Abstract
The interactions between flowering plants and insect pollinators shape ecological communities and provide one of the best examples of coevolution. Although these interactions have received much attention in both ecology and evolution, their temporal aspects are little explored. Here we review studies on the circadian organization of pollination-related traits in bees and flowers. Research, mostly with the honeybee, Apis mellifera, has implicated the circadian clock in key aspects of their foraging for flower rewards. These include anticipation, timing of visits to flowers at specified locations and time-compensated sun-compass orientation. Floral rhythms in traits such as petal opening, scent release and reward availability also show robust daily rhythms. However, in only few studies was it possible to adequately determine whether these oscillations are driven by external time givers such as light and temperature cycles, or endogenous circadian clocks. The interplay between the timing of flower and pollinator rhythms may be ecologically significant. Circadian regulation of pollination-related traits in only few species may influence the entire pollination network and thus affect community structure and local biodiversity. We speculate that these intricate chronobiological interactions may be vulnerable to anthropogenic effects such as the introduction of alien invasive species, pesticides or environmental pollutants.
This article is part of the themed issue ‘Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals’.
Keywords: circadian rhythms, pollination, flower, bee, foraging behaviour, network
1. Introduction
During the Cretaceous Period, there was a remarkable radiation of insects along with the flowering plants (angiosperms) that they pollinated and fed on. Although many insect species pollinate plants, we focus here on bees, which are the most important and best-studied pollinators. Bees are also the principal crop pollinators in agricultural settings; most of the 75% of cultivated plant species that rely on insects for pollination depend on bees [1]. Worryingly, many recent studies (reviewed in [2]) show evidence of large-scale global declines in pollinator richness and density, lending an urgency to studies of plant–pollinator interactions. The coevolution of bees and angiosperms involving numerous modifications in the morphology, phenology and anatomy of both bees and flowers [3–5] is also of interest as one of the best paradigms for studying co-adaptation. The diets of larvae and adults of most bee species depend exclusively on pollen and nectar from flowers [6–8]. Bees show notable behavioural adaptations allowing them to effectively find flowers, learn their location and morphology, and develop means to manipulate them to proficiently harvest their pollen and nectar [9]. In parallel, plants have developed traits to promote and facilitate these plant–pollinator interactions.
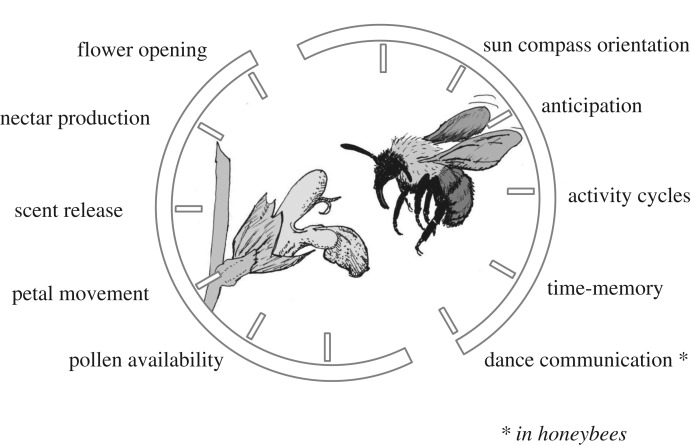
Successful pollination of plants by insects requires both spatial and temporal coordination of both partners. The scales of temporal coordination can range from annual rhythms ensuring that pollinators are active at the same time of year in which flowers are available, to very short interval timing on the scale of minutes and seconds [10]. In this review, we focus on daily rhythms which are the best studied in both bees and plants. Although daily events can be coordinated by changes in environmental variables such as light intensity and temperature, numerous studies in both plants and animals have shown that many of these processes are also influenced by internal, approximately 24 h (circadian, which are distinguished from diel rhythms which are driven by external day–night oscillations) clocks that will free-run under constant conditions and are reset (entrained) by environmental changes [11]. In the context of this review, it is important to note that though the circadian clocks of plants and insects and other organisms do free-run they may be compromised by stimuli such as chemical contaminants, light pollution and other environmental insults (e.g. [12,13]). In general, it has been suggested that having a circadian clock may confer a selective advantage by allowing an organism to anticipate and prepare for predictable daily changes in its environment rather than reacting to changes. Moreover, the circadian system plays an important role in coordinating internal metabolic processes [14]. In recent years, rapid progress in molecular biology and physiology techniques has facilitated mechanistic and evolutionary investigations of the circadian clock of both plants and bees [15]. Below we review evidence showing how circadian systems are part of the toolkit regulating pollination in bees and flowers (figure 1), the implications of both plant and bee rhythms for ecological communities and the possibilities of interactions between the circadian clocks of the two taxa. We focus on chronobiological mechanisms that are driven by molecular, physiological and neuronal (in the case of pollinators) processes, and on how interplay between timing processes in bees and flowers may affect ecological processes. We also discuss what is known about the evolutionary processes that could have shaped this temporal interplay.
Figure 1.
Pollination-promoting traits that vary with approximately 24 h rhythms in plants and bees.
2. Circadian control of pollination-promoting traits in plants
Plants have developed many traits to promote and facilitate pollination by insects and other animals. These traits can be separated into three categories: those that attract pollinators, such as flower colour and scent, those that reward pollinators, such as nectar and pollen production, and the physical traits that enable pollination to occur, such as flower opening [16–19]. Given that many of these pollination-promoting traits are accompanied by a high metabolic cost [20] and possible water-loss, control mechanisms, such as the circadian system, to ensure they only occur at the correct times of day, are clearly valuable.
The ability to anticipate daily changes in their environment is particularly important for sessile plants, which cannot avoid unfavourable conditions (e.g. by contrast to animals, which can opt to move to a different environment, or stay in protected domiciles), and the plant circadian system regulates numerous physiological and molecular processes including chlorophyll biosynthesis, photosynthesis, starch metabolism, growth, leaf movements, stomatal opening and the expression of a large percentage of the genome [21–23]. The plant clock also modulates ‘gating’, the mechanism which ensures that the plant is sensitive to particular signals only at certain times of day; for example, plants may be more sensitive to the stress hormone ABA in the afternoon [24]. Plants also have seasonal rhythms, such as flowering time in many species, that may be controlled by the circadian system, via the photoperiodic pathway.
In this section of the review, we highlight what is known about circadian control of various pollination-promoting traits. Perhaps surprisingly, given the importance of the regulation of pollination to both agriculture and the environment, in recent years there have been relatively few in-depth studies of the circadian regulation of most plant processes involved in pollination. This possibly reflects the current focus for circadian rhythm studies on the model plant Arabidopsis thaliana, which has tiny flowers and, with an outcrossing rate of less than 0.3% in the wild, is predominantly self-pollinating. However, a dearth of reports of circadian rhythms in flowers does not mean that they do not exist; the absence of evidence is not evidence of absence. We are increasingly realizing that the circadian system affects almost all aspects of plant life and, to the best of our current understanding, every cell in a plant has a circadian clock [25]. Thus, it is likely that many of the diel rhythms reported in flowers, including those described below, are under circadian control.
(a). Floral scent
Scent is a complex composition of volatile organic compounds (VOCs); so far, more than 1700 VOCs have been identified. The composition of VOCs can vary greatly, not only between even closely related species, but also between floral organs, flowers of different ages and at various times of day [26–29]. Given that controlled experiments testing circadian, rather than diel, regulation are available for only a few species, it is difficult to generalize at this stage, but the importance of the circadian system in regulating metabolic processes suggests that many more species may show at least partial circadian control of VOCs. In the plants that have so far been studied, it appears that scent production in night-pollinated flowers such as petunia (Petunia hybrida), wax plant (Hoya carnosa), flowering tobacco (Nicotiana sylvestris) and night-blooming jasmine (Cestrum nocturnum) [30–33] is more commonly controlled by the circadian system while emission of volatiles in day-flowers such as white clover (Trifolium repens) [34] tends to be regulated directly by light, suggesting diel control. However, there are exceptions, for example, in the day-blooming common snapdragon (Antirrhinum majus), scent emission is controlled by the circadian system [35]. VOC production may also vary within a flower: while the most abundant VOCs in flowering tobacco, benzyl alcohol and methyl benzoate, are under circadian control, levels of at least one major VOC, caryophyllene, do not oscillate under constant conditions [32].
The temporal expression of scent may be primarily regulated at the level of transcription of genes encoding metabolic pathway components [36]. The circadian system is clearly involved; the timing of volatile emissions in petunia was altered by mis-expression of LATE ELONGATED HYPOCOTYL, a key circadian oscillator gene [37]. However, temporal regulation is complex. Around 30% of the A. thaliana genes are under circadian control and twice as many genes cycle in diel conditions [38], so it is likely that many genes and pathways regulating scent production may be affected both by the circadian system and directly by changes in light or temperature. Adding complexity, the regulation of VOC emissions has been shown in roses (Rosa hybrida) to be under multiple layers of control; the transcription of the enzyme involved in synthesizing the scent component geranyl acetate is under a circadian control but levels of its substrate is light-regulated [39]. Intriguingly, overexpression of a key VOC metabolism transcription factor in roses altered the composition of the scent bouquet and affected the response of honeybees [40]. Thus, although more work is required to understand the extent to which the circadian oscillator regulates VOC emissions in different species, the circadian system may be an important aspect of the plant repertoire for regulating the timing and duration of pollinator visits.
(b). Nectar and pollen
Nectar and pollen may also serve as attractants for bees (see above). Nectar is an aqueous solution composed mainly of sugars with amino acids, proteins, lipids and a range of secondary metabolites. The timing of nectar secretion has been studied in several species. For example, squash (Cucurbita pepo) flowers show robust diel rhythms of nectar volume and concentration, with highest levels during the day when bees are active [41]. However, very few studies have addressed the question of whether the secretion is also under circadian control. One such study used wax plant flowers [42] and showed that nectar production oscillated under continuous light conditions with a maximum during the subjective night which corresponded with the pattern of floral scent emission and the presence of nocturnal pollinators [31]. Diel regulation of pollen production has been observed, especially in anemophilous (wind pollinated) plants, such as varieties of grasses (Poaceae), Roman wormwood (Ambrosia artemesiifolia) and maize (Zea mays) [43–45]. To the best of our knowledge, however, there are no published reports of circadian rhythms of pollen release under constant environmental conditions.
(c). Organ movements
Although plants are sessile, plant organs demonstrate a surprising degree of movement. Circadian-regulated leaf movements were first reported by de Mairan in 1729 [46] and are still routinely used as markers for studying circadian rhythms in plants. In 1880, Darwin & Darwin published a book describing circumnutations, the elliptical bending movements executed by plant organs, including roots, hypocotyls, branches and flower stalks as they grow [47], proposing that these movements are controlled by an internal apparatus. Much more recently, heliotropism (solar tracking) in the shoot apices (tips) of young sunflower (Helianthus annuus) has been shown to be controlled by the circadian system [48]. The apices shift from east-facing in the morning to west-facing in the evening and then back, and disruption of circadian control of heliotropism results in a loss of biomass. In older sunflowers, at anthesis (flower opening and anther dehiscence) the apices stop solar tracking and permanently face east. The authors of the study examined the implications of floral direction by comparing flowers set to face east or west and showed that east-facing flowers warm up more rapidly in the early morning and that this warming is associated with a fivefold increase in the number of pollinator visits.
The flowers of many plants open and then stay open permanently. However, in a range of species belonging to a large number of families, flowers open and close to match environmental conditions. Pollination success may be strongly dependent on the timing of flower opening and closure [49], which determines the pollinator's ability to access the reward and the reproductive organs of the flower. Although most research has focused on flower opening in diel conditions, circadian rhythms have also been demonstrated for several species [19] including day bloomers such as field marigold (Calendula arvensis), common daisy (Bellis perennis) and kalanchoe (Kalanchoë blossfeldiana), and night bloomers such as night-blooming jasmine [33,50–53]. In at least some plants, entrainment of flower opening is flower-autonomous [16,19,50,51]. For example, in field marigold the opening and closing follow the light/dark regime that each flower is subjected to, and leaf and buds on the same plant can be differentially entrained. Importantly, pollinators can have a role in setting the closure pattern of flowers; pollination of smooth hawksbeard (Crepis capillaris) causes rapid (within 1–2 h) flower closing; young flowers will reopen the following day [54].
Circadian petal movement can be a result of differential growth or, possibly less commonly [19], of cell expansion and contraction meditated by ion uptake. In kalanchoe [55], there is uptake of potassium ions during the day, which elevates cell osmolarity and increases turgor pressure resulting in cell expansion in the upper epidermis and petal opening. At night, ion levels decrease leading to low cell pressure and flower closure. By contrast, in marigolds circadian flower opening is caused by differential growth of petal parts [16,56]. Plant growth is controlled by hormones such as methyl jasmonate, ethylene, auxin and giberellins and, in a number of different species, the circadian system has been shown to regulate hormone synthesis, signal reception and processing [57]. Not surprisingly, hormones can also play a role in circadian-controlled petal opening. For example, in kalanchoe, application of methyl jasmonate causes a shorter period of flower opening [58].
(d). Coordination of pollination traits in flowers
In some plants, more than one trait has been shown to be under circadian control. For example, in coyote tobacco (Nicotiana attenuata) the circadian clock controls the movements of petals and the flower stalks (the pedicel), and the emission of the major volatile, benzyl acetone, and these are disrupted by mutations in key circadian clock genes [59]. Similarly, in night-blooming jasmine, the circadian system synchronizes flower opening and scent production. At times when the flowers open, the scent emission is at a maximum. Conversely, when the flowers are closed, no odour is emitted [33]. Thus, the circadian system can function both to allow plants to anticipate and react to predictable daily changes in their environment (for example, the end of the night and the arrival of early pollinators), and to coordinate pollination traits involving a range of processes from gene expression to movements.
The limited studies carried out to date have demonstrated circadian-influenced pollinator-attracting traits in many plant species from a range of families (figure 1). Given the already proved centrality of the circadian system to plants and the potential resource saving capacity of circadian control, we predict that many animal-pollinated flowers will be found to have at least one pollination trait that is under circadian control. The most likely candidates for circadian control are probably scent and nectar production, as these can be affected by circadian mediation of metabolic gene expression, and a large percentage of the plant genome is under circadian control, and substrate availability.
3. Circadian control of pollination-related activities in bees
(a). Anticipation and foraging activity
Most bee species are day active (diurnal) [60]. Relying on the circadian clock to anticipate the time of sunset and sunrise may enable diurnal bees to most efficiently exploit the hours with sufficient sunlight for foraging. Moreover, there can be competitive advantages for bees in arriving early at flowers that have high amounts of nectar and pollen (see below). Consistent with the idea that internal clocks influence activity rhythms in bees are the observations that individually isolated foragers of bumblebees and honeybees typically show strong circadian rhythms in locomotor activity with higher levels of activity during the day even when kept under constant laboratory conditions [15,61–63]. Under more natural conditions, foragers of several species of social bees, including the Western honeybee (Apis mellifera) [64], the stingless bees Partamona helleri [65], Scaptotrigona depilis [66] and Melipona bicolor [67], and the large earth bumblebee (Bombus terrestris) [62], show increased locomotor activity just before sunrise (anticipation) when still inside their nest cavity. Such anticipation has been observed even for cavity-dwelling species with tightly thermo-regulated nests. This strong diurnal pattern of activity is consistent with the premise that an internal clock regulates their morning anticipation.
In solitary bees which do not live in a climatic-controlled nest environment it is more difficult to exclude the role of changes in ambient temperature or light intensity in the regulation of activity rhythms. One of the few studies in which the regulation of activity patterns was studied for a solitary bee focused on the large carpenter bee, Xylocopa (Proxylocopa) olivieri. The pronounced crepuscular (twilight, immediately after dawn and before dusk) activity pattern of this species was suggested to create a distinct temporal niche allowing the bee to forage at times of high floral rewards but low competition, and therefore allocate most of the daytime to nest defence [68]. Given that they start to forage before sunrise or sunset, it seems unlikely that foraging onset is triggered by light. Moreover, visits finish at the same times irrespective of temperature or season. Reward depletion can also be ruled out as the cause because the bees do not extend their foraging time to later hours in flowering patches in which the reward is artificially increased. All of which suggest that carpenter bee foraging may, at least in part, be under circadian control. Another study monitored the daily visit times of fragrance-collecting male orchid bees (Euglossine) to artificial chemical baits [69]. In the early morning, the number of visits was affected mainly by air temperature, but later, activity patterns correlated with time of day and not with ambient temperature, suggesting a potential circadian influence.
(b). Time-memory
Historically, the first involvement of the circadian clock in foraging behaviour to be discovered in bees (published as early as 1900) was the timing of visits to flowers to correspond with periods of maximal reward availability [70]. A few years later, August Forel noted that bees arrived day after day at the same time to feed on his marmalade while he ate breakfast on his patio. The bees continued to arrive at the same time even on later days on which there was no marmalade reward available, leading him to suggest that bees have a ‘time-memory’ (Zeitgedächtnis) [71]. The discovery of time-memory in honeybees inspired a set of elegant studies by von Frisch and his colleagues who showed that foragers can learn multiple daily times of food reward (typically sugar syrup) availability, and can associate the time of reward with a specific location (time-place learning) [72–76]. Forager honeybees can learn to arrive at a specified location at any time of the day and can learn as many as nine time points with intervals of only 45 min between feeder availability [77]. One of the first convincing pieces of evidence that time-memory is controlled by an internal clock came from a jet lag experiment in which honeybees that were trained in Long Island, New York, were flown overnight to Davis, California. On their first day in California these bees foraged according to the New York time rather than using local time cues such as the Sun position in the sky [78]. Additional studies established that time-memory is under circadian control: it free-runs under constant conditions, is entrained by light : dark cycles, can be phase shifted and has a narrow range of entrainment (20 to 26 h cycles), similar to other circadian rhythms (reviewed in [61]).
It is now commonly accepted that time-memory (and time-place learning) is different from entrainment. Whereas entrainment is resetting the clock to a new phase, time-memory occurs when phase information (time of day) derived from an internal oscillator is ‘stamped’ in memory as a contextual feature to form associations with other contextual features [75]. More recent studies with honeybees show that the time of day can be associated with complex cognitive functions. For example, honeybees can retrieve different memories and learn to perform different tasks at different times of day [79,80]. Time-memory learning is not limited to honeybees. Stingless bees have also been trained to arrive at artificial feeders at a certain time during the day. After training, the stingless bees anticipated the time of reward availability and arrived at the site even when the feeders were no longer available [81–83]. Time-memory was also suggested for the two solitary bee species Tetraglossula ventralis (Colletidae) and Heterosarellus sp. (Andrenidae) arriving at Ludwigia elegans flowers before the time of full anthesis [84]. Given that petal opening in this plant typically occurs 30–60 min before full anthesis and may serve as a sign that there will be reward soon, additional studies are needed for establishing circadian regulation in this system. Nevertheless, the authors report that the bees typically left the flowers before the reward was depleted, suggesting that the circadian clock influenced their entire daily schedule. It should be noted, however, that the regulation of the temporal pattern of foraging activity is probably a complex process which may be affected by multiple internal and external factors rather than strictly determined by the bee's circadian system.
(c). Sun-compass orientation and dance communication
Honeybees use the position of the Sun as a celestial compass to find the way over relatively large distances to known food sources, and when homing back to their nests; they orientate themselves by maintaining a fixed angle to the Sun [73,85–88]. Even when it is overcast they are still able to detect light polarization which is correlated with the position of the Sun in the sky [73,89]. The continuous daily and seasonal changes of the Sun position relative to the horizon pose a challenge to animals that use the Sun as a compass, but they can compensate for the Sun's movement over time by ‘consulting’ their circadian clocks. Phase shift experiments, including Renner's [78] classical jet lag experiments (described above), have confirmed that the circadian clock is key for time compensation. Time-compensated sun-compass orientation has also been reported for the stingless bee Trigona spinipes [90]. Recent studies suggest that bumblebees can detect polarized light [91] and have neuroanatomical structures similar to those found in other insects that use polarized light for navigation [92], but, to the best of our knowledge, direct behavioural evidence for time-compensated sun-compass orientation is still missing for bumblebees as well as for species of solitary bees. The Sun's position in the sky also guides recruitment using waggle dance communication in honeybees (reviewed in [73]). The angle between the straight line going through the waggling part of dance and the line pointing opposite to the centre of gravity denotes the angle between the line connecting the hive and the Sun position and the direct path to the food source (or putative nest site in scouts hunting for new nests). Foragers that stay inside the nest for long periods (e.g. owing to bad weather) rely on their clock to shift the direction of their waggle dance with a remarkable correlation to the Sun's path in the sky [72,73].
4. Synchronization between plant and bee circadian systems
Clearly, the circadian system of both plants and bees will be entrained by abiotic environmental signals such as light and temperature, but can they also entrain each other? Despite the importance of the topic for both crop production and ecosystem diversity, surprisingly little attention has been paid to the significance of circadian systems in shaping plant–pollinator interactions.
Pollinator visits might potentially affect flower rhythms. Floral opening and closing are possible points of synchronization between flower and bee circadian rhythms. However, most floral responses to pollination are probably too slow to affect rhythms of flower opening [93,94] and those plants that are known to respond to pollination within a few hours, for example, Geraniaceae [95], have not been shown to have circadian regulation of floral parts, or their circadian regulation is insignificant compared with other factors such as light and temperature [19]. Nevertheless, the possibility that pollinator visits may entrain floral opening rhythms should still be considered. If this interaction does occur, it may have ecological implications: it has been shown that a successful visit by an insect pollinator can significantly advance the time of flower closure and shape the temporal pattern of floral availability and the entire pollination network [54]. Flowers of the chicory subfamily (Cichorioideae) typically open in the early morning and close 1–2 h after pollination. Although to the best of our knowledge there is no evidence that the opening and closing of Cichorioideae flowers is regulated by an internal circadian clock rather than by environmental factors, this study is important because it highlights the potential importance of the temporal programme of dominant plants for ecological communities.
In contrast with the scarcity of evidence for pollinator influence on flower rhythms, it is well established that flowers can modulate the temporal organization of pollinator activity, and this may be mediated, at least in part, by the circadian clock. For example, in the stingless bee Melipona fasciata the daily onset of foraging activity is influenced by previous experience; successful foraging results in earlier departure on the following day [96]. It would be interesting to know whether this represents a genuine entrainment of the circadian clock, or a form of time-memory (see above) in which a memorized event is associated with a certain phase of the circadian cycle. We suggest that time-memory rather than entrainment is more commonly used by bees for timing visits to flowers. First, time-memory does not require shifting the phase of the circadian clock, which is also involved in multiple other processes, including those related to social organization. Second, time-memory allows the flowering times for several flower species and locations to be recorded.
It is tempting to speculate that plant–pollinator coevolution included circadian system modifications. Wild petunia flowers and their pollinators potentially provide an example of such coevolution. The violet petunia (Petunia integrifolia) is naturally pollinated by day-visiting bees (mostly by Leioproctus spp., subgenus Hexantheda, members of the plasterer bee family Colletidae) while the sympatric large white petunia (Petunia axillaris) flowers are visited by night-active moths such as hawkmoths (Manduca sp.). Controlled laboratory experiments under diel and constant light conditions show that the large white petunias release benzenoid VOCs with a circadian rhythm and an emission peak at night, which is absent in violet petunias. When odours from flowers of different petunias are applied directly to the excised antennae of female hawkmoths (Manduca sexta) the large white petunia odours elicit stronger responses than violet petunia odours, and the response is stronger for night-collected fragrance [97]. Thus, this study is consistent with the premise that a pollinator species has evolved to respond specifically to odours of specific flowers it commonly visits. Similarly, the antennae of honeybee foragers show elevated responsiveness to flower odours during the day [98]. Additional studies will be needed to explicitly test the hypothesis that plant–pollinator coevolution shaped properties of their circadian systems.
5. The potential influences of plant and pollinator circadian rhythms on ecological communities
Given the key roles of plants in shaping ecological communities and the mutual dependence of flowering plants and pollinators, the temporal organization of their interactions may have broad implications on ecological systems. For example, the temporal organization of flower reward availability and bee foraging activity may affect the degree of competition, and may lead sympatric plant and/or pollinator species to either exclusion or coexistence. In plants, temporal partitioning of pollinator attraction times may minimize pollen loss due to transfer to heterospecific flowers. The most basic concept in this context is the ‘temporal niche’ which means that time-based variation in resource consumption rates can allow two or more competitors to coexist while limited by the same resource [99,100]. In the context of pollination ecology, this may mean that sympatric plant species that share the same pollinators, but partition the time during which they provide pollen or nectar rewards, can coexist in the same community despite the competition for pollinators.
(a). Plant diversity
Temporal partitioning is expected to be beneficial to plants because it reduces competition, and to visiting pollinators because they can more effectively exploit rewards provided by multiple flower species at different times during the same day. Consistent with this premise, Armbruster & Herzig [101] described two sympatric species of Dalechampia plants that differ in flower opening time during the day. The inflorescences of Dalechampia heteromorpha are open and produce pollen and resins early in the morning, whereas in Dalechampia scandens this occurs only during the afternoon. Solitary bee pollinators of the genus Hypanthodium and highly eusocial stingless bees of the genus Trigona exploit the first species during morning hours and then switch to the other during the afternoon, hence minimizing the competition between the two plant species, as well as heterospecific pollen transfer. Similarly, records of pollen presence indicate clear temporal separation of pollen availability in flowers of sympatric Acacia species which bloom in the same season. Although the visitor assemblages of the different species are overall different, there are some pollinators, particularly a small group of solitary bee species of the family Megachilidae, that visit four different Acacia species, and switch between them in line with the time of maximal pollen reward [102,103]. It is possible that colour or odour changes in the Acacia flowers during pollen dehiscence are being used as cues by the bees or that the bees rely on time–place learning but use the cues from the trees to fine-tune the temporal organization of their foraging activity. Interestingly, gum arabic trees (Acacia senegal) bloom later and their pollen dehiscence time is more synchronous in communities where they grow together with a related species, A. zazibarica, than in communities where A. zanzibarica is absent [103], suggesting a competitive character displacement and convergent selection of pollen dehiscence time at the population level. A temporal partitioning of blooming time was also described for two sympatric Mexican Acacia species, with evidence that this minimizes both competition and unwanted heterospecific pollen transfer [104]. Despite the paucity of studies, we suggest that temporal partitioning may be important for plant–pollinator relationships, and therefore, more common than currently acknowledged. Given the evidence for circadian influences on pollination-related traits in insects, we speculate that selection acting on genes involved in rhythm generation or entrainment may contribute to temporal portioning, at least in some systems. This is an important topic for future research in eco-chronobiology.
(b). Pollinator diversity
Temporal partitioning can also reduce competition between pollinators and allow for coexistence of species exploiting similar resources. Pollination networks provide an excellent tool for assessing both temporal (time of day in this context) and trophic (the food sources they consume) niches of species coexisting in the same habitat. A network analysis for six sympatric neotropical carpenter bees (Xylocopa spp.) on mixed crop agricultural land in northern Brazil showed that bees are more strongly separated in the temporal niche dimension than in the dietary dimension [105]. The results further suggest that the relative importance of the dietary (trophic) and temporal niches is not fixed and may change with variables such as time of year or climate. For example, in a dry tropical forest in northern Brazil the overlap in activity time increases, and temporal niche partitioning decreases, when bees can forage only during the few hours a day in which the temperatures are not too high [106]. An important implication of this temporal influence on pollination is that the pollination networks at the same location could vary with time of day, for example, being significantly different before and after noon.
The presence of ecologically dominant species with a strong temporal flowering time can have significant influence on the pollination network and local biodiversity. Studies on meadows in Germany that are dominated by morning-flowering Cichorioideae plants reveal the extent to which Cichorioideae plant species dominate the habitat and shape the dynamics of interactions among pollinators within the course of a day, including the number of pollinator visits recorded on flowers of other plant species. Some flowers, such as the common yarrow (Achillea millefolium), are mainly visited during the afternoon in networks with a high proportion of Cichorioideae, but during the morning in networks with a low proportion of Cichorioideae. These observations suggest that the visitation rate to common yarrow is reduced during the morning owing to competition for pollinators from Cichorioideae [54]. Additional studies, however, are needed to determine whether the observed oscillations are driven by ambient time givers or internal circadian clocks. The importance of this study is that it shows that the temporal organization of flowering in even a small number of plant species can have a profound effect on the times at which other plants in the same habitat are visited. Thus, circadian influence on pollination-attracting traits in even a few species may have a great influence on the entire pollination network, and accordingly, on pollinator biodiversity.
A fitness benefit of the increase in number and diversity of pollinators owing to temporal niche partitioning among pollinators may be an increase of plant fecundity. There is evidence that an increase in number or diversity of pollinators can improve seed and fruit set (the proportion of a plant's ovules and flowers that develop into mature seeds and fruits, respectively [107]). The improved fecundity is thought to stem from the ‘complementary effects’ of pollinators with diverse body size, morphology and behaviour [108]. The idea is that different pollinator types complement each other by reaching flowers in different parts of the plant, at different points in time, or by contacting different parts within the same flower. For example, in pumpkins (Cucurbita moschata), seed set was better correlated with the number of visiting bee species than with the total number of bee visits [109]. On a larger scale, an analysis of data collected worldwide from 600 fields of 41 crop systems shows that an increase in wild insect visitation enhances fruit set by twice as much as an equivalent increase in honeybee visitation and that the influence of honeybee pollination is complementary to that of wild bees [107]. For example, pollinator richness may improve within-flower pollen dispersal if early-visiting large bees carry a large amount of pollen between flowers, whereas later-arriving smaller bees may distribute pollen more effectively within flowers. A more specific contribution of complementary time of pollinators active during different times during the day was shown for the hedgehog cactus (Echinopsis chiloensis). More seeds were produced by plants visited by both bees during the day and hawkmoths during the night, compared with plants for which pollinator visits were prevented during either day or night [110]. The increase in pollinator diversity and its temporal complementary effect on plant fecundity may explain why many plants invest in offering floral reward over many hours during the day, or at several different times of the same day (e.g. [110–112]). The hypothesis that pollinators that visit flowers at different times during the day complement each other and by that improve fruit quantity or quality has an obvious economic value and should be tested for key crops.
6. Conclusion and future perspectives
Both plants and insects show temporal organization of pollination-related activities. The circadian system has been implicated in the regulation of various aspects of foraging behaviour of individual bees as well as in social processes that determine the proportion of bees a colony allocates to foraging activities (see above and [15,61]). In plants, there is evidence for circadian regulation of pollination-related activities such as flower opening, scent release and reward (e.g. pollen and nectar) availability.
However, despite the potential importance of circadian rhythms for plant–pollinator interactions, our review highlights significant gaps in our knowledge. On the pollinator side, most of the research has focused on a single species, the European (or Western) honeybee. We do not know the extent to which time-memory and time-compensated sun-compass navigation are important for the foraging behaviour of non-Apis pollinators. Honeybee societies are large, typically composed of thousand to tens of thousands of individuals, and forage over large areas for which efficient orientation, visit timing and recruitment (e.g. waggle dance communication) are functionally significant. By contrast, most bee species are solitary or live in simple societies, and forage over much shorter distances. Thus, it is not clear the extent to which the findings for honeybees (and to some extent bumblebees and stingless bees) can be generalized to ecological or agricultural systems that are not dominated by honeybees (or more generally, social bees).
Circadian rhythms have been well characterized in many plant tissues and organs, but floral circadian rhythms are poorly understood; few attempts have been made to distinguish rhythms driven by the endogenous circadian clock rather than by the environment. The few species in which floral rhythms have been studied do not allow us to provide even a crude estimate of the extent to which floral rhythms are under circadian, as opposed to diel, control. We can speculate that the potential of the circadian system for coordinating molecular, metabolic and physiological responses and for allowing the plant to anticipate environmental changes and, possibly, pollinator visits, as opposed to just reacting to the environment, means that in the future we will find that many diel rhythms are also regulated by circadian clocks.
Given that circadian rhythms in pollination-facilitating traits have been well characterized for only a few species of plants and pollinators, caution should be exercised when attempting to estimate the influence of the interplay between the timing systems of plants and pollinators at the ecological level. Nonetheless, the second part of our review lends credence to this idea by showing that chronobiological interactions between pollinators and flowers may influence diverse ecological processes. Plants and pollinators can have profound influence on the temporal niche of each other; the interplay between their daily rhythms can affect biodiversity, interspecific competition and interaction networks. On an evolutionary scale, the interplay between temporal processes in plants and pollinators could lead to coevolutionary modifications in the circadian system or in other systems controlling the response to environmental cycles (for example, in photoperiod or temperature cycles). Nevertheless, our review suggests that even if pollination-related traits are circadianly regulated in only a few species of plants, this may have profound influence on the entire pollination network, which in turn can affect biodiversity, and community structure.
Future studies are also needed to explore the mutual influences of pollinators and flowers on each other's circadian clock systems. Can the time of flower reward availability entrain the clock of bees and other visitors, and can the time of pollinator visit shift the rhythms of flower opening or closing, or the time flowers provide pollen or nectar? Bees can indeed be trained to arrive at flowers at specific times of reward availability, but very little is known about the neuronal mechanisms underlying this fascinating behaviour. How and where in the central nervous system is time of day information associated with information on floral rewards? Are there special cells in the brain circadian network that are in charge of this task? Studies such as those with sympatric Acacia species, described above, suggest that the phase of flowering rhythms can be influenced by the presence of other flowering plants that may compete for pollinator visits. Moreover, we do not understand what mediates temporal plant–pollinator interactions at both ecological and evolutionary time scales. To what extent and how do plants adjust their flowering time during the day to minimize potentially harmful competition? Clearly, the chronobiology of plants and pollinators deserves more attention from ecologists studying plant communities, species diversity and pollination networks.
Given the evidence that the circadian clock is crucial for foraging behaviour in honeybees and other bee species (and probably other pollinators), it is important to assess influences of environmental stressors on their circadian functions. For example, insecticides such as neonicotinoids influence many neuronal and behavioural functions in insects [113] and it is reasonable to suspect that neuroactive chemicals may also affect the brain circadian network and the behaviours it controls. Invasive species may also change the temporal pattern of pollination networks, which in turn, can pose strong competitive challenges to local species with narrow temporal niches. Our unpublished results suggest that invasive (or range-extending) bumblebees (Bombus terrestris) arrive at flowers during the early and cold morning and deplete non-replenished pollen from many flowers (N. Bar-Shai and G. Bloch 2016, unpublished results; figure 2). Later-arriving native solitary bees may suffer reduced pollen availability in areas into which bumblebees have recently extended their distribution range. Species with specialized temporal niches such as the crepuscular Xylocopa (Proxilocopa) olivierri [68] may be particularly vulnerable to invasive species that can exploit resources available during their restricted time of activity. Similarly, invasive plant species can dominate habitats and attract many pollinators, hence reducing the pollination services available to native species during at least part of the day [54].
Figure 2.
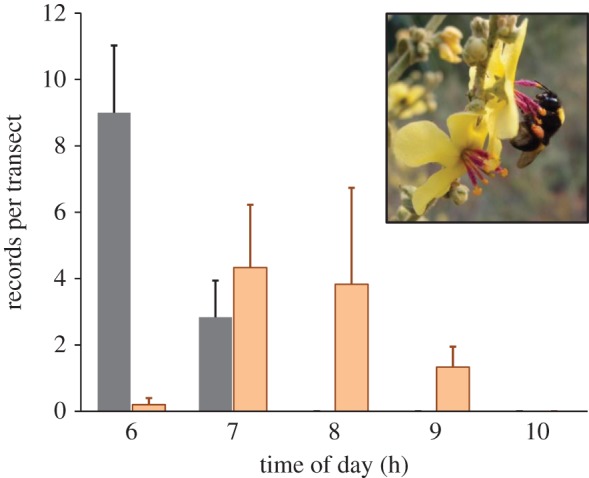
Bumblebees arrive earlier in the morning to pollen producing flowers. The plot shows the number of visit records of bumblebees (Bombus terrestris; black bars) and native solitary bee species (orange bars) on Verbascum sinuatum flowers. The flowers of this herbaceous perennial plant are open merely during morning hours and provide only pollen as reward to visiting bees. Visits were recorded along a fixed route (transect) and are summed as number of records per transect. Observations were performed on a weekly bases throughout the blooming period on June and July 2013, in the Judean Hills, Israel. The inset shows a B. terrestris worker on a V. sinuatum flower (photo credit: Noam Bar-Shai).
In summary, although the interplay between the circadian clocks of bees and flowers has been little explored, we believe that the evidence summarized above is sufficient to convince chronobiologists, ecologists, entomologists and botanists that this theme is promising. There is solid evidence that pollination-related traits of both plants and bees are under circadian control, and evidence that diurnal rhythms can affect various ecological processes. Importantly, the ecological importance of circadian clocks does not require that all, or even most pollinators or flowers show strong circadian rhythms—even a few species with strong circadian rhythms in pollination-related traits may have profound influence on the entire pollination network, and consequently, on the structure of ecological communities and biodiversities. In the future, we predict that understanding the mechanisms by which circadian clocks influence plant–pollinator systems, and their vulnerability to environmental stressors, may improve crop production and help mitigate decline of pollinators.
Acknowledgement
We thank Achik Dorchin and two anonymous reviewers for helpful comments on an earlier version of this manuscript.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Research in the Bloch lab relevant to this paper has been supported by grants from the Israel Science Foundation (ISF), the Binational Agricultural Research & Development Fund (BARD), The U.S.–Israel Binational Science Foundation (BSF), the Ring Foundation and the Israel Taxonomy Initiative (ITI). Research in the Green lab relevant to this paper has been supported by grants from the ISF and BSF.
References
- 1.Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomann M, Imbert E, Devaux C, Cheptou PO. 2013. Flowering plants under global pollinator decline. Trends Plant Sci. 18, 353–359. ( 10.1016/j.tplants.2013.04.002) [DOI] [PubMed] [Google Scholar]
- 3.Regal PJ. 1977. Ecology and evolution of flowering plant dominance. Science 196, 622–629. ( 10.1126/science.196.4290.622) [DOI] [PubMed] [Google Scholar]
- 4.Crepet WL. 1979. Insect pollination: a paleontological perspective. Bioscience 29, 102–108. ( 10.2307/1307746) [DOI] [Google Scholar]
- 5.Crepet WL. 1984. Advanced (constant) insect pollination mechanisms: pattern of evolution and implications vis-à-vis angiosperm diversity. Ann. MO Bot. Gar. 71, 607–630. ( 10.2307/2399041) [DOI] [Google Scholar]
- 6.Willmer PG, Stone GN. 2004. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv. Stud. Behav. 34, 347–466. ( 10.1016/s0065-3454(04)34009-x) [DOI] [Google Scholar]
- 7.Willmer P. 2011. Pollination and floral ecology, p. 775 Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.Michener CD. 2000. Bees of the world. Baltimore, MD: John Hopkins University Press. [Google Scholar]
- 9.Macior LW. 1974. Behavioral aspects of coadaptations between flowers and insect pollinators. Ann. MO Bot. Gar. 61, 760–769. ( 10.2307/2395027) [DOI] [Google Scholar]
- 10.Boisvert MJ, Sherry DF. 2006. Interval timing by an invertebrate, the bumble bee Bombus impatiens. Curr. Biol. 16, 1636–1640. ( 10.1016/j.cub.2006.06.064) [DOI] [PubMed] [Google Scholar]
- 11.Dunlap JC, Loros JJ, Decoursey PJ. 2004. Chronobiology: biological timekeeping, p. 406 Sunderland, MA: Sinauer Associates. [Google Scholar]
- 12.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18 664–18 669. ( 10.1073/pnas.1008734107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanil D, Shetty NJ. 2012. The effect of sublethal exposure to temephos and propoxur on reproductive fitness and its influence on circadian rhythms of pupation and adult emergence in Anopheles stephensi Liston—a malaria vector. Parasitol. Res. 111, 423–432. ( 10.1007/s00436-012-2857-2) [DOI] [PubMed] [Google Scholar]
- 14.Yerushalmi S, Green RM. 2009. Evidence for the adaptive significance of circadian rhythms. Ecol. Lett. 12, 970–981. ( 10.1111/j.1461-0248.2009.01343.x) [DOI] [PubMed] [Google Scholar]
- 15.Bloch G. 2010. The social clock of the honeybee. J. Biol. Rhythms 25, 307–317. ( 10.1177/0748730410380149) [DOI] [PubMed] [Google Scholar]
- 16.van Doorn WG, van Meeteren U. 2003. Flower opening and closure: a review. J. Exp. Bot. 54, 1801–1812. ( 10.1093/jxb/erg213) [DOI] [PubMed] [Google Scholar]
- 17.Sheehan H, Hermann K, Kuhlemeier C. 2012. Color and scent: how single genes influence pollinator attraction. Cold Spring Harb. Symp. Quant. Biol. 77, 117–133. ( 10.1101/sqb.2013.77.014712) [DOI] [PubMed] [Google Scholar]
- 18.Simpson BB, Neff JL. 1983. Evolution and diversity of floral rewards. In Handbook of experimental pollination biology (eds Jones CE, Little RJ), pp. 142–159. New York, NY: Van Nostrand Reinhold. [Google Scholar]
- 19.van Doorn WG, Kamdee C. 2014. Flower opening and closure: an update. J. Exp. Bot. 65, 5749–5757. ( 10.1093/jxb/eru327) [DOI] [PubMed] [Google Scholar]
- 20.Vogel S. 1983. Ecophysiology of zoophilic pollination. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 21.Nagel DH, Kay SA. 2012. Complexity in the wiring and regulation of plant circadian networks. Curr. Biol. 22, R648–R657. ( 10.1016/j.cub.2012.07.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113. ( 10.1126/science.290.5499.2110) [DOI] [PubMed] [Google Scholar]
- 23.Yakir E, Hilman D, Harir Y, Green RM. 2007. Regulation of output from the plant circadian clock. FEBS J. 274, 335–345. ( 10.1111/j.1742-4658.2006.05616.x) [DOI] [PubMed] [Google Scholar]
- 24.Robertson FC, Skeffington AW, Gardner MJ, Webb AA. 2009. Interactions between circadian and hormonal signalling in plants. Plant Mol. Biol. 69, 419–427. ( 10.1007/s11103-008-9407-4) [DOI] [PubMed] [Google Scholar]
- 25.Endo M, Shimizu H, Nohales MA, Araki T, Kay SA. 2014. Tissue-specific clocks in Arabidopsis show asymmetric coupling. Nature 515, 419–422. ( 10.1038/nature13919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudsen JT, Eriksson R, Gershenzon J, Stahl B. 2006. Diversity and distribution of floral scent. Bot. Rev. 72, 1–120. ( 10.1663/0006-8101(2006)72%5B1:DADOFS%5D2.0.CO;2) [DOI] [Google Scholar]
- 27.Raguso RA, Levin RA, Foose SE, Holmberg MW, McDade LA. 2003. Fragrance chemistry, nocturnal rhythms and pollination ‘syndromes’ in Nicotiana. Phytochemistry 63, 265–284. ( 10.1016/S0031-9422(03)00113-4) [DOI] [PubMed] [Google Scholar]
- 28.Dotterl S, Jurgens A. 2005. Spatial fragrance patterns in flowers of Silene latifolia: lilac compounds as olfactory nectar guides? Plant Syst. Evol. 255, 99–109. ( 10.1007/S00606-005-0344-2) [DOI] [Google Scholar]
- 29.Pichersky E, Raguso RA, Lewinsohn E, Croteau R. 1994. Floral scent production in Clarkia (Onagraceae).1. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol. 106, 1533–1540. ( 10.1104/pp.106.4.1533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng S, Fu X, Mei X, Zhou Y, Du B, Watanabe N, Yang Z. 2016. Regulation of biosynthesis and emission of volatile phenylpropanoids/benzenoids in Petunia × hybrida flowers by multi-factors of circadian clock, light, and temperature. Plant Physiol. Biochem 107, 1–8. ( 10.1016/j.plaphy.2016.05.026) [DOI] [PubMed] [Google Scholar]
- 31.Altenburger R, Matile P. 1988. Circadian rhythmicity of fragrance emission in flowers of Hoya carnosa R. Br. Planta 174, 248–252. ( 10.1007/Bf00394778) [DOI] [PubMed] [Google Scholar]
- 32.Loughrin JH, Hamiltonkemp TR, Andersen RA, Hildebrand DF. 1991. Circadian-rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens. Physiol. Plantarum 83, 492–496. ( 10.1034/J.1399-3054.1991.830324.X) [DOI] [Google Scholar]
- 33.Overland L. 1960. Endogenous rhythm in opening and odor of flowers of Cestrum nocturnum. Am. J. Bot. 47, 378–382. ( 10.2307/2439225) [DOI] [Google Scholar]
- 34.Jakobsen HB, Olsen CE. 1994. Influence of climatic factors on emission of flower volatiles in situ. Planta 192, 365–371. ( 10.1007/BF00198572) [DOI] [Google Scholar]
- 35.Kolosova N, Gorenstein N, Kish CM, Dudareva N. 2001. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 13, 2333–2347. ( 10.1105/Tpc.13.10.2333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenske MP, Imaizumi T. 2016. Circadian rhythms in floral scent emission. Front. Plant Sci. 7, 462 ( 10.3389/fpls.2016.00462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenske MP, Hewett Hazelton KD, Hempton AK, Shim JS, Yamamoto BM, Riffell JA, Imaizumi T. 2015. Circadian clock gene LATE ELONGATED HYPOCOTYL directly regulates the timing of floral scent emission in Petunia. Proc. Natl Acad. Sci. USA 112, 9775–9780. ( 10.1073/pnas.1422875112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mockler TC, Michael TP, Priest HD, Shen R, Sullivan CM, Givan SA, McEntee C, Kay SA, Chory J. 2007. The Diurnal project: diurnal and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb. Symp. Quant. Biol. 72, 353–363. ( 10.1101/sqb.2007.72.006) [DOI] [PubMed] [Google Scholar]
- 39.Hendel-Rahmanim K, Masci T, Vainstein A, Weiss D. 2007. Diurnal regulation of scent emission in rose flowers. Planta 226, 1491–1499. ( 10.1007/S00425-007-0582-3) [DOI] [PubMed] [Google Scholar]
- 40.Zvi MM, Shklarman E, Masci T, Kalev H, Debener T, Shafir S, Ovadis M, Vainstein A. 2012. PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 195, 335–345. ( 10.1111/j.1469-8137.2012.04161.x) [DOI] [PubMed] [Google Scholar]
- 41.Edge AA, van Nest BN, Johnson JN, Miller SN, Naeger N, Boyd SD, Moore D. 2012. Diel nectar secretion rhythm in squash (Cucurbita pepo) and its relation with pollinator activity. Apidologie 43, 1–16. ( 10.1007/s13592-011-0087-8) [DOI] [Google Scholar]
- 42.Matile P. 2006. Circadian rhythmicity of nectar secretion in Hoya carnosa. Bot. Helv. 116, 1–7. ( 10.1007/S00035-006-0740-4) [DOI] [Google Scholar]
- 43.van Hout R, Chamecki M, Brush G, Katz J, Parlange MB. 2008. The influence of local meteorological conditions on the circadian rhythm of corn (Zea mays L.) pollen emission. Agric. For. Meteorol. 148, 1078–1092. ( 10.1016/J.Agrformet.2008.02.009) [DOI] [Google Scholar]
- 44.Martin MD, Chamecki M, Brush GS. 2010. Anthesis synchronization and floral morphology determine diurnal patterns of ragweed pollen dispersal. Agric. For. Meteorol. 150, 1307–1317. ( 10.1016/J.Agrformet.2010.06.001) [DOI] [Google Scholar]
- 45.Reddi CS, Reddi NS, Janaki BA. 1988. Circadian patterns of pollen release in some species of Poaceae. Rev. Palaeobot. Palynol. 54, 11–42. ( 10.1016/0034-6667(88)90003-6) [DOI] [Google Scholar]
- 46.Pittendrigh CS, Bruce V. 1959. Daily rhythms as coupled oscillator systems and their relationship to thermoperiodism and photoperiodism. In Photoperiodism and related phenomena in plants and animals (ed. Withrow RB.), pp. 475–505. Washington, DC: American Association for the Advancement of Science. [Google Scholar]
- 47.Darwin CA, Darwin F. 1880. The power of movement in plants. London, UK: John Murray. [Google Scholar]
- 48.Atamian HS, Creux NM, Brown EA, Garner AG, Blackman BK, Harmer SL. 2016. Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science 353, 587–590. ( 10.1126/science.aaf9793) [DOI] [PubMed] [Google Scholar]
- 49.Miyake T, Yahara T. 1999. Theoretical evaluation of pollen transfer by nocturnal and diurnal pollinators: when should a flower open? Oikos 86, 233–240. ( 10.2307/3546441) [DOI] [Google Scholar]
- 50.Stoppel R. 1910. Über den Einfluss des Lichtes auf das Öffnen und Schliessen einiger Blüten. Z. Bot. 2, 367–453 (in German). [Google Scholar]
- 51.Stoppel R, Kniep H. 1910. Weitere Untersuchungen über das Öffnen und Schliessen der Blüten. Z. Bot. 3, 369–399 (in German). [Google Scholar]
- 52.Bünsow R. 1953. Endogene Tagesrhythmik und Photoperiodismus bei Kalenchoë blössfeldiana. Planta 42, 220–252. ( 10.1007/BF01938571) [DOI] [Google Scholar]
- 53.Bünsow R. 1953. Uber den Einfluss der Lichtmenge auf die endogene Tagesrhythmik bei Kalenchoë blössfeldiana. Biologisches Zentralbl. 72, 465–477 (in German). [Google Scholar]
- 54.Frund J, Dormann CF, Tscharntke T. 2011. Linnés floral clock is slow without pollinators – flower closure and plant-pollinator interaction webs. Ecol. Lett. 14, 896–904. ( 10.1111/J.1461-0248.2011.01654.X) [DOI] [PubMed] [Google Scholar]
- 55.Schrempf M. 1977. Studies of circadian-rhythm of petal movement in Kalanchoe blossfeldiana. J. Interdiscipl. Cycle 8, 396–400. ( 10.1080/09291017709359611) [DOI] [Google Scholar]
- 56.Kerner von Marilaun A. 1891. Pflanzenleben. Leipzig, Germany: Verlag des Bibliographisches Institut. [Google Scholar]
- 57.Atamian HS, Harmer SL. 2016. Circadian regulation of hormone signaling and plant physiology. Plant Mol. Biol. 91, 691–702. ( 10.1007/s11103-016-0477-4) [DOI] [PubMed] [Google Scholar]
- 58.Engelmann W, Sommerkamp A, Veit S, Hans J. 1997. Methyl-jasmonate affects the circadian petal movement of Kalanchoe flowers. Biol. Rhythm Res. 28, 377–390. ( 10.1076/Brhm.28.4.377.13115) [DOI] [Google Scholar]
- 59.Yon F, Joo Y, Cortes Llorca L, Rothe E, Baldwin IT, Kim SG. 2016. Silencing Nicotiana attenuata LHY and ZTL alters circadian rhythms in flowers. New Phytol. 209, 1058–1066. ( 10.1111/nph.13681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehmann M, Gustav D, Galizia CG. 2011. The early bee catches the flower - circadian rhythmicity influences learning performance in honey bees, Apis mellifera. Behav. Ecol. Sociobiol. 65, 205–215. ( 10.1007/s00265-010-1026-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore D. 2001. Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J. Insect. Physiol. 47, 843–857. ( 10.1016/s0022-1910(01)00057-9) [DOI] [Google Scholar]
- 62.Yerushalmi S, Bodenhaimer S, Bloch G. 2006. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J. Exp. Biol. 209, 1044–1051. ( 10.1242/jeb.02125) [DOI] [PubMed] [Google Scholar]
- 63.Chittka L, Stelzer RJ, Stanewsky R. 2013. Daily changes in ultraviolet light levels can synchronize the circadian clock of bumblebees (Bombus terrestris). Chronobiol. Int. 30, 434–442. ( 10.3109/07420528.2012.741168) [DOI] [PubMed] [Google Scholar]
- 64.Woyke J. 1992. Diurnal flight activity of African bees Apis mellifera adansonii in different seasons and zones of Ghana. Apidologie 23, 107–117. ( 10.1051/apido:19920203) [DOI] [Google Scholar]
- 65.Azevedo GG. 1997. Atividade de vôo e determinação do número de ínstares larvais em Partamona helleri (Friese): (Hymenoptera, Apidae, Meliponinae). Viçosa, Brazil: Universidade Federal de Viçosa, MG. [Google Scholar]
- 66.Bellusci S, Marques MD. 2001. Circadian activity rhythm of the foragers of a eusocial bee (Scaptotrigona aff depilis, Hymenoptera, Apidae, Meliponinae) outside the nest. Biol. Rhythm Res. 32, 117–124. ( 10.1076/brhm.32.2.117.1351) [DOI] [Google Scholar]
- 67.Hilario S, Gimenes M, Imperatriz-Fonseca VL. 2003. The influence of colony size in diel rhythms of flight activity of Melipona bicolor Lepeletier (Hymenoptera, Apidae, Meliponini). In Neotropical Apoidea: in honour of the 90th birthday of Jesus Santiago Moure (eds Melo GAR, Alves dos Santos I), pp. 191–197. Criciúma, Brazil: Editora UNESC. [Google Scholar]
- 68.Gottlieb D, Keasar T, Shmida A, Motro U. 2005. Possible foraging benefits of bimodal daily activity in Proxylocopa olivieri (Lepeletier) (Hymenoptera: Anthophoridae). Environ. Entomol. 34, 417–424. ( 10.1603/0046-225X-34.2.417) [DOI] [Google Scholar]
- 69.Armbruster WS, McCormick KD. 1990. Diel foraging patterns of male euglossine bees—ecological causes and evolutionary response by plants. Biotropica 22, 160–171. ( 10.2307/2388409) [DOI] [Google Scholar]
- 70.von Buttel-Reepen HB. 1900. Sind die Bienen Reflexmaschinen? Experimentelle Beitrage zur Biologie der Honigbiene. Biol. Zbl. 20, 177–193 (in German). [Google Scholar]
- 71.Forel VA. 1910. Das sinnesleben der insekten: eine sammlung von experimentellen und kritischen studien ueber insektenpsychologie, p. 393 Muenchen, Germany: Reinhardt; (in German). [Google Scholar]
- 72.Lindauer M. 1961. Communication among social bees. Cambridge, MA: Harvard University Press. [Google Scholar]
- 73.von Frisch K. 1967. The dance language and orientation of bees. Cambridge, MA: Harvard University Press. [Google Scholar]
- 74.Wahl O. 1932. Neue Untersuchungen über das Zeitgedächtnis der Bienen. J. Comp. Physiol. 16, 529–589 (in German). [Google Scholar]
- 75.Mulder CK, Gerkema MP, Van der Zee EA. 2013. Circadian clocks and memory: time-place learning. Front. Mol. Neurosci. 6, 8 ( 10.3389/fnmol.2013.00008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beling I. 1929. Über das Zeitgedächtnis der Bienen Zeitschrift für Vergleichende. Physiologie 9, 259–388 (in German). [Google Scholar]
- 77.Koltermann R. 1971. Circadian memory rhythm after scent and colour training with honey-bees. Z. Vergleichende Physiol. 75, 49–68. ( 10.1007/bf00335137) [DOI] [Google Scholar]
- 78.Renner M. 1959. Über ein weiteres Versetzungsexperiment zur Analyse des Zeitsinnes und der Sonnenorientierung der Honigbiene. J. Compar. Physiol. 42, 449–483 (in German). ( 10.1007/BF00297804) [DOI] [Google Scholar]
- 79.Pahl M, Zhu H, Pix W, Tautz J, Zhang S. 2007. Circadian timed episodic-like memory – a bee knows what to do when, and also where. J. Exp. Biol. 210, 3559–3567. ( 10.1242/jeb.005488) [DOI] [PubMed] [Google Scholar]
- 80.Zhang S, Schwarz S, Pahl M, Zhu H, Tautz J. 2006. Honeybee memory: a honeybee knows what to do and when. J. Exp. Biol. 209, 4420–4428. ( 10.1242/jeb.02522) [DOI] [PubMed] [Google Scholar]
- 81.Breed MD, Stocker EM, Baumgartner LK, Vargas SA. 2002. Time-place learning and the ecology of recruitment in a stingless bee, Trigona amalthea (Hymenoptera, Apidae). Apidologie 33, 251–258. ( 10.1051/apido:2002018) [DOI] [Google Scholar]
- 82.Murphy CM, Breed MD. 2008. Time-place learning in a neotropical stingless bee, Trigona fulviventris Guerin (Hymenoptera: Apidae). J. Kansas Entomol. Soc. 81, 73–76. ( 10.2317/jkes-704.23.1) [DOI] [Google Scholar]
- 83.de Jesus T, Venturieri GC, Contrera FAL. 2014. Time-place learning in the bee Melipona fasciculata (Apidae, Meliponini). Apidologie 45, 257–265. ( 10.1007/s13592-013-0245-2) [DOI] [Google Scholar]
- 84.Gimenes M, BeneditoSilva AA, Marques MD. 1996. Circadian rhythms of pollen and nectar collection by bees on the flowers of Ludwigia elegans (Onagraceae). Biol. Rhythm Res. 27, 281–290. ( 10.1076/Brhm.27.3.281.12971) [DOI] [Google Scholar]
- 85.von Frisch K, Lindauer M. 1956. The ‘language’ and orientation of the honey bee. Annu. Rev. Entomol. 1, 45–58. ( 10.1146/annurev.en.01.010156.000401) [DOI] [Google Scholar]
- 86.Green RF, Nunez AT. 1986. Central-place foraging in a patchy environment. J. Theor. Biol. 123, 35–43. ( 10.1016/S0022-5193(86)80233-8) [DOI] [Google Scholar]
- 87.Dyer FC. 1998. Spatial cognition: lessons from central-place foraging insects. In Animal cognition in nature. The convergence of psychology and biology in laboratory and field (eds Balda R, Pepperberg I, Kamil A), pp. 119–154. London, UK: Academic Press. [Google Scholar]
- 88.Dickinson JA. 1994. Bees link local landmarks with celestial compass cues. Naturwissenschaften 81, 465–467. ( 10.1007/BF01136652) [DOI] [Google Scholar]
- 89.Wehner R. 1981. Spatial vision in arthropods. In Vision in invertebrates, handbook of sensory physiology (ed. Autrum H.), pp. 287–616. Berlin, Germany: Springer. [Google Scholar]
- 90.Kerr WE. 1973. Sun compass orientation in the stingless bees Trigona (Trigona) spinipes (Fabricius, 1793) (Apidae). An. Acad. Bras. Cienc. 45, 301–308. [Google Scholar]
- 91.Foster JJ, Sharkey CR, Gaworska AVA, Roberts NW, Whitney HM, Partridge JC. 2014. Bumblebees learn polarization patterns. Curr. Biol. 24, 1415–1420. ( 10.1016/j.cub.2014.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pfeiffer K, Kinoshita M. 2012. Segregation of visual inputs from different regions of the compound eye in two parallel pathways through the anterior optic tubercle of the bumblebee (Bombus ignitus). J. Comp. Neurol. 520, 212–229. ( 10.1002/cne.22776) [DOI] [PubMed] [Google Scholar]
- 93.Clark MJ, Husband BC. 2007. Plasticity and timing of flower closure in response to pollination in Chamerion angustifolium (Onagraceae). Int. J. Plant Sci. 168, 619–625. ( 10.1086/513486) [DOI] [Google Scholar]
- 94.He YP, Duan YW, Liu JQ, Smith WK. 2005. Floral closure in response to temperature and pollination in Gentiana straminea Maxim. (Gentianaceae), an alpine perennial in the Qinghai-Tibetan Plateau. Plant Syst. Evol. 256, 17–33. ( 10.1007/s00606-005-0345-1) [DOI] [Google Scholar]
- 95.Fitting H. 1911. Untersuchungen uber die vorzeitige Entblatterund von Bluten. Jahrd. Wiss. Bot. 49, 187–263 (in German). [Google Scholar]
- 96.Biesmeijer JC, van Nieuwstadt MGL, Lukacs S, Sommeijer MJ. 1998. The role of internal and external information in foraging decisions of Melipona workers (Hymenoptera: Melipona). Behav. Ecol. Sociobiol. 42, 107–116. ( 10.1007/s002650050418) [DOI] [Google Scholar]
- 97.Hoballah ME, Stuurman J, Turlings TCJ, Guerin PM, Connetable S, Kuhlemeier C. 2005. The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta. Planta 222, 141–150. ( 10.1007/s00425-005-1506-8) [DOI] [PubMed] [Google Scholar]
- 98.Nagari M, Szyszka P, Galizia G, Bloch G. 2017. Task-related phasing of circadian rhythms in antennal responsiveness to general odorants and pheromones in honeybees. J. Biol. Rhyth. (in press). [DOI] [PubMed] [Google Scholar]
- 99.Abrams P. 1984. Variability in resource consumption rates and the coexistence of competing species. Theor. Popul. Biol. 25, 106–124. ( 10.1016/0040-5809(84)90008-x) [DOI] [Google Scholar]
- 100.Loreau M. 1989. Coexistence of temporally segregated competitors in a cyclic environment. Theor. Popul. Biol. 36, 181–201. ( 10.1016/0040-5809(89)90029-4) [DOI] [Google Scholar]
- 101.Armbruster WS, Herzig AL. 1984. Partitioning and sharing of pollinators by four sympatric species of Dalechampia (Euphorbiaceae) in Panama. Ann. MO Bot. Gar. 71, 1–16. ( 10.2307/2399053) [DOI] [Google Scholar]
- 102.Stone G, Willmer P, Nee S. 1996. Daily partitioning of pollinators in an African Acacia community. Proc. R. Soc. Lond. B 263, 1389–1393. ( 10.1098/rspb.1996.0203) [DOI] [Google Scholar]
- 103.Stone GN, Willmer P, Rowe JA. 1998. Partitioning of pollinators during flowering in an African Acacia community. Ecology 79, 2808–2827. ( 10.1890/0012-9658(1998)079%5B2808:POPDFI%5D2.0.CO;2) [DOI] [Google Scholar]
- 104.Raine NE, Pierson AS, Stone GN. 2007. Plant–pollinator interactions in a Mexican Acacia community. Arthropod Plant Interac. 1, 101–117. ( 10.1007/s11829-007-9010-7) [DOI] [Google Scholar]
- 105.Carvalho DM, Aguiar CM. L, Santos GMM. 2013. Food niche overlap among neotropical carpenter bees (Hymenoptera: Apidae: Xylocopini) in an agricultural system. Sociobiology 60, 283–288. ( 10.13102/sociobiology.v60i3.283-288) [DOI] [Google Scholar]
- 106.Santos GMDM, de Carvalho CAL, Aguiar CML, Macedo LSSR, Mello MAR. 2013. Overlap in trophic and temporal niches in the flower-visiting bee guild (Hymenoptera, Apoidea) of a tropical dry forest. Apidologie 44, 64–74. ( 10.1007/s13592-012-0155-8) [DOI] [Google Scholar]
- 107.Garibaldi LA, et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608–1611. ( 10.1126/science.1230200) [DOI] [PubMed] [Google Scholar]
- 108.Bluethgen N, Klein A-M. 2011. Functional complementarity and specialisation: the role of biodiversity in plant–pollinator interactions. Basic Appl. Ecol. 12, 282–291. ( 10.1016/j.baae.2010.11.001) [DOI] [Google Scholar]
- 109.Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I. 2008. Functional group diversity of bee pollinators increases crop yield. Proc. R. Soc. B 275, 2283–2291. ( 10.1098/rspb.2008.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lemaitre AB, Pinto CF, Niemeyer HM. 2014. Generalized pollination system: are floral traits adapted to different pollinators? Arthropod Plant Interact. 8, 261–272. ( 10.1007/s11829-014-9308-1) [DOI] [Google Scholar]
- 111.Stone GN, Gilbert F, Willmer P, Potts S, Semida F, Zalat S. 1999. Windows of opportunity and the temporal structuring of foraging activity in a desert solitary bee. Ecolog. Entomol. 24, 208–221. ( 10.1046/j.1365-2311.1999.00181.x) [DOI] [Google Scholar]
- 112.Stone GN. 1994. Activity patterns of females of the solitary bee Anthophora plumipes in relation to temperature, nectar supplies and body-size. Ecolog. Entomol. 19, 177–189. ( 10.1111/j.1365-2311.1994.tb00408.x) [DOI] [Google Scholar]
- 113.van der Sluijs JP, Simon-Delso N, Goulson D, Maxim L, Bonmatin JM, Belzunces LP. 2013. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 5, 293–305. ( 10.1016/j.cosust.2013.05.007) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.