Abstract
Many group-living animals cooperatively signal to defend resources, but what stops deceptive signalling to competitors about coalition strength? Cooperative-signalling species include mated pairs of birds that sing duets to defend their territory. Individuals of these species sometimes sing ‘pseudo-duets’ by mimicking their partner's contribution, but it is unknown if these songs are deceptive, or why duets are normally reliable. We studied pseudo-duets in Australian magpie-larks, Grallina cyanoleuca, and tested whether multimodal signalling constrains deception. Magpie-larks give antiphonal duets coordinated with a visual display, with each sex typically choosing a different song type within the duet. Individuals produced pseudo-duets almost exclusively during nesting when partners were apart, but the two song types were used in sequence rather than antiphonally. Strikingly, birds hid and gave no visual displays, implying deceptive suppression of information. Acoustic playbacks showed that pseudo-duets provoked the same response from residents as true duets, regardless of whether they were sequential or antiphonal, and stronger response than that to true duets consisting of a single song type. By contrast, experiments with robot models showed that songs accompanied by movements of two birds prompted stronger responses than songs accompanied by movements of one bird, irrespective of the number of song types or singers. We conclude that magpie-larks used deceptive pseudo-duets when partners were apart, and suppressed the visual display to maintain the subterfuge. We suggest that the visual component of many species' duets provides the most reliable information about the number of signallers and may have evolved to maintain honesty in duet communication.
Keywords: pseudo-duets, deception, multimodal signals, magpie-lark, robotic birds, duets
1. Introduction
In many bird species, members of mated pairs sing coordinated duets to cooperatively defend their territory [1–4]. By singing together, partners demonstrate that their territory is defended by an alliance of two individuals [5–7]. Therefore, during territorial conflicts duets generally elicit stronger responses than solo songs, and partners threatened by territorial intrusion are more likely to duet than to sing independently [8–10]. In addition to signalling about the number of defenders, many duets have superb temporal coordination, which adds an extra message to cooperative signalling [11,12]. High coordination can reveal pair longevity, and therefore commitment and ability to defend the territory [11,13]. In addition to producing temporally coordinated songs, duetting birds typically come close together to sing, reinforcing the signal that the pair is ready for cooperative defence [3,14].
Although of benefit when together, duetting when spatially separated is likely to be ineffective, which could select for deceptive ‘duetting’ by single birds. Duetting while apart reveals that members of the pair are not together for united defence [3], and separation can also reduce the tempo and perceived temporal coordination of duets [15,16]. Consistent with these limitations of duetting, individuals can avoid duetting if they are further apart [17,18]. Spatial separation is sometimes unavoidable, however, such as during incubation and brooding, and the alternative of solo singing could reveal that the partner is absent or the pair is not united. To overcome these limitations, a bird might imitate the contribution of its partner in addition to its own song, creating a deceptive pseudo-duet [19]. A cheater would benefit by signalling deceitfully the readiness of a pair for cooperative defence, while receivers would incur a cost by losing any opportunity for territorial intrusion. Early reports do show that one individual may imitate duets when their partner is absent or not singing [19–23]. In Laniarius aethiopicus and captive pairs of Copsychus malabaricus, for example, individuals sang the whole duet pattern if their partner was absent [20,23], and in captive Cossypha heuglini, such pseudo-duets were given at a higher rate when true duet rates were low [19]. Despite these observations, however, there has been no detailed study of pseudo-duetting, and nothing is known about its function, including whether it is deceptive. It is also possible that pseudo-duetting is non-adaptive, perhaps reflecting a neural template for complete duets and not merely individual contributions [24].
A potential constraint on deceptive pseudo-duetting is that many duetting species produce a multimodal display in which duets are accompanied by synchronized body movements [10,25–30]. Vision provides more precise spatial information than sound, and synchronized movements affect auditory perception [31]. These movements therefore link songs with singers, so that a listener is certain who is singing, including being able to verify that two individuals contribute to a duet. Multimodal displays could therefore evolve to enhance signal reliability, as long as pairs usually duet from visible locations [5]. Consistent with this expectation, in Heuglin's robin chats, C. heuglini, and spotted palm-thrushes, Cichladusa guttata, for example, duets prompted by a vocal challenge by rivals are accompanied by conspicuous movements of the male and female much more frequently than spontaneous duets [26,30]. This suggests that birds use a visual component to enhance duet reliability. If pseudo-duets were deceptive, therefore, cheaters would have to sing from concealment and avoid visual displays.
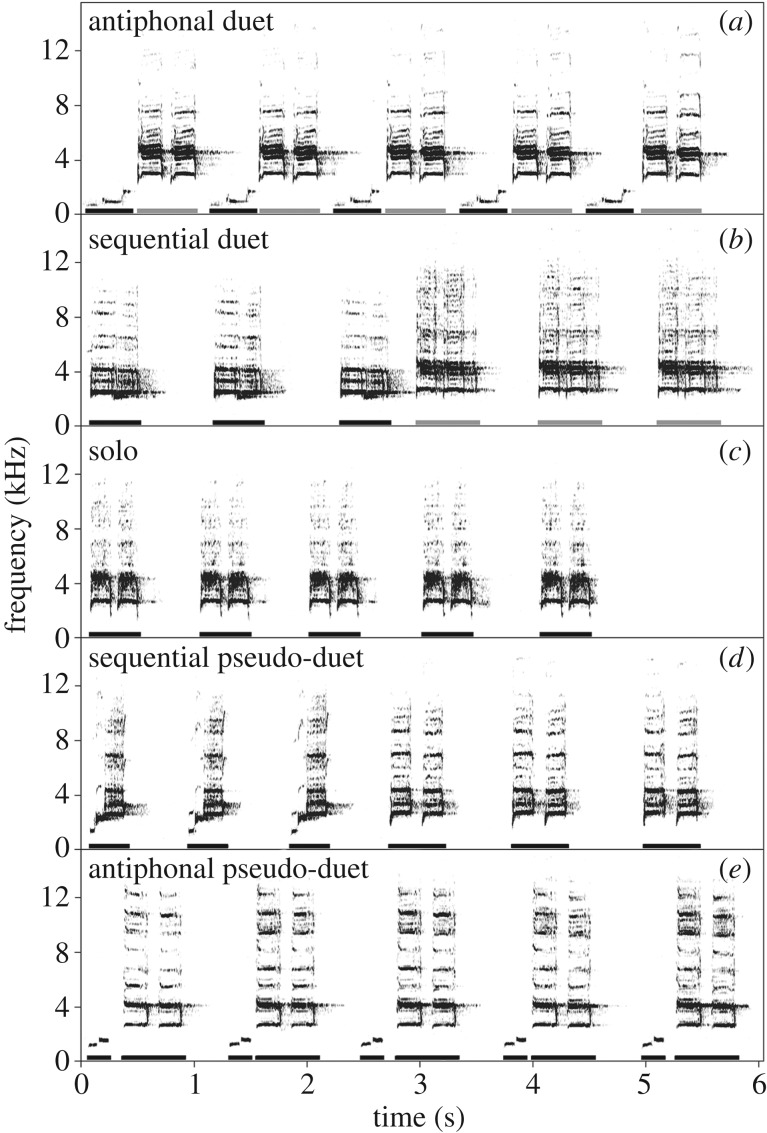
We examined pseudo-duetting in Australian magpie-larks, Grallina cyanoleuca. Members of pairs have overlapping repertoires of three to six song types, each composed of a series of short motifs (300–600 ms) of the same type, given at a tempo of approximately 1 motif s−1 (figure 1). Motif types differ in the number of elements or their acoustic structure, and no type is sex-specific. Each motif type can be given in solo songs or with their partner in highly coordinated antiphonal (or sometimes sequential) duets that function to defend the territory [8,11] (figure 1). Although solo songs are composed from a single motif type, individuals contribute a different motif type to their partner when forming a duet [15,28]. Birds come together when duetting, and are less likely to answer a partner to form a duet when further apart [17]. Birds almost always coordinate both solo and duet songs with visual signals, including movements of the wings or whole body, to produce conspicuous multimodal displays on prominent song posts [25,28]. The visual component of the display might coordinate vocal duets within pairs [28], but its role in between-pair communication has not been studied. Sometimes also one bird produces a song consisting of two different motif types in succession, thereby forming a pseudo-duet (figure 1), but there are no previous descriptions of pseudo-duetting in the literature on this species.
Figure 1.
Sonograms of natural magpie-lark songs: (a) antiphonal duet, (b) sequential duet, (c) solo song, (d) sequential pseudo-duet, and (e) antiphonal pseudo-duet. Individual contributions are denoted with black and grey bars. All song types can be produced and initiated by both sexes, with 48% and 52% of pseudo-duets given by females and males, respectively (Results section).
We carried out natural observations and playback experiments to test whether pseudo-duetting could be deceptive. Observations tested the prediction that if pseudo-duetting was deceptive, it would be used primarily during the nesting period, when partners were constrained to be apart, and would be given cryptically, rather than combined with visual displays on song posts. Audio playback experiments then tested the prediction that listeners could not distinguish pseudo-duets from true duets by sound alone. Finally, audio-visual playbacks with robotic birds tested whether the visual display provides information on the number of singers, and thus normally ensures honest signalling when birds are in sight and displaying.
2. Material and methods
(a). Study site and species
We studied a colour-banded population of magpie-larks in and near the Australian National University campus in Canberra. Magpie-larks are endemic to Australia and the surrounding islands, and hold breeding territories near water in open habitats with scattered trees [32]. In the study area these trees include native eucalyptus, and introduced conifers and deciduous trees. Males and females have distinct plumage, and pairs defend territories throughout the year, breeding primarily from August to February [8]. There are no known acoustic differences between male and female song types, yet playbacks suggest that magpie-larks can distinguish the sex of unfamiliar birds from their solo song [8,33].
Magpie-lark pairs commonly sing duets, and these are usually accompanied by visual displays. Either sex can initiate duets, and about half the songs initiated by each sex are answered by their partner to form duets, while the others remain as solo songs [15] (figure 1). Most commonly, the duet initiator and responder alternate motifs antiphonally, on average six times, with each using a different motif type [15,28], but sometimes the response is delayed so that the songs form sequential duets (figure 1). Most duets are accompanied by synchronized wing or body movements [28], but in contrast to song types, partners usually use the same movement within a duet [28]. Solo songs are accompanied by these visual displays as often as songs answered to form duets (99% of duets and 96% of solos; Results section). Individuals sometimes include two motif types within a song, thereby forming a pseudo-duet (above).
(b). Context of pseudo-duetting
We quantified the context in which magpie-larks gave pseudo-duets to test two predictions of deceptive duetting. First, pseudo-duets should be relatively more frequent when the bird's partner is on the nest, when they cannot come together to duet. Previous studies revealed that the proportion of song initiations answered to form duets decreased during the egg-laying period [15], and when partners were further apart [17]. Magpie-lark partners incubate eggs and brood hatchlings alternately and almost constantly [34]. Second, if pseudo-duets are given deceptively, they should be given without movements and avoiding conspicuous song posts. Obvious visual displays highlight both the number of birds and their locations, which is inconsistent with deception. Duets and solos are generally given from exposed perches, presumably attracting the attention of receivers [25].
To test our predictions, we observed 17 pairs of magpie-larks before nest-building, during nesting and after chicks left the nest. We observed the same nine males and eight females from August 2015 to February 2016. Breeding is not synchronous, so observations were made at different dates for different pairs. Birds were observed for 30 min during each period, 1–2 h after sunrise. Nesting period watches were taken only when the partner of the focal bird was on the nest and paused when the focal bird was on the nest. We counted solos, pseudo-duets and duets initiated by the focal bird, and noted any visual display. We also noted if solo songs and pseudo-duets produced during the nesting period were given from the top of a tree, so that the bird could be observed from a distance; or from within the crown of a tree, so that the bird could be observed only from a short distance. Three pairs were excluded from these observations because the structure of vegetation in their territories precluded assessment of birds' positions.
(c). Playback experiment design
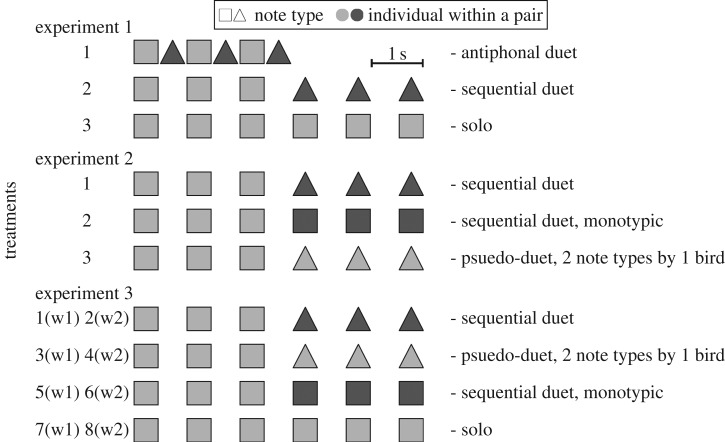
We carried out three playback experiments in January and February 2016 to test whether pseudo-duets could be deceptive signals, and whether the visual displays affect the assessment of songs (figure 2). The first two experiments used acoustic playback alone, to test the importance of song timing, song type and sex in response to duets. The third experiment used both acoustic playback and robotic models to examine the importance of the visual display in the response to song.
Figure 2.
The design and timeline of treatments during experiments. In experiment 3, each acoustic treatment was carried out twice: once when a single robotic bird displayed its wings (w1; electronic supplementary material, movie S1) and once when both did so (w2; electronic supplementary material, movie S2), so there was a total of eight treatments.
The design of all experiments was similar. Each was carried out on a different set of 12 focal pairs. Playbacks originated from high-quality recordings of duets from 36 pairs, and were fully replicated, with each focal pair receiving a unique set of exemplars from unfamiliar birds. Recordings were from a Canon XA20 HD Camcorder and Sennheiser ME66 microphone, sampling wave files at 48 kHz and 16 bits, and then edited using the Avisoft-SASLab Pro software. Every playback contained six motifs, and lasted approximately 3 s for antiphonal duets and 6 s for sequential duets and solos, which are natural timings [25]. In every experiment, half of each duet type started with each sex, and half solos and pseudo-duets were of each sex. In all cases treatment order was balanced, to avoid order or carry-over effects confounding results. The population is of high density and pairs interact with each other regularly every day, so the number of playbacks is unlikely to cause a lasting disturbance.
(i). Experiment 1: are sequential duets treated as duets or solo song?
We tested whether sequential duetting is treated as duetting or solo singing. Each focal pair received an antiphonal duet, a sequential duet and a solo song on a single day (figure 2). Duet playbacks were composed by pasting male and female motifs into a wave file, either in alternation or sequentially, while solo playbacks consisted of either male or female motifs. Following typical usage, male and female motif types were different in duets, but solos contained only a single motif type.
(ii). Experiment 2: does the number of motif types signal a duet?
We examined whether the number of motif types within a song signals the number of singers (figure 2). Focal birds received three treatments on the same day (figure 2). Two were sequential duets, which differed in whether the male and female motifs were the same or different. The third was a pseudo-duet with two motifs, each given by the same individual. If the number of motif types determines the focal birds' response, then responses to the pseudo-duet will be similar to the two-motif sequential duet and greater than the single-motif sequential duet. In that case, pseudo-duets could be deceptive. By contrast, if listeners respond to the sex or individuality of the singer, the response to the two sequential duets would be the same, and greater than to the pseudo-duet.
(iii). Experiment 3: do wing movements affect response to songs?
We used taxidermic robotic birds in combination with acoustic playbacks to examine whether wing movements affect the response to songs (figure 2). We used a male–female pair of robotic magpie-larks to imitate wing movements, the most common movement that is synchronized with song [28]. Each bird was animated with two servo-motors (Power HD, Analog Micro Servo HD-1440A), attached to humeri of the wings, which moved the outstretched wings up and down. The movements were controlled by an Arduino circuit board (Arduino Micro; http://www.arduino.cc), enabling precisely timed wing movements and vocalizations. Robotic birds were perched next to each other facing the same direction, and enclosed in a cage of fine black netting to protect them from damage.
The robotic birds were programed to reproduce a natural temporal pattern of movement and song [25,28]. The beginnings of movement and acoustic playback were synchronized and movement continued for 0.5 s up and 0.5 s down. The motif finished before the end of respective wing movement and the length of this gap depended on the length of the motif. This sequence of movements and motifs was repeated six times, with one model bird moving six times or two model birds moving three times each sequentially, depending on the treatment. Both robotic birds were present during all treatments, to ensure that the mere presence of individuals did not confound results.
Each focal pair was exposed to two treatments per day over 4 days, for a total of eight treatments (figure 2). The eight treatments were made up of four different playbacks, combined with the wing display of either one or both robot birds. Treatments on different days had different vocal components: a sequential duet, a monotypic sequential duet, a pseudo-duet or a solo. Treatments on the same day consisted of the same vocal component but different visual components, in which six motifs were synchronized with six movements given either by both the robotic birds (three by each bird, in sequence) or by one robotic bird with the other being motionless. Two treatments carried out on the same day were either male- or female-initiated, but over the experiment there were equal numbers of each type. Each playback day was separated by 3 days without playback, to minimize the risk of carry-over effects.
(d). Playback field methods
Playbacks were carried out between 06.20 and 11.20 h on days without rain or strong wind. Treatments on the same day were preceded by an 8 min control period followed by 8 min periods for each treatment, separated by 15 min intervals. Playbacks were initiated only if partners were together and within 25 m of the speaker, and if they had not engaged in any interaction with neighbours during the minute before each treatment. During each treatment, birds received six playbacks, which by default started at 0, 30, 60, 90, 120, 150 s, but a playback was delayed a few seconds if birds were singing at these times. Playbacks were broadcast from a Mipro MA-101A amplified loudspeaker (45 W, frequency range 60–15 000 Hz). Songs were broadcast at natural amplitudes of 66–70 dB SPL at 10 m, measured by a RadioShack Sound Level Meter (see also [28,33]).
We measured the response to playback in the 8 min period starting with the first playback. This sample for each treatment was longer than the period of playbacks because birds usually continued any response for several minutes after the last playback. We followed previous studies of this species by measuring the territorial response to playback as the number of songs by the focal male, including both solos and duets [11]. This is because male song rate is the most sensitive measure of response to territorial threat [8].
(e). Statistical analysis
We used generalized estimating equations (GEEs) to compare the number of duets, solos and pseudo-duets produced during natural observations before, during and after the nesting period. This procedure is appropriate for repeated measures, and particularly for non-normally distributed data [35]. Fisher's LSD method was used in the GEE to create confidence intervals for differences between means. We also used the Dunn-Bonferroni (D-B) test as a non-parametric post hoc procedure for multiple comparisons following a Friedman's test [36].
To compare the effects of treatments in experiments, we used repeated measures ANOVA, and Tukey's HSD method to compare means among treatments. Control periods were not included in models as separate factor levels because it is likely that any playback of conspecifics would elicit a response above the baseline level. Instead, we used control measurements as covariates within models to control for the effect of initial differences among subjects. In the analysis for experiment 3, we used the mean from the four control measurements for each subject as the covariate. The assumptions of normality and sphericity were tested with the Shapiro–Wilk and Mauchly's tests, respectively. The analyses were conducted using the SPSS 23 software. All p-values were two-tailed, and means ± s.e.m. are given unless stated otherwise. The data reported in this paper are available in the electronic supplementary material, dataset S1.
3. Results
(a). Context of pseudo-duetting
Pseudo-duets were more frequent during nesting, as predicted if substituting for normal duets when partners were apart (figure 3). Although birds produced a similar number of total songs before, during and after nesting (GEE: Wald 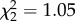 , p = 0.59), the absolute number and proportion of pseudo-duets was much greater during nesting (figure 3; GEE: Wald
, p = 0.59), the absolute number and proportion of pseudo-duets was much greater during nesting (figure 3; GEE: Wald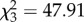 , p < 0.0001). Furthermore, the proportion of songs answered to form duets decreased during the nesting period, suggesting that pseudo-duets were produced more often when their partner was less likely to respond and form a duet (before 0.42 ± 0.05, during 0.22 ± 0.04, after 0.51 ± 0.07; Friedman's test: χ22 = 9.97, p = 0.007; D-B test: before–during: p = 0.02; before–after: p = 0.61; during–after: p = 0.005). Consistent with the importance of spatial proximity, during the nesting period all 13 duets were given from the nesting tree, where birds come together to swap over during parental care, whereas all 26 pseudo-duets were given away from the nesting tree, when partners were separated by 15–120 m (mean = 55 m). Among the 27 pseudo-duets given before, during or after nesting, two were antiphonal and 25 sequential. Males and females were equally likely to produce pseudo-duets (mean number in 30 min: males, 1.56 ± 0.53; females, 1.63 ± 0.53; Mann–Whitney U-test: U = 35, p = 0.96).
, p < 0.0001). Furthermore, the proportion of songs answered to form duets decreased during the nesting period, suggesting that pseudo-duets were produced more often when their partner was less likely to respond and form a duet (before 0.42 ± 0.05, during 0.22 ± 0.04, after 0.51 ± 0.07; Friedman's test: χ22 = 9.97, p = 0.007; D-B test: before–during: p = 0.02; before–after: p = 0.61; during–after: p = 0.005). Consistent with the importance of spatial proximity, during the nesting period all 13 duets were given from the nesting tree, where birds come together to swap over during parental care, whereas all 26 pseudo-duets were given away from the nesting tree, when partners were separated by 15–120 m (mean = 55 m). Among the 27 pseudo-duets given before, during or after nesting, two were antiphonal and 25 sequential. Males and females were equally likely to produce pseudo-duets (mean number in 30 min: males, 1.56 ± 0.53; females, 1.63 ± 0.53; Mann–Whitney U-test: U = 35, p = 0.96).
Figure 3.
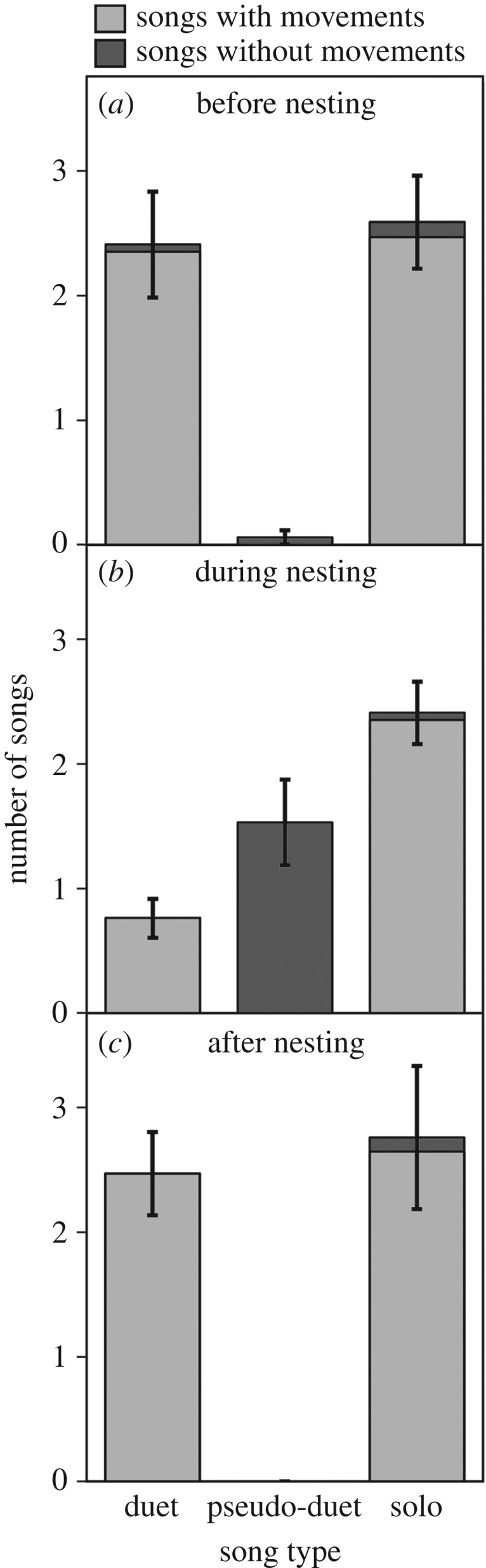
The number of duets, pseudo-duets and solo songs given by magpie-larks (n = 8 males and 7 females) in 30 min (a) before, (b) during and (c) after the nesting period. Duets were songs given by the male and female; pseudo-duets were songs consisting of two motif types but given by one individual; and solos were songs given by one individual and consisting of one motif type. The boxes for songs with and without movements for a given song type are additive. The boxes show mean ± s.e.m.
Birds behaved cryptically during pseudo-duetting, consistent with deception (figure 3). Birds never gave visual displays while pseudo-duetting, but almost always did so during true duets and solos, regardless of the stage of breeding (95 of 96 duets and 127 of 132 solos). Specifically during the nesting period, when the three types of songs were common, pseudo-duets differed from both solo songs and true duets in their lack of visual display (figure 3; Friedman's test: χ22 = 20.46, p < 0.001; D-B test: solo–pseudo-duet: p = 0.005; duet–pseudo-duet: p = 0.002; solo–duet: p = 0.80). In addition to suppressing visual display, pseudo-duetting individuals never sang from exposed perches, while they usually did so during solo songs (0 out of 26 pseudo-duets and 25 out of 29 solos by 14 pairs; Wilcoxon-matched pairs: Z = 3.06, p = 0.002).
(b). Experiment 1: are sequential duets treated as duets or solo songs?
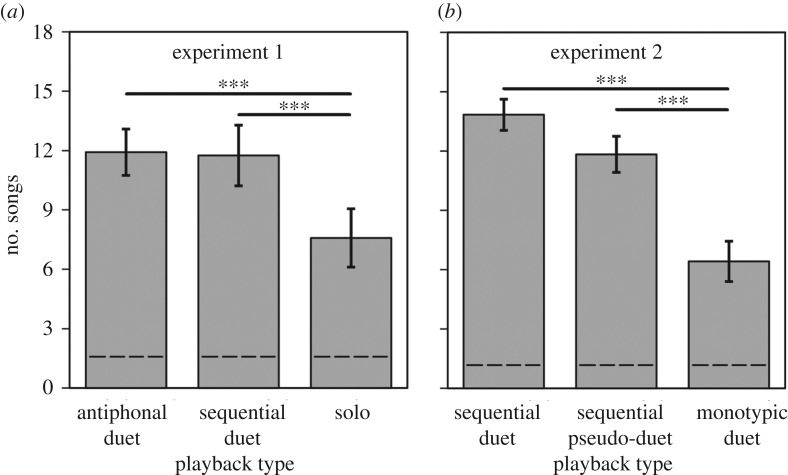
Birds responded to sequential duets as duets and not solo songs. Sequential duets prompted a similar territorial response to antiphonal duets, and greater than that to solo songs (figure 4a; repeated measures (RM) ANOVA: F2,20 = 5.60, p = 0.012, partial η2 = 0.36). This effect was independent of the baseline number of songs, recorded in the 8 min control period before playback began (RM ANOVA: F2,20 = 0.11, p = 0.894, partial η2 = 0.01).
Figure 4.
The number of songs given by males during (a) experiment 1 and (b) experiment 2. The treatments carried out in experiment 1 were: antiphonal duet (alternating male and female motifs), sequential duet (sequential male and female motifs) and solo (male or female solo song). The treatments for experiment 2 were: sequential duet, pseudo-duet (male or female solo song with two motif types played sequentially) and monotypic duet (male and female motifs of the same type played sequentially). Numbers of songs given before playbacks (control) are indicated with the dashed lines. Significant differences are indicated with thick black lines (n = 12; Tukey HSD, *** p < 0.001). The boxes show mean ± s.e.m.
(c). Experiment 2: does the number of motif types signal a duet?
The number of motif types affected the response to playback, whereas the number of callers did not. Pseudo-duets and sequential duets both contained two motif types and prompted similar responses, which were greater than those to monotypic duets, which contained one motif type yet two callers (figure 4b; RM ANOVA: F2,20 = 12.02, p = 0.0004, partial η2 = 0.55). The differences between treatments were independent of the baseline conditions (RM ANOVA: F2,20 = 0.70, p = 0.51, partial η2 = 0.07), and there was no effect of the sex of the caller on response (RM ANOVA: F2,22 = 1.95, p = 0.17, partial η2 = 0.15).
Comparison of responses in experiments 1 and 2 also supports the importance of the number of motifs and not callers. The solo song playbacks in experiment 1 prompted similar responses to monotypic duets in experiment 2, despite the difference in the number of callers (figure 4; t-test: t22 = −0.65, p = 0.52), a weaker response to pseudo-duets in experiment 2 (figure 4; t-test: t22 = 2.45, p = 0.023).
(d). Experiment 3: do wing movements affect response to songs?
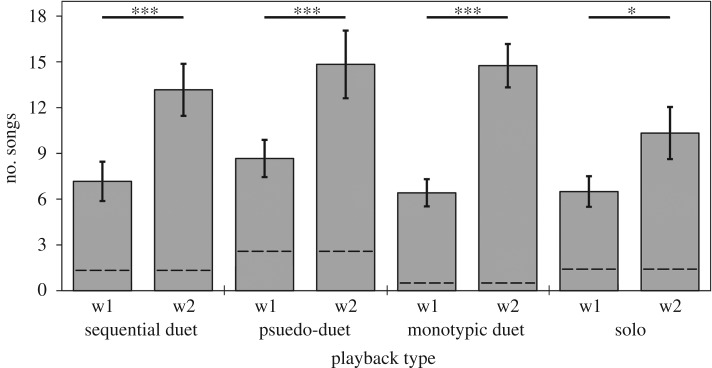
During audio-visual playbacks, visual information on the numbers of callers overrode acoustic information. When robotic birds were present, focal males always produced more songs in response to treatments with two moving birds than to treatments with one moving bird, independently of the acoustic component (figure 5; RM ANOVA: wing movement, F1,10 = 35.32, p = 0.0001, partial η2 = 0.78; song type, F3,30 = 0.72, p = 0.546, partial η2 = 0.07; interaction between wing movement and song type: F3,30 = 0.49, p = 0.695, partial η2 = 0.05). This difference persisted even for solo songs, suggesting that the number of moving birds can be used to discriminate between a duet and solo song. The differences between treatments were independent of the baseline conditions (RM ANOVA covariate effect: F3,30 = 0.93, p = 0.439, partial η2 = 0.09).
Figure 5.
Number of songs given by males during eight treatments: sequential duet, pseudo-duet, monotypic (sequential) duet and solo, accompanied by one (w1) or two (w2) birds moving their wings. Numbers of songs given by males prior to playbacks (control) are indicated by dashed lines. Significant differences between songs with one and two moving birds are indicated with thick black lines (n = 12; Tukey HSD, *** p < 0.001, * p = 0.01–0.05). The boxes show mean ± s.e.m.
4. Discussion
Our observations and experiments together show that magpie-larks use deceptive pseudo-duets when members of the pair cannot be together. Individuals produced pseudo-duets almost exclusively when their partner was confined to the nest and the pair could not come together to give a cooperative multimodal display. Remarkably, pseudo-duets were sung from concealment and never accompanied by a visual display, suggesting deceptive suppression of information. Playback experiments support this interpretation. If magpie-lark models were in view, listeners used the visual display to judge whether a song was a duet, but when not in view they used the number of motifs. Therefore, while the visual display normally guarantees the honesty of duets, individuals can deceptively mimic a duet by being visually inconspicuous, and using two motif types within their pseudo-duet.
The context of singing suggests that pseudo-duetting is used when true duetting would betray spatial separation of partners. Pseudo-duets were most common during the nesting stage, when their partner was on the nest. This increase during nesting was not owing to a general increase in vocal activity, because the number of solo songs remained constant over the nesting cycle. Instead, the increase in pseudo-duetting compensated for a lower number of true duets, which were confined to times when birds came together in the nesting tree. Overall, individuals were less likely during the nesting period to answer their partner's song to form duets. These observations suggest that pseudo-duetting emerges because there is a trade-off between cooperative signalling, which entails being together [17,30,37], and nesting, which requires spatial separation [15,38,39].
In striking contrast to solo songs and true duets, pseudo-duets were never accompanied by synchronized body movements, and were produced from concealment, suggesting that birds deceptively suppress information that the pseudo-duet originates from one individual. Magpie-larks gave visual displays during over 97% of solo songs and true duets, mostly from conspicuous song posts, whereas pseudo-duetting individuals suppressed visual display before they initiated singing, not simply because their partner failed to respond to form a duet. Visual displays are likely to draw the attention of distant observers within the magpie-lark's relatively open habitat, and allow attribution of motifs to individual birds. Such a function has also been suggested for the wing display of duetting spotted palmthrushes, Ci. guttata [26]. The behaviours associated with pseudo-duets therefore very likely hinder the identification of singers and make deception more effective. Such deception may be difficult to detect because not all true duetters will be within the line of sight, and because even nesting magpie-larks produced some true duets.
Deceptive communication faces the general problem that it is ineffective if used too often or is perceptually inaccurate, particularly if there is a high cost of being deceived [40–42]. For example, fork-tailed drongos, Dicrurus adsimilis, use deceptive alarm calls to scare heterospecifics and steal food, but deception becomes less effective with repeated use of the same alarm call [43]. Drongos solve this problem by switching to accurate, mimicked alarm calls so that any one call is used infrequently and is difficult to discriminate from the copied species' alarm calls [43,44]. Furthermore, the net cost of being deceived is low, because victims also get compensatory benefits from true alarm calls [45]. Magpie-lark pseudo-duetting might be effective for comparable reasons. Pseudo-duets are used infrequently compared to true duets, because they are restricted to a specific period of the nesting cycle, during which true duets can also occur. Pseudo-duets maintain perceived accuracy by being produced from concealment and suppressing the tell-tale visual display, and we predict that individuals would not use pseudo-duetting when rivals are close or during an ongoing territorial interaction. Furthermore, the short-term cost of being deceived is small—perhaps reduced opportunities to intrude onto a neighbouring territory to collect food—compared to the longer-term cost of ignoring true duets, which would include wasteful aggression between neighbours [46,47].
Although most magpie-lark duets are antiphonal while most of their pseudo-duets are sequential, this difference surprisingly did not affect the response to playback, meaning that antiphonal timing does not protect against deceptive duetting in this species. Pseudo-duets may still be effective because true duets are occasionally sequential or because some pseudo-duets are antiphonal (figure 1). Although both types of duets are effective, it is possible that pseudo-duets are rarely antiphonal because they are challenging for a single bird to mimic. Antiphonal duets are often precisely coordinated, with a tempo twice that of solo songs, a perfect alternation of motifs, and a high synchrony of movements and motifs [9,11,25,27,32]. A sequential rather than antiphonal structure might also be a safer option for deception because it starts out with the same structure as a solo song, with a single motif repeated at a 1 s tempo. If a partner unexpectedly responds with a temporally coordinated song, the result is a true duet rather than exposure of deception, which would occur if the initial bird started with an antiphonal pseudo-duet and was joined by its partner. Starting with a solo structure also gives the singer more time to ensure that it is not being observed.
When physical models were motionless, magpie-larks responded more to playbacks consisting of two motif types than a single motif type, independently of the number of singers. Using the number of motifs to classify songs as duets would normally be reliable, because birds almost always use different motifs from their partners within duets, but a single motif type within solo songs [15]. Magpie-larks did not recognize or use information within songs on individuality or sex when assessing the number of callers. Although there are no obvious sex differences in song [15,25], magpie-larks can respond differently to the playback of an unfamiliar male and female solo song [33], and identifying the sex of callers would have allowed discrimination of true duets from pseudo-duets. Perhaps recognizing the sex of contributors within duets is more difficult than recognizing the sex of a solo singer, or conspicuous motif differences override subtle sex or individual differences, potentially explaining why magpie-larks usually avoid using the same motif within duets [25]. Indeed, partners in most species contribute different song types within duets [1,5], suggesting a general system of communicating the number of callers. Regardless of the cause, identifying duets from the number of motif types means that pseudo-duets send deceptive information on the number of callers.
Our experiment using a robotic pair of magpie-larks revealed that the visual display, when available, was the primary source of information on the number of singers. The wing display of both birds prompted stronger responses than that of a single bird regardless of the type of song or the number of motifs. The visual component of the song display therefore overrides acoustic information on the number of callers. Movements similar to magpie-lark's are present among species with varying duet structure, suggesting that visual displays are broadly important in duet evolution. For example, black-breasted wood-quail, Odontophorus leucolaemus, are like magpie-larks in that their movements accompany highly synchronized antiphonal duets [48], whereas rufous-naped wrens, Campylorhynchus rufinucha, produce movements that accompany duets in which partners sing simultaneously [49]. The function of these conspicuous visual components of duets is generally unknown, but they are likely to produce different responses from those to vocal signals alone. For example, Heuglin's robin chat duets are generally given on exposed perches and accompanied by rhythmical body, tail and wing movements [30], and even the presentation of closely perched taxidermic models affected the responses of birds to subsequent vocal duets [37]. This result suggests that vocal duetting is more effective when combined with visual information on pair cooperation. Clearly, we need further research on the presence and function of the visual components of cooperative displays.
We suggest, further, that the visual component of duet displays may have evolved in birds as the most reliable of multiple possible ways to enhance signal honesty and reduce the risk of deceptive pseudo-duetting (see also [37]). Ways to enhance reliability are likely to vary with habitat and signal properties. In open habitat, like that of magpie-larks, visual displays should often provide a reliable measure of the number and location of singers. However, duet structure, sex and individual specificity, and singing locations may all enhance signal reliability, especially in visually occluded habitats. Any feature of duet structure that is difficult for one individual to sing would constrain pseudo-duetting. In magpie-larks, antiphonal pseudo-duets are rare, perhaps because they are more difficult for individuals to mimic than sequential duets (above). If they are indeed difficult to mimic, and other individuals did not treat sequential duets as duets at all, then antiphonal structure itself could constrain deception. This might be true in other species, or even in magpie-larks if pseudo-duetting was common. Duetting in which individuals sing in unison might also constrain deception, although the dual structure of the avian syrinx means that an individual can produce two sounds simultaneously [50]. In birds that sing in pairs, deceptive pseudo-duetting would also be recognized if there were easily identified and invariant differences in songs or voice quality between the sexes, although individuals of some species can occasionally produce song types normally given by the other sex [51]. Similarly, distinct individual differences in voice or repertoire might also allow recognition of true duets, although the net cost of discrimination could be too high with a low probability of deception [52]. If individuals were always spatially separate when duetting, then spatial separation could help distinguish duets from pseudo-duets. However, spatial separation is likely to carry costs for cooperative defence [53], and can lead to poorly coordinated duets (above). Furthermore, many species, including those such as the black-bellied wren, Pheugopedius fasciatoventris, that inhabit dense vegetation generally approach a distant partner before replying to form a duet [1,18]. Finally, duets potentially have multiple functions [1,3], and deception may not be a problem if its function entails no conflict of interest between signaller and intended recipient, which may even be its partner [3].
Supplementary Material
Acknowledgements
We thank Joanna Rusin for assistance in the field, and Tomasz Osiejuk, Michał Budka, Andrew Cockburn, Brani Igic, Naomi Langmore, Jessica McLachlan and the Avian Ecology group at the ANU for discussion and insightful comments on the manuscript.
Ethics
This work was carried out with permission from the Australian Bird and Bat Banding Scheme, Environment ACT, Australian University Ethics Committee (A2014/17).
Authors' contributions
P.R. and R.M. conceived the study and contributed to writing the manuscript. P.R. conducted the study.
Competing interests
We have no competing interests.
Funding
This work was supported by a Polish Ministry of Science and Higher Education ‘Mobility Plus’ scholarship to P.R., National Science Centre, Poland (grant no. 2015/18/E/NZ8/00477), and the Research School of Biology at the Australian National University.
References
- 1.Hall ML. 2009. A review of vocal duetting in birds. Adv. Stud. Behav. 40, 67–121. ( 10.1016/S0065-3454(09)40003-2) [DOI] [Google Scholar]
- 2.Farabaugh SM. 1982. The ecological and social significance of duetting. In Acoustic communication in birds (eds Kroodsma DE, Miller EH), pp. 85–124. New York, NY: Academic Press. [Google Scholar]
- 3.Hall ML. 2004. A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430. ( 10.1007/s00265-003-0741-x) [DOI] [Google Scholar]
- 4.Benedict L. 2008. Occurrence and life history correlates of vocal duetting in North American passerines. J. Avian Biol. 39, 57–65. ( 10.1111/j.0908-8857.2008.04103.x) [DOI] [Google Scholar]
- 5.Todt D, Naguib M. 2000. Vocal interactions in birds: the use of song as a model in communication. Adv. Stud. Behav. 29, 247–296. ( 10.1016/S0065-3454(08)60107-2) [DOI] [Google Scholar]
- 6.Krebs JR. 1977. The significance of song repertoires: the Beau Geste hypothesis. Anim. Behav. 25, 475–478. ( 10.1016/0003-3472(77)90022-7) [DOI] [Google Scholar]
- 7.Wickler W. 1980. Vocal dueting and the pair bond. I. Coyness and partner commitment. A hypothesis. Z. Tierpsychol. 52, 201–209. ( 10.1111/j.1439-0310.1980.tb00711.x) [DOI] [Google Scholar]
- 8.Hall ML. 2000. The function of duetting in magpie-larks: conflict, cooperation, or commitment? Anim. Behav. 60, 667–677. ( 10.1006/anbe.2000.1517) [DOI] [PubMed] [Google Scholar]
- 9.Koloff J, Mennill DJ. 2013. The responses of duetting antbirds to stereo duet playback provide support for the joint territory defence hypothesis. Ethology 119, 462–471. ( 10.1111/eth.12084) [DOI] [Google Scholar]
- 10.Von Seibt U, Wickler W. 1977. Duettieren als revier-anzeige bei vögeln. Z. Tierpsychol. 43, 180–187. ( 10.1111/j.1439-0310.1977.tb00067.x) [DOI] [Google Scholar]
- 11.Hall ML, Magrath RD. 2007. Temporal coordination signals coalition quality. Curr. Biol. 17, R406–R407. ( 10.1016/j.cub.2007.04.022) [DOI] [PubMed] [Google Scholar]
- 12.Kovach KA, Hall ML, Vehrencamp SL, Mennill DJ. 2014. Timing isn't everything: responses of tropical wrens to coordinated duets, uncoordinated duets and alternating solos. Anim. Behav. 95, 101–109. ( 10.1016/j.anbehav.2014.06.012) [DOI] [Google Scholar]
- 13.Rivera-Cáceres KD, Quirós-Guerrero E, Araya-Salas M, Searcy WA. 2016. Neotropical wrens learn new duet rules as adults. Proc. R. Soc. B 283, 20161819 ( 10.1098/rspb.2016.1819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunkel P. 1974. Mating systems of tropical birds: the effects of weakness or absence of external reproduction-timing factors, with special reference to prolonged pair bonds. Z. Tierpsychol. 34, 265–307. ( 10.1111/j.1439-0310.1974.tb01802.x) [DOI] [Google Scholar]
- 15.Hall ML. 2006. Convergent vocal strategies of males and females are consistent with a cooperative function of duetting in Australian magpie-larks. Behaviour 143, 425–449. ( 10.1163/156853906776240623) [DOI] [Google Scholar]
- 16.Logue DM. 2005. Cooperative defence in duet singing birds. Cogn. Brain Behav. 9, e510. [Google Scholar]
- 17.Hall ML, Magrath RD. 2000. Duetting and mate-guarding in Australian magpie-larks (Grallina cyanoleuca). Behav. Ecol. Sociobiol. 47, 180–187. ( 10.1007/s002650050009) [DOI] [Google Scholar]
- 18.Logue DM, Gammon DE. 2004. Duet song and sex roles during territory defence in a tropical bird, the black-bellied wren, Thryothorus fasciatoventris. Anim. Behav. 68, 721–731. ( 10.1016/j.anbehav.2003.10.026) [DOI] [Google Scholar]
- 19.Hultsch H. 1983. Behavioural significance of duet interactions: cues from antiphonal duetting between males (Cossypha heuglini H.). Behaviour 86, 89–99. ( 10.1163/156853983X00589) [DOI] [Google Scholar]
- 20.Thorpe WH, North MEW. 1965. Origin and significance of the power of vocal imitation: with special reference to the antiphonal singing of birds. Nature 208, 219–222. ( 10.1038/208219a0) [DOI] [Google Scholar]
- 21.Zapletal ML. 1982. Pseudoduette von Cossypha heuglini. Z. Tierpsychol. 60, 119–126. ( 10.1111/j.1439-0310.1982.tb00493.x) [DOI] [Google Scholar]
- 22.Wickler W, Seibt U. 1982. Song splitting in the evolution of dueting. Z. Tierpsychol. 59, 127–140. ( 10.1111/j.1439-0310.1982.tb00334.x) [DOI] [Google Scholar]
- 23.Gwinner E, Kneutgen J. 1962. Über die biologische Bedeutung der ‘zweckdienlichen’ Anwendung erlernter laute bei Vögeln. Z. Tierpsychol. 19, 692–696. ( 10.1111/j.1439-0310.1962.tb00799.x) [DOI] [Google Scholar]
- 24.Fortune ES, Rodríguez C, Li D, Ball GF, Coleman MJ. 2011. Neural mechanisms for the coordination of duet singing in wrens. Science 334, 666–670. ( 10.1126/science.1209867) [DOI] [PubMed] [Google Scholar]
- 25.Tingay S. 1974. Antiphonal song of the magpie lark. Emu 74, 11–17. ( 10.1071/MU974011) [DOI] [Google Scholar]
- 26.Todt D, Fiebelkorn A. 1980. Display, timing and function of wing movements accompanying antiphonal duets of Cichladusa guttata. Behaviour 72, 82–106. ( 10.1163/156853980X00069) [DOI] [Google Scholar]
- 27.Zimmer KJ, Whittaker A, Oren DC. 2001. A cryptic new species of flycatcher (Tyrannidae: Suiriri) from the cerrado region of central South America. Auk 118, 56–78. ( 10.1642/0004-8038(2001)118%5B0056:acnsof%5D2.0.co;2) [DOI] [Google Scholar]
- 28.Ręk P, Magrath RD. 2016. Multimodal duetting in magpie-larks: how do vocal and visual components contribute to a cooperative signal's function? Anim. Behav. 117, 35–42. ( 10.1016/j.anbehav.2016.04.024) [DOI] [Google Scholar]
- 29.Malacarne G, Cucco M, Camanni S. 1991. Coordinated visual displays and vocal duetting in different ecological situations among Western Palearctic non-passerine birds. Ethol. Ecol. Evol. 3, 207–219. ( 10.1080/08927014.1991.9525369) [DOI] [Google Scholar]
- 30.Todt D, Hultch H, Duvall FP. 1981. Behavioural significance and social function of vocal and non-vocal displays in the monogamous duet-singer Cossypha heuglini. H. Zool. Beitr. 27, 421–448. [Google Scholar]
- 31.Stein BE. 2012. The new handbook of multisensory processing. Cambridge, MA: MIT Press. [Google Scholar]
- 32.Peter JM, Cowling SJ, Higgins PJ. 2006. Handbook of Australian, New Zealand & Antarctic birds. Volume 7, Boatbill to starlings. Melbourne, Australia: Oxford University Press. [Google Scholar]
- 33.Mulder RA, Bishop H, Cooper M, Dennis S, Koetsveld M, Marshall J, Saunders BL, Langmore NE. 2003. Alternate functions for duet and solo songs in magpie-larks, Grallina cyanoleuca. Aust. J. Zool. 51, 25–30. ( 10.1071/ZO02060) [DOI] [Google Scholar]
- 34.Hall ML. 1999. The importance of pair duration and biparental care to reproductive success in the monogamous Australian magpie-lark. Aust. J. Zool. 47, 439– 454 ( 10.1071/ZO99037) [DOI] [Google Scholar]
- 35.Hardin JW, Hilbe JM. 2002. Generalized estimating equations. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 36.Dunn OJ. 1964. Multiple comparisons using rank sums. Technometrics 6, 241–252. ( 10.2307/1266041) [DOI] [Google Scholar]
- 37.Hultsch H, Todt D. 1984. Spatial proximity between allies: a territorial signal tested in the monogamous duet singer Cossypha heuglini. Behaviour 91, 286–293. ( 10.1163/156853984X00119) [DOI] [Google Scholar]
- 38.Thorpe WH, Hall-Craggs J, Hooker B, Hooker T, Hutchison R. 1972. Duetting and antiphonal song in birds: its extent and significance. Behaviour. Supplement, III–197.
- 39.Topp SM, Mennill DJ. 2008. Seasonal variation in the duetting behaviour of rufous-and-white wrens (Thryothorus rufalbus). Behav. Ecol. Sociobiol. 62, 1107–1117. ( 10.1007/s00265-007-0538-4) [DOI] [Google Scholar]
- 40.Ruxton GD, Sherratt TN, Speed M. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press. [Google Scholar]
- 41.Lindström L, Alatalo RV, Mappes J. 1997. Imperfect Batesian mimicry: the effects of the frequency and the distastefulness of the model. Proc. R. Soc. Lond. B 264, 149–153. ( 10.1098/rspb.1997.0022) [DOI] [Google Scholar]
- 42.Dalziell AH, Welbergen JA. 2016. Mimicry for all modalities. Ecol. Lett. 19, 609–619. ( 10.1111/ele.12602) [DOI] [PubMed] [Google Scholar]
- 43.Flower TP, Gribble M, Ridley AR. 2014. Deception by flexible alarm mimicry in an African bird. Science 344, 513–516. ( 10.1126/science.1249723) [DOI] [PubMed] [Google Scholar]
- 44.Flower T. 2011. Fork-tailed drongos use deceptive mimicked alarm calls to steal food. Proc. R. Soc. B 278, 1548–1555. ( 10.1098/rspb.2010.1932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radford AN, Bell MBV, Hollén LI, Ridley AR. 2011. Singing for your supper: sentinel calling by kleptoparasites can mitigate the cost to victims. Evolution 65, 900–906. ( 10.1111/j.1558-5646.2010.01180.x) [DOI] [PubMed] [Google Scholar]
- 46.Budka M, Osiejuk TS. 2013. Neighbour–stranger call discrimination in a nocturnal rail species, the Corncrake Crex crex. J. Ornithol. 154, 685–694. ( 10.1007/s10336-013-0933-8) [DOI] [Google Scholar]
- 47.Osiejuk TS. 2014. Differences in frequency of shared song types enables neighbour-stranger discrimination in a songbird species with small song repertoire. Ethology 120, 893–903. ( 10.1111/eth.12260) [DOI] [Google Scholar]
- 48.Hale AM. 2006. The structure, context and functions of group singing in black-breasted wood-quail (Odontophorus leucolaemus). Behaviour 143, 511–533. ( 10.1163/156853906776240614) [DOI] [Google Scholar]
- 49.Bradley DW, Mennill DJ. 2009. Solos, duets and choruses: vocal behaviour of the Rufous-naped Wren (Campylorhynchus rufinucha), a cooperatively breeding neotropical songbird. J. Ornithol. 150, 743–753. ( 10.1007/s10336-009-0393-3) [DOI] [Google Scholar]
- 50.Catchpole CK, Slater PJB. 2008. Bird song: biological themes and variations, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 51.Rogers AC. 2005. Male and female song structure and singing behaviour in the duetting eastern whipbird, Psophodes olivaceus. Aust. J. Zool. 53, 157–166. ( 10.1071/ZO04083) [DOI] [Google Scholar]
- 52.Ręk P. 2014. Do aggressive signals evolve towards higher reliability or lower costs of assessment? J. Evol. Biol. 27, 2605–2613. ( 10.1111/jeb.12512) [DOI] [PubMed] [Google Scholar]
- 53.Rogers A, Ferguson J, Harrington H, Mcdowell S, Miller A, Panagos J. 2004. Use of stereo duet playback to investigate traditional duet playback methods and mechanisms of cooperative territorial defence in magpie-larks. Behaviour 141, 741–753. ( 10.1163/1568539042245169) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.