Abstract
Unravelling the multiple interacting drivers of host–pathogen coexistence is crucial in understanding how an apparently stable state of endemism may shift towards an epidemic and lead to biodiversity loss. Here, we investigate the apparent coexistence of the global amphibian pathogen Batrachochytrium dendrobatidis (Bd) with Bombina variegata populations in The Netherlands over a 7-year period. We used a multi-season mark–recapture dataset and assessed potential drivers of coexistence (individual condition, environmental mediation and demographic compensation) at the individual and population levels. We show that even in a situation with a clear cost incurred by endemic Bd, population sizes remain largely stable. Current environmental conditions and an over-dispersed pathogen load probably stabilize disease dynamics, but as higher temperatures increase infection probability, changing environmental conditions, for example a climate-change-driven rise in temperature, could unbalance the current fragile host–pathogen equilibrium. Understanding the proximate mechanisms of such environmental mediation and of site-specific differences in infection dynamics can provide vital information for mitigation actions.
Keywords: chytridiomycosis, demographic compensation, endemism, Europe, prevalence
1. Introduction
Emerging wildlife diseases are a key driver of global biodiversity loss (e.g. [1]). The impact of an infectious disease on a wildlife population is evident when the population crashes during a mass mortality event [2]. However, the impact on population persistence may be equally significant, but less obvious and more difficult to assess, when the pathogen has a long-term presence in the population [3]. Overall, the impact of wildlife diseases is highly variable and most often depends on a complex interplay between multiple host, pathogen and environmental factors [4].
Chytridiomycosis, caused by the amphibian chytrid fungus Batrachochytrium dendrobatidis (Bd) is a particularly relevant globally emerging wildlife disease, detected in more than 500 amphibian species [5]. Yet within this global context, there is no universal inter- or intraspecific response to Bd infection. In Australia and the Neotropics, chytridiomycosis outbreaks have led to species extinctions [6,7]. Conversely, many amphibian species across the globe persist in spite of recurrent or ongoing Bd infections. In Europe, Bd outbreaks have been limited in number and are variable in extent [8–10]. In particular, in northern Europe, Bd was shown to have been present at least since the late 1990s and is widespread, but mass mortalities and consistent negative effects on population trends have not been observed [11]. In northern Europe, Bd seems to have reached a state of endemism and the build-up of lethal Bd infection-loads is hypothesized to be precluded by unfavourable environmental conditions [11]. However, the true nature of this hypothesized coexistence and its driving mechanisms are not known.
Ecological theory and empirical evidence suggest a range of different mechanisms may facilitate coexistence. Firstly, the pathogen may not impact the hosts' survival or recruitment rate, instead behaving as a commensal [12]. Secondly, the pathogen may be parasitic but have compensatory, rather than additive, effects on the vital rates of individual hosts; that is, the individuals that die of disease would also have had higher mortality rates in the absence of the pathogen [13]. Alternatively, the parasitic pathogen may inflict additive mortality, but this is compensated at the host population level, for example through increased recruitment and a shift in age structure [4,14,15]. Understanding whether, and by what mechanisms, host populations truly coexist with a pathogen is crucial for predicting and eventually managing the potential impacts of a disease.
Here, we analyse a multiple-year dataset of Bd infection dynamics and demographic data in two populations (multiple-pond systems) of B. variegata, a locally endangered amphibian species hypothesized to coexist with Bd [16]. This dataset allowed us a rare possibility to study the drivers of coexistence in the absence of obvious mortality effects at the population level. Rather than focusing on a single potential coexistence mechanism, we simultaneously addressed the role of individual body condition, environmental mediation and demographic compensation in shaping individual- and population-level effects of Bd infection. We show that in our study system, despite potential negative effects at the individual level, coexistence at the population level is most probably maintained by environmental conditions and demographic compensation. Key to long-term population persistence is the maintenance of high-quality habitat to safeguard the compensatory mechanism as climate change and positive feedback between host demography and pathogen prevalence may threaten the stability of this equilibrium.
2. Material and methods
(a). Study species and area
The yellow-bellied toad (B. variegata) is a species of high conservation concern in the European Union [17]. The species exhibits slow development (toads usually become sexually mature in their third summer), high longevity (a lifespan of over 20 years) and relatively small clutch sizes for an amphibian (up to 130 eggs per female, deposited in several clutches over a prolonged period between April and September: [18] and references therein). In The Netherlands, the natural range of the yellow-bellied toad only covers the southernmost part of the country, where it occurs in seven isolated populations [19].
The magnitude of Bd-induced mortality for this species in the wild seems limited [16]. While prevalence varies between 4.5% and 32%, infection intensities are generally low [11,14,16,20–24]. Nonetheless, lethal chytridiomycosis has been observed in the Apennine (continental Italy) subspecies of yellow-bellied toad, B. variegata pachypus [25]. Also, Woodhams et al. [26] found that post-metamorphic survival of the yellow-bellied toad was substantially reduced after an infection experiment with the Swiss lineage Bd TG739. A confirmed chytridiomycosis disease outbreak in captive juvenile yellow-bellied toads shortly after metamorphosis resulted in a 50% mortality rate (15/30 animals died). Mortality ceased after initiation of antifungal treatment (F. Pasmans 2017, unpublished data).
We monitored two populations of yellow-bellied toads in The Netherlands over a 7-year period (2010–2016). The populations, relatively small but apparently stable, occupy two separate sites, ‘Groeve ’t Rooth’ (hereafter Rooth; 15.8 ha) and ‘Wahlwiller’ (3.9 ha), 11 km apart in a straight line. In 1982 six yellow-bellied toads were brought from Rooth to Wahlwiller [19]. Rooth is a marl quarry that has been actively operated since 1938, whereas Wahlwiller is a southern exposed hill slope with extensive agricultural activity (pasture and vineyard). Within the sites, there are respectively 35 and 19 artificial, irregularly maintained ponds, of which respectively 31 and 17 ponds are suitable for reproduction by yellow-bellied toads.
Between 2010 and 2016 we visited each site on one (2015) or more occasions (all other years except 2014 when no surveys were carried out), totalling up to 22 visits to Rooth and 24 visits to Wahlwiller. We caught and ‘marked’ each individual by photographing the unique belly pattern. We classified photographs using the program AMPHIDENT, an automatic algorithm to identify individuals on the basis of ventral spot patterns [27–29]. We classified each individual in one of four life stages: larvae, juveniles (metamorphosed toadlets before their first hibernation), sub-adults (stage between the first hibernation and breeding condition) and adults (sexually mature) [11]. In contrast to other studies [18], we found that the belly patterns of juvenile toads often changed considerably until the sub-adult life stage, making recognition possibly subject to error. Therefore, we compiled multi-season capture–recapture data only from the sub-adult life stage onwards. For the juvenile stages we compiled count data and mark–recapture data only for the single season of the first capture. Animals were also weighed to the nearest 0.1 g using a digital spoon scale (Konsait Electronic Spoon weight Scale, Stainless LCD Display 0.1 g/500 g) and snout to vent length was measured to the nearest millimetre using a regular ruler. We calculated body condition using the ‘scaled mass index’ (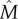 ), a method that takes account of the scaling between body components and body size [30].
), a method that takes account of the scaling between body components and body size [30].
We obtained a skin sample from every individual at each capture, using aluminium sterile cotton-tipped dry swabs (rayon-Dacron, COPAN, UNSPSC CODE 41104116) following the procedure and biosecurity measures described in Spitzen-van der Sluijs et al. [11]. All swabs tested were stored at −20°C until DNA extraction. For the quantification of Bd-DNA, we first extracted all swabs following the protocol of Boyle et al. [31]. Quantitative PCR was carried out with a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). Extractions were diluted 1/10 and all assays were performed in duplicate. Samples were considered positive for Bd when there was a clear log-linear amplification and both repeats were above the detection limit >0.1 mean genomic equivalents (GE), which for analyses we treated as mean infection intensity. Infection intensity was measured as GE/swab.
We obtained weather data from the weather station nearest to the sampling locations (Maastricht; 3 km (Rooth) and 14 km (Wahlwiller); Royal Netherlands Meteorological Institute, www.knmi.nl). For each sampling occasion we calculated daily mean temperature, the minimum temperature and the maximum temperature (all in 0.1°C) estimated over two periods: 7 and 30 days prior to each site visit [11]. We measured pond pH using HI 2211 pH/ORP Meter (Hanna® instruments, Temse, Belgium), and water temperature at each visit 1 m from the shore—or in the centre of the pond if the pond had a diameter smaller than 1 m—using an HI 98311 DiSTt5 EC/TDS/temperature tester (Hanna Instruments, Nieuwegein, The Netherlands) to the nearest 0.1°C repeatedly at the same position and depth (10 cm) at each site.
(b). Statistical analyses
To understand the mechanisms of coexistence between B. variegata and Bd, we estimated first the survival and transmission dynamics at the individual level and their individual and environmental predictors, and then survival, recruitment and Bd prevalence at the population level. To this end we modelled three datasets: (i) individual mark–recapture data of individuals older than 2 years (grouping together sub-adults and adults), using a Jolly–Seber model; (ii) individual mark–recapture data of juveniles during their first year, using a Cormack–Jolly–Seber model; and (iii) counts of juveniles for each year, using a state-space model.
(i). Adult mark–recapture data
We built an open-population Jolly–Seber model [32] with four states (‘not entered’, ‘alive, not infected’, ‘alive, infected’, ‘dead’). State transitions are described as survival (φ, allowed to differ between infected and non-infected individuals), probability of infection (ϑ, transition from non-infected to infected), probability of clearing infection (χ, transition from infected to non-infected), and probability of entry (γ, probability that a new individual enters the pool of individuals available for capture).
We began our analysis with a full model, in which the transition probabilities φ, ϑ and χ were estimated as a logistic regression of individual body condition, the mean minimum air temperature in the week before the survey—after preliminary analyses suggested this was the most supported predictor over mean and maximum temperatures, over 7 or 30 days prior to the survey—an interaction between those two covariates, and a site-specific fixed effect. We included temperature to reflect the potential influence of environmental conditions on infection dynamics [33,34], and body condition to assess whether the endemic infection was more likely to affect individuals with a lower body condition, as reflected by the transition probabilities. Since the body condition is unobserved every time an individual is not caught, we imputed missing values randomly from the lognormal distribution of observed values, after preliminary modelling suggested no time-, site-, sex- or individual-specific pattern in body conditions. Similarly, for infected individuals we included the infection load (log genomic equivalent) as a predictor of survival, assessing the possibility of pathogen overdispersion. We followed the same procedure as above for imputation of unobserved infection loads. We used uninformative priors for these three coefficients and centred all covariates.
We modelled the probability of entry (γ) to vary between the first and subsequent occasions every year. This reflects our belief that, given the marked isolation of sites is likely to prevent immigration, γ can be interpreted as recruitment only. Therefore, we expect entries to occur at the beginning of each sampling season: that is, sub-adults captured during a season are most likely to have entered at the beginning of that season (after their first hibernation). We expressed this belief using different prior distributions, respectively an uninformative uniform (0, 1) for the first occasion and an informative uniform (0, 0.05) for subsequent occasions in every year. We allowed the probability of individual detection p to vary temporally but not by site, since ponds are largely similar and effort identical, and to be independent of the infection state, since we observed no differences in behaviour or external features between infected and non-infected individuals.
We fitted the model in JAGS [35]. We ran each model for 50 000 iterations, after discarding the first 25 000 as a burn-in and applying a thinning rate of 10. We ran three Markov chains with overdispersed initial values and assessed convergence by visual inspection of the chain histories, and through the R-hat statistic. We progressively simplified the model by removing covariate predictors of φ, ϑ and χ for which the 95% credible intervals of the coefficient's posterior distribution encompassed zero [36].
(ii). Juvenile mark–recapture data
Although we found that individuals could not be tracked with certainty between their first and second year, we were still able to model individual mark–recaptures during their first year (between metamorphosis and before or just following their first hibernation). We fitted a Cormack–Jolly–Seber in JAGS, following the same procedure and predictors as for the adult data; the main difference being that the Cormack–Jolly–Seber model conditions on first capture and does not allow estimation of new entries and population sizes. We modelled the progressive change of belly patterns and effective loss of marking using an exponential decay function for the probability of individual recapture over time. In other words, the model estimated survival of juveniles within the first year accounting for the fact that as individuals grew after metamorphosis, it would become increasingly unlikely that their photographs could be matched to those of the previous captures.
(iii). Juvenile count data
Finally, we modelled the maximum number of juveniles counted at any occasion in every year as the outcome of a binomial observation process (to account for imperfect detection) and a Poisson fecundity process, where the mean fecundity rate per adult was modelled as a function of population density in the current year, using a log link. We assumed each female to lay between 40 and 70 eggs, with a maximum of 130, and a mean 10% survival from egg to metamorphosis [37,38]; the number of non-breeding females is compensated by the number of females that lay a second clutch, approximately 12% according to [38]. We ran the model in JAGS using the same settings and diagnostics described above.
(iv). Derived parameters
After obtaining the final versions of the three models above, we combined them to derive population-level parameters of interest. For the first survey occasion of every year we calculated the number of individuals in each age class. For juveniles, the age class size was estimated directly; for sub-adults and adults, by summing the number of individuals estimated to be alive in the respective latent states. Similarly, we calculated the prevalence of Bd infection as the proportion of the individuals alive at any given occasion that were in the latent state ‘infected’. We calculated adult survival as the proportion of adults alive at year t − 1 that were still alive at t; juvenile survival and recruitment as the number of new entries in the adult class on the first occasion of year t, divided respectively by the number of juveniles at year t − 1, and the number of adults alive on the first occasion of year t − 1; and population growth as the ratio of population sizes on the first occasions of years t and t − 1. All ratios were rescaled by the length of the intervals between first occasions (in days). We excluded the first survey of the study from the calculation of recruitment, since γ and p were not identifiable separately on the first occasion [36]. Finally, we calculated the density of adults by dividing the size of this class by the total area of each site, and the density of juveniles by dividing their estimated number by the total surface of water bodies at each site (reflecting the respective use of both terrestrial and aquatic habitats, for adults, and of water bodies and immediate surroundings only, for juveniles). We were not able to directly estimate the relationship between Bd patterns (infection, prevalence) and host density, the inclusion of which in the model led to poor fit and wide estimates; therefore, we were forced to limit our analysis to a visual assessment of the correspondence between host density and prevalence patterns.
3. Results
(a). Survey results
We made 1008 captures of 608 uniquely marked yellow-bellied toads (326 individuals in Rooth, and 282 in Wahlwiller; 400 adults, 97 sub-adults and 111 juveniles) throughout the study period, including multiple captures for 206 individuals. Forty individuals changed infection state at least once (became infected or recovered) and 11 changed state twice, with none recaptured in an infected state after recovery. The strongest infection intensity recorded prior to recovery was 3500 GE/swab, while the highest overall infection intensity was 29 600 GE/swab in a larva (second highest load: 26 800 in a juvenile toad). The observed prevalence of Bd in yellow-bellied toads (all age classes, both sites) fluctuated between years, ranging from 0.5% (in 2011) to 22.2% in 2012 and 2016. When observed over the entire study period, the younger life stages showed higher prevalence rates than the older life stages (larvae: 18.7%, juveniles: 40%, sub-adults: 5.5%; adults, 6.6%). The observed infection intensity varied by life stage and particularly by site, with maximum loads for adults and juveniles respectively one and two orders of magnitude greater at Rooth (adults: at Rooth median 34.9 GE, range 3.44–10 820; at Wahlwiller median 28.8 GE, range 5.36–276; juveniles: at Rooth median 1724 GE, range 4.74–26 800; at Wahlwiller median 17.55 GE, range 11.30–6680) (table 1).
Table 1.
Observed Bd prevalence in two B. variegata populations (Rooth and Wahlwiller) between 2010 and 2016.
| P/N (SQ mean; SQ range) | fraction infected ponds | P/N (SQ mean; SQ range) per age class |
|||||
|---|---|---|---|---|---|---|---|
| adult | subadult | juvenile | larvae | ||||
| Wahlwiller | 2010 | 1/65 (15.68; –) | 1/8 | 1/46 (15.68; –) | 0/12 (–;–) | 0/7 (–;–) | n.a. |
| 2011 | 1/143 (276; –) | 1/11 | 0/107 (–;–) | 1/36 (276; –) | n.a. | n.a. | |
| 2012 | 34/287 (233.7; 4.1–6680) | 3/13 | 10/186 (43.1; 19.5–166.2) | 0/6 (–;–) | 1/26 (6680) | 23/69 (36.6; 4.1–129.2) | |
| 2013 | 0/73 (–;–) | 0/8 | 0/69 (–;–) | 0/2 (–;–) | 0/2 (–;–) | n.a. | |
| 2015 | 21/73 (471.2; 3.4–7520) | 5/6 | 1/7 (33.2) | 1/2 (20.2) | 18/36 (545.4; 3.4–7520) | 1/28 (24.8) | |
| 2016 | 10/67 (56.03; 5.4–367) | 2/4 | 1/29 (5.36; –) | 1/2 (367; –) | 8/33 (23.5; 11.3–70) | 0/3 (–;–) | |
| Rooth | 2010 | 0/40 (–;–) | 0/12 | 0/33 (–;–) | 0/6 (–;–) | 0/1 (–;–) | n.a. |
| 2011 | 0/60 (–;–) | 0/14 | 0/53 (–;–) | 0/1 (–;–) | n.a. | 0/6 (–;–) | |
| 2012 | 123/420 (2241.9; 4.7–29600) | 9/22 | 23/96 (439.0; 7.3–4200) | 3/22 (3654.4; 23–10 820) | 53/92 (3332.7;4.7–26 800) | 44/210 (1774.2; 23.2–29 600) | |
| 2013 | 15/131 (30.6; 3.4–224) | 7/19 | 13/91 (33.3; 3.4–224) | 2/39 (13; 9.0–17.1) | 0/1 (–;–) | n.a. | |
| 2015 | 3/35 (73.5; 8–204) | 2/6 | 1/2 (8.02; –) | 0/1 (–;–) | n.a. | 2/32 (106.3; 8.6–204) | |
| 2016 | 1/88 (49; –) | 1/6 | 0/41 (–;–) | 1/17 (49; –) | 0/4 (–;–) | 0/26(–;–) | |
(b). Individual infection patterns
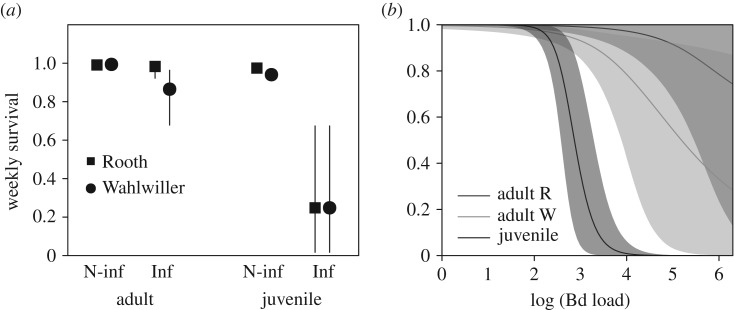
The survival of infected individuals was lower than that of non-infected individuals, both for adults and juveniles (figure 1a). Weekly survival for non-infected adults was φ(A)NI = 0.993 (95% CRI: 0.990, 0.993) at Rooth and φ(A)NI = 0.994 (0.992, 0.994) at Wahlwiller (figure 1a). Survival of infected individuals decreased markedly with higher infection loads, particularly for juveniles, although considerable uncertainty surrounded estimates for high infection loads (figure 1b). For adults with the median infection load of 23.2 GE, φ(A)I = 0.989 (0.949, 0.999) at Rooth and φ(A)I = 0.921 (0.827, 0.976) at Wahlwiller. Weekly survival for non-infected juveniles was φ(A)NI = 0.975 (95% CRI: 0.959, 0.987) at Rooth and φ(A)NI = 0.940 (0.910, 0.964) at Wahlwiller (figure 1a). For infected juveniles, we found no significant difference between sites; for juveniles with the median observed load of 351 GE, weekly survival was φ(A)I = 0.248 (0.017, 0.674). We found no evidence for an effect of body condition or temperature on survival, with the posterior distribution of regression coefficients for these covariates always encompassing zero.
Figure 1.
(a) Estimated survival over a 7-day period for adult (more than 2 years old) and juvenile (first-year) B. variegata, in different infection states (not infected ‘N-inf’, and infected ‘Inf’) at two sites. For infected toads, values indicate survival for the median of the observed infection loads in the respective age classes (adults = 23.2 GE; juveniles = 351 GE). Bars indicate 95% credible intervals. (b) Estimated survival over a 7-day period as a function of the infection load at t expressed in GE, for adult toads at each site and juvenile toads at both sites. Shaded areas indicate 95% credible intervals. R, Rooth; W, Wahlwiller.
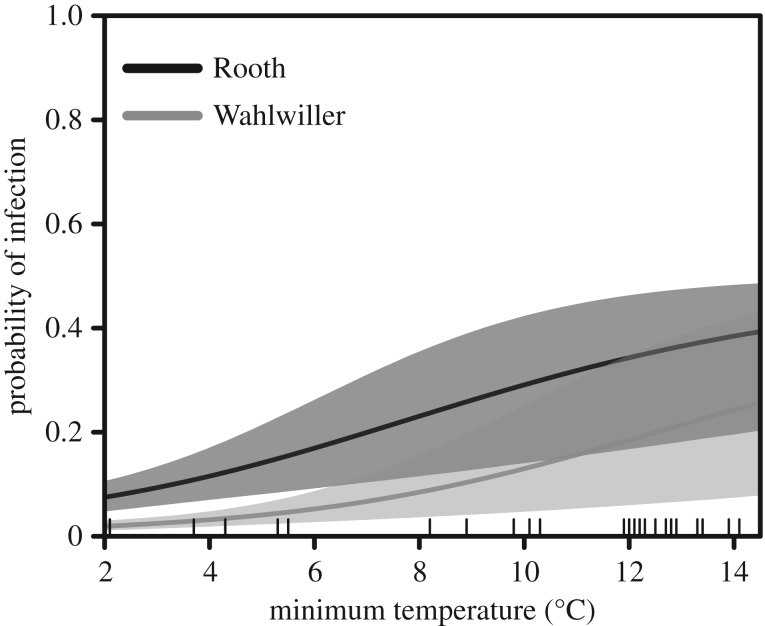
The probability of an adult becoming infected increased with higher minimum temperatures in the week preceding the survey and was always higher at Rooth than Wahlwiller (figure 2), and was not correlated to body condition. In average observed conditions (median of the observed temperatures) the probability of infection was 0.69 (0.338, 0.929) at Rooth and 0.37 (0.121, 0.705) at Wahlwiller. We found no evidence of the probability of an adult clearing infection being correlated to either body condition or temperature, with a mean value of χ(A) = 0.738 (95% CRI: 0.504, 0.966). For juveniles, the mean probability of becoming infected was ϑ(J) = 0.11 (0.037, 0.209) and the mean probability of clearing the infection was χ(J) = 0.791 (0.413, 0.992); neither were strongly correlated to body condition or temperature, with the posterior distribution of regression coefficients for these covariates always encompassing zero.
Figure 2.
Probability of becoming infected over a 7-day period for adult B. variegata, as a function of site and the mean minimum temperature in the week prior to the survey. The shaded area indicates 95% credible intervals.
(c). Population-level patterns
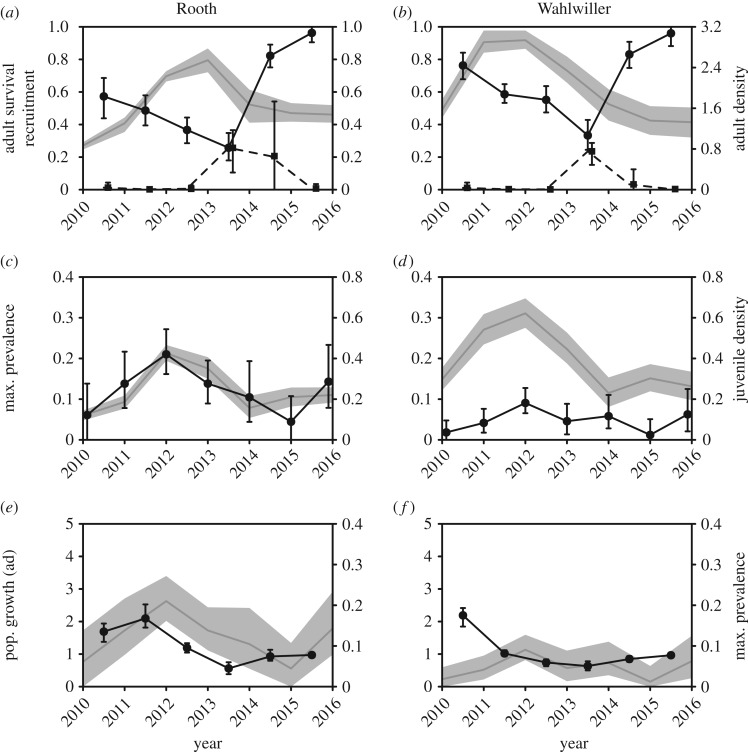
The two sites showed a markedly similar pattern in adult survival, recruitment and Bd prevalence, although the latter showed greater magnitude of change at Rooth. The survival of adults decreased between 2010 and 2013 at both sites, then increased in 2014 and 2015 (figure 3a,b; we refer to survival in a given year as the survival from that year to the next). In the year of minimum survival (2013), both sites showed a marked increase in recruitment (figure 3a,b). At Rooth, the decrease in adult survival in 2010–2013 matched a constant increase in adult population density; at Wahlwiller, the low adult survival in 2013 occurred after a decline in density had already begun in 2012. The number of juveniles produced per adult remained mostly constant throughout the study period, as highlighted by the correspondence between adult and juvenile densities (figure 3a–d). The density of adults, calculated over the total site area, was generally higher at Wahlwiller than at Rooth (figure 3a,b), but the density of juveniles, calculated over the surface of water bodies at each site, was largely comparable (figure 3c,d).
Figure 3.
Patterns of host demography and Bd prevalence patterns at two sites during the study period. The left y-axis indicates the line and error bars; the right y-axis indicates the curve surrounded by the shaded area. In (a,b), the solid line indicates adult survival, the dashed line indicates recruitment. Values that fall exactly on the year are estimated during the sampling season (i.e. density in 2014); values that fall between years indicate the value from one year to the other (i.e. survival between 2013 and 2014 indicates the proportion of adults alive at the start of 2013 that were still alive at the start of 2014). Bars and shaded areas indicate 95% credible intervals. Juvenile density is calculated over the total surface of water bodies at each site; adult density is calculated over the total area of sites (15.8 and 3.9 ha respectively), scaled by 10−1 for ease of presentation.
At Rooth, the trend in the prevalence of Bd in the adult population closely matched that of the density of juveniles in ponds (figure 3c). The growth rate of the population, particularly of the adult age class, also matched the trend in prevalence: in particular, prevalence appeared to follow the population growth in the previous year (figure 3e). At Wahlwiller, juvenile density showed greater variability, but Bd prevalence remained lower throughout the study period, although the year of highest juvenile density was also the year of highest Bd prevalence (figure 3d). The trend in population growth suggested a more stable population (figure 3f); although a growth rate of λ = 2 was estimated in the first year, Wahlwiller was only surveyed once in 2010 and the number of new entries in 2011 might therefore have been overestimated.
4. Discussion
Our results highlight the complexity behind apparent host–pathogen coexistence. In our study region Bd is historically present but has not been associated with declines in amphibian populations [11]. In the absence of obvious disease-driven mass mortality for our B. variegata populations, we found a potential cost of endemic Bd at the individual host level. The parasitic fungus randomly infects individuals and lowers the survival of infected individuals; however, this additive mortality does not noticeably translate to a negative effect at the level of the population, and the size of both our focal populations appeared relatively stable over the study period.
(a). Individual-level patterns
At the individual level, environmental mediation represented the most likely mechanism of host–pathogen coexistence. The survival of individual toads was significantly reduced by Bd infection, in line with previous observations for this species [25,26] and with two separate cases of chytridiomycosis-induced mortality in captive yellow-bellied toads that were recorded in Belgium (F. Pasmans 2017, unpublished data). We found no evidence that body condition correlated significantly with probability of infection or survival of either healthy or infected individuals. Within the limitations of our analysis, the individual probability of infection appeared random, and survival was clearly reduced for infected individuals, suggesting a potential true cost of Bd infection for B. variegata.
Infection intensity may determine transmission probability and pathogenicity; the presence of an over-dispersed pathogen load distribution (where most infections are weak, and only a small number of hosts are heavily infected) might therefore have a stabilizing effect on host–pathogen dynamics [39]. Our results appear to support this possibility, suggesting high infection loads, uncommon within the studied populations, are correlated with a marked decrease in survival. Uncertainty surrounding survival at high infection loads reflects their rare occurrence and the need to impute a large proportion of unobserved (latent) covariates.
The probability of an individual toad becoming infected clearly increased with temperature at both sites, suggesting environmental conditions play an important role in infection dynamics. Temperatures vary seasonally, and this seasonality largely coincides with the host activity patterns, potentially confounding the Bd–temperature relationship. Density-dependent transmission may become more likely as temperatures increase during the season, first with breeding activity and later with increasing numbers of juveniles in and near the ponds. However, the multi-season analysis showed Bd prevalence did not vary consistently within each season; rather, higher Bd prevalence occurred consistently in warmer years, while host activity remained largely similar (highlighted, for example, by consistent recapture probabilities and numbers of offspring per female), suggesting the relationship between Bd infection and temperature is not simply an indirect result of modified host behaviour. As longer time series become available, the model can be adapted to separate these effects.
A marked site-specific difference in the probability of infection also supported a role of environmental mediation, although the exact dynamics are more difficult to interpret than temperature patterns. Moreover, high infection loads were more common for Rooth than for Wahlwiller for both age classes, although the model did not suggest a significant site-specific difference in survival for a given load. These results suggest unidentified factors disrupt pathogen transmission and subsequent build up at Wahlwiller more than at Rooth, but once a given load is reached its negative effect on individual survival is similar. The topographic and physical structure of the two sites is largely similar and they host identical amphibian species assemblages. Although water temperature at Wahlwiller was consistently 1–2°C higher than at Rooth, both remain within the suitable thermal conditions for Bd growth for most of the Bombina breeding season [40,41], although other biotic interactions may be playing a role. For example, [42] found a strong relationship between seasonality and experimental Bd infection in Eleutherodactylus coqui, reflecting seasonal changes in the microbial community of the host species. The populations studied are close genetically, Wahlwiller having been established from a recent translocation from Rooth, although from a small founder population that may have magnified different levels of genetically determined disease tolerance. Identifying the proximate cause of these site differences represents a priority for future research, given the potential to inform mitigation actions.
(b). Population-level patterns
Further mechanisms for coexistence may operate at the population level, such that even observable negative effects of Bd on the individual host do not automatically translate to negative effects at the population level [4,43]. In our case, both populations appeared relatively stable over the study period. The observed pattern of adult survival and recruitment suggests compensatory recruitment might occur in years of particularly low adult survival. Importantly, this apparent compensation occurred in the same year at both sites, under different Bd prevalence but following a similar trend in adult and juvenile density, suggesting a process independent of Bd prevalence and infection load.
Also, the site with a more stable demographic pattern showed the smaller fluctuations in prevalence, suggesting some level of feedback between the two. At both sites, the maximum prevalence was reached in the year of maximum density of juveniles in ponds. The correspondence between Bd prevalence and juvenile density was particularly close at the site that appeared most favourable for Bd transmission (Rooth). The higher prevalence and intensity of infection in juveniles may reflect less developed immune responses or immunosuppression, following the stress of metamorphosis [44], increasing both transmission probabilities and pathogen growth on infected individuals. Late-stage tadpoles and early metamorphs may thus provide an infection reservoir in and around the waterbodies, increasing the probability of infection for adults that move to the water for reproduction [45,46]. Moreover, since new recruits (sub-adults) do not breed in the first year, their contact rate would be smaller than older, breeding-age adults; this explains how, in spite of compensatory recruitment in 2014, juvenile density and therefore prevalence showed a 1-year lag before increasing again in 2016 (when those replacement recruits would have entered the breeding age class). These observations are consistent with a classic infectious disease pattern, where higher density leads to higher transmission rates and ultimately higher population costs. Given the role of environmental mediation, where favourable conditions occur (either reflecting site suitability or weather/climate patterns), Bd prevalence can amplify the underlying density-dependent dynamics in a positive feedback cycle.
The altering global pattern in precipitation and temperature shifts is likely to impact local environmental conditions and through this increase the infection probability of an individual host and overall infection prevalence [47]. However, feedbacks between environmental mediation, infection and host demography such as we have observed for B. variegata increase the difficulty of predicting the ultimate effect on population trends. In the face of such uncertainty, maintaining the capacity of host populations to compensate reduced survival (regardless of its cause) through recruitment is crucial for their long-term persistence. These considerations have been made for amphibian hosts in epizootic situations [4,48,49], but they also hold for endemic, temporarily stable host–pathogen systems, particularly where environmental factors that currently mediate coexistence might change in the future. To counter this risk, several studies have suggested that long-term population coexistence may be promoted by actively optimizing the environmental conditions, for example through habitat manipulation [49–51].
5. Conclusion
Our results reinforce the findings of previous studies about the role of environmental conditions and demography in regulating Bd infection in amphibian hosts [4,11,14,15]. Furthermore, by explicitly modelling both individual- and population-level effects, we could use our results to support the initial hypothesis of coexistence between Bd and B. variegata at population level, despite a potential cost of Bd infection for individual toads, and to suggest this equilibrium is most probably maintained by environmental mediation. Positive feedbacks between host demography (particularly juvenile density) and pathogen prevalence suggest changes in the mediating environmental factors, for example because of climate change, might lead to a breakdown of the current equilibrium. Future research should focus on the effect of those factors on pathogen and host ecology, as well as on the proximate causes of site-specific differences in infection dynamics, given both have potential for informing disease mitigation actions. Most importantly, the complexity of the system we have described, and the implications for conservation in the face of environmental change, highlight why situations of apparent host–pathogen coexistence still require monitoring and analysis, even in the absence of obvious mass mortalities.
Acknowledgements
We cordially thank Staatsbosbeheer, Limburgs Landschap and Sibelco Europe for providing access to the study sites, and M. Blooi, S. Bogaerts and M. Starkey for fieldwork assistance.
Ethics
No animals were harmed in conducting this study. Surveys and sample collections were conducted in accordance with the landowners. The collection of skin swabs in the field was conducted under permit FF/75A/2016/015. To prevent research-mediated spread of Bd between populations, all field research equipment that contacted water or amphibians was disinfected with 1% Virkon S solution.
Data accessibility
All data have been deposited in the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.1m207 [52].
Authors' contributions
All authors contributed ideas, A.S.-v.d.S. assembled the field data, S.C. conducted statistical analyses, A.M. conducted the laboratory analyses and all authors wrote the article. All authors agreed to submission of the manuscript and accept the responsibility for the accuracy and integrity of the manuscript.
Competing interests
We have no competing interests.
Funding
S.C. is supported by the Research Foundation Flanders (FWO16/PDO/019).
References
- 1.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 2.Price SJ, Garner TWJ, Nichols RA, Balloux F, Ayres C, de Alba A Mora-Cabello, Bosch J. 2014. Collapse of amphibian communities due to an introduced ranavirus. Curr. Biol. 24, 2586–2591. ( 10.1016/j.cub.2014.09.028) [DOI] [PubMed] [Google Scholar]
- 3.Scheele BC, et al. 2017. After the epidemic: ongoing declines, stabilizations and recoveries in amphibians afflicted by chytridiomycosis. Biol. Conserv. 206, 37–46. ( 10.1016/j.biocon.2016.12.010) [DOI] [Google Scholar]
- 4.Muths E, Scherer RD, Pilliod DS. 2011. Compensatory effects of recruitment and survival when amphibian populations are perturbed by disease. J. Appl. Ecol. 48, 873–879. ( 10.1111/j.1365-2664.2011.02005.x) [DOI] [Google Scholar]
- 5.Olson DH, et al. 2013. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS ONE 8, e56802 ( 10.1371/journal.pone.0056802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lips KR, et al. 2006. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl Acad. Sci. USA 103, 3165–3170. ( 10.1073/pnas.0506889103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skerratt LF, et al. 2016. Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildl. Res. 43, 105–120. ( 10.1071/wr15071) [DOI] [Google Scholar]
- 8.Bosch J, Martínez-Solano I, García-París M. 2001. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol. Conserv. 97, 331–337. ( 10.1016/s0006-3207(00)00132-4) [DOI] [Google Scholar]
- 9.Simoncelli F, Fagotti A, Dall'Olio R, Vagnetti D, Pascolini R, Rosa ID. 2005. Evidence of Batrachochytrium dendrobatidis infection in water frogs of the Rana esculenta complex in Central Italy. Ecohealth 2, 307–312. ( 10.1007/s10393-005-8337-8) [DOI] [Google Scholar]
- 10.Rosa GM, Anza I, Moreira PL, Conde J, Martins F, Fisher MC, Bosch J. 2012. Evidence of chytrid-mediated population declines in common midwife toad in Serra da Estrela, Portugal. Anim. Conserv. 16, 306–315. ( 10.1111/j.1469-1795.2012.00602.x) [DOI] [Google Scholar]
- 11.Spitzen-van der Sluijs A, Martel A, Hallmann CA, Bosman W, Garner TWJ, Van Rooij P, Jooris R, Haesebrouck F, Pasmans F. 2014. Environmental determinants of recent endemism of Batrachochytrium dendrobatidis infections in amphibian assemblages in the absence of disease outbreaks. Conserv. Biol. 28, 1302–1311. ( 10.1111/cobi.12281) [DOI] [PubMed] [Google Scholar]
- 12.Briggs CJ, Knapp RA, Vredenburg VT. 2010. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc. Natl Acad. Sci. USA 107, 9695–9700. ( 10.1073/pnas.0912886107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolles AE, Etienne RS, Olff H. 2006. Independent and competing disease risks: implications for host populations in variable environments. Am. Nat. 167, 745–757. ( 10.1086/503055) [DOI] [PubMed] [Google Scholar]
- 14.Scheele BC, Hunter DA, Skerratt LF, Brannelly LA, Driscoll DA. 2015. Low impact of chytridiomycosis on frog recruitment enables persistence in refuges despite high adult mortality. Biol. Conserv. 182, 36–43. ( 10.1016/j.biocon.2014.11.032) [DOI] [Google Scholar]
- 15.McDonald JL, Bailey T, Delahay RJ, McDonald RA, Smith GC, Hodgson DJ. 2016. Demographic buffering and compensatory recruitment promotes the persistence of disease in a wildlife population. Ecol. Lett. 19, 443–449. ( 10.1111/ele.12578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner N, Neubeck C, Guicking D, Finke L, Wittich M, Weising K, Geske C, Veith M. 2017. No evidence for effects of infection with the amphibian chytrid fungus on populations of yellow-bellied toads. Dis. Aquat. Organ. 123, 55–65. ( 10.3354/dao03090) [DOI] [PubMed] [Google Scholar]
- 17.Kuzmin S, et al. 2009. Bombina variegata . The IUCN Red List of Threatened Species 2009. e.T54451A11148290. ( ) [DOI]
- 18.Gollmann B, Gollmann G.. 2012. Die Gelbbauchunke. Von der Suhle zur Radspur. Laurenti-Verlag, Bielefeld, 176 pp.
- 19.Bosman W, Laan RM, Van Delft JJCW. 2009. Geelbuikvuurpad Bombina variegata. In De amfibieën en reptielen van Nederland. Nederlandse fauna 9 (eds Creemers RCM, van Delft JJCW), pp. 142–153. Leiden, The Netherlands: Nationaal Natuurhistorisch Museum Naturalis, European Invertebrate Survey. [Google Scholar]
- 20.Sztatecsny M, Glaser F.. 2011. From the eastern lowlands to the western mountains: first records of the chytrid fungus Batrachochytrium dendrobatidis in wild amphibian populations from Austria. Herpetol. J. 21, 87–90. [Google Scholar]
- 21.Civiš P, Vojar J, Literák I, Baláž V. 2012. Current state of Bd occurrence in the Czech Republic. Herpetol. Rev. 43, 150–159. [Google Scholar]
- 22.Gál JT, Szabó K, Vörös J. 2012. Survey on Batrachochytrium dendrobatidis in an amphibian community in Bakony Mountains, Hungary. Állattani Közlemények 97, 47–59. [Google Scholar]
- 23.Ohst T, Gräser Y, Plötner J. 2013. Batrachochytrium dendrobatidis in Germany: distribution, prevalences, and prediction of high risk areas. Dis. Aquat. Organ. 107, 49–59. ( 10.3354/dao02662) [DOI] [PubMed] [Google Scholar]
- 24.Vörös J, Bosch J, Dán Á, Hartel T. 2013. First record of Batrachochytrium dendrobatidis on amphibians in Romania. North-West J. Zool. 9, 446–449. [Google Scholar]
- 25.Stagni G, Dall'olio R, Fusini U, Mazzotti S, Scoccianti C, Serra A. 2004. Declining populations of Apennine yellow-bellied toad Bombina pachypus in the northern Apennines (Italy): Is Batrachochytrium dendrobatidis the main cause? Ital. J. Zool. 71, 151–154. ( 10.1080/11250000409356625) [DOI] [Google Scholar]
- 26.Woodhams DC, et al. 2014. Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE 9, e96375 ( 10.1371/journal.pone.0096375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthé M, Schönbrodt T, Berger G. 2008. Computergestützte Bildanalyse von Bauchfleckenmustern des Kammmolchs (Triturus cristatus). Z. Feldherpetol. 15, 89–94. [Google Scholar]
- 28.Matthé M, Sannolo M, Winiarsk K, Spitzen-van der Sluijs A, Goedbloed D, Steinfartz S, Stachow U. 2017. Comparison of photo-matching algorithms commonly used for photographic capture–recapture studies. Ecol. Evol. 7, 5861–5872. ( 10.1002/ece3.3140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drechsler A, Helling T, Steinfartz S. 2014. Genetic fingerprinting proves cross-correlated automatic photo-identification of individuals as highly efficient in large capture-mark-recapture studies. Ecol. Evol. 5, 141–151. ( 10.1002/ece3.1340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 31.Boyle D, Boyle D, Olsen V, Morgan J, Hyatt A. 2004. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Organ. 60, 141–148. ( 10.3354/dao060141) [DOI] [PubMed] [Google Scholar]
- 32.Lebreton J-D, Burnham KP, Clobert J, Anderson DR. 1992. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 62, 67–118. ( 10.2307/2937171) [DOI] [Google Scholar]
- 33.Daskin JH, Bell SC, Schwarzkopf L, Alford RA. 2014. Cool temperatures reduce antifungal activity of symbiotic bacteria of threatened amphibians—implications for disease management and patterns of decline. PLoS ONE 9, e100378 ( 10.1371/journal.pone.0100378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raffel TR, Halstead NT, McMahon TA, Davis AK, Rohr JR.. 2015. Temperature variability and moisture synergistically interact to exacerbate an epizootic disease. Proc. R. Soc. B 282, 20142039 ( 10.1098/rspb.2014.2039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plummer M. 2003. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. DSC 2003 Working Papers; 2003-03-20 to 2003-03-22; Technische Universität Wien in Vienna, Austria: Austrian Association for Statistical Computing (AASC), R Foundation for Statistical Computing.
- 36.Kéry M, Schaub M. 2011. Bayesian population analysis using WinBUGS/OpenBUGS—a hierarchical perspective. New York, NY: Academic Press. [Google Scholar]
- 37.Morand A. 1997. Stabilité relative des habitats de développement larvaire et de reproduction de Bombina variegata et Bufo calamita: l'insuffisance des Modèles r-K et r-K-A. Geobios 30, 23–36. ( 10.1016/s0016-6995(97)80064-2) [DOI] [Google Scholar]
- 38.Barandun J, Reyer H-U, Anholt B. 1997. Reproductive ecology of Bombina variegata: aspects of life history. Amphib-reptil. 18, 347–355. ( 10.1163/156853897X00404) [DOI] [Google Scholar]
- 39.Grogan LF, Phillott AD, Scheele BC, Berger L, Cashins SD, Bell SC, Puschendorf R, Skerratt LF. 2016. Endemicity of chytridiomycosis features pathogen overdispersion. J. Anim. Ecol. 85, 806–816. ( 10.1111/1365-2656.12500) [DOI] [PubMed] [Google Scholar]
- 40.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219 ( 10.2307/3761366) [DOI] [Google Scholar]
- 41.Piotrowski JS, Annis SL, Longcore JE. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96, 9 ( 10.2307/3761981) [DOI] [PubMed] [Google Scholar]
- 42.Longo AV, Zamudio KR. 2017. Environmental fluctuations and host skin bacteria shift survival advantage between frogs and their fungal pathogen. ISME J. 11, 349–361. ( 10.1038/ismej.2016.138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobler U, Borgula A, Schmidt BR. 2012. Populations of a susceptible amphibian species can grow despite the presence of a pathogenic chytrid fungus. PLoS ONE 7, e34667 ( 10.1371/journal.pone.0034667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warne RW, Crespi EJ, Brunner JL. 2011. Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct. Ecol. 25, 139–146. ( 10.1111/j.1365-2435.2010.01793.x) [DOI] [Google Scholar]
- 45.Medina D, Garner T, Carrascal L, Bosch J. 2015. Delayed metamorphosis of amphibian larvae facilitates Batrachochytrium dendrobatidis transmission and persistence. Dis. Aquat. Organ. 117, 85–92. ( 10.3354/dao02934) [DOI] [PubMed] [Google Scholar]
- 46.Valencia-Aguilar A, Toledo LF, Vital MVC, Mott T. 2016. Seasonality, environmental factors, and host behavior linked to disease risk in stream-dwelling tadpoles. Herpetologica 72, 98–106. ( 10.1655/herpetologica-d-15-00013) [DOI] [Google Scholar]
- 47.Clare FC, et al. 2016. Climate forcing of an emerging pathogenic fungus across a montane multi-host community. Phil. Trans. R. Soc. B 371, 20150454 ( 10.1098/rstb.2015.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheele BC, Guarino F, Osborne W, Hunter DA, Skerratt LF, Driscoll DA. 2014. Decline and re-expansion of an amphibian with high prevalence of chytrid fungus. Biol. Conserv. 170, 86–91. ( 10.1016/j.biocon.2013.12.034) [DOI] [Google Scholar]
- 49.Heard GW, Scroggie MP, Ramsey DSL, Clemann N, Hodgson JA, Thomas CD. 2017. Can habitat management mitigate disease impacts on threatened amphibians? Conserv. Lett. ( 10.1111/conl.12375) [DOI] [Google Scholar]
- 50.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA 107, 9689–9694. ( 10.1073/pnas.0914111107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savage AE, Sredl MJ, Zamudio KR. 2011. Disease dynamics vary spatially and temporally in a North American amphibian. Biol. Conserv. 144, 1910–1915. ( 10.1016/j.biocon.2011.03.018) [DOI] [Google Scholar]
- 52.Spitzen-van der Sluijs A, Canessa S, Martel A, Pasmans F. 2017. Data from: Fragile coexistence of a global chytrid pathogen with amphibian populations is mediated by environment and demography Dryad Digital Repository. ( 10.5061/dryad.1m207) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Spitzen-van der Sluijs A, Canessa S, Martel A, Pasmans F. 2017. Data from: Fragile coexistence of a global chytrid pathogen with amphibian populations is mediated by environment and demography Dryad Digital Repository. ( 10.5061/dryad.1m207) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data have been deposited in the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.1m207 [52].