Abstarct
The potential disturbance of dolphins from tourism boats has been widely discussed in the literature, in terms of both physical vessel presence and associated underwater noise. However, less attention has been paid to the potential impact of non-tourism vessels, despite these being much more widespread and occurring in greater numbers throughout coastal dolphin habitats. The Indo-Pacific bottlenose dolphin (T. aduncus) community using the Fremantle Inner Harbour, Western Australia, is exposed to high levels of vessel traffic. To investigate whether behavioural responses could be occurring, a non-invasive combination of visual and acoustic monitoring was conducted using a theodolite and an autonomous acoustic logger. Dolphins significantly increased their average movement speeds in high vessel densities, but only for some activity states. Behavioural budgets also changed in the presence of vessels, with animals spending greater time travelling and less time resting or socialising. Finally, multiple whistle characteristics varied with rising levels of broadband noise, and other contextual variables. Despite being acoustically specialised for higher frequencies, dolphins had the strongest acoustic variation during low-frequency noise. This study highlights the complexity of disturbance responses in this species, confirming the need for consideration of both surface and acoustic behaviour alongside appropriate contextual data.
Introduction
In coastal marine environments, vessel traffic is the most ubiquitous anthropogenic activity with the potential to disturb marine wildlife1,2. With wildlife-watching tours becoming increasingly popular, tourism vessels have been the focus of concerns regarding animal disturbance, particularly for coastal species such as bottlenose dolphins (Tursiops sp.) whose core habitats overlap with human activities and are relatively accessible for research programmes3–17. The literature reports varying degrees of tourism-related disturbance to dolphins – from relatively subtle changes in vocal behaviour to broad-scale animal movements away from the affected area. However, comparatively little attention has been paid to the potential impacts of recreational vessels, despite predictions of significant growth in vessel traffic over coming years18. Recreational vessels already occur in high numbers in some areas19–23, and thus have the potential to elicit dolphin behavioural responses despite not being actively engaged in wildlife-watching activities. This is again of particular concern with regard to coastal dolphin species, who are exposed to higher levels of human activity as well as the potential risk of cumulative impacts from repeated, long-term exposure to vessel traffic24–26.
Previous studies of vessel disturbance effects have often focused on dolphin displacement or changes in occurrence/site occupancy4,6,27–29. However, such an approach ignores the complex array of factors determining animal habitat-use in human-dominated seascapes30. Some studies have taken this next step and yielded evidence that dolphins may alter their movement patterns, for example by changing travel direction to orient away from/move around approaching vessels, beginning to travel erratically, or significantly increasing their travelling speeds9,17,23,24,31,32. Changes to activity states or behavioural budgets have also been reported, with travelling behaviour generally increasing with vessel presence, whilst resting and socialising become more infrequent3,8,9,12,15. Foraging is less predictable, with some studies reporting greater foraging activity in the vicinity of boats11, whilst others suggest the contrary3,8,33.
Less obvious to human observers are potential changes in dolphin acoustic behaviour in response to vessel traffic and associated underwater noise. Dolphins have been observed to adjust some characteristics of their whistles in elevated noise conditions or in the presence of tourism vessels, for instance by modifying their frequency range5,13,34–37, duration5,13,35, or the number of frequency modulations36. Increases in whistle production rates have also been reported7,13,19. These changes presumably stem from impaired communication efficiency as a result of vessel noise38. However, changes in acoustic behaviour can also occur in association with other factors, for example different surface behaviours, group sizes, group compositions, or even geographic location5,13,39–45. The plasticity of dolphin whistles requires consideration of context when investigating potential acoustic responses to human activities.
Yet, few studies control for additional explanatory variables to provide suitable context, or recognize that behavioural and acoustical responses are not necessarily mutually exclusive. In practice, it can be difficult to disentangle the effects of physical boat presence and associated noise28,46, with results also being confounded by the fact that behavioural studies are frequently conducted from research vessels that could themselves elicit responses12,13,34. Moreover, the way in which one population responds to vessel traffic does not necessarily mean that all populations will respond in the same manner46. Dolphin communities vary in terms of their vessel exposure histories, home range environmental conditions, and survival pressures such as prey availability or predator densities. Thus, dolphin communities should be examined on a “case by case” basis, especially when considering the conservation of small communities or populations particularly susceptible to threats due to their small numbers and low reproductive rates25,26. There is a need for studies investigating a range of dolphin-vessel contexts with regard to recreational vessel traffic, from both behavioural and acoustical perspectives, preferably conducted from land-based research platforms.
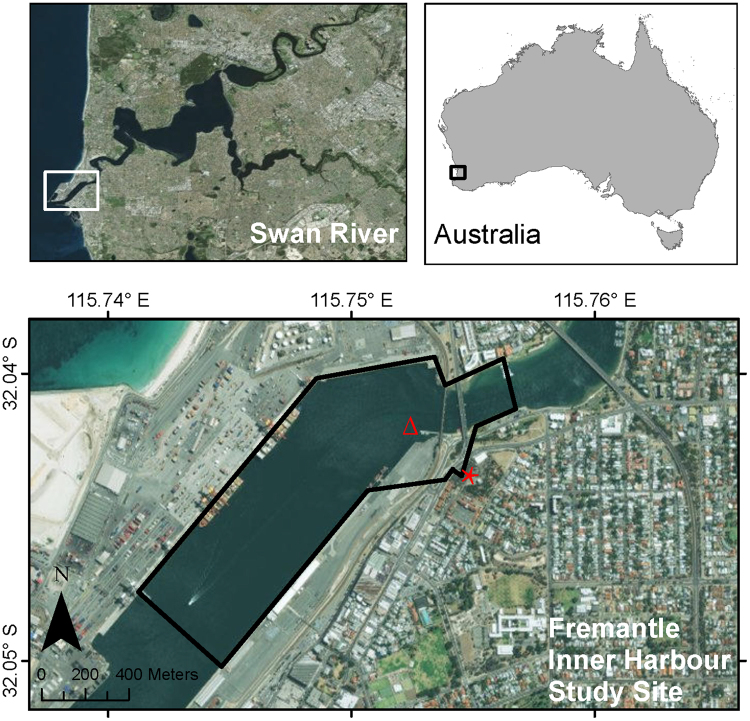
Here, we consider the behavioural and acoustical responses of a dolphin community in Western Australia to vessel traffic and underwater noise. Recreational vessel traffic is on the rise in Australia, with the country currently experiencing rapidly growing recreation use of marine resources. Between 1999 and 2009, there was a 44% increase in the number of recreational vessels registered in Western Australia47. There are currently approximately 95,000 recreational vessels registered in the state, with over half of these within the state capital of Perth (J. Nunn; personal communication, 21st January 2014). Consequently, the Swan-Canning River system, which flows through the centre of Perth, receives high levels of vessel traffic22. The Fremantle Inner Harbour (Fig. 1) is part of an industrial port at the river mouth, and acts as a gateway between the Swan River and the Indian Ocean. It has been identified as the anthropogenically-noisiest site in the river, with transits of up to 56 vessels per hour22,48. But despite being a busy, noisy site, the Fremantle Inner Harbour is frequently used by the river’s resident community of Indo-Pacific bottlenose dolphins (T. aduncus). This community consists of approximately 18 adults, plus several juveniles and calves, which use the harbour as one of several “hotspots”49–52. Dolphins have been shown to remain within the Fremantle Inner Harbour during periods of high vessel traffic, with no alterations to their presence/absence, number of sightings, or duration of time spent in this area in relation to vessel density22. However, behavioural responses could be occurring at a finer scale, despite the lack of site avoidance. Since the manner in which dolphins respond to vessel traffic could have implications to their health, the effects of vessel traffic and underwater noise must be quantified.
Figure 1.
Map of the Fremantle Inner Harbour study area, showing the location of the theodolite hill-top station (red star) and acoustic logger deployment (Δ). Maps were created in ArcGIS® (version 10.1) by ESRI (www.esri.com) using the World Imagery basemap (sources: Esri, DigitalGlobe, Earthstar Geographics, CNES/Airbus DS, GeoEye, USDA FSA, USGS, Getmapping, Aerogrid, IGN, IGP, and the GIS User Community; http://goto.arcgisonline.com/maps/World_Imagery).
This study aims to investigate the behavioural responses of bottlenose dolphins to vessel traffic and acoustical behaviour in varying underwater noise conditions in the Fremantle Inner Harbour. To achieve this, dolphin movement speeds, activity states and whistle characteristics in varying levels of vessel traffic and underwater noise were examined. Data were collected using autonomous acoustic recordings and land-based visual observations to minimise bias from the presence of a research vessel. Additionally, contextual covariates (such as behaviour, group size, and calf presence) were recorded for inclusion in analyses. The resulting knowledge on fine-scale behavioural response to vessels will improve management practices and conservation outcomes.
Results
A total of 217 h of visual observations were undertaken during 83 surveys over three years. Surveys ranged in duration from 0.5 to 4.1 h, and yielded a total of 185 dolphin group sightings in the study area. Of the cumulative 83 h of 5-min samples collected, 28% of dolphin observations occurred in high vessel-density conditions (i.e. a median of over three vessels per 5-min sample).
Dolphin Movement Speed
Average dolphin group speeds ranged from 0.03 to 2.67 m/s per 5-min sample (n = 782), and differed significantly among the five activity states, as defined in Supplementary Table S1 (Kruskal-Wallis Test; X 2 = 77.381, df = 4, p < 0.001).
Average speeds for travelling were significantly higher than during all other activity states (Mann-Whitney U Tests; p < 0.01), whilst averaging speeds during resting were significantly lower than during all other activity states (p < 0.05; Fig. 2a). There were no significant differences (p > 0.05) between average speed during foraging, milling and socialising.
Figure 2.
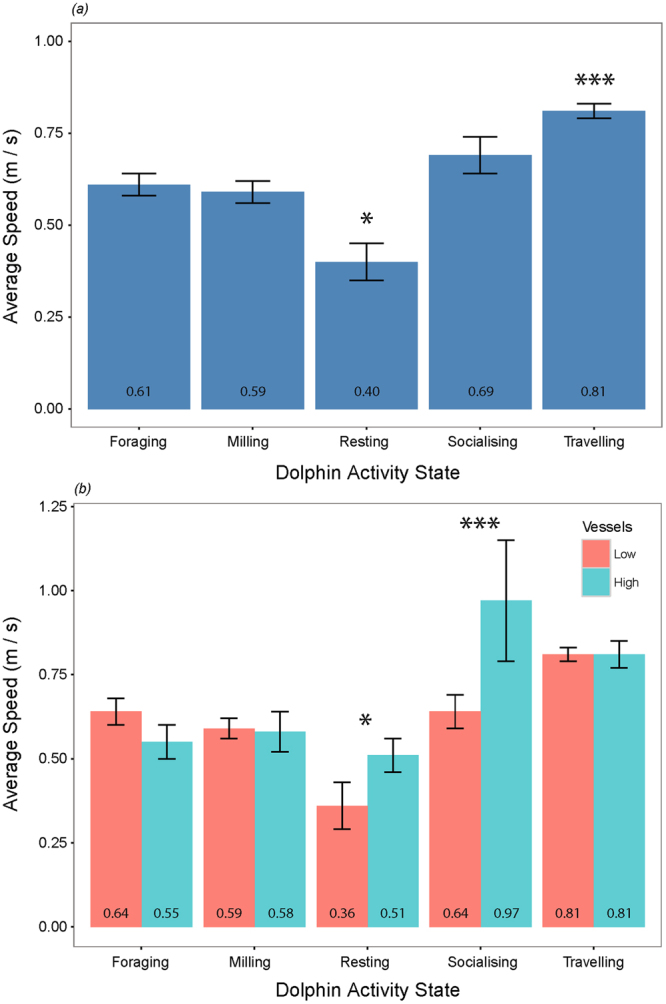
Average dolphin group movement speed according to (a) activity state, and (b) high and low vessel densities for each activity state. Definitions for each activity state are provided in Supplementary Table S1. Numbers represent the average speed in m/s. Error bars represent the standard error. Significance level: ***≤0.001; **≤0.01; *≤0.05.
Activity states were considered independently for different levels of vessel traffic (low or high). Mann-Whitney U Tests showed no significant differences (p > 0.05) in average speed between vessel contexts for travelling, foraging or milling. Both resting and socialising average speeds significantly increased with increasing vessel densities (respectively, U = 176.5, p = 0.01673; U = 503, p = 0.04216; Fig. 2a). However, these results should be interpreted with caution given the low sample sizes associated with high vessel densities (high n = 10 and 11, respectively).
Dolphin Activity State
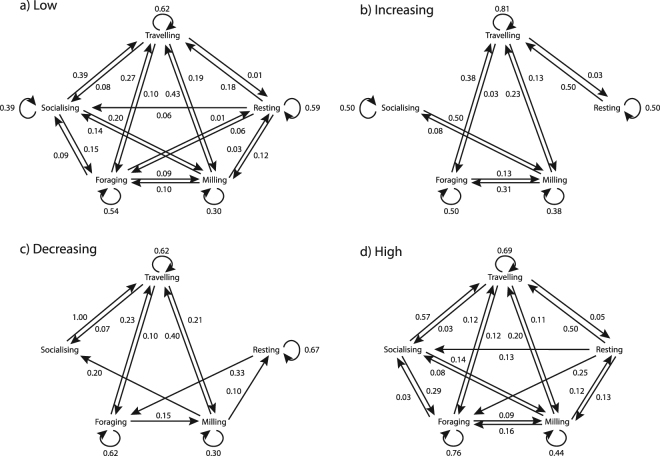
A total of 714 activity state transitions were extracted from sequences of two adjacent 5-min samples. Transitions included 462 in Low vessel traffic, 139 in High vessel traffic, 56 in Increasing vessel traffic, and 57 transitions in Decreasing traffic conditions.
In any vessel traffic context, transition probabilities indicated that dolphins were most likely to continue travelling or foraging if that was the behaviour they were engaged in previously (with some exceptions, Fig. 3). At High vessel densities, resting was most likely to transition to travelling, as was socialising. For Decreasing vessel densities, milling and resting animals were both most likely to begin travelling. By contrast, at Increasing vessel densities, resting and socialising were as likely to continue as they were to transition into travelling or milling activities.
Figure 3.
Markov chains representing transition probabilities between preceding and succeeding activity states in four different vessel contexts: (a) Low; (b) Increasing; (c) Decreasing; and (d) High vessel traffic. Definitions for each activity state are provided in Supplementary Table S1.
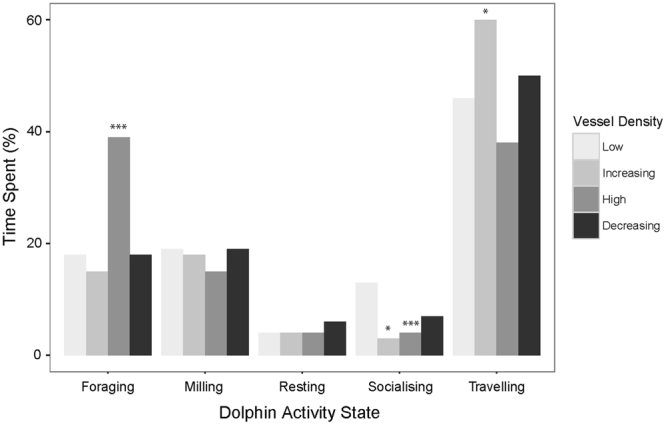
Small sample sizes for some behaviours and vessel contexts prevented these from being statistically tested. Out of 75 possible activity state comparisons between the Low vessel density and the other vessel impact conditions, 12 statistical comparisons were possible. For Low versus Increasing, activity state comparisons were made between travelling-to-travelling and milling-to-milling transition probabilities. For Low versus High, activity state comparisons were travelling-travelling, travelling-milling, travelling-foraging, milling-travelling, milling-milling, milling-foraging, and foraging-foraging. Finally, for Low versus Decreasing, activity state comparisons were travelling-travelling, travelling-milling and foraging-foraging. Of these 12 possible comparisons, only three were significantly different from the Low vessel context. When vessel density was Increasing, the probability of travelling-to-travelling (i.e. no transition in travelling behaviour) increased (z = 2.1, p = 0.038). When vessel density was High, the probability of milling transitioning to travelling decreased (z = 2.1, p = 0.036) and the probability of foraging to continue (foraging-to-foraging transition) increased (z = 2.2, p = 0.028).
Behavioural budget comparisons indicated that dolphins spent a greater proportion of time travelling (z = 2, p = 0.0476) and less time socialising (z = 2.2, p = 0.0292) when exposed to Increasing vessel densities than in Low conditions (Fig. 4). When vessel density was High, the proportion of time spent socialising was also significantly reduced (z = 3, p = 0.0029), whereas foraging significantly increased relative to Low vessel densities (z = 5.2, p < 0.001). The proportion of time spent in different activity states was not significantly different between Low and Decreasing vessel densities (all p > 0.05).
Figure 4.
Behavioural budgets of dolphins in four different vessel contexts (Low, Decreasing, Increasing and High). Results of Z-Test comparisons between Low and other vessel contexts are indicated: ***≤0.001; **≤0.01; *≤0.05.
Whistle Characteristics
All of the whistle characteristics measured were associated with mean broadband noise level (NL_BB) and dolphin activity state (Supplementary Table S2), and to a lesser extent with group size and the presence of calves. GAMs were fitted to 161 of the 164 whistles, after three were removed as outliers. GAMs included all whistle characteristic measures as response variables, except for the number of breaks or saddles due to small sample sizes (<20 whistles).
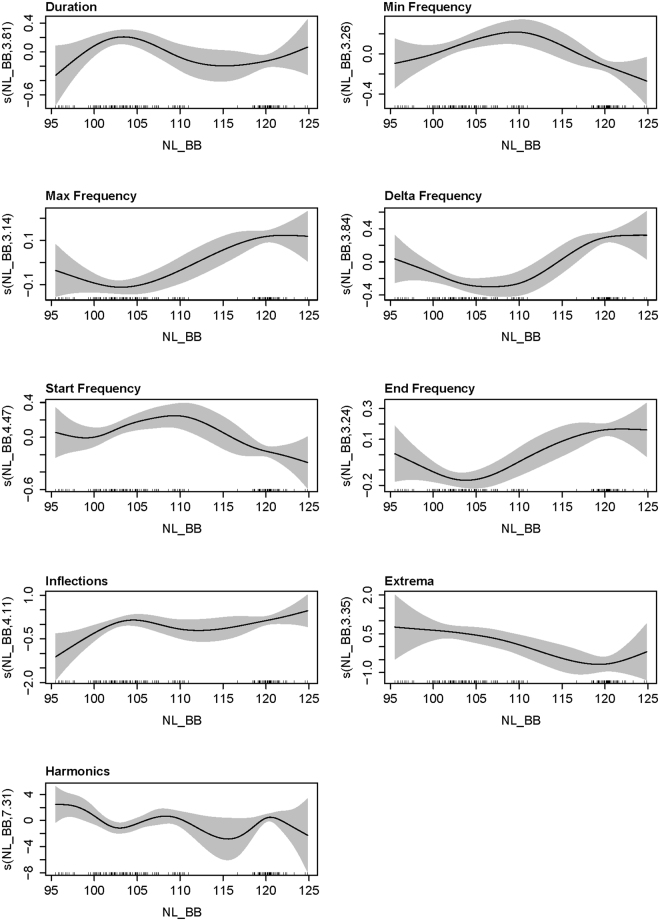
NL_BB over the whole recording period were approximately 110 dB re 1 µPa rms (10 Hz–48 kHz). Whistle duration, minimum frequency, start frequency, number of extrema and presence of harmonics decreased with increasing NL_BB (Fig. 5). In comparison, maximum frequency, end frequency, and change in frequency (delta frequency) typically increased with increasing noise.
Figure 5.
Results of the nine whistle characteristic GAMs which selected broadband noise level (NL_BB) as a significant explanatory variable.
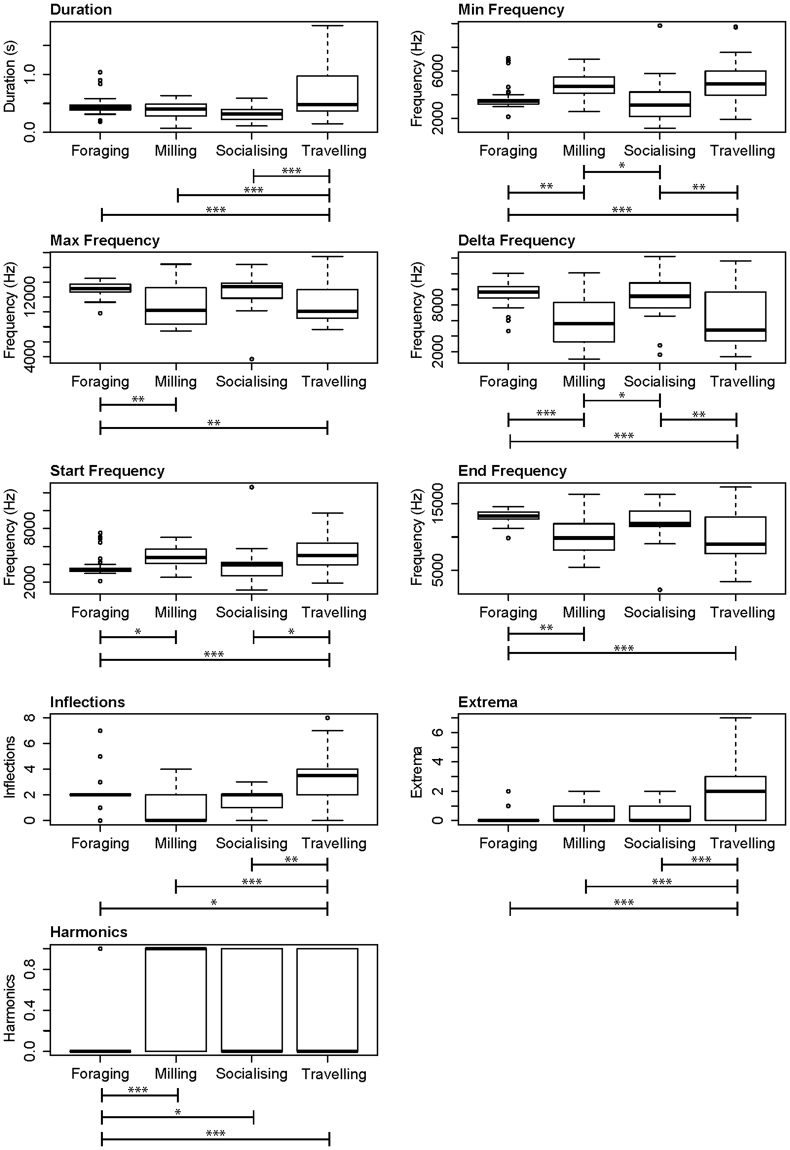
The majority of the whistles were produced when the predominant activity state of the group was travelling (51%), followed by foraging (25%), milling (13%) and socialising (10%). No high-quality whistles were obtained whilst animals were resting. Differences in all whistle characteristics occurred between travelling and foraging dolphins (Supplementary Table S2; Tukey post-hoc tests, Fig. 6). All frequency measurements and harmonics were significantly different between milling and foraging dolphins, representing two-thirds of all whistle characteristics. Similarly, two-thirds of whistle characteristics significantly differed between travelling and socialising dolphins, namely: duration, minimum frequency, delta frequency, start frequency, inflection points, and extrema. Fewer differences existed between the remaining activity state pairs. Differences included those in duration, inflection points, and extrema between travelling and milling; minimum and delta frequencies between socialising and milling; and the presence of harmonics between socialising and foraging.
Figure 6.
Results of the nine whistle characteristic GAMs which selected dolphin activity state as a significant explanatory variable. Post-hoc tests were used to test for differences between behavioural states. Significance level: ***≤0.001; **≤0.01; *≤0.05.
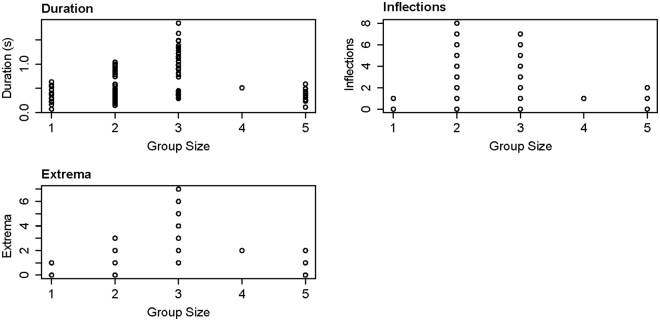
Whistles were all produced in the presence of single groups composed of 1 to 5 dolphins (mean = 2.4). Group size was only significantly related to whistle duration, maximum frequency, delta frequency, inflection points and extrema (Supplementary Table S2; Fig. 7). Values for these characteristics were typically highest for group sizes of two or three animals.
Figure 7.
Results of the three whistle characteristic GAMs which selected dolphin group size as a significant explanatory variable.
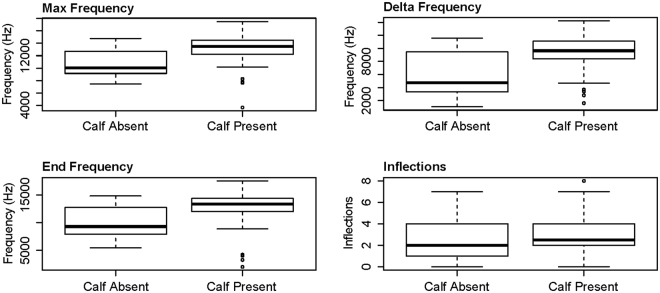
Of the whistles analysed, 41% were produced in the presence of dolphin calves. The presence of a calf significantly affected the maximum, delta and end frequency characteristics and inflection points (Supplementary Table S2; Fig. 8), with all mean values increasing.
Figure 8.
Results of the four whistle characteristic GAMs which selected the presence of dolphin calves as a significant explanatory variable.
Investigation into noise levels at specific frequency levels revealed associations between whistle characteristics and twelve octave-band levels (OBLs). However, the bands showed high collinearity, and only three (with VIFs < 5) were accordingly included in GAMs: 1 kHz, 16 kHz and 32 kHz. While dolphins have been reported to show sensitivity to noise at 100 Hz53,54 and even to produce sounds with fundamental frequencies as low as 260 Hz55, only the three OBLs listed above were included as they are within the frequency range of most dolphin whistles recorded. The 1 kHz OBL (NL_1000) was associated with all whistle characteristics in the best fitting models (Supplementary Table S3, Supplementary Figure S4). Whistle duration, minimum frequency, start frequency, extrema and harmonics generally declined when NL_1000 was high, whereas the remaining frequency characteristics typically increased. Inflections showed variable results. The 16 kHz OBL (NL_16000) was associated with maximum frequency, inflections and harmonics, which all decreased as NL_16000 increased (Supplementary Table S3, Supplementary Figure S5). Finally, the 32 kHz OBL (NL_32000) was associated with six whistle characteristics; typically with increasing duration, extrema and harmonics, decreasing delta frequency and end frequency, and displaying mixed results for inflections (Supplementary Tables S3 and S6). Based on the number of characteristics affected, lower-frequency noise generally was overall more strongly associated with dolphin whistle characteristics than higher-frequency noise.
Discussion
This study showed significant changes in bottlenose dolphin behaviour in Fremantle Inner Harbour in association with high vessel densities and underwater noise. Animals’ responses to stressors can take a variety of forms. For instance, whilst a previous study conducted in Fremantle Inner Harbour documented reduced dolphin detection rates during pile-driving activity56, more recent research at the same site found no alteration in the occurrence of dolphins in heavy vessel traffic22. Here, dolphins did appear to change movement speeds and activity states in response to vessel density, whilst whistle characteristics differed in varying underwater noise conditions.
While the average travel rates of dolphin groups were a function of activity state, their movement speed was also affected by vessel traffic. Animals moved fastest during travelling and slowest during resting activities. Resting and socialising groups significantly increased their average speed when vessel density was high. Captive studies suggest that higher swim speeds induce greater metabolic rates57,58. The risk of impact may be particularly high for resting animals, in that they expend more energy than they would otherwise and their quality of rest is reduced. Resting is important for overall animal health. Disruption to resting activities can induce stress as well as reduce energy reserves, which ultimately affect an animal’s condition and immune function12,59–62. Thus an increase in dolphin movement speed whilst resting could have both physiological and energetic consequences at the individual-level.
Resting and socialising activity states were mainly observed during low vessel densities. While there was an observed increase in resting after high vessel densities, the increase was not statistically significant. This may ensue from the limited power of the analysis caused by small sample sizes for this behaviour. Resting occurring during low vessel densities could reflect animals that were capitalising on less-busy periods to rest following exposure to high vessel traffic. In addition, female dolphins mainly nurse their calves while resting9. The slight increase in resting behaviour after exposure to high vessel densities could therefore represent an opportunity for nursing. There were no differences between behavioural budgets during low and decreasing vessel contexts for any of the activity states. Thus, dolphins may make use of low or decreasing vessel density periods to return to their original activities.
Time spent socialising significantly shortened during increasing and high vessel densities. Socialising behaviour is important for the maintenance of conspecific relationships, calf play, and mating opportunities. If these behaviours are repeatedly interrupted there could be long-term, population-level consequences. For example, fewer successful mating attempts could result in lower reproduction rates and thus influence population dynamics. Play behaviour has been shown to be important to the development of dolphin calves as a method of learning physical manoeuvres, object manipulation, problem-solving skills, social behaviours, and foraging methods63–65. But play behaviour is less likely to occur in stressful situations66, and could also be disrupted if socialising events become less frequent. Decreased socialising opportunities for calves may therefore impact reproductive or foraging success when they become adults.
Dolphins spent more time travelling whilst vessel density was increasing. Travelling typically occurs at greater average movement speeds than other activity states, so may lead to increased energetic demands. Time spent travelling also leaves fewer opportunities to engage in other vital behaviours such as foraging or resting. Once vessel density was high, dolphins increased the time they spent foraging. Christiansen et al.11 also reported an increase in foraging when bottlenose dolphins encountered tour boats; yet many other studies have provided evidence to the contrary3,8,33. Increased foraging activity could be an attempt to compensate for energy lost during disturbance, or could alternatively reflect changes in prey behaviours. For example, fish may scatter in response to vessel transits, making them easier to capture. The observed foraging behaviour may also have been erroneously reported. What was perceived as foraging behaviour could have been an increase in transitions to diving in the presence of tour boats, which has previously been hypothesised to represent vessel avoidance by utilising a different part of the water column15. Because high vessel densities characterised approximately 28% of the dolphin behavioural samples collected, dolphins could be expected to spend a significant proportion of time expending energy by altering their activities. If these indeed result in increased energy requirements, reduced fitness at both individual and population levels may occur17.
Different vocal behaviours were also observed in contrasting noise conditions associated with presence of vessel traffic (among other sources). When noise levels were relatively high (≥110 dB re 1 µPa rms [10 Hz–48 kHz]), whistles were typically short with a wide frequency range and had multiple inflection points and fewer extrema (similar to ‘complex’ whistles). At higher noise levels, harmonics were more frequently absent, though this could be the result of masking by noise at overlapping frequencies. Noise at lower frequencies was associated with the broadest differences in whistles, as differences were observed in all characteristics.
Whilst whistle characteristics varied with noise levels, they also varied according to activity state, group size and calf presence. The whistles of foraging and socialising groups were typically associated with whistles of short duration and wide frequency range, starting low and ending high, with few inflections and extrema (similar to ‘upsweeps’). Whistles recorded in the presence of milling dolphins were also relatively short but had a smaller frequency range, with low numbers of inflection points and extrema, though they generally included harmonics. Travelling groups exhibited longer-duration whistles that spanned a narrower frequency range but contained the highest numbers of inflections and extrema (similar to ‘sine’ whistles). Whistles recorded when dolphin duos or trios were present were typically of longer duration, with higher maximum and delta frequency values (but unchanged minimum frequency) and contained a greater number of inflections and extrema compared to singletons and group of four and five animals. And finally, whistles recorded when calves were present typically started at a lower frequency and ended at a higher frequency (giving a correspondingly high delta-frequency), plus had lower minimum and higher maximum frequency values and a greater number of inflection points.
Although the whistle sample size was relatively large, the number of unique dolphin groups from which the samples were derived was very limited (n = 11). Merely three of these groups produced the majority of whistles analysed in this study, with remaining groups contributing < 20 whistles each. These three groups were engaged in one of two predominant activities (foraging or socialising), and were of specific group sizes (2 or 3 individuals) and compositions (two groups without calves, one with a calf). In this context, and despite the lack of apparent collinearity between noise levels and activity state, group size or presence of calves, it remains unclear whether noise is ultimately a proximate driver of the observed variation in whistle characteristics. Future research should focus on disentangling the effects of group from activity state, group size and calf presence. Furthermore, it was not possible to identify which individuals were producing the whistles. It is therefore possible that some individuals were re-sampled in this study, and that signature whistles unique to individual dolphins were captured multiple times. Bottlenose dolphins’ whistles are highly plastic, which facilitates adaptation to dynamic environmental conditions35 and communication requirements. In the future, it would be beneficial to examine group composition in terms of which individual dolphins are present with regards to alterations in whistle characteristics in association with noise levels. This will help to clarify the most important drivers of whistle variations.
Other recommended future work involves quantifying energetic consequences of disturbance, collecting finer-scale information on behavioural and acoustical responses, comparing dolphin responses within the Fremantle Inner Harbour with those of other Swan River sites, and determining which vessel characteristics may elicit responses. Previous studies have found that dolphin metabolic rates increase during periods of sound production, and may take several minutes to return to resting values67. Similarly, if increased vessel density causes increases in travelling or foraging behaviours that are associated with greater energy demands for communication, the effects from boat traffic could be potentially magnified. This not only carries implications for dolphin energetic requirements but also highlights the complexity of disturbance responses in this species. Quantifying the impacts of vessel traffic on dolphin metabolism from a conservation physiology perspective would also be further enhanced by obtaining behavioural response information at an even finer resolution, e.g. by monitoring surfacing patterns, dive times, inter-animal spacing, within-site movements, whistle rates and individual behavioural events7,9,13,16,17,19,24,68,69. How the responses of Swan River dolphins vary between sites, as well as in comparison with other dolphin communities, could highlight areas of conservation priority. For example, some locations may be mainly used for travelling or foraging, whereas others may act as resting or nursing grounds with an inherently higher risk of disruption. Individuals may even select to use ‘quieter’ sites for resting or socialising to compensate for decreases in these activity states at busier or noisier sites. Some populations may naturally be less energetically-stressed than others, thus being able to better cope with greater energy demands during disturbance. Sites also vary with regard to their use by vessels. Finally, considering the range of vessel types observed within the Fremantle Inner Harbour22, it would be beneficial to investigate dolphin responses in terms of vessel manoeuverability, speed and noise characteristics. This could inform guidelines for boat operations within important dolphin habitats, and would be particularly relevant should dolphin-watch tourism activities (which are not currently present in the Swan River) commence. Tourism operations could result in behavioural responses occurring more frequently and intensely, and represent a broader range of responses than those observed in this study. Overall, this study expands upon previous behavioural response work on this dolphin community22, 56, and sets the scene for future research.
In conclusion, dolphins within Fremantle Inner Harbour changed their movement speeds and activity states in increasing vessel traffic. Whistle characteristics varied with different underwater noise conditions, although whether these were changes in response to noise remains unclear. While dolphins’ behaviour changed, they did not avoid the site altogether in response to high vessel densities22. The behavioural responses observed in this study raises concerns regarding the potential for increased energetic demands for dolphins, through increased movement speeds, greater time spent travelling, less time resting/socialising, and increased vocal effort. However, further work is needed to determine whether some of these energy requirements could be offset by the energy gained foraging at this site. The capture probability of prey responding to transiting vessels may even be improved. Further research is required to examine dolphin target prey species, prey responses to vessel traffic, and the possibility of vertical avoidance strategies by dolphins within the Fremantle Inner Harbour. The complexity of disturbance responses in this species justifies the inclusion of contextual information in future behavioural response studies.
Methods
Data Collection
Land-based visual surveys were conducted from a 32 m vantage point (32.0436°S, 115.7542° E) overlooking the Fremantle Inner Harbour (Fig. 1). Surveys occurred in May – June 2012, February – June 2014 and April – June 2015, covering months which coincided with a period of peak dolphin sightings in this area52. Surveys were only undertaken in good weather conditions (low glare, high visibility, Beaufort <4, temperatures <35 °C).
A surveyor’s theodolite (TopCon GTS-603 AF Electronic Total Station) was used to obtain positions of dolphin groups and vessels, with data recorded in real-time using the Vadar (vr 2.00.01b) software (E. Kniest, University of Newcastle). When dolphins were sighted, an initial theodolite position was taken, whilst group size, composition and activity state were noted by observers using 7 × 50 mm Bushnell binoculars. A “group” was defined as an association of dolphins in close proximity engaged in the same direction of travel and/or general activity. Five activity states were used: foraging, milling, resting, socialising, and travelling. These activity states were mutually exclusive, and similar to those used in other studies15,70. The predominant activity state was considered as the activity based on the most frequently observed behaviour. A theodolite position was taken at every surfacing interval while within the study area, with the exception of times when the surfacing interval was too brief to obtain an accurate reading. Vessels were tracked continuously during their time in the study area (irrespective of dolphin presence), using the same theodolite. Vessel identity was noted based on either the name or a relevant physical description (hull/canopy colour, crew, distinguishing features, etc.) to allow continual tracking, regardless of the number of vessels present.
Each survey was divided into 5-min samples, starting at integer multiples of 5 min. For each 5-min behavioural sample, the dolphins’ predominant activity state, group size and composition were noted. To determine the context of vessel traffic, vessel densities were calculated based on the number of vessels present in the study area during any 5-min sample. Vessel densities were then categorised according to a cut-off value based on the median number of vessels observed in any 5-min sample across the whole study period (median = 3). This resulted in ‘low’ and ‘high’ vessel traffic respectively being 0–3 and 4–16 vessels per 5-min sample.
Acoustic data collection took place concurrently with the April – June 2015 visual surveys. A high-frequency autonomous acoustic logger, equipped with a programmable 16-bit digital sound recorder (made by Wildlife Acoustics Inc) and an external hydrophone (Reson TC4033-1, sensitivity −202.8 dB re 1 µPa/V), was deployed within the Fremantle Inner Harbour (32.0420 S, 115.7528°E; Fig. 1). The hydrophone entered the housing via a bulkhead connector to an impedance matching pre-amplifier with 20 dB gain. Digitised recordings were written to four 128 GB SD cards. Before deployment, the logger was calibrated by applying white noise of known power spectral density. An 8 Hz high-pass filter was employed to filter out high levels of low-frequency noise, thus enhancing the dynamic range of the recorder at the frequencies of interest. The logger recorded at a duty cycle of 10 min every 15 min at a sampling frequency of 96 kHz.
Movement Speeds
Vadar automatically calculates a target’s speed of travel based on the time and location of the previous versus the current theodolite position. Only samples with a minimum of two theodolite measurements were used, to ensure sufficient observations for speed calculations. This also ensured reliable speed estimates for animals ‘looping’ around the area. The average speed across all theodolite positions was then calculated for each 5-min sample, and paired with the corresponding dolphin activity state and level of vessel traffic, as defined above.
Analyses were undertaken by using a Kruskal-Wallis Test to determine whether activity state was associated with average speed. Subsequent Mann-Whitney U Tests were used to identify the source of differences. A series of Mann-Whitney U Tests were then conducted comparing average speeds during low and high vessel traffic for each activity state. These non-parametric tests were applied because a Shapiro-Wilk Test showed that the data were not normally distributed. All tests were conducted in R using the stats package71.
Activity States
Assessing variation in animal behaviour is difficult due to the inherent temporal dynamics of activity states and changing contextual conditions. To quantify this temporal dependence and how context may change behaviour, Markov chains were employed to model dolphin responses to vessel density based on surface behaviour. First-order Markov chains were chosen, as these describe events which depend on the immediately preceding event72; thus, each activity state was examined with regard to the immediately preceding behaviour. The following methodology is an adaptation of Lusseau15, where the background statistics are extensively described.
Vessel traffic conditions were categorised as either: (1) Low, (2) High, (3) Increasing, or (4) Decreasing. These definitions considered the preceding and succeeding samples, and were based on the cut-off value of 3 vessels per 5-min sample as described above. For example, if both the preceding and succeeding behaviours occurred at low vessel densities, conditions were classed as ‘Low’. However, if the preceding and succeeding behaviours occurred at low and high vessel densities respectively, conditions were classed as ‘Increasing’.
A “transition” occurred when a dolphin group changed from a preceding activity state i to a succeeding activity state j, under a given vessel context. These transitions were recorded in a three-way contingency table, considering preceding versus succeeding activity state for each vessel traffic condition. Based on this table, transition probabilities were computed to detect the effect of changing vessel densities on dolphin activity states. The transition probability was calculated by dividing the number of transitions by the total number of times i was seen as the preceding behaviour:
where a ij is the number of transitions observed from i to j, and p ij is the transition probability from i to j. The transition probabilities from the Low chain were then compared to those of the three other vessel traffic conditions using a Z-Test for proportions via the online EpiTools calculator (http://epitools.ausvet.com.au) where cell sample size was greater than five.
The activity budgets of dolphins in each vessel traffic condition were computed based on these same transition probabilities. These were arranged in four matrices, which were used to calculate the left eigenvectors of the dominant eigenvalues of each matrix. Calculations were performed in Matlab (Version 2014a, The Mathworks Inc.) based on code provided in Caswell72. Behavioural budgets for Low vessel contexts were compared with the other three contexts via a Z-test for proportions.
Whistle Characteristics
Concurrent visual and acoustic records during May/June 2015 totalled approximately 35 h, during which 336 dolphin whistles were recorded. Marley et al.73 describes how dolphin whistles were identified and characterised based on these recordings. The current study uses 164 of these whistles, which were deemed to be ‘high quality’73, to investigate the relationship between underwater noise and dolphin whistles.
Separate Generalised Additive Models (GAMs) with contextual covariates were fitted for each of the 11 whistle characteristics described in Marley et al.73. The explanatory variables initially considered for inclusion in each model were: activity state, group size, calf presence, and broadband noise levels. To attribute these data, whistles were first paired with a visual observation record of the dolphins, based on the temporally closest theodolite data. If there was no theodolite observation within 10 min, the whistle was excluded from analysis. Secondly, for each whistle the broadband noise levels (NL_BB) were calculated as the root-mean-square sound pressure level over the 2 s period immediately prior to each whistle. There is no literature regarding the time delay required before increased noise levels induce an acoustic change in dolphin whistles. However, studies of the Lombard effect in bats suggest that acoustic alteration to vocal emissions may occur at the neural level (<2 s)74,75. With no other studies to refer to, a 2 s period was selected and the NL_BB calculated for this period immediately prior to each whistle. Although short, this period is longer than the average dolphin whistle recorded in the Fremantle Inner Harbour76.
GAMs were selected as they allow smoothing functions to be fitted to explanatory variables, generating curves which can flow more freely than a straight line for describing data. Although activity state and calf presence were included as factors and group size as a linear term, explanatory analyses indicated that NL_BB required fitting as a smoothed nonlinear term. A GAM with a logit-link binomial distribution was fitted for predicting the presence/absence of harmonics, whilst GAMs with a log-link Poisson distribution were fitted to predict counts of inflection points, extrema, breaks and saddles. The remaining whistle characteristics represented continuous data, and so were fitted with a log-link gamma distribution. These characteristics were whistle duration, minimum frequency, maximum frequency, delta frequency, start frequency and end frequency.
The appropriateness and assumptions for each model were assessed in R71. To verify independence of model errors, semi-variograms of standardised residuals were checked to ensure no temporal autocorrelation existed77. Variance inflation factors (VIFs78) were used to confirm the absence of concurvity (i.e. the non-parametric analogue of multi-collinearity) between explanatory variables. The absence of overdispersion was confirmed using the sum of squared Pearson residuals divided by sample size, minus the number of parameters.
Only the best fitting explanatory variables, chosen using the second-order Akaike information criterion corrected for small sample sizes (AICc79), were included in the final model for each whistle characteristic. Models with a difference of <2 AICc units are equally plausible as the best model to explain observed patterns in the data79. In such situations, the Akaike’s Information Criterion weight (wAICc) was calculated; the model with the greatest weight was then selected79. The QQ-plots, histograms, and percentage of deviance explained were examined to quantify each model’s goodness-of-fit80. To further investigate where differences existed between activity states for each whistle characteristic, post-hoc tests with Tukey contrasts were calculated from the fitted model to conduct pairwise comparisons.
All analyses of whistle characteristics were conducted in R71, using the packages: car 81; MASS 82; mgcv 83; multcomp 84; and MuMIn 85.
Data Availability
The acoustic dataset analysed during the current study is available as a data descriptor under review in Scientific Data (SDATA-17-00084) doi:10.1038/sdata.2017.126. The visual datasets analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors would like to thank the Swan River Trust and the Department of Parks and Wildlife (DPaW) for continuing support of dolphin research in the Swan-Canning Rivers. The Fremantle City Council and Fremantle Ports kindly allowed access to their sites for data collection. Financial assistance was provided by the Holsworth Wildlife Research Endowment - Equity Trust Fund & Ecological Society of Australia. We would also like to thank the following people: Eric Kniest for supporting theodolite and Vadar software set-up; Angela Recalde Salas and Phil Bouchet for assistance in field site set-up and statistical discussions; Dave Minchin for technical support; Nick Riddoch, Leila Fouda and Jamie McWilliam for their assistance with logger deployment and recovery; and Mathew Bentley for assistance with data review. This study received extraordinary support from over 60 volunteer observers, without whom data collection would not be possible. Of these, special thanks go to: Philippa Adamson, Chella Armstrong, Mariana Barbosa, Jordon Claytor, Ana Costa, Caroline Delaisse, Michael Dwyer, David Grose, Adam Koziol, Damien Morales, Jacob Noonan, Tamar Orly, Lucy Rudd, Charlotte Teckenoff, Eloisa Wainwright, Jessica Walker, Alessandra Wasserman, Alice Watt, and Katie Werndly. Finally, we would also like to extend our appreciation for the time and effort of the two anonymous reviewers, whose comments undoubtedly improved this manuscript.
Author Contributions
S.M. led the project and was involved in all stages of study design, data collection, data processing and analysis, and drafting of the manuscript. C.S.K. assisted in the design of the study, data analysis and commented on the manuscript. C.E. assisted in the design of the study, data collection, and commented on the manuscript. I.P. assisted in data collection, data analysis, and commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13252-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bittencourt L, Carvalho RR, Lailson-Brito J, Azevedo AF. Underwater noise pollution in a coastal tropical environment. Mar. Pollut. Bull. 2014;83:331–336. doi: 10.1016/j.marpolbul.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Kelly C, Glegg GA, Speedie CD. Management of marine wildlife disturbance. Ocean Coast. Manag. 2004;47:1–19. doi: 10.1016/j.ocecoaman.2004.03.001. [DOI] [Google Scholar]
- 3.Arcangeli A, Crosti R. The short-term impact of dolphin-watching on the behavior of bottlenose dolphins (Tursiops truncatus) in Western Australia. J. Mar. Anim. their Ecol. 2009;2:3–9. [Google Scholar]
- 4.Bejder L, et al. Decline in Relative Abundance of Bottlenose Dolphins Exposed to Long-Term Disturbance. Conserv. Biol. 2006;20:1791–1798. doi: 10.1111/j.1523-1739.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- 5.May-Collado LJ, Quiñones-Lebrón SG. Dolphin changes in whistle structure with watercraft activity depends on their behavioral state. J. Acoust. Soc. Am. 2014;135:EL193–EL198. doi: 10.1121/1.4869255. [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Jorge S, et al. Effects of nature-based tourism and environmental drivers on the demography of a small dolphin population. Biol. Conserv. 2016;197:200–208. doi: 10.1016/j.biocon.2016.03.006. [DOI] [Google Scholar]
- 7.Scarpaci C, Bigger SW, Corkeron PJ, Nugegoda D. Bottlenose dolphins (Tursiops truncatus) increase whistling in the presence of‘ swim-with-dolphin’ tour operations. J. Cetacean Res. Manag. 2000;2:183–185. [Google Scholar]
- 8.Steckenreuter A, Möller L, Harcourt R. How does Australia’s largest dolphin-watching industry affect the behaviour of a small and resident population of Indo-Pacific bottlenose dolphins? J. Environ. Manage. 2012;97:14–21. doi: 10.1016/j.jenvman.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Stensland E, Berggren P. Behavioural changes in female Indo-Pacific bottlenose dolphins in response to boat-based tourism. Mar. Ecol. Prog. Ser. 2007;332:225–234. doi: 10.3354/meps332225. [DOI] [Google Scholar]
- 10.Symons J, Pirotta E, Lusseau D. Sex differences in risk perception in deep-diving bottlenose dolphins leads to decreased foraging efficiency when exposed to human disturbance. J. Appl. Ecol. 2014;51:1584–1592. doi: 10.1111/1365-2664.12337. [DOI] [Google Scholar]
- 11.Christiansen F, Lusseau D, Stensland E, Berggren P. Effects of tourist boats on the behaviour of Indo-Pacific bottlenose dolphins off the south coast of Zanzibar. Endanger. Species Res. 2010;11:91–99. doi: 10.3354/esr00265. [DOI] [Google Scholar]
- 12.Constantine R, Brunton DH, Dennis T. Dolphin-watching tour boats change bottlenose dolphin (Tursiops truncatus) behaviour. Biol. Conserv. 2004;117:299–307. doi: 10.1016/j.biocon.2003.12.009. [DOI] [Google Scholar]
- 13.Guerra M, Dawson SM, Brough TE, Rayment WJ. Effects of boats on the surface and acoustic behaviour of an endangered population of bottlenose dolphins. Endanger. Species Res. 2014;24:221–236. doi: 10.3354/esr00598. [DOI] [Google Scholar]
- 14.Heiler J, Elwen SH, Kriesell HJ, Gridley T. Changes in bottlenose dolphin whistle parameters related to vessel interaction, surface behaviour and group composition. Anim. Behav. 2016;117:167–177. doi: 10.1016/j.anbehav.2016.04.014. [DOI] [Google Scholar]
- 15.Lusseau D. Effects of Tour Boats on the Behavior of Bottlenose Dolphins: Using Markov Chains to Model Anthropogenic Impacts. Conserv. Biol. 2003;17:1785–1793. doi: 10.1111/j.1523-1739.2003.00054.x. [DOI] [Google Scholar]
- 16.Lusseau D. Male and female bottlenose dolphins Tursiops spp. have different strategies to avoid interactions with tour boats in Doubtful Sound, New Zealand. Mar. Ecol. Prog. Ser. 2003;257:267–274. doi: 10.3354/meps257267. [DOI] [Google Scholar]
- 17.Lusseau D. The Short-Term Behavioral Reactions of Bottlenose Dolphins To Interactions With Boats in Doubtful Sound, New Zealand. Mar. Mammal Sci. 2006;22:802–818. doi: 10.1111/j.1748-7692.2006.00052.x. [DOI] [Google Scholar]
- 18.McCarthy, E. International regulation of underwater sound: establishing rules and standards to address ocean noise pollution. (Kluwer Academic Publishers, 2004).
- 19.Buckstaff K. Effects of Watercraft Noise on the Acoustic Behavior of Bottlenose Dolphins, Tursiops Truncatus, in Sarasota Bay, Florida. Mar. Mammal Sci. 2004;20:709–725. doi: 10.1111/j.1748-7692.2004.tb01189.x. [DOI] [Google Scholar]
- 20.Davenport J, Davenport JL. The impact of tourism and personal leisure transport on coastal environments: A review. Estuar. Coast. Shelf Sci. 2006;67:280–292. doi: 10.1016/j.ecss.2005.11.026. [DOI] [Google Scholar]
- 21.Lloret J, Zaragoza N, Caballero D, Riera V. Impacts of recreational boating on the marine environment of Cap de Creus (Mediterranean Sea) Ocean Coast. Manag. 2008;51:749–754. doi: 10.1016/j.ocecoaman.2008.07.001. [DOI] [Google Scholar]
- 22.Marley, S. A., Salgado Kent, C. P. & Erbe, C. Occupancy of bottlenose dolphins (Tursiops aduncus) in relation to vessel traffic, dredging and environmental variables within a highly-urbanised estuary. Hydrobiologia792, 243 10.1007/s10750-016-3061-7 (2016).
- 23.Mattson MC, Thomas J, St. Aubin D. Effects of Boat Activity on the Behavior of Bottlenose Dolphins (Tursiops truncatus) in Waters Surrounding Hilton Head Island, South Carolina. Aquat. Mamm. 2005;31:133–140. doi: 10.1578/AM.31.1.2005.133. [DOI] [Google Scholar]
- 24.Nowacek SM, Wells RS, Solow AR. Short-term effects of boat traffic on bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar. Mammal Sci. 2001;17:673–688. doi: 10.1111/j.1748-7692.2001.tb01292.x. [DOI] [Google Scholar]
- 25.Ross, G. J. B. Review of the Conservation Status of Australia’s Smaller Whales and Dolphins. Report to the Australian Government10.1080/08905760600696445 (2006).
- 26.Wilson B, Hammond PS, Thompson PM. Estimating size and assessing trends in a coatal bottlenose dolphin population. Ecol. Appl. 1999;9:288–300. doi: 10.1890/1051-0761(1999)009[0288:ESAATI]2.0.CO;2. [DOI] [Google Scholar]
- 27.Lusseau D. Residency pattern of bottlenose dolphins Tursiops spp. in Milford Sound, New Zealand, is related to boat traffic. Mar. Ecol. Prog. Ser. 2005;295:265–272. doi: 10.3354/meps295265. [DOI] [Google Scholar]
- 28.Pirotta E, et al. Dredging displaces bottlenose dolphins from an urbanised foraging patch. Mar. Pollut. Bull. 2013;74:396–402. doi: 10.1016/j.marpolbul.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Rako N, et al. Leisure boating noise as a trigger for the displacement of the bottlenose dolphins of the Cres-Loinj archipelago (northern Adriatic Sea, Croatia) Mar. Pollut. Bull. 2013;68:77–84. doi: 10.1016/j.marpolbul.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Llaneza L, Lopez-Bao JV, Sazatornil V. Insights into wolf presence in human-dominated landscapes: The relative role of food availability, humans and landscape attributes. Divers. Distrib. 2012;18:459–469. doi: 10.1111/j.1472-4642.2011.00869.x. [DOI] [Google Scholar]
- 31.Au D, Perryman W. Movement and speed of dolphin schools responding to an approaching ship. Fish. Bull. 1982;80:371–379. [Google Scholar]
- 32.Lemon M, Lynch TP, Cato DH, Harcourt RG. Response of travelling bottlenose dolphins (Tursiops aduncus) to experimental approaches by a powerboat in Jervis Bay, New South Wales, Australia. Biol. Conserv. 2006;127:363–372. doi: 10.1016/j.biocon.2005.08.016. [DOI] [Google Scholar]
- 33.Pirotta E, Merchant ND, Thompson PM, Barton TR, Lusseau D. Quantifying the effect of boat disturbance on bottlenose dolphin foraging activity. Biol. Conserv. 2015;181:82–89. doi: 10.1016/j.biocon.2014.11.003. [DOI] [Google Scholar]
- 34.Heiler J, Elwen SH, Kriesell HJ, Gridley T. Changes in bottlenose dolphin whistle parameters related to vessel presence, surface behaviour and group composition. Anim. Behav. 2016;117:167–177. doi: 10.1016/j.anbehav.2016.04.014. [DOI] [Google Scholar]
- 35.May-Collado LJ, Wartzok D. A Comparison of Bottlenose Dolphin Whistles in the Atlantic Ocean: Factors Promoting Whistle Variation. J. Mammal. 2008;89:1229–1240. doi: 10.1644/07-MAMM-A-310.1. [DOI] [Google Scholar]
- 36.Morisaka T, Shinohara M, Nakahara F, Akamatsu T. Effects of Ambient Noise on the Whistles of Indo-Pacific Bottlenose Dolphin Populations. J. Mammal. 2005;86:541–546. doi: 10.1644/1545-1542(2005)86[541:EOANOT]2.0.CO;2. [DOI] [Google Scholar]
- 37.Rako Gospić N, Picciulin M. Changes in whistle structure of resident bottlenose dolphins in relation to underwater noise and boat traffic. Mar. Pollut. Bull. 2016;105:193–198. doi: 10.1016/j.marpolbul.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 38.Jensen FH, et al. Vessel noise effects on delphinid communication. Mar. Ecol. Prog. Ser. 2009;395:161–175. doi: 10.3354/meps08204. [DOI] [Google Scholar]
- 39.Acevedo-Gutiérrez A, Stienessen SC. Bottlenose Dolphins (Tursiops truncatus) Increase Number of Whistles When Feeding. Aquat. Mamm. 2004;30:357–362. doi: 10.1578/AM.30.3.2004.357. [DOI] [Google Scholar]
- 40.Díaz López B. Whistle characteristics in free-ranging bottlenose dolphins (Tursiops truncatus) in the Mediterranean Sea: Influence of behaviour. Mamm. Biol. 2011;76:180–189. doi: 10.1016/j.mambio.2010.06.006. [DOI] [Google Scholar]
- 41.Esch HC, Sayigh LS, Blum JE, Wells RS. Whistles as Potential Indicators of Stress in Bottlenose Dolphins (Tursiops truncatus) J. Mammal. 2009;90:638–650. doi: 10.1644/08-MAMM-A-069R.1. [DOI] [Google Scholar]
- 42.Hernandez EN, Solangi M, Kuczaj Sa. Time and frequency parameters of bottlenose dolphin whistles as predictors of surface behavior in the Mississippi Sound. J. Acoust. Soc. Am. 2010;127:3232–3238. doi: 10.1121/1.3365254. [DOI] [PubMed] [Google Scholar]
- 43.Jones GJ, Sayigh LS. Geographic Variation in Rates of Vocal Production of Free-Ranging Bottlenose Dolphins. Mar. Mammal Sci. 2002;18:374–393. doi: 10.1111/j.1748-7692.2002.tb01044.x. [DOI] [Google Scholar]
- 44.Quick NJ, Janik VM. Whistle rates of wild bottlenose dolphins (Tursiops truncatus): Influences of group size and behaviour. J. Comp. Psychol. 2008;122:305–311. doi: 10.1037/0735-7036.122.3.305. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins ER. Geographic variations in the whistles of bottlenose dolphins (Tursiops aduncus) along the east and west coasts of Australia. J. Acoust. Soc. Am. 2010;128:924–935. doi: 10.1121/1.3459837. [DOI] [PubMed] [Google Scholar]
- 46.Ellison WT, Southall BL, Clark CW, Frankel AS. A New Context-Based Approach to Assess Marine Mammal Behavioral Responses to Anthropogenic Sounds. Conserv. Biol. 2012;26:21–28. doi: 10.1111/j.1523-1739.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 47.Burgin S, Hardiman N. The direct physical, chemical and biotic impacts on Australian coastal waters due to recreational boating. Biodivers. Conserv. 2011;20:683–701. doi: 10.1007/s10531-011-0003-6. [DOI] [Google Scholar]
- 48.Marley SA, Erbe C, Salgado-Kent CP, Parsons MJG, Parnum IM. Spatial and temporal variation in the acoustic habitat of bottlenose dolphins (Tursiops aduncus) within a highly urbanised estuary. Front. Mar. Sci. 2017;4:197. doi: 10.3389/fmars.2017.00197. [DOI] [Google Scholar]
- 49.Beidatsch, K. Machine Learning for Species Distribution Modelling: Evaluation of a Novel Method. (Curtin University Honours Thesis, 2012).
- 50.Chabanne D, Finn H, Salgado-Kent C, Bedjer L. Identification of a resident community of bottlenose dolphins (Tursiops aduncus) in the Swan Canning Riverpark, Western Australia, using behavioural information. Pacific Conserv. Biol. 2012;18:247–262. doi: 10.1071/PC120247. [DOI] [Google Scholar]
- 51.Moiler, K. Bottlenose Dolphins (Tursiops sp.) – a Study of Patterns in Spatial and Temporal use of the Swan River, Western Australia. (Curtin University Honours Thesis, 2008).
- 52.Swan River Trust (SRT). Dolphin Watch Annual Report 2014-15. (2015).
- 53.Johnson, C. S. In Marine Bioacoustics (ed. Tavolga, W.) 247–260 (Pergamon, New York, 1967).
- 54.Turl CW. Low-frequency sound detection by a bottlenose dolphin. J. Acoust. Soc. Am. 1993;94:3006. doi: 10.1121/1.407333. [DOI] [PubMed] [Google Scholar]
- 55.Schultz KW, Cato DH, Corkeron PJ, Bryden MM. Low frequency narrow-band sounds produced by bottlenose dolphins. Mar. Mammal Sci. 1995;11:503–509. doi: 10.1111/j.1748-7692.1995.tb00673.x. [DOI] [Google Scholar]
- 56.Paiva EG, Salgado Kent CP, Gagnon MM, McCauley R, Finn H. Reduced Detection of Indo-Pacific Bottlenose Dolphins (Tursiops aduncus) in an Inner Harbour Channel During Pile Driving Activities. Aquat. Mamm. 2015;41:455–468. doi: 10.1578/AM.41.4.2015.455. [DOI] [Google Scholar]
- 57.Williams TM, et al. Travel at low energetic cost by swimming and wave-riding bottlenose dolphins. Nature. 1992;355:821–3. doi: 10.1038/355821a0. [DOI] [PubMed] [Google Scholar]
- 58.Yazdi P, Kilian A, Culik BM. Energy expenditure of swimming bottlenose dolphins (Tursiops truncatus) Mar. Biol. 1999;134:601–607. doi: 10.1007/s002270050575. [DOI] [Google Scholar]
- 59.Bishop CM. The maximum oxygen consumption and aerobic scope of birds and mammals: getting to the heart of the matter. Proc. R. Soc. London B. 1999;266:2275–2281. doi: 10.1098/rspb.1999.0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacArthur Ra, Johnston RH, Geist V. Factors influencing heart rate in free-ranging bighorn sheep: a physiological approach to the study of wildlife harassment. Can. J. Zool. 1979;57:2010–2021. doi: 10.1139/z79-265. [DOI] [Google Scholar]
- 61.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med. Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Tyne JA, Johnston DW, Rankin R, Loneragan NR, Bejder L. The importance of spinner dolphin (Stenella longirostris) resting habitat: Implications for management. J. Appl. Ecol. 2015;52:621–630. doi: 10.1111/1365-2664.12434. [DOI] [Google Scholar]
- 63.Kuczaj S, Makecha R, Trone M, Paulis RD, Ramos J. Role of Peers in Cultural Innovation and Cultural Transmission: Evidence from the Play of Dolphin Calves. Int. J. Comp. Psychol. 2006;19:223–240. [Google Scholar]
- 64.Mann J, Smuts B. Behavioral Development in Wild Bottlenose Dolphin Newborns (Tursiops sp.) Behaviour. 1999;136:529–566. doi: 10.1163/156853999501469. [DOI] [Google Scholar]
- 65.Pace DS. Fluke-made bubble rings as toys in bottlenose dolphin calves (Tursiops truncatus) Aquat. Mamm. 2000;26:57–64. [Google Scholar]
- 66.Pellis DM. How motivationally distinct is play? A prelimary case study. Anim. Behav. 1991;42:851–853. doi: 10.1016/S0003-3472(05)80129-0. [DOI] [Google Scholar]
- 67.Noren DP, Holt MM, Dunkin RC, Williams TM. The metabolic cost of communicative sound production in bottlenose dolphins (Tursiops truncatus) J. Exp. Biol. 2013;216:1624–1629. doi: 10.1242/jeb.083212. [DOI] [PubMed] [Google Scholar]
- 68.Hastie GD, Wilson B, Tufft LH, Thompson PM. Bottlenose dolphins increase breathing synchrony in response to boat traffic. Mar. Mammal Sci. 2003;19:74–84. doi: 10.1111/j.1748-7692.2003.tb01093.x. [DOI] [Google Scholar]
- 69.Janik VM, Thompson PM. Changes in surfacing patterns of bottlenose dolphins in response to boat traffic. Mar. Mammal Sci. 1996;12:597–602. doi: 10.1111/j.1748-7692.1996.tb00073.x. [DOI] [Google Scholar]
- 70.Shane SH, Wells RS, Würsig B. Ecology, behavior and social organization of the bottlenose dolphin: A review. Mar. Mammal Sci. 1986;2:34–63. doi: 10.1111/j.1748-7692.1986.tb00026.x. [DOI] [Google Scholar]
- 71.R Core Team, L. R: A language and environment for statistical computing at http://www.r-project.org/ (2015).
- 72.Caswell, H. Matrix Population Models. (Sinauer Associates, 2001).
- 73.Marley, S. A., Erbe, C. & Salgado-Kent, C. P. Underwater recordings of the whistles of bottlenose dolphins in Fremantle Inner Harbour, Western Australia. Sci. Data4, 170126 (2017). [DOI] [PMC free article] [PubMed]
- 74.Gillam EH, Ulanovsky N, McCracken GF. Rapid jamming avoidance in biosonar. Proc. Biol. Sci. 2007;274:651–60. doi: 10.1098/rspb.2006.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hase K, Miyamoto T, Kobayasi KI, Hiryu S. Rapid frequency control of sonar sounds by the FM bat, Miniopterus fuliginosus, in response to spectral overlap. Behav. Processes. 2016;128:126–133. doi: 10.1016/j.beproc.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Ward R, Parnum I, Erbe C, Salgado-Kent C. Whistle Characteristics of Indo-Pacific Bottlenose Dolphins (Tursiops aduncus) in the Fremantle Inner Harbour, Western Australia. Acoust. Aust. 2016;44:159–169. doi: 10.1007/s40857-015-0041-4. [DOI] [Google Scholar]
- 77.Zuur, A., Ieno, E. N., Walker, N., Saveliev, A. A. & Smith, G. S. Mixed effects models and extensions in ecology with R. (Springer Science and Business Media, 2009).
- 78.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010;1:3–14. doi: 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 79.Burnham KP, Anderson DR. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004;33:261–304. doi: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 80.Zuur AF, Ieno EN. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016;7:636–645. doi: 10.1111/2041-210X.12577. [DOI] [Google Scholar]
- 81.Fox, J. & Weisberg, S. An R Companion to Applied Regression. (Thousand Oaks; California, 2011).
- 82.Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. (Springer, New York, 2002).
- 83.Wood, S. N. Generalized Additive Models: An Introduction with R. (Chapman and Hall / CRC, 2006).
- 84.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 85.Barton, K. MuMIn: Multi-Model Inference (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The acoustic dataset analysed during the current study is available as a data descriptor under review in Scientific Data (SDATA-17-00084) doi:10.1038/sdata.2017.126. The visual datasets analysed during the current study are available from the corresponding author on reasonable request.