Abstract
Under natural conditions, many aspects of the abiotic and biotic environment vary with time of day, season or even era, while these conditions are typically kept constant in laboratory settings. The timing information contained within the environment serves as critical timing cues for the internal biological timing system, but how this system drives daily rhythms in behaviour and physiology may also depend on the internal state of the animal. The disparity between timing of these cues in natural and laboratory conditions can result in substantial differences in the scheduling of behaviour and physiology under these conditions. In nature, temporal coordination of biological processes is critical to maximize fitness because they optimize the balance between reproduction, foraging and predation risk. Here we focus on the role of peripheral circadian clocks, and the rhythms that they drive, in enabling adaptive phenotypes. We discuss how reproduction, endocrine activity and metabolism interact with peripheral clocks, and outline the complex phenotypes arising from changes in this system. We conclude that peripheral timing is critical to adaptive plasticity of circadian organization in the field, and that we must abandon standard laboratory conditions to understand the mechanisms that underlie this plasticity which maximizes fitness under natural conditions.
This article is part of the themed issue ‘Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals’.
Keywords: chronobiology, seasonality, clocks, peripheral clocks, timing, circadian
1. Introduction
The temporal programme of behaviours and physiology expressed by an organism is driven by a vast network of clocks and rhythms distributed across tissues throughout the body [1]. The interactions within this network and its response to external time cues have been intensively studied in laboratory experiments. Here we want to assess how this network would operate under natural conditions, where a plethora of potential time cues are—often simultaneously—acting on it.
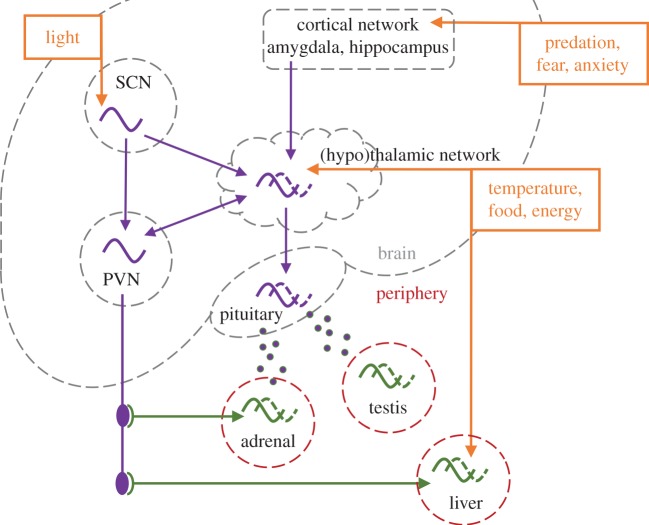
The hierarchical view of the circadian system in mammals is that the clock in the suprachiasmatic nucleus (SCN) of the hypothalamus is entrained by the environmental light–dark conditions, and that this timing information is transmitted to clocks in other brain regions and tissues of the body (figure 1). All non-SCN clocks are collectively known as the ‘peripheral timing system’, and we know that almost every cell in the body can expresses a circadian clock, making the peripheral timing system a large and complex system that expresses many rhythms with different phases. The current hierarchical view that the SCN acts as the conductor of this orchestra of peripheral clocks underplays the independence of most peripheral clocks [2], which can autonomously entrain to many non-photic timing cues (Zeitgebers) such as food availability [3,4], temperature [5], arousal [6] and internal glucocorticoid levels [7], independently of SCN derived timing.
Figure 1.
Schematic representation of the biological timing system relevant to this paper. PVN, paraventricular nucleus; SCN, suprachiasmatic nucleus.
Perhaps one of the most intriguing questions is how timing of all the clocks in the peripheral timing system can be mapped against the abundance in rhythms in physiology and behaviour. Several clocks will contribute to the timing of a single process, which is, for example, clear from the simultaneous expression of both light and food driven timing of behavioural activity [8,9]. In laboratory conditions, photic and non-photic cues may be altered independently, whereas in the field they are not always independent. For example, daily temperature cycles can be offered in anti-phase with the light–dark cycle in the laboratory, but in nature temperature is typically higher during the light phase due to the heat that radiates from the sun. Similarly, laboratory experiments offering food exclusively during the rest phase have taught us a lot about the food entrainable oscillator (FEO), but under natural conditions food intake will mostly happen in the active phase, and timing of food availability may in fact be one of the reasons why an organism is active at that time. These dependencies among Zeitgebers in nature are complex, and our laboratory experiments do not necessarily take these complex relationships into account, which reduces the translational value of our laboratory experiments.
An added complication is that phase relationships between Zeitgebers can be variable in nature. For instance, predation risk for small rodents may be inflicted by both nocturnal and diurnal predators, and depending on season, vegetation cover or habitat, high nocturnal predation risk may be replaced by high diurnal predation risk. Such changes may also lead to adaptations in prey species, leading to changed phase angles of peripheral oscillators in the body. In general, Zeitgebers to which peripheral clocks entrain, such as food intake, light exposure, temperature and arousal can differ in their temporal relationships due to factors such as predation, seasons, climate, social interactions and ongoing day-to-day variation. In addition, animals can often self-regulate light exposure in the field by retreating into burrows, a condition that is not always available in laboratory conditions. Adaptation to, and anticipation of these timed events requires a flexible phenotype that responds to daily, seasonal and annual changes in the environment, and given that different peripheral clocks (in e.g. the liver, heart and adipose tissue) may respond differently to each of the Zeitgebers, this flexible phenotype must rely heavily on the peripheral timing system.
The complexity of the changes in this peripheral, multi-clock timing system that result from altering phase relationships between photic and non-photic cues, are nicely demonstrated by studies on humans and rodent models in the laboratory, in which photic and non-photic cues are misaligned in conditions such as shift work and sleep restriction. For instance, shift work in mice or humans leads to severe and complex disruption of timing of gene transcription, and the timing of gene expression may not simply shift in line with a single oscillator, but exposes genes in which rhythmicity is lost or altered in phase and amplitude, and new rhythms even appear where transcription was non-rhythmic before [10,11]. This leads to the view of the biological timing system as a four-dimensional landscape of loosely delineated, resonating tissue clocks and rhythms which combine to drive timing of behaviour and physiology. Perturbations in this system may be associated with several adverse health conditions such as obesity, diabetes, cancer, problems with cognitive performance and mood disorders in humans, but almost nothing is known about the implications of this complex system on timing and survival of non-humans in nature.
Here, we hypothesize that in natural conditions the peripheral timing system is key to the flexible phenotype, and that this flexibility cannot be attributed to the dominance of a single ‘master’ oscillator in the brain. We provide evidence for this hypothesis in four aspects of the temporal niche in the field: reproduction, endocrine activity, feeding and chronotype, and argue that, given this evidence, we must abandon standard laboratory conditions if we are to understand the regulatory mechanisms that underlie flexible timing in the field, where it is essential to survival.
2. Mechanisms of orchestrating peripheral timing in the field
Endogenous circadian rhythms and their alignment with the external world provide an adaptive advantage when they help animals to predict upcoming events or conditions in their (a)biotic environment. Such anticipation of environmental risks and opportunities can increase fitness in many ways such as limiting predation risk, generating offspring at times of plentiful food availability, and by increasing energy efficiency. In nature, however, the temporal occurrence of Zeitgebers may be irregular, and can vary among Zeitgebers such as light, food and predation, which can cause a reduction in fitness due to misalignment of endogenous and environmental rhythms. Moreover, such misalignment may not only exist between the environment and the endogenous timing system as a whole, but because clocks in different tissues synchronize (entrain) to different Zeitgebers, misalignment may also occur among oscillators within the body. Therefore, the process of maximizing the temporal niche in nature faces two challenges: (i) how to align circadian rhythms of multiple tissues correctly towards each other, and (ii) what is the most beneficial internal alignment pattern for any given environmental temporal niche? These questions remain mostly unanswered, and we will discuss these by taking a bottom-up approach, starting at the cellular circadian oscillator.
3. The molecular circadian clockwork is expressed in almost all cells
A key feature of circadian rhythms is that they are endogenously driven with a period close to 24 h in constant conditions. To a large extent, these rhythms are considered to be generated by cellular molecular oscillators, which are observed in almost every nucleated cell of the body. These oscillators drive widespread temporal gene expression patterns that are tissue specific [12], forming the foundation of rhythmic physiology and behaviour. In mammals, this clockwork consists of two interlocking transcription–translation feedback loops in which proteins serve as negative feedback on their own RNA transcription. This molecular oscillator can explain the rhythmic expression of numerous tissue-specific genes through rhythmic interactions with the E-Box, D-Box, and Rev response elements (RREs) in the promotor regions of many genes throughout the genome [13]. However, this molecular oscillator can also have more subtle effects on downstream gene expression, for example through opening up the chromatin structure, which ‘gates’ the ability of circadian transcription factors to bind to the promotor region of target genes [14–16]. It is easily conceivable that many clock controlled genes are influenced in their final phase of expression by the combined actions of these rhythmic core transcription factors [17].
It is estimated that the cellular molecular clock drives thousands of different protein coding genes to exhibit daily rhythms in expression [12]. These circadian transcripts contribute significantly to the physiological functions of a cell. However, because circadian rhythmicity is so widespread, the origin of circadian rhythmicity in a given processes can be complex. For example, the response to a constant signal (e.g. a stable plasma glucose level) may still be rhythmic if there is a daily rhythm in the sensitivity of a cell due to a daily rhythm in receptor expression. In line with the different physiological roles of different cell types and tissues, we typically observe different combinations of transcripts of clock controlled genes (CCGs) across tissues, and therefore each tissue possesses its own, unique rhythmic transcriptome [12,18]. Moreover cellular clocks and their accompanying rhythmic transcriptome are sensitive to entrainment by Zeitgeber in a tissue-specific way; the liver is primarily entrained by food intake, whereas the SCN of the hypothalamus is almost exclusively entrained by environmental light–dark cycles.
4. The suprachiasmatic nucleus as central light entrainable clock
In mammals, neurons in the SCN display a robust and high amplitude circadian rhythm in neuronal activity, with high frequency action-potential trains during daytime and relatively few neurons firing at night [19,20]. Neurons of the SCN are highly coupled, increasing both the robustness as well as the accuracy of the generated rhythms (for review see [21,22]). Light is the main Zeitgeber that determines the phase, period and amplitude of the SCN (for review see [23]). The SCN is often regarded as the main circadian pacemaker that sets the phase of peripheral oscillators throughout the body, but—while this might be valid under stable laboratory conditions—natural conditions might reveal the SCN's role as less prominent. SCN lesions in rodents lead to a loss of circadian rhythmicity in physiology and behaviour in constant environments, a finding that first led to the (now outdated) title of ‘master clock’ for the SCN [24–26]. However, housing animals in more natural rhythmic environments, in which factors such as light-level, food abundance, social cues, ambient temperature, or exposure to rewarding or fearful experiences show daily variation, can reinstate rhythms in behaviour and physiology in SCN-lesioned individuals [8,27,28]. These rhythmic phenotypes in SCN lesioned animals are testimony to the fact that circadian responses that animals display can (in part) be independent of SCN timing, and that rhythmicity in physiology and behaviour may also be a direct response to environmental cues (masking [29], also see [30]) or may even trigger extra-SCN circadian oscillators to drive rhythmicity in the body [8,31]. Rhythmicity in peripheral organs therefore does not require an intact SCN, and instead, it seems more appropriate to denote the SCN as an entity which provides the body and brain with an internal representation of the external light–dark cycle, rather than a ‘master’ clock.
5. Linking the suprachiasmatic nucleus and peripheral timing
Under constant laboratory conditions, the (dorsal) SCN provides a phase reference through direct neuronal projections, releasing glutamate, GABA, AVP and other factors. These pathways regulate target (thalamic and hypothalamic) brain regions through neuronal projections [32–34] and paracrine output [35]. A major neuronal output pathway is the SCN's projection to the paraventricular nucleus (PVN), driving the autonomous nervous system that reaches peripheral organs such as liver, kidney and adrenal gland [36,37]. The SCN-PVN-adrenal projection offers a potentially important role for glucocorticoids to mediate circadian organization in the body [7,37,38], although they can also encode stressful events.
Glucocorticoids have been shown to be key Zeitgebers for peripheral tissues [7], which can interfere with other Zeitgebers such as food availability [39] as described below. There are also several interactions between circadian and glucocorticoid systems during early development that are still not well understood [40]. For example, fetuses exposed to increased maternal glucocorticoid concentrations display phase-advances in locomotor activity rhythms later in life (citations in [40]). This effect is likely mediated through glucocorticoid receptors, which are present in the fetal SCN [41]. Further blood-borne Zeitgebers could include several cytokines—including TNF-α and IL-6, which were suggested to have regulatory effects on peripheral clocks [42,43]. As these anti-inflammatory cytokines are also produced by glial cells, it remains to be tested whether glial communication might also be involved in the principles of entraining and maintaining rhythmicity in neuronal tissues (a theory already proposed more than 20 years ago [44]).
6. Modulation of suprachiasmatic nucleus rhythmicity by peripheral feedback
The SCN seems primarily entrained by photic input (both directly through the RHT and indirectly through the geniculohypothalamic–neuropeptide Y (GHT–NPY) pathway). In addition to this, non-photic inputs can also modulate the circadian properties of the SCN clock (see for review [45]). Inducing activity during the day was shown to increase NPY release from IGL terminals onto SCN cells at the time of induced running [46]. Indeed, running wheel access and scheduled activity can have considerable impact on light entrained activity patterns in rodents and also in humans [45]. Another level of SCN modulation by peripheral feedback may be through sex hormones such as androgens and oestrogens, for which the SCN expresses receptors [47,48], as is described in §11.
7. Non-suprachiasmatic nucleus derived Zeitgebers of peripheral clocks
Physiological output rhythms of one tissue (e.g. body temperature or corticosterone release) can serve as rhythmic input to other cellular clocks. For example, all peripheral tissues can entrain to body temperature cycles in vitro [5]. Often, peripheral tissue clocks are sensitive to (rhythmic) inputs from multiple sources (e.g. expressing both melatonin and glucocorticoid receptors and receiving both sympathetic and parasympathetic input). In addition, it is well known that behavioural rhythms such as feeding and fasting can have a strong effect on the circadian phase of local tissue clock-gene rhythms. The wide diversity of signalling routes makes it a complex and daunting task to understand the phase relationship between cellular clocks in multiple tissues, and grasp the flexibility between circadian rhythms in the SCN versus those in non-SCN tissues. For most tissues we still need to establish which specific inputs determine the phase of the local cellular clock. Local clock-phase is likely the result of checks and balances between multiple inputs collectively, and the relative contributions of the involved signals may be altered depending on the environmental or internal condition at the time that the Zeitgebers are perceived.
A flexible timing system would be essential for evolutionary reasons, as circadian organization may need to alter alignment when the environment or the animal's internal state changes over time, requiring adaptive phasing of behaviour and physiology. We are only just starting to comprehend the flexibility between the phase of the SCN and the phase of organ clocks. Furthermore, in order to obtain a useful concept of this flexibility, it is important to understand the dynamics and variability of the natural habitats. Understanding the relevance and limitations of circadian flexibility may bear highly relevant insights into human health (e.g. shift work, jet-lag recovery, performance and productivity, timed medicine, and healthy lifestyles). Similarly, understanding how the daily timing system can incorporate past experiences into future predictions would be a mayor insight helpful to conservation biology, for instance by allowing predictions on how species can adapt with changing habitat conditions such as caused by global warming.
8. Contribution of peripheral clocks to flexible reproductive timing in the field
Reproductive activity is typically observed during specific seasons, and circadian timing of Zeitgebers such as dusk and dawn (photoperiod) predictably vary between these seasons. Indeed, in the laboratory, rodents from the temperate zone often respond robustly to short photoperiod by suppression of reproductive physiology and activity, and respond to long photoperiod by stimulating reproduction [49,50]. These photoperiodic responses depend upon nocturnal secretion of melatonin by the pineal, driven by a functioning central circadian clock [51]. In the field, however, the patterns can be more complex than explainable by reference solely to melatonin and the central clock [52]. For example, in some species of Peromyscus, long photoperiods in the laboratory trigger fertility and breeding [49], but the same species—and often the same populations in the field—exhibit a summer, long-day hiatus in breeding [52–56]. The causes of the summer interruption in breeding are debated, with lines of evidence emerging that parasites, disease and food availability all have significant roles in this behaviour [55,57–59].
The mismatches between laboratory and field data are conceivably due to (i) different inputs to the central clock in the SCN, (ii) difference in other environmental factors that act as gatekeepers for the reproductive axis by affecting hypothalamic reproductive neurons, and (iii) environmental factors that act on peripheral clocks in reproductive tissues. The presence of robust peripheral circadian rhythms has been implicated in reproductive function of the hypothalamus [60], pituitary, gonads and reproductive organs [61,62]. The single exception is the testis, which exhibits relatively weak circadian rhythms [61,63–66]. In the testis, the peripheral clock is blocked by the testis-specific repressor PASD1 acting against CLOCK:BMAL1 [67]. However, testicular Leydig cells and their secretory rhythm of testosterone appear to be controlled at least partially by a peripheral clock [68]. Disruption of peripheral clocks may contribute to infertility [62,68,69], suggesting that these peripheral clocks are potential targets for environmental modification of reproduction.
9. A role for peripheral clocks in incorporating food availability and disease-loads into reproductive functions
In males, even in the absence of strong peripheral clocks in the testis, peripheral clocks have the potential to alter fertility. Dissociating feeding cycles from the light–dark cycle (known as temporal food restriction; described in §10) has been reported to cause changes in androgen-dependent male reproductive tissues, including changes that inhibit fertility [70]. In wild mammals, this response to temporal food restriction could inhibit fertility when unusual conditions force a male to feed outside of the normal activity period. This response to temporal food restriction may increase fitness by reducing the costs of reproduction until conditions return a male to a normal feeding cycle.
Peripheral clocks appear to be present in reproductive tissues across mammals, birds, other vertebrates and invertebrates [31]. Some of these clocks may have little direct effect on reproduction; instead, for example, they could be coordinating a daily cycle of nutrient input from gut and liver with body-wide cycles of nutrient demand [71–73]. However, there is evidence for relevance of these peripheral clocks to fertility. There is a growing body of evidence that peripheral circadian clocks in the ovaries of vertebrate females can adjust the sensitivity of the ovary to luteinizing hormone (LH), thereby modifying the LH surge that causes ovulation [31,74,75]. The surge in LH induces a surge in oestrogen, which induces mating behaviours in many species. Therefore, peripheral clocks have the potential to alter the timing of ovulation, mating and conception in mammals [31,74] and birds [75]. The timing of mating may affect the risk of predation during mating, while alterations in the timing of conception may alter the timing of birth. Therefore, peripheral ovarian clocks that regulate mating and pregnancy may serve an important ecological function for reproductive timing.
Ovarian clocks may affect both the development of embryos and sex steroid secretion. After fertilization, the oviduct supports the developing embryo for a period of days, and the timing of mating affects the proportion of morphologically normal embryos through embryonic development. An influence of the timing of mating on the frequency of morphologically defective embryos in the oviduct suggests that a peripheral clock in the oviduct could affect fertility [76]. Elsewhere in the ovary, clock gene knockouts restricted to steroidogenic tissues disrupt the secretion of steroids, altering stages of the female reproductive cycle from ovulation to parturition [77], further indicating the importance of peripheral clocks for fertility and reproduction. Following conception, the corpus luteum produces progesterone to maintain the uterus in a condition to support embryos. Clock and Bmal1 knockouts that are either systemic, ovary-specific, or even clock-gene knockdowns within the ovary, can all result in implantation failures. These failures may be caused by a deficient clock in the corpus luteum, which can contribute to reduced progesterone secretion and failure of implantation or reabsorption of an embryo [31,77–80]. In nature, disruption of a peripheral clock in the corpus luteum could be an ecologically relevant mechanism for post-fertilization blockage of pregnancy, in response to an environment hostile to raising offspring. Finally, uterine myometrial deletion of Bmal1 can alter the timing of parturition [81], providing further evidence for peripheral clocks as modulators of reproductive events. In mammals, the timing of birth is often linked to specific times of day that minimize risk to mother and offspring during this vulnerable period, suggesting an important ecological role for a myometrial uterine clock.
10. How the reproductive state might affect peripheral rhythms of other organs
Peripheral reproductive clocks respond to ecologically relevant signals that include availability of metabolic fuels. These include glucose-sensing [82] and responses to free fatty acids [83] by specific aspects of the molecular cellular clock. Peripheral clocks in the adrenal glands, and elsewhere, have been shown to contribute to the control of blood glucose [82]. Neurons in the central circadian clock alter function in response to glucose-sensing [84], and glucose-sensing alters the function of peripheral clocks in the liver [85] and isolated fibroblasts [86]. There is evidence for feedback from reproductive tissues to the liver, as pregnancy alters circadian expression of clock genes in the liver [87], raising the intriguing possibility that synchronization between peripheral clocks in the liver and placenta are a normal part of pregnancy. One function of peripheral reproductive clocks may be to stimulate or inhibit activity in reproductive organs based upon availability of metabolic fuels as well as to stimulate other peripheral clocks to increase nutrient availability.
Fertility is affected by parasites [88] and disease, and there are ecological trade-offs between immunity and fertility [89,90]. It seems likely that there are interactions between parasites, disease and inflammation with peripheral clocks involved in fertility. Peripheral clocks exist in immune tissues (see table 1 in [91]), and the immune system has multiple mechanisms of signalling to the circadian system [92]. These local responses may be a particularly rich area for investigation. Pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) are major molecular inputs to pattern-recognition receptors in signalling pathways that respond to damage or disease [93]. Peripheral clocks can control the timing of expression of receptors for DAMPs or PAMPs (e.g. [94]), and immune cells that respond to DAMPs and PAMPs produce circulating signals that can affect peripheral clocks in other tissues [93,95,96]. For example, specific regions of the ovary, oviduct and uterus (all of which contain peripheral clocks) might respond to local signals of damage by modifying their clock function. Firstly, peripheral clocks in reproductive tissues might be impacted by regions of damage to reduce, for example, the receptivity of dysfunctional areas within a uterus to implantation. Secondly, systemic signalling induced by parasites or disease may affect peripheral reproductive clocks. Systemic effects of rhythms in the peripheral immune system include the circadian rhythm of TNF-α, driven in part by peripheral clocks in lymphocytes of the immune system [97], as well as proinflammatory cytokines regulated by the clock protein CRYPTOCHROME [98]. The role of cytokine signalling from peripheral clocks to the reproductive system is not well studied, but there are indications that cytokines affect fertility. Local proinflammatory signals may be a normal aspect of mammalian implantation [99], while excessive proinflammatory signals may disrupt fertility and pregnancy [100,101] and contribute to infertility in males [102,103]. Here, there are profound gaps: could PAMPS and DAMPS affect peripheral clocks and rhythms in the reproductive system and fertility, either directly, or indirectly via signals from clock-driven lymphocytes? It seems reasonable that circadian clocks may integrate information about damage or parasite infestation to cause adaptive, localized responses that allow the reproductive system to bypass areas of damage, and direct activities and embryos to areas that are undamaged. One could speculate that signals from the immune system modify peripheral clocks involved in pregnancy, in order to alter the rate of development during responses to parasites or disease. In such a case, peripheral clocks may permit adaptive changes in fertility in response to systemic signals of parasitism or disease.
It is plausible that these peripheral clocks are sensitive to additional environmental inputs that in nature either enhance or suppress fertility to increase fitness. For example, high levels of androgens from either endogenous or exogenous sources may disrupt peripheral clocks and alter fertility [104]. Both central and peripheral clocks contain elements for glucose sensing [85] and free fatty acid sensing [83], suggesting that peripheral clocks in reproductive tissues might modify fertility based upon the availability of nutrients. Decreases in Leydig cell metabolism may be linked via the cell energy-sensing protein SIRT-1 to the peripheral clock and reduced secretion of testosterone, suggesting a potential link between the nutritional environment and fertility in males [105]. Variation in nutritional input may be due to external ecological factors, such as drought or competition for resources, or due to internal signals, such as nutritional modulation by social cues, parasites or disease.
It is clear from laboratory studies that peripheral clocks are necessary for normal fertility, and that modulation of peripheral clocks may enhance or inhibit fertility. However, whether such involvement of peripheral timing benefits fertility and reproduction in field condition remains mostly unclear. As it stands, there is insufficient evidence arguing either for or against such benefits, but given the many roles of peripheral clocks in regulating fertility, particularly in females, it is reasonable to expect that environmental signals act on fertility in many animals in part by modifying peripheral clocks.
11. Contributions of sex hormones to flexibility timing in the field
Reproductive hormones such as sex steroids convey information on biotic or abiotic variation in the environment of an organism to the internal central and peripheral clock systems. It is well established that social and environmental factors can modulate plasma concentrations of sex steroids both in the field and in the laboratory [106,107], as well as cause ubiquitous changes in many other hormones related to reproduction in vertebrates [108–110]. For example, in the majority of seasonal vertebrate species, circulating concentrations of sex steroids increase dramatically when the reproductive axis becomes activated as a result of photic stimulation. Such elevated levels of sex steroids serve to promote the expression of behaviours associated with reproduction, including territorial aggression, courtship, competitive and mating behaviour [111–114]. Not only photic signals, but also social stimuli can be potent modulators of sex steroid concentrations [115,116].
In many species, plasma sex steroid concentrations undergo diel variations, with testosterone typically being elevated at night and/or during the early morning [117–119]. While such diel rhythms in sex steroid concentrations are clearly present in the laboratory, at present we lack evidence for their existence under natural conditions (though diel rhythms in glucocorticoids are present in wild populations [120,121]). As a result, specific characteristics of diel rhythms in sex steroids in wild populations such as phase angles and amplitudes, as well as their variation with season, local environmental conditions and social circumstances still have not received much attention, and remain mostly unknown. Partly, this lack of knowledge may arise from logistical issues associated with sampling wild populations repeatedly at specific times of day. However, non-invasive techniques such as hormone analyses from excrements and new technologies including implantable biosensors may open up new opportunities for closing this gap [122,123]; but see [124].
A topic that has received more attention, at least in select species, are the actions of sex steroids on circadian timing. In various vertebrates, experimental manipulation of sex steroid concentrations and/or their rhythms has profound effects on circadian characteristics, with consequences for entrainment properties including altered phase angles and activity timing [125]. For example, in male mice a reduction of testosterone concentrations through gonadectomy results in a lengthening of the free-running period (τ) of circadian activity rhythms and reduces the precision of activity onset as well as overall activity levels, while androgen replacement restores these changes [47,126]. Oestrogens also can affect circadian properties in rodents [127]. For example, natural increases in oestrogen concentrations during oestrous as well as experimental oestrogen administration phase-advances behavioural activity rhythms in some rodent species [128–130]. Oestrogens can also increase overall behavioural locomotor activity, and oestrogens may also modulate the effectiveness of non-photic stimuli mediated through, for example, wheel-running on circadian rhythms [131]. However, the proximate mechanism underlying these oestrogenic actions in female rodents is still debated and may involve changes in activity levels, arousal, sleep and responsiveness to non-photic stimuli rather than changes in τ, although species differences may also account for some divergences in mechanisms [48,130,132].
In terms of mechanism, the effects of androgens on τ in rodents are likely mediated by androgen receptors that are present in the SCN [47]. The SCN also expresses oestrogen receptors, although only sparsely in female rodents [48]. Hence, effects of oestrogens on circadian functioning may occur primarily through peripheral sites outside the SCN, and mechanisms of actions may differ between the two sexes [48]. Both androgen and oestrogen receptors have been located in brain regions that communicate with the SCN, as well as other parts of the body including the gonads [133].
Taken together, the available evidence from laboratory-based studies suggests that sex steroids can have substantial modulatory effects on circadian rhythms, although there exist large variations in these effects and the mechanisms they employ among taxa. It has been hypothesized that these actions are adaptive, serving to fine-tune activity times to match altered needs during the reproductive period. For example, altered phase-angles during oestrous may promote encounter rates between males and females, thus increasing mating opportunities [128]. Rigid field tests of this appealing idea are thus far still lacking. However, some recent field studies in avian species have corroborated fitness benefits associated with altered circadian function during the reproductive period [134]. For example, in blue and great tits, males with earlier activity and song onset during the mating period gain more extra-pair fertilizations than males that become active later [135,136]. Further, experimentally induced delays in male activity onset increase the rate of cuckoldry of focal individuals, suggesting that mate-guarding abilities are also impaired by mistimed activity [137]. It is tempting to speculate that sex steroids, which are increased during the reproductive season, are involved in this circadian reorganization, but direct tests of this idea have not yet been carried out.
Clearly, more work under natural conditions is needed to understand why there exists variation in the effects of sex steroids on circadian functioning among taxa and between the sexes. Likewise, the functional reason for the existence of diel variations in circulating sex steroid concentrations is also still unclear. Why is testosterone elevated at night in diurnal taxa such as birds? Does that pattern represent a phylogenetic constraint, since it appears to be present across taxa or does it have specific functions, for example for the reorganization of circadian patterns?
12. Contribution of peripheral food entrainment to flexible timing in the field
Food availability has long been known as the ‘other’ circadian Zeitgeber [138] which serves side-by-side with the light–dark Zeitgeber as the dominant entraining cue for the circadian timing system. The feeding ecology of most animals in the field includes temporal restrictions on food availability for reasons such as daily prey availability/predation risk, environmental darkness and of course one's own sleep–wake cycle. Daily feeding constraints lead to demonstrable changes in timing of overt behaviour in laboratory rodents, most notably as daily bouts of food anticipatory activity (FAA) [139]. Many studies have exposed that ability of food to entrain circadian feeding patterns in, for example, rodents [140], omnivores [141], herbivores [142], carnivores [143], primates [144], marsupials [145,146] and birds [147], but the ‘strength’ of feeding as a timing cue for FAA varies between species and studies.
It has been firmly established in laboratory conditions that food timing directly entrains molecular peripheral clocks and the transcriptome of tissues such as in the liver, adipose tissue, gastrointestinal tract, kidney, heart and pancreas, without shifting the clock in the SCN [3,4,148,149], which means that food entrainment is restricted to the peripheral timing system, including non-SCN brain regions [139,150]. Food entrainment has been conceptualized to be driven by a ‘food entrainable oscillator’, but the exact nature and location of this oscillator is not fully understood [139]. Even though it is unclear what the critical clocks are, the mechanisms that encode food timing into cellular circadian oscillators in mammals are being identified as bi-directional interactions between the genes and proteins involved with the circadian clock and biochemical markers of cellular cat- and anabolism. Known examples include intracellular (i) NAD+, (ii) AMP, (iii) haem and (iv) reactive oxygen species (ROS) which participate in molecular interactions with clock genes, thereby altering their expression and protein stability [151,152]. (i) BMAL1 and PER2 are subject to NAD+ dependent deacetylation by SIRT1, altering their stability and thereby altering their transcriptional control over other clock genes [153]. (ii) AMP-activated protein kinase phosphorylation destabilizes CRY1, altering the repressive feedback on Clock and Bmal1 [154]. (iii) REV-ERBα non-covalently binds haem, which promotes binding of REV-ERBα with a co-repressor complex resulting in repression of Bmal1 expression [155]. (iv) Hyperoxidized moieties of peroxiredoxins (PRX-SO2/3H) exhibit circadian rhythms, reflecting ROS production, which is proposed as a novel, non-genetic circadian clock [156].
In field conditions, food availability is paramount to survival, especially for species that feed only at limited times of the day such as hunting species. However, many species, including grazers, have been shown to forage at several, and not a single, specific times of day, which can be synchronized among conspecifics within the same group [157]. Animals eat in many different feeding patterns, which are often multi-modal, with two or more ‘meals’ per day [158]. Such multi-modal patterns in feeding are different from what is normally assumed and/or enforced in laboratory conditions and therefore the translation of laboratory studies to field conditions is constrained. The clock in the liver can entrain to a sequence of several meals, rather than a single meal, possibly taking into account both timing and size of each meal [159,160]. However, the consequences of several meals on peripheral timing has been reported to dissociate timing of rhythms such as ghrelin and cortisol, as well as the phase of several peripheral brain clocks [161].
Bi, or tri-modal feeding patterns are distinctly different from ultradian feeding patterns, which are defined by semi-equidistant meals throughout day and night. In the field, ultradian feeding and sleep cycles are particularly evident in the common vole (Microtus arvalis) [162]. This herbivore hindgut fermenter feeds every 2–3 h throughout the day and night, while simultaneously expressing weak circadian modulation of behaviour [9]. In the vole liver, there are no detectable circadian expression patterns of clock genes, while in the very same animals these genes exhibit clear circadian expression rhythms in the SCN [6]. Imposing daily, 8 h fasting episodes results in strengthened circadian timing of behaviour in the vole and the emergence of circadian cycles of clock gene expression in the liver [6]. Interestingly, it was recently shown that widespread ultradian gene expression patterns in the mouse liver transcriptome in vivo, as well as fibroblast in cell culture in vitro, are strongly associated with cellular metabolism [163], suggesting a relevance of ultradian timing of metabolism irrespective of feeding patterns.
Next to the multimodal feeding patterns often observed in nature, a second constraint in translating our laboratory-based data to the field is the daily variation in feeding time. For example, in a group of Kerry cows, day-to-day variation in feeding time is larger than between-individual variation [164], but for many species these levels of variation are unknown. The ability of the food entrainment system to deal with such daily variability is mostly unexplored, although Escobar et al. showed that rats receiving daily changes in food access were able to shift their FAA each day corresponding to the food availability the day before [165].
Food entrainment can even serve to drive behavioural and physiological cycles that are independent of the SCN, or light-entrained activity patterns [8,9,166], which indicates that besides the primary benefit of anticipating food availability and post-prandial anabolic metabolism, food entrainment may also aid entrainment in conditions where the light–dark cycle is a weak or absent Zeitgeber. Food cycles hasten re-entrainment to a changing phase in the light–dark cycle [167,168], which may aid entrainment of species that live in covered or underground habitats such as the ground squirrel [169,170]. It has also been suggested that food availability can substitute for photoperiod as the primary signal driving seasonal timing at latitudes where the seasonal change in photoperiod is minimal, or in temperate ecosystems where food is more critical than season for initiating reproductive effort [171].
One interesting aspects of food entrainment of the peripheral timing system is the relationship between diet and chronotype (temporal niche), for example in fish, in which major shifts in daily timing and chronotype occur with changes in diet and juvenile and adult phase [172], and the cheetah, which varies the number and duration of feeding bouts in response to the lunar cycle (visibility) and wet versus dry season [173]. These changes in chronotype are directly related to availability of the food in terms of accessibility and abundance, but have also been suggested to relate to energy balance and thermoregulation.
13. Temporal niche switching
When daily activity patterns of several species have been measured in the field, these patterns appear to be decidedly different from the nocturnal activity patterns of these same animals in the laboratory [174]. A landmark study that exposed switches in the temporal niche of overt behaviour in the golden hamster (Mesocricetus auratus), reported that in their native habitat in Turkey these animals exhibited crepuscular activity patterns, which contrasts the laboratory-conditions in which they are almost completely nocturnal [175]. Similarly, when laboratory mice were housed in large outdoor enclosures exposing them to natural weather conditions they departed from their initial nocturnal behavioural activity rhythm, and showed multiple temporal niche switches between nocturnality and diurnality over the 2 year study period [176]. The functional factors underlying temporal niche switching in these studies are mostly unknown but multiple studies have suggested changes in predation risk [177,178], interspecific competition [179] and challenges to the energetic balance [176,180] as driving forces.
The effect of energetic challenges on the daily timing of behaviour in mice has been shown under controlled laboratory conditions. Challenging mice by housing them at lowered ambient temperatures and/or letting them run in a wheel to earn food pellets results in phase advances of the active behavioural phase, where the magnitude of the shift into the light phase depends on the severity of the energetic challenge [181–183]. Phase-shifting the light–dark cycle results in a corresponding shift of activity, demonstrating continued light–dark entrainment under energetically challenging conditions [183]. The phase-shifted behavioural active phase does not depend on a phase shift of the central circadian clock in the SCN [183] but likely results from a shifted downstream mechanism controlling the timing of behaviour. Such an altered coupling of behavioural timing to the SCN phase suggests a role for the SCN as an internal representation of the external light–dark cycle, with other mechanisms linking behavioural timing to the SCN. Such a mechanism would be beneficial to animals that spend large parts of the day hiding in dark locations, to distribute physiological and behavioural activity into the appropriate daily phase without the need to constantly assess the external light–dark cycle. A further benefit of using the SCN as an internal representation of the light–dark cycle is that it can make the daily timing of activity more robust to daily variability in light exposure. The self-sustained nature of the SCN clock makes it robust against day-to-day variability in light exposure, thereby preventing dramatic shifts in daily activity timing as a result of daily variability in the timing of light exposure.
14. The role of the peripheral timing system in temporal niche switching
The behavioural shift to diurnality in response to energetic challenges is mirrored by simultaneous phase advances of peripheral oscillations [183]. Peripheral rhythms of corticosterone and Period2 clock gene expression in the liver and adrenal are shifted by 4–6 h under conditions of simulated food shortage [183], resulting in an internal phase angle between SCN and peripheral clocks that is similar to that seen in diurnal rodents [184,185]. The shifted phase of peripheral oscillators likely reflects a shift of the overall physiology to remain synchronized with the shifted timing of activity in energetically challenged mice. The mechanisms responsible for the circadian reorganization of internal clocks and behaviour in response to energetic challenges are, however, unknown. The shift in the phase of peripheral clocks under these conditions is likely a direct consequence of the shift in behavioural timing and clocks in the brain, and not dependent on altered characteristics of peripheral oscillators.
Peripheral clocks are entrained by the combined influence of multiple factors that act as Zeitgebers to peripheral oscillators, and under natural conditions, all of these timing signals are expected to peak at roughly the same time of day since environmental and brain-controlled rhythms will be synchronized to the stable environmental day–night cycle. The brain mechanisms involved in resynchronizing the timing of behaviour and timing cues originating from SCN phase are unknown. An oscillator downstream of the SCN that alters its phase relationship with the SCN depending on the energetic state of an animal and controls the timing of behaviour and systemic timing signals would be sufficient to optimize the timing of physiology and behaviour to the encountered environmental conditions. However, at the current time it is unclear whether a change in timing of peripheral clocks precedes, or follows, the shift in behavioural timing.
15. Ultimate consequences of temporal niche switching
Avoiding prolonged periods of negative energy balance is an important and often challenging requirement for animals living under natural conditions in the field. Maintaining energy balance requires balancing energy intake and expenditure, and both of these components are influenced by the daily timing of activity and rest. A substantial part of energy expenditure of small endotherms living in temperate climates is used for thermoregulation [186]. The circadian thermo-energetics hypothesis proposes that endotherms can reduce thermoregulatory costs by shifting activity to the day [180]. Because ambient temperatures are high during the day and low at night and the resting phase is associated with energy saving strategies, being active during the warmer day allows animals to optimize the energetic benefits of insulation and reduce daily energy expenditure. Quantification of different environmental factors modulating energy expenditure identifies thermal buffering provided by a sheltered nesting location as the dominant factor in determining the energetic consequences of temporal niche switching [187]. The daily temperature cycle inside a sheltered nesting location has a reduced amplitude compared to the outside ambient temperature cycle. Diurnality therefore allows animals to use the difference in nest and outside temperatures to encounter the higher nest temperature during the night and go outside during the warmer day, thereby encountering higher ambient temperature during both day and night [9]. Daily energy expenditure of small endotherms living in temperate climates is therefore expected to be reduced by 6–10% as a result of a shift of the active phase to the day [187].
Optimizing the daily timing of activity in relation to the timing of other animals is a second major factor in determining the ultimate consequences of a selected temporal niche [188]. One obvious example of the importance of finding the appropriate temporal niche is during the search for a mate, since a mate can only be found when both animals are active at the same time. Similarly, avoiding activity at times when predators are present can increase survival, while activity should be synchronized with prey species. An illustrative example of how temporal niche selection depends on the activity timing of both predator and prey has been documented in two species of hawks [189]. This study recorded the activity timing of the sharp-shinned hawk (Accipiter striatus) and the larger Cooper's hawks (Accipiter cooperii) and their relation to the activity patterns of their primary prey, small songbirds [189]. The activity and most dense period of attacks of the Cooper's hawks followed the peak in prey abundance in the hours around sunrise and sunset. For the smaller A. striatus the morning peak of hunting activity was largely absent, the hawks leaving their roosts after the time of densest prey abundance. Of the 12 sharp-shinned hawks that were killed by predators by the end of the study, one fell prey to a Cooper's hawk and the remaining 11 were caught by nocturnal owls. Hence, the presence of higher order predators might relieve some of the predation pressures on the small songbirds in this habitat, indicating that predator–prey relationships might operate directly and indirectly between the different species that occupy a certain habitat. In general, there is not a specific temporal niche that will minimize predation risks and maximize food availability for all habitats, but whenever daily rhythms in predation risk/food availability are present it is to be expected that a specific temporal niche exists that would be optimal. Plasticity in the daily timing of activity allows animals to respond to environmental changes and be able to cope with different ecological niches.
16. Selecting the temporal niche in a complex environment that maximizes fitness
In order to increase fitness, temporal niche selection should ultimately optimize the sum of costs and benefits of all possible fitness components, such as energy expenditure, survival, reproduction and other interspecific interactions [190]. These different fitness components are influenced by rhythms in environmental factors such as ambient temperature, predation risk and the availability of food and mates. Since the optimal time of day to be active will often be different for different environmental factors (e.g. predation risk might be lowest at night but this is also the energetically worst time to be active), trade-offs between fitness components have to be made. The mechanisms involved in making this trade-off are unknown but are important for understanding temporal niche selection in the field.
Although the ultimate benefits of temporal niche switching might be reductions in energy expenditure or predation risks, the proximate mechanisms responsible for these shifts can be unrelated to the specific benefits. In order to assess the roles of the light–dark and ambient temperature cycles for selecting a temporal niche, energetically challenged mice were housed under laboratory conditions with temperature cycles either in phase or in anti-phase with the light–dark cycle, which altered their daily distribution of activity, including a switch from nocturnality to diurnality [183]. Similarly, common voles (Microtus arvalis) exposed to constant high ambient temperatures during lactation shifted their nursing behaviour to the night [191]. Both of these examples illustrate that it is the light–dark cycle and not the temperature cycle that is used to determine the energetically optimal timing of activity and rest. Since daytime temperatures are reliably higher compared to the night (diurnality is energetically beneficial on 95–98% of days [187]), the light–dark cycle can be used as a proxy for the energetically optimal time of day to be active. The high predictability of higher temperatures during the light phase thus makes more complex regulatory mechanisms involving direct feedback from the ambient temperature cycles unnecessary.
Identification of the proximate factors responsible for temporal niche switching in response to changes in predation risk is hampered by the difficulties in systematically studying predator–prey interactions. Field studies assessing changes in daily activity timing in response to changes in (perceived) predation risk have shown that prey species can respond to increased night-time predation by becoming diurnal [177,178] and vice versa following increased daytime predation [192]. This suggests that prey species, at the very least, incorporate the temporal niche of the predator in their temporal niche selection. The greater variability in the temporal organization of predation risk compared to the high predictability of ambient temperature rhythms would be an argument for more complex regulatory mechanisms being responsible for temporal niche switching in response to changes in predation risk.
17. Putting it all together
Under natural conditions, animals are exposed to a variety of environmental opportunities, challenges and threats that require adaptive responses to maximize fitness. Some of these environmental variables will exhibit predictable variation over the day (e.g. light, temperature), some will be constantly present (e.g. food for grazers), and some will have erratic timing over the day or season (e.g. rain, drought). Moreover, the diurnal patterns of variables such as predation risk can vary strongly between days. Thus, in order to maximize fitness, animals must find the optimal balance between foraging, predation risk and reproduction, and finding such a balance requires maximizing the orchestration of timing of these behaviours across the day.
The mammalian SCN provides an internal representation of the light–dark cycle [193] and therefore provides an essential timing signal for target tissues in the central nervous system and peripheral organs (figure 1). The SCN has therefore been historically coined as the ‘conductor’ of the clocks in the rest of the body [2]. However, this paradigm is primarily based on evidence gathered under laboratory conditions, when most peripheral Zeitgebers are aligned with the light–dark cycle. Under natural conditions not all relevant environmental variables show stable, aligned daily cycles, and the notion of the SCN as orchestrator is stretched to its limits. In fact, maximization of fitness is reliant on plasticity in the timing of these peripheral rhythms, as well as behavioural activity that is associated with non-photic cycles and patterns. These real-life considerations suggest that an SCN-centric view is in direct contradiction with maximizing fitness.
We have shown here that timing of behavioural activity associated with, for example, the endocrine system, food availability and other factors is associated with peripheral clocks and rhythms. Moreover, there are many examples of non-photic Zeitgebers driving clocks and rhythms in the periphery, often dissociated from the light–dark cycle. Even a lack of food per se will have a dramatic impact on overt and endogenous rhythms. Similarly, ambient temperature and predation risk can lead to substantial changes in rhythmicity in mice, and voles seem especially adapted to change their rhythmicity from more circadian to more ultradian forms, whereby a noticeable shift to more diurnal activity occurs especially when it is cold and food is scarce.
Based on these tangible, real-life considerations—rather than those originating from laboratory-based observations—it does not seem appropriate to describe the SCN as the conductor of the orchestra of body tissues. It seems much more pertinent to consider the SCN primarily as the internal representation of the external light–dark cycle, providing a signal that can be consulted by other brain areas and peripheral tissues (figure 1). This change in viewpoint provides a prominent role for peripheral tissues, also because the degree to which peripheral clocks follow the SCN signal or other signals depends—at least in part—on the state of each of these individual clocks. The palette of hormonal and neurotransmitter receptors expressed by each tissue in the body, including hypothalamic nuclei downstream from the SCN, will therefore determine their phase angle relative to that of the SCN, as well as their response to environmental cues.
Some functions of peripheral tissues, however, require a tight phase angle with the light–dark cycle by means of a tight coupling with the SCN. Melatonin production may be one of them. The direct coupling of the melatonin producing pinealocytes to the SCN [32,33] can be functionally understood from its role in the photoperiodic response, driving seasonal gonadal development (see for a brief review [134]). The peripheral clock in the ovaries, for example, will detect the carefully timed hypothalamic drive on gonadotropin release by the pituitary as a response to photoperiod [69]. This hypothalamic drive depends on the measurement of photoperiod, requiring the SCN–melatonin axis and circadian clocks in the pars tuberalis to precisely work together [194]. A series of circadian clock systems need to be precisely tuned to the environment and to each other to get successful ovulation: day length > SCN > melatonin > pars tuberalis > pituitary > ovary. But even here it is clear that internal state (fat reserves) and environmental variables (e.g. temperature and food) play important roles in modifying the hypothalamic drive to the pituitary, although the precise mechanisms by which these modifiers act remain unclear [195].
In the natural world, there is no single optimum phenotype for the interacting central and peripheral clocks because environments vary spatially and change over time. All animals inherit the clocks of ancestors whose fitness was based on their specific environment: the specific location and time in which they lived. Much of the spatial variation in environment among individuals is not predictable, and the exact timing of seasonal change, daily temperature change, and other variables is not predictable. Thus, selection is acting on clock systems that exist in a cyclical world with significant unpredictable variation. Natural selection cannot favour an optimum for all existing environments; natural selection can only favour alleles that tend to be successful in most environments. Offspring of individuals who happen to have clocks that function well in one year and one location, will disperse to new and different locations, and those offspring will grow and mature in different days/seasons/years in which those same clocks may, by chance, decrease fitness. The fitness landscape is complex, and that landscape becomes even more complex when there are multiple interacting components: the many different peripheral clocks in animals.
Understanding the adaptive value of the mammalian circadian system thus requires a better understanding of the mechanisms by which peripheral clocks are timed by the environmental cues in combination with SCN derived signals. To obtain such insights, we must expand beyond the standard 12 h light : 12 h dark regimes at room temperature, because these artificial conditions hide, or mask, the independence of peripheral clocks. The plasticity of circadian organization, as provided by the contribution of peripheral clocks under a range of real environmental conditions, should be studied to provide insight into the heterogeneity of regulatory mechanisms in circadian organization. Eventually we should be able to understand the variety of circadian patterns observed under natural conditions, how they evolve, to which signals they respond, what the functions of those responses are, and how they contribute to increasing survival and reproduction. We can then see a range of mechanisms by which the environment connects with a complex internal circadian landscape to allow timing relationships between the SCN and multiple peripheral clocks that maximize fitness under natural conditions.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study. RAH obtained financial support from NWO-STW (Nederlandse Organisatie voor Wetenschappelijk onderzoek/Stichting voor de Technische Wetenschappen 10.13039/501100003958) OnTime Program Grant (project 12185).
References
- 1.Albrecht U. 2012. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74, 246–260. ( 10.1016/j.neuron.2012.04.006) [DOI] [PubMed] [Google Scholar]
- 2.Davidson AJ, Yamazaki S, Menaker M. 2003. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found. Symp. 253, 110–121; discussion 121–115, 281–114 ( 10.1002/0470090839.ch9) [DOI] [PubMed] [Google Scholar]
- 3.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. 2000. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961. ( 10.1101/gad.183500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. 2001. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493. ( 10.1126/science.291.5503.490) [DOI] [PubMed] [Google Scholar]
- 5.Buhr ED, Yoo S-H, Takahashi JS. 2010. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385. ( 10.1126/science.1195262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Veen DR, Minh NL, Gos P, Arneric M, Gerkema MP, Schibler U. 2006. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc. Natl Acad. Sci. USA 103, 3393–3398. ( 10.1073/pnas.0507825103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347. ( 10.1126/science.289.5488.2344) [DOI] [PubMed] [Google Scholar]
- 8.Stephan FK, Swann JM, Sisk CL. 1979. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav. Neural Biol. 25, 545–554. ( 10.1016/S0163-1047(79)90332-7) [DOI] [PubMed] [Google Scholar]
- 9.van der Veen DR, Saaltink DJ, Gerkema MP. 2011. Behavioral responses to combinations of timed light, food availability, and ultradian rhythms in the common vole (Microtus arvalis). Chronobiol. Int. 28, 563–571. ( 10.3109/07420528.2011.591953) [DOI] [PubMed] [Google Scholar]
- 10.Archer SN, et al. 2014. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl Acad. Sci. USA 111, E682–E691. ( 10.1073/pnas.1316335111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM, Lehnert H, Oster H. 2012. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS ONE 7, e37150 ( 10.1371/journal.pone.0037150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. 2014. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16 219–16 224. ( 10.1073/pnas.1408886111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Wang H, Liu Y, Shao C. 2008. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput. Biol. 4, e1000193 ( 10.1371/journal.pcbi.1000193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fustin JM, O'Neill JS, Hastings MH, Hazlerigg DG, Dardente H. 2009. Cry1 circadian phase in vitro: wrapped up with an E-box. J. Biol. Rhythms 24, 16–24. ( 10.1177/0748730408329267) [DOI] [PubMed] [Google Scholar]
- 15.Menet JS, Pescatore S, Rosbash M.. 2014. Clock: BMAL1 is a pioneer-like transcription factor. Genes Dev. 28, 8–13. ( 10.1101/gad.228536.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. 2012. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. Elife 2012, 1–25. ( 10.7554/eLife.00011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korenčič A, Košir R, Bordyugov G, Lehmann R, Rozman D, Herzel H. 2014. Timing of circadian genes in mammalian tissues. Sci. Rep. 4, 5782 ( 10.1038/srep05782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. 2009. Harmonics of circadian gene transcription in mammals. PLoS Genet. 5, e1000442 ( 10.1371/journal.pgen.1000442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inouye ST, Kawamura H. 1979. Persistence of circadian rhythmicity in a mammalian hypothalamic ‘island’ containing the suprachiasmatic nucleus. Proc. Natl Acad. Sci. USA 76, 5962–5966. ( 10.1073/pnas.76.11.5962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaap J, Pennartz CMA, Meijer JH. 2003. Electrophysiology of the circadian pacemaker in mammals. Chronobiol. Int. 20, 171–188. ( 10.1081/CBI-120019311) [DOI] [PubMed] [Google Scholar]
- 21.Mohawk JA, Takahashi JS. 2011. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 34, 349–358. ( 10.1016/j.tins.2011.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh DK, Takahashi JS, Kay SA. 2010. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 72, 551–577. ( 10.1146/annurev-physiol-021909-135919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colwell CS. 2011. Linking neural activity and molecular oscillations in the SCN. Nat. Rev. Neurosci. 12, 553–569. ( 10.1038/nrn3086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter C. 1967. Sleep and activity: their relationship to the 24-hour clock. Res. Publ. Ass. Nerv. Ment. Dis. 45, 8–29. [PubMed] [Google Scholar]
- 25.Moore RY, Eichler VB. 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206. ( 10.1016/0006-8993(72)90054-6) [DOI] [PubMed] [Google Scholar]
- 26.Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl Acad. Sci. USA 69, 1583–1586. ( 10.1073/pnas.69.6.1583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCoursey PJ, Krulas JR. 1998. Behavior of SCN-lesioned chipmunks in natural habitat: a pilot study. J. Biol. Rhythms 13, 229–244. ( 10.1177/074873098129000075) [DOI] [PubMed] [Google Scholar]
- 28.Pellman BA, Kim E, Reilly M, Kashima J, Motch O, de la Iglesia HO, Kim JJ. 2015. Time-specific fear acts as a non-photic entraining stimulus of circadian rhythms in rats. Sci. Rep. 5, 14916 ( 10.1038/srep14916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mrosovsky N. 1999. Masking: history, definitions, and measurement. Chronobiol. Int. 16, 415–429. ( 10.3109/07420529908998717) [DOI] [PubMed] [Google Scholar]
- 30.Helm B, Visser ME, Schwartz W, Kronfeld-Schor N, Gerkema M, Piersma T, Bloch G. 2017. Two sides of a coin: ecological and chronobiological perspectives of timing in the wild. Phil. Trans. R. Soc. B 372, 20160246 ( 10.1098/rstb.2016.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. 2010. Circadian organization is governed by extra-SCN pacemakers. J. Biol. Rhythms 25, 432–441. ( 10.1177/0748730410385204) [DOI] [PubMed] [Google Scholar]
- 32.Buijs RM, Kalsbeek A. 2001. Hypothalamic integration of central and peripheral clocks. Nat. Rev. Neurosci. 2, 521–526. ( 10.1038/35081582) [DOI] [PubMed] [Google Scholar]
- 33.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. 2006. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 21, 458–469. ( 10.1177/0748730406293854) [DOI] [PubMed] [Google Scholar]
- 34.Vujovic N, Gooley JJ, Jhou TC, Saper CB. 2015. Projections from the subparaventricular zone define four channels of output from the circadian timing system. J. Comp. Neurol. 523, 2714–2737. ( 10.1002/cne.23812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver R, LeSauter J, Tresco PA, Lehman MN. 1996. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–813. ( 10.1038/382810a0) [DOI] [PubMed] [Google Scholar]
- 36.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. 2004. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 24, 7604–7613. ( 10.1523/JNEUROSCI.5328-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oster H, Damerow S, Hut RA, Eichele G. 2006. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J. Biol. Rhythms 21, 350–361. ( 10.1177/0748730406293053) [DOI] [PubMed] [Google Scholar]
- 38.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. 2006. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 4, 163–173. ( 10.1016/j.cmet.2006.07.002) [DOI] [PubMed] [Google Scholar]
- 39.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. 2001. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 20, 7128–7136. ( 10.1093/emboj/20.24.7128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cagampang FR, Poore KR, Hanson MA. 2011. Developmental origins of the metabolic syndrome: body clocks and stress responses. Brain Behav. Immun. 25, 214–220. ( 10.1016/j.bbi.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 41.Rosenfeld P, Van Eekelen JA, Levine S, De Kloet ER. 1988. Ontogeny of the type 2 glucocorticoid receptor in discrete rat brain regions: an immunocytochemical study. Brain Res. 470, 119–127. ( 10.1016/0165-3806(88)90207-6) [DOI] [PubMed] [Google Scholar]
- 42.Dudek M, et al. 2016. The intervertebral disc contains intrinsic circadian clocks that are regulated by age and cytokines and linked to degeneration. Ann. Rheum. Dis. 76, 576–584. ( 10.1136/annrheumdis-2016-209428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. 2007. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc. Natl Acad. Sci. USA 104, 12 843–12 848. ( 10.1073/pnas.0701466104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Den Pol AN, Dudek FE. 1993. Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience 56, 793–811. ( 10.1016/0306-4522(93)90128-3) [DOI] [PubMed] [Google Scholar]
- 45.Wams EJ, Riede SJ, van der Laan I, ten Bulte T, Hut RA. 2017. Mechanisms of non-photic entrainment. In Biological timekeeping: clocks, rhythms and behaviour (ed. Kumar V.), pp. 395–404. New Delhi, India: Springer. [Google Scholar]
- 46.Glass JD, Guinn J, Kaur G, Francl JM. 2010. On the intrinsic regulation of neuropeptide Y release in the mammalian suprachiasmatic nucleus circadian clock. Eur. J. Neurosci. 31, 1117–1126. ( 10.1111/j.1460-9568.2010.07139.x) [DOI] [PubMed] [Google Scholar]
- 47.Model Z, Butler MP, LeSauter J, Silver R. 2015. Suprachiasmatic nucleus as the site of androgen action on circadian rhythms. Horm. Behav. 73, 1–7. ( 10.1016/j.yhbeh.2015.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. 2011. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J. Neurosci. 31, 16 107–16 116. ( 10.1523/jneurosci.4175-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prendergast BJ, Kriegsfeld LJ, Nelson RJ. 2001. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs and functions. Q. Rev. Biol. 76, 293–325. ( 10.1086/393989) [DOI] [PubMed] [Google Scholar]
- 50.Follett BK. 2015. ‘Seasonal changes in the neuroendocrine system’: some reflections. Front. Neuroendocrinol. 37, 3–12. ( 10.1016/j.yfrne.2014.11.003) [DOI] [PubMed] [Google Scholar]
- 51.Coomans CP, Ramkisoensing A, Meijer JH. 2015. The suprachiasmatic nuclei as a seasonal clock. Front. Neuroendocrinol. 37, 29–42. ( 10.1016/j.yfrne.2014.11.002) [DOI] [PubMed] [Google Scholar]
- 52.Bronson FH, Heideman PD. 1994. Seasonal regulation of reproduction in mammals. In The physiology of reproduction (eds Knobil E, Neill JD), pp. 541–584, 2nd edn New York, NY: Raven Press. [Google Scholar]
- 53.Boyer TS, Terman CR. 1993. Comparing reproductive inhibition in female white-footed mice from Michigan and Virginia. J. Mammal. 74, 813–818. ( 10.2307/1382419) [DOI] [Google Scholar]
- 54.Terman CR. 1998. Early-summer reproductive curtailment in wild white-footed mice and reproductive recovery in the laboratory. J. Mammal. 79, 320–325. ( 10.2307/1382868) [DOI] [Google Scholar]
- 55.Terman CR. 1999. Early-summer inhibition of reproduction in wild white-footed mice (Peromyscus leucopus noveboracensis): influence of supplemental food. Res. Popul. Ecol. 41, 299–304. ( 10.1007/s101440050035) [DOI] [Google Scholar]
- 56.Heideman PD, Bruno TA, Singley JW, Smedley JV. 1999. Genetic variation in photoperiodism in Peromyscus leucopus: geographic variation in an alternative life-history strategy. J. Mammal. 80, 1232–1242. ( 10.2307/1383173) [DOI] [Google Scholar]
- 57.Pedersen AB, Greives TJ. 2008. The interaction of parasites and resources cause crashes in a wild mouse population. J. Anim. Ecol. 77, 370–377. ( 10.1111/j.1365-2656.2007.01321.x) [DOI] [PubMed] [Google Scholar]
- 58.Vandegrift KJ, Raffel TR, Hudson PJ. 2008. Parasites prevent summer breeding in white-footed mice, peromyscus leucopus. Ecology 89, 2251–2258. ( 10.1890/07-1935.1) [DOI] [PubMed] [Google Scholar]
- 59.Vandegrift KJ, Hudson PJ. 2009. Could parasites destabilize mouse populations? The potential role of Pterygodermatites peromysci in the population dynamics of free-living mice, Peromyscus leucopus. Int. J. Parasitol. 39, 1253–1262. ( 10.1016/j.ijpara.2009.02.025) [DOI] [PubMed] [Google Scholar]
- 60.Chassard D, Bur I, Poirel V-J, Mendoza J, Simonneaux V. 2015. Evidence for a putative circadian kiss-clock in the hypothalamic AVPV in female mice. Endocrinology 156, 2999–3011. ( 10.1210/en.2014-1769) [DOI] [PubMed] [Google Scholar]
- 61.Richards J, Gumz ML. 2012. Advances in understanding the peripheral circadian clocks. FASEB J. 26, 3602–3613. ( 10.1096/fj.12-203554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sellix MT. 2013. Clocks underneath: the role of peripheral clocks in the timing of female reproductive physiology. Front. Endocrinol. 4, 1–6. ( 10.3389/fendo.2013.00091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu S, Cai Y, Sothern RB, Guan Y, Chan P. 2007. Chronobiological analysis of circadian patterns in transcription of seven key clock genes in six peripheral tissues in mice. Chronobiol. Int. 24, 793–820. ( 10.1080/07420520701672556) [DOI] [PubMed] [Google Scholar]
- 64.Mazzoccoli G, et al. 2012. Clock gene expression in mouse kidney and testis: analysis of periodical and dynamical patterns. J. Biol. Regul. Homeost. Agents 26, 303. [PubMed] [Google Scholar]
- 65.Meyer V, Lerchl A. 2014. Evidence for species-specific clock gene expression patterns in hamster peripheral tissues. Gene 548, 101–111. ( 10.1016/j.gene.2014.07.019) [DOI] [PubMed] [Google Scholar]
- 66.Singh D, Rani S, Kumar V. 2013. Daily expression of six clock genes in central and peripheral tissues of a night-migratory songbird: evidence for tissue-specific circadian timing. Chronobiol. Int. 30, 1208–1217. ( 10.3109/07420528.2013.810632) [DOI] [PubMed] [Google Scholar]
- 67.Michael AK, Harvey SL, Sammons PJ, Anderson AP, Kopalle HM, Banham AH, Partch CL. 2015. Cancer/testis antigen PASD1 silences the circadian clock. Mol. Cell 58, 743–754. ( 10.1016/j.molcel.2015.03.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. 2008. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J. Biol. Rhythms 23, 26–36. ( 10.1177/0748730407311254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellix MT, Yoshikawa T, Menaker M. 2010. A circadian egg timer gates ovulation. Curr. Biol. 20, R266–R267. ( 10.1016/j.cub.2010.01.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Go EH, Lee S-H. 2014. Effect of long term reverse feeding on the reproductive and non-reproductive tissues in male mice. Dev. Reprod. 18, 161–166. ( 10.12717/DR.2014.18.3.161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krueger KC, Feldman BJ. 2013. Adipose circadian clocks: coordination of metabolic rhythms by clock genes, steroid hormones, and PPARs. Horm. Mol. Biol. Clin. Investig. 14, 15–24. ( 10.1515/hmbci-2013-0011) [DOI] [PubMed] [Google Scholar]
- 72.Gerhart-Hines Z, Lazar MA. 2015. Circadian metabolism in the light of evolution. Endocr Rev. 36, 289–304. ( 10.1210/er.2015-1007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zwighaft Z, Reinke H, Asher G. 2016. The liver in the eyes of a chronobiologist. J. Biol. Rhythms 31, 115–124. ( 10.1177/0748730416633552) [DOI] [PubMed] [Google Scholar]
- 74.Sen A, Sellix MT. 2016. The circadian timing system and environmental circadian disruption: from follicles to fertility. Endocrinology 157, 3366–3373. ( 10.1210/en.2016-1450) [DOI] [PubMed] [Google Scholar]
- 75.Nakao N, et al. 2007. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology 148, 3031–3038. ( 10.1210/en.2007-0044) [DOI] [PubMed] [Google Scholar]
- 76.Kennaway DJ. 2005. The role of circadian rhythmicity in reproduction. Hum. Reprod. Update 11, 91–101. ( 10.1093/humupd/dmh054) [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, et al. 2014. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl Acad. Sci. USA 111, 14 295–14 300. ( 10.1073/pnas.1209249111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. 2004. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr. Biol. 14, 1367–1373. ( 10.1016/j.cub.2004.07.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ratajczak CK, Boehle KL, Muglia LJ. 2009. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology 150, 1879–1885. ( 10.1210/en.2008-1021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li R, Cheng S, Wang Z. 2015. Circadian clock gene plays a key role on ovarian cycle and spontaneous abortion. Cell. Physiol. Biochem. 37, 911–920. ( 10.1159/000430218) [DOI] [PubMed] [Google Scholar]
- 81.Ratajczak CK, Asada M, Allen GC, McMahon DG, Muglia LM, Smith D, Bhattacharyya S, Muglia LJ. 2012. Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod. Fertil. Dev. 24, 759–767. ( 10.1071/RD11164) [DOI] [PubMed] [Google Scholar]
- 82.Barclay JL, Tsang AH, Oster H. 2012. Chapter 10—Interaction of central and peripheral clocks in physiological regulation. In Progress in brain research (eds Kalsbeek A, Merrow M, Roenneberg T, Foster RG), pp. 163–181. Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed] [Google Scholar]
- 83.Furutani A, et al. 2015. Fish oil accelerates diet-induced entrainment of the mouse peripheral clock via GPR120. PLoS ONE 10, e0132472 ( 10.1371/journal.pone.0132472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oosterman JE, Belsham DD. 2016. Glucose alters Per2 hythmicity independent of AMPK, whereas AMPK inhibitor compound C causes profound repression of clock genes and AgRP in mHypoE-37 hypothalamic neurons. PLoS ONE 11, e0146969 ( 10.1371/journal.pone.0146969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaasik K, et al. 2013. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab. 17, 291–302. ( 10.1016/j.cmet.2012.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. 2002. Glucose down-regulates Per1 and Per2mRNA levels and induces circadian gene expression in cultured rat-1 fibroblasts. J. Biol. Chem. 277, 44 244–44 251. ( 10.1074/jbc.M206233200) [DOI] [PubMed] [Google Scholar]
- 87.Wharfe M, Mark P, Waddell B. 2011. Circadian variation in placental and hepatic clock genes in rat pregnancy. Endocrinology 152, 3552–3560. ( 10.1210/en.2011-0081) [DOI] [PubMed] [Google Scholar]
- 88.Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, Martin M, Kaplan H, Gurven M. 2015. Helminth infection, fecundity, and age of first pregnancy in women. Science 350, 970–972. ( 10.1126/science.aac7902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 90.Lochmiller RL, Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98. ( 10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 91.Martinez-Bakker M, Helm B. 2015. The influence of biological rhythms on host–parasite interactions. Trends Ecol. Evol. 30, 314–326. ( 10.1016/j.tree.2015.03.012) [DOI] [PubMed] [Google Scholar]
- 92.Geiger SS, Fagundes CT, Siegel RM. 2015. Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology 146, 349–358. ( 10.1111/imm.12525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Labrecque N, Cermakian N. 2015. Circadian clocks in the immune system. J. Biol. Rhythms 30, 277–290. ( 10.1177/0748730415577723) [DOI] [PubMed] [Google Scholar]
- 94.Silver AC, Arjona A, Walker WE, Fikrig E. 2012. The circadian clock controls Toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36, 251–261. ( 10.1016/j.immuni.2011.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scheiermann C, Kunisaki Y, Frenette PS. 2013. Circadian control of the immune system. Nat. Rev. Immunol. 13, 190–198. ( 10.1038/nri3386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Juan A, Druzd D, Ince L, Scheiermann C. 2016. Regulation of immunity by the circadian clock. In Circadian clocks: role in health and disease (ed. Gumz LM.), pp. 251–266. New York, NY: Springer. [Google Scholar]
- 97.Hashiramoto A, et al. 2010. Mammalian clock gene cryptochrome regulates arthritis via proinflammatory cytokine TNF-α. J. Immunol. 184, 1560–1565. ( 10.4049/jimmunol.0903284) [DOI] [PubMed] [Google Scholar]
- 98.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. 2012. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl Acad. Sci. USA 109, 12 662–12 667. ( 10.1073/pnas.1209965109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mathew DJ, Lucy MC, D Geisert R. 2016. Interleukins, interferons, and establishment of pregnancy in pigs. Reproduction 151, R111–R122. ( 10.1530/rep-16-0047) [DOI] [PubMed] [Google Scholar]
- 100.Banerjee P, Jana SK, Pasricha P, Ghosh S, Chakravarty B, Chaudhury K. 2013. Proinflammatory cytokines induced altered expression of cyclooxygenase-2 gene results in unreceptive endometrium in women with idiopathic recurrent spontaneous miscarriage. Fertil. Steril. 99, 179–187.e172. ( 10.1016/j.fertnstert.2012.08.034) [DOI] [PubMed] [Google Scholar]
- 101.Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F. 2016. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum. Reprod. Update 22, 104–115. ( 10.1093/humupd/dmv044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fraczek M, Kurpisz M. 2015. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 108, 98–104. ( 10.1016/j.jri.2015.02.001) [DOI] [PubMed] [Google Scholar]
- 103.Leisegang K, Bouic PJD, Henkel RR. 2016. Metabolic syndrome is associated with increased seminal inflammatory cytokines and reproductive dysfunction in a case-controlled male cohort. Am. J. Reprod. Immunol. 76, 155–163. ( 10.1111/aji.12529) [DOI] [PubMed] [Google Scholar]
- 104.Amaral FG, Castrucci AM, Cipolla-Neto J, Poletini MO, Mendez N, Richter HG, Sellix MT. 2014. Environmental control of biological rhythms: effects on development, fertility and metabolism. J. Neuroendocrinol. 26, 603–612. ( 10.1111/jne.12144) [DOI] [PubMed] [Google Scholar]
- 105.Baburski AZ, Sokanovic SJ, Bjelic MM, Radovic SM, Andric SA, Kostic TS. 2016. Circadian rhythm of the Leydig cells endocrine function is attenuated during aging. Exp. Gerontol. 73, 5–13. ( 10.1016/j.exger.2015.11.002) [DOI] [PubMed] [Google Scholar]
- 106.Nelson RJ. 2011. An introduction to behavioral endocrinology, 4th edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 107.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 108.Ball GF, Bentley GE. 2000. Neuroendocrine mechanisms mediating the photoperiodic and social regulation of seasonal reproduction in birds. In Reproduction in context (eds Wallen K, Schneider JE), pp. 129–158. Cambridge, MA: MIT Press. [Google Scholar]
- 109.Bronson FH. 1989. Mammalian reproductive biology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 110.Stevenson TJ, Hahn TP, MacDougall-Shackleton SA, Ball GF. 2012. Gonadotropin-releasing hormone plasticity: a comparative perspective. Front. Neuroendocrinol. 33, 287–300. ( 10.1016/j.yfrne.2012.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ball GF, Balthazart J. 2004. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol. Behav. 83, 329–346. ( 10.1016/j.physbeh.2004.08.020) [DOI] [PubMed] [Google Scholar]
- 112.Woolley SC, Sakata JT, Crews D. 2004. Evolutionary insights into the regulation of courtship behavior in male amphibians and reptiles. Physiol. Behav. 83, 347–360. ( 10.1016/j.physbeh.2004.08.021) [DOI] [PubMed] [Google Scholar]
- 113.Floody OR. 1983. Hormones and aggression in female mammals. In Hormones and aggressive behavior (ed. Svare BB.), pp. 39–90. New York, NY: Plenum Press. [Google Scholar]
- 114.Monaghan EP, Glickman SE.. 1992. Hormones and aggressive behavior. In Behavioral endocrinology (eds Becker JB, Breedlove SM, Crews D), pp. 261–285. Cambridge, MA: MIT Press. [Google Scholar]
- 115.Wingfield JC, Hegner RE, Dufty AMJ, Ball GF. 1990. The ‘challenge-hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846. ( 10.1086/285134) [DOI] [Google Scholar]
- 116.Hirschenhauser K, Oliveira RF. 2006. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 71, 265–277. ( 10.1016/j.anbehav.2005.04.014) [DOI] [Google Scholar]
- 117.Hau M, Romero MR, Brawn JD, Van't Hof TJ. 2002. Effect of polar day on plasma profiles of melatonin, testosterone and estradiol in high-Arctic Lapland longspurs. Gen. Comp. Endocrinol. 126, 101–112. ( 10.1006/gcen.2002.7776) [DOI] [PubMed] [Google Scholar]
- 118.Laucht S, Dale J, Mutzel A, Kempenaers B. 2011. Individual variation in plasma testosterone levels and its relation to badge size in house sparrows Passer domesticus: it's a night-and-day difference. Gen. Comp. Endocrinol. 170, 501–508. ( 10.1016/j.ygcen.2010.11.007) [DOI] [PubMed] [Google Scholar]
- 119.Kriegsfeld LJ, LeSauter J, Hamada T, Pitts SM, Silver R. 2002. Circadian rhythms in the endocrine system. In Hormones, brain and behavior (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT), pp. 33–91. New York, NY: Academic Press. [Google Scholar]
- 120.Tarlow E, Hau M, Anderson DJ, Wikelski M. 2003. Diel changes in plasma melatonin and corticosterone concentrations in tropical Nazca boobies (Sula granti) in relation to moon phase and age. Gen. Comp. Endocrinol. 133, 297–304. ( 10.1016/S0016-6480(03)00192-8) [DOI] [PubMed] [Google Scholar]
- 121.Murray CM, Heintz MR, Lonsdorf EV, Parr LA, Santymire RM. 2013. Validation of a field technique and characterization of fecal glucocorticoid metabolite analysis in wild chimpanzees (Pan troglodytes). Am. J. Primatol. 75, 57–64. ( 10.1002/ajp.22078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N. 2003. Concentrations of four fecal steroids in wild baboons: short-term storage conditions and consequences for data interpretation. Gen. Comp. Endocrinol. 132, 264–271. ( 10.1016/S0016-6480(03)00093-5) [DOI] [PubMed] [Google Scholar]
- 123.Gumus A, Lee S, Ahsan SS, Karlsson K, Gabrielson R, Guglielmo CG, Winkler DW, Erickson D. 2015. Lab-on-a-bird: biophysical monitoring of flying birds. PLoS ONE 10, e0123947 ( 10.1371/journal.pone.0123947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goymann W. 2012. On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol. Evol. 3, 757–765. ( 10.1111/j.2041-210X.2012.00203.x) [DOI] [Google Scholar]
- 125.Turek FW, Gwinner E.. 1982. Role of hormones in the circadian organization of vertebrates. In Vertebrate circadian systems (eds Aschoff J, Daan S, Groos G), pp. 173–182. Berlin, Germany: Springer. [Google Scholar]
- 126.Daan S, Damassa D, Pittendrigh CS, Smith ER. 1975. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc. Natl Acad. Sci. USA 72, 3744–3747. ( 10.1073/pnas.72.9.3744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Blattner MS, Mahoney MM. 2015. Changes in estrogen receptor signaling alters the timekeeping system in male mice. Behav. Brain Res. 294, 43–49. ( 10.1016/j.bbr.2015.07.060) [DOI] [PubMed] [Google Scholar]
- 128.Morin LP, Fitzgerald KM, Zucker I. 1977. Estradiol shortens the period of hamster circadian rhythms. Science 196, 305–307. ( 10.1126/science.557840) [DOI] [PubMed] [Google Scholar]
- 129.Albers EE, Gerall AA, Axelson JF. 1981. Effect of reproductive state on circadian periodicity in the rat. Physiol. Behav. 26, 21–25. ( 10.1016/0031-9384(81)90073-1) [DOI] [PubMed] [Google Scholar]
- 130.Labyak SE, Lee TM. 1995. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol. Behav. 58, 573–585. ( 10.1016/0031-9384(95)00096-2) [DOI] [PubMed] [Google Scholar]
- 131.Legan SJ, Peng X, Yun C, Duncan MJ. 2015. Effect of arousing stimuli on circulating corticosterone and the circadian rhythms of luteinizing hormone (LH) surges and locomotor activity in estradiol-treated ovariectomized (ovx + EB) Syrian hamsters. Horm. Behav. 72, 28–38. ( 10.1016/j.yhbeh.2015.04.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan L, Silver R. 2016. Neuroendocrine underpinnings of sex differences in circadian timing systems. J. Steroid Biochem. Mol. Biol. 160, 118–126. ( 10.1016/j.jsbmb.2015.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dart DA, Waxman J, Aboagye EO, Bevan CL.. 2013. Visualising androgen receptor activity in male and female mice. PLoS ONE 8, e71694 ( 10.1371/journal.pone.0071694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hau M, Dominoni D, Casagrande S, Buck CL, Wagner G, Hazlerigg D, Greives T, Hut RA. 2017. Timing as a sexually selected trait: the right mate at the right moment. Phil. Trans. R. Soc. B 372, 20160249 ( 10.1098/rstb.2016.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kempenaers B, Borgstroem P, Loes P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 136.Poesel A, Kunc HP, Foerster K, Johnsen A, Kempenaers B. 2006. Early birds are sexy: male age, dawn song and extrapair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Anim. Behav. 72, 531–538. ( 10.1016/j.anbehav.2005.10.022) [DOI] [Google Scholar]
- 137.Greives TJ, et al. 2015. Costs of sleeping in: circadian rhythms influence cuckoldry risk in a songbird. Funct. Ecol. 29, 1300–1307. ( 10.1111/1365-2435.12440) [DOI] [Google Scholar]
- 138.Stephan FK. 2002. The ‘other’ circadian system: food as a Zeitgeber. J. Biol. Rhythms 17, 284–292. ( 10.1177/074873002129002591) [DOI] [PubMed] [Google Scholar]
- 139.Mistlberger RE. 2009. Food-anticipatory circadian rhythms: concepts and methods. Eur. J. Neurosci. 30, 1718–1729. ( 10.1111/j.1460-9568.2009.06965.x) [DOI] [PubMed] [Google Scholar]
- 140.Hatori M, Panda S. 2015. Chapter 7—Response of peripheral rhythms to the timing of food intake. In Methods in enzymology (ed. Amita S.), pp. 145–161. New York, NY: Academic Press. [DOI] [PubMed] [Google Scholar]
- 141.Ware JV, Nelson OL, Robbins CT, Jansen HT. 2012. Temporal organization of activity in the brown bear (Ursus arctos): roles of circadian rhythms, light, and food entrainment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R890–R902. ( 10.1152/ajpregu.00313.2012) [DOI] [PubMed] [Google Scholar]
- 142.Tucker CB. 2009. Chapter 11: Behaviour of cattle. In The ethology of domestic animals (ed. Jensen P.), pp. 151–160. Wallingford, UK: CABI Publishing. [Google Scholar]
- 143.Zielinski WJ. 1986. Circadian rhythms of small carnivores and the effect of restricted feeding on daily activity. Physiol. Behav. 38, 613–620. ( 10.1016/0031-9384(86)90254-4) [DOI] [PubMed] [Google Scholar]
- 144.Boulos Z, Frim DM, Dewey LK, Moore-Ede MC. 1989. Effects of restricted feeding schedules on circadian organization in squirrel monkeys. Physiol. Behav. 45, 507–515. ( 10.1016/0031-9384(89)90066-8) [DOI] [PubMed] [Google Scholar]
- 145.O'Reilly H, Armstrong SM, Coleman GJ. 1986. Restricted feeding and circadian activity rhythms of a predatory marsupial, Dasyuroides byrnei. Physiol. Behav. 38, 471–476. ( 10.1016/0031-9384(86)90413-0) [DOI] [PubMed] [Google Scholar]
- 146.Kennedy GA, Coleman GJ, Armstrong SM. 1996. Daily restricted feeding effects on the circadian activity rhythms of the stripe-faced dunnart, Sminthopsis macroura. J. Biol. Rhythms 11, 188–195. ( 10.1177/074873049601100301) [DOI] [PubMed] [Google Scholar]
- 147.Hau M, Gwinner E. 1992. Circadian entrainment by feeding cycles in house sparrows, Passer domesticus. J. Comp. Physiol. A 170, 403–409. ( 10.1007/bf00191457) [DOI] [PubMed] [Google Scholar]
- 148.Hoogerwerf WA, Hellmich HL, Cornélissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. 2007. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133, 1250–1260. ( 10.1053/j.gastro.2007.07.009) [DOI] [PubMed] [Google Scholar]
- 149.Zvonic S, et al. 2006. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55, 962–970. ( 10.2337/diabetes.55.04.06.db05-0873) [DOI] [PubMed] [Google Scholar]
- 150.Mistlberger RE. 2011. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 104, 535–545. ( 10.1016/j.physbeh.2011.04.015) [DOI] [PubMed] [Google Scholar]
- 151.Asher G, Schibler U. 2011. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 13, 125–137. ( 10.1016/j.cmet.2011.01.006) [DOI] [PubMed] [Google Scholar]
- 152.Bass J. 2012. Circadian topology of metabolism. Nature 491, 348–356. ( 10.1038/nature11704) [DOI] [PubMed] [Google Scholar]
- 153.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. 2008. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340. ( 10.1016/j.cell.2008.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lamia KA, et al. 2009. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–440. ( 10.1126/science.1172156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yin L, et al. 2007. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318, 1786–1789. ( 10.1126/science.1150179) [DOI] [PubMed] [Google Scholar]
- 156.O'Neill JS, Reddy AB. 2011. Circadian clocks in human red blood cells. Nature 469, 498–503. ( 10.1038/nature09702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA.. 2008. The emergence of leaders and followers in foraging pairs when the qualities of individuals differ. BMC Evol. Biol. 8, 1 ( 10.1186/1471-2148-8-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rijnsdorp A, Daan S, Dijkstra C. 1981. Hunting in the kestrel, Falco tinnunculus, and the adaptive significance of daily habits. Oecologia 50, 391–406. ( 10.1007/bf00344982) [DOI] [PubMed] [Google Scholar]
- 159.Luby MD, Hsu CT, Shuster SA, Gallardo CM, Mistlberger RE, King OD, Steele AD.. 2012. Food anticipatory activity behavior of mice across a wide range of circadian and non-circadian intervals. PLoS ONE 7, e37992 ( 10.1371/journal.pone.0037992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kuroda H, et al. 2012. Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Sci. Rep. 2, 711 ( 10.1038/srep00711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Patton DF, et al. 2014. Circadian mechanisms of food anticipatory rhythms in rats fed once or twice daily: clock gene and endocrine correlates. PLoS ONE 9, e112451 ( 10.1371/journal.pone.0112451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Daan S, Slopsema S. 1978. Short-term rhythms in foraging behaviour of the common vole, Microtus arvalis. J. Comp. Physiol. 127, 215–227. ( 10.1007/bf01350112) [DOI] [Google Scholar]
- 163.van der Veen DR, Gerkema MP.. 2016. Unmasking ultradian rhythms in gene expression. FASEB J. 31, 743–750. ( 10.1096/fj.201600872R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Linnane MI, Brereton AJ, Giller PS. 2001. Seasonal changes in circadian grazing patterns of Kerry cows (Bos taurus) in semi-feral conditions in Killarney National Park, Co. Kerry, Ireland. Appl. Anim. Behav. Sci. 71, 277–292. ( 10.1016/S0168-1591(00)00188-X) [DOI] [PubMed] [Google Scholar]
- 165.Escobar C, Martinez-Merlos MT, Angeles-Castellanos M, del Carmen Minana M, Buijs RM. 2007. Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. Eur. J. Neurosci. 26, 2804–2814. ( 10.1111/j.1460-9568.2007.05893.x) [DOI] [PubMed] [Google Scholar]
- 166.Krieger DT, Hauser H, Krey LC. 1977. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science 197, 398–399. ( 10.1126/science.877566) [DOI] [PubMed] [Google Scholar]
- 167.Angeles-Castellanos M, Amaya JM, Salgado-Delgado R, Buijs RM, Escobar C. 2011. Scheduled food hastens re-entrainment more than melatonin does after a 6-h phase advance of the light-dark cycle in rats. J. Biol. Rhythms 26, 324–334. ( 10.1177/0748730411409715) [DOI] [PubMed] [Google Scholar]
- 168.Carneiro BT, Araujo JF.. 2011. Influence of scheduled restricted feeding on reentrainment of motor activity rhythm after a 6-h light-dark advance in rats. Psychol. Neurosci. 4, 317 ( 10.3922/j.psns.2011.3.004) [DOI] [Google Scholar]
- 169.Hut RA, Mrosovsky N, Daan S. 1999. Nonphotic entrainment in a diurnal mammal, the European ground squirrel (Spermophilus citellus). J. Biol. Rhythms 14, 409–419. ( 10.1177/074873099129000812) [DOI] [PubMed] [Google Scholar]
- 170.Hut RA, van Oort BE, Daan S. 1999. Natural entrainment without dawn and dusk: the case of the European ground squirrel (Spermophilus citellus). J. Biol. Rhythms 14, 290–299. ( 10.1177/074873099129000704) [DOI] [PubMed] [Google Scholar]
- 171.Bronson FH. 2009. Climate change and seasonal reproduction in mammals. Phil. Trans. R. Soc. B 364, 3331–3340. ( 10.1098/rstb.2009.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Amundsen PA, Bergersen R, Huru H, Heggberget TG. 1999. Diel feeding rhythms and daily food consumption of juvenile Atlantic salmon in the River Alta, northern Norway. J. Fish Biol. 54, 58–71. ( 10.1111/j.1095-8649.1999.tb00612.x) [DOI] [Google Scholar]
- 173.Broekhuis F, Grünewälder S, McNutt JW, Macdonald DW. 2014. Optimal hunting conditions drive circalunar behavior of a diurnal carnivore. Behav. Ecol. 25, 1268–1275. ( 10.1093/beheco/aru122) [DOI] [Google Scholar]
- 174.Calisi RM, Bentley GE. 2009. Lab and field experiments: are they the same animal? Horm. Behav. 56, 1–10. ( 10.1016/j.yhbeh.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 175.Gattermann R, et al. 2008. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol. Lett. 4, 253–255. ( 10.1098/rsbl.2008.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Daan S, et al. 2011. Lab mice in the field: unorthodox daily activity and effects of a dysfunctional circadian clock allele. J. Biol. Rhythms 26, 118–129. ( 10.1177/0748730410397645) [DOI] [PubMed] [Google Scholar]
- 177.Bakker ES, Reiffers RC, Olff H, Gleichman JM. 2005. Experimental manipulation of predation risk and food quality: effect on grazing behaviour in a central-place foraging herbivore. Oecologia 146, 157–167. ( 10.1007/s00442-005-0180-7) [DOI] [PubMed] [Google Scholar]
- 178.Fenn MGP, Macdonald DW. 1995. Use of middens by red foxes: risk reverses rhythms of rats. J. Mammal. 76, 130–136. ( 10.2307/1382321) [DOI] [Google Scholar]
- 179.Levy O, Dayan T, Kronfeld-Schor N. 2007. The relationship between the golden spiny mouse circadian system and its diurnal activity: an experimental field enclosures and laboratory study. Chronobiol. Int. 24, 599–613. ( 10.1080/07420520701534640) [DOI] [PubMed] [Google Scholar]
- 180.Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. 2012. In search of a temporal niche: environmental factors. Prog. Brain Res. 199, 281–304. ( 10.1016/B978-0-444-59427-3.00017-4) [DOI] [PubMed] [Google Scholar]
- 181.Hut RA, Pilorz V, Boerema AS, Strijkstra AM, Daan S. 2011. Working for food shifts nocturnal mouse activity into the day. PLoS ONE 6, e17527 ( 10.1371/journal.pone.0017527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Perrigo G. 1987. Breeding and feeding strategies in deer mice and house mice when females are challenged to work for their food. Anim. Behav. 35, 1298–1316. ( 10.1016/S0003-3472(87)80002-7) [DOI] [Google Scholar]
- 183.van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA. 2014. Cold and hunger induce diurnality in a nocturnal mammal. Proc. Natl Acad. Sci. USA 111, 15 256–15 260. ( 10.1073/pnas.1413135111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. 2005. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus). J. Biol. Rhythms 20, 206–218. ( 10.1177/0748730405275135) [DOI] [PubMed] [Google Scholar]
- 185.Lambert CM, Weaver DR. 2006. Peripheral gene expression rhythms in a diurnal rodent. J. Biol. Rhythms 21, 77–79. ( 10.1177/0748730405281843) [DOI] [PubMed] [Google Scholar]
- 186.Speakman J. 1997. Factors influencing the daily energy expenditure of small mammals. Proc. Nutr. Soc. 56, 1119–1136. ( 10.1079/PNS19970115) [DOI] [PubMed] [Google Scholar]
- 187.van der Vinne V, Gorter JA, Riede SJ, Hut RA. 2015. Diurnality as an energy-saving strategy: energetic consequences of temporal niche switching in small mammals. J. Exp. Biol. 218, 2585–2593. ( 10.1242/jeb.119354) [DOI] [PubMed] [Google Scholar]
- 188.Kronfeld-Schor N, Dayan T. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 153–181. ( 10.1146/annurev.ecolsys.34.011802.132435) [DOI] [Google Scholar]
- 189.Roth TC II, Lima SL. 2007. The predatory behavior of wintering Accipiter hawks: temporal patterns in activity of predators and prey. Oecologia 152, 169–178. ( 10.1007/s00442-006-0638-2) [DOI] [PubMed] [Google Scholar]
- 190.Kronfeld-Schor N, Visser ME, Salis L, van Gils JA. 2017. Chronobiology of interspecific interactions in a changing world. Phil. Trans. R. Soc. B 372, 20160248 ( 10.1098/rstb.2016.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.van der Vinne V, Simons MJ, Reimert I, Gerkema MP. 2014. Temporal niche switching and reduced nest attendance in response to heat dissipation limits in lactating common voles (Microtus arvalis). Physiol. Behav. 128, 295–302. ( 10.1016/j.physbeh.2014.01.019) [DOI] [PubMed] [Google Scholar]
- 192.Kitchen AM, Gese EM, Schauster ER. 2000. Changes in coyote activity patterns due to reduced exposure to human persecution. Canadian J. Zool. 78, 853–857. ( 10.1139/z00-003) [DOI] [Google Scholar]
- 193.Riede SJ, van der Vinne V, Hut RA. 2017. The flexible clock: predictive and reactive homeostasis, energy balance and the circadian regulation of sleep–wake timing. J. Exp. Biol. 220, 738–749. ( 10.1242/jeb.130757) [DOI] [PubMed] [Google Scholar]
- 194.Dardente H, Wyse CA, Birnie MJ, Dupré SM, Loudon ASI, Lincoln GA, Hazlerigg DG. 2010. A molecular switch for photoperiod responsiveness in mammals. Curr. Biol. 20, 2193–2198. ( 10.1016/j.cub.2010.10.048) [DOI] [PubMed] [Google Scholar]
- 195.Hut RA, Dardente H, Riede SJ. 2014. Seasonal timing: how does a hibernator know when to stop hibernating? Curr. Biol. 24, R602–R605. ( 10.1016/j.cub.2014.05.061) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.