Abstract
Interactions between multiple anthropogenic environmental changes can drive non-additive effects in ecological systems, and the non-additive effects can in turn be amplified or dampened by spatial covariation among environmental changes. We investigated the combined effects of night-time warming and light pollution on pea aphids and two predatory ladybeetle species. As expected, neither night-time warming nor light pollution changed the suppression of aphids by the ladybeetle species that forages effectively in darkness. However, for the more-visual predator, warming and light had non-additive effects in which together they caused much lower aphid abundances. These results are particularly relevant for agriculture near urban areas that experience both light pollution and warming from urban heat islands. Because warming and light pollution can have non-additive effects, predicting their possible combined consequences over broad spatial scales requires knowing how they co-occur. We found that night-time temperature change since 1949 covaried positively with light pollution, which has the potential to increase their non-additive effects on pea aphid control by 70% in US alfalfa. Our results highlight the importance of non-additive effects of multiple environmental factors on species and food webs, especially when these factors co-occur.
Keywords: aphid, climate change, Coccinellidae, light pollution, predator–prey interactions, VIIRS
1. Background
The majority of research addressing abiotic effects on ecological communities focuses on single environmental drivers, such as temperature or precipitation [1,2], and relatively little is known about the interactive effects of multiple environmental drivers [3,4]. Indeed, only 2% of published experiments addressing climate change effects on trophic interactions manipulated more than one abiotic factor [5]. Furthermore, none of these studies included consumer-free control treatments (i.e. no predator or no herbivore), making it impossible to disentangle the direct effects of abiotic factors from the indirect effects arising from altered interspecific interactions. This highlights that experiments in which multiple abiotic factors are explicitly manipulated are needed, because interactions between species, such as predators and their prey, can lead to non-additive dynamics in which the responses of predators and prey to one abiotic factor are contingent on the strength of a second [5–7]. Therefore, multiple abiotic factors must be studied in concert to predict the consequences of global change on predator–prey systems [2–6,8,9].
When non-additive dynamics make the responses of predators and prey to one abiotic factor contingent on a second, predicting the effects of multiple abiotic factors requires knowledge of spatio-temporal covariance [10–12]. For example, locations experiencing an increase in temperature may simultaneously experience a decrease in precipitation if changes in temperature and precipitation negatively covary at that location. Covarying changes in abiotic factors may result in more-frequent worst-case or best-case scenarios across landscapes, and although ‘much progress has been made in understanding the consequences of single drivers, … elucidating interacting drivers remains a challenge’ [13, p. 388]. Anticipating the consequences of multiple environmental factors requires a broad-scale approach to understand how they covary in space and time [14].
Here, we present a study of the combined effects of night-time warming and anthropogenic light on an insect herbivore and two of its predators. Warming and light pollution are two widespread components of environmental change, and they co-occur especially near urbanized areas [15]. While much effort has been made to understand the effects of warming [16–18], we know little about the population- and ecosystem-level effects of light pollution [19–21] and have only begun to speculate about their interactive effects [9]. Pea aphids (Acyrthosiphon pisum) are common pests in gardens, where they attack peas and beans, and also in commercial pea, bean, and alfalfa (lucerne) fields. Pea aphids are attacked by a suite of natural enemies that can exert strong top-down control and prevent pea aphid outbreaks [22–24]. We examined two of the most common ladybeetles in these agricultural landscapes, Coccinella septempunctata and Coleomegilla maculata. Like many mobile insect predators, both ladybeetle species increase their foraging activity at higher temperatures [25–27]. However, while both species can hunt nocturnally, Col. maculata does not use visual cues to find prey, whereas Coc. septempunctata does and is therefore less effective in the dark [16,28]. We predicted that Col. maculata should respond only to our experimental temperature manipulation. By contrast, Coc. septempunctata should only have increased predation rates on pea aphids with night warming when there was also light pollution, producing a non-additive indirect effect of these two factors on pea aphid abundance.
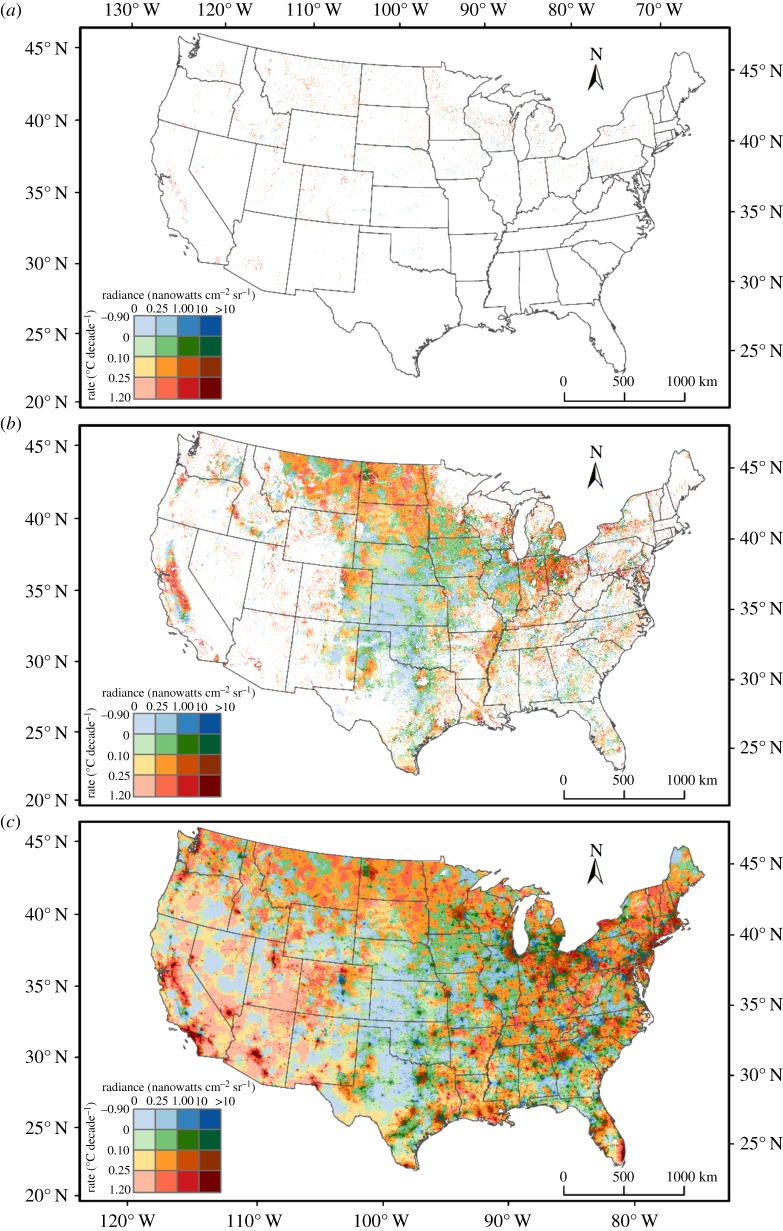
In many parts of the United States (US), including our Wisconsin study site, night-time temperatures are increasing faster than daytime temperatures [29–31]. While this day-night asymmetry in warming is generally ignored in ecological research, the asymmetry may be important when comparing species with different diel activity patterns [32,33]. Nocturnal species may experience greater impacts of global warming than diurnal species. Furthermore, while the day-night asymmetry occurs at broad spatial scales, it is particularly acute in urban areas where radiation absorbed during the day into anthropogenic heat sinks (e.g. roads, buildings) is emitted at night [30,34]. Similarly, light pollution is likely to be strongest in cities [35]. Therefore, at a continental scale, we would expect positive covariation between night warming and light pollution. However, non-urban cropland is where the bulk of insect pests occur, and also where facilitation of nocturnal predators may have the greatest economic benefits through biological control. To compare all land area with cropland and with alfalfa crops, we mapped night warming and light pollution for the total contiguous US, US cropland, and US alfalfa to determine whether the distributions of night warming and light pollution covary spatially, leading to a greater-than-expected area with high levels of both.
2. Material and methods
(a). Field and laboratory experiment
We conducted the field experiment at the University of Wisconsin Arlington Agricultural Research Station in Arlington, Wisconsin, USA, between 28 July and 6 August 2014. We constructed 48 enclosures using insect netting supported by cylindrical-shaped wire frames (base: 0.1 m2; circumference: 1.1 m; height: 0.75 m). To minimize colonization of enclosures by arthropods, we installed enclosures 1 day after alfalfa was harvested when field densities of arthropods were low. Enclosures were placed over alfalfa plants (5–10 cm tall), arranged in eight rows of six cages and spaced 3–5 m from each other. The experimental design consisted of 12 treatment combinations, crossing light pollution (yes or no), night warming (yes or no) and predator type (none, two adult Col. maculata, or two adult Coc. septempunctata) in a factorial design that we replicated four times. Treatments were randomly assigned within a block design, with two neighbouring rows of enclosures constituting a block.
After installing the enclosures, we waited 6 days before stocking any insects and beginning the experiment. This allowed us to ensure that the enclosures were secure and allowed the plants to recover from the recent harvest. We visually scanned inside enclosures and removed all visible arthropods before beginning the experiment. We initiated the experiment by stocking all enclosures with 20 adult pea aphids obtained from a laboratory colony (5AR; red/pink colour). Predator treatment enclosures were stocked with two unsexed adult lady beetles, which is at the high range of field densities but consistent with similar global change experiments [22,36].
We simulated light pollution using two commercially available, solar powered LED lights at the top of each light treatment enclosure (manufactured by ASC, Inc.). The LED lights included photosensitive triggers that automatically turned lights on during periods of darkness, and both lights combined produced approximately 1500 nanowatts cm−2, as measured with a handheld light meter (Li-cor, Model LI-250). This light pollution treatment is roughly equivalent to a lighted carpark at night and dimmer than normal street level illuminance [19], and approximately 30× that of a full moon [37]. Night warming was created using water-filled black plastic bags. We filled contractor-grade black rubbish bags with approximately 19 l of water and positioned them next to warming treatment cages. The bags absorbed solar energy during the day and then radiated the heat throughout the night. At sunset, we covered all cages with another opaque plastic bag, which we secured to the ground with stakes. In control treatments, bags blocked all ambient light (e.g. anthropogenic or extraterrestrial light) creating ‘no light’ control treatments. In light pollution treatments, covers blocked all external light and triggered the LED lights to turn on, standardizing light levels among treatments. Finally, in warming cages the covers served as insulation, trapping the heat of the warmed water. We removed covers at sunrise each morning. This methodology created the asymmetric day-night pattern of warming similar to that forecasted for the region, increasing the temperature of the warming treatments by 2.1°C at night and 1.5°C during the day (electronic supplementary material, figure S1).
After 9 days, we removed all alfalfa and arthropods from within each enclosure. We cut alfalfa at the soil surface, and placed the plants and any attached insects in a bag for transportation. We collected any aphids that dropped from the plants during removal with aspirators and counted them on site. Because adult ladybeetles are quick to fly away when disturbed, we were unable to consistently capture and count predators remaining at the end of the experiment. The following day in a controlled laboratory environment, we sorted the alfalfa and counted the number of aphids from each enclosure. We summed the number of aphids aspirated in the field and the number of aphids counted in the laboratory to determine total aphid abundance for each enclosure.
We conducted feeding trials with three levels of lighting to determine how light affects feeding rates of Coc. septempunctata and Col. maculata using methods similar to those used by Harmon et al. [16]. Predators were starved for 24 h and then placed in clear plastic containers (0.6 l) containing 20 adult aphids and sealed with nylon mesh lids. We used the same clonal line of aphids that was used in the field experiment. Containers were immediately placed within cardboard boxes (25 cm L × 18 cm W × 22 cm H) that contained either 0, 1 (centred; approximately 800 nanowatts cm−2 sr−1), or 3 (equally spaced; approximately 2000 nanowatts cm−2 sr−1) solar powered LED lights (same brand and model as used in the field experiment) suspended 10 cm above the top of the plastic containers. We placed two containers in each box, one containing each predator species, and left them in a dark room. After 1 h, we counted the number of aphids remaining, which we subtracted from 20 (initial aphid count) to determine the number of aphids consumed during the experiment. We replicated the experiment 13 times for each species. We used a linear model to analyse the effect of predator species (P) and lights (L) on the number of aphids consumed using the model log10(aphids + 1) = P × L.
(b). Remote sensing
To investigate the co-occurrence of night warming and light pollution, we used long-term gridded meteorological data and remotely sensed night-time light levels to map the combined magnitudes of night temperature change and light pollution across the contiguous USA. Rather than focus narrowly on our specific study system, we instead analysed the night-time light data in the broader context of insect predator–prey systems. We used the crop-type map produced by the National Agricultural Statistics Services, US Department of Agriculture (USDA), which is derived chiefly from Landsat imagery [38]. The map has been validated using USDA-collected ground-truth data, and the overall accuracy is higher than 85% [38]. We downloaded the data for 2014 from CropScape, a web-based application for exploring USA crop data layers [39] with 30 m spatial resolution. We masked the non-crop lands (e.g. forest, urban and water) and extracted the croplands by merging the areas of different crop types.
To derive the spatial pattern for night-time warming, we applied the gridded monthly minimum temperatures from 1949 to 2010 generated by Maurer et al. [40], which is based on weather station observations well for the conterminous United States [40]. We calculated mean minimum temperatures from 1949 to 2010 for the growing season period (May–September), and calculated the rate of temperature change using linear regression. We reclassified the map of rates of temperature change into four categories using quantile classification: −0.90 to 0, 0 to 0.10, 0.10 to 0.25, and 0.25 to 1.20°C decade−1.
To assess light pollution, we used the night-time light data from the Visible Infrared Imaging Radiometer Suite (VIIRS) Day/Night Band. The VIIRS data are superior to the US Air Force Defense Meteorological Satellite Program (DMSP) Operational Linescan System (OLS) data for mapping night-time lights [41], although the DMSP-OLS night-time lights have longer time series (1992–2013) and are widely used for estimating global socio-economic parameters [42]. The VIIRS night-time light data are monthly average radiance composite images excluding the impacts of stray light, lightning, lunar illumination and cloud cover, and are available in 15 arc-second (approx. 500 m) geographical grids.
We reclassified VIIRS data into four categories that are relative to the irradiance of a full moon. We did this for two reasons. First, confirming the precise VIIRS values in crop fields would require extensive field measures that were beyond the scope of the study. Second, there is no comprehensive compilation of the abilities of insect predators to see at night, and therefore interpreting VIIRS generated irradiance values would be difficult. Thus, we scaled our assessment of light pollution according to an ecologically-meaningful metric, the brightness of a full moon. We selected this brightness scale because visually foraging nocturnal insects would, at a minimum, have to be able to see at night under a full moon, which is the largest naturally occurring source of nocturnal light. Insects with superposition compound eyes [43] can see at light levels well less than full moon [44]. For example, the nocturnal hawk moth Deilephila elpenor can distinguish flower colour at light levels corresponding to dim starlight [45]. By contrast, ladybeetles have focal apposition compound eyes [46] and generally limited visual abilities in dim light [44]. However, Coc. septempunctata can forage in full moonlight; its visual sensitivity has been measured to 5 nanowatts cm−2 within the visible spectrum [47]. These differences in eye structure may result in different effects of light pollution among species.
To cover the range of night vision abilities found among insects, we divided remotely sensed reflected night light levels into four categories, <0.25, 0.25–1, 1–10, >10 nanowatts cm−2. There is much variation in the literature reports of full moon irradiance, with many values—although probably incorrect—as high as 320 nanowatts cm−2 (2.2 lux) [37]. We used a full moon irradiance value of 100 nanowatts cm−2 (approx. 0.683 lux), which is at the high side of realistic estimates [37]. Given that 1 watts sr−1 is approximately 10 watts (actual value = 12.6 w, isotropic), the satellite-derived radiance, 10 nanowatts cm−2 sr−1, is close to the largest measures of full moon irradiance. Further, by using a relatively large value for full moon irradiance, our results are conservative estimates of the magnitude of light pollution. These classification thresholds corresponded roughly to wildlands (<0.25 nanowatts cm−2 sr−1), urban fringes (0.25–1 and 1–10 nanowatts cm−2 sr−1) and urban areas (>10 nanowatts cm−2 sr−1).
To combine the three maps (crop, night-time temperature change and night-time light maps) with different spatial resolutions, we derived the pixel coordinates for crop map and extracted the class properties from the other two maps at exactly the same location. The combination of different classes for night-time temperature change and light pollution constituted a new classification scheme as indicated in figure 3.
Figure 3.
Spatial pattern of the combined effects of night-time warming and light pollution across the contiguous United States for (a) alfalfa fields only, (b) all croplands and (c) all lands. White areas indicate (a) non-alfalfa or (b) non-crop fields.
3. Results and discussion
(a). Field experiment on interactions between night warming and light pollution
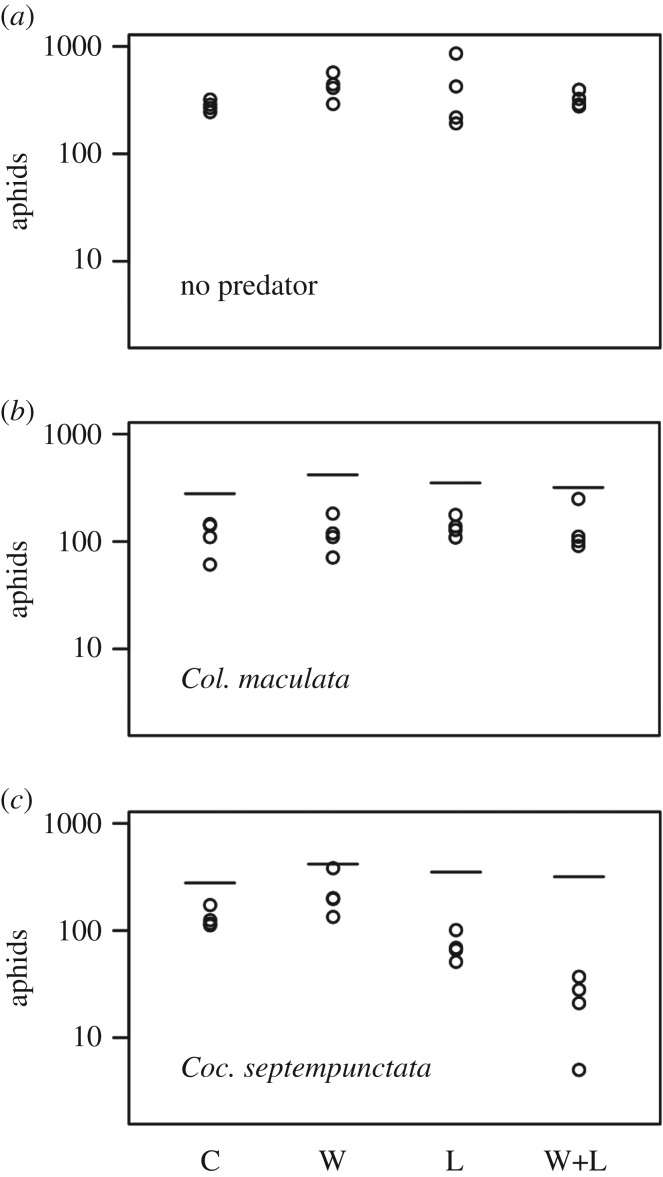
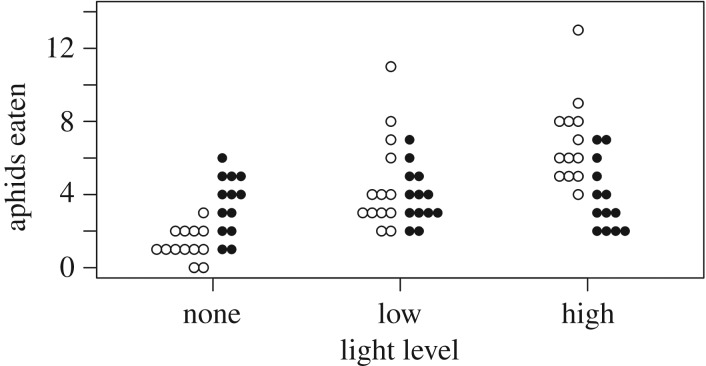
The field experiment revealed significant statistical interactions among night warming, light pollution and predation (figure 1; F2,36 = 15.71, p < 0.001; table 1). In no-predator treatments, night warming increased aphid abundance, while light pollution had no measurable effect (figure 1a). In treatments with no warming and no lights, both predators significantly reduced the final abundance of aphids relative to the no-predator control (ANOVA: Col. maculata, p = 0.0007; Coc. septempunctata, p = 0.003; figure 1b,c). In the presence of Col. maculata, the addition of warming and lights both singly or in combination did not alter aphid abundance. We did not expect an effect of light on predation rates, and the absence of an effect of warming was probably caused by both aphid growth rates and predation rates increasing with temperature, and balancing each other out. However, in the presence of Coc. septempunctata, there was a statistical interaction between night warming and light pollution: night warming alone increased aphid abundance, whereas night warming in combination with light pollution greatly decreased aphid abundance (F1,12 = 12.28, p = 0.004; table 2; figure 1c). There was a strong statistical interaction between light levels and predator species (F5,72 = 16.12, p < 0.001; electronic supplementary material, table S1); the number of aphids consumed by Col. maculata did not differ among light levels, but for Coc. septempunctata predation increased with increasing light (figure 2). This increase also shows that Coc. septempunctata benefitted from even higher light levels than were used in the field experiment.
Figure 1.
Interactions between night-time warming and simulated light pollution for the three predator treatments ((a) no predators, (b) Col. maculata and (c) Coc. septempunctata) shown by the numbers of aphids remaining at the end of the 9-day field experiment. Points give numbers from the four replicates per treatment, and in the Col. maculata and Coc. septempunctata panels the mean log10 numbers of aphids from the no-predator controls are given by horizontal lines. Warming and light treatments had no effect on aphid density in the no-predator and Col. maculata treatments, but had significant individual and interactive effects in the presence of Coc. septempunctata. (C = control; W = warming treatment; L = light treatment). Statistics are presented in table 1.
Table 1.
Interactions between night warming and light pollution for the different predator treatments (no predators, Col. maculata and Coc. septempunctata) in the field experiment. (Each treatment was replicated four times, for a total of 2 × 2 × 3 × 4 = 48 experimental units. Statistical analyses were performed on the log10 abundance of pea aphids at the end of the 9-day experiment.)
| d.f. | SS (type II) | F | p | |
|---|---|---|---|---|
| predator | 2 | 0.38 | 5.36 | 0.009 |
| warming | 1 | 0.06 | 1.74 | 0.20 |
| light | 1 | 0.02 | 0.58 | 0.45 |
| pred : warm | 2 | 0.04 | 0.58 | 0.56 |
| pred : light | 2 | 0.18 | 2.57 | 0.091 |
| warm : light | 1 | 0.05 | 1.36 | 0.25 |
| pred : warm : light | 2 | 0.3 | 4.3 | 0.021 |
| residuals | 36 | 1.28 |
Table 2.
For the Coc. septempunctata treatment only (16 experimental units), interactions between night warming and light. (Statistical analyses were performed on the log10 abundance of pea aphids at the end of the 9-day experiment.)
| d.f. | SS (type II) | F | p | |
|---|---|---|---|---|
| warming | 1 | 0.14 | 2.6 | 0.13 |
| light | 1 | 1.79 | 34.5 | 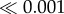 |
| warm : light | 1 | 0.64 | 12.3 | 0.004 |
| residuals | 12 | 0.62 |
Figure 2.
Effects of light level on aphid consumption by the two predators during laboratory feeding trials. Predators were allowed to forage for 20 aphids for 1 h with three different light levels (none, low or high). The number of aphids consumed by Col. maculata (closed circles) was not affected by light level. However, the number of aphids consumed by Coc. septempunctata (open circles) increased with light level. Each point is the number of aphids consumed during one of 13 replicated trials. Statistics are presented in the electronic supplementary material, table S1.
(b). Covariation in night warming and light pollution
Across the contiguous USA, monthly minimum temperatures have increased in 82% of the area, with 55% increasing more rapidly than 0.1°C decade−1 (table 3a). In croplands, night warming has been slightly less extensive (80% of all croplands, 47% with >0.1°C decade−1, table 3b). For light pollution, 49.6%, 10.8% and 2.6% of area had light levels equivalent to 2.5–10%, 10–100% and >100% full moon levels, and for cropland the values were 60.9%, 12.2% and 0.86% respectively. Thus, cropland had greater area of moderate light pollution (2.5–10% and 10–100% full moonlight) than the landscape in general, but smaller area of strong light pollution (>100% full moonlight). This pattern of large areas of moderate light pollution was even stronger in alfalfa fields, with 66.9% and 17.4% having light pollution equivalent to 2.5–10% and 10–100% full moonlight (table 3c).
Table 3.
Across the (a) contiguous USA, (b) US cropland, and (c) US alfalfa (lucerne) fields, the percentages for different classes of combined night-time temperature change and light pollution; the expected values if there were no covariance are given in parentheses. (The rows show different classes of night-time temperature change (°C decade−1), while the columns indicate different degrees of light pollution (nanowatts cm−2 sr−1).)
| <0.25 | 0.25–1 | 1–10 | >10 | row total | |
|---|---|---|---|---|---|
| (a) | |||||
| −0.90–0 | 8.2 (6.5) | 7.7 (8.8) | 1.5 (1.9) | 0.36 (0.47) | 17.66 |
| 0–0.10 | 9.1 (10.0) | 14.2 (13.5) | 3.1 (2.9) | 0.69 (0.72) | 27.14 |
| 0.10–0.25 | 11.3 (13.5) | 19.7 (18.1) | 4.5 (3.9) | 1.1 (1.0) | 36.49 |
| 0.25–1.20 | 8.5 (6.9) | 8.0 (9.3) | 1.7 (2.0) | 0.52 (0.49) | 18.72 |
| column total | 37.01 | 49.56 | 10.80 | 2.64 | |
| (b) | |||||
| −0.90–0 | 8.0 (5.2) | 10.3 (12.2) | 1.6 (2.4) | 0.12 (0.17) | 20.0 |
| 0–0.10 | 8.8 (8.3) | 19.2 (19.5) | 3.7 (3.9) | 0.25 (0.28) | 32.1 |
| 0.10–0.25 | 6.9 (9.5) | 24.0 (19.5) | 5.2 (4.4) | 0.35 (0.31) | 36.4 |
| 0.25–1.20 | 2.3 (3.0) | 7.4 (7.0) | 1.6 (1.4) | 0.13 (0.10) | 11.5 |
| column total | 26.0 | 60.9 | 12.2 | 0.86 | |
| (c) | |||||
| −0.90–0 | 3.55 (2.11) | 8.70 (9.61) | 2.00 (2.50) | 0.10 (0.14) | 14.36 |
| 0–0.10 | 3.48 (3.50) | 16.12 (16.00) | 4.10 (4.17) | 0.18 (0.24) | 23.89 |
| 0.10–0.25 | 3.99 (5.76) | 27.66 (26.24) | 7.25 (6.84) | 0.33 (0.39) | 39.23 |
| 0.25–1.20 | 3.64 (3.30) | 14.41 (15.07) | 4.09 (3.93) | 0.39 (0.23) | 22.52 |
| column total | 14.67 | 66.90 | 17.44 | 1.00 | |
To describe the pattern of co-occurrence in night warming and light pollution, we compared the expected proportion of area in each warming and light category to the observed proportions (table 3). For the contiguous USA (figure 3c), there was a slight positive co-occurrence, with the observed (expected) percentage of area for the highest categories (temperature increase >0.25°C decade−1, light >100% full moonlight) of 0.52% (0.49%). For areas with temperature increase >0.1°C decade−1 and light >10% full moonlight, the observed and expected values were 7.8% (7.4%), or roughly 5% more than expected. For croplands (figure 3b), the corresponding values were 0.13% (0.10%) and 7.3% (6.3%), which correspond to increases over the expected by 30% and 15% respectively. For alfalfa (figure 3a), the values were 0.39% (0.23%) and 12.06% (11.39%), which corresponds to increases over the expected by 70% and 6% respectively.
Although the areas with larger increases in night temperatures (>0.1 or 0.25°C decade−1) and light pollution (>10% or 100% full moonlight) are still relatively small compared to the total contiguous USA, US cropland and US alfalfa, our results show that there is considerable co-occurrence in these two environmental drivers. In the context of our field experiment on pea aphids and ladybeetle predators, this co-occurrence could be important: for Coc. septempunctata, the visual predator, high light levels were required for an effect of higher temperatures on night-time foraging, and in alfalfa co-occurrence between night warming and light pollution increases the area of highest warming and greatest light pollution by 70%. These two factors probably cause a non-additive effect on Coc. septempunctata predation, leading to much lower densities of pea aphids in our experimental cages. To illustrate this effect (with the caveat that our small-scale experiments used high ladybeetle densities and did not include many other factors that can also affect pea aphid populations), we note that the experimental increase in night temperature (2.1°C) corresponds roughly to the predicted change of >0.25°C decade−1 between 1949 and 2030 (>2°C), and that our simulated light pollution treatment increased levels by >100% of moonlight. In this combination of night temperature and light, Coc. septempunctata reduced pea aphid population growth to near zero, implying strong top-down population control of this pest. Assuming further that night temperatures and light pollution only affect Coc. septempunctata at these high levels, the positive co-occurrence of night warming and light pollution would increase the total area of effective top-down control of pea aphids in US alfalfa by 70%.
The key point in this simple illustration is that, if the effects of night temperature and light pollution were additive, then the non-independent co-occurrence patterns of night-time warming and light pollution would have no effect. This is a consequence of the fact that, if xi and yj are additive responses to levels i and j of environmental factors x and y, then the average response to both environmental factors is 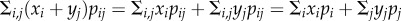 , where pij is the joint distribution of the two environmental factors, and pi and pj are their respective marginal distributions. In words, the combined effect of both environmental factors is the sum of their independent effects, regardless of whether they covary. But because there is a non-additive effect of night-time warming and light pollution on the interactions between pea aphids and Coc. septempunctata, the co-occurrence of the two anthropogenic changes is important: the co-occurrence patterns scale with the magnitude of non-additive effects of environmental factors on ecological systems.
, where pij is the joint distribution of the two environmental factors, and pi and pj are their respective marginal distributions. In words, the combined effect of both environmental factors is the sum of their independent effects, regardless of whether they covary. But because there is a non-additive effect of night-time warming and light pollution on the interactions between pea aphids and Coc. septempunctata, the co-occurrence of the two anthropogenic changes is important: the co-occurrence patterns scale with the magnitude of non-additive effects of environmental factors on ecological systems.
4. Conclusion
Our results showed a clear non-additive interaction between night-time warming and light pollution on Coc. septempunctata suppression of pea aphid densities. The mechanism behind this interaction is that Coc. septempunctata cannot take advantage of higher night temperatures in the dark ([16,28], this study). The absence of a non-additive effect for Col. maculata emphasizes that the non-additive effect of multiple environmental drivers may vary among species, depending on their natural history. It is important to note that our experimental light pollution treatments were at the far high end of real light pollution levels and would be within the >100% full moonlight category. As noted elsewhere, the literature is plagued by inconsistent measures of moonlight and ‘a definitive publication is needed of long-term ‘typical’ values of moonlit nights’ which would help ecologists construct realistic experiments [37]. However, global change studies commonly rely on treatments with larger magnitudes than observed or predicted in order to detect effects at reasonable sample sizes and time scales [48]. While this study has taken an important first step in evaluating the interactive effects of light and warming, a laudable next step would be to evaluate these factors under more realistic levels. An additional limitation of our study is that we have focused entirely on the top-down control of aphids, but light pollution and warming could also have bottom-up effects. While our factorial experimental design allowed us to test our hypotheses about changes to top-down control, future studies may benefit by integrating the bottom-up effects of light pollution and warming as well.
In general, not enough is known about either the visual sensitivity of insect predators or their behavioural reliance on vision for night foraging. Additionally, it may be difficult to separate the effects of light on a given predator's circadian rhythm as opposed to hunting efficiency, as some species do have a diurnal or crepuscular circadian rhythm, which may be affected by high levels of light pollution [49]. This will be an important consideration for future work, especially given the general pattern of night-time dominated climate warming, as the interactions between warming and circadian rhythms is poorly understood [33]. Nonetheless, given the large number of predatory beetle species and the occurrence of night-adapted superposition compound eyes in at least some of them (e.g. fireflies), we suspect that many insect predators are aided by anthropogenic light at night.
More generally, non-additive effects from multiple environmental changes will probably affect many species, trophic interactions and ecosystems. Whenever there are non-additive responses to multiple environmental changes, it is also necessary to know how these changes covary. Non-additive effects make predictions about species responses to environmental changes difficult, because the response to multiple environmental factors cannot be predicted by studying each separately. Similarly, non-independent distributions of environmental changes can amplify or dampen the non-additive species responses; therefore, making predictions requires information about the covariation between environmental changes. Non-independent changes in environmental factors, and non-additive effects on species, are probably the rule rather than the exception, yet there are few studies (e.g. [11,50]) that explicitly consider multiple environmental drivers on trophic systems and how they may covary. Thus, the results of this study, and paucity of existing literature, demonstrate that much work remains to be done if we are to accurately predict how ecological interactions will change in the face of anthropogenic-driven environmental changes.
Supplementary Material
Supplementary Material
Acknowledgements
We thank J. Alfonso, J. Breuer, E. Green, W. Matzke and H. Miller for assistance with fieldwork and E. Spalding and G. Ervin for assistance quantifying our light treatments.
Ethics
All work presented here was in compliance with the University of Wisconsin's policies on insect research.
Data accessibility
Data collected during this study are available on Dryad: http://dx.doi.org/10.5061/dryad.h7k26 [51]. Script for analysing remote sensing data and creating maps were written in Python and ran in a Linux environment, and are included as the electronic supplemental material.
Authors' contributions
B.T.B., L.Z., V.C.R., K.M.O., J.P.H. and A.R.I. designed the research; C.R.M., B.T.B. and L.Z. performed the research; B.T.B., L.Z. and A.R.I. analysed data; C.R.M., B.T.B., L.Z., V.C.R., K.M.O., J.P.H. and A.R.I. wrote the paper.
Competing interests
We have no competing interests.
Funding
This work was funded by NSF-DEB-Dimensions 1240804 to A.R.I., J.P.H., K.M.O. and V.C.R.; the NASA Biodiversity and Ecological Forecasting program; a University of Wisconsin-Madison Department of Integrative Biology Undergraduate Research grant to C.R.M.; and a Welton Summer Sophomore Apprenticeship grant to C.R.M.
References
- 1.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 2.Hoover SE, Tylianakis JM. 2012. Species interactions. In Behavioural responses to a changing world: mechanisms and consequences (eds Candolin U, Wong BB), pp. 138–139. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Jamieson MA, Trowbridge AM, Raffa KF, Lindroth RL. 2012. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 160, 1719–1727. ( 10.1104/pp.112.206524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunderson AR, Armstrong EJ, Stillman JH. 2016. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Annu. Rev. Mar. Sci. 8, 357–378. ( 10.1146/annurev-marine-122414-033953) [DOI] [PubMed] [Google Scholar]
- 5.Rosenblatt AE, Schmitz OJ. 2014. Interactive effects of multiple climate change variables on trophic interactions: a meta-analysis. Clim. Chang. Resp. 1, 8 ( 10.1186/s40665-014-0008-y) [DOI] [Google Scholar]
- 6.Laws AN, Joern A. 2015. Predator–prey interactions are context dependent in a grassland plant–grasshopper–wolf spider food Chain. Environ. Entomol. 44, 519–528. ( 10.1093/ee/nvv033) [DOI] [PubMed] [Google Scholar]
- 7.Rosenblatt AE, Smith-Ramesh LM, Schmitz OJ. In press. Interactive effects of multiple climate change variables on food web dynamics: modeling the effects of changing temperature, CO2, and water availability on a tri-trophic food web. Food Webs. ( 10.1016/j.fooweb.2016.10.002) [DOI] [Google Scholar]
- 8.Scherber C. 2015. Insect responses to interacting global change drivers in managed ecosystems. Curr. Opin. Insect Sci. 11, 56–62. ( 10.1016/j.cois.2015.10.002) [DOI] [PubMed] [Google Scholar]
- 9.Gaston KJ, Duffy JP, Gaston S, Bennie J, Davies TW. 2014. Human alteration of natural light cycles: causes and ecological consequences. Oecologia 176, 917–931. ( 10.1007/s00442-014-3088-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benton MJ. 2009. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732. ( 10.1126/science.1157719) [DOI] [PubMed] [Google Scholar]
- 11.Holdo RM, Holt RD, Fryxell JM. 2009. Opposing rainfall and plant nutritional gradients best explain the wildebeest migration in the Serengeti. Am. Nat. 173, 431–445. ( 10.1086/597229) [DOI] [PubMed] [Google Scholar]
- 12.Smith DM, Murphy JM. 2007. An objective ocean temperature and salinity analysis using covariances from a global climate model. J. Geophys. Res. Oceans 112, C02022 ( 10.1029/2005jc003172) [DOI] [Google Scholar]
- 13.Turner MG, Gardner RH. 2015. Landscape ecology in theory and practice, 2nd edn New York, NY: Springer. [Google Scholar]
- 14.Chown SL, Gaston KJ. 2015. Macrophysiology–progress and prospects. Funct. Ecol. 30, 330–334. ( 10.1111/1365-2435.12510) [DOI] [Google Scholar]
- 15.Neil K, Wu J. 2006. Effects of urbanization on plant flowering phenology: a review. Urban Ecosyst. 9, 243–257. ( 10.1007/s11252-006-9354-2) [DOI] [Google Scholar]
- 16.Harmon JP, Losey JE, Ives A. 1998. The role of vision and color in the close proximity foraging behavior of four coccinellid species. Oecologia 115, 287–292. ( 10.1007/s004420050518) [DOI] [PubMed] [Google Scholar]
- 17.Barton BT, Ives AR. 2014. Direct and indirect effects of warming on aphids, their predators, and ant mutualists. Ecology 95, 1479–1484. ( 10.1890/13-1977.1) [DOI] [PubMed] [Google Scholar]
- 18.Eigenbrode SD, Davis TS, Crowder DW, Björkman C, Niemelä P. 2015. Climate change and biological control in agricultural systems: principles and examples from North America. In Climate change and insect pests (eds Bjorkman C, Niemala P), pp. 119–135. Wallingford, UK: CABI. [Google Scholar]
- 19.Gaston KJ, Bennie J, Davies TW, Hopkins J. 2013. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. 88, 912–927. ( 10.1111/brv.12036) [DOI] [PubMed] [Google Scholar]
- 20.Sanders D, Kehoe R, Tiley K, Bennie J, Cruse D, Davies TW, van Veen FF, Gaston KJ. 2015. Artificial nighttime light changes aphid-parasitoid population dynamics. Sci. Rep. 5, 15232 ( 10.1038/srep15232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennie J, Davies TW, Cruse D, Inger R, Gaston KJ. 2015. Cascading effects of artificial light at night: resource-mediated control of herbivores in a grassland ecosystem. Phil. Trans. R. Soc. B 370, 20140131 ( 10.1098/rstb.2014.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton BT, Ives AR. 2014. Species interactions and a chain of indirect effects driven by reduced precipitation. Ecology 95, 486–494. ( 10.1890/13-0044.1) [DOI] [PubMed] [Google Scholar]
- 23.Cardinale BJ, Harvey CT, Gross K, Ives AR. 2003. Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 6, 857–865. ( 10.1046/j.1461-0248.2003.00508.x) [DOI] [Google Scholar]
- 24.Ives AR, Schooler SS, Jagar VJ, Knuteson SE, Grbic M, Settle WH. 1999. Variability and parasitoid foraging efficiency: a case study of pea aphids and Aphidius ervi. Am. Nat. 154, 652–673. ( 10.1086/303269) [DOI] [PubMed] [Google Scholar]
- 25.Mack T, Smilowitz Z. 1982. Using temperature-mediated functional response models to predict the impact of Coleomegilla maculata (DeGeer) adults and 3rd-instar larvae on green peach aphids. Environ. Entomol. 11, 46–52. ( 10.1093/ee/11.1.46) [DOI] [Google Scholar]
- 26.Xia J, Van der Werf W, Rabbinge R. 1999. Temperature and prey density on bionomics of Coccinella septempunctata (Coleoptera: Coccinellidae) feeding on Aphis gossypii (Homoptera: Aphididae) on cotton. Environ. Entomol. 28, 307–314. ( 10.1093/ee/28.2.307) [DOI] [Google Scholar]
- 27.Rauf M, Khan J, Rehman A, Gillani WA, Ali A. 2013. Biology and predatory potential of Coccinella septempunctata Linn. on Schizaphis graminum aphid under controlled conditions. Pak. J. Agricult. Res. 26, 124–129. [Google Scholar]
- 28.Nakamuta K. 1984. Visual orientation of a ladybeetle, Coccinella septempunctata L, (Coleoptera: Coccinellidae), toward its prey. Appl. Entomol. Zool. 19, 82–86. ( 10.1303/aez.19.82) [DOI] [Google Scholar]
- 29.Karl TR, Kukla G, Razuvayev VN, Changery MJ, Quayle RG, Heim RR, Easterling DR, Fu CB. 1991. Global warming: evidence for asymmetric diurnal temperature change. Geophys. Res. Lett. 18, 2253–2256. ( 10.1029/91GL02900) [DOI] [Google Scholar]
- 30.Schatz J, Kucharik CJ. 2015. Urban climate effects on extreme temperatures in Madison, Wisconsin, USA. Environ. Res. Lett. 10, 094024 ( 10.1088/1748-9326/10/9/094024) [DOI] [Google Scholar]
- 31.Impacts WIOCC. 2011. Wisconsin's changing climate: impacts and adaptation. Nelson Institute for Environmental Studies, University of Wisconsin-Madison, and Wisconsin Department of Natural Resources, Madison, USA.
- 32.Kutz S, Hoberg EP, Polley L, Jenkins E. 2005. Global warming is changing the dynamics of Arctic host–parasite systems. Proc. R. Soc. B 272, 2571–2576. ( 10.1098/rspb.2005.3285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speights CJ, Harmon JP, Barton BT. 2017. Contrasting the potential effects of daytime versus nighttime warming on insects. Curr. Opin. Insect Sci. 35, 792–804. ( 10.1016/j.cois.2017.06.005) [DOI] [PubMed] [Google Scholar]
- 34.Pachauri RK, et al. 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.
- 35.Gaston KJ, Visser ME, Hölker F. 2015. The biological impacts of artificial light at night: the research challenge. Phil. Trans. R. Soc. B 370, 20140133 ( 10.1098/rstb.2014.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murrell EG, Barton BT. 2017. Warming alters prey density and biological control in conventional and organic agricultural systems. Integr. Comp. Biol. 57, icx006. [DOI] [PubMed] [Google Scholar]
- 37.Kyba C, Mohar A, Posch T. 2017. How bright is moonlight? Astron. Geophys. 58, 1.31–31.32. ( 10.1093/astrogeo/atx025) [DOI] [Google Scholar]
- 38.Boryan C, Yang Z, Mueller R, Craig M. 2011. Monitoring US agriculture: the US department of agriculture, national agricultural statistics service, cropland data layer program. Geocarto Int. 26, 341–358. ( 10.1080/10106049.2011.562309) [DOI] [Google Scholar]
- 39.Han W, Yang Z, Di L, Mueller R. 2012. CropScape: a web service based application for exploring and disseminating US conterminous geospatial cropland data products for decision support. Comput. Electron. Agric. 84, 111–123. ( 10.1016/j.compag.2012.03.005) [DOI] [Google Scholar]
- 40.Maurer E, Wood A, Adam J, Lettenmaier D, Nijssen B. 2002. A long-term hydrologically based dataset of land surface fluxes and states for the conterminous United States. J. Clim. 15, 3237–3251. ( 10.1175/1520-0442(2002)015%3C3237:ALTHBD%3E2.0.CO;2) [DOI] [Google Scholar]
- 41.Elvidge CD, Baugh KE, Zhizhin M, Hsu F-C. 2013. Why VIIRS data are superior to DMSP for mapping nighttime lights. Proc. Asia-Pacific Adv. Netw. 35, 62–69. ( 10.7125/APAN.35.7) [DOI] [Google Scholar]
- 42.Doll CH, Muller J.-P, Elvidge CD. 2000. Night-time imagery as a tool for global mapping of socioeconomic parameters and greenhouse gas emissions. AMBIO 29, 157–162. ( 10.1579/0044-7447-29.3.157) [DOI] [Google Scholar]
- 43.Autrum H, et al. 1979. Apposition and superposition eyes. In Comparative physiology and evolution of vision in invertebrates (ed. Autrum H.), pp. 441–502. Berlin, Germany: Springer. [Google Scholar]
- 44.Warrant E, Dacke M. 2011. Vision and visual navigation in nocturnal insects. Ann. Rev. Entomol. 56, 239–254. ( 10.1146/annurev-ento-120709-144852) [DOI] [PubMed] [Google Scholar]
- 45.Kelber A. 2002. Pattern discrimination in a hawkmoth: innate preferences, learning performance and ecology. Proc. R. Soc. Lond. B 269, 2573–2577. ( 10.1098/rspb.2002.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin J-T. 1993. Identification of photoreceptor locations in the compound eye of Coccinella septempunctata Linnaeus (Coleoptera, Coccinellidae). J. Insect. Physiol. 39, 555–562. ( 10.1016/0022-1910(93)90037-R) [DOI] [Google Scholar]
- 47.Agee HR, Mitchell ER, Flanders R. 1990. Spectral sensitivity of the compound eye of Coccinella septempunctata (Coleoptera: Coccinellidae). Ann. Entomol. Soc. Am 83, 817–819. ( 10.1093/aesa/83.4.817) [DOI] [Google Scholar]
- 48.Newman JA, Gedalof ZE, Hunt SL, Anand M, Henry HA. 2011. Climate change biology. Wallingford, UK; Cambridge, MA: CAB Intl. [Google Scholar]
- 49.Nakamuta K. 1987. Diel rhythmicity of prey-search activity and its predominance over starvation in the lady beetle, Coccinella septempunctata bruckii. Physiol. Entomol. 12, 91–98. ( 10.1111/j.1365-3032.1987.tb00727.x) [DOI] [Google Scholar]
- 50.Couture JJ, Meehan TD, Kruger EL, Lindroth RL. 2015. Insect herbivory alters impact of atmospheric change on northern temperate forests. Nat. Plants 1, 15016 ( 10.1038/nplants.2015.16) [DOI] [PubMed] [Google Scholar]
- 51.Miller CR, Barton BT, Zhu L, Radeloff VC, Oliver KM, Harmon JP, Ives AR. 2017. Data from: Combined effects of night warming and light pollution on predator–prey interactions Data Dryad Repository. ( 10.5061/dryad.h7k26) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Miller CR, Barton BT, Zhu L, Radeloff VC, Oliver KM, Harmon JP, Ives AR. 2017. Data from: Combined effects of night warming and light pollution on predator–prey interactions Data Dryad Repository. ( 10.5061/dryad.h7k26) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data collected during this study are available on Dryad: http://dx.doi.org/10.5061/dryad.h7k26 [51]. Script for analysing remote sensing data and creating maps were written in Python and ran in a Linux environment, and are included as the electronic supplemental material.