Abstract
Introduction
Several reports have described the usefulness of a high-flow nasal cannula (HFNC). However, the physiological mechanisms of this system are unclear. In the current study, various methods were used to investigate the physiological mechanisms of an HFNC in healthy volunteers.
Methods
The physiological mechanisms of the constant-flow and constant-pressure models of HFNC were studied in 10 healthy volunteers by the oesophageal balloon method, the electrical impedance method and the forced oscillation technique (FOT).
Results
The tidal volume (TV) increased markedly during HFNC (off, 30 L/min, 50 L/min: 685.6±236.5 mL, 929.8±434.7 mL, 968.8±451.1 mL). The end-inspiratory oesophageal pressure (EIOP) was not significantly different, but there was a tendency for it to decrease. HFNC 30 L/min and 50 L/min, the increment in TV and the difference in EIOP showed strong negative correlations (p=0.0025, 0.003). The end-expiratory oesophageal pressure (EEOP) increased. The respiratory system reactance at 5 Hz (X5) by FOT decreased significantly. There was a flow rate-dependent EEOP increase, and the positive end-expiratory pressure (PEEP) effect of HFNC was confirmed. There was a correlation between the difference in X5 and the difference in EEOP during HFNC 30 L/min and 50 L/min, with correlation coefficients of 0.534 and 0.404 (p=0.112, 0.281). The amount of change in EEOP and the fluctuation in X5 were positively correlated.
Conclusions
The PEEP effect of HFNC was confirmed by the electrical impedance method and FOT. The increment in TV and the difference in EIOP of HFNC showed strong negative correlations.
Keywords: ambulatory oxygen therapy, assisted ventilation, COPD exacerbations, Non invasive ventilation, respiratory measurement
Introduction
A high-flow nasal cannula (HFNC) is an oxygen therapy system that can deliver oxygen at a high flow rate of up to 60 L/min.1 The interface of the HFNC is even more non-invasive than non-invasive positive pressure ventilation (NPPV), and a large-scale randomised controlled trial reported its usefulness in patients with acute respiratory failure.2 In addition, several recent reports have shown the usefulness of HFNC for respiratory management in terms of avoiding intubation after extubation and respiratory management.3 4
In terms of respiratory physiology, HFNC is reported to have benefits, such as a decrease in dead space by flushing out the expired gas accumulated in the anatomical dead space of the nasopharynx and mucociliary movement of the respiratory tract.2 5–7 It has also been reported that intra-airway and alveolar pressures increase by flowing oxygen at a high flow rate, resulting in a positive end-expiratory pressure (PEEP)-like effect.5–7 Parke et al compared an HFNC and a face mask at an oxygen flow rate of 35 L/min in 15 patients after cardiac surgery.6 They reported that, with the mouth closed, mean airway pressure was higher with the HFNC (2.7 cmH2O) than with the face mask (0.2 cmH2O).
Corley et al used HFNCs postcardiac surgery and measured the pharyngeal pressure and end-expiratory lung volume by electrical impedance tomography.7 They found that the pharyngeal pressure increased from 0.3±0.9 to 2.7±1.2 cmH2O, while the end-expiratory lung impedance increased from 419±213 to 1936±213 units. However, they did not evaluate the tidal volume (TV) itself.
The physiological mechanism of HFNC remains unclear. We measured the change in TV ex vivo using the ExSpiron 1Xi (Respiratory Motion, Waltham, Massachusetts, USA), which uses the electrical impedance method, during HFNC use at a constant flow rate and constant pressure. In the present study, a constant flow rate and a constant pressure difference, which have not been considered so far, were examined. In addition, it was possible to determine the relationship between the intrathoracic pressure and the change in TV during HFNC by the oesophageal balloon method, and total expiratory resistance was measured by the forced oscillation technique (FOT). In the current study, the findings of various methods for examining the physiological mechanisms of HFNC are presented.
Subjects and methods
Ten healthy adult Japanese men were enrolled. They had been confirmed to have no pre-existing conditions on the basis of individual questioning, and so on, and they had no allergies to the tests or drugs necessary for the tests. They had each provided their written informed consent to participate in the study. The study was approved by the Hirakata Kohsai Hospital Institutional Review Board. Before the examination, respiratory function was evaluated.
The study was carried out at the Federation of National Public Service Personnel Mutual Aid Association Hirakata Kohsai Hospital. After obtaining the subjects’ informed consent, each subject’s respiratory function was measured with a Multifunctional Spirometer HI-801 (Chest MI, Tokyo, Japan). The obtained TV was used to increase the accuracy of the ventilation metre at the time of the TV measurement with the ExSpiron 1Xi. The subject was in the supine position, with the head elevated 30°. The TCM/TOSCA (Radiometer, Copenhagen, Denmark) was attached to an earlobe to measure the transcutaneous carbon dioxide PaO2 (PtcCO2) and transcutaneous arterial blood oxygen saturation (SpO2). L-shaped electrode pads were attached to the sternum and ribs of the subjects undergoing ExSpiron 1Xi examination. In order to measure the oesophageal pressure, an oesophageal balloon (Avea Smart Cath Nasogastric Pressure Monitoring Tube Set, 16 F Adult) was placed in the oesophagus and connected to the AVEA ventilator (Care Fusion, San Diego, California, USA). First, the oesophageal balloon was inserted into the stomach via a nostril. The oesophageal balloon was then pulled back into the oesophagus until a pressure wave-shaped heartbeat artefact was recognised; it was immobilised at about 45 cm from the nostril. The subject was in the supine position, with the head elevated 30°. The following three models were used as machines that deliver oxygen at a high flow-rate with a humidifier and a cannula with the oxygen concentration (fractional inspired oxygen (FiO2)) was set at 21%:
Steady air (Atom Medical, Tokyo, Japan), constant flow type
AIRVO 2, Optiflow (Fisher & Paykel, Auckland, New Zealand), constant flow type
Vivo 50 (Breas Medical AB, Mölnlycke, Sweden) + PMH 7000 plus (Pacific Medico, Tokyo, Japan) nasal cannula No. 3 (Pacific Medico), constant pressure type.
The three machines were used in the order of 1, 2, and 3 at 30 L/min for 5 min, followed by washout for at least 10 min by spontaneous breathing. Then, the HFNC was applied at 50 L/min for 5 min, and the oxygen saturation, percutaneous blood carbon dioxide concentration, oesophageal pressure, TV, minute ventilation (MV) volume and respiratory rate were recorded at the time of resting and spontaneous breathing and during HFNC application. Then, the FOT was used to investigate the change in ventilation dynamics during constant pressure type HFNC with the MostGraph-02 (Chest MI), which can measure the pulmonary impedance and reactance, which is the reciprocal of compliance. For the respiratory system resistance (Rrs), Rrs at 5 Hz was abbreviated to R5 (cmH2O/L/s), Rrs at 20 Hz was abbreviated to R20 (cmH2O/L/s) and the difference between R5 and R20 was abbreviated to R5–R20 (cmH2O/L/s). For the respiratory system reactance (Xrs), Xrs at 5 Hz was abbreviated to X5 (cmH2O/L/s), and the resonance frequency at which X=0 was defined as the resonance frequency (Fres). The integrated value of the reactance up to the oscillation frequency was defined as the low-frequency reactance area (ALX) (cmH2O/L/S*Hz).
Statistics
All statistical analyses were performed with EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna. Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.8
The results are shown as means±SD. Intergroup comparisons of HFNC flow rates of 30 L/min and 50 L/min and comparisons of groups with each model were tested by repeated measures analysis of variance, followed by Bonferroni’s test. p<0.05 was considered significant. Testing between two groups was performed using a paired t-test.
Results
Table 1 shows the characteristics of the subjects. All subjects were Japanese men with normal respiratory function.
Table 1.
Subjects’ characteristics (mean±SD)
| Characteristic | ||||
| Responder | Non-responder | Total | p Value | |
| Age (years) | 33.4±7.1 | 31.5±9.2 | 33.0±7.0 | 0.75 |
| Height (cm) | 171.9±7.4 | 168.00±4.3 | 171.1±6.9 | 0.51 |
| Weight (kg) | 64.0±12.5 | 60.5±3.5 | 63.3±11.2 | 0.71 |
| BMI (kg/m2) | 21.5±2.6 | 21.5±2.3 | 21.5±2.4 | 1.00 |
| VC (L) | 4.6±0.4 | 3.78±0.30 | 4.43±0.51 | 0.03 |
| %VC (%pred) | 97.7±7.9 | 82.6±0.6 | 94.7±9.5 | 0.03 |
| FVC (L) | 4.53±0.48 | 3.75±0.49 | 4.38±0.56 | 0.08 |
| %FVC (%pred) | 98.0±10.2 | 83.0±3.4 | 95.0±11.1 | 0.09 |
| FEV1.0 (L) | 3.82±0.40 | 3.19±0.88 | 3.70±0.53 | 0.14 |
| FEV1.0 (%pred) | 84.7±8.0 | 84.2±12.4 | 84.6±6.8 | 0.94 |
| FRC (L) | 3.35±0.27 | 3.25±0.22 | 3.33±0.26 | 0.68 |
| VT (L) | 0.77±0.35 | 0.42±0.18 | 0.70±0.34 | 0.22 |
n=10.
BMI, body mass index; FEV1.0, forced expiratory volume in 1.0 s; FRC,functional residual capacity; FVC, forced vital capacity; VC, vital capacity.
During Nasal High-Flow Oxygen Therapy System (NHF-OTS), subjects who showed an increased TV were defined as responders (n=8), while those with no increase were defined as non-responders (n=2). t-Tests revealed significant differences between the two groups for vital capacity (VC), %VC and forced vital capacity (FVC).
Changes in ventilator parameters
HFNC was performed using three systems: the constant flow rate AIRVO 2, steady air constant rate and the constant pressure type Vivo 50. As seen in table 2, there were no significant differences among the systems for the TV, PtcCO2, RR, end-inspiratory oesophageal pressure (EIOP) and end-expiratory oesophageal pressure (EEOP) parameters.
Table 2.
Comparison of the three systems
| AIRVO 2 Constant flow |
Steady air Constant flow |
Vivo 50 Constant pressure |
p Values | |
| Difference in tidal volume (mL) | 221.40±263.92 | 257.30±315.06 | 244.24±268.63 | p>0.99 |
| Difference in PtcCO2 (mm Hg) | −0.50±2.37 | −0.30±1.06 | −0.60±2.32 | p>0.05 |
| Difference in RR (bpm) | −3.26±2.14 | −3.17±4.93 | −4.18±2.68 | p>079 |
| Difference in EIOP (cmH2O) | −3.18±4.70 | −2.04±1.74 | −1.77±2.56 | p>0.85 |
| Difference in EEOP (cmH2O) | −0.51±2.80 | 0.71±2.34 | 1.02±3.99 | p>0.92 |
p Values in comparison between NHF-OTS 30 L/min and baseline.
difference EEOP, difference in end-expiration oesophageal pressure; difference EIOP, difference in end-inspiration oesophageal pressure; PtcCO2, transcutaneous carbon dioxide; RR, respiratory rate.
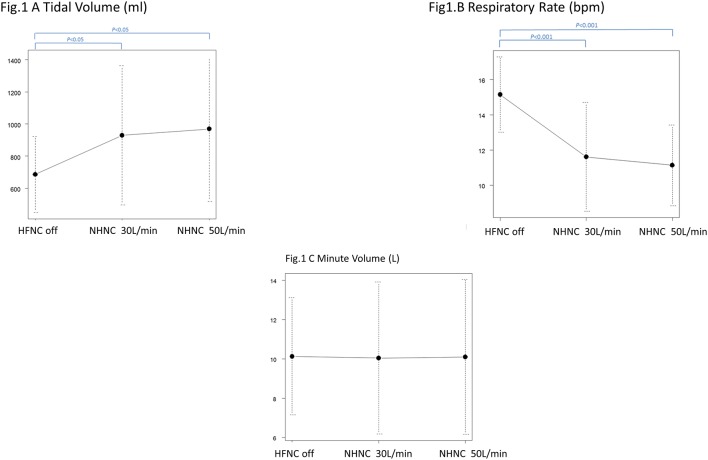
Table 3 shows the statistical analysis results for each parameter at each oxygen flow rate. The TV increased markedly (off, 30 L/min, 50 L/min: 685.6±236.5 mL, 929.8±434.7 mL, 968.8±451.1 mL), while RR decreased (off, 30 L/min, 50 L/min: 15.1±2.14 bpm, 11.6±3.08 bpm, 11.1±2.29 bpm). Accordingly, there were no significant differences in the MV (off, 30 L/min, 50 L/min: 9.77±0.62 L/min, 10.05±3.87 L/min, 10.10±3.94 L/min) (figure 1A–C).
Table 3.
Changes in ventilator parameters
| NHF-off | NHF-OTS 30 L/min | NHF-OTS 50 L/min | |
| TV | |||
| Mean (mL)±SD | 685.6±236.6 | 929.8±434.7 | 968.8±451.1 |
| p Value from NHF-off | <0.05 | <0.05 | |
| MV | |||
| Mean (L)±SD | 10.1±3.0 | 10.1±3.9 | 10.1±4.0 |
| p Value from NHF-off | p=1.00 | p=1.00 | |
| RR | |||
| Mean (bpm)±SD | 15.1±2.1 | 11.6±3.1 | 11.1±2.3 |
| p Value from NHF-off | p<0.01 | p<0.01 | |
| PtcCO2 | |||
| Mean (mm Hg)±SD | 37.3±2.8 | 36.6±3.6 | 36.4±5.0 |
| p Value from NHF-off | p>0.05 | p>0.05 |
p Value compared with NHF-OTS off.
n=30.
MV, minute ventilation; PtcCO2, transcutaneous carbon dioxide; RR, respiratory rate; TV, tidal volume.
Figure 1.
HFNC off is compared with HFNC 30 L/min and HFNC 50 L/min for tidal volume. HFNC off and HFNC 30 L/min and HFNC 50 L/min differ significantly. The HFNC group is significant, but 30 L/min and 50 L/min show no significant differences (HFNC off, HFNC 30 L/min, HFNC 50 L/min: 685.6±236.5 mL, 929.8±434.7 mL, 968.8±451.1 mL). HFNC off is compared with HFNC 30 L/min and HFNC 50 L/min for respiratory rate. HFNC off and HFNC 30 L/min and HFNC 50 L/min differ significantly. The high flow group is significantly different, but no significant difference is found between 30 L/min and 50 L/min (high Flow off, HFNC 30 L/min, HFNC 50 L/min: 15.1±2.14 bpm, 11.6±3.08 bpm, 11.1±2.29 bpm). HFNC off is compared with HFNC 30 L/min and HFNC 50 L/min for minute ventilation. High flow off does not differ significantly from high flow 30 L/min or 50 L/min (HFNC off, HFNC 30 L/min, HFNC 50 L/min: 9.77±0.62 L/min, 10.05 ±3.87 L/min, 10.10 ±3.94 L/min). HFNC, high-flow nasal cannula.
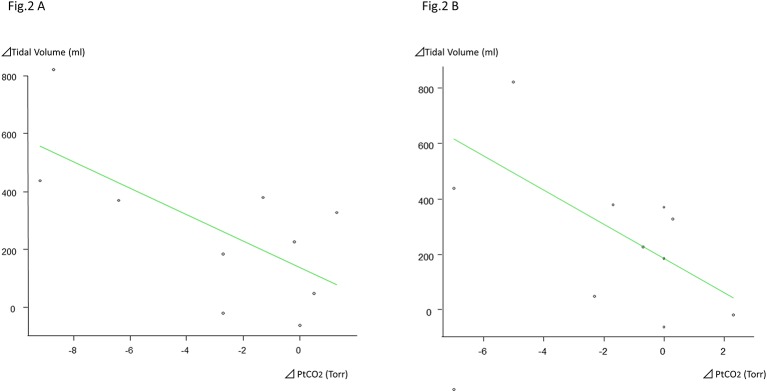
The rapid shallow breathing index (RSBI) showed a significant tendency to decrease with the HFNC, compared with HFNC off (off, 30 L/min, 50 L/min: 24.6±9.2 bpm, 15.6±8.70 bpm, 14.6±8.9 bpm). On HFNC at 30 L/min and 50 L/min, the difference between the increment in TV and the PtcCO2 showed negative correlations, with correlation coefficients of –0.461 and –0.652 (p=0.18, 0.04) (figure 2A and B).
Figure 2.
The amount of change in the NHF-OTS HFNC 30 L/min tidal volume and Δ PtCO2. At HFNC 30 L/min, the difference between the increment in vital capacity and the Δ PtCO2 shows a tendency for a weak negative correlation, with a correlation coefficient of –0.461, but it is not significant (p=0.18). The amount of change in the NHF-OTS HFNC 50 L/min tidal volume and Δ PtCO2. At HFNC 50 L/min, the increment in the vital capacity and the difference in the Δ PtCO2 show a correlation coefficient of –0.652, with a 95% CI of –0.909 to –0.0376, and p=0.0412. HFNC, high-flow nasal cannula; PtcCO2, transcutaneous carbon dioxide.
Oesophageal pressure during HFNC
EIOP, that is, the end-inspiratory thoracic pressure, decreased with HFNC, and a significant difference was observed between 30 L/min and HFNC off (off, 30 L/min, 50 L/min: 4.1±2.8 cmH2O, 1.8±3.6 cmH2O, 2.4±4.2 cmH2O). EEOP, that is, the end-expiratory thoracic pressure, increased. There was no significant difference between the 30 L/min and 50 L/min groups, but the tendency to increase was stronger in the 50 L/min group (off, 30 L/min, 50 L/min: 8.3±2.8 cmH2O, 8.7±3.0 cmH2O, 9.9±3.1 cmH2O) (table 4).
Table 4.
Changes in oesophageal pressure
| NHF-off | NHF-OTS 30 L/min | NHF-OTS 50 L/min | |
| End-inspiratory oesophageal pressure | |||
| Mean (cmH2O)±SD | 4.1±2.8 | 1.8±3.6 | 2.4±4.2 |
| p Value from NHF-off | <0.05 | >0.05 | |
| End-expiratory oesophageal pressure | |||
| Mean (cmH2O)±SD | 8.3±2.8 | 8.7±3.0 | 9.9±3.1 |
| p Value from NHF-off | >0.05 | >0.05 | |
| Δ Oesophageal pressure | |||
| Mean (cmH2O)±SD | 4.2±1.9 | 6.9±2.9 | 7.48±3.1 |
| p Value from NHF-off | <0.05 | <0.05 | |
| Ccw | |||
| Mean (mL/cmH2O)±SD | 175.9±55.0 | 138.4±30.9 | 136.8±51.5 |
| p Value from NHF-off | <0.05 | >0.05 |
p Value compared with the NHF-OTS off n=30.
Ccw, chest wall compliance.
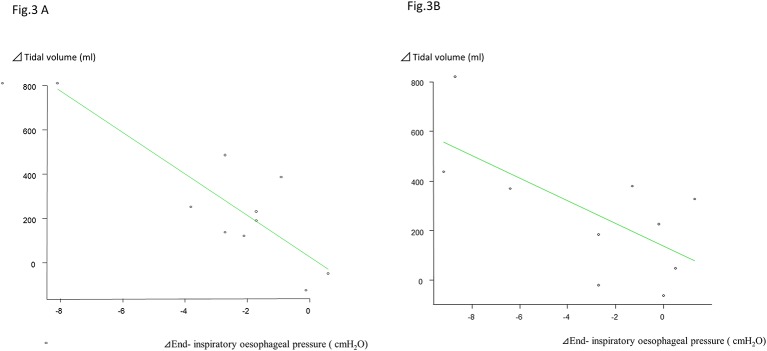
On HFNC 30 L/min, there were strong negative correlations between the increment in TV and the difference in fluctuation in EIOP, with correlation coefficients of –0.837 and −0.68 (p=0.0025, 0.03) (figure 3A and B).
Figure 3.
Correlation between Δ tidal volume and the end-inspiratory oesophageal pressure at NHF-OTS HFNC 30 L/min. At HFNC 30 L/min, the difference in the increment in vital capacity and the difference in the maximal inspiratory oesophageal pressure show a strong negative correlation, with a correlation coefficient of –0.84, and the correlation is significant (p=0.003). Correlation between Δ tidal volume and the end inspiratory oesophageal pressure at NHF-OTS HFNC 50 L/min. At HFNC 0 L/min, the difference in the increment in vital capacity and the difference in the maximal inspiratory oesophageal pressure show a strong negative correlation, with a correlation coefficient of –0.68, and the correlation is significant (p=0.003). HFNC, high-flow nasal cannula.
Forced oscillation technique
During HFNC, there were no significant differences in resistance at 5 Hz and 20 Hz (R5 and R20). However, the Xrs at 5 Hz (X5) decreased significantly (off, 30 L/min, 50 L/min: 0.06±0.15 cmH2O/L/s, 0.02±0.19 cmH2O/L/s, –0.12±0.17 cmH2O/L/s) (table 5).
Table 5.
Changes in forced oscillation technique
| NHF-off | NHF-OTS 30 L/min | NHF-OTS 50 L/min | |
| Resistance at 5 Hz | |||
| Mean (cmH2O/L/s)±SD | 2.15±0.77 | 2.26±1.03 | 2.35±1.08 |
| >0.05 | >0.05 | ||
| Resistance at 20 Hz | R20 | ||
| Mean (cmH2O/L/s)±SD | 1.96±0.75 | 2.01±0.92 | 2.00±0.86 |
| p Value from NHF-off | >0.05 | >0.05 | |
| X5 | |||
| Mean (cmH2O/L/s)±SD | 0.06±0.15 | 0.02±0.19 | −0.12±0.17 |
| p Value from NHF-off | >0.05 | <0.05 | |
| Resonant frequency (Fres) (Hz) | |||
| Mean±SD | 4.80±0.81 | 5.03±1.37 | 6.42±1.67 |
| p Value from NHF-off | >0.05 | <0.05 | |
| ALX | |||
| Mean (cmH2O/L/s*Hz)±SD | 0.15±0.18 | 0.30±0.41 | 0.61±0.50 |
| p Value from NHF-off | >0.05 | <0.05 |
p Value compared with the NHF-OTS off n=10.
ALX, low-frequency reactance area; X5, respiratory system reactance at 5 Hz.
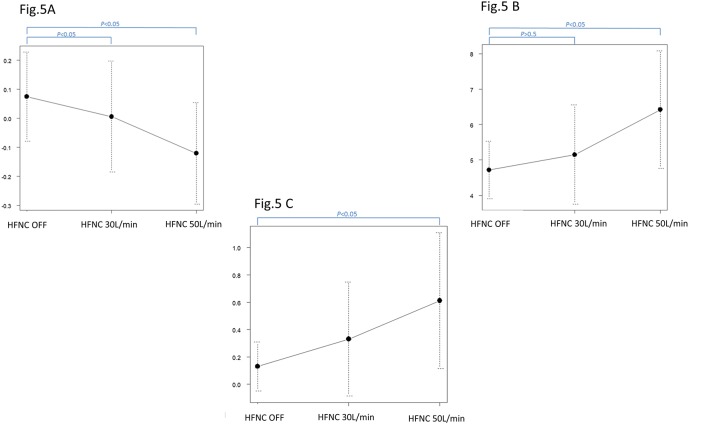
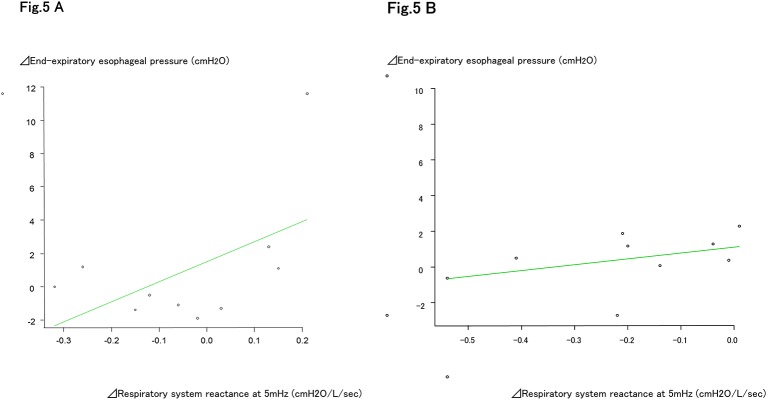
On HFNC 50 L/min, both the Fres and ALX increased significantly (Fres: off, 30 L/min, 50 L/min: 4.80±0.81 cmH2O/L/s, 5.03±1.37 cmH2O/L/s, 6.42±0.67 cmH2O/L/s) (ALX: off, 30 L/min, 50 L/min: 0.15±0.18 cmH2O/L/s, 0.30±0.41 cmH2O/L/s, –0.61±0.50 cmH2O/L/s) (table 5, figure 4A–C). The amount of change in EEOP and the fluctuation in X5 determined by the oesophageal balloon method showed weak positive correlations with both HFNC 30 L/min and 50 L/min, with correlation coefficients of 0.534 (95% CI –0.144 to 0.871, and p=0.112) and 0.404 (95% CI –0.356 to 0.842, and p=0.281) (figure 5A, B).
Figure 4.
Respiratory system resistance (Xrs) at 5 Hz (cmH2O/L/s). HFNC off is compared with HFNC 30 L/min and HFNC 50 L/min for X5. HFNC off and HFNC 30 L/min show significant differences from HFNC 50 L/min. The high flow group is significantly different, but no significant difference is found between 30 L/min and 50 L/min (high flow off, HFNC 30 L/min, HFNC 50 L/min: 0.06±0.15, 0.02±0.19, –0.12±0.17 cmH2O/L/s). HFNC off is compared with HFNC 30 L/min and HFNC 50 L/min for Fres. HFNC off and HFNC 30 L/min show significant differences from HFNC 50 L/min. The high flow group is significantly different, but no significant difference is found between 30 L/min and 50 L/min (high flow off, HFNC 30 L/min, HFNC 50 L/min: 4.80±0.81, 5.03±1.37, 6.42±0.67 cmH2O/L/s). HFNC off is compared with HFNC 30 L/min and HFNC 50 L/min for ALX. HFNC off and HFNC 30 L/min show significant differences from HFNC 50 L/min. The high flow group is significantly different, but no significant difference is found between 30 L/min and 50 L/min (high flow off, HFNC 30 L/min, HFNC 50 L/min: 0.15±0.18, 0.30±0.41, 0.61±0.50 cmH2O/L/s). ALX, low-frequency reactance area; HFNC, high-flow nasal cannula; X5, respiratory system reactance at 5 Hz.
Figure 5.
The amount of change in end-expiratory oesophageal pressure and the amount of change in X5. At HFNC30 L/min, the difference in the increment in vital capacity and the difference in the end-expiratory oesophageal pressure shows a tendency for a weak negative correlation, with a correlation coefficient of –0.534, but it is not significant (p=0.112). The amount of change in end-expiratory oesophageal pressure and the amount of change in X5. At HFNC50 L/min, the difference in the increment in vital capacity and the difference in the end expiratory oesophageal pressure shows a tendency for a weak negative correlation, with a correlation coefficient of –0.404, but it is not significant (p=0.281). ALX, low-frequency reactance area; HFNC, high-flow nasal cannula; X5, respiratory system reactance at 5 Hz.
Discussion
The present study generated two important findings. First, the EIOP was not significantly different, but there was a tendency for it to decrease, and there was a significant positive correlation between the amount of that change and the amount of change in TV. EEOP was dependent on the oxygen flow rate, thus confirming the PEEP effect of HFNC. RR decreased markedly, and RSBI decreased. These effects did not differ significantly between the constant flow model and the constant pressure model.
Next, the results of the FOT studies showed that HFNC did not cause any change in expiratory airway resistance, but it did cause flow rate-dependent increases in the elastic resistance and inertial resistance of the lungs. The amounts of change in EEOP and X5, which is an indicator of the peripheral capacitive reactance, were determined by the oesophageal balloon method and were shown to have a significant positive correlation. The PEEP effect of the HFNC was thus confirmed. This result suggests the possibility that the effects of HFNC can be confirmed by FOT, without depending on the invasive oesophageal balloon method.
Many reports have shown the usefulness of HFNC in the intensive care unit for type I acute respiratory failure,4 9 10 acute respiratory failure due to super-flu,11 prevention of reintubation after extubation3 12 and so on, but the respiratory physiological effects have not been clarified.
To date, the respiratory physiological effects of HFNC have been reported in regard to its effect in flushing out dead spaces, decreasing nasal and pharyngeal mucosal resistance, PEEP effect (1–3 cmH2O), increasing alveolar ventilation, improving warming and humidification, improving the accuracy of the inspired oxygen concentration and so on.3 4 12 13 Corley et al, Mauri et al and so on reported that HFNC use improved lung volume measured by the electrical impedance method.
In the present study, changes in the TV and MV were measured with an ExSpiron 1Xi. The ExSpiron 1Xi is a device that can externally and non-invasively measure TV, MV, and RR by detecting impedance changes in the thorax and respiratory muscles.14 15 It was found that the TV correlated with the oxygen flow during HFNC use and increased similarly to that reported by Corley et al 7 and Mauri et al.16
Bräunlich et al and Mündel et al used an elastic sensor belt for polysomnography to measure TV in healthy volunteers.17 18 Interestingly, the two papers provided different results in healthy volunteers. Bräunlich’s paper stated that TV decreased in healthy people. The present results differ, perhaps because the sex ratio differed (10 males in the present study, while they studied 6 males and 10 females), so that inspiratory reserve volume and functional residual capacity, and so on, may have been different. In addition, ages were very different between chronic obstructive pulmonary disease (COPD)/idiopathic pulmonary fibrosis (IPF) patients and healthy individuals in their study, and this was stated as one of the limitations of that study.17 Mündel did not mention the sex of the subjects. Bräunlich et al and Mündel et al used a polysomnograph for data measurement.17 18 Since we were using the electrical impedance method, the measured results may have differed. In addition, each of the papers, including the present one, had a small sample number of around 10; further studies with larger sample sizes are warranted. Finally, Bräunlich et al did not mention the mechanism by which the decrease in TV is supposed to occur in healthy people.17
Mündel et al also stated that, in healthy people during wakefulness, TV decreased with HFNC use, and the respiratory rate decreased. Those findings are consistent with the present results.18
Even in the present report, RR decreased markedly, as reported previously,19 and RSBI decreased.17 20 21 Based on these findings, HFNC use appears to reduce the work of breathing.
Earlier reports concluded that PEEP increased ventilation.7 22 In the present study, the EIOP, that is, the end-inspiratory thoracic pressure, was not significantly different, but there was a tendency for it to decrease with HFNC use, and there was a strong, significant negative correlation between the TV increment and the difference in EIOP (figure 3A and B). The reasons for this are thought to be that, by delivering inspiratory gas at a rate exceeding the patient’s inspiratory flow, the HFNC reduces intake resistance due to collapse of the nasopharynx, and spontaneous respiration is promoted by the high-pressure inspiratory gas, thereby decreasing the end-inspiratory thoracic pressure. As a result, the increment in TV is also thought to increase, because the pressure of the inspiratory gas increases as the flow rate becomes higher. This can be thought to support the earlier report that, as the flow-rate becomes higher, it becomes one factor in improving the patient’s ventilation, even when FiO2 is the same.8
The causes of the increase in end-expiratory oesophageal pressure are thought to be stretching of the alveoli and PEEP due to the HFNC. It can be surmised that, because the alveoli expanded, the intrathoracic volume became narrower, and EEOP increased.
It was thought that this extension of the alveoli became alveolar recruitment and contributed to improved oxygenation by HFNC use.
It was also reported that the work of breathing is reduced during HFNC use because thoracoabdominal synchrony becomes better than with a face mask.23 Accordingly, it is thought that HFNC use is not merely an oxygen therapy, and it exerts a spontaneous ventilatory assistance effect in terms of the respiratory physiology.
Most earlier studies on nasal high flow-rate oxygen therapy have used a constant flow type (AIRVO). In the present study, there was no significant difference in the ventilatory assistance effects between the constant flow and constant pressure types. (table 1) Based on that finding, it seems that a HFNC using the NPPV device is also advantageous, in that NPPV and a HFNC can be used together in the same circuit. It was also thought that, for pathological conditions in which assisted ventilation cannot be achieved with an HFNC alone, its combination with biphasic cuirass ventilation, which is also negative-pressure-assisted ventilation, may be useful.
As described above, HFNC, which increases TV, suppresses rebreathing of carbon dioxide, improves tachypnoea and can be expected to exert a PEEP effect, can be thought to be useful for COPD patients with type II respiratory failure. Reports of this are starting to appear.19 24 25
The advantages of respiratory management of COPD by HFNCs are an improved positive end-expiratory pressure phenomenon (autoPEEP) due to a PEEP effect, increased TV due to decreased EIOP resulting from improved inspiratory resistance, decreased RR, a constant inspired oxygen concentration because of the high flow rate and so on. The usefulness of NPPV has been established for acute exacerbations of COPD.26 27 However, due to the interface, an HFNC is reported to be more comfortable than NPPV, and better patient tolerability is reported.9 25 An HFNC was also reported to be more useful than NPPV in the treatment of immunodeficient patients with respiratory failure, because it improves airway mucosal clearance.27 The reason for acute exacerbation of COPD is often infection, and an HFNC appears useful from that perspective as well.
We earlier reported that HFNC use improved CO2 narcosis in acute COPD exacerbations and improved sleep alveolar hypoventilation with PSG.24 Bräunlich et al reported night-time use of an HFNC for 6 weeks in patients with stable hypercapnic COPD, but there was no significant difference from NPPV. Based on the present study, as well, it may be possible to prevent the onset of pulmonary hypertension and improve the prognosis of patients with chronic phase COPD by using HFNCs, which show high tolerability.19
Sztrymf et al 4 applied HFNCs for 48 hours in acute respiratory failure. PaO2 improved at 15 min after starting, and RR, respiratory distress, respiration pattern and heart rate also improved significantly. COPD patients with hypercapnia also showed improvement in RR and PtcCO2 in only 20 min.25 In the responder group in the present study as well, RR tended to decrease 2 min after starting HFNC, and steady breathing was observed after about 5 min. This suggests that, with HFNC use, RR, a few minutes after the start of treatment, may serve as an indicator for judging whether the patient is a responder to HFNC use and that if tachypnoea does not improve, the patient is a non-responder.
The FOT is a respiratory function test method that can carry out measurements non-invasively at resting ventilation. With FOT vibration, waves are loaded on the airway during resting ventilation, and the airflow and pressure in the oral cavity are measured over time. Such indicators of mechanics as Rrs and Xrs can be measured.28 29 MostGraph-02 is an instrument that uses the FOT and successively and continuously measures the respiratory resistance and reactance from 4 to 35 Hz.30 31
With HFNC use, there was no significant difference between resistance at 5 Hz and resistance at 20 Hz, which represent the airway resistances in the lungs. However, the Xrs at 5 Hz (X5), which is an indicator of peripheral capacitive reactance, decreased significantly. That is, it is thought that the PEEP effect of HFNC use causes hyperinflation of the lungs, and the alveoli become difficult to expand (table 5). In other words, as in the case of COPD lungs, it is thought that both the elastic resistance and the inertial resistance of the lung are increased due to the expansion of the lung by PEEP due to HFNC treatment (figure 5A–C). Based on the FOT results, there was no change in respiratory resistance, but the PEEP-like effect of HFNC was proven. It is thought that the reasons why the expiratory resistance did not improve were that the subject was a healthy person and that there was an incentive. If endogenous PEEP occurs as in COPD, the PEEP of HFNC use may decrease expiratory resistance.
Both Fres and ALX, which represent the elastic resistance of the lungs, increased, and thorax compliance determined from the oesophageal pressure decreased. These results were the same as in COPD.31
The oesophageal balloon method and the FOT showed that PEEP was applied to the lungs. Therefore, FOT may be useful for judging the effects of HFNC, since it is a method that can non-invasively measure, during resting ventilation, the overall respiratory resistance, without using the invasive oesophageal balloon method.
The limitations of this research are that it enrolled only healthy subjects, the number of subjects was small because the examination was invasive and the duration of HFNC use was short. In healthy subjects, the lung ventilatory parameters were stabilised after 5 min, but patients with impaired respiratory function may need a slightly longer period of evaluation.
Conclusion
The present study elucidated the physiological characteristics of HFNC use in healthy volunteers. EIOP was not significantly different but was a tendency for it to decrease, and the change correlated significantly with TV. EEOP increase was dependent on the oxygen flow rate, and the PEEP effect of HFNC use was confirmed. Similarly, RR decreased markedly, and RSBI decreased. The effect did not differ significantly between the constant flow type and the constant pressure type.
The results of the FOT indicated that HFNC use did not change expiratory airway resistance, but it became clear that it increased elastic resistance of the lungs in a flow-dependent manner. The amount of change in EEOP determined by the oesophageal balloon method and X5 showed a significant positive correlation. These results indicated that the effects of HFNC can be confirmed by FOT, without depending on the invasive oesophageal balloon method. In addition, the present demonstration that FOT yields similar results to findings from the oesophageal balloon method represents new and useful information.
Footnotes
Contributors: MO, NT, FK and RN designed the study and wrote the initial draft of the manuscript. KN, TK, YKa, YKi and YO contributed to analysis and interpretation of data and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation and critically reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Hirakata Kohsai Hospital Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Gotera C, Díaz Lobato S, Pinto T, et al. Clinical evidence on high flow oxygen therapy and active humidification in adults. Rev Port Pneumol 2013;19:217–27. doi:10.1016/j.rppneu.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 2. Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185–96. doi:10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 3. Rittayamai N, Tscheikuna J, Rujiwit P. High-flow nasal cannula versus conventional oxygen therapy after endotracheal extubation: a randomized crossover physiologic study. Respir Care 2014;59:485–90. doi:10.4187/respcare.02397 [DOI] [PubMed] [Google Scholar]
- 4. Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med 2011;37:1780–6. doi:10.1007/s00134-011-2354-6 [DOI] [PubMed] [Google Scholar]
- 5. Ritchie JE, Williams AB, Gerard C, et al. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care 2011;39:1103–10. [DOI] [PubMed] [Google Scholar]
- 6. Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth 2009;103:886–90. doi:10.1093/bja/aep280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011;107:998–1004. doi:10.1093/bja/aer265 [DOI] [PubMed] [Google Scholar]
- 8. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452–8. doi:10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roca O, Riera J, Torres F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care 2010;55:408–13. [PubMed] [Google Scholar]
- 10. Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care 2011;56:265–70. doi:10.4187/respcare.00801 [DOI] [PubMed] [Google Scholar]
- 11. Rello J, Pérez M, Roca O, et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care 2012;27:434–9. doi:10.1016/j.jcrc.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 12. Kernick J, Magarey J. What is the evidence for the use of high flow nasal cannula oxygen in adult patients admitted to critical care units? A systematic review. Aust Crit Care 2010;23:53–70. doi:10.1016/j.aucc.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 13. Hasani A, Chapman TH, McCool D, et al. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis 2008;5:81–6. doi:10.1177/1479972307087190 [DOI] [PubMed] [Google Scholar]
- 14. Voscopoulos CJ, MacNabb CM, Freeman J, et al. Continuous noninvasive respiratory volume monitoring for the identification of patients at risk for opioid-induced respiratory depression and obstructive breathing patterns. J Trauma Acute Care Surg 2014;77:S208–S215. doi:10.1097/TA.0000000000000400 [DOI] [PubMed] [Google Scholar]
- 15. Holley K, MacNabb CM, Georgiadis P, et al. Monitoring minute ventilation versus respiratory rate to measure the adequacy of ventilation in patients undergoing upper endoscopic procedures. J Clin Monit Comput 2016;30:33–9. doi:10.1007/s10877-015-9674-y [DOI] [PubMed] [Google Scholar]
- 16. Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2017;195:1207–15. doi:10.1164/rccm.201605-0916OC [DOI] [PubMed] [Google Scholar]
- 17. Bräunlich J, Beyer D, Mai D, et al. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration 2013;85:319–25. doi:10.1159/000342027 [DOI] [PubMed] [Google Scholar]
- 18. Mündel T, Feng S, Tatkov S, et al. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol 2013;114:1058–65. doi:10.1152/japplphysiol.01308.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bräunlich J, Seyfarth HJ, Wirtz H. Nasal High-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med 2015;10:27 doi:10.1186/s40248-015-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biselli PJ, Kirkness JP, Grote L, et al. Nasal high-flow therapy reduces work of breathing compared with oxygen during sleep in COPD and smoking controls: a prospective observational study. J Appl Physiol 2017;122:82–8. doi:10.1152/japplphysiol.00279.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pisani L, Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. COPD 2017;14:346–50. doi:10.1080/15412555.2017.1315715 [DOI] [PubMed] [Google Scholar]
- 22. Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 2007;20:126–31. doi:10.1016/j.aucc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 23. Itagaki T, Okuda N, Tsunano Y, et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients. Respir Care 2014;59:70–4. doi:10.4187/respcare.02480 [DOI] [PubMed] [Google Scholar]
- 24. Okuda M, Kashio M, Tanaka N, et al. Nasal high-flow oxygen therapy system for improving sleep-related hypoventilation in chronic obstructive pulmonary disease: a case report. J Med Case Rep 2014;8:341 doi:10.1186/1752-1947-8-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraser JF, Spooner AJ, Dunster KR, et al. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax 2016;71:759–61. doi:10.1136/thoraxjnl-2015-207962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995;333:817–22. doi:10.1056/NEJM199509283331301 [DOI] [PubMed] [Google Scholar]
- 27. Frat JP, Ragot S, Girault C, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med 2016;4:646–52. doi:10.1016/S2213-2600(16)30093-5 [DOI] [PubMed] [Google Scholar]
- 28. Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther 2001;14:341–50. doi:10.1006/pupt.2001.0310 [DOI] [PubMed] [Google Scholar]
- 29. Mikamo M, Fujisawa T, Oyama Y, et al. Clinical significance of forced Oscillation Technique for evaluation of Small Airway Disease in Interstitial lung diseases. Lung 2016;194:975–83. doi:10.1007/s00408-016-9949-1 [DOI] [PubMed] [Google Scholar]
- 30. Kitaguchi Y, Yasuo M, Hanaoka M. Comparison of pulmonary function in patients with COPD, asthma-COPD overlap syndrome, and asthma with airflow limitation. Int J Chron Obstruct Pulmon Dis 2016;11:991–7. doi:10.2147/COPD.S105988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karayama M, Inui N, Mori K, et al. Respiratory impedance is correlated with morphological changes in the lungs on three-dimensional CT in patients with COPD. Sci Rep 2017;7:41709 doi:10.1038/srep41709 [DOI] [PMC free article] [PubMed] [Google Scholar]