Abstract
Genitalia are morphologically variable across many taxa and in physical contact during intromission, but little is known about how variation in form correlates with function during copulation. Marine mammals offer important insights into the evolutionary forces that act on genital morphology because they have diverse genitalia and are adapted to aquatic living and mating. Cetaceans have a fibroelastic penis and muscular vaginal folds, while pinnipeds have a baculum and lack vaginal folds. We examined copulatory fit in naturally deceased marine mammals to identify anatomical landmarks in contact during copulation and the potential depth of penile penetration into the vagina. Excised penises were artificially inflated to erection with pressurized saline and compared with silicone vaginal endocasts and within excised vaginas in simulated copulation using high-resolution, diffusible iodine-based, contrast-enhanced computed tomography. We found evidence suggestive of both congruent and antagonistic genital coevolution, depending on the species. We suggest that sexual selection influences morphological shape. This study improves our understanding of how mechanical interactions during copulation influence the shape of genitalia and affect fertility, and has broad applications to other taxa and species conservation.
Keywords: copulatory fit, genitalia, functional morphology, sexual selection, marine mammal, dice-CT
1. Introduction
Male genitalia are known to be one of the most phenotypically diverse and rapidly evolving morphological structures across taxa [1–3]. The extensive variation in male intromittent organ morphology is largely attributed to mechanisms of post-copulatory sexual selection [4–6]. The shape or size of an intromittent organ can influence a male's ability to deposit sperm and secure paternity [7–10]. Although female genitalia have received comparatively sparse attention [11,12], recent studies have revealed that female reproductive anatomy is more variable and evolves more rapidly than previously expected [13–17]. As the mechanical contact of genitalia during copulation is the most direct evolutionary interaction between the sexes, male and female genitalia are expected to coevolve together [6]. While it may seem intuitive that the penis fits inside the vagina, little is known about which features of male and female genitalia interact and how these interactions affect fertility.
The biomechanics and details of the anatomical fit of genitalia during intromission can be quite complex and have seldom been explored, particularly in vertebrates. However, such data can inform which aspects of genitalia may be under direct selection because of their mechanical function and yield information on the evolutionary pressures that have led to their diversification. In a few recent papers on arthropods, copulating males and females were flash-frozen in liquid nitrogen, and resulting micro-CT (computed tomography) scans or scanning electron microscopy images were used to examine how anatomical landmarks interacted between males and females (e.g. common fruit flies, Drosophila melanogaster [18]; tsetse flies, genus Glossina [19,20]; millipedes, Antichiropus variabilis [21]; grasshoppers, Melanoplus rotundipennis [22]; seed beetles, Callosobruchus maculatus [17]). These techniques have yielded evidence of both congruent and sexually antagonistic coevolution of genitalia. For example, the vaginal teeth of female common fruit flies interdigitated with male claspers, suggesting congruent coevolution, while the aedeagus tip perforated through and damaged the vaginal wall, indicating sexual conflict [18]. In seed beetles, the aedeagus spines contacted the thickest part of the female reproductive tract, suggesting antagonistic coevolution between males and females [17].
In vertebrates, copulatory fit was explored in anole lizards (Anolis carolinensis) by submerging them in liquid nitrogen during copulation [23], but this technique is not feasible for larger vertebrates. Model vaginas have been used in studies of live-trained captive waterfowl to visualize how the penis may function inside the female [24]. A technique was recently developed to inflate male penises directly inside the female reproductive tracts of plains garter snakes (Thamnophis sirtalis) and rats (Rattus rattus) before using micro-CT scans to visualize which features of male and female genitalia are in contact during simulated copulation [12]. These techniques demonstrate both a close fit between male and female genitalia [12,23] and an antagonistic mechanical interaction derived from sexual conflict, where female genital features act as physical barriers against penile eversion [24].
Here we expand the use of these techniques using marine mammals as a model system to explore genital interactions during simulated copulation. Aquatically mating mammals have extreme and unique copulatory adaptations influenced by sexual selection and environmental constraints. Cetaceans (whales, dolphins and porpoises) mate exclusively in aquatic environments and have unusual genital morphological characteristics shared only among cetartiodactyls (cetaceans and even-toed ungulates; [25]). The cetacean vagina contains muscular protrusions of the vaginal wall into the vaginal lumen that vary in number, shape, size and positioning across species [16]. These vaginal folds are of unknown function(s), although their morphological diversity may be driven by sexual selection [16]. In addition, the folds may serve as a mechanism to prevent seawater from entering the female reproductive tract, as seawater is lethal to sperm in at least one species of cetacean (common bottlenose dolphin, Tursiops truncatus; [26]).
The cetacean penis is fibroelastic; erectile tissue is filled with collagen and elastin fibres instead of the spongy tissue found in the vascular penis of most mammals [27]. Thus the cetacean penis is generally maintained in a turgid state within the body cavity and distends further with an influx of blood into the erectile tissue [27]. The penis in cetaceans does not have an inflatable terminal glans, but in some species it ends in a terminal cone (pars intrapreputialis; [27]; figure 1). In alpaca (Vicugna pacos), which are part of the group of terrestrial artiodactyls closely related to cetaceans, this terminal cone or tip can go through the cervix and reach directly into the uterus during copulation [28]. However, in cetaceans, it is not known whether the terminal cone or tip also penetrates through the cervix. The pinniped (seals, sea lions, fur seals and walruses) penis is vascular and not fibroelastic, and it contains a baculum (os penis bone) within its glans [29]. While pinnipeds lack the extensive vaginal folds unique to cetaceans and artiodactyls, they have a hymen (a membrane that covers all or part of the vaginal opening) that may function to prevent the incursion of seawater and debris into the reproductive tract [30].
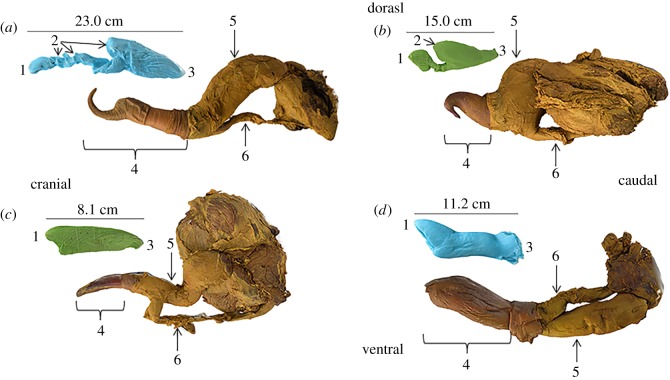
Figure 1.
Shape correspondence of male and female genitalia. The silicone vaginal endocast is lined up with the inflated penis tip in sexually mature (a) harbour porpoises (Phocoena phocoena), (b) common bottlenose dolphins (Tursiops truncatus), (c) short-beaked common dolphins (Delphinus delphis) and (d) harbour seals (Phoca vitulina). Male and female genital are aligned in the positioning and angle that would enable the deepest penile penetration. Anatomical landmarks identified on the vaginal endocast include the (1) proximate cervix, (2) vaginal folds and (3) vaginal opening. Anatomical landmarks identified on the penis include the (4) penis tip or glans to sheath, (5) penis shaft and (6) penis retractor muscle.
Our first objective was to determine the shape compatibility of marine mammal genitalia. Specifically, we assessed whether there is a close fit between the sexes and/or evidence that females may pose barriers to penile penetration by comparing species with and without conspicuous vaginal folds. Secondly, we examined the potential depth of penile penetration into the vagina, as it is unclear whether and how the penis navigates through complex vaginal folds, spirals, and recesses to deposit sperm deep in the female reproductive tract to facilitate fertilization. Specifically, we assessed the potential for the penis tip to reach though the cervix. Our third objective was to infer the copulatory position that would result in the best genital fit between the sexes. Finally, we aimed to develop a technique to assess copulatory fit in large vertebrates.
2. Material and methods
(a). Specimen collection
The excised reproductive tracts of naturally deceased marine mammals were collected opportunistically from marine mammal stranding networks along the US coastline. Intact reproductive tracts consisted of the external urogenital slit through to the caudal attachment of the root of the penis or crurae to the pelvis bones, and the external urogenital slit through to the ovaries, in males and females, respectively. Specimens were collected from fresh (less than 24 h post-mortem), sexually mature, male and female harbour porpoises (P. phocoena), common bottlenose dolphins (T. truncatus), short-beaked common dolphins (D. delphis), and harbour seals (P. vitulina). The species investigated are spontaneous ovulators [30,31]. Specimens were frozen immediately and shipped to necropsy facilities located at Mount Holyoke College. The one best quality male and female specimen within each species was selected for further analysis. While an attempt was made to pair males and females within a species to the same population, the opportunistic nature of specimen acquisition was a hindrance. However, Orbach et al. [32] did not find strong evidence of inter-population variation in dolphin vaginal morphology.
(b). Penis inflations
Each excised male reproductive tract was cleaned to remove extraneous tissue, photographed, measured and weighed. Photographs were collected using a Canon G16 powershot camera from a bird's-eye-view angle in sagittal (left and right) and coronal (anterior and posterior) planes. Measurements were collected from the distal limit of the pelvic bone to the distal penis tip in sagittal planes and from the sheath to penis tip (figure 1) in all planes. The penis was then filled to distention with pressurized saline to simulate erection. Saline was pressurized by pumping nitrogen into an 11.4 l Corco keg filled with physiological saline; fluid was injected into penile vascular spaces under variable pressure, depending on the resistance encountered during injection. In general, larger penises required higher pressure. Saline was pumped into the corpus cavernosa through the proximal ends of the crura using a 18 gauge needle connected to a 5 ml syringe. Injection continued until the tissue was turgid and backflow was achieved. Saline was next introduced into the corpus spongiosum by inserting the syringe on either side of the urethra until backflow of fluids was achieved. Penises were ligated distal to the crurae with cotton string to maintain turgidity. They were then re-measured, weighed and submersed in 10% buffered formalin to fix the tissue in its distended form.
(c). Copulatory fit and shape compatibility
Flaccid female reproductive tracts were frozen–thawed. Vaginal endocasts were made of the lumen of female reproductive tracts using Mold Star® 16 FAST or Elite HD™ light body dental silicone. The resulting silicone moulds were carefully extracted from the vaginas to prevent tearing of the mould or tissue. Duplicate moulds were made of the vaginal lumens to ensure shape consistency and identify potential artefacts (e.g. additional invaginations). As the lengths, shapes and positions of vaginal folds in the duplicate endocasts were consistent with the original endocast, the original endocast was used.
Each inflated formalin-fixed penis was inserted into the frozen–thawed flaccid vagina of a female of the same species as deeply as possible to simulate intromission. The alignment of the genitalia (e.g. ventral-to-ventral, dorsal-to-ventral) was determined based on the best shape compatibility of the vaginal endocast to the inflated penis tip (figure 1) without a priori consideration of natural copulatory positions, as intromission has not been observed in most species of marine mammals [33]. The interacting penis and vagina were then sewn together to prevent alignment shifts and formalin-fixed in 10% buffered formalin. The ‘copulating’ genitalia were submerged in a bucket of 10% iodine ethanol solution (resublimed, crystallized VWR iodine (BDH4620)) for two weeks following Gignac & Kley [34], and rotated daily to prevent iodine from precipitating. Diffusible, iodine-based, contrast-enhanced computed tomography (Dice-CT) substantially augments the ability of X-ray CT scans to define subtle differences in soft-tissue [35]. The specimens were positioned in sternal (anterior) recumbency on a standard CT-Table (Toshiba Aquilion 16 slice) at Tufts University Cummings School of Veterinary Medicine. Volume data of the specimens were acquired with slice thickness of 2.0 mm. Images were reconstructed in bone and soft-tissue algorithms in sagittal, transverse and coronal planes. Three-dimensional volume rendering protocols were generated using standard DICOM viewers (EFilm v. 3.4, Merge HealthCare, IBM and Carestream Health PACS 11.4).
In order to test how well the male and female genitalia fit together, we produced three-dimensional models of the penis and vaginal lumen (endocast) using Autodesk ReMake! (v.17.25.3.1). We collected up to 153 photographs per specimen using a Canon G16 powershot camera. The female models were trimmed to a standardized length using linear measurements derived from the CT scan volume reconstructions in sagittal plane (EFilm version 3.4, Merge HealthCare, IBM). Straight-line measurements were taken down the midline of the vaginal lumen from the cranial (deep) midpoint of the largest vaginal fold to the distal vaginal opening. Male models were cropped at the edge of the sheath. Models were exported as STL files for analysis in Geomagic Studio 2014. For each species, we imported the male and female models together and used the maximum volume overlap between the models to predict copulatory position. In harbour porpoises and common bottlenose dolphins, consideration was taken of the orientation needed for the penis tip to fit into the small lumen of the cranial vagina. For short-beaked common dolphins and harbour seals, the best fit orientation included apposition of the urethral opening and the cervix. The best fit orientation was assigned as 0° rotation. We then rotated the male penis model 90°, 180° and 270° from the 0° alignment, and adjusted the position to fit as well as possible each time. Finally, we quantified both the total volume overlap between the male and female models, and the percentage of the model that did not fit for each sex. In the harbour seal, the penis model was much larger than the vaginal endocast. Accordingly, we enlarged the endocast diameter to match the penis diameter, as vaginal tissue stretching would likely occur during copulation. Since male volumes protruded from the base of the female models by a variable amount in all species, the model fit is not comparable across species.
3. Results
We found a range of morphological variation in the genitalia of both sexes (electronic supplementary material, table S1). Male harbour porpoises (P. phocoena) have a long penis shaft and tip while females have complex vaginas consisting of a deep flap-like recess and a cranial series of spiralling vaginal folds (figure 1a). Male common bottlenose dolphin (T. truncatus) have a short penis shaft and tip while females have intermediate vaginal complexity, consisting of one large recess and no other folds (figure 1b). In both harbour porpoises and common bottlenose dolphins, there is a natural bend in the penis, which appears to correspond with kinks in the vaginal wall shape (figure 1). Male short-beaked common dolphins (D. delphis) have a terminal penis tip while females have one small vaginal fold and no large recesses (figure 1c). Male harbour seals (P. vitulina) have a baculum and blood vascular penis with a terminal glans while females lack vaginal folds but possess hymens. In all species, vaginal folds were consistently deepest (extended outwardly) on the dorsal side of the female.
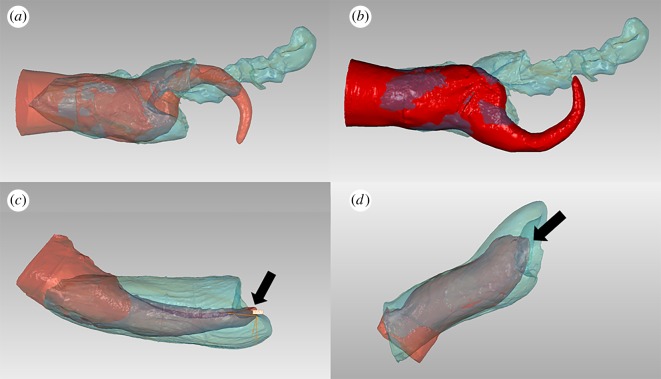
In all four species, male and female reproductive anatomy had the best shape correspondence and would enable the deepest penile penetration of both the shaft and penis tip in a dorsal penis to ventral vagina orientation (figures 2 and 3). Any deviation from this best fit orientation (at 90°, 180° or 270°) would result in the penis tip becoming trapped along the vaginal wall or in a higher percentage of male or female shapes not overlapping (electronic supplementary material, table S2). In harbour porpoises and common bottlenose dolphins, the shaft of the penis fits into the caudal large vaginal chamber and further penetration appears to be prevented by the prominent vaginal fold (figures 2 and 3). The penis tip must be positioned bending upwards to fit the narrowing cranial vaginal lumen and deposit sperm proximate to the egg (figure 2a). Thus harbour porpoises and common bottlenose dolphins seem to be examples of an antagonistic fit, where the female vaginal fold appears to act as a barrier to penile penetration. In short-beaked common dolphins and harbour seals, the two species with comparatively simple genitalia and lacking elongated penis tips, the best fit orientation included apposition of the male urethral opening with the female cervical opening, which was lost when models were rotated from 0° (figure 2c,d). Short-beaked common dolphins and harbour seals seem to exemplify a congruent fit due to the lack of apparent physical barriers (electronic supplementary material, table S2).
Figure 2.
Three-dimensional model fit of male (red) inside female (blue) genitalia: (a) The best fit in harbour porpoises (P. phocoena) mimics a dorsoventral copulatory position and includes the potential for the penis tip to continue moving cranially into the reduced vaginal lumen. (b) The harbour porpoise penis model has been rotated 180° within the female, demonstrating that a ventral–ventral copulatory position would prevent the penis tip from reaching the cranial vaginal lumen. (c) In short-beaked common dolphins (D. delphis) and in (d) harbour seals (P. vitulina), the penis can rotate freely around the vagina without physical barriers, although alterations from a dorsoventral copulatory position reduce the apposition between the urethral opening and the cervical opening. The arrows point to the urethral openings.
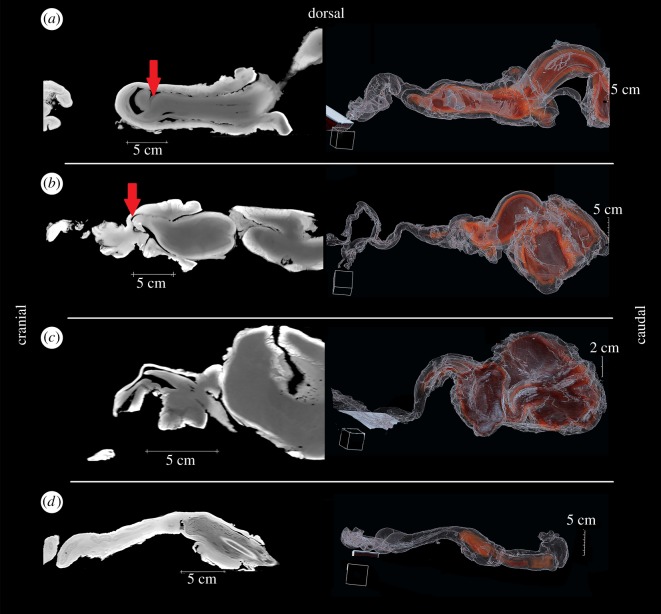
Figure 3.
Reconstructions of CT scan images of penises inside vaginas. Images are reconstructed in sagittal plane for: (a) harbour porpoises (P. phocoena), (b) common bottlenose dolphins (T. truncatus), (c) short-beaked common dolphins (D. delphis), and (d) harbour seals (P. vitulina). Images on the left are two-dimensional volume reconstructions using sharp lung and smooth soft tissue algorithms. The red arrows point to vaginal folds that prevent deeper penetration of the penis. Images on the right are three-dimensional volume rendered (VOLR) protocols. Erectile tissue is red while non-erectile tissue is transparent.
Reconstructions of CT scan data confirm that the penis shaft and tip comprise primarily erectile tissue (figure 3). In females, erectile tissue is limited to the clitoris. Only the portion of the penis distal to its sheath is able to penetrate the vagina. The tip of the penis did not penetrate the cervix in any species (figure 3). The CT scan data are congruent with the three-dimensional model rotation data. In harbour porpoises (figure 3a) and common bottlenose dolphins (figure 3b), the penis tip can be bent backwards by the largest vaginal fold, which protrudes into the vaginal lumen and prevents the penis shaft from penetrating deeper. In short-beaked common dolphins (figure 3c) and harbour seals (figure 3d), there is no evident physical barrier imposed by vaginal fold tissue.
4. Discussion
In the species examined, the genitalia of male and female marine mammals appear to have closely coevolved. The tip of the penis or shape of the terminal portion of the shaft and the lumen of the vagina strongly suggest morphological covariation. We suggest both congruent and antagonistic coevolution of male and female genitalia can occur among marine mammals. Congruent coevolution is apparent in short-beaked common dolphins (D. delphis) and harbour seals (P. vitulina), where the vagina did not present physical barriers to obstruct the penis, and the depth of penetration may be limited only by the length of the penis. The shape of the female lumen and the male penis are very similar, and both species lack a filiform penis tip. In contrast, the well-developed vaginal fold(s) in the harbour porpoise (P. phocoena) and common bottlenose dolphin (T. truncatus) seem to curtail the depth of penile penetration through physical obstructions, and therefore appear to be sexually antagonistic in nature. In these last two species, there is a conspicuous penis tip (clearly distinct from the shaft) that is bent at the level of the recess created by the largest vaginal fold.
Our technique resulted in full inflation of the penis shaft, but only partial inflation of the penis tip or cone. Therefore, in common bottlenose dolphins and harbour porpoises, it is possible that the distal filamentous penis tip may be able to navigate through the remaining cranial vaginal folds, as it appears to be under voluntary control in live cetaceans (D.N.O., personal observation). However, the penis tip does not appear to be long enough to penetrate the cervix as in some other artiodactyls [28], and therefore sperm ejaculated into the vagina would remain subject to the extensive chemical defences, anti-microbial defences and immune response mechanisms of the cervix [36]. Our data fail to support the functional hypothesis that that the presence of a baculum may help males enter the cervix, as the penis tip did not penetrate the cervix in harbour seals, consistent with the findings for rats (R. rattus; [12]).
Although we present data on only one representative male and female from four focal species, we believe our reported patterns are robust as clear inter-population variation was not found in the reproductive tract morphology of female common bottlenose dolphins [32]. We selected only the specimens in the best preservation state that were sexually mature. Additionally, endocast shapes are conserved within species, and inflated penis measurements and shapes closely correspond with those collected on live males (unpublished data). However, our small sample size is a limitation of this study, and future work examining genital coevolution with larger sample sizes would be fruitful.
In ducks, vaginal complexity is the consequence of sexual coercion by males that are able to use their long penises to quickly inseminate females despite female resistance [37]. Vaginal folds in cetaceans may similarly function as a mechanism of post-copulatory control of paternity. The extensive variation in vaginal fold morphology across cetacean species is not well-explained by phylogenetic or allometric patterns [16]. As cetaceans do not hold each other in place with appendages during copulation, and as females have the ability to evade males in a three-dimensional space, the body positioning of the male relative to the female during sexual approach may be a critical determinant of his fertilization success. Our data indicate the best genital shape correspondence and deepest possible penile penetration is a dorsal penis to ventral vagina orientation (figure 2). As female cetaceans are generally evasive of prospective mates [38], subtle shifts in body alignment during copulation may enable females to divert the penises of undesirable males into blind end vaginal recesses thereby creating longer distances and more obstacles for sperm to traverse to reach her egg. Thus vaginal folds may present females with a mechanism of cryptic female choice [39], as female cetaceans may incur substantial direct costs to resisting copulations including physical injury, harassment, lost foraging opportunities and infanticide [40–42].
Although mating has not been observed in most species of cetaceans and aquatically mating pinnipeds owing to the logistical challenges of opportunistically observing copulations (e.g. offshore habitats, submerged and out of view of observers; [33]), future studies that document the body alignment of free-swimming cetaceans during copulation are warranted to verify that our simulated copulatory positions reflect real copulatory patterns. In harbour porpoises and occasionally bottlenose dolphin, males have been observed hooking their penises around the female in a dorsal penis-to-ventral vagina orientation (D.N.O., 2016 unpublished data), consistent with our predictions for genital shape correspondence. Since sex can serve several non-conceptive functions among cetaceans (e.g. social learning, play, establishment of social bonds and dominance relationships; [43]), not all copulation events are expected to be associated with the optimal body alignment predicted for conceptive sexual intercourse. However, the techniques we developed to explore copulatory fit can be broadly applied to other taxa where copulations explicitly function in conception. Specifically, our techniques can help predict which natural copulations will lead to fertilization based on body alignment.
Our technique of inflating post-mortem penises and inserting them into vaginas revealed that marine mammal genitalia vary in their shape correspondence and in the possible mechanical interactions that may occur during non-simulated copulation. Other techniques to artificially inflate flaccid post-mortem intromittent organs have been developed for insects [44,45], snakes [12], alligators [46], armadillos [47], bats [48] and rats [12]. However, as the cetacean penis is fibroelastic rather than vascular, a novel inflation approach was necessary to overcome the resistance of dense collagenous and fibrous tissue. Our pressurized saline pump design can be used to explore functional genital morphology in males and genital coevolution in a variety of animals that have remained enigmatic due to the density of their erectile tissue, or to the large size of their intromittent organs. Our techniques may be particularly relevant to captive breeding programmes interested in species conservation. For example, the reconstructed CT scan images revealed that the penis shaft and tip pressed against the vaginal wall and that there were mechanical interactions between male and female anatomical landmarks (figure 3). It is possible that mechanical stimulation of particular landmarks of the vagina could enhance fertilization success. The development of vaginal endocasts to explore genital shape could be applied to build biomimetic vaginas that stimulate a higher volume or better quality ejaculate from males during semen collection procedures.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Linda Kinney for assistance with CT scans, Thomas Houle for provisioning us with a nitrogen tank, and Mount Holyoke College students (Mariesa Mazzone, Charlotte Knopp, Andrea Corbett, Pooja Arumugam and Camila Mirow) for assisting with dissections or making endocasts. Duncan Irshcick (Digital Life) kindly provided us with use of photography equipment. Dan Pulaski performed the rotational analysis of genital fit and Betsy Dumont shared her laboratory resources funded through NSF grant IOS 1354240. Two anonymous reviewers kindly provided insightful suggestions. We thank Alaska Veterinary Pathology Services (particularly Kathy Burek and the Prescott grant), International Fund for Animal Welfare (particularly Misty Niemeyer), Long Marine Lab Stranding Network (particularly Robin Dunkin), Oregon State University particularly (particularly Jim Rice), Virginia Aquarium Stranding Response Program (particularly Kristy Phillips), and the Whale Museum (particularly Jennifer Olson) for providing specimens.
Ethics
Specimens were collected under a National Marine Fisheries Services (NMFS) salvage permit letter to two of the authors (D.N.O. and P.L.R.B.).
Data accessibility
All data are included in the article or in the electronic supplementary material.
Authors' contributions
P.L.R.B. conceived the project idea. D.N.O. collected and dissected the reproductive tracts and prepared the endocasts. D.A.K. developed the pressurized saline pump. D.N.O., P.L.R.B. and D.A.K. inflated the penises. M.S. conducted the CT scans and generated the CT-3D models. D.N.O. analysed the data, wrote the initial manuscript and developed figures with input from all authors. All authors provided substantial edits on the final manuscript.
Competing interests
The authors declare they have no competing interests.
Funding
This work was supported by a faculty grant from Mount Holyoke College and the University of Massachusetts Amherst and from a Killam Postdoctoral Fellow research grant from Dalhousie University.
References
- 1.Eberhard WG. 1985. Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Eberhard WG. 2010. Rapid divergent evolution of genitalia. In The evolution of primary sexual characters in animals (eds Leonard J, Cordoba-Aguilar A), pp. 40–78. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Klaczko J, Ingram T, Losos J. 2015. Genitals evolve faster than other traits in Anolis lizards. J. Zool. 295, 44–48. ( 10.1111/jzo.12178) [DOI] [Google Scholar]
- 4.Hosken DJ, Stockley P. 2004. Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93. ( 10.1016/j.tree.2003.11.012) [DOI] [PubMed] [Google Scholar]
- 5.Simmons LW. 2014. Sexual selection and genital evolution. Aust. Entomol. 53, 1–17. ( 10.1111/aen.12053) [DOI] [Google Scholar]
- 6.Brennan PLR, Prum RO. 2015. Mechanisms and evidence of genital coevolution: the roles of natural selection, mate choice and sexual conflict. Cold Spring Harb. Perspect. Biol. 7, a017749 ( 10.1101/cshperspect.a017749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnqvist G, Danielsson I. 1999. Copulatory behavior, genital morphology, and male fertilization success in water striders. Evolution 53, 147–156. ( 10.2307/2640927) [DOI] [PubMed] [Google Scholar]
- 8.House CM, Simmons LW. 2003. Genital morphology and fertilization success in the dung beetle Onthophagus taurus: an example of sexually selected male genitalia. Proc. R. Soc. Lond. B 270, 447–455. ( 10.1098/rspb.2002.2266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takami Y. 2003. Experimental analysis of the effect of genital morphology on insemination success in the ground beetle Carabus insulicola (Coleoptera Carabidae). Ethol. Ecol. Evol. 15, 51–61. ( 10.1080/08927014.2003.9522690) [DOI] [Google Scholar]
- 10.Wenninger EJ, Averill AL. 2006. Influence of body and genital morphology on relative male fertilization success in oriental beetle. Behav. Ecol. 17, 656–663. ( 10.1093/beheco/ark013) [DOI] [Google Scholar]
- 11.Ah-King M, Barron AB, Herberstein ME. 2014. Genital evolution: why are females still understudied? PLoS Biol. 12, e1001851 ( 10.1371/journal.pbio.1001851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan PLR. 2016. Studying genital coevolution to understand intromittent organ morphology. Integr. Comp. Biol. 56, 669–681. ( 10.1093/icb/icw018) [DOI] [PubMed] [Google Scholar]
- 13.Puniamoorthy N, Kotrba M, Meier R. 2010. Unlocking the ‘Black box’: internal female genitalia in Sepsidae Diptera evolve fast and are species-specific. BMC Evol. Biol. 10, 275 ( 10.1186/1471-2148-10-275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Showalter I, Todd B, Brennan PLR. 2013. Intraspecific and interspecific variation of the vagina in two species of water snakes. Biol. J. Linn. Soc. Lond. 111, 183–191. ( 10.1111/bij.12184) [DOI] [Google Scholar]
- 15.Yassin A, Orgogozo V. 2013. Coevolution between male and female genitalia in the Drosophila melanogaster species subgroup. PLoS ONE 8, e57158 ( 10.1371/journal.pone.0057158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orbach DN, Marshall CD, Mesnick SL, Würsig B. 2017. Patterns of cetacean vaginal folds yield insights into functionality. PLoS ONE 12, e0175037 ( 10.1371/journal.pone.0175037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougherty LR, van Lieshout E, McNamara KB, Moschilla JA, Arnqvist G, Simmons LW. 2017. Sexual conflict and correlated evolution between male persistence and female resistance traits in the seed beetle Callosobruchus maculatus. Proc. R. Soc. B 284, 20170132 ( 10.1098/rspb.2017.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattei AL, Riccio ML, Avila FW, Wolfner MF. 2015. Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro-computed tomography scanning. Proc. Natl Acad. Sci. USA 112, 8475–8480. ( 10.1073/pnas.1505797112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briceño RD, Eberhard WG, Robinson AS. 2007. Copulation behaviour of Glossina pallidipes (Diptera: Muscidae) outside and inside the female, with a discussion of genitalic evolution. Bull. Entomol. Res. 97, 471–488. ( 10.1017/S0007485307005214) [DOI] [PubMed] [Google Scholar]
- 20.Briceño RD, Eberhard WG, Chinea-Cano E, Wegrzynek D, dos Santos Rolo T. 2016. Species-specific differences in the behavior of male tsetse fly genitalia hidden in the female during copulation. Ethol. Ecol. Evol. 28, 53–76. ( 10.1080/03949370.2014.1002114) [DOI] [Google Scholar]
- 21.Wojcieszek JM, Austin P, Harvey MS, Simmons LW. 2012. Micro-CT scanning provides insight into the functional morphology of millipede genitalia. J. Zool. 287, 91–95. ( 10.1111/j.1469-7998.2011.00892.x) [DOI] [Google Scholar]
- 22.Woller DA, Song H. 2017. Investigating the functional morphology of genitalia during copulation in the grasshopper Melanoplus rotundipennis (Scudder, 1878) via correlative microscopy. J. Morphol. 278, 334–359. ( 10.1002/jmor.20642) [DOI] [PubMed] [Google Scholar]
- 23.Conner J, Crews D. 1980. Sperm transfer and storage in the lizard, Anolis carolinensis. J. Morphol. 163, 331–348. ( 10.1002/jmor.1051630307) [DOI] [PubMed] [Google Scholar]
- 24.Brennan PLR, Clark C, Prum R. 2010. Explosive eversion and functional morphology of waterfowl penis supports sexual conflict in genitalia. Proc. R. Soc. B 277, 1309–1314. ( 10.1098/rspb.2009.2139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pabst DA, Rommel SA, McLellan WA. 1998. Evolution of thermoregulatory function in cetacean reproductive systems. In The emergence of whales: evolutionary patterns in the origin of Cetacea (ed. Thewissen JGM.), pp. 379–397. New York, NY: Springer. [Google Scholar]
- 26.Schroeder JP, Keller KV. 1989. Seasonality of serum testosterone levels and sperm density in Tursiops truncatus. J. Exp. Zool. 249, 316–321. ( 10.1002/jez.1402490310) [DOI] [PubMed] [Google Scholar]
- 27.Slijper EJ. 1966. Functional morphology of the reproductive system in cetacean. In Whales, dolphins, and porpoises (ed. Norris NS.), pp. 277–319. Berkeley, CA: University of California Press. [Google Scholar]
- 28.Bravo PW, Moscoso J, Ordonez C, Alarcon V. 1996. Transport of spermatozoa and ova in female alpaca. Anim. Reprod. Sci. 43, 173–179. ( 10.1016/0378-4320(95)01465-9) [DOI] [Google Scholar]
- 29.Harrison RJ, Matthews LH, Roberts JM. 1952. Reproduction in some pinnipedia. Trans. Zool. Soc. Lond. 27, 437–540. ( 10.1111/j.1096-3642.1952.tb00001.x) [DOI] [Google Scholar]
- 30.Atkinson S. 1997. Reproductive biology of seals. Rev. Reprod. 2, 175–194. ( 10.1530/ror.0.0020175) [DOI] [PubMed] [Google Scholar]
- 31.Kirby VL, Ridgway SH. 1984. Hormonal evidence of spontaneous ovulation in captive dolphins, Tursiops truncatus and Delphinus delphis. In Reproduction in whales, dolphins and porpoises (eds Perrin WF, Brownell RL Jr, DeMartin DP.), pp. 459–464. Cambridge, UK: Rep. Int. Whal. Comm. [Google Scholar]
- 32.Orbach DN, Marshall CD, Würsig B, Mesnick SL. 2016. Variation in female reproductive tract morphology of the common bottlenose dolphin (Tursiops truncatus). Anat. Rec. 299, 520–537. ( 10.1002/ar.23318) [DOI] [PubMed] [Google Scholar]
- 33.Lanyon JM, Burgess EA. 2014. Methods to examine reproductive biology in free-ranging, fully-marine mammals. In Reproductive sciences in animal conservation: progress and prospects (eds Holt WV, Brown JL, Comizzoli P), pp. 241–274. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 34.Gignac PM, Kley NJ. 2014. Iodine-enhanced micro-CT imaging: methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. J. Exp. Zool. B Mol. Dev. Evol. 322, 166–176. ( 10.1002/jez.b.22561) [DOI] [PubMed] [Google Scholar]
- 35.Gignac PM, et al. 2016. Diffusible iodine-based contrast-enhanced computed tomography (diceCT): an emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues . J. Anat. 228, 889–909. ( 10.1111/joa.12449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suarez SS, Pacey AA. 2006. Sperm transport in the female reproductive tract. Hum. Reprod. Update 12, 23–37. ( 10.1093/humupd/dmi047) [DOI] [PubMed] [Google Scholar]
- 37.Brennan PLR, Prum R. 2012. The limits of sexual conflict in the narrow sense: new insights from waterfowl biology. Phil. Trans. R. Soc. B 367, 2324–2338. ( 10.1098/rstb.2011.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orbach DN, Packard JM, Kirchner T, Würsig B. 2015. Evasive behaviours of female dusky dolphins (Lagenorhynchus obscurus) during exploitative scramble competition. Behaviour 152, 1953–1977. ( 10.1163/1568539X-00003310) [DOI] [Google Scholar]
- 39.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 40.Connor RC, Smolker RA. 1996. ‘Pop’ goes the dolphin: a vocalization male bottlenose dolphins produce during consortships. Behaviour 133, 643–662. ( 10.1163/156853996X00404) [DOI] [Google Scholar]
- 41.Scott EM, Mann J, Watson-Capps JJ, Sargeant BL, Connor RC. 2005. Aggression in bottlenose dolphins: evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-rake marks and behaviour. Behaviour 142, 21–44. ( 10.1163/1568539053627712) [DOI] [Google Scholar]
- 42.Watson JJ. 2005. Female mating behavior in the context of sexual coercion and female ranging behavior of bottlenose dolphins (Tursiops sp.) in Shark Bay, Western Australia. Ph.D. Thesis, Georgetown University, Washington, DC. [Google Scholar]
- 43.Mann J. 2006. Establishing trust: socio-sexual behaviour and the development of male–male bonds among Indian Ocean bottlenose dolphins. In Homosexual behaviour in animals: an evolutionary perspective (eds Sommer V, Vasey PL), pp. 107–130. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 44.Matthews M. 1998. The CSIRO vesica everter: a new apparatus to inflate and harden eversible and other weakly sclerotised structures in insect genitalia. J. Nat. Hist. 32, 317–327. ( 10.1080/00222939800770161) [DOI] [Google Scholar]
- 45.Hünefeld F, Brehm G, Pohl H. 2013. A simple ‘hands-off’ apparatus to inflate concealed soft parts of the genitalia of small insect specimens. Microsc. Res. Tech. 76, 258–262. ( 10.1002/jemt.22161) [DOI] [PubMed] [Google Scholar]
- 46.Kelly DA. 2013. Penile anatomy and hypotheses of erectile function in the American alligator (Alligator mississippiensis): muscular eversion and elastic retraction. Anat. Rec. 296, 488–494. ( 10.1002/ar.22644) [DOI] [PubMed] [Google Scholar]
- 47.Kelly DA. 1999. Expansion of the tunica albuginea during penile inflation in the nine-banded armadillo (Dasypus novemcinctus). J. Exp. Biol. 202, 253–265. [DOI] [PubMed] [Google Scholar]
- 48.Herdina AN, Kelly DA, Jahelková H, Lina PH, Horáček I, Metscher BD. 2015. Testing hypotheses of bat baculum function with 3D models derived from microCT. J. Anat. 226, 229–235. ( 10.1111/joa.12274) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the article or in the electronic supplementary material.