Abstract
Lifespan in poikilothermic organisms, such as Caenorhabditis elegans, can be substantially increased simply by decreasing growth temperature. To gain insights into the mechanistic underpinnings of this effect, we investigated the effects of temperature in development and adulthood on C. elegans lifespan. We found that worms exposed to 25 °C during development and shifted to 15 °C in adulthood exhibited an even longer lifespan than animals constantly kept at 15 °C. Analysis of the in vivo redox status demonstrated that at 25 °C, C. elegans larvae have a more reduced redox state and higher Prdx-2 expression levels than animals raised at 15 °C. Worms lacking prdx-2 fail to show the additional lifespan extension upon shift from 25 °C to 15 °C and reveal a lifespan similar to prdx-2 worms always kept at 15 °C. These results suggest that transiently altering the in vivo redox state during development can have highly beneficial long-term consequences for organisms.
Abbreviations: C. elegans, Caenorhabditis elegans; FUdR, 5-Fluoro-2′-deoxyuridine; Grx1, human glutaredoxin-1; roGFP2, redox sensitive GFP; NGM, Nematode Growth Medium; Prdx-2, peroxiredoxin-2; qRT-PCR, quantitative real-time reverse transcription polymerase chain reaction; SEM, standard error of the mean
Keywords: Aging, Temperature, C. elegans, Oxidants, Peroxiredoxin
Graphical abstract
Highlights
-
•
Development at 25 °C extends adult lifespan at 15 °C in C. elegans.
-
•
Lower oxidant level in C. elegans larvae grown at 25 °C compared to worms at 15 °C.
-
•
Increased peroxiredoxin-2 level in larvae raised at 25 °C.
-
•
Peroxiredoxin-2 mutants lack developmental temperature-induced lifespan extension.
1. Introduction
The free radical theory of aging proposes that endogenous reactive oxygen species cause deterioration of cells and organisms [1]. Its validity has been primarily tested by studying the stress response and lifespan of organisms following genetic manipulation of antioxidant expression level or in response to oxidant or antioxidant exposure. While some studies have found evidence in favor of the free radical theory of aging, others have produced inconclusive or even contradictory results. Therefore, the debate about the role of oxidants in aging is still ongoing [2].
Previous work from our lab addressed the question as to when and to what extent model organisms such as Caenorhabditis elegans encounter endogenous oxidants during their life cycle [3]. We therefore generated transgenic C. elegans expressing the ratiometric peroxide sensor protein HyPer [4] in the body wall muscle cells, and analyzed endogenous peroxide levels in individual worms of a synchronized population over their lifetime. These experiments revealed that animals grown at 15 °C not only encounter increased levels of endogenous oxidants as they age, but also experience highly elevated peroxide levels transiently during their development [3]. Unexpectedly, by analyzing short- and long-lived mutants of the insulin signaling pathway, we noticed a correlation between the extent of and recovery from developmental oxidant levels and their subsequent adult lifespan: the long-lived daf-2 mutant strain recovered significantly earlier from developmental peroxide accumulation, and displayed lower oxidant levels in young adults than the short-lived daf-16 animals [3]. These results suggested that events very early in life might define C. elegans lifespan, a conclusion that was in good agreement with studies showing that the timed manipulation of components of the mitochondrial electron transport chain specifically during development extends the adult lifespan of C.elegans [5], [6].
It is well-established that the lifespan of C. elegans decreases with increasing environmental temperatures [7]. We report here the unexpected finding that the growth temperature during the development has significant effects on the endogenous redox levels and the adult lifespan. These results provide further evidence that oxidant levels during development play a potentially crucial role in the longevity of C. elegans.
2. Material & methods
A detailed description can be found in the supplement.
C. elegans strains were cultured at 15 °C or 25 °C on NGM agar plates. Alkaline hypochlorite-mediated lysis was used to release the eggs from gravid adult worms. Eggs were then incubated in M9 buffer without a food source which allows hatching of eggs but causes L1 arrest thereby creating a synchronized population of animals. The exit from the L1 larval arrest was performed sequentially to allow simultaneous imaging of the L3 larval stage. Aliquots of the Grx1-roGFP2 sub populations grown at the two temperatures were imaged at L3 stage and during day 1 and day 3 of adulthood using an Olympus BX61 upright microscope with a UPlan S-Apo 20x objective (NA 0.75) and excitation filters 420/40x, 500/20x, and emission filter 535/30x. Imaging of the HyPer sensor and quantification of the HyPer ratio using ImageJ and MATLAB Wormsuite are detailed in [3]. Fluorescence intensity of the Grx1-roGFP2 images was analyzed in a similar manner as the HyPer images with the exception that the Grx ratio oxidized to reduced was determined as fluorescence intensity of “420 nm excitation” divided by fluorescence intensity of “500 nm excitation”.
Starting from L4 stage animals were cultivated on NGM Agar plates containing 20 mg/L 5-Fluoro-2′-deoxyuridine (FUdR, Sigma-Aldrich, St. Louis, MO, Cat #: F0503–1G) to prevent progeny production and maintain synchronicity.
For the lifespan assays animals were incubated at either 15 °C or 25 °C, or shifted to the opposite temperature after L4 stage. Animals that did not move after tapping of the plate and which did not respond to gentle prodding with a platinum wire were scored as dead. Lifespan data were plotted and analyzed using GraphPadPrism and a log-rank test was used for statistical analysis.
Peroxiredoxin-2 expression level of wildtype animals grown at either 15 °C or 25 °C were determined at L3 larval stage by quantitative RT-PCR. Relative mRNA level were calculated using the Comparative Ct method.
3. Results
3.1. High temperatures during development significantly extend 15 °C lifespan of C. elegans
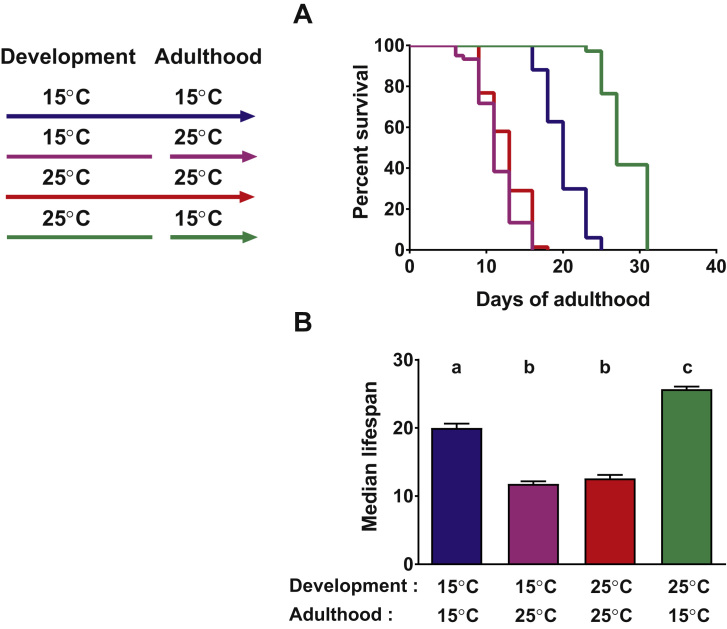
It has previously been shown that C. elegans lifespan is highly dependent on environmental temperatures [7]. In agreement with these results, we found that the lifespan of worms grown at 25 °C during development and adulthood was significantly shorter than the lifespan of animals constantly reared at 15 °C (Fig. 1, compare red and blue). To dissect the effects of specific temperatures during development on the adult lifespan, we grew wildtype C. elegans at either 15 °C or 25 °C until the end of the L4 larval stage, and then switched them to the opposing temperature during adulthood. Animals exposed to 15 °C during development (approximately three days at this temperature) and then shifted to 25 °C displayed a similarly short lifespan than worms continuously grown at 25 °C (Fig. 1, compare pink and red). In contrast, however, we found that animals exposed to 25 °C during their development (approximately two days) and then shifted to 15 °C during adulthood had a lifespan that was even longer than the cohort continuously exposed to 15 °C (Fig. 1, compare green and blue). These results were consistent with recent studies that showed that transient exposure of C. elegans to 25 °C during development increases subsequent adult lifespan at 20 °C compared to worms incubated at 15 °C during development [8]. They furthermore suggested that short-term exposure of worms to 25 °C during development is sufficient to positively influence the adult animals’ lifespan. These beneficial effects, however, appear to be masked by detrimental effects induced by persistent high temperature during adult lifespan, ultimately resulting in a shortened lifespan in animals reared constantly at 25 °C (Fig. 1, red).
Fig. 1.
Exposure ofC. eleganslarvae to 25 °C further extends adult lifespan at 15 °C.C. elegans grown at 25 °C and shifted to 15 °C in the late L4 larvae stage (green line) have an extended adult lifespan compared to animals constantly grown at 15 °C (blue line). In contrast, the lifespan of worms constantly grown at 25 °C (red line) or of animals shifted from 15 °C to 25 °C (pink line) are similarly short. A representative lifespan assay is shown in panel A. The average of the median lifespans of all five experiments and the SEM are shown in panel B. The four subpopulations were compared with each other and means that are not significantly different share the same letter (ANOVA Tukey's multiple comparisons test, p<0.0001). Number of animals in each experiment for 15 °C: 67, 16, 50, 88, 78; for 25 °C: 69, 106, 42, 86, 81; for the 25 °C − 15 °C switch: 72, 89, 51, 81, 80; and for the 15 °C − 25 °C switch: 60, 56, 44, 84, 81. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Developmental redox states in C. elegans are altered by growth temperature
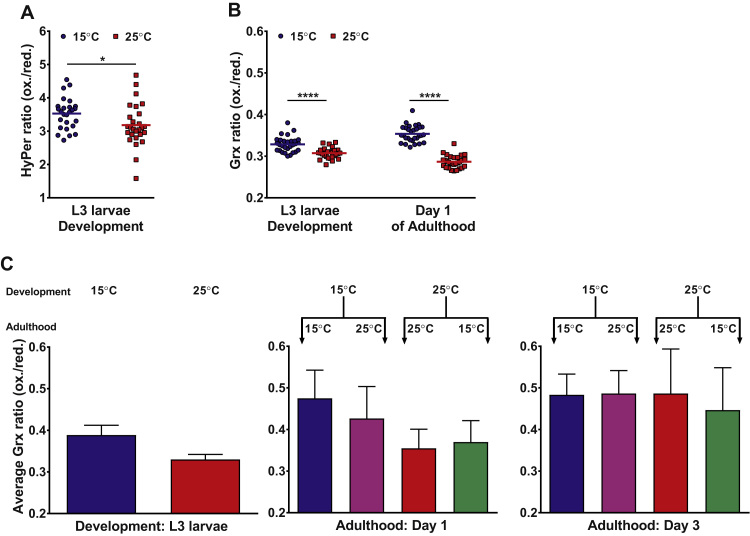
To investigate whether environmental growth temperatures affect the in vivo peroxide levels in larval animals, we determined the HyPer ratio in the body wall muscle cells of our C. elegans strain grown at either 15 °C or 25 °C (Fig. 2A). These studies revealed that C. elegans cultivated at 25 °C, a temperature that significantly shortens the lifespan of worms compared to growth at 15 °C, had substantially lower hydrogen peroxide level than worms raised at 15 °C. These results were unexpected because our previous study suggested a correlation between lower in vivo peroxide level and extended lifespan. Since the HyPer fluorescence read-out is affected by changes in pH [9], [10], which in turn might be affected by temperature [11], we decided to test a second C. elegans strain that ubiquitously expresses the pH independent ratiometric redox sensor protein Grx1-roGFP2 instead [12]. This probe responds directly to altered oxidized and reduced glutathione levels, with higher Grx1-roGFP2 ratios corresponding to higher endogenous redox potentials and peroxide levels [10]. To test whether the growth temperature affects the Grx ratio in developing and young adult C. elegans, we synchronized the worms as before and raised the worms at either 15 °C or 25 °C. We removed aliquots of the respective populations at defined time points during development and early adulthood and determined the in vivo Grx ratio in individual animals. We decided not to test any later time points since animals raised at these two temperatures show markedly different average lifespans (i.e., ~20 days at 15 °C versus ~12 days at 25 °C), which makes direct comparisons at later ages difficult to interpret. Similar to our results with the HyPer worms, we found that worms raised at 25 °C had much lower Grx1-roGFP2 ratios (i.e., lower endogenous oxidant levels) at both L3 larval stage and during day 1 of adulthood than the corresponding worms grown at 15 °C (Fig. 2B). By day 3 of adulthood, however, Grx1-roGFP2 ratios seemed to be similar regardless of the incubation temperature (Fig. 2C).
Fig. 2.
In vivo redox levels are higher in animals cultivated at lower (life-prolonging) temperatures. A, B, C.elegans expressing the HyPer sensor in the body wall muscle (A) or the Grx1-roGFP2 sensor ubiquitously (B) were cultured at either 15 °C (blue circles) or 25 °C (red squares). The HyPer ratios or Grx ratios were determined at the L3 larval stage and during early adulthood (day 1, for the Grx1-roGFP2 sensor) with each symbol representing an individual animal. *: p<0.05, ****: p<0.0001, unpaired t-test. C, The average Grx ratios of animals either continuously grown at 15 °C (blue) or 25 °C (red) and of animals shifted at the end of L4 larval stage (green, pink) from independent experiments (L3: n=7, Day 1: n=4, Day 3: n=3) and the SEM are displayed. At day 1 of adulthood the average Grx ratio of animals exposed to the new temperature seems to be similar to the Grx ratio of animals that were not switched. The Grx ratios at day 3 of adulthood do not seem to differ from each other. Note: Y-axis starts at 0.2 for the Grx1-roGFP2 sensor. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Since exposure to elevated temperatures during development appeared to cause more reducing redox environments during the early stages of life and extends adult lifespan at 15 °C (Fig. 1), we tested whether the developmental growth temperature could affect the Grx ratio in adult animals upon shifting them to the opposing temperature at late L4 stage. While at day 1 of adulthood (i.e., one day of exposure to the new temperature), the Grx1-roGFP2 ratios of the shifted cohorts are still closer to the ratios observed during development, at day 3 of adulthood there seems to be no difference in Grx ratios between the four groups. We concluded from these results that transient changes in the endogenous redox state during development might be sufficient to affect C. elegans lifespan.
3.3. Role of developmental Prdx-2 levels in C. elegans lifespan
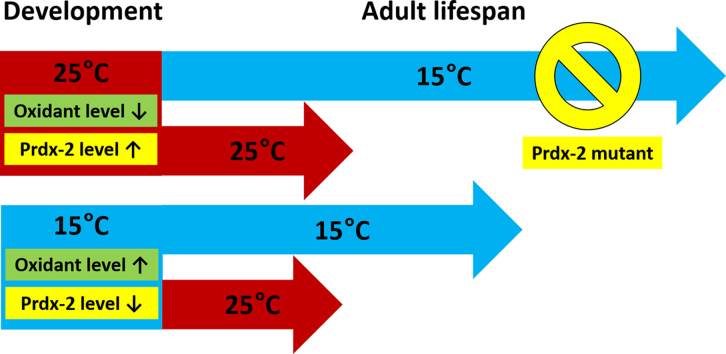
Our data suggested that elevated temperatures during C. elegans development result in a more reducing redox environment. This could be due to either decreased production of reactive oxygen species or increased levels of antioxidant gene expression [3]. Since elevated temperatures rather increase metabolism and hence enhance ROS production [13], [14], we tested whether the expression of antioxidant genes is affected by temperature. We chose to focus on the expression levels of the peroxide scavenger protein peroxiredoxin 2 (prdx-2), a very highly abundant antioxidant protein in C. elegans [15], which consumes hydrogen peroxide and hence contributes to a more reducing redox environment. Indeed, we found that the mRNA levels of Prdx-2 in L3 larvae grown at 25 °C were significantly higher compared to the expression level of animals grown at 15 °C (Fig. 3), suggesting that increased temperatures during development elevate the antioxidant capacity of C. elegans.
Fig. 3.
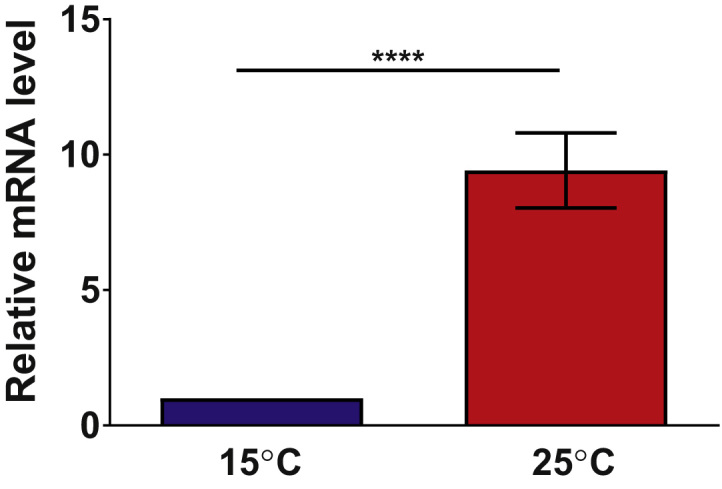
Peroxiredoxin 2 gene expression is influenced by environmental growth temperatures.C.elegans L3 larvae grown at 15 °C (blue) or 25 °C (red) were tested for peroxiredoxin 2 gene expression using qRT-PCR. Animals grown at 25 °C had higher levels of prdx-2 expression. The mean of 7 experiments and the SEM are displayed. ****: p<0.0001, unpaired t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
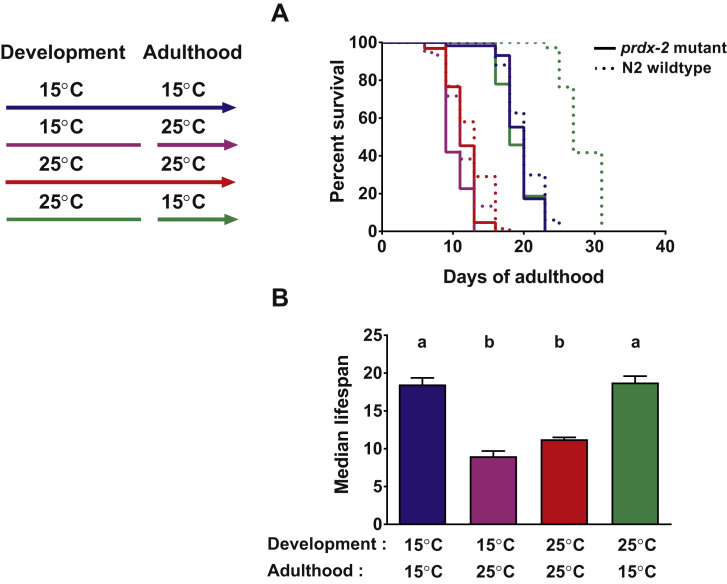
To directly test whether prdx-2 is responsible for the observed lifespan extension upon shift from developmental 25 °C to 15 °C in adulthood, we conducted the same temperature shift experiments in prdx-2 mutants. In contrast to earlier reports in which the same prdx-2 strains showed a measurable lifespan defect when cultivated at 15 °C [16], the lifespan of wildtype and prdx-2 were not significantly different. This result is likely due to the fact that in contrast to the published studies, our lifespan measurements were conducted in the presence of FUdR, a compound that is commonly used to prevent progeny production but which can extend lifespan in some mutant worms [17]. In either case, our results with prdx-2 mutants that were continuously grown at either 15 °C or 25 °C during both development and adulthood were very similar to our wildtype results (Fig. 4, compare red and blue). The striking difference to wildtype, however, was that the prdx-2 mutants grown at 15 °C during adulthood no longer benefited from a developmental exposure to 25 °C (Fig. 4, compare solid green and dashed green). These results suggested that the overexpression of Prdx-2 during development at 25 °C contributes to the extended lifespan of C. elegans upon shift to 15 °C.
Fig. 4.
Peroxiredoxin-2 mutants no longer benefit from developmental cultivation at 25 °C. A, Wildtype (dashed lines) and prdx-2 mutants (solid lines) were grown at 25 °C or 15 °C (as in Fig. 1) and shifted to the opposing temperatures as late L4 larvae. The animals that remained at 25 °C during development and adulthood (red line) had a shorter lifespan than animals kept constantly at 15 °C (blue line). In contrast to wildtype animals (dashed green line), prdx-2 mutants shifted from 25 °C to 15 °C at the end of L4 larval stage (solid green line) do not show an extended adult lifespan. A representative lifespan assay is shown. B, The average of the median lifespan of the prdx-2 mutants of all 4 experiments and the SEM are shown. The four subpopulations were compared with each other and means that are not significantly different, share the same letter (ANOVA Tukey multiple comparisons test, p<0.0001). Number of animals in each experiment for 15 °C, N2: 67, 16, 88, 78 and prdx-2: 58, 60, 83, 54; for 25 °C, N2: 69, 106, 86, 81 and prdx-2: 64, 60, 67, 83; for the 25 °C − 15 °C switch, N2: 72, 89, 81, 80 and prdx-2: 59, 64, 84, 64; and for the 15 °C − 25 °C switch, N2: 60, 56, 84, 81 and prdx-2: 62, 57, 83, 86. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
If and how changes in oxidant levels affect lifespan has been under investigation for over 50 years. Our studies now show that during development, exposure to 25 °C, a temperature that significantly accelerates aging in adult C. elegans, is highly beneficial for subsequent survival at 15 °C. We discovered that L3 larvae and young adults had a more reducing redox environment at 25 °C compared to cohorts raised at 15 °C. Consistent with this result, we found that the expression level of the oxidant scavenger protein Prdx-2 was massively elevated in animals raised at 25 °C. Based on the finding that animals that were exposed to 25 °C during development and then shifted to 15 °C at the end of larval development had an even longer lifespan compared to animals continuously reared at 15 °C, we now propose that an adaptation to stressful conditions during development could become beneficial once these stressors are gone. This result expands on previous findings suggesting that events early in life determine adult lifespan. For instance, it has been shown that disrupting mitochondrial respiratory components specifically during development is necessary and sufficient to extend the lifespan of C. elegans [5]. We now demonstrated that simply exposing worms to elevated temperatures during their development can positively affect lifespan. These findings support the notion that the development represents a critical time window in which nematode lifespan can be both positively and negatively modulated [8].
Our results demonstrate that animals raised at 25 °C have a more reduced redox state and increased expression levels of the 2-cysteine peroxiredoxin-2, an enzyme that is responsible for detoxifying peroxide and restoring redox homeostasis. Based on previous studies that revealed that oxidant levels increase with increasing temperatures [13], we now propose that peroxide levels increase at 25 °C, causing induction of the oxidative stress response, the accumulation of Prdx-2 during development and an adjustment to more reducing redox environments during development. These results agree well with earlier studies that showed that long-lived C.elegans mutants are able to recover from developmental peroxide accumulation earlier and more efficiently than short-lived mutants [3]. It became clear that Prdx-2 plays a direct role in the observed lifespan extension upon shift from 25 °C to 15 °C when we tested the prdx-2 mutant worms, which failed to respond to the increased temperature during development. The exact mechanism of how developmental prdx-2 accumulation could benefit the adult lifespan is still open for investigation. Taken together, our study suggests that transient events very early in the lifetime of an organism can robustly influence the subsequent lifespan by modulating gene expression and endogenous redox levels.
5. Conclusion
Aging of C.elegans inversely correlates with the environmental growth temperature with warmer temperatures resulting in shorter lifespan. A transient exposure to 25 °C during development, however, significantly extends adult lifespan at 15 °C compared to constant exposure to 15 °C. Moreover, developmental growth at 25 °C leads to a more reducing redox environment and causes elevated expression of the antioxidant peroxiredoxin-2. Interestingly, the lack of peroxiredoxin-2 abolishes the lifespan extension indicative of a role of antioxidants in temperature-induced lifespan extension. These findings provide further evidence for the intriguing possibility that events occurring very early in life could have potential aging-modulating effects long into adulthood.
Acknowledgement
This work was supported by the National Institute of Health Grants AG027349 and R35-GM-122506. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We are grateful to Bart Braeckman and Patricia Back for providing us with the Grx1-roGFP2 strain N2 (jrIs1[Prpl-17::Grx1-roGFP2]). We thank Rohan Ved for experimental help, and Carol Mousigian and Daphne Bazopoulou for help in generating transgenic animals.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.10.003.
Contributor Information
Ursula Jakob, Email: ujakob@umich.edu.
Daniela Knoefler, Email: knoefler@umich.edu.
Appendix A. Supplementary material
Supplementary material
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Vina J. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid. Redox Signal. 2013;19(8):779–787. doi: 10.1089/ars.2012.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knoefler D. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol. Cell. 2012;47(5):767–776. doi: 10.1016/j.molcel.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belousov V.V. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods. 2006;3(4):281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 5.Dillin A. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298(5602):2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 6.Rea S.L., Ventura N., Johnson T.E. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5(10):e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klass M.R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 1977;6(6):413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B. Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep. 2015;11(9):1414–1424. doi: 10.1016/j.celrep.2015.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilan D.S., Belousov V.V. HyPer family probes: state of the art. Antioxid. Redox Signal. 2016;24(13):731–751. doi: 10.1089/ars.2015.6586. [DOI] [PubMed] [Google Scholar]
- 10.Gutscher M. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods. 2008;5(6):553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 11.Wang T., Jackson D.C. How and why pH changes with body temperature: the alpha-stat hypothesis. J. Exp. Biol. 2016;219(Pt 8):1090–1092. doi: 10.1242/jeb.139220. [DOI] [PubMed] [Google Scholar]
- 12.Back P. Exploring real-time in vivo redox biology of developing and aging Caenorhabditis elegans. Free Radic. Biol. Med. 2012;52(5):850–859. doi: 10.1016/j.freeradbiomed.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Bai Z., Harvey L.M., McNeil B. Elevated temperature effects on the oxidant/antioxidant balance in submerged batch cultures of the filamentous fungus Aspergillus niger B1-D. Biotechnol. Bioeng. 2003;83(7):772–779. doi: 10.1002/bit.10726. [DOI] [PubMed] [Google Scholar]
- 14.Van Voorhies W.A., Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc. Natl. Acad. Sci. USA. 1999;96(20):11399–11403. doi: 10.1073/pnas.96.20.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumsta C., Thamsen M., Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antioxid. Redox Signal. 2011;14(6):1023–1037. doi: 10.1089/ars.2010.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olahova M. A redox-sensitive peroxiredoxin that is important for longevity has tissue- and. Proc. Natl. Acad. Sci. USA. 2008;105(50):19839–19844. doi: 10.1073/pnas.0805507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Raamsdonk J.M., Hekimi S. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mech. Ageing Dev. 2011;132(10):519–521. doi: 10.1016/j.mad.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material