Abstract
An emerging hypothesis of animal personality posits that animals choose the habitat that best fits their personality, and that the match between habitat and personality can facilitate population differentiation, and eventually speciation. However, behavioural plasticity and the adjustment of behaviours to new environments have been a classical explanation for such matching patterns. Using a population of dunnocks (Prunella modularis), we empirically tested whether personality or behavioural plasticity is responsible for the non-random distribution of shy and bold individuals in a heterogeneous environment. We found evidence for bold individuals settling in areas with high human disturbance, but also that birds became bolder with increasing age. Importantly, personality primarily determines the distribution of individuals, and behavioural adjustment over time contributes very little to the observed patterns. We cannot, however, exclude a possibility of very early behavioural plasticity (a type of developmental plasticity) shaping what we refer to as ‘personality’. Nonetheless, our findings highlight the role personality plays in shaping population structure, lending support to the theory of personality-mediated speciation. Moreover, personality-matching habitat choice has important implications for population management and conservation.
Keywords: animal personality, dispersal, genotype-environment covariance, habitat selection, human disturbance, repeatability
1. Introduction
Animals have evolved striking phenotypic adaptations to their environments via natural selection [1–3]. Within species, evolved phenotypic adaptations usually refer to morphological or physiological traits as opposed to behavioural traits (e.g. [4,5]). This is because animals are traditionally assumed to be flexible and to adjust their behaviours according to their environments [6]. However, recent theoretical and empirical work on animal personality challenges this view [7].
Animal personality is defined as behavioural differences among individuals that are consistent over time and across situations (e.g. [6,8,9]), and has been found in a broad variety of traits and species [10,11]. Importantly, behavioural consistency is accompanied by limited behavioural plasticity [12,13]. Consequently, personality traits may restrict an individual's ability to cope with its environment (e.g. predator abundance) and thus may lead to a non-random distribution of behavioural phenotypes across different environments [14], generating a phenotype–environment covariance.
Phenotype–environment covariance can be explained by the recently proposed personality-matching habitat choice hypothesis (hereafter ‘personality-matching hypothesis’; cf. [15]). The personality-matching hypothesis posits that individuals settle in a habitat that best suits their own personality [16,17] to reduce stress and to avoid costly adjustment of behaviours [6]. While phenotype–environment covariances have been observed (e.g. [15,18,19]), to the best of our knowledge, the personality-matching hypothesis has not been explicitly tested and empirically teased apart from other alternative mechanisms.
Behavioural plasticity is one alternative mechanism that has been used to explain spatial sorting of phenotypes in heterogeneous environments [20,21]. Behavioural plasticity implies that the expression of behavioural traits is flexible and that individuals adjust their behaviour according to their environment (e.g. [22]). Given that environmental conditions are stable over time (e.g. within generations), behavioural adjustment to such stable environments (e.g. habituation, [23,24]) can also generate covariance between behavioural types and environments [15] (hereafter referred to as ‘behavioural plasticity hypothesis’).
In this study, we examine whether an individual's personality (consistent between-individual differences) or behavioural plasticity within individuals is the main factor that leads to the spatial distribution of behavioural phenotypes within a wild population of dunnocks (Prunella modularis). Specifically, we test whether an individual's risk-taking behaviour (i.e. boldness) covaries with the level of human disturbance in an individual's habitat, and also examine whether individuals alter their risk-taking behaviour relative to disturbance level over time. For this purpose, we measured flight-initiation distance (FID) as a proxy for shyness–boldness of individual dunnocks over a period of 3 years. Moreover, we quantified the level of human disturbance in each individual's territory by counting the number of pedestrians that visited the study area. In urban habitats, humans represent a frequent stressor, which can cause time-allocation trade-offs between, for instance, an individual's foraging and antipredator behaviours (e.g. escape [25]). Furthermore, a human's physical presence may trigger physiological responses, such as increased production of stress hormones [26,27], which in turn can lead to low reproductive success [28].
Under both the personality-matching and the behavioural-plasticity hypotheses, we expect that individuals with the highest tolerance (i.e. short FIDs) will be found in the most disturbed areas. These two hypotheses, however, predict different patterns in the between-individual variance (VB) and within-individual variance (VW) of FID. Specifically, the personality-matching hypothesis assumes that individuals settle non-randomly and are consistent in their FIDs over time. Accordingly, if personality is the underlying mechanism, we expect VB to be high and constant over the length of our study. Moreover, we expect VW to be consistently low, because individuals should always respond in a similar way when repeatedly confronted with human disturbance. Therefore, we expect a consistently high repeatability of FIDs across time. On the other hand, the behavioural-plasticity hypothesis predicts that individuals settle randomly and adjust their behaviour to disturbance levels as time passes and/or also to the levels of real-time disturbance. Although it is difficult to predict how VB would change over time, we expect that VW changes over time (e.g. individuals respond plastically). Thus, we should observe inconsistent repeatability values in FIDs at different time points of an individual's life.
2. Material and methods
(a). Study area and study species
We studied an individually colour-banded population of wild dunnocks (Prunella modularis), inhabiting an urban park, the Dunedin Botanic Garden, New Zealand (45°86′ S, 170°52′ E). The 7.2 ha study area is a frequently visited tourist site, and attracts between 40 440 and 187 550 visitors per month (for details, see electronic supplementary material, S1). We divided the study area into a grid of 141 squares (each measuring 25 m × 25 m or 0.0625 ha; note that some squares at the margins of our study area did not completely overlap with the boundaries of the study area; electronic supplementary material, figure S2) to be able to assign specific values of behavioural and demographic measurements to each square (see below).
In our study area dunnocks occupy small territories (approx. 0.24 ha per breeding group/pair [29]), which they defend all year round. We collected data in three breeding seasons (September–January of 2012, 2013 and 2014) and two non-breeding seasons (April–August of 2013 and 2014). For more details on demographic information, see electronic supplementary material S2; note that in our population, as in others [30], dunnocks showed very strong affinity to the breeding territories where they initially settled.
(b). Individual age and individual sampling interval
We aged all dunnocks used in this study (N[total] = 99, age range: 0–6 years); birds were considered to be in year ‘zero’ until their first breeding season. For 25% of the birds, we knew their exact age, because they were banded as nestlings or fledglings in our study population (N[fledglings] = 24). Immigrants coming from outside to breed in the study area were banded (usually within one week after arrival) and considered to be first time breeders (age of 1; N[new breeders] = 52), following Davies [30]. The rationale for this decision is that dunnocks are extremely sedentary and only disperse directly after the fledgling period or shortly before their first breeding season to acquire their own breeding territory (B.H., E.S.A.S., C.E.L. & S.N. 2009–2014, personal observations) [30]. As our banding efforts started in 2009, dunnocks banded in 2009 were considered to be 1 year old at that time (N[2009 birds] = 23 included in this study; for more details on these birds, and for a sensitivity analysis with birds from 2009 considered to be 2 years old, see electronic supplementary material S3).
We used age as a proxy to test if birds altered their behaviour over their lifetimes (i.e. over the long term). For each dunnock, we also determined an individual sampling interval, calculated as the time (in days) between the first FID measurement (day zero) and each of the following FID measurements (total range from 0 to 761 days). Therefore, individual sampling intervals functioned as a covariate to test whether individuals altered their behaviour over the course of the study (i.e. over short to intermediate terms; see also electronic supplementary material S6).
(c). Flight-initiation distance
We repeatedly measured flight-initiation distance (FID) as a proxy for boldness [6] of all adult dunnocks that were present in our study area during the breeding and non-breeding seasons (2012–2014; mean interval between measurements ± s.d., 44.36 ± 67.97 days). After identifying an individual that was feeding or resting on the open lawn or footpath by its colour bands, the observer started walking towards the focal individual from a standardized distance of 15–20 m. The observer approached a focal individual by walking a direct line at a constant speed of approx. 1.5 m s−1. When the focal individual escaped (either by foot or by flight), the observer immediately stopped walking and measured the distance between their location and the position from which the bird escaped, as well as the distance between place of escape and the nearest vegetation (i.e. cover) using a rangefinder (±1 m, Bushnell Sport 850; FIDs < 1 m were all recorded as 0.5 m). One single observer (B.H.) collected the majority (over 91%) of FID measurements. The three other observers, which were trained by B.H., collected 7%, 1.5% and 0.5% of the FID measurements, respectively. Since observer ID had negligible effects on FIDs, we conducted our analyses without including observer ID (for more details, see electronic supplementary material S4).
(d). Levels of human disturbance
To quantify the levels of human disturbance in the study area, we counted the number of visitors in each of the 141 squares. In total, we conducted three surveys (February 2014, August 2014, February 2015), each over a period of 24 consecutive days, which were divided into a first and second interval (12 days each). On each day, we counted all visitors (once per day) by walking a pre-defined set of seven transects (equally distributed) that covered all of the 141 squares of our study area (electronic supplementary material, figure S2). Due to the mobility of pedestrians, we assigned all visitors to the square where we first encountered them. For each of the 12 days of the first interval, we started our visitor count at a different time between 07.00 and 18.00 hours (on every full hour, in a random order). After 12 days of counting, we repeated the same procedure for another 12 days (second interval), but walked all transects in reversed direction, resulting in a total of 72 counts per square.
(e). Statistical analyses
(i). Consistency of flight-initiation distance
We used Bayesian linear mixed models (BLMMs) with Markov chain Monte Carlo (MCMC) methods implemented in the MCMCglmm package in R v. 3.3.2 [31,32] to evaluate the consistency of FID (N = 99 individuals and 857 observations). In total, we fitted 15 BLMMs to obtain unadjusted, adjusted and conditional estimates of repeatability (i.e. intra-class correlation, ICC; sensu [33]). For all models, we square-root-transformed the response variable (FID) to achieve normality of residuals. Therefore, we ran all BLMMs with a Gaussian error structure with identity link (for the details of MCMC specifications and model priors, see electronic supplementary material S5).
First, we fitted a ‘null’ mixed model (intercept-only) along with individual-specific intercepts (i.e. the random factor, individual identity) to calculate unadjusted repeatability of FID. This model served as a basis for the 14 remaining models (see below) for which we also fitted ‘observation period’ (five levels, including three breeding and two non-breeding seasons) as a random factor to account for seasonal effects upon FID (e.g. [34]).
Second, to test whether repeatability of FID changed with increasing age or over individual sampling intervals, we calculated adjusted repeatabilities by fitting two models including either age or sampling interval as a fixed effect. In addition, we fitted six random-slope mixed models for age as a fixed effect, as well as random slopes for individuals. In each of the six models the reference points were adjusted (age 1, 2, 3, 4, 5 and 6), so that resulting between- and within-individual variances could be used to obtain conditional repeatabilities at the specific age points [33]. Similarly, we ran six random-slope mixed models with the ‘sampling interval’ (see above) as a fixed effect, with random slopes to obtain conditional repeatabilities at different time points of the sampling period (days 100, 200, 300, 400, 500 and 600). We calculated all repeatabilities as the proportion of the total variance that is explained by the between-individual variance according to Nakagawa & Schielzeth [33].
(ii). Spatial correlation of human disturbance, flight-initiation distance and age
We created heat maps, using the package ggplot2 [35] in R to visualize the distribution patterns of human disturbance, FID and bird age in the study area (N = 99 individuals over 3 years). The level of human disturbance showed an almost identical distribution pattern for the three surveys (see above). Also, the amount of disturbance in each of the 141 squares was highly correlated between all surveys (Spearman's correlations between 0.81 and 0.87; electronic supplementary material, table S3). Thus, we combined all surveys by calculating one mean visitor score for each of the 141 squares and plotted one overall heat map of human disturbance.
To be able to map the distribution of FIDs, we allocated one specific value of FID to each of the 141 squares. For that reason, we first calculated individual mean FIDs, and then averaged the mean values of the birds that occupied the same square (i.e. birds have overlapping territories), separately for each of the three study years (including breeding and non-breeding seasons). Similarly, we estimated one age value per square, by averaging the ages of the birds that shared the same squares during each breeding season. To make different types of values comparable, we z-transformed the human disturbance score, FID and age. We determined the correlation between human disturbance and FID, as well as between human disturbance and age, using Spearman's rank correlations.
(iii). Effects of human disturbance and behavioural plasticity on flight-initiation distances
We acknowledge that the simple correlations in the preceding sub-section may result in a type of pseudo-replication, inflating type I error, because statistical significance is influenced by the number of squares (i.e. 141) that we determined. Therefore, in an alternative set of analyses, we modelled the relationships among FIDs, human disturbance and other variables, using each measurement of FID as the unit of analysis (N = 99 individuals and 857 observations). Particularly, we fitted BLMMs with FID (square-root transformed) as the response variable, and a Gaussian error structure with identity link. Individual identity and observation period were fitted as random effects. In the same model, we included five fixed effects: (i) human disturbance (i.e. mean number of visitors of all squares used by a particular bird during the breeding season, log-transformed), (ii) bird age, (iii) sampling interval (in days), (iv) distance to cover and (v) sex (for alternative specifications of fixed effects, see electronic supplementary material, S6–S8). We included sex because male and female dunnocks differ in FIDs in our study population [36]. Further, we fitted the sampling interval as random slope to obtain a precise estimate of the fixed term, sampling interval [37]. We standardized the continuous variables level of human disturbance, bird age, sampling interval, and distance to cover (by centring each variable on its mean and by dividing each variable by its standard deviation) to generate comparable regression coefficients [38] (see also [39]). The binary variable sex was coded as females = −1 and males = 1 so that sex has a mean and standard deviation of close to 0 and 1, respectively (for the details of MCMC specifications and model priors, see electronic supplementary material S5). We report the estimates of all regression coefficients (β) as posterior means with their 95% credible intervals (CIs), and considered effects statistically significant when the 95% CIs did not overlap zero. Additionally, as sensitivity analysis (electronic supplementary material S9), we re-ran all models using a reduced dataset that included 51 or 23 individuals (of 99) for which we measured FIDs relatively shortly after they settled.
3. Results
(a). Consistency of flight-initiation distance
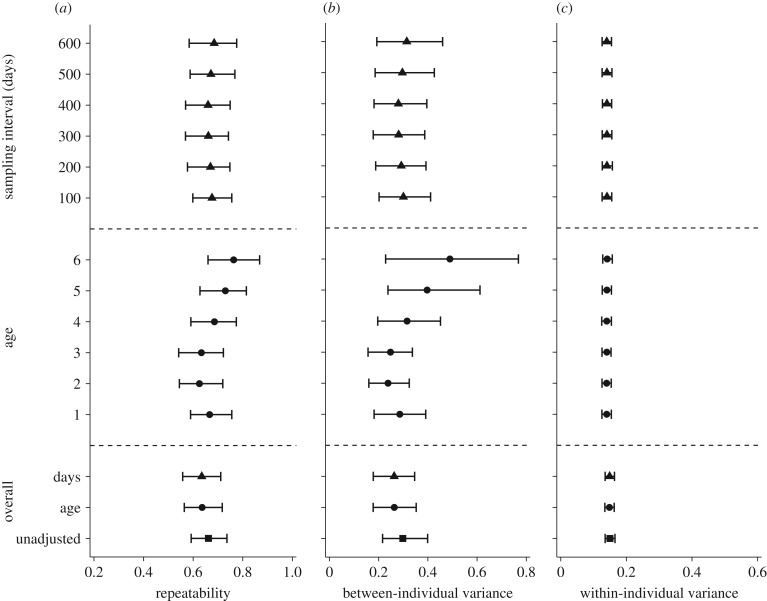
In total, we collected 857 FID measurements from 99 individuals. Observed FIDs ranged from 0.5 m to 19 m (population mean ± s.d., 6.40 ± 3.20 m) and were highly repeatable (unadjusted R = 0.66, 95% credible interval (CI) = 0.59 to 0.74; for more details see below, and figure 1a). Overall, dunnocks showed highly consistent FIDs over time (for both age and sampling interval), with point estimates of repeatability between 0.63 and 0.76 (all 95% CIs between 0.54 and 0.87; figure 1a). For all estimated repeatabilities, the high values resulted from a combination of moderate between-individual variance (VB; figure 1b), and low within-individual variance (VW; figure 1c). Although VB increased slightly with age (figure 1b), repeatabilities among different sampling intervals and age classes did not significantly differ from each other (electronic supplementary material, tables S4 and S5). The increase of VB at the age classes 5 and 6 resulted in slightly higher repeatabilities than for the other ages (figure 1a). The VW, however, showed very little change at the different time scales, indicating highly consistent within-individual variation in FID across all age classes and individual sampling intervals.
Figure 1.
Repeatabilities of flight-initiation distance (FID) in dunnocks measured over a period of 3 years (2012–2014). (a) From bottom to top of panel: unadjusted (square), adjusted (age and days; circle and triangle) and conditional repeatabilities for specific ages (1–6; circles) and time points (100–600 days; triangles) with 95% credible intervals (CIs). Panel (b) shows the corresponding between-individual variances with 95% CIs, and panel (c) shows the corresponding within-individual variances with 95% CIs. The analyses included 857 measurements from 99 individuals: age and day categories had uneven numbers of birds (age: N[Age 1] = 54, N[Age 2] = 39, N[Age 3] = 24, N[Age 4] = 31, N[Age 5] = 25, N[Age 6] = 12; and days: N[Days 100] = 69, N[Days 200] = 61, N[Days 300] = 58, N[Days 400] = 34, N[Days 500] = 26, N[Days 600] = 25).
(b). Relationships between human disturbance and flight-initiation distance, and human disturbance and age
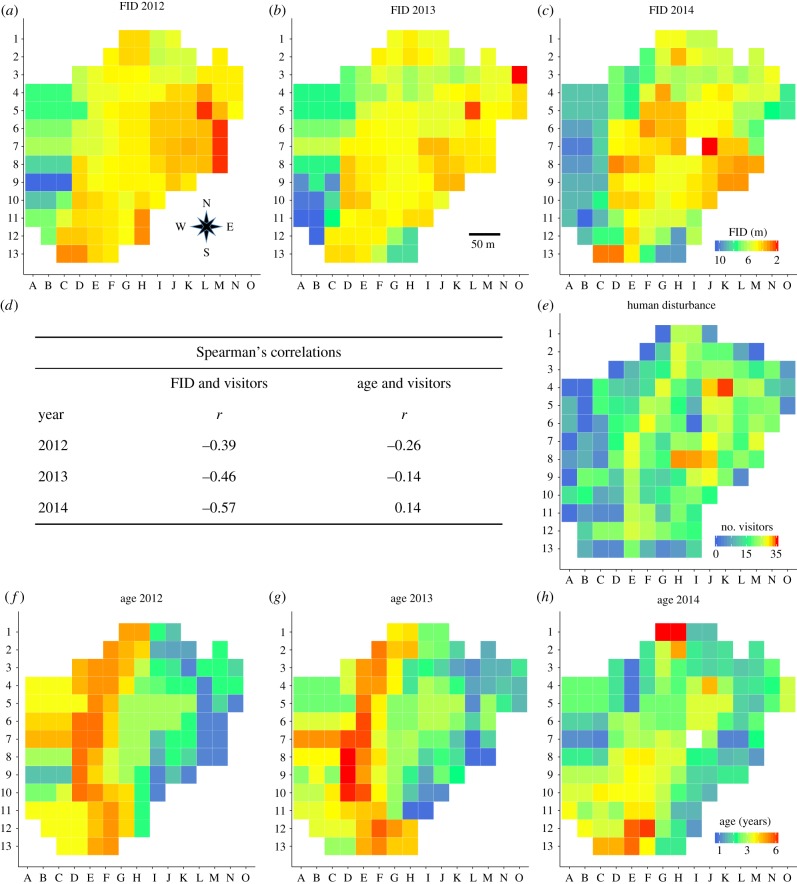
The distributions of FIDs showed somewhat similar but non-random patterns for all 3 years (2012–2014; figure 2a–c). In relation to the distribution of visitors (figure 2e), we found shy individuals (long FIDs) mainly in areas with low levels (blue and green squares in figure 2e), and bold individuals (short FIDs) in areas with high levels of human disturbance (orange and red squares). In agreement with the visual patterns in figure 2, the distributions of FIDs and human disturbance were negatively and significantly correlated to a medium to strong degree (sensu [40]; Spearman's correlation coefficients, rs = −0.37 to −0.57, figure 2d) for all 3 years. In 2012 and 2013, the age structure showed a similar pattern; young individuals were mainly found in the eastern part, while older birds were found in the western part of the study area (figure 2f–g). For both years, we also found non-significant negative correlations between age and human disturbance (rs = −0.14 to −0.26; figure 2d). In 2014, however, the age distribution exhibited a more diverse pattern (figure 2h). In contrast to the two previous years (2012 and 2013), we found a non-significant positive relationship (rs = 0.14; figure 2d) between age and human disturbance in 2014. These non-significant and inconsistent spatial patterns suggest that age may be of little importance with regard to the level of human disturbance. We note, however, that a strong spatial correlation would only occur when individuals move to more disturbed areas as they get older. Therefore, the lack of a spatial correlation between age and human disturbance does not necessarily mean that there is no effect of age on FIDs (see below).
Figure 2.
Distributions of flight-initiation distance FID (a–c) and age (f–h) of dunnocks in the study area between 2012 and 2014. Data were not available for white squares. (e) The number of visitors estimated from three visitor surveys (February 2014, August 2014 and February 2015). (d) Spearman's correlations between FID & human disturbance, and age & human disturbance for the 3 years 2012, 2013 and 2014. (Online version in colour.)
(c). Effects of human disturbance and behavioural plasticity on flight-initiation distance
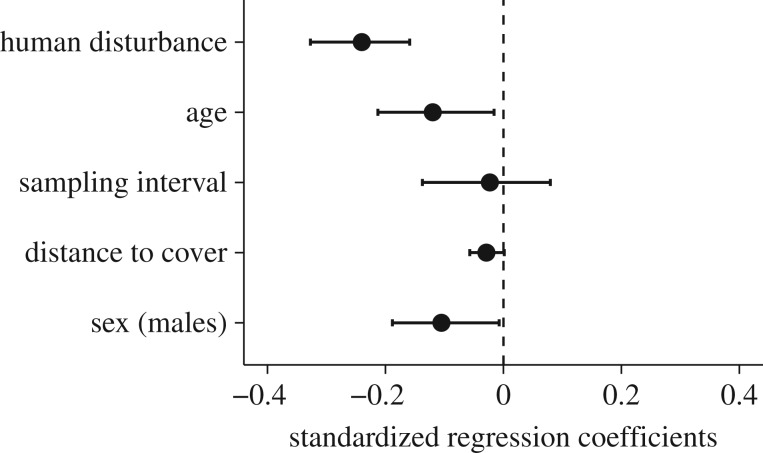
Levels of human disturbance negatively and significantly predicted FIDs (BLMM: standardized regression coefficient, β[human disturbance] = −0.24, 95% CI = −0.33 to −0.16; figure 3; electronic supplementary material, table S6); on average, an increase of 20 visitors per square correlated with a decrease of 1.5 m in FID. Moreover, individuals became slightly bolder (shorter FIDs) as they grew older (BLMM: β[age] = −0.12, 95% CI = −0.23 to −0.02; i.e. support for ‘long-term’ behavioural change; figure 3; see also electronic supplementary material, figures S5 and S6). However, the estimated effect size was relatively small or half of the effect size of human disturbance (see above). On average, the FID of an individual decreased by 0.56 m per 1 year of age (for an alternative interpretation of age-dependent change, see electronic supplementary material S8). There was little evidence that this observed age effect was the result of higher mortality of shy individuals or due to habitat-specific selection on FID (electronic supplementary material S10). Consistent with this idea, an alternative analysis showed that the age effect was due to within-individual and not between-individual changes (electronic supplementary material S7).
Figure 3.
Effects of human disturbance, age, sampling interval (days), distance to cover and sex on flight-initiation distance (FID). We present standardized regression coefficients estimates from a linear mixed model, BLMM, with their 95% credible intervals (CIs). Statistically significant negative effect sizes for visitors and age indicate that dunnocks in highly disturbed areas as well as older birds have shorter flight-initiation distances (are bolder) in comparison with birds in undisturbed areas and younger birds. For the fixed factor sex, a statistically significant negative effect size illustrates that males have shorter FIDs (are bolder) than females. The vertical dashed line indicates zero effect.
In the main, the results on the effect of human disturbance and age on FIDs were congruent with those from the correlation analysis (see section above and figure 2). In contrast, FID was not significantly affected by increasing length of the sampling interval (BLMM: β[number of days] = −0.02, 95% CI = −0.14 to 0.08; figure 3), indicating that individuals did not alter their behaviour over intermediate or over short time scales (for even shorter time scales, i.e. within days, see electronic supplementary material, S6). Also, individuals had shorter FIDs the further they stayed away from cover. This effect, however, was not significant (BLMM: β[distance to cover] = −0.03, 95% CI = −0.06 to 0.01). Finally, there was a small but significant effect of sex (BLMM: β[sex (males)] = −0.10, 95% CI = −0.19 to −0.01; figure 3), with males having shorter FIDs than females (see above). For the results of models with alternative fixed effect specifications, see electronic supplementary material, tables S6–S8.
4. Discussion
In this study, we investigated whether variation and spatial distribution of flight-initiation distance (FID, a proxy for boldness) is better explained by personality-matching habitat choice or behavioural plasticity using a population of wild dunnocks in the Dunedin Botanic Garden. We found support for both hypotheses. For example, bolder birds inhabited areas of high human disturbance, while shy birds avoided disturbed areas. At the same time, the boldness of dunnocks was highly consistent across all age classes (1–6) and over the entire study period (figure 1), indicating that personality plays an important role. Individuals became bolder with age, which is expected from the behavioural-plasticity hypothesis, but this effect was weak in comparison with the relationship between FIDs and human disturbance (figure 3). Taken together, our results show that personality contributes to a greater extent to the observed relationship between boldness and human disturbance than behavioural adjustments over time. Below we explore these patterns in more detail and discuss potential underlying mechanisms, as well as theoretical and practical implications.
(a). Personality-matching hypothesis
Theory suggests that individuals with similar personality types share the same habitat preferences, leading to an aggregation of individuals with similar behavioural phenotypes (i.e. phenotype–environment covariance [16,41,42]). In agreement with this theory, we found that bold individuals occupied territories with high human disturbance, whereas shy individuals were found in areas with low numbers of pedestrians. Our results are similar to previous research on the impact of disturbance that showed that burrowing owls (Athene cunicularia) with short FIDs bred in closer proximity to roads than individuals with long FIDs [15]. Such patterns, however, could also be generated by behavioural change over time. Hence, more importantly, our results suggest that individuals settle in an area that best matches their own behavioural phenotype and then stay in their initially chosen habitats. Indeed, dunnocks showed strong fidelity to their breeding territories (i.e. individual reused more than 70% of the area they used in the previous breeding season; electronic supplementary material S2), and consequently also an affinity to specific environmental features (e.g. human disturbance; but see electronic supplementary material S11).
Strong theoretical and empirical support exists for a substantial part of personality traits being caused by genetic components [43,44]. In such a case, phenotype–environment covariance, often found in association with personality traits, can be interpreted as genotype–environment covariance [16,45]. Consequently, individuals are likely to mate with conspecifics that share a similar genotype, and thereby potentially restrict the gene flow within a population, termed the multiplier effect (sensu [46]; see also [47]). For instance, within a population of three-spined sticklebacks (Gasterosteus aculeatus), individuals that differed in their habitat preference (stream versus lake) also differed genetically from each other, despite living in close proximity [48]. Therefore, it has been theorized that personality-dependent habitat selection can maintain different personality types within populations [42,49], and also can lead to speciation over time [50–52]. Although speciation is likely to happen on larger spatial and much longer temporal scales, our findings lend support to the potential for micro-evolutionary and ecological differentiation over time [53,54].
(b). Behavioural plasticity hypothesis
Along with the strong evidence for personality-matching habitat selection, we also found some indication that individual dunnocks became slightly bolder with increasing age (i.e. individuals exhibited behavioural plasticity over long-term; 0.56 m shorter FIDs per year). Moreover, dunnocks varied slightly in their response to humans during the day (electronic supplementary material S6). A previous study on blackbirds (Turdus merula) showed evidence of habituation, with individuals adjusting their risk-taking behaviour with regards to daily fluctuations in pedestrian numbers [55]. A meta-analysis also showed that individuals reduce their FIDs when frequently exposed to a predator [56]. In contrast, the lack of change in FIDs for short and intermediate time scales (among days; electronic supplementary material S9) suggests that behavioural change does not occur rapidly, but is a gradual process over time (for alternative interpretations of age-dependent change in FIDs, see electronic supplementary material S8). Notable in our study is that the age effect on FID was relatively minor (half the size; figure 3) in comparison with the relationship between FID and human disturbance. Therefore, the strong observed correlation between behavioural phenotype and habitat in dunnocks most likely originated from a personality-matched habitat choice [16] rather than from the adjustment of behaviour over time (electronic supplementary material S9).
An alternative mechanism that can explain non-random distribution patterns of phenotypes, and may not be mutually exclusive from habituation, is risk allocation (e.g. [55,57]). The risk-allocation hypothesis [58] posits that individuals adjust their antipredator behaviour according to fluctuating degrees of risk. Given that dunnocks show high temporal consistency in FIDs, and that minor adjustments in FIDs were similar for areas with low and high disturbance levels, it is unlikely that risk allocation explains our findings.
(c). Difficulty in separating personality from behavioural plasticity
Various studies have suggested that apart from genetic factors, individual experience during early development (ontogenetic experience) could substantially influence personality formation and adaptive personality differences among individuals (e.g. [49,59]). For instance, individuals that grow up in highly disturbed habitats might develop different strategies to cope with humans than individuals that grow up in less disturbed areas (e.g. natal habitat preference or learned antipredator response [59,60]). The effect of early experience could explain the non-random distribution of behavioural phenotypes. Importantly, such a scenario does not preclude the personality-matching hypothesis being an explanation for our findings, because personality seems to dictate where individuals will settle [15,16], thereby generating the phenotype-environment covariance (note that the correlation between genotype and environment may no longer exist in this case).
Alternatively, it is also possible that the different levels of human disturbance in our study area shaped the ‘boldness’ (or FID) of individuals within a few days of settlement, resulting in the observed relationship between boldness and human disturbance. Such rapid behavioural change (e.g. within a few days) would be a very interesting case of behavioural plasticity, which is akin to ‘developmental plasticity’ [49]. In our study it was not possible to collect a sufficient amount of data to examine this alternative, as the majority of fledglings leave the study area to settle somewhere else, and because of low turnover rates of birds in general (but see electronic supplementary material S9). While our data cannot discriminate between the above alternatives, an additional analysis suggested that bold individuals could be found in both low- and high-disturbance areas, lending support to the personality-matching hypothesis, but not the plasticity hypothesis (see electronic supplementary material S12). Nonetheless, this finding cannot refute the possibility that ontogenetic or short-term experiences (e.g. exposure to humans) have strong effects on the development of personality traits. Future research is required to thoroughly investigate if the boldness of an individual is determined before or immediately after settlement.
(d). Additional implications: population management and conservation
Our study indicates that an individual's personality could play a decisive role in selecting a suitable habitat. In the face of the current unprecedented human-induced rapid environmental change and associated habitat loss [50,61,62], an optimal habitat for a particular personality type might not always be available [13]. For example, human-modified environments are often characterized by monotonous landscapes (e.g. urbanization, agriculture), which may favour only specific behavioural phenotypes. Commonly, bold individuals flourish in urban, human-disturbed habitats, whereas shy individuals predominantly thrive in undisturbed rural areas [63,64]. In consequence, the composition of phenotypes and genotypes of populations may be reduced [65,66], leading ultimately to local extinction. Thus, understanding the full extent of personality-related habitat selection may be the key to establish conservation strategies for species restricted to specific habitats.
Supplementary Material
Acknowledgements
We thank Jolyn Chia, Matthew Jarvis, Hannah Millar, Freya Moore and Simon Tomkins for help with fieldwork. We are grateful to Bruce Robertson, Sheri Johnson and two anonymous reviewers for insightful comments, which helped to improve the manuscript. We wish to thank the Dunedin Botanic Garden, its staff and especially Barbara Wheeler for providing access to the study site.
Ethics
This study was carried out under the licence of the Animal Ethics Committee of the University of Otago (permit no. 49/12 and 89/14) and the New Zealand Department of Conservation national permit no. 36716-FAU.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t8t25 [67].
Authors' contributions
B.H. and S.N. conceived and designed the study. B.H., E.S.A.S. and C.E.L. collected the data. B.H. with help from S.N. analysed the data. B.H. and S.N. wrote the manuscript with contributions from E.S.A.S. and C.E.L. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
B.H. was supported by a University of Otago Doctoral Scholarship. S.N. was funded by a Rutherford Discovery Fellowship (New Zealand) and a Future Fellowship (Australia, FT130100268).
References
- 1.Endler JA. 1977. Geographical variation, speciation and clines. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 4.Smith TB, Skulason S. 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 27, 111–133. ( 10.1146/annurev.ecolsys.27.1.111) [DOI] [Google Scholar]
- 5.Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey DC, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. ( 10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 6.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 7.Carere C, Maestripieri D. 2013. Animal personalities: behavior, physiology, and evolution. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 8.Dall SRX, Houston AI, McNamara JM. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739. ( 10.1111/j.1461-0248.2004.00618.x) [DOI] [Google Scholar]
- 9.Sih A, Bell, Alison M., Johnson JC, Ziemba Robert E. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 10.Gosling SD. 2001. From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86. ( 10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- 11.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 13.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 14.Cote J, Clobert J, Brodin T, Fogarty S, Sih A. 2010. Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065–4076. ( 10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrete M, Tella JL. 2010. Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol. Lett. 6, 167–170. ( 10.1098/rsbl.2009.0739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelaar P, Siepielski AM, Clobert J. 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 2462–2472. ( 10.1111/j.1558-5646.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- 17.Jacob S, Bestion E, Legrand D, Clobert J, Cote J. 2015. Habitat matching and spatial heterogeneity of phenotypes: implications for metapopulation and metacommunity functioning. Evol. Ecol. 29, 851–871. ( 10.1007/s10682-015-9776-5) [DOI] [Google Scholar]
- 18.Duckworth RA, Badyaev AV. 2007. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl Acad. Sci. USA 104, 15 017–15 022. ( 10.1073/pnas.0706174104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin JG.A, Réale D. 2008. Animal temperament and human disturbance: implications for the response of wildlife to tourism. Behav. Processes 77, 66–72. ( 10.1016/j.beproc.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 20.Sol D, Lapiedra O, González-Lagos C. 2013. Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. ( 10.1016/j.anbehav.2013.01.023) [DOI] [Google Scholar]
- 21.Beaman JE, White CR, Seebacher F. 2016. Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 31, 237–249. ( 10.1016/j.tree.2016.01.004) [DOI] [PubMed] [Google Scholar]
- 22.Tuomainen U, Candolin U. 2011. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. ( 10.1111/j.1469-185X.2010.00164.x) [DOI] [PubMed] [Google Scholar]
- 23.Thompson RF, Spencer WA. 1966. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 73, 16–43. ( 10.1037/h0022681) [DOI] [PubMed] [Google Scholar]
- 24.Rankin CH, et al. 2009. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 92, 135–138. ( 10.1016/j.nlm.2008.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frid A, Dill LM. 2002. Human-caused disturbance stimuli as a form of predation risk. Ecol. Soc. 6, 11 ( 10.5751/ES-00404-060111) [DOI] [Google Scholar]
- 26.Steven R, Pickering C, Guy Castley J. 2011. A review of the impacts of nature based recreation on birds. J. Environ. Manag. 92, 2287–2294. ( 10.1016/j.jenvman.2011.05.005) [DOI] [PubMed] [Google Scholar]
- 27.Coetzee BW.T, Chown SL. 2015. A meta-analysis of human disturbance impacts on Antarctic wildlife. Biol. Rev. 91, 578–596. ( 10.1111/brv.12184) [DOI] [PubMed] [Google Scholar]
- 28.Beale CM, Monaghan P. 2004. Human disturbance: people as predation-free predators? J. Appl. Ecol. 41, 335–343. ( 10.1111/j.0021-8901.2004.00900.x) [DOI] [Google Scholar]
- 29.Santos ES.A, Nakagawa S. 2013. Breeding biology and variable mating system of a population of introduced dunnocks (Prunella modularis) in New Zealand. PLoS ONE 8, e69329 ( 10.1371/journal.pone.0069329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies NB. 1992. Dunnock behaviour and social evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 31.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 32.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 33.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 34.Cooper WE. 1999. Tradeoffs between courtship, fighting, and antipredatory behavior by a lizard, Eumeces laticeps. Behav. Ecol. Sociobiol. 47, 54–59. ( 10.1007/s002650050649) [DOI] [Google Scholar]
- 35.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 36.Holtmann B, Grosser S, Lagisz M, Johnson SL, Santos ES.A., Lara CE, Robertson BC, Nakagawa S. 2016. Population differentiation and behavioural association of the two ‘personality’ genes DRD4 and SERT in dunnocks (Prunella modularis). Mol. Ecol. 25, 706–722. ( 10.1111/mec.13514) [DOI] [PubMed] [Google Scholar]
- 37.Schielzeth H, Forstmeier W. 2009. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. ( 10.1093/beheco/arn145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. ( 10.1111/j.2041-210X.2010.00012.x) [DOI] [Google Scholar]
- 39.Gelman A. 2008. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 27, 2865–2873. ( 10.1002/sim.3107) [DOI] [PubMed] [Google Scholar]
- 40.Cohen J. 1988. Statistical power analysis for the behavioural sciences, 2nd edn Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 41.Dingemanse NJ, Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958. ( 10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf M, Weissing FJ. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968. ( 10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Oers K, de Jong G, van Noordwijk AJ, Kempenaers B, Drent PJ. 2005. Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1185–1206. ( 10.1163/156853905774539364) [DOI] [Google Scholar]
- 44.Van OK, Sinn DL. 2013. Quantitative and molecular genetics of animal personality. In Animal personalities: behaviour, physiology, and evolution (eds Carere C, Maestripieri D), pp. 149–200. Chicago, IL: Chicago University Press. [Google Scholar]
- 45.Dingemanse NJ, Kazem AJN, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 46.McNamara JM, Dall SRX. 2011. The evolution of unconditional strategies via the ‘multiplier effect’. Ecol. Lett. 14, 237–243. ( 10.1111/j.1461-0248.2010.01576.x) [DOI] [PubMed] [Google Scholar]
- 47.Edelaar P, Bolnick DI. 2012. Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665. ( 10.1016/j.tree.2012.07.009) [DOI] [PubMed] [Google Scholar]
- 48.Bolnick DI, Snowberg LK, Patenia C, Stutz WE, Ingram T, Lau OL. 2009. Phenotype-dependent native habitat preference facilitates divergence between parapatric lake and stream stickleback. Evolution 63, 2004–2016. ( 10.1111/j.1558-5646.2009.00699.x) [DOI] [PubMed] [Google Scholar]
- 49.Stamps JA, Groothuis TGG. 2010. Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Phil. Trans. R. Soc. B 365, 4029–4041. ( 10.1098/rstb.2010.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sih A, Stamps J, Yang LH, McElreath R, Ramenofsky M. 2010. Behavior as a key component of integrative biology in a human-altered world. Integr. Comp. Biol. 50, 934–944. ( 10.1093/icb/icq148) [DOI] [PubMed] [Google Scholar]
- 51.Ingley SJ, Johnson JB. 2014. Animal personality as a driver of reproductive isolation. Trends Ecol. Evol. 29, 369–371. ( 10.1016/j.tree.2014.04.008) [DOI] [PubMed] [Google Scholar]
- 52.Blumstein DT. 2016. Habituation and sensitization: new thoughts about old ideas. Anim. Behav. 120, 255–262. ( 10.1016/j.anbehav.2016.05.012) [DOI] [Google Scholar]
- 53.Garant D, Kruuk LEB, Wilkin TA, McCleery RH, Sheldon BC. 2005. Evolution driven by differential dispersal within a wild bird population. Nature 433, 60–65. ( 10.1038/nature03051) [DOI] [PubMed] [Google Scholar]
- 54.Garroway CJ, Radersma R, Sepil I, Santure AW, De Cauwer I, Slate J, Sheldon BC. 2013. Fine-scale genetic structure in a wild bird population: the role of limited dispersal and environmentally based selection as causal factors. Evolution 67, 3488–3500. ( 10.1111/evo.12121) [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Prieto I, Fernández-Juricic E, Martín J, Regis Y. 2009. Antipredator behavior in blackbirds: habituation complements risk allocation. Behav. Ecol. 20, 371–377. ( 10.1093/beheco/arn151) [DOI] [Google Scholar]
- 56.Stankowich T, Blumstein DT. 2005. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B 272, 2627–2634. ( 10.1098/rspb.2005.3251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirza RS, Mathis A, Chivers DP. 2006. Does temporal variation in predation risk influence the intensity of antipredator responses? A test of the risk allocation hypothesis. Ethology 112, 44–51. ( 10.1111/j.1439-0310.2006.01111.x) [DOI] [Google Scholar]
- 58.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. ( 10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 59.Stamps JA, Davis JM. 2006. Adaptive effects of natal experience on habitat selection by dispersers. Anim. Behav. 72, 1279–1289. ( 10.1016/j.anbehav.2006.03.010) [DOI] [Google Scholar]
- 60.Griffin AS. 2004. Social learning about predators: a review and prospectus. Anim. Learn. Behav. 32, 131–140. ( 10.3758/bf03196014) [DOI] [PubMed] [Google Scholar]
- 61.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. ( 10.1016/j.tree.2005.11.019) [DOI] [PubMed] [Google Scholar]
- 62.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088. ( 10.1016/j.anbehav.2013.02.017) [DOI] [Google Scholar]
- 63.Møller AP. 2008. Flight distance of urban birds, predation, and selection for urban life. Behav. Ecol. Sociobiol. 63, 63–75. ( 10.1007/s00265-008-0636-y) [DOI] [Google Scholar]
- 64.Samia DS.M., Nakagawa S, Nomura F, Rangel TF, Blumstein DT. 2015. Increased tolerance to humans among disturbed wildlife. Nat. Commun. 6, 8877 ( 10.1038/ncomms9877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arroyo B, Mougeot F, Bretagnolle V. 2017. Individual variation in behavioural responsiveness to humans leads to differences in breeding success and long-term population phenotypic changes. Ecol. Lett. 20, 317–325. ( 10.1111/ele.12729) [DOI] [PubMed] [Google Scholar]
- 66.Smith BR, Blumstein DT. 2013. Animal personality and conservation biology: the importance of behavioral diversity. In Animal personalities: behaviour, physiology, and evolution (eds Carere C, Maestripieri D), pp. 381–413. Chicago, IL: Chicago University Press. [Google Scholar]
- 67.Holtmann B, Santos ESA, Lara CE, Nakagawa S. 2017. Data from: Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype–environment covariance Dryad Digital Repository. ( 10.5061/dryad.t8t25) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Holtmann B, Santos ESA, Lara CE, Nakagawa S. 2017. Data from: Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype–environment covariance Dryad Digital Repository. ( 10.5061/dryad.t8t25) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t8t25 [67].