Abstract
Seasonal change in daylength (photoperiod) is widely used by insects to regulate temporal patterns of development and behaviour, including the timing of diapause (dormancy) and migration. Flexibility of the photoperiodic response is critical for rapid shifts to new hosts, survival in the face of global climate change and to reproductive isolation. At the same time, the daily circadian clock is also essential for development, diapause and multiple behaviours, including correct flight orientation during long-distance migration. Although studied for decades, how these two critical biological timing mechanisms are integrated is poorly understood, in part because the core circadian clock genes are all transcription factors or regulators that are able to exert multiple effects throughout the genome. In this chapter, we discuss clocks in the wild from the perspective of diverse insect groups across eco-geographic contexts from the Antarctic to the tropical regions of Earth. Application of the expanding tool box of molecular techniques will lead us to distinguish universal from unique mechanisms underlying the evolution of circadian and photoperiodic timing, and their interaction across taxonomic and ecological contexts represented by insects.
This article is part of the themed issue ‘Wild clocks: integrating chronobiology and ecology to understand timekeeping in free-living animals’.
Keywords: insect photoperiodism, diapause, migration, clock genes, seasonal adaptations, climate change
1. Introduction
Seasonal environments with periods of adversity offer insects two options: escape to a more favourable site during the inimical season or remain in place and enter diapause, a dormant state characterized by arrested development, suppressed metabolism and enhanced stress responses [1–3]. The ‘escape’ strategy does not negate a diapause response. Most insects that migrate in advance of an unfavourable season enter diapause upon arrival at their new location; migration is a preparatory component of the diapause syndrome. But escaping in time or space is not a simple reaction to unfavourable environmental conditions. Insects normally enter diapause or begin migratory flights before the advent of the unfavourable season, implying that insects anticipate upcoming adverse conditions and take countermeasures well in advance. Daylength is the dominant environmental signal used by insects to predict onset of an unfavourable season, most commonly winter at temperate latitudes [1,4]. Daylength overshadows other potential environmental cues due to its reliability in predicting seasonal change through evolutionary time.
Though a few insects respond to the actual change in daylength, the more common scenario is for insects to enter diapause when a threshold daylength has been crossed [1]. To use such information to programme diapause implies the ability to distinguish a short day (usually diapause-inducing) from a long day (usually diapause-averting) during a photosensitive stage. Since diapause normally is not an immediate response, photoperiodic information must be stored in the brain and then acted upon at a later stage of development. How photoperiodic information is perceived, stored and acted upon during insect development remains largely unknown [4]. This is hampered in part because most model insects lack a robust diapause response, while those species with a strong diapause lack the full range of genetic and molecular tools required to comprehensively address the question [5]. Yet the recent sequencing and transcript profiling of many species with a diapause, combined with the power of new genetic knock-down and knock-out tools for non-model insects, bodes well for future progress in this exciting field.
Here we provide a brief overview of how insect clocks are engaged in photoperiodic responses in natural, ecological contexts. We also highlight recent advances in insect diapause and migration that provide new and future directions based on our understanding of clock mechanisms, molecular events that underpin the diapause phenotype, and critical adaptations of insect clocks to accommodate host-plant shifts and a rapidly warming climate.
2. Clocks and the diapause response
The circadian clock that drives daily rhythms in insect feeding, sleep–wake cycles, eclosion time, mating, oviposition and other physiological and behavioural activities requires precision that is well understood at the molecular level. One might assume that a mechanism already in place and capable of distinguishing the time of day would also be employed to provide the seasonal information demanded of a photoperiodic timer. A functional clock that employs clock genes such as period, timeless and others is indeed essential for the photoperiodic response in numerous insects [2,6]. Knock-down experiments that render the circadian clock inoperable and a wealth of classic experiments using the Nanda–Hamner protocol and Bünsow night-interruption experiments implicate circadian rhythmicity in the diapause response [4]. And even circannual rhythms, the rather unusual multi-year synchronized rhythms of development known for a few insects such as the varied carpet beetle Anthrenus verbasci and several species of ants, appear to engage circadian systems [7].
But a photoperiodic timer adds an additional feature of discernment that requires distinguishing short days from long days, and how this information is determined is still not clear. It is reasonable to assume that the circadian clock and photoperiodic timer are somehow linked, but the exact nature of their relationship remains to be demonstrated [8].
The downstream hormonal events controlling diapause are well known [9], but less is known about connections between the circadian clock or the photoperiodic timer and hormonal control mechanisms. One important link that helps explain how the diverse features of diapause are generated is the transcription factor FOXO, regulated by insulin signalling and juvenile hormone [10]. In the mosquito Culex pipiens, FOXO is linked to a network of downstream genes that regulate key features of diapause such as metabolic depression, stress tolerance, lifespan extension and cell cycle arrest [11], suggesting that activation of a single transcription factor can regulate many of the diverse features comprising the complex diapause phenotype. Direct links between clock genes and the insulin/FOXO pathway [12] offer promise for eventually connecting the photoperiodic clock to the developmental switch leading to diapause.
3. When photoperiodic clocks are not needed
(a). Obligate diapauses circumvent the need for photoperiodism
Insects that complete only one generation each year frequently enter diapause at a fixed developmental stage regardless of prevailing environmental cues [1]. Such a diapause is genetically programmed and is referred to as an obligate diapause. Obligate diapause does not require a mechanism to measure daylength for diapause induction, but environmental cues remain essential for regulating the timing of diapause termination and the onset of development. This mechanism for terminating diapause at the appropriate time dictates the active window of the insect's life. As we discuss later, selection on the timing of genetically determined obligate diapause facilitated adaptive radiation of Rhagoletis fruit flies, and their associated parasitoid wasps, thereby synchronizing their univoltine life cycles with the fruiting times of an array of host plants.
(b). Substituting thermoperiod for photoperiod
Low temperatures, especially during the dark, frequently enhance an insect's diapause response. In addition, experiments with the parasitoid wasp Nasonia vitripennis [4] and several species of moths [13] show that thermoperiod alone can generate a robust diapause response. This indicates that alternative pathways can provide input and even substitute for photoperiod as environmental cues regulating onset of diapause.
(c). Low and high latitude responses that lack photoperiodic input
Although insect clocks are amazingly precise, the slight changes in daylength within 5 degrees north or south of the equator are probably too small for insects to perceive [14]. This does not, however, mean that insects at low latitudes do not have the capacity for diapause. Diapause is widespread in tropical species, but photoperiod is supplanted as the dominant cue and other cues such as temperature, moisture and changes in food quality dictate the induction of diapause [14]. Thus, the physiological mechanisms for entering diapause are intact, but manifestation of the response is generated by conduits other than photoperiod.
At polar latitudes seasonal differences in photoperiod are extreme. Some species retain a photoperiodic response and clock genes cycle, resulting in diapause programming (e.g. Drosophila montana), albeit at critical photoperiods (more than 22 h light/day) considerably longer than for insects at lower latitudes [15]. At the other extreme, the midge Belgica antarctica has a short window of activity in Antarctica, and it remains fully active day and night as long as prevailing temperatures permit development. Although the midge has the full complement of circadian clock genes, the genes fail to show the cyclic pattern of expression seen in species from temperate latitudes [16]. Thus, diapause and other seasonal adaptations may or may not be separated from the photoperiodic timer in these extreme environments.
(d). Overriding the clock with maternal effects
Among some Diptera, Hymenoptera and Lepidoptera, photoperiodic information garnered by the mother can dictate the diapause fate of her progeny, and in a few cases the mother's diapause history alters her progeny's capacity for diapause [2,17]. For example, in the flesh fly Sarcophaga bullata, if the mother has overwintered in pupal diapause, her progeny are unable to enter pupal diapause even if they are exposed to strong diapause-inducing environmental conditions [18]. This mechanism is an adaptive response allowing early spring emergence of the adults without the risk of an untimely entrance into diapause by her progeny that would be exposed to short daylengths of spring and early summer. This result implies an uncoupling of the photoperiodic mechanism that programmes diapause from the downstream effector mechanism initiating diapause, a response that effectively overrides the photoperiodic timer and shuts off the capacity for diapause. Such maternal effects provide an unexploited opportunity to separate functions of the circadian clock, presumably fully active in flies whose photoperiodic initiation of diapause is overridden by maternal effects, from downstream mechanisms that may translate circadian clock function into the diapause programme. Another well-recognized example of a maternal effect in the aphid Megoura viciae reveals a maternal override of the photoperiodic response to determine the developmental fate (parthenogenetic or sexual forms) of the female's progeny [19].
4. Role of circadian clocks and clock genes in insect migration
A number of insects living in temperate climates, including locusts, butterflies, moths and dragonflies, use photoperiodic cues to trigger seasonal long-distance migration in anticipation of upcoming unfavourable changes in the environment [20], a response akin to that of many birds [21]. Yet how insects sense photoperiodic changes that induce the physiological and behavioural migratory programme is largely unknown. In particular, involvement of circadian clocks or clock genes in a photoperiodic timer triggering migration has yet to be explored.
(a). The monarch butterfly: an emerging model for examining clock control of migration
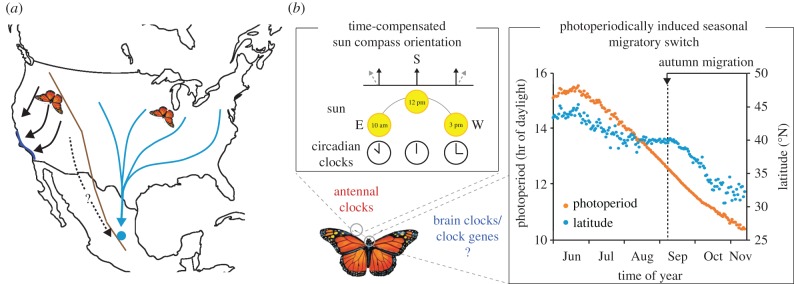
Among insect migratory species, few are currently suited for mechanistically dissecting links between biological clocks, clock genes and migration. The monarch butterfly, Danaus plexippus, has emerged as a tractable model to start addressing this question. Our current knowledge of monarch natural history indicates that its migratory behaviour and associated reproductive diapause are genetically or epigenetically encoded seasonally induced traits [22]. The eastern North American monarch migratory cycle involves several generations of migratory and non-migratory forms, all equipped with the same genome. Monarchs emerging in late summer are programmed for diapause and fly southward to make the autumn journey from Canada to overwintering grounds in Mexico (figure 1a). In the spring, coincident with rising temperatures and longer daylengths, migrants break diapause, mate and re-migrate northwards, with the females depositing eggs on milkweed plants across the southern United States. The northward progression of the monarch is staggered, requiring two to three subsequent generations to repopulate the northern range of the eastern monarch population. Progress has been made characterizing the monarch molecular circadian clock [23], a draft genome is available [24], and reverse genetic tools using engineered nucleases have yielded monarch clock gene knockouts that can be used to determine whether the circadian clock as a functional unit or individual clock genes function as part of the photoperiodic timer [25,26].
Figure 1.
Timing insect migration: from flight orientation to photoperiod-induced migratory switch. (a) Autumn migratory routes of western and eastern monarch butterflies on each side of the Rocky Mountains (brown lines), from their breeding to their overwintering sites in Mexico and California (light blue circle and dark blue line, respectively); (modified from Reppert et al. [22]). (b) Circadian clock control of monarch migration. Left, Circadian clocks in the antennae allow autumn migrants to time compensate their sun compass orientation to maintain a constant flight bearing to the south. The black arrows denote the southward orientation taken by migrants at any time of the day, and the grey dashed arrows denote the default direction the monarch would take in absence of time compensation. Right, Circadian clocks or clock genes in the brain may be part of a photoperiodic timer sensing a photoperiodic decrease that coincides with the onset of autumn southward movement. The blue dots depict the average latitude of eastern adult monarchs sighted at a given day between 1997 and 2013 (observations from Journey North, http://www.learner.org/jnorth/; sightings include the ‘First monarch butterfly’ and ‘Monarch first migration sighting’ for August–November; sightings from Florida were removed as they may be from a resident population). The orange dots depict the average photoperiod experienced by sighted adult monarchs at a given day.
(b). Circadian clock control of migratory flight orientation
Independent of their possible function in triggering migration, circadian clocks play a critical role in the navigational abilities of monarchs during both south migration and remigration north [22]. Migratory monarchs primarily use the sun as a compass cue for flight orientation but adjust their orientation relative to daily changes of the sun's azimuthal position to maintain a constant flight bearing. Flight orientation experiments on monarchs released in the wild or flown in a flight simulator have shown that circadian clocks provide the timing component necessary to compensate for the sun's movement in the sky over the course of the day to ultimately allow monarchs to maintain the proper migratory direction and maximize distance travelled (figure 1b) [27–29]. Circadian clock control of migratory flight orientation has not yet been reported in other insects, but given that other long-distance migrating insects like desert locusts use a sky compass for navigation [30], it would not be surprising if circadian clock-mediated time compensation is a conserved navigational strategy in insects.
Unexpectedly, clocks relevant to time compensation in monarchs reside in the butterfly's antennae, not the brain [31], therefore opening new avenues to investigate neural and/or humoral pathways relaying timing information to the sun compass integration centre in the brain. Unlike autumn migrants and spring remigrants, summer monarchs do not exhibit oriented flight [32]. Antennal clocks in summer butterflies are, however, probably functional since rhythms of core circadian clock genes are found in the antennae of wild-type monarchs reared under summer-like conditions in the laboratory [25]. This observation indicates seasonal plasticity in the clock-compass network that could be altered in response to changes in photoperiod. With the advent of genome editing tools, tagging clock genes with fluorescent proteins in vivo is now feasible, and mapping the clock circuitry in the monarch brain and antennae should reveal neural correlates of time-compensation and degrees of plasticity between seasons.
(c). Tracking the seasons: from timekeeping mechanisms to migratory output
The precisely timed departure of migratory monarchs year after year from their breeding grounds in the autumn and from their overwintering sites in the spring strongly suggests that photoperiodic changes drive the migratory switch leading to reproductive diapause, increased lifespan and the urge to fly directionally. Monarch sightings throughout the United States documented by citizen scientists for more than a decade indicate that migrants in the autumn start travelling south (i.e. towards lower latitudes) when the photoperiod they experience is below a threshold (approx. 12.5 h of daylight; figure 1b). Whether migratory monarchs respond to decreasing daylengths or a certain threshold needs to be formally tested. With the availability of an artificial diet to raise monarchs from eggs to adults [25], testing which photoperiodic conditions induce diapause and flight orientation behaviour can now be accomplished.
How do monarchs sense photoperiodic change? Despite the need for migratory monarch populations to track seasons for entering diapause and engaging in migratory behaviour, little is known about how the timing mechanisms work. Evidence from other insect species suggests that circadian clocks or clock genes in the brain play a pleiotropic role in the photoperiodic timer responsible for diapause induction [5,8]. In monarchs, the only indication to date that circadian clock genes might be involved in diapause induction comes from a recent study of whole-genome sequencing of migratory and non-migratory monarch populations in which timeless (tim) and cullin-3, whose gene product controls TIM oscillations in Drosophila, were found among the genomic regions strongly associated with migration [33]. The advent of CRISPR/Cas9-mediated targeted mutagenesis in the monarch [26] makes it possible to knock out critical players of both the positive and negative arms of the circadian transcription-translation feedback loop (e.g. clock, bmal1, period, timeless and cryptochrome2). Subjecting knockouts to short and long photoperiods in the laboratory should help determine whether the circadian clock or individual clock genes are necessary for the induction of diapause and oriented flight behaviour exhibited by migratory butterflies.
An equally important question is how photoperiod, once integrated by a timekeeping mechanism, is translated into a long- or short-day signal to the brain, and how that signal activates the downstream pathways responsible for the migratory switch both physiologically and behaviourally. The hormonal events downstream of the photoperiodic timer known to regulate diapause in other adult insects, i.e. decreases in insulin and juvenile hormone (JH) signalling [9], are probably conserved in monarchs. However, the molecular events leading to migratory oriented flight must diverge from those controlling diapause because topical application of a JH analogue on diapausing wild-caught migrants breaks diapause without disrupting the ability of monarchs to exhibit oriented flight [32]. The level at which these two pathways bifurcate may occur either downstream of a common master transcription factor (TF) or through pathways regulated by different TFs. Comparing genome-wide gene expression between wild-type and clock gene mutants raised in laboratory conditions under long and short days, as well as between wild-caught migrants and non-migrants using RNA-seq should not only identify these TFs but also reveal the set of downstream regulated genes underlying the photoperiodically induced migratory state. Performing these studies at a fine temporal resolution over the course of the 24-h day could also provide insights into the mode of photoperiod encoding in insects (i.e. photoperiod-dependent changes in the amplitude or peak duration of rhythmic signals).
5. Shifts in diapause timing facilitate reproductive isolation
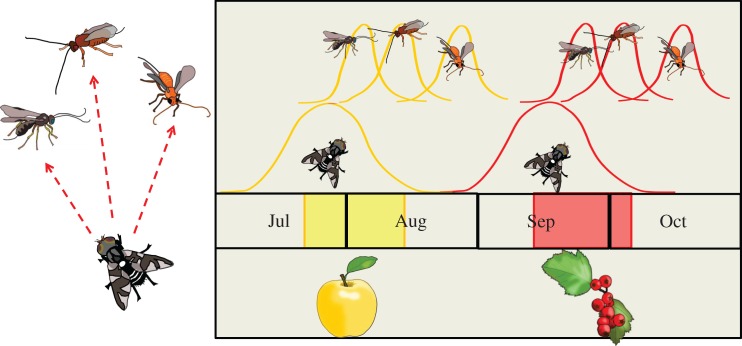
Apple maggot flies, Rhagoletis pomonella, typify how obligate overwintering diapause timing can facilitate ecological speciation [34]. Though circadian patterns are commonly used to coordinate activities of flowering plants and their insect pollinators (see [35]), the evolutionary events unfolding with the apple maggot reflect, not circadian events, but timing patterns that operate on a seasonal scale. In the mid-1800s, flies shifted from attacking fruits of the native downy hawthorn, Crataegus mollis, to domesticated apples (figure 2). Apple and hawthorn races of R. pomonella are differentiated at loci across the genome, despite gene flow (3–4%) [34,36]. Flies are univoltine and use timing of their obligate pupal diapause to synchronize short-lived adults with host fruit phenology. Because apples fruit earlier than hawthorns where both occur, colonizing apples selected for apple flies with a shortened pupal diapause and consequently an earlier spring emergence [34]. Genetic association, quantitative trait loci and common-garden studies rearing flies among host fruits show diapause timing is highly heritable, but with a diffuse polygenic architecture [34,36].
Figure 2.
Differences in seasonal phenology between the ancestral hawthorn host plant and introduced domestic apples has driven speciation by allochronic reproductive isolation in Rhagoletis fruit flies. Radiation of univoltine Rhagoletis flies onto apples has also created a new temporal niche that allowed sequential speciation of a community of univoltine parasitoid wasps that attack these flies. Arrows radiating from flies to wasps signify that changes in the timing of fly seasonality to match their host fruits have also driven concomitant shifts in the seasonal timing of their parasitoid wasps. In both flies and their wasp parasites, regulation of diapause timing serves to synchronize hosts and consumers across trophic levels, driving genetic divergence by allochronic isolation.
Other flies in the Rhagoletis complex are also univoltine and synchronized with their host plants by diapause timing [36]. In the midwestern United States where they co-occur, the blueberry maggot R. mendax emerges first, synchronized with blueberry fruit, followed by apple race R. pomonella, then hawthorn race R. pomonella, with flowering dogwood (Cornus florida) flies emerging last [37]. The recent shift in diapause timing that allowed colonization of apples is probably indicative of deeper patterns of adaptive radiation by diapause phenology that facilitates host-plant specialization across this group. Furthermore, each Rhagoletis fly is attacked by 1–3 parasitoid wasp species that are also univoltine and are using the timing of their own diapause to sequentially diversify along with their fly hosts (figure 2) [38]. Thus, diversification of diapause timing in flies has driven further diversification of diapause timing across trophic levels to include co-speciation by univoltine parasitoids feeding on those flies. While the roles of the circadian clock in these shifts in seasonal timing in either the flies or their parasitoid wasps are not currently known, circadian genes are differentially expressed at diapause termination in these flies [39], and there is polymorphism in clock genes, including period (Berlocher et al. 2017).
European corn borer moths, Ostrinia nubialis, exemplify allochronic isolation by facultative diapause timing [40]. Two genetically distinct European corn borer forms in the United States are undergoing ecological speciation, the E- and Z-pheromone strains. Three major traits differentiate these strains: (i) pheromone blend, (ii) early versus late mating within a night, and (iii) seasonal flight timing set by larval diapause duration. Despite the importance of pheromone blend and circadian partitioning of mating times, Dopman et al. [33] showed that seasonal flight timing, driven by larval diapause, makes the greatest contribution to reproductive isolation.
Larval diapause of the European corn borer is cued by photoperiod and temperature, and diapause duration predicts adult emergence time [41]. In New York, the E-strain is bivoltine with one early flight from larvae terminating diapause early in spring, followed by progeny of the first flight individuals in a second flight whose larvae enter diapause, overwinter and form the early flight the next year. But Z-strain larvae from New York terminate diapause approximately 1 month later than E-strain larvae, have only one flight, and their progeny enter larval diapause before the second E-strain flight. QTL studies associate diapause duration with a single locus on the sex chromosome (Z-chromosome, Lepidoptera males are homogametic ZZ and females heterogametic ZW) [40]. Compellingly, a similar Z-linked QTL is associated with voltinism and speciation by divergence in pupal diapause timing through effects on timing of adult flights in tiger swallowtail butterflies [42].
The circadian clock genes possibly contribute to divergence in diapause timing. The clock gene period occurs in the target region of the Z-chromosome, showing polymorphism associated with long (E-strain) versus short diapause (Z-strain) [43], and differences in levels of gene expression between the strains are also evident for several circadian transcripts, including period [44]. In the midwestern United States, voltinism exhibits a geographical cline: populations in northern Minnesota have 1 generation per year, southern Minnesota 1–2 generations, Iowa 2 generations and Missouri has 2–3 generations. This latitudinal cline in voltinism shows a pattern where E- versus Z-strain alleles of period alternate as predicted if selection is maximizing the number of successful generations in a growing season by matching diapause duration (short versus long) with local conditions. This alternating cline in period alleles may be due to linkage with the larval diapause-duration locus. Interestingly, clinal oscillations in E- versus Z-strain alleles of the autosomal gene cryptochrome 1 are correlated with those for period [43].
6. Photoperiodic adaptation as an evolutionary response to climate change
Altering the timing of diapause onset and termination is not only critical for switching to new hosts but also for enabling insects to respond to climate change. Entering diapause or breaking out of an overwintering diapause too early or too late exacts an obvious cost [45,46]. In a warming environment photoperiod remains unchanged but temperatures are elevated, resulting in a longer growing season and possible asynchrony between the insect and its host plants. In this section we summarize the light conditions that define daylength in nature and discuss how this information may be integrated by the insect to adapt to climate change.
A day is assumed to start and end some time during morning and evening twilights, respectively. It is during twilight, especially from an angle of the sun between 90° (mid-civil twilight) and 102° (end of nautical twilight) from directly overhead (zenith), that incident light is declining exponentially and exhibits the least variation in intensity due to wavelength, due to direction towards, away from, or perpendicular to the sun, and due to day-to-day variation in atmospheric conditions [47]. Hence, light intensity at some point during twilight will provide the most reliable cue for when a day begins and when a day ends, defining daylength for an organism.
But it is difficult to know the light environment of a mobile insect during the period of their life cycle when they are sensitive to daylength and making the go/no-go switch between continued development and diapause. Resolving this problem is possible with an insect that is captive in a fixed location if one can define both the wavelength and the intensity to which the insect is sensitive and can determine the wavelength and intensity of light during twilight in their fixed location. These conditions are met with the mosquito Wyeomyia smithii, which throughout its range from Florida to Canada (30–50°N) completes its entire pre-adult development within the water-filled leaves of the purple pitcher plant, Sarracenia purpurea. The onset, maintenance and termination of diapause in W. smithii are determined while larvae are captive within their stationary host leaf. In W. smithii, photoperiodic action spectra varying in both wavelength and intensity reveal the same peak sensitivity at both dawn and dusk in the blue region of the spectrum, with a shoulder of lesser sensitivity in the green, and exponentially declining sensitivity at longer wavelengths (yellow, orange and red). Like other plants, pitcher-plant leaves absorb blue light used in photosynthesis and transmit green light so that twilight inside a pitcher-plant leaf is richer in green and sparser in blue light [48]. The net result inside a leaf is that blue and green light are equally effective in stimulating the photoperiodic response. By overlaying the photoperiodic action spectra with the spectra of light available inside a pitcher plant leaf during actual twilight, the critical daylength defining 50% development and 50% diapause begins at a solar angle of 96° from vertical and also ends at a solar angle of 96°, i.e. at the onset of civil twilight in the dawn and at the end of civil twilight in the dusk [48].
(a). Predicting seasonality from critical photoperiod
Does knowing the astronomic units that define a day enable prediction of seasonal development in nature from critical photoperiods determined under controlled conditions? Like most insects, critical photoperiod in W. smithii increases with latitude and altitude of origin [49]. After combining the spectral properties of photoperiodic response and light-availability spectra in pitcher-plant leaves, critical photoperiods determined under controlled conditions [49] accurately predict the actual timing of diapause in nature over a wide geographical scale [50].
(b). Responding to rapid climate change
Is photoperiodic response adaptive in a temperate seasonal environment? Given the close correlation between geography and photoperiodic response, the answer to this question may appear obvious. However, to be adaptive in the evolutionary sense, one must show that the trait under consideration first is genetically based, second responds to directional selection and third achieves greater fitness in the selected environment than in the ancestral environment. First and second, critical photoperiod is genetically based and responds to selection under controlled conditions and in the wild [45,50]. When confronted with recent rapid climate change, photoperiodic response in W. smithii exhibited a dramatic genetic shift towards a more southern genotype (shorter critical photoperiods) that can be observed over as short a period as 5 years [45]. This pattern is repeated in genetically based shifts in seasonal timing in other insects, plants, birds and mammals [45,50,51]. In none of these organisms was there shown to be a genetic change in thermal optimum or thermal tolerance over the same period of time. These results indicate that the length of the growing season is the selective force driving these genetic shifts and indicate that the immediate target of selection during recent rapid climate change has been on photoperiodic response.
Third, photoperiodic response can be shown to be adaptive by transplanting northern populations of W. smithii to southern climates under controlled conditions. The power of using W. smithii is that it can be reared within intact leaves of pitcher plants and fed insect prey on a schedule replicating the actual age-dependent prey capture of leaves in nature. Experiments can be run in computer-controlled environment rooms where daily and annual cycles of light, temperature, and humidity can be varied independently, and accurately replicate any environment from the equatorial to the polar regions of the Earth. Experiments can either duplicate or completely contrast precise seasonal patterns observed in the field, thereby revealing the relative importance of thermal versus photoperiodic adaptation. The resulting data show that when northern W. smithii are reared in a programmed, year-long northern climate, they achieve the expected fitness of a northern population. However, when northern populations are exposed to a southern thermal year but maintain their northern photic year, fitness (R = year-long, per capita population growth rate integrated over all four seasons) increases by 47% above the northern baseline; i.e. transplanted northern populations actually achieve higher fitness when they experience a warmer southern climate. By contrast, when these same northern populations are exposed to both a southern thermal year and a southern photic year, fitness declines by 88% [50]. Fitness of northern populations improves in a warmer climate, but only if the organism's genetically programmed response to daylength is appropriate for that warmer climate. Incorrect photic cues are lethal and can quickly lead to extinction of populations within several generations. Climate change from the point of view of actual organisms in the temperate regions of the Earth results in milder winters, earlier springs, later autumns, warmer, more favourable summer temperatures and longer growing seasons. As a consequence, the most immediate evolutionary (genetic) response of insects, as well as plants, birds and mammals to recent rapid climate change has involved altered seasonal timing, principally through genetic shifts in the photoperiodic response [45,50,51].
(c). Evolution of photoperiodic response independent of circadian clock
From the above, photoperiodism, a physiological adaptation that relies on day- or nightlength as cued by dark–light and light–dark transitions during twilight, enables plants and animals to organize their seasonal patterns of activities, growth, development, reproduction, dormancy and migration. These same light transitions enable the circadian clocks of plants and animals to organize daily patterns of behaviour, physiology and biochemistry. Hence, light transitions are important factors in the input of the environment to both the seasonal photoperiodic timer and the daily circadian clock. The question remains as to the mechanistic relationship between them. Elucidating the exact physiological role of circadian clock genes is complicated by the fact that the core circadian clock genes are transcription factors or transcription regulators and can exert pleiotropic effects, making it challenging to distinguish clock from non-clock functions of these canonical clock genes [8].
One way of disentangling this confound is by comparing patterns across latitudes with those across elevation. At any specific latitude on the same day of the year, regardless of altitude, daylength is the same today as it was 10 000 years ago and will be 10 000 years into the future. With increasing latitude, length of the growing season declines while length of day during the summer growing season increases [52]. Consequently, it could be that correlations between photoperiodism and circadian rhythmicity over a latitudinal gradient might be due to parallel adaptation of the circadian clock to varying summer daylength and the photoperiodic timer to length of the growing season, and not necessarily reflect a causal relationship between these physiological mechanisms [53,54]. By contrast, at any given latitude, summer daylength remains constant regardless of altitude, but the length of the growing season declines as one ascends in altitude. Hence, comparisons between formal properties of the circadian clock and the photoperiodic timer over altitudinal gradients (varying seasonality) at the same latitude (holding summer daylength constant) can be used to direct future research into mechanistic connections between the daily circadian clock and the seasonal photoperiodic timer. In W. smithii, critical photoperiod increases with latitude and altitude [49]. When both altitude as well as latitude are taken into account, experiments imposing variable nightlengths following a long or a short fixed daylength (Nanda–Hamner) or experiments interrupting an otherwise long night with 1 h light pulses (Bünsow) fail to show covariation of either the photoperiodic timer or counter with period, amplitude, or phase of a purported causal circadian clock [53]. These results led to the conclusion that in W. smithii, photoperiodic response and circadian rhythmicity have evolved independently of one another. This conclusion is supported by the fact that antagonistic selection reverses the genetic correlation between circadian and photoperiodic properties within populations in both Drosophila littoralis [55] and W. smithii [54].
Independent evolution of timing mechanisms over geographical gradients in nature does not, in itself, preclude circadian rhythmicity playing a role in photoperiodic time measurement within a population. However, as shown by antagonistic selection [56], any putative interaction that links the circadian clock and the photoperiodic timer must be heritable and able to respond to selection. It is clear that even though properties of overt circadian rhythms can change over latitudinal gradients [52], photoperiodic response exhibits much more dramatic covariation with latitude and altitude, as well as adaptive response to rapid climate change. The conundrum is genetically how to effect rapid adaptive response of the seasonal photoperiodic timer to range expansion [51] or climate change [45,50] without disrupting daily circadian organization.
One possible solution to this problem would be if the two physiological mechanisms were independent and connected through one to a few pleiotropic genes in the circadian clock that the photoperiodic timer co-opts as a time-reference point [53,57,58] (figure 3). In this case, the circadian clock would maintain daily temporal coordination of the organism while the photoperiodic timer would maintain a phase relationship, determined by local seasonal selection, with a single or a few circadian clock proteins. An altered seasonal environment can then exert selection on the photoperiodic timer, thereby altering the phase relationship of the photoperiodic timer entirely without affecting the circadian clock. One or a few circadian clock genes would then function principally in circadian organization and pleiotropically as a phase reference point for the photoperiodic timer, completely independently of and incidentally to their role in the circadian clock. This model would explain the ability of the photoperiodic timer to respond independently to seasonal selection without disrupting circadian organization in an invariant 24 h daily environment. Both the composition and evolutionary rates of core circadian clock genes vary within and between orders of insects [58]. There is then an even broader potential for different and independent evolutionary connections between the daily circadian clock and the seasonal photoperiodic timer through the co-option of different circadian clock genes by different taxa.
Figure 3.
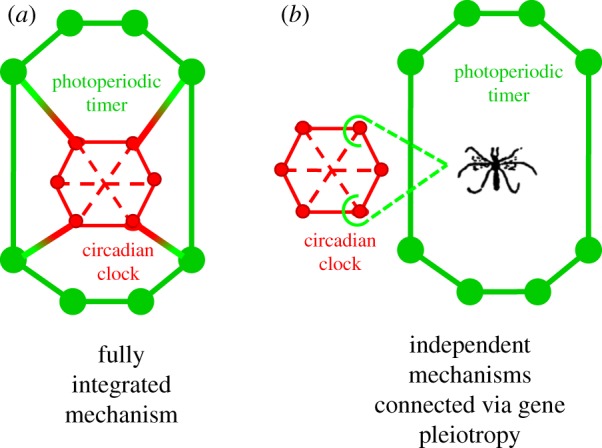
Alternative models for the relationship between the daily circadian clock and the seasonal photoperiodic timer. (a) Fully integrated mechanism in which the circadian clock (red) is viewed as an integrated unit that also provides the mechanistic basis of the photoperiodic timer (green). (b) Independent mechanisms in which the circadian clock and the photoperiodic timer are distinct physiological mechanisms.
The above discussion defines daylength in nature from a biological perspective and demonstrates experimentally that response to daylength (photoperiodism) is an adaptive physiological process as implied by its geographical variation. Critical photoperiod is a heritable trait, responds to selection, and exhibits a genetically based response to climate change in its native or introduced habitat. Finally, photoperiodic response undergoes more rapid evolution than either thermal or morphological traits when organisms are confronted with novel climates in time or space and is able to evolve entirely independently of the circadian clock.
7. Concluding remarks
In spite of substantial advances in insect photoperiodism, we still lack a detailed pathway leading from photoreception to expression of the diapause and/or migration phenotype. How are short days distinguished from long days, and how is this critical information stored in the brain to be acted upon at a later stage or even in the following generation? Epigenetic mechanisms are likely to emerge as important regulators of the diapause response, but we remain in an early phase of understanding how such mechanisms contribute to the decision to enter, maintain and terminate diapause. Though a functional circadian clock appears essential for the diapause response, it is not at all clear how the circadian clock and the photoperiodic timer are integrated. This integration is further complicated by the fact that the canonical core circadian clock genes are all transcription factors or transcription regulators that can exert clock-independent pleiotropic effects throughout the genome. The circadian clock has also been implicated in migratory flight orientation and time compensation, but how this clock function is linked to the diapause response and how the wiring of the clock-compass network changes throughout the season remains unclear. Likewise, links between thermoperiodism and photoperiodism remain poorly understood. Temperature and light cycles appear to offer complementary and/or alternative environmental inputs for the programming of diapause in some insects, but how these two distinct pathways merge is not known.
Equally as important as deciphering the mechanisms of photoperiodic responses is being able to understand how such responses can evolve over time, especially in response to climate change and the availability of new hosts. What are the actual genetic targets of selection that alter the photoperiodic response, thereby enabling insects to exploit longer growing seasons in a warmer Earth or to synchronize their life cycles in concordance with new or altered timing of host resources? Artificial laboratory-based experiments can benefit from more focus on biological timing in actual or carefully simulated natural environments under controlled conditions. Although most insects enter diapause at some point in their life cycle, insects that currently offer the best models for genetic research lack a robust diapause or photoperiodic response. Further development of genetic tools for non-model species, including both loss and gain-of-function mutations, are urgently needed to advance the exciting field of insect photoperiodism. Wild clocks offer both a curse and a blessing. While wild clocks lack the genetic tools readily available in model systems, the natural world offers an incredibly rich diversity of biological clocks that can be probed for understanding the timing of seasonal activity.
Acknowledgements
We thank Journey North for access to monarch sightings data, Samantha Iiams for producing figure 1, Tom Powell for producing figure 2 and members of our laboratories for thoughtful comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed equally to the writing, and all authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
D.L.D. was supported by grants from USDA-AFRI (2015-67013-23416) and NSF (IOS-1354377), D.A.H. from NSF (IOS-1257298), the Florida Ag Experiment Station, and the FAO/IAEA CRP Dormancy Management to Enable Mass-rearing, C.M. from NSF (IOS-1456985), and W.E.B. and C.M.H. from NSF (DEB-0917827, IOS-1255628, OPUS-1455506).
References
- 1.Tauber MJ, Tauber CA, Masaki S. 1986. Seasonal adaptations in insects. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Denlinger DL. 2002. Regulation of diapause. Annu. Rev. Entomol. 47, 93–122. ( 10.1146/annurev.ento.47.091201.145137) [DOI] [PubMed] [Google Scholar]
- 3.Hahn DA, Denlinger DL. 2011. Energetics of insect diapause. Annu. Rev. Entomol. 56, 103–121. ( 10.1146/annurev-ento-112408-085436) [DOI] [PubMed] [Google Scholar]
- 4.Saunders DS. 2004. Insect clocks, 3rd edn Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 5.Meuti ME, Denlinger DL. 2013. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr. Comp. Biol. 53, 131–143. ( 10.1093/icb/ict023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Numata H, Miyazaki Y, Ikeno T. 2015. Common features in diverse insect clocks. Zoological Lett. 1, 10 ( 10.1186/s40851-014-0003-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazaki Y, Nisimura T, Numata H. 2014. Circannual rhythms in insects. In Annual, lunar, and tidal clocks (eds Numata H, Helm B) pp. 333–352. Tokyo, Japan: Springer. [Google Scholar]
- 8.Emerson KJ, Bradshaw WE, Holzapfel CM. 2009. Complications of complexity: integrating environmental, genetic and hormonal control of diapause. Trends Genet. 25, 217–225. ( 10.1016/j.tig.2009.03.009) [DOI] [PubMed] [Google Scholar]
- 9.Denlinger DL, Yocum GD, Rinehart JP. 2012. Hormonal control of diapause. In Insect endocrinology (ed. Gilbert LI.), pp. 430–463. San Diego, CA: Academic Press. [Google Scholar]
- 10.Sim C, Denlinger DL. 2008. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens . Proc. Natl Acad. Sci USA 105, 6777–6781. ( 10.1073/pnas.0802067105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sim C, Kang DS, Kim S, Bai X, Denlinger DL. 2015. Identification of FOXO targets that generate the diverse features of the diapause phenotype in the mosquito Culex pipiens . Proc. Natl Acad. Sci. USA 112, 3811–3816. ( 10.1073/pnas.1502751112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng X, Sehgal A. 2010. AKT and TOR signaling set the pace of the circadian pacemaker. Curr. Biol. 20, 1203–1208. ( 10.1016/j.cub.2010.05.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck SD. 1983. Insect thermoperiodism. Annu. Rev. Entomol. 28, 91–108. ( 10.1146/annurev.en.28.010183.000515) [DOI] [Google Scholar]
- 14.Denlinger DL. 1986. Dormancy in tropical insects. Annu. Rev. Entomol. 31, 239–264. ( 10.1146/annurev.en.31.010186.001323) [DOI] [PubMed] [Google Scholar]
- 15.Kauranen H, Ala-Honkola O, Kankare M, Hoikkala A. 2016. Circadian clock of Drosophila montana is adapted to high variation in summer day lengths and temperatures prevailing at high latitudes. J. Insect Physiol. 89, 9–18. ( 10.1016/j.jinsphys.2016.03.005) [DOI] [PubMed] [Google Scholar]
- 16.Kobelkova A, Goto SG, Peyton JT, Ikeno T, Lee RE Jr, Denlinger DL. 2015. Continuous activity and no cycling of clock genes in the Antarctic midge during the polar summer. J. Insect Physiol. 81, 90–96. ( 10.1016/j.jinsphys.2015.07.008) [DOI] [PubMed] [Google Scholar]
- 17.Mousseau TA, Dingle H. 1991. Maternal effects in insect life histories. Annu. Rev. Entomol. 36, 511–534. ( 10.1146/annurev.en.36.010191.002455) [DOI] [Google Scholar]
- 18.Henrich VC, Denlinger DL. 1982. A maternal effect that eliminates pupal diapause in progeny of the flesh fly, Sarcophaga bullata. J. Insect Physiol. 28, 881–884. ( 10.1016/0022-1910(82)90102-0) [DOI] [Google Scholar]
- 19.Lees AD. 1960. The role of photoperiod and temperature in the determination of parthenogenetic and sexual forms in the aphid Megoura viciae Bukton—II. The operation of the ‘interval timer’ in young clones. J. Insect Physiol. 4, 154–175. ( 10.1016/0022-1910(60)90078-0) [DOI] [Google Scholar]
- 20.Chapman JW, Reynolds DR, Wilson K. 2015. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 18, 287–302. ( 10.1111/ele.12407) [DOI] [PubMed] [Google Scholar]
- 21.Åkesson S, Ilieva M, Karagicheva J, Rakhimberdiev E, Tomotani B, Helm B. 2017. Timing avian long-distance migration: from internal clock mechanisms to global flights. Phil. Trans. R. Soc. B 372, 20160252 ( 10.1098/rstb.2016.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reppert SM, Guerra PA, Merlin C. 2016. Neurobiology of monarch butterfly migration. Annu. Rev. Entomol. 61, 25–42. ( 10.1146/annurev-ento-010814-020855) [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, Emery P, Reppert SM. 2008. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 6, e4 ( 10.1371/journal.pbio.0060004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan S, Merlin C, Boore JL, Reppert SM. 2011. The monarch butterfly genome yields insights into long-distance migration. Cell 147, 1171–1185. ( 10.1016/j.cell.2011.09.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merlin C, Beaver LE, Taylor OR, Wolfe SA, Reppert SM. 2013. Efficient targeted mutagenesis in the monarch butterfly using zinc-finger nucleases. Genome Res. 23, 159–168. ( 10.1101/gr.145599.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markert MJ, Zhang Y, Enuameh MS, Reppert SM, Wolfe SA, Merlin C. 2016. Genomic access to monarch migration using TALEN and CRISPR/Cas9-mediated targeted mutagenesis. G3 6, 905–915. ( 10.1534/g3.116.027029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez SM, Taylor OR, Jander R. 1997. A sun compass in monarch butterflies. Nature 387, 29 ( 10.1038/387029a0) [DOI] [Google Scholar]
- 28.Mouritsen H, Frost BJ. 2002. Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc. Natl. Acad. Sci. USA 99, 10 162–10 166. ( 10.1073/pnas.152137299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froy O, Gotter AL, Casselman AL, Reppert SM. 2003. Illuminating the circadian clock in monarch butterfly migration. Science 300, 1303–1305. ( 10.1126/science.1084874) [DOI] [PubMed] [Google Scholar]
- 30.Homberg U. 2015. Sky compass orientation in desert locusts—evidence from field and laboratory studies. Front. Behav. Neurosci. 9, 346 ( 10.3389/fnbeh.2015.00346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merlin C, Gegear RJ, Reppert SM. 2009. Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325, 1700–1704. ( 10.1126/science.1176221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu H, Gegear RJ, Casselman A, Kanginakudru S, Reppert SM. 2009. Defining behavioral and molecular differences between summer and migratory monarch butterflies. BMC Biol. 7, 14 ( 10.1186/1741-7007-7-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhan S, et al. 2014. The genetics of monarch butterfly migration and warning colouration. Nature 514, 317–321. ( 10.1038/nature13812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feder JL, Xie XF, Rull J, Velez S, Forbes A, Leung B, Dambroski H, Filchak KE, Aluia M. 2005. Mayr, Dobzhansky, and Bush and the complexities of sympatric speciation in Rhagoletis. Proc. Natl Acad. Sci. USA 102, 6573–6580. ( 10.1073/pnas.0502099102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulla M, Oudman T, Bijleveld AI, Piersma T, Kyriacou CP. 2017. Marine biorhythms: bridging chronobiology and ecology. Phil. Trans. R. Soc. B 372, 20160253 ( 10.1098/rstb.2016.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egan SP, Ragland GJ, Assour L, Powell THQ, Hood GR, Emerich S, Nosil P, Feder JL. 2015. Experimental evidence of genome-wide impact of ecological selection during early stages of speciation-with-gene-flow. Ecol. Lett. 18, 817–825. ( 10.1111/ele.12460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell THQ, Hood GR, Murphy MO, Heilveil JS, Berlocher SH, Nosil P, Feder JL. 2013. Genetic divergence along the speciation continuum: the transition from host race to species in Rhagoletis (Diptera: Tephritidae). Evolution 67, 2561–2576. ( 10.1111/evo.12209) [DOI] [PubMed] [Google Scholar]
- 38.Hood GR, Forbes AA, Powell THQ, Egan SP, Hamerlinck G, Smith JJ, Feder JL. 2015. Sequential divergence and the multiplicative origin of community diversity. Proc. Natl Acad. Sci. USA 112, E5980–E5989. ( 10.1073/pnas.1424717112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ragland GJ, Egan SP, Feder JL, Berlocher SH, Hahn DA. 2011. Developmental trajectories of gene expression reveal candidates for diapause termination: a key life-history transition in the apple maggot fly Rhagoletis pomonella . J. Exp. Biol. 214, 3948–3959. ( 10.1242/jeb.061085) [DOI] [PubMed] [Google Scholar]
- 40.Dopman E.B, Robbins PS, Seaman A. 2010. Components of reproductive isolation between North American pheromone strains of the European corn borer. Evolution 64, 881–902. ( 10.1111/j.1558-5646.2009.00883.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadsworth CB, Woods WA, Hahn DA, Dopman EB. 2013. One phase of the dormancy developmental pathway is critical for the evolution of insect seasonality. J. Evol. Biol. 26, 2359–2368. ( 10.1111/jeb.12227) [DOI] [PubMed] [Google Scholar]
- 42.Kunte K, Shea C, Aardema ML, Scriber JM, Juenger TE, Gilbert LE, Kronforst MR. 2011. Sex chromosome mosaicism and hybrid speciation among tiger swallowtail butterflies. PLoS Genet. 7, e1002274 ( 10.1371/journal.pgen.1002274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy RC, Kozak GM, Wadsworth CB, Coates BS, Dopman EB. 2015. Explaining the sawtooth: latitudinal periodicity in a circadian gene correlates with shifts in generation number. J. Evol. Biol. 28, 40–53. ( 10.1111/jeb.12562) [DOI] [PubMed] [Google Scholar]
- 44.Wadsworth CB, Dopman EB. 2015. Transcriptome profiling reveals mechanisms for the evolution of insect seasonality. J. Exp. Biol. 218, 3611–3622. ( 10.1242/jeb.126136) [DOI] [PubMed] [Google Scholar]
- 45.Bradshaw WE, Holzapfel CM. 2006. Evolutionary response to rapid climate change. Science 312, 1477–1478. ( 10.1126/science.1127000) [DOI] [PubMed] [Google Scholar]
- 46.van Asch M, Visser ME. 2007. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu. Rev. Entomol. 52, 37–55. ( 10.1146/annurev.ento.52.110405.091418) [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg GV. 1966. Twilight. A study in atmospheric optics. New York, NY: Plenum Press. [Google Scholar]
- 48.Bradshaw WE, Phillips DL. 1980. Photoperiodism and the photic environment of the pitcher-plant mosquito, Wyeomyia smithii. Oecologia 44, 311–316. ( 10.1007/BF00545233) [DOI] [PubMed] [Google Scholar]
- 49.Bradshaw WE, Quebodeaux MC, Holzapfel CM. 2003. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive response to the photic environment or correlated response to the seasonal environment? Am. Nat. 161, 735–748. ( 10.1086/374344) [DOI] [PubMed] [Google Scholar]
- 50.Bradshaw WE, Holzapfel CM. 2010. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 72, 147–166. ( 10.1146/annurev-physiol-021909-135837) [DOI] [PubMed] [Google Scholar]
- 51.Urbanski J, Mogi M, O'Donnell D, DeCotiis M, Toma T, Armbruster P. 2012. Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 179, 490–500. ( 10.1086/664709) [DOI] [PubMed] [Google Scholar]
- 52.Hut RA, Paolucci S, Dor R, Kyriacou CP, Daan S. 2013. Latitudinal clines: an evolutionary view on biological rhythms. Proc. R. Soc. B 280, 20130433 ( 10.1098/rspb.2013.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradshaw WE, Holzapfel CM. 2010. What season is it anyway? Circadian tracking vs. photoperiodic anticipation in insects. J. Biol. Rhythms 25, 155–165. ( 10.1177/0748730410365656) [DOI] [PubMed] [Google Scholar]
- 54.O'Brien C, Bradshaw WE, Holzapfel CM. 2011. Testing for causality in covarying traits: genes and latitude in a molecular world. Mol. Ecol. 20, 2471–2476. ( 10.1111/j.1365-294X.2011.05133.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lankinen P, Forsman P. 2006. Independence of genetic geographical variation between photoperiodic diapause, circadian eclosion rhythm, and the Thr-Gly repeat region of the period gene in Drosophila littoralis . J. Biol. Rhythms 21, 3–12. ( 10.1177/0748730405283418) [DOI] [PubMed] [Google Scholar]
- 56.Bradshaw WE, Emerson KJ, Holzapfel CM. 2012. Genetic correlations and the evolution of photoperiodic time measurement within a local population of the pitcher-plant mosquito, Wyeomyia smithii. Heredity 108, 473–479. ( 10.1038/hdy.2011.108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradshaw WE, Holzapfel CM. 2010. Circadian clock genes, ovarian development and diapause. BMC Biol. 8, 115 ( 10.1186/1741-7007-8-115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tormey D, Colbourne JK, Mockaitis K, Choi J-H, Lopez J, Burkhart J, Bradshaw W, Holzapfel C. 2015. Evolutionary divergence of core and post-translational circadian clock genes in pitcher-plant mosquito, Wyeomyia smithii . BMC Genomics 16, 754 ( 10.1186/s12864-015-1937-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.