeping. After initiating migration, when red knots can more successfully predict phenological conditions on the breeding grounds, they may modify timing. For example, if the spring is late, they may postpone arrival on the tundra and/or delay the timing of breeding [112]. The timing of the post-breeding moult depends on the dates of departure from the breeding grounds, which in turn depends on breeding success, since failed breeders migrate earlier [113].
Thus, patterns converged between the seasonal trends in the behaviour of free-living knots and in the rigidity of life-cycle stage timing in captive knots. Based on this, Karagicheva et al. [14] suggested that in red knots, components of an annual cycle that require punctuality are largely based on endogenous rhythms, while others may allow for greater environmental influence. The authors proposed that this long-distance migrating shorebird differentially prioritizes the information received from several components of its timekeeping system: (i) endogenous clock(s), (ii) a photoperiod-response system, and (iii) systems that perceive and process a variety of non-photic information, depending on the required (evolutionarily informed) balance between flexibility and rigidity of timing. When punctuality is beneficial (or there is no apparent benefit from flexibility), birds fully rely on endogenous timekeeping, whereas when the environment provides more useful information, birds give priority to these cues. Future studies of other migrating bird species will need to investigate whether it is a general pattern that parts of the annual cycle are rigid and others flexible, as suggested earlier for songbirds and shorebirds [98,107], or whether long-distance migrating waders with their very intense annual schedule are an extreme example. In the red knots discussed here, we can now ask more advanced questions, for example whether, or how, this system of circannually programmed life-history stages connected to an internal clock tracks global changes in the environment [114]. Whether any such adjustments are possible through canalized evolutionary responses [115], and/or individual adjustment during ontogeny [114] is a timely research question in view of ongoing climate change, and the particular pressures it exerts on red knots and other Arctic breeding migratory birds [76].
(b). CASE STUDY 2: songbirds—endogenous spatio-temporal programmes and navigation mechanisms
Key questions: How are ecophysiological and behavioural mechanisms for navigation and barrier crossings encoded in the endogenous migration programme in individually migrating songbirds? In what ways are migration programmes modified when new routes evolve?
As described in the introduction, in individually migrating songbirds timing, distance and directions of the first migration are encoded as part of a circannual time programme [5,116]. These detailed programmes allow birds to follow direct routes between breeding sites and wintering areas crossing landmasses and seas, or more complex migration routes circumventing barriers [117]. The endogenous programme influences decisions on fuelling, fasting and flight periods, as well as handling of winds and navigation. In turn, timekeeping determines the pay-offs and costs of alternative migration strategies and associated physiological adaptations for coping with challenging migration routes including extended barrier crossings. But how can migratory naive birds predict a barrier crossing and how do they prepare for it? A long-distance nocturnal passerine migrant, the thrush nightingale Luscinia luscinia, responds to information from the geomagnetic field triggering extensive fuelling at the right time of year before an extensive barrier crossing during the migration to eastern Africa [118]. To manage the crossing of the Sahara desert successfully, the birds then need to time their flights to explore favourable winds and avoid overheating. The crossing of the Sahara was initially suggested to be performed by a single well-timed several-day-long flight by Palaearctic-African bird migrants [119,120]. While this behaviour is supported for some species, the evidence is mixed. Several studies reported that songbirds use an intermittent flight strategy during the Sahara crossing by which they time their migrations to fly at night and rest on the ground at daytime [121–124]. The strategy may save energy and avoid the risk of heat stress in the hot desert environment [124]. Birds may, however, respond opportunistically to winds on migration across the Sahara resulting in extended flight periods in tailwind conditions [125–127]. Winds and food availability seem to be important for common swifts Apus apus crossing the Sahara [128]. The timing of spring passage for this species is highly synchronous between populations as compared to autumn when populations differ in departure date and time taken for the passage. The northernmost breeding populations initiate the passage latest in autumn and take the shortest time for the crossing [128], suggesting they use the journey to catch up with their more southern conspecifics.
In order for detailed programmes to work, birds need to maintain their inherited spatio-temporal course during migration. To do so birds have access to three biological compasses, based on information from the sun and the skylight polarization pattern, stars and the geomagnetic field [129,130]. Interactions between these compasses may lead to recalibrations during migration [131,132], and during ontogeny, a combined experience of geomagnetic information and a rotating star pattern is crucial for songbirds to express a relevant population-specific migratory direction [133]. In the wild, tracking studies increasingly reveal complex course changes throughout annual migration periods [134,135]. Such complex routes, involving one or more shifts during migration [135,136], lead to the question how course shifts are encoded relative to the circannual programme in different species and populations of birds. We find experimental support both for course shifts expressed at expected times under constant environmental conditions [12], and shifts expressed after exposure to relevant geomagnetic information predicted to be met en route [137]. In both cases, the endogenous circannual time programme seems to be strongly involved in controlling the timing of shifts. Whether complex course changes throughout the annual cycle are encoded as a sequence of vectors relative to geographical north and expressed only when exposed to local geomagnetic information predicted to be met en route, or simply in a sequence relative to the circannual time programme, however, remains to be shown.
Range expansions and the evolution of new migration routes are especially interesting to study as they may reveal limitations stemming from time programmes and associated navigation mechanisms. A promising study species for addressing such questions is the paddyfield warbler (Acrocephalus agricola), which has expanded its breeding range from Central Asia to the western Black Sea coast during the last century [138]. Despite this considerable westwards expansion, all populations still winter in Pakistan and India [139] (figure 3). Such wintering site fidelity is not entirely surprising because apparent sub-optimal migratory routes after range expansion have been observed in other long-distance passerine migrants [117]. Expanding migration distance as a consequence of an expanded breeding range, however, comes with challenges especially for the most distant populations. The total time spent on migration will increase for the expanded population, and this in turn probably will affect the timing of arrival on wintering sites, arrival at the breeding sites, and time available for breeding (see discussion case study 1). An additional complication for the paddyfield warblers from the most western part of the breeding range arises from the geography, with the Black Sea as an obstacle on the direct route to the wintering areas. Consequently, they seem to follow the route of the range expansion circumventing the Black Sea and the Caspian Sea to the north [138] (figure 3). This new migration route requires the evolution of a new direction of migration, of a subsequent shift in the direction after bypassing the barrier, and of timing of regular stopovers for fuelling (figure 3). Data from other Palaearctic warblers suggest that such changes could happen surprisingly rapidly [141,142]. In other species, in the far north, range expansion after the most recent glaciation period has led to genetic adaptations involving the control of orientation and migration routes around the Baltic Sea, for example in two subspecies of the willow warbler Phylloscopus trochilus trochilus and P. t. acredula [27], migrating to different wintering areas in west and southeastern Africa, respectively [143]. The case of the paddyfield warbler circumventing barriers is, thus, no exception.
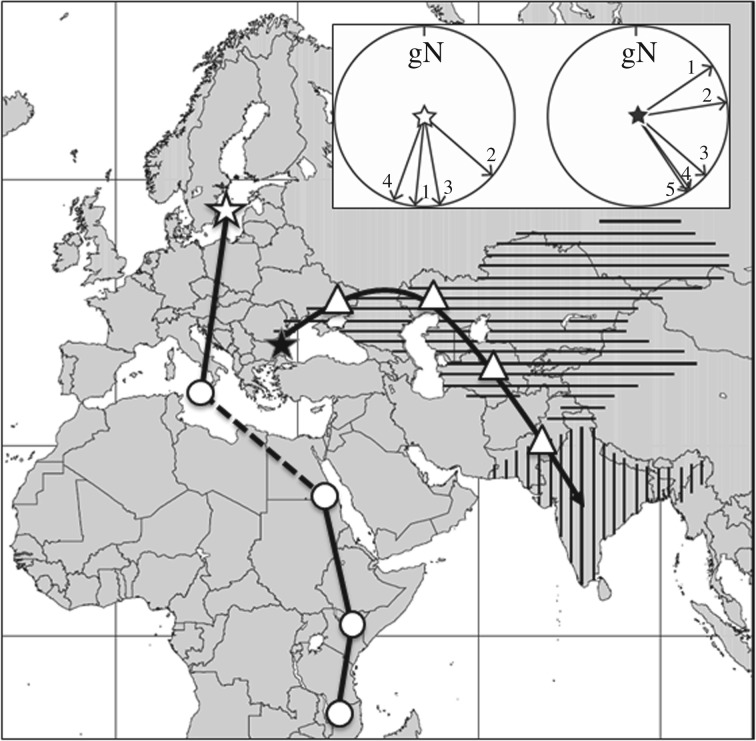
Figure 3.
Hypothetical migration route of the westernmost population of the paddyfield warbler, Acrocephalus agricola, in autumn, compared to a migration route including stopovers of one adult thrush nightingale, Luscinia luscinia tracked by light-level geolocation by Stach et al. [140]. The broken line indicates equinox period when the route is unknown, stars indicate the breeding areas (filled star: paddyfield warbler, open star: thrush nightingale). Open circles indicate recorded locations of stopover sites for the thrush nightingale, while open triangles show hypothetical stopover sites for the paddyfield warbler. The stopover locations have been used for calculations of day length shifts presented in case study 2. Flight directions along the routes of the species are presented in the two circular inlay diagrams. Breeding range and wintering distribution of the paddyfield warbler are marked by horizontal and vertical hatching, respectively.
An associated, possible challenge for the paddyfield warblers is the crossing of longitudes on migration, leading to a predictable, but substantial time shift (3 h 20 min) during autumn migration, combined with the need to adjust the migration course as the journey progresses from approximately 56° relative to geographical north at departure to 146° at the end of the migration (figure 3). This flight route requires repeated, considerable resetting of the circadian rhythm as longitudes are crossed. In comparison, for example, the journey of the thrush nightingale south from Scandinavia to southeastern Africa in autumn [140] requires much smaller adjustments of time (1 h 40 min) and direction (131° to 199°) (figure 3). Substantial north-south movements, including an equator crossing, on the other hand, will involve substantial changes of day length and of movement patterns of celestial bodies across the sky [144], as well as a shifted geomagnetic inclination by 180° [145]. Although we understand some of the adaptations needed to meet these chronobiological and spatial challenges during the first migration, this field of research needs further attention to resolve in what ways time programmes are flexible, and where we may find limitations in how programmes may encode new migration routes.
Detours are commonly observed in long-distance passerine migrants associated with the Palaearctic-African migration system [146]. Recently, geolocator data have shown that Eastern European and Middle Eastern populations of several species pass through the Arabian Peninsula and the Middle East on their spring migration. These routes probably offer more suitable habitats for refuelling after the desert crossing than the more direct routes from African wintering sites [147–149], and possibly also favourable winds, resulting in a longer but energetically more economical route [150]. In this way, being at the right time at the right place, the birds would be able to use the temporarily available resources, despite the disadvantage of needing to travel further. Given such delicate trade-offs, however, unusual environmental events might have significant influence on the timing of migration, as for example the severe drought at the Horn of Africa, which caused a long delay in the spring arrival at the breeding grounds of the long-distance migrants passing through this area [151]. This example shows that endogenous time programmes are sufficiently flexible to account for unexpected delays en route. However, it is still unclear what consequences such delays may have for subsequent life-history events and to what degree songbirds are able to compensate for introduced delays in their circannual programme, as compared to the waders presented in case study 1.
(c). CASE STUDY 3: a globetrotting lifestyle in terrestrial and marine migrants
Key questions: How do birds track resources that vary over time across large geographical areas? How has the circannual programme adapted to track shifts over time and space?
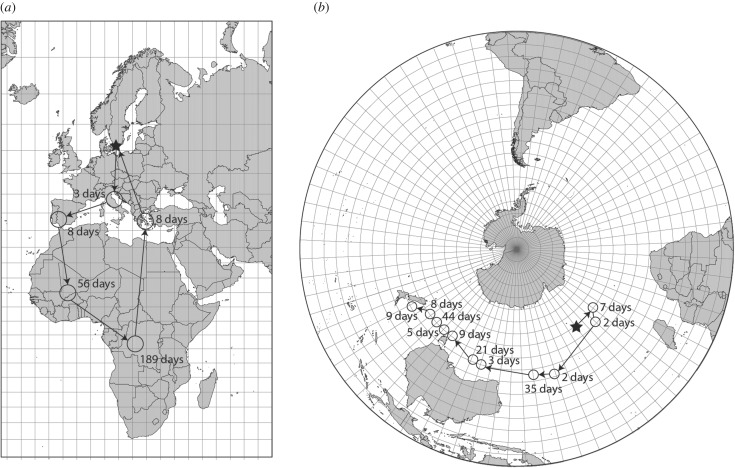
For birds showing high movement capacity and a mobile lifestyle, such as seabirds exploring resources in the open sea and insectivorous terrestrial aerial foragers like swifts, timing their movements relative to local availability of food is central. They also need means to navigate across substantial geographical range. Here we will first discuss seabirds, whose movement patterns may involve a range of behaviours from sedentary with or without roaming exploration flights, to well-timed long directed movements to specific ocean areas [152–154]. As a main example we use the wandering albatross (Diomedea exulans), which explores the Southern Ocean for breeding and foraging. Tracking of wandering albatrosses breeding at the Crozet Islands (approx. 46°S) has revealed a sex-specific movement pattern that was similar for juvenile as well as adult birds, suggesting that the spatial locations at sea are hard-wired and encoded in an endogenous circannual programme for this species (figure 4b) [153]. The sex-specific pattern involves more than twice the movement distance in male compared to female wandering albatrosses (on average ca 3600 km and 1500 km, respectively), probably as a result of competitive exclusion in the past [153,155]. The young albatrosses left by the parents selectively departed from the breeding island to the northeast in tailwind conditions [153]. However, the cues used by the albatrosses to navigate across the Southern Ocean still remain largely unknown [153,156,157]. Tracking individual birds from different breeding islands (Crozet and Kergulean islands) has further revealed at least four movement strategies for the wandering albatross, suggesting substantial variation in the spatio-temporal programme for this species [154]. Wandering albatrosses further enjoy a sabbatical year between breeding events [72], and extensive periods of sexual immaturity before the first breeding, stretching the temporal adaptations of the circannual programme even further. It is interesting to note the large variation in migration phenotypes for a species of seabird inhabiting a substantial part of the Southern Ocean [154], where navigation is especially challenging [156], and where the circadian system needs to be continuously adjusted as longitudes are passed during long movements across open sea (figure 4b). The example of a juvenile wandering albatross tracked during the initial part of its first migration presented in figure 4b shows a gradual movement pattern, with intermediate periods of local residency and segments of faster transportation flights. These temporary stops may be important not only for foraging, but for adjusting the internal time programme to the local day-night light regime at a particular longitude in order to navigate. How the spatio-temporal programme functions, and what information is encoded for navigation in seabirds engaged in continuous circumpolar movements, however, needs to be explained, especially for juvenile birds, and for navigation under particularly challenging conditions around the Antarctic continent [153].
Figure 4.
Examples of complete and partial migration routes of (a) an adult common swift tracked by light-level geolocation for 1 year, and (b) a juvenile wandering albatross tracked by satellite telemetry, illustrating periods of residency (open circles) during migration and migration routes. Stars illustrate starting points corresponding to breeding locations in South Sweden and at Crozet Islands, for the respective species. Data presented modified after Åkesson et al. [138] (a) and Åkesson & Weimerskirch [152] (b).
Many terrestrial birds, such as storks, passerines and swifts, also perform movements to multiple areas where they remain sedentary for some time throughout the annual cycle. They time these movements relative to the availability of food resources involving stationary exploration of several wintering locations and fast transportation between them [55,134,135,158] (figure 4a). The movement of the Intertropical Convergence Zone (ITCZ) causes rainfall to vary with latitude across the year in Africa, and these rainfall patterns result in strong seasonal variations in vegetation growth and insect numbers [159]. Recent tracking of Palaearctic-African migrating birds has demonstrated the gradual movement of birds from initial stopovers in the Sahel Zone [160,161] and nearby areas in early autumn, to wintering areas further south later in winter [83,134,140] (figure 4a). This movement pattern is highly synchronous and was initially proposed based on ringing data [158,162]. The example of a common swift tracked by a miniature geolocator (GLS) shown in figure 4a illustrates this latitudinal movement pattern in winter. Gradual shifts to wintering areas further to the south in late winter are shown by several species, also including for example raptors [163].
Studies of captive birds using Zugunruhe have also demonstrated the flexible reactivation of circannual programmes in winter (e.g. [60]), suggesting that this mechanism is hard-wired and could prepare birds to cope with environmental variations in foraging conditions. These studies suggest that it is the currently deteriorating foraging situation, rather than the expected situation and timing of improvement at the destination, that triggers movement shifts across the winter quarters. At present, it is unclear how birds can predict where the best foraging conditions are. A mobile lifestyle may be challenging for birds exploring the ocean environments, or tropical wintering areas on land for which local food resources vary across time. To fully understand how birds cope with a mobile lifestyle, we need to investigate the intricate interactions between variations in the environment and the resulting timing of birds' movements, including potential influences of endogenous programmes and of the physiological state of the birds [164]. To resolve these questions we will need to record repeated individual tracks of migrants as well as perform controlled experiments in the laboratory, where foraging regimes and migratory restlessness may be experimentally manipulated and measured.
4. Conclusion and future directions
In this review, we have exposed the main timing challenges migratory birds face during the annual cycle. We have drawn on ecological and chronobiological research to outline how the different challenges are tackled. Clearly, our understanding is still in its infancy, but we believe that bird migration research is at an exciting stage when several fields come together to provide answers, and when fast advancement of tracking technology rapidly sheds light on migration strategies and timing in wild populations. To make the most of this opportunity, we see an urgent need for combined efforts of laboratory and field-based approaches. Development of tracking technology provides ever more detail from free-living birds [16], and overall, the data indicate a good match with carefully interpreted data from captive studies [15]. For example, in the study of Bäckman et al. [20] (figure 1), a year-round recording of a free-flying red-backed shrike (Lanius collurio) showed similar time patterns in nocturnal activity as earlier group recordings of captive conspecifics [21]. The field data provide intriguing interpretational context for the Zugunruhe patterns: the long, drawn-out period of autumn Zugunruhe overlapped three separate migration stretches of the free-flying conspecific. During the period when the captive birds moulted, the wild bird remained largely residential, and in both studies, birds started subsequent spring migration almost simultaneously. The wild shrike showed relatively few nights of migratory flights, in contrast to months of Zugunruhe in captivity, which however was averaged over 10 birds. Overall, this comparison supports an interpretation of Zugunruhe as a proxy for a readiness of birds to migrate, but cautions against considering it a direct reflection of migration flight [15].
This example indicates that future tracking studies will guide an understanding of how regulatory mechanisms, inferred from captivity, translate to behaviour in the wild, and how environmental factors influence the outcome. Likewise, we expect that the interpretation of field data can be guided by growing insights into timing mechanisms gathered from captive studies. A particularly promising avenue are genetic approaches [5,116,142,165–169]. These are increasingly used to identify mechanistic processes, specific features and evolutionary consequences of migration. For example, several recent studies compared gene expression, either globally or for candidate genes, between migrants and non-migrants, or between migrants during and outside migration phases [142,165–168]. These studies give important leads to identifying the physiological organization of the timing of migration and the interactive roles of different physiological systems. Other studies have looked at genetic differentiation of populations that differed in migratory phenotype, such as the timing or direction of their journeys [27,169,170]. Several studies indicate evolutionary changes that are associated with the timing of migration [25,171]. It is now entirely feasible to combine tracking data with molecular information, such as differences in candidate genes that based on mechanistic studies in chronobiology are predicted to influence migratory timing, even if suspected links currently are still often tenuous [170]. A concurrent challenge, however, for efficient data-mining of bird tracking information, is development of sound theoretical frameworks within migration biology (e.g. [59,68,172]). The vast amount of data generated with new techniques call, more than ever, for modelling approaches that can generate clear predictions and help integrate information on spatio-temporal movements. Despite these challenges, the fields that we reviewed are already well on their way towards integrated studies of migratory timing.
From this review, many key follow-up questions arise, of which we highlight only a few. A worrying consequence of the intricate timing challenges faced by migratory birds are the potential implications of rapid global change for their fitness. We still know little about how reported changes (or lack thereof) in the timing of migration could affect fitness, in particular because species or populations show widely different behavioural responses. Despite repeatabilities and conservatisms described above, several studies report changes in timing of avian migration caused by climate change and other human interventions (e.g. [74,79,92,173], and references therein). It is poorly known whether there are associated changes in survival and reproductive success [79], and what the potential consequences of mismatches across annual cycle stages exist (but see, [76,80] for examples in birds, and [174,175] for examples in mammals). Some studies suggest that a lack of advancement of arrival at the breeding grounds would be detrimental and correlated with patterns of population declines [176,177] as birds would be constrained to adjust their breeding time in relation to the local conditions [79] (but see [178]). The lack of clarity is partly caused by the difficulty of collecting individual-based long-term data on both phenology and fitness around the annual cycle, which could identify mismatches between timing and optimal environmental conditions [78,79]. Another issue is a bias of greater data collection during breeding compared to other phases of the annual cycle [81], a situation that is improving with the development and miniaturization of tracking devices [179]. Finally, improved knowledge on the mechanistic basis of the relationship between migration phenology and environment is important to forecast species responses to conditions they are not currently exposed to but may be with environmental change [33].
Many open questions that need further attention are related to endogenous programmes and timing of songbird migration. We still lack complete understanding on how clock mechanisms are interacting with external information to encode complex routes throughout the annual cycle (e.g. [134,135]), including what compass birds may use during migration and if a timekeeper is turned on or off during active flight [47,50]. Another open question concerns the degree to which spatial and time programmes in different species of birds allow for flexibility (see also case study 2) [180]. Generally, as explained above, circannual time programmes prevent long-distance migrants from developing reproductive competence in the winter quarters [29–31], explaining for example ‘why Bobolinks don't breed in Brazil’ [30]. Instead, these programmes trigger breeding migrations, sometimes at population-specific times and from sites where related species begin to breed [17–19]. However, the mechanisms that underlie biological rhythms [181,182] can also shed light on the rare occasions when long-distance migrants begin to breed in the winter quarters [182,183]. Biological rhythms synchronize to Zeitgebers (see case study 1) in ways that change across the annual cycle [181]. Reproductive competence is initially inactivated in long-distance migrants, enabling them to leave locations when other birds begin to breed. However, the circannual programme subsequently reduces the inhibition and becomes sensitive to synchronizing cues [30,31]. In this way, rhythms become synchronized to local conditions [181]. Hence, a full phase shift of the entire annual cycle is expected if trans-equatorial migrants stay at the winter quarters until their sensitivity to breeding cues is restored [182]. In a recent, remarkable event, such a reversal has occurred in North American barn swallows (Hirundo rustica) that have started to breed in South American winter quarters. A new study by Winkler et al. [183] reports a full inversion of the annual cycle of the colonizing population, including a reversed direction of migration. It is possible that the swallows' gregarious behaviour may have facilitated delayed departure from the wintering grounds, which in turn phase-shifted their annual cycle. The new study on barn swallows is also an excellent example of the power of intelligent miniature tracking technology [20,184] for revealing information on migration performance in wild songbirds, including route choice, stopover use and flight strategies. If paired with rapidly developing molecular tools [165–170] some long-standing questions of bird migration research can finally be addressed.
We hope to have shown that the spatio-temporal challenges birds are facing when globally tracking fluctuating resources are formidable, in particular at times when environments undergo rapid change, but that birds have also evolved enormous diversity in how they tackle these challenges as exemplified by our three case studies. Understanding the challenges and the scope of responses is more pressing than ever. Migratory species are particularly vulnerable and show severe global declines [93]. Their capacity to adapt to changing conditions will determine their chance to survive, but also has important implications for humans. For example, changes in migration timing, routes and wintering areas can alter ecosystem services and generate conflict with human land use [185,186]. Moreover, migratory birds have been implicated in transmission dynamics of infectious diseases, for which the spatio-temporal characteristics of their movements are of central importance [187,188]. Finally, however, we believe that a better understanding of migratory timing will provide important fundamental insights, as well as inspiration, in view of the fascination that animal movements instil on their human observers.
Acknowledgements
The authors acknowledge Bill Schwartz for support when preparing the manuscript, and Johan Bäckman and Marielle van Toor for sharing data and help with figures. Two anonymous referees helped us considerably.
Ethics
Research projects using animals referred to in this paper adhere to local guidelines and were performed under the appropriate ethical approval and licences in respective country.
Data accessibility
There are no datasets supporting this article.
Authors' contributions
All co-authors contributed to the concept and content of the review, S.Å. and B.H. drafted the introduction with support from B.M.T., J.K. and E.R. drafted case study 1, M.I. and S.Å. drafted case study 2, S.Å. drafted case study 3, B.H. and S.Å. drafted the conclusions and future directions and synthesized the manuscript. All co-authors provided comments on the text. Final approval of the version to be published was given by all co-authors.
Competing interests
We have no competing interests.
Funding
Funding was received to S.Å. from the Swedish Research Council (621-2013-4361) and the Centre for Animal Movement Research (CAnMove) funded by a Linnaeus grant from the Swedish Research Council (349-2007-8690) and Lund University. B.M.T. received a doctorate grant from CNPq (237790/2012-2). J.K. and E.R. were supported by the Spinoza Premium 2014 awarded by NOW to Theunis Piersma. E.R. was additionally supported by a grant to Theunis Piersma from Waddenfonds (‘Metawad’, WF209925).
References
- 1.Rowan W. 1926. On photoperiodism, reproductive periodicity, and the annual migrations of birds and certain fishes. Proc. Boston Soc. Nat. Hist. 38, 147–189. [Google Scholar]
- 2.Aschoff J. 1955. Jahresperiodik der Fortpflanzung bei Warmblütern. Stud. Gen. 8, 742–776. [Google Scholar]
- 3.Stanley CQ, MacPherson M, Fraser KC, McKinnon EA, Stutchbury BJM. 2012. Repeat tracking of individual songbirds reveals consistent migration timing but flexibility in route. PLoS ONE 7, e40688 ( 10.1371/journal.pone.0040688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gwinner E. 1996. Circannual clocks in avian reproduction and migration. Ibis 138, 47–63. ( 10.1111/j.1474-919X.1996.tb04312.x) [DOI] [Google Scholar]
- 5.Berthold P. 1996. Control of bird migration. Berlin, Germany: Springer. [Google Scholar]
- 6.Birkhead TR. 2008. The wisdom of birds. An illustrated history of ornithology. London, UK: Bloomsbury. [Google Scholar]
- 7.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press. [Google Scholar]
- 8.Kramer G. 1957. Experiments on bird orientation and their interpretation. Ibis 99, 196–227. ( 10.1111/j.1474-919X.1957.tb01947.x) [DOI] [Google Scholar]
- 9.Gwinner E. 1967. Circannuale Periodik der Mauser und der Zugunruhe bei einem Vogel. Naturwissenschaften 54, 447 ( 10.1007/BF00603157) [DOI] [PubMed] [Google Scholar]
- 10.Gwinner E. 1986. Circannual rhythms. Endogenous annual clocks in the organization of seasonal processes. Berlin, Germany: Springer. [Google Scholar]
- 11.Wiltschko W, Gwinner E. 1974. Evidence for an innate magnetic compass in Garden Warblers. Naturwissenschaften 61, 406 ( 10.1007/BF00622630) [DOI] [PubMed] [Google Scholar]
- 12.Gwinner E, Wiltschko W. 1978. Endogenously controlled changes in migratory direction of the Garden Warbler Sylvia borin. J. Comp. Physiol. 125, 267–273. ( 10.1007/BF00656605) [DOI] [Google Scholar]
- 13.Maggini I, Bairlein F. 2010. Endogenous rhythms of seasonal migratory body mass changes and nocturnal restlessness in different populations of northern wheatear Oenanthe oenanthe. J. Biol. Rhythms 25, 268–276. ( 10.1177/0748730410373442) [DOI] [PubMed] [Google Scholar]
- 14.Karagicheva J, Rakhimberdiev E, Dekinga A, Brugge M, Koolhaas A, ten Horn J, Piersma T. 2016. Seasonal time keeping in a long-distance migrating shorebird. J. Biol. Rhythms. 31, 509–521. ( 10.1177/0748730416655929) [DOI] [PubMed] [Google Scholar]
- 15.Van Doren B, Liedvogel M, Helm B. 2016. Programmed and flexible: long-term Zugunruhe data highlight the many axes of variation in avian migratory behaviour. J. Avian Biol. 48, 155–172. ( 10.1111/jav.01348) [DOI] [Google Scholar]
- 16.Dominoni D, Åkesson S, Klaassen R, Spoelstra K, Bulla M. 2017. Methods in field chronobiology. Phil. Trans. R. Soc. B 372, 20160247 ( 10.1098/rstb.2016.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conklin JR, Battley PF, Potter MA, Fox JW. 2010. Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat. Commun. 1, 67 ( 10.1038/ncomms1072) [DOI] [PubMed] [Google Scholar]
- 18.Altshuler DL, Cockle KL, Boyle WA. 2013. North American ornithology in transition. Biol. Lett. 9, 20120876 ( 10.1098/rsbl.2012.0876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briedis M, Hahn S, Gustafsson L, Henshaw I, Träff J, Král M, Adamík P. 2016. Breeding latitude leads to different temporal but not spatial organization of the annual cycle in a long-distance migrant. J. Avian Biol. 47, 743–748. ( 10.1111/jav.01002) [DOI] [Google Scholar]
- 20.Bäckman J, Andersson A, Alerstam T, Pedersen L, Sjöberg S, Thorup K, Tøttrup AP. 2017. Activity and migratory flights of individual free-flying songbirds throughout the annual cycle: method and first case study. J. Avian Biol. 48, 309–319. ( 10.1111/jav.01068) [DOI] [Google Scholar]
- 21.Gwinner E, Biebach H. 1977. Endogene Kontrolle der Mauser und der Zugdisposition bei südfinnischen und südfranzösischen Neuntötern (Lanius collurio). Vogelwarte 29, 56–63. [Google Scholar]
- 22.Alerstam T, Hedenström A, Åkesson S. 2003. Long-distance migration: evolution and determinants. Oikos 103, 247–260. ( 10.1034/j.1600-0706.2003.12559.x) [DOI] [Google Scholar]
- 23.Helbig AJ. 2003. Evolution of bird migration: a phylogenetic and biogeographic perspective. In Avian migration (eds Berthold P, Gwinner E, Sonnenschein E), pp. 3–20. Berlin, Germany: Springer. [Google Scholar]
- 24.Piersma T, Pérez-Tris J, Mouritsen H, Bauchinger U, Bairlein F. 2005. Is there a ‘migratory syndrome’ common to all migrant birds? Ann. N.Y. Acad. Sci. 1046, 282–293. ( 10.1196/annals.1343.026) [DOI] [PubMed] [Google Scholar]
- 25.Bearhop S, Fiedler W, Furness RW, Votier SC, Waldron S, Newton J, Bowen GJ, Berthold P, Farnsworth K. 2005. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science 310, 502–504. ( 10.1126/science.1115661) [DOI] [PubMed] [Google Scholar]
- 26.Buehler DM, Baker AJ, Piersma T. 2006. Reconstructing palaeoflyways of the late Pleistocene and early Holocene red knot (Calidris canutus). Ardea 94, 485–498. [Google Scholar]
- 27.Bensch S, Grahn M, Müller N, Gay L, Åkesson S. 2009. Genetic, morphological, and feather isotope variation of migratory willow warblers show gradual divergence in a ring. Mol. Ecol. 18, 3087–3096. ( 10.1111/j.1365-294X.2009.04210.x) [DOI] [PubMed] [Google Scholar]
- 28.Åkesson S, Hedenström A. 2007. How migrants get there: migratory performance and orientation. BioScience 57, 123–133. ( 10.1641/B570207) [DOI] [Google Scholar]
- 29.Helm B, Schwabl I, Gwinner E. 2009. Circannual basis of geographically distinct bird schedules. J. Exp. Biol. 212, 1259–1269. ( 10.1242/jeb.025411) [DOI] [PubMed] [Google Scholar]
- 30.Hamner WM, Stocking J. 1970. Why don't Bobolinks breed in Brazil? Ecology 51, 743–751. ( 10.2307/1934060) [DOI] [Google Scholar]
- 31.Gwinner E. 1988. Photorefractoriness in equatorial migrants. In Proc. 19th Intern. Ornithol. Cong. (ed. Ouellet H.), pp. 626–633. Ottawa, Canada: University of Ottawa Press. [Google Scholar]
- 32.Ramenofsky M, Cornelius J, Helm B. 2012. Physiological and behavioral responses of migrants to environmental cues. J. Ornithol. 153, 181–191. ( 10.1007/s10336-012-0817-3) [DOI] [Google Scholar]
- 33.Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. 2010. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Phil. Trans. R. Soc. B 365, 3113–3127. ( 10.1098/rstb.2010.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler DW, et al. 2014. Cues, strategies, and outcomes: how migrating vertebrates track environmental change. Mov. Ecol. 2, 10 ( 10.1186/2051-3933-2-10) [DOI] [Google Scholar]
- 35.Helm B, Gwinner E, Trost L. 2005. Flexible seasonal timing and migratory behavior. Results from Stonechat breeding programs. Ann. N. Y. Acad. Sci. 1046, 216–227. ( 10.1196/annals.1343.019) [DOI] [PubMed] [Google Scholar]
- 36.Paxton E, Cohen EB, Németh Z, Zenzal T, Paxton KL, Diehl RH, Moore FR. 2015. Spring resource phenology and timing of songbird migration across the Gulf of Mexico. In Studies in avian biology (eds Wood EM, Kellermann JL), pp. 63–82. Boca Raton, FL: CRC Press. [Google Scholar]
- 37.Coppack T, Becker SF, Becker PJJ. 2008. Circadian flight schedules in night-migrating birds caught on migration. Biol. Lett. 4, 619–622. ( 10.1098/rsbl.2008.0388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartell PA, Gwinner E. 2005. A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J. Biol. Rhythms 20, 538–549. ( 10.1177/0748730405281826) [DOI] [PubMed] [Google Scholar]
- 39.Moore FR. 1987. Sunset and the orientation behaviour of migratory birds. Biol. Rev. 62, 65–86. ( 10.1111/j.1469-185X.1987.tb00626.x) [DOI] [Google Scholar]
- 40.Åkesson S, Hedenström A. 2000. Wind selectivity of migratory flight departures in birds. Behav. Ecol. Sociobiol. 47, 140–144. ( 10.1007/s002650050004) [DOI] [Google Scholar]
- 41.Tsvey A, Bulyuk VN, Kosarev V. 2007. Influence of body condition and weather on departures of first-year European robins, Erithacus rubecula, from an autumn migratory stopover site. Behav. Ecol. Sociobiol. 61, 1665–1674. ( 10.1007/s00265-007-0397-z) [DOI] [Google Scholar]
- 42.Bolshakov CV, et al. 2007. Time of nocturnal departures in European robins, Erithacus rubecula, in relation to celestial cues, season, stopover duration and fat stores. Anim. Behav. 74, 855–865. ( 10.1016/j.anbehav.2006.10.024) [DOI] [Google Scholar]
- 43.Sjöberg S, Alerstam T, Åkesson S, Schltz A, Weidauser A, Coppack T, Muheim R. 2015. Weather and fuel reserves determine departure and flight decisions in passerine migrants across the Baltic Sea. Anim. Behav. 104, 59–68. ( 10.1016/j.anbehav.2015.02.015) [DOI] [Google Scholar]
- 44.Zúñiga D, Falconer J, Fudickar AM, Jensen M, Schmidt A, Wikelski M, Partecke J. 2016. Abrupt switch to migratory night flight in a wild migratory songbird. Sci. Rep. 6, 34207 ( 10.1038/srep34207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt-Koenig K. 1990. The sun compass. Experientia 46, 336–342. ( 10.1007/BF01952166) [DOI] [Google Scholar]
- 46.Schmidt-Koenig K, Ganzhorn JU, Ranvaud R. 1991. The sun compass. In Orientation in birds (ed. Berthold P.), pp. 1–15. Basel, Switzerland: Birkhäuser. [PubMed] [Google Scholar]
- 47.Alerstam T, Pettersson S-G. 1991. Orientation along great circles by migrating birds using a sun compass. J. Theor. Biol. 152, 191–202. ( 10.1016/S0022-5193(05)80452-7) [DOI] [Google Scholar]
- 48.Alerstam T, Gudmundsson GA, Green M, Hedenström A. 2001. Migration along orthodromic sun compass routes by arctic birds. Science 291, 300–303. ( 10.1126/science.291.5502.300) [DOI] [PubMed] [Google Scholar]
- 49.Wiltschko W, Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62–64. ( 10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- 50.Kiepenheuer J. 1984. The magnetic compass mechanism of birds and its possible associations with the shifting course direction of migrants. Behav. Ecol. Sociobiol. 14, 81–99. ( 10.1007/BF00291900) [DOI] [Google Scholar]
- 51.Åkesson S, Bianco G. 2016. Assessing vector navigation in birds. Behav. Ecol. 27, 865–875. ( 10.1093/beheco/arv231) [DOI] [Google Scholar]
- 52.Åkesson S, Bianco G. 2017. Route simulations, compass mechanisms and long-distance migration flights in birds. J. Comp. Physiol. A 203, 475–490. ( 10.1007/s00359-017-1171-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helbig AJ. 1991. Inheritance of migratory direction in a bird species: a cross-breeding experiment with SE-and SW-migrating blackcaps (Sylvia atricapilla). Behav. Ecol. Sociobiol. 28, 9–12. ( 10.1007/BF00172133) [DOI] [Google Scholar]
- 54.Delmore KE, Irwin DE. 2014. Hybrid songbirds employ intermediate routes in a migratory divide. Ecol. Lett. 17, 1211 ( 10.1111/ele.12326) [DOI] [PubMed] [Google Scholar]
- 55.Gudmundsson GA, Benvenuti S, Alerstam T, Papi F, Lilliendahl K, Åkesson S. 1995. Examining the limits of flight and orientation performance: satellite tracking of brent geese migrating across the Greenland ice-cap. Proc. R. Soc. Lond. B 261, 73–79. ( 10.1098/rspb.1995.0119) [DOI] [Google Scholar]
- 56.Berthold P, Kaatz M, Querner U. 2004. Long-term satellite tracking of white stork (Ciconia ciconia) migration: constancy versus variability. J. Ornithol. 145, 356–359. ( 10.1007/s10336-004-0049-2) [DOI] [Google Scholar]
- 57.Fuller MR, Seegar WS, Schueck LS. 1998. Routes and travel rates of migrating Peregrine Falcons Falco peregrinus and Swainson's Hawks Buteo swainsoni in the Western Hemisphere. J. Avian Biol. 29, 433–440. ( 10.2307/3677162) [DOI] [Google Scholar]
- 58.Gill RE, et al. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc. R. Soc. B 276, 447–457. ( 10.1098/rspb.2008.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hedenström A, Alerstam T. 1997. Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J. Theor. Biol. 189, 227–234. ( 10.1006/jtbi.1997.0505) [DOI] [PubMed] [Google Scholar]
- 60.Gwinner E, Dittami J, Beldhuis HJ. 1988. The seasonal development of photoperiodic responsiveness in an equatorial migrant, the garden warbler (Sylvia borin). J. Comp. Physiol. A 162, 389–396. ( 10.1007/BF00606125) [DOI] [Google Scholar]
- 61.Goymann W, Spina F, Ferri A, Fusani L. 2010. Body fat influences departure from stopover sites in migratory birds: evidence from whole-island telemetry. Biol. Lett. 6, 478–481. ( 10.1098/rsbl.2009.1028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gwinner E, Schwabl-Benzinger I, Schwabl H, Dittami J. 1993. Twenty-four hour melatonin profiles in a nocturnally migrating bird during and between migratory seasons. Gen. Comp. Endocrinol. 90, 119–124. ( 10.1006/gcen.1993.1066) [DOI] [PubMed] [Google Scholar]
- 63.Fusani L, Gwinner E. 2004. Simulation of migratory flight and stopover affects night levels of melatonin in a nocturnal migrant. Proc. R. Soc. Lond. B 271, 205–211. ( 10.1098/rspb.2003.2561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wingfield JC. 2008. Organization of vertebrate annual cycles: implications for control mechanisms. Phil. Trans. R. Soc. B 363, 425–441. ( 10.1098/rstb.2007.2149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Visser ME, Holleman LJM, Caro SP. 2009. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331. ( 10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornelius JM, Boswell T, Jenni-Eiermann S, Breuner CW, Ramenofsky M. 2013. Contributions of endocrinology to the migration life history of birds. Gen. Comp. Endocrinol. 190, 47–60. ( 10.1016/j.ygcen.2013.03.027) [DOI] [PubMed] [Google Scholar]
- 67.Moore FR, Smith RJ, Sandberg R. 2005. Stopover ecology of intercontinental migrants: en route problems and consequences for reproductive performance. In Birds of two worlds—the ecology and evolution of migration (eds Greenberg R, Marra P), pp. 251–261. Baltimore, MD: John Hopkins Press. [Google Scholar]
- 68.Hedenström A, Barta Z, Helm B, Houston AI, McNamara JM, Jonzén N. 2007. Migration speed and scheduling of annual events by migrating birds in relation to climate change. Clim. Res. 35, 79–91M. ( 10.3354/cr00715) [DOI] [Google Scholar]
- 69.Williams CT, Klaassen M, Barnes BM, Buck CL, Arnold W, Giroud S, Vetter SG, Ruf T. 2017. Seasonal reproductive tactics: annual timing and the capital-to-income breeder continuum. Phil. Trans. R. Soc. B 372, 20160250 ( 10.1098/rstb.2016.0250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jenni L, Winkler R. 1994. Moult and ageing of European passerines. London, UK: Academic Press. [Google Scholar]
- 71.McKinnon EA, Stanley CQ, Stutchbury BJM. 2015. Carry-over effects of nonbreeding habitat on start-to-finish spring migration performance of a songbird. PLoS ONE 10, e0141580 ( 10.1371/journal.pone.0141580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weimerskirch H, Wilson RP. 2000. Oceanic respite for wandering albatrosses. Nature 406, 955–956. ( 10.1038/35023068) [DOI] [PubMed] [Google Scholar]
- 73.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 74.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 75.Visser ME, Perdeck AC, van Balen JH, Both C. 2009. Climate change leads to decreasing bird migration distances. Glob. Change Biol. 15, 1859–1865. ( 10.1111/j.1365-2486.2009.01865.x) [DOI] [Google Scholar]
- 76.van der Jeugd HP, Eichhorn G, Litvin KE, Stahl J, Larsson K, Van der Graaf AJ, Drent RH. 2009. Keeping up with early springs: rapid range expansion in an avian herbivore incurs a mismatch between reproductive timing and food supply. Glob. Change Biol. 15, 1057–1071. ( 10.1111/j.1365-2486.2008.01804.x) [DOI] [Google Scholar]
- 77.van Gils JA, Lisovski S, Meissner W, Lok T, Ożarowska A, de Fouw J, Soloviev M, Piersma T, Klaassen M. 2016. Body shrinkage due to Arctic warming reduces red knot fitness in tropical wintering range. Science 352, 2016 ( 10.1126/science.aad6351) [DOI] [PubMed] [Google Scholar]
- 78.Crick HQP, Dudley C, Glue DE, Thomson DL. 1997. UK birds are laying eggs earlier. Nature 388, 526 ( 10.1038/41453) [DOI] [Google Scholar]
- 79.Gienapp P, Leimu R, Merilä J. 2007. Responses to climate change in avian migration time—microevolution versus phenotypic plasticity. Clim. Res. 35, 25–35. ( 10.3354/cr00712) [DOI] [Google Scholar]
- 80.Both C, Visser ME. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298. ( 10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- 81.Marra PP, Francis CM, Mulvihill RS, Moore FR. 2005. The influence of climate on the timing and rate of spring bird migration. Oecologia 142, 307–315. ( 10.1007/s00442-004-1725-x) [DOI] [PubMed] [Google Scholar]
- 82.Ouwehand J, Both C. 2016. African departure rather than migration speed determines variation in spring arrival in pied flycatchers. J. Anim. Ecol. 86, 88–97. ( 10.1111/1365-2656.12599) [DOI] [PubMed] [Google Scholar]
- 83.Norevik G, Åkesson S, Hedenström A. 2017. Migration strategies and annual space-use in an Afro-Palaearctic aerial insectivore—the European nightjar Caprimulgus europaeus. J. Avian Biol. 48, 738–747. ( 10.1111/jav.01071) [DOI] [Google Scholar]
- 84.Tøttrup AP, Thorup K, Rainio K, Yosef R, Lehikoinen E, Rahbek C. 2008. Avian migrants adjust migration in response to environmental conditions en route. Biol. Lett. 4, 685–688. ( 10.1098/rsbl.2008.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Both C. 2010. Flexibility of timing of avian migration to climate change masked by environmental constraints en route. Curr. Biol. 20, 243–248. ( 10.1016/j.cub.2009.11.074) [DOI] [PubMed] [Google Scholar]
- 86.Crozier LG, Hendry AP, Lawson PW, Quinn TP, Mantua NJ, Battin J, Shaw RG, Huey RB. 2008. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol. Appl. 1, 252–270. ( 10.1111/j.1752-4571.2008.00033.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahola M, et al. 2004. Variation in climate warming along the migration route uncouples arrival and breeding dates. Glob. Change Biol. 10, 1610–1617. ( 10.1111/j.1365-2486.2004.00823.x) [DOI] [Google Scholar]
- 88.Valtonen A, Latja R, Leinonen R, Pöysä H. 2017. Arrival and onset of breeding of three passerine birds in Eastern Finland tracks climatic variation and phenology of insects. J. Avian Biol. 48, 785–795. ( 10.1111/jav.01128) [DOI] [Google Scholar]
- 89.Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569. ( 10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Small-Lorenz SL, Culp LA, Ryder TB, Will TC, Marra PP. 2013. A blind spot in climate change vulnerability assessments. Nat. Clim. Chang. 3, 91–93. ( 10.1038/nclimate1810) [DOI] [Google Scholar]
- 91.Marra PP, Cohen EB, Loss SR, Rutter JE, Tonra CM. 2015. A call for annual cycle research in animal ecology. Biol. Lett. 11, 20150552 ( 10.1098/rsbl.2015.0552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charmantier A, Gienapp P. 2014. Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evol. Appl. 7, 15–28. ( 10.1111/eva.12126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Both C, van Turnhout CAM, Bijlsma RG, Siepel H, van Strien AJ, Foppen RPB. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 277, 1259–1266. ( 10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bairlein F. 2016. Migratory birds under threat. Science 354, 547–548. ( 10.1126/science.aah6647) [DOI] [PubMed] [Google Scholar]
- 95.Helms CW. 1963. The annual cycle and Zugunruhe in birds In Proc. XIII Intern. Ornithol. Congr., pp. 925–939. Ithaca, NY, USA: American Ornithologists' Union. [Google Scholar]
- 96.Wingfield JC. 2005. Flexibility in annual cycles of birds: implications for endocrine control mechanisms. J. Ornithol. 146, 291–304. ( 10.1007/s10336-005-0002-z) [DOI] [Google Scholar]
- 97.McNamara JM, Houston AI. 2008. Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. B 363, 301–319. ( 10.1098/rstb.2007.2141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Conklin JR, Battley PF, Potter MA. 2013. Absolute consistency: individual versus population variation in annual-cycle schedules of a long-distance migrant bird. PLoS ONE 8, e54535 ( 10.1371/journal.pone.0054535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piersma T. 1987. Hop, skip or jump? Constraints on migration of arctic waders by feeding, fattening and flight speed. Limosa 60, 185–194. Dutch with English summary. [Google Scholar]
- 100.Conklin JR, Senner NR, Battley PF, Piersma T. 2017. Extreme migration and the individual quality spectrum. J. Avian Biol. 48, 19–36. ( 10.1111/jav.01316) [DOI] [Google Scholar]
- 101.Battley PF, et al. 2012. Contrasting extreme long-distance migration patterns in bartailed godwits Limosa lapponica. J. Avian Biol. 43, 21–32. ( 10.1111/j.1600-048X.2011.05473.x) [DOI] [Google Scholar]
- 102.Piersma T, Gill RE Jr. 1998. Guts don't fly: small digestive organs in obese bar-tailed godwits. Auk 115, 196–203. ( 10.2307/4089124) [DOI] [Google Scholar]
- 103.Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K. 2000. Empirical evidence for differential organ reductions during trans–oceanic bird flight. Proc. R. Soc. Lond. B 267, 191–195. ( 10.1098/rspb.2000.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buehler DM, Piersma T. 2008. Travelling on a budget: predictions and ecological evidence for bottlenecks in the annual cycle of long-distance migrants. Phil. Trans. R. Soc. B 363, 247–266. ( 10.1098/rstb.2007.2138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bauer S, Gienapp P, Madsen J. 2008. The relevance of environmental conditions for departure decision changes en route in migrating geese. Ecology 89, 1953–1960. ( 10.1890/07-1101.1) [DOI] [PubMed] [Google Scholar]
- 106.Jenni L, Schaub M. 2003. Behavioural and physiological reaction to environmental variation in bird migration: a review. In Bird migration (eds Berthold P, Gwinner E, Sonnenschein E), pp. 155–171. Berlin, Germany: Springer. [Google Scholar]
- 107.Helm B. 2006. Zugunruhe of migratory and non-migratory birds in a circannual context. J. Avian Biol. 37, 533–540. ( 10.1111/j.2006.0908-8857.03947.x) [DOI] [Google Scholar]
- 108.Helm B, Ben-Shlomo R, Sheriff MJ, Hut RA, Foster R, Barnes BM, Dominoni D. 2013. Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc. R. Soc. B. 280, 20130016 ( 10.1098/rspb.2013.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hazlerigg DG, Lincoln GA. 2011. Hypothesis: cyclical histogenesis is the basis of circannual timing. J. Biol. Rhythms 26, 471–485. ( 10.1177/0748730411420812) [DOI] [PubMed] [Google Scholar]
- 110.Piersma T, Brugge M, Spaans B, Battley PF. 2008. Endogenous circannual rhythmicity in body mass, molt, and plumage of great knots (Calidris tenuirostris). Auk 125, 140–148. ( 10.1525/auk.2008.125.1.140) [DOI] [Google Scholar]
- 111.Piersma T. 2007. Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide. J. Ornithol. 148, S45–S59. ( 10.1007/s10336-007-0240-3) [DOI] [Google Scholar]
- 112.Morrison RIG, Davidson NC, Piersma T. 2005. Transformations at high latitudes: why do red knots bring body stores to the breeding grounds? Condor 107, 449–457. ( 10.1650/7614) [DOI] [Google Scholar]
- 113.Dietz MW, Rogers KG, Piersma T. 2013. When the seasons don't fit: speedy molt as a routine carry-over cost of reproduction. PLoS ONE 8, e53890 ( 10.1371/journal.pone.0053890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piersma T. 2011. Flyway evolution is too fast to be explained by the modern synthesis: proposals for an ‘extended’ evolutionary research agenda. J. Ornithol. 152(Suppl. 1), S151–S159. ( 10.1007/s10336-011-0716-z) [DOI] [Google Scholar]
- 115.Berthold P, Pulido F. 1994. Heritability of migratory activity in a natural bird population. Proc. R. Soc. Lond. B 257, 311–315. ( 10.1098/rspb.1994.0131) [DOI] [Google Scholar]
- 116.Liedvogel M, Åkesson S, Bensch S. 2011. The genetics of migration on the move. Trends Ecol. Evol. 26, 561–569. ( 10.1016/j.tree.2011.07.009) [DOI] [PubMed] [Google Scholar]
- 117.Sutherland WJ. 1998. Evidence for flexibility and constraint in migration systems. J. Avian Biol. 29, 441–446. ( 10.2307/3677163) [DOI] [Google Scholar]
- 118.Fransson T, Jakobsson S, Johansson P, Kullberg C, Lind J, Vallin A. 2001. Bird migration: magnetic cues trigger extensive refuelling. Nature 414, 35–36. ( 10.1038/35102115) [DOI] [PubMed] [Google Scholar]
- 119.Moreau RE. 1961. Problems of Mediterranean – Saharan migration. Ibis 103, 373–427. [Google Scholar]
- 120.Moreau RE. 1972. Palaearctic-African bird migration systems. London, UK: Academic Press. [Google Scholar]
- 121.Biebach H, Friedrich W, Heine G. 1986. Interaction of body bass, fat, foraging and stopover period in trans-Saharan migrating passerine birds. Oecologia 69, 370–379. ( 10.1007/BF00377059) [DOI] [PubMed] [Google Scholar]
- 122.Bairlein F. 1988. How do migratory songbirds cross the Sahara? Trends Ecol. Evol. 3, 191–194. ( 10.1016/0169-5347(88)90005-5) [DOI] [PubMed] [Google Scholar]
- 123.Biebach H. 1990. Strategies of trans-Sahara migrants. In Bird migration, pp. 352–367. Berlin, Germany: Springer. [Google Scholar]
- 124.Schmaljohann H, Liechti F, Bruderer B. 2007. Songbird migration across the Sahara: the non-stop hypothesis rejected!. Proc. R. Soc. B 274, 735–739. ( 10.1098/rspb.2006.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmaljohann H, Liechti F, Bruderer B. 2007. Daytime passerine migrants over the Sahara—are these diurnal migrants or prolonged flights of nocturnal migrants? Ostrich J. Afr. Ornithol. 78, 357–362. ( 10.2989/OSTRICH.2007.78.2.38.118) [DOI] [Google Scholar]
- 126.Adamík P, et al. 2016. Barrier crossing in small avian migrants: individual tracking reveals prolonged nocturnal flights into the day as a common migratory strategy. Sci. Rep. 6, 21560 ( 10.1038/srep21560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ouwehand J, Both C. 2016. Alternate non-stop migration strategies of pied flycatchers to cross the Sahara desert. Biol. Lett. 12, 20151060 ( 10.1098/rsbl.2015.1060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Åkesson S, Bianco G, Hedenström A. 2016. Negotiating an ecological barrier: crossing the Sahara in relation to winds by common swifts. Phil. Trans. R. Soc. B 371, 20150393 ( 10.1098/rstb.2015.0393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Able KP. 1980. Mechanisms of orientation, navigation and homing. In Animal migration, orientation and navigation (ed. Gauthreaux SA., Jr), pp. 283–373. New York, NY: Academic Press. [Google Scholar]
- 130.Åkesson S, Boström J, Liedvogel M, Muheim R. 2014. Animal navigation. In Animal movement across scales (eds Hansson L-A, Åkesson S), pp. 151–178. Oxford, UK: Oxford University Press. [Google Scholar]
- 131.Muheim R, Phillips JB, Åkesson S. 2006. Polarized light cues underlie compass calibration in migratory songbirds. Science 313, 837–839. ( 10.1126/science.1129709) [DOI] [PubMed] [Google Scholar]
- 132.Åkesson S, Odin C, Hegedüs R, Ilieva M, Sjöholm C, Farkas A, Horváth G. 2015. Testing avian compass calibration: comparative experiments with diurnal and nocturnal passerine migrants in South Sweden. Biol. Open 4, 35–47. ( 10.1242/bio.20149837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weindler P, Wiltschko R, Wiltschko W. 1996. Magnetic information affects the stellar orientation of young bird migrants. Nature 383, 158–160. ( 10.1038/383158a0) [DOI] [Google Scholar]
- 134.Åkesson S, Klaassen R, Holmgren J, Fox JW, Hedenström A. 2012. Migration routes and strategies in a highly aerial migrant, the common swift Apus apus, revealed by light-level geolocators. PLoS ONE 7, e41195 ( 10.1371/journal.pone.0041195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Willemoes M, Strandberg R, Klaassen RH, Tøttrup AP, Vardanis Y, Howey PW, Thorup K, Wikelski M, Alerstam T. 2014. Narrow-front loop migration in a population of the common cuckoo Cuculus canorus, as revealed by satellite telemetry. PLoS ONE 9, e83515 ( 10.1371/journal.pone.0083515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Helbig AJ, Berthold P, Wiltschko W. 1989. Migratory orientation in blackcaps (Sylvia atricapilla): population-specific shifts of direction during the autumn. Ethology 82, 307–315. ( 10.1111/j.1439-0310.1989.tb00510.x) [DOI] [Google Scholar]
- 137.Beck W, Wiltschko W. 1988. Magnetic factors control the migratory direction of Pied flycatchers (Ficedula albicollis PALLAS). In Ouellet, H. (ed.). Acta XIX Congr. Int. Orn. Ottawa 1986, S. 1955–1962.
- 138.Zehtindjiev P, Ilieva M, Åkesson S. 2010. Autumn orientation behaviour of paddyfield warblers Acrocephalus agricola from a recently expanded breeding range on the western Black Sea coast. Behav. Process. 85, 167–171. ( 10.1016/j.beproc.2010.07.003) [DOI] [PubMed] [Google Scholar]
- 139.Cramp S, Brooks DJ. 1992. Handbook of the birds of Europe, the Middle East and North Africa. The birds of the western Palearctic, vol. VI. Warblers, pp. 396–405. Oxford, UK: Oxford University Press. [Google Scholar]
- 140.Stach R, Jakobsson S, Kullberg C, Fransson T. 2012. Geolocators reveal three consecutive wintering areas in the Thrush Nightingale. Anim. Migr. 1, 1–7. ( 10.2478/ami-2012-0001) [DOI] [Google Scholar]
- 141.Berthold P, Helbig AJ, Mohr G, Querner U. 1992. Rapid microevolution of migratory behaviour in a wild bird species. Nature 360, 668–670. ( 10.1038/360668a0) [DOI] [Google Scholar]
- 142.Boss J, et al. 2016. Gene expression in the brain of a migratory songbird during breeding and migration. Mov. Ecol. 4, 4 ( 10.1186/s40462-016-0069-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chamberlain CP, Bensch S, Feng X, Åkesson S, Andersson T. 2000. Stable isotopes examined across a migratory divide in Scandinavian willow warblers (Phylloscopus trochilus trochilus and Phylloscopus trochilus acredula) reflect their African winter quarters. Proc. R. Soc. Lond. B 267, 43–48. ( 10.1098/rspb.2000.0964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wehner R. 1998. Navigation in context: grand theories and basic mechanisms. J. Avian Biol. 29, 370–386. ( 10.2307/3677156) [DOI] [Google Scholar]
- 145.Wiltschko W, Wiltschko R. 1992. Migratory orientation: magnetic compass orientation of garden warblers (Sylvia borin) after a simulated crossing of the magnetic equator. Ethology 91, 70–74. ( 10.1111/j.1439-0310.1992.tb00851.x) [DOI] [Google Scholar]
- 146.Briedis M, Träff J, Hahn S, Ilieva M, Král M, Peev S, Adamík P. 2016. Year-round spatiotemporal distribution of the enigmatic Semi-collared Flycatcher Ficedula semitorquata. J. Ornithol. 157, 895–900. [Google Scholar]
- 147.Alerstam T. 2001. Detours in bird migration. J. Theor. Biol. 209, 319–331. ( 10.1006/jtbi.2001.2266) [DOI] [PubMed] [Google Scholar]
- 148.Tøttrup AP, et al. 2012. The annual cycle of a trans-equatorial Eurasian–African passerine migrant: different spatio-temporal strategies for autumn and spring migration. Proc. R. Soc. B 279, 1008–1016. ( 10.1098/rspb.2011.1323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koleček J, et al. 2016. Cross-continental migratory connectivity and spatiotemporal migratory patterns in the great reed warbler. J. Avian Biol. 47, 756–767. ( 10.1111/jav.00929) [DOI] [Google Scholar]
- 150.Kranstauber B, Weinzierl R, Wikelski M, Safi K. 2015. Global aerial flyways allow efficient travelling. Ecol. Lett. 18, 1338–1345. ( 10.1111/ele.12528) [DOI] [PubMed] [Google Scholar]
- 151.Tøttrup AP, Klaassen RHG, Kristensen MW, Strandberg R, Vardanis Y, Lindström Å, Rahbek C, Alerstam T, Thorup K. 2012. Drought in Africa caused delayed arrival of European songbirds. Science 338, 1307 ( 10.1126/science.1227548) [DOI] [PubMed] [Google Scholar]
- 152.Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JR. 2010. Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc. Natl Acad. Sci. USA 107, 2078–2081. ( 10.1073/pnas.0909493107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Åkesson S, Weimerskirch H. 2014. Evidence for sex-segregated ocean distributions of first-winter wandering albatrosses at Crozet Islands. PLoS ONE 9, e86779 ( 10.1371/journal.pone.0086779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weimerskirch H, Delord K, Guitteaud A, Phillips RA, Pinet P. 2015. Extreme variation in migration strategies between and within wandering albatross populations during their sabbatical year, and their fitness consequences. Sci. Rep. 5, 8853 ( 10.1038/srep08853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Clobert J, Danchin E, Dhondt AA, Nichols JD. 2001. Dispersal. Oxford, UK: Oxford University Press. [Google Scholar]
- 156.Åkesson S, Alerstam T. 1998. Oceanic navigation: are there any feasible geomagnetic bi-coordinate combinations for albatrosses? J. Avian Biol. 29, 618–625. ( 10.2307/3677182) [DOI] [Google Scholar]
- 157.Boström JE, Åkesson S, Alerstam T. 2012. Where on earth can animals use a geomagnetic bi-coordinate map for navigation? Ecography 35, 1039–1047. ( 10.1111/j.1600-0587.2012.07507.x) [DOI] [Google Scholar]
- 158.Pearson DJ, Backhurst GC. 1976. The southward migration of Palaearctic birds over Ngulia, Kenya. Ibis 118, 78–105. ( 10.1111/j.1474-919X.1976.tb02012.x) [DOI] [Google Scholar]
- 159.Sinclair ARE. 1978. Factors affecting the food supply and breeding season of resident birds and movements of Palaearctic migrants in a tropical African savannah. Ibis 120, 480–497. ( 10.1111/j.1474-919X.1978.tb06813.x) [DOI] [Google Scholar]
- 160.Morel G. 1973. The Sahel zone as an environment for Palaearctic migrants. Ibis 115, 413–417. ( 10.1111/j.1474-919X.1973.tb01979.x) [DOI] [Google Scholar]
- 161.Zwarts L, Bijlsma RG, van der Kamp J, Wymenga E. 2009. Living on the edge: wetlands and birds in a changing Sahel, pp. 472–479. Zeist, The Netherlands: KNNV Publishing. [Google Scholar]
- 162.Hedenström A, Bensch S, Hasselquist D, Lochwood M, Ottosson U. 1993. Migration, stopover and moult of the great reed warbler Acrocephalus arundinaceus in Ghana, West Africa. Ibis 135, 177–180. ( 10.1111/j.1474-919X.1993.tb02829.x) [DOI] [Google Scholar]
- 163.Trierweiler C, Mullie WC, Drent RH, Exo KM, Komdeur J, Bairlein F, Harouna A, de Bakker M, Koks BJ. 2013. A Palaearctic migratory raptor species tracks shifting prey availability within its wintering range in the Sahel. J. Anim. Ecol. 82, 107–120. ( 10.1111/j.1365-2656.2012.02036.x) [DOI] [PubMed] [Google Scholar]
- 164.Goymann W, Lupi S, Kaiya H, Cardinale M, Fusani L. 2017. Ghrelin affects stopover decisions and food intake in a long-distance migrant. Proc. Natl Acad. Sci. USA 114, 1946–1951. ( 10.1073/pnas.1619565114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rastogi A, Kumari Y, Rani S, Kumar V. 2011. Phase inversion of neural activity in the olfactory and visual systems of a night-migratory bird during migration. Eur. J. Neurosci. 34, 99–109. ( 10.1111/j.1460-9568.2011.07737.x) [DOI] [PubMed] [Google Scholar]
- 166.Rastogi A, Kumari Y, Rani S, Kumar V. 2013. Neural correlates of migration: activation of hypothalamic clock(s) in and out of migratory state in the black-headed bunting (Emberiza melanocephala). PLoS ONE 8, e70065 ( 10.1371/journal.pone.0070065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fudickar AM, Peterson MP, Greives TJ, Atwell JW, Bridge ES, Ketterson ED. 2016. Differential gene expression in seasonal sympatry: mechanisms involved in diverging life histories. Biol. Lett. 12, 20160069 ( 10.1098/rsbl.2016.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Johnston RA, Paxton KL, Moore FR, Wayne RY, Smit TB. 2016. Seasonal gene expression in a migratory bird. Mol. Ecol. 25, 5680–5691. ( 10.1111/mec.13879) [DOI] [PubMed] [Google Scholar]
- 169.Ergen AG, Chernetsov N, Lundberg M, Åkesson S, Bensch S. 2017. The use of molecular diagnostics to infer migration directions of Willow Warblers in the southeast Baltic. J. Ornithol. 158, 737–743. ( 10.1007/s10336-017-1434-y) [DOI] [Google Scholar]
- 170.Bazzi G, et al. 2016. Clock gene polymorphism, migratory behaviour and geographic distribution: a comparative study of trans-Saharan migratory birds. Mol. Ecol. 25, 6077–6091. ( 10.1111/mec.13913) [DOI] [PubMed] [Google Scholar]
- 171.Ketterson ED, Fudickar AM, Atwell JW, Greives TJ. 2015. Seasonal timing and population divergence: when to breed, when to migrate. Curr. Opin. Behav. Sci. 6, 50–58. ( 10.1016/j.cobeha.2015.09.001) [DOI] [Google Scholar]
- 172.McNamara JM, Welham RK, Houston AI. 1998. The timing of migration within the context of an annual routine. J. Avian Biol. 29, 395–404. ( 10.2307/3677160) [DOI] [Google Scholar]
- 173.Jenni L, Kéry M. 2003. Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants. Proc. R. Soc. Lond. B 270, 1467–1471. ( 10.1098/rspb.2003.2394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485. ( 10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moyes K, Nussey DH, Clements MN, Guinness FE, Morris A, Morris S, Pemberton JM, Kruuk LEB, Clutton-Brock TH. 2011. Advancing breeding phenology in response to environmental change in a wild red deer population. Glob. Change Biol. 17, 2455–2469. ( 10.1111/j.1365-2486.2010.02382.x) [DOI] [Google Scholar]
- 176.Møller AP, Rubolini D, Lehikoinen E. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16 195–16 200. ( 10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Both C, Bouwhuis S, Lessells CM, Visser ME. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83. ( 10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 178.Goodenough AE, Hart AG, Elliot SL. 2011. What prevents phenological adjustment to climate change in migrant bird species? Evidence against the ‘arrival constraint’ hypothesis. Int. J. Biometeorol. 55, 97–102. ( 10.1007/s00484-010-0312-6) [DOI] [PubMed] [Google Scholar]
- 179.Stutchbury BJM, Tarof SA, Done T, Gow E, Kramer PM, Tautin J, Fox JW, Afanasyev V. 2009. Tracking long-distance songbird migration by using geolocators. Science 323, 896 ( 10.1126/science.1166664) [DOI] [PubMed] [Google Scholar]
- 180.Vardanis Y, Nilsson J-Å, Klaassen RH, Strandberg R, Alerstam T. 2016. Consistency in long-distance bird migration: contrasting patterns in time and space for two raptors. Anim. Behav. 113, 177–187. ( 10.1016/j.anbehav.2015.12.014) [DOI] [Google Scholar]
- 181.Helm B, Visser ME, Schwartz W, Kronfeld-Schor N, Gerkema M, Piersma T, Bloch G. 2017. Two sides of a coin: ecological and chronobiological perspectives of timing in the wild. Phil. Trans. R. Soc. B 372, 20160246 ( 10.1098/rstb.2016.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gwinner E, Helm B. 2003. Circannual and circadian contributions to the timing of avian migration. In Avian migration (eds Berthold P, Gwinner E, Sonnenschein E), pp. 81–95. Berlin, Germany: Springer. [Google Scholar]
- 183.Winkler DW, Gandoy FA, Areta JI, Iliff MJ, Rakhimberdiev E, Kardynal KJ, Hobson KA. 2017. Long-distance range expansion and rapid adjustment of migration in a newly established population of barn swallows breeding in Argentina. Curr. Biol. 27, 1080–1084. ( 10.1016/j.cub.2017.03.006) [DOI] [PubMed] [Google Scholar]
- 184.Hedenström A, Norevik G, Warfinge K, Andersson A, Bäckman J, Åkesson S. 2016. Annual 10-month aerial life-phase in the common swift Apus apus. Curr. Biol. 26, 3066–3070. ( 10.1016/j.cub.2016.09.014) [DOI] [PubMed] [Google Scholar]
- 185.Tombre IM, Eythórsson E, Madsen J. 2013. Towards a solution to the goose-agriculture conflict in North Norway, 1988–2012: the interplay between policy, stakeholder influence and goose population dynamics. PLoS ONE 8, e71912 ( 10.1371/journal.pone.0071912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kronfeld-Schor N, Visser ME, Salis L, van Gils JA. 2017. Chronobiology of interspecific interactions in a changing world. Phil. Trans. R. Soc. B 372, 20160248 ( 10.1098/rstb.2016.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bauer S, Lisovski S, Hahn S. 2015. Timing is crucial for consequences of migratory connectivity. Oikos 125, 605–612. ( 10.1111/oik.02706) [DOI] [Google Scholar]
- 188.Hill NJ, Ma EJ, Meixell BW, Lindberg MS, Boyce WM, Runstadler JA. 2016. Transmission of influenza reflects seasonality of wild birds across the annual cycle. Ecol. Lett. 19, 915–925. ( 10.1111/ele.12629) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no datasets supporting this article.