Abstract
Significance: Impaired wound healing is a major complication of diabetes, and can lead to development of chronic foot ulcers in a significant number of patients. Despite the danger posed by poor healing, very few specific therapies exist, leaving patients at risk of hospitalization, amputation, and further decline in overall health.
Recent Advances: Redox signaling is a key regulator of wound healing, especially through its influence on the extracellular matrix (ECM). Normal redox signaling is disrupted in diabetes leading to several pathological mechanisms that alter the balance between reactive oxygen species (ROS) generation and scavenging. Importantly, pathological oxidative stress can alter ECM structure and function.
Critical Issues: There is limited understanding of the specific role of altered redox signaling in the diabetic wound, although there is evidence that ROS are involved in the underlying pathology.
Future Directions: Preclinical studies of antioxidant-based therapies for diabetic wound healing have yielded promising results. Redox-based therapeutics constitute a novel approach for the treatment of wounds in diabetes patients that deserve further investigation. Antioxid. Redox Signal. 27, 823–838.
Keywords: : diabetes, wound healing, reactive oxygen species, extracellular matrix, collagen
Introduction
Diabetes is widespread in the United States, and its complications have devastating effects on health and quality of life (1, 2, 174). One of the most serious complications of diabetes is impaired wound healing, which leads to the development of chronic wounds in the lower extremities in 15–25% of diabetes patients (27, 28, 30, 167, 194). Chronic wounds significantly decrease mobility, social functioning, and overall health, and are the leading cause of hospitalization and limb amputation in diabetes patients (28, 144, 167, 174). In addition, management of diabetic wounds is a major economic burden, generating $13 billion in healthcare costs per year in the United States (28, 194). Conventional wound care practices can be effective in diabetes patients, but a large fraction of diabetic ulcers still persist (10–15%) or lead to amputation (5–24%) 6–18 months after diagnosis (8, 27, 119, 128). Novel therapeutic strategies must focus on the pathological mechanisms underlying impaired healing in diabetes to improve patient outcomes.
Aberrant redox signaling and increased oxidative stress are widely accepted contributors to the development of diabetic complications, including cardiovascular disease, nephropathy, and retinopathy (12, 33, 68, 112, 145, 161). Oxidative stress also plays a significant role in regulating normal wound healing by facilitating hemostasis, inflammation, wound closure, and development and maturation of the extracellular matrix (ECM) (53, 149, 152–154, 156). The ECM is an important mediator of healing—it provides structure, coordinates cell–matrix and cell–cell interactions, and facilitates signal transduction in the wound. This review will examine the role of oxidative stress in the etiology of impaired healing in diabetes, with a particular focus on the ECM, and discuss the development of treatment strategies based on these principles.
Wound Healing in Diabetes
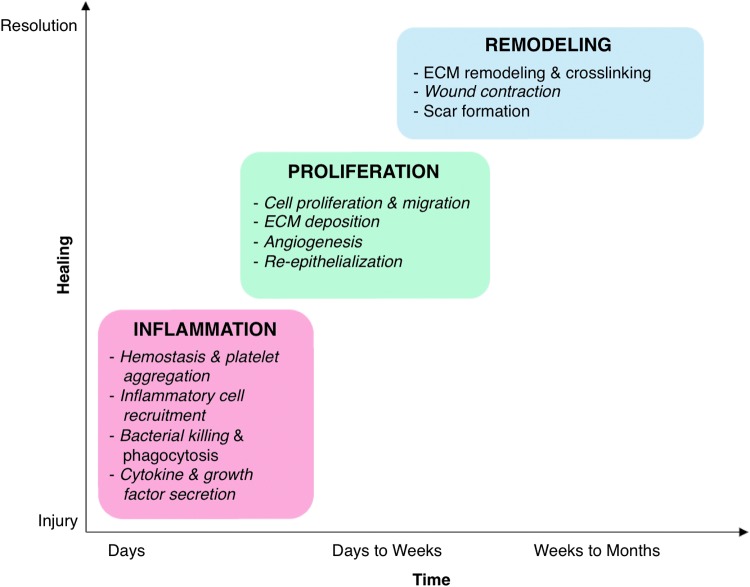
Dermal wound healing [reviewed extensively in Refs. (19, 72, 160, 185)] is a highly coordinated process that occurs in three overlapping phases: (i) inflammation, which includes hemostasis, inflammatory cell recruitment, and cytokine and growth factor secretion; (ii) proliferation, which is characterized by formation of the provisional matrix, angiogenesis, and re-epithelialization; and (iii) remodeling, in which granulation tissue is reorganized and the mature scar is formed (Fig. 1). In the diabetic wound, each of these phases is compromised, disrupting and delaying the orderly progression of healing (Fig. 2) (26, 59, 167). The bulk of controlled studies involving diabetic wounds have been performed in animal models. It is widely accepted that these do not fully recapitulate the human disease, so researchers often employ multiple animal models to study wound healing (91). This review is largely based on studies of diabetic wound healing in animals, coupled with data from controlled human studies when available.
FIG. 1.
Redox control of dermal wound healing. Normal wound healing occurs in three overlapping phases: inflammation, proliferation, and remodeling. Progression through these phases is highly regulated and coordinated by several mechanisms, including redox signaling. Both generation and scavenging of ROS, particularly H2O2, are critical to normal healing. The major processes regulated by redox signaling in each phase of healing are indicated in italics. ECM, extracellular matrix; H2O2, hydrogen peroxide; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
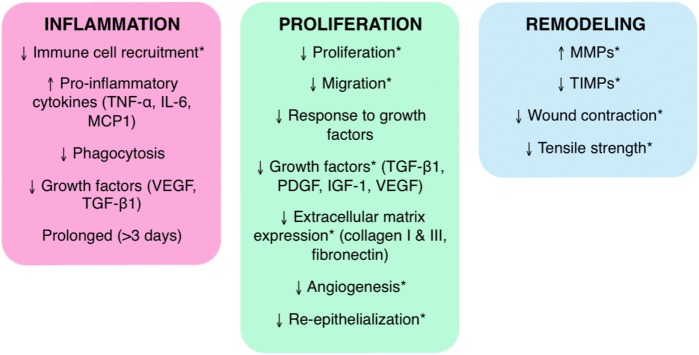
FIG. 2.
Wound healing in diabetes. In contrast to normal healing, wound healing in diabetes is uncoordinated and spatiotemporally disorganized. Chronic diabetic wounds do not progress smoothly through inflammation, proliferation, and remodeling; they are instead characterized by an extended inflammation phase, a limited proliferation phase, and irregular remodeling. The critical changes in each phase of healing in diabetes are identified. Healing processes that involve ECM, a critical facilitator of healing because of its role as structural support and a mediator of cellular interactions, are indicated by asterisk (*). IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; MCP-1, macrophage chemoattractant protein-1; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; TGF-β1, transforming growth factor-β1; TIMP, tissue inhibitors of metalloproteinase; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The inflammation phase in diabetic wound healing is prolonged but ineffective (3, 163, 167, 183). Diabetes is characterized by chronic systemic inflammation, evidenced by increased baseline expression of inflammatory markers, including macrophage chemoattractant protein-1, tumor necrosis factor (TNF), interleukin-6 (IL-6), and soluble P- and E-selectins, in blood collected from diabetes patients (87, 125, 181). This pattern of expression of proinflammatory factors influences inflammation in response to injury, and is characteristic of chronic ulcers in a variety of settings (52, 195). After injury in diabetes, neutrophils and macrophages are slowly recruited to the wound, but remain in the wound bed in large numbers for an extended period of time (3, 65, 106, 182, 183). This creates an environment that is particularly enriched in proinflammatory cytokines (such as IL-1β, IL-6, and TNF-α) and reactive oxygen species (ROS), which further damage the tissue and stall proliferation of fibroblasts and keratinocytes essential for the later phases of healing (3, 167, 183). Macrophages in diabetic wounds also exhibit reduced phagocytic capacity, which allows bacteria and debris to accumulate and decreases expression of growth factors, such as vascular endothelial growth factor (VEGF) (26, 59, 88, 167). These defects limit angiogenesis and progression to the proliferation phase (3, 167, 183).
In diabetic wounds, the proliferation phase is characterized by impaired granulation tissue formation. Granulation tissue comprises an ECM produced by fibroblasts and new blood vessels formed by invading endothelial cells and serves as a scaffold for keratinocyte migration and wound closure. Decreased expression of growth factors (such as VEGF and TGF-β) diminishes the proliferation, migration, and differentiation of fibroblasts, endothelial cells, and keratinocytes (26, 78). Diabetic wound fibroblasts also have abnormal morphology, decreased adhesion, diminished response to growth factors and cytokines, and decreased production of collagens and fibronectin (FN) (9, 71, 94, 189). This results in abnormal ECM structure and composition (Fig. 3) (163). Moreover, the ECM is damaged by overexpression of matrix metalloproteinases (MMPs) and decreased expression of their inhibitors (tissue inhibitors of metalloproteinases [TIMPs]) (26, 29, 102, 103). The MMP/TIMP imbalance also leads to growth factor degradation, which interrupts signaling vital to endothelial cell and keratinocyte migration, and therefore impairs angiogenesis and re-epithelialization (15, 26, 37, 59, 81).
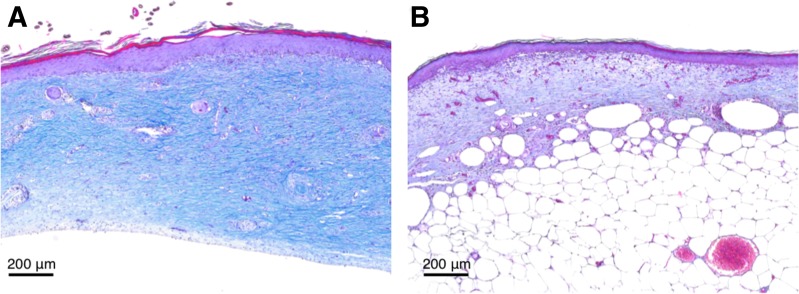
FIG. 3.
ECM deposition is reduced in diabetes. Reduced deposition of ECM is characteristic of wound healing in diabetes. Masson's trichrome staining of mouse granulation tissue of healthy C57BL/6 mice (A) and diabetic db/db mice (B) reveals significantly reduced collagen deposition and maturation (blue). Wounds were explanted 14 days postinjury.
The remodeling phase is similarly impaired by the proteolytic environment in diabetic wounds. Excessive breakdown of ECM proteins and the formation of abnormal protein–protein bonds disrupt normal formation of the mature collagen matrix and permanent scar (11, 26, 117, 167). This can lead to decreased scar thickness (depth), as is observed in the type 2 diabetic Zucker rat and other animal models; such changes in ECM deposition in the scar may reduce tensile strength and make the skin more susceptible to damage and reinjury (117, 150, 163). Decreased wound contraction during remodeling also contributes to reinjury risk; in the Zucker rat, a greater portion of healed skin is composed of scar tissue, which is significantly weaker than normal skin (163). Chronic wound development in diabetes is influenced by myriad defects in signaling, cell function, and ECM structure throughout the healing process.
ECM in Diabetic Wound Healing
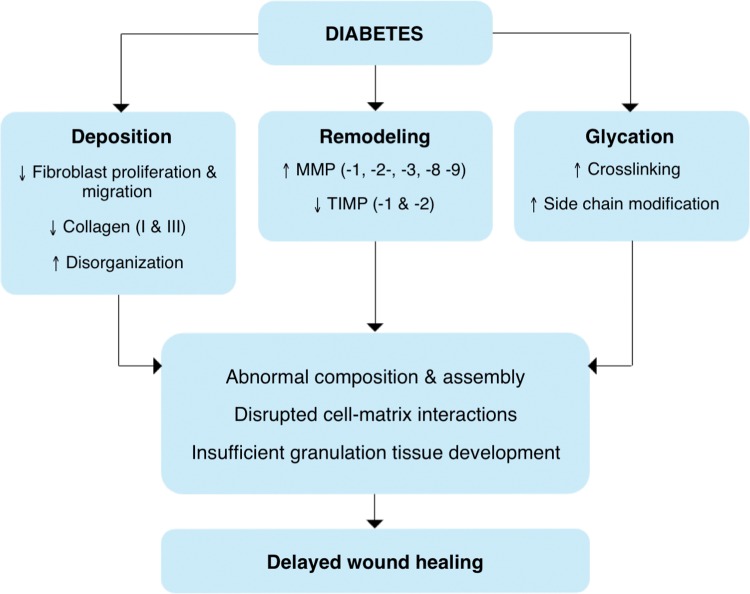
ECM is a critical facilitator of wound healing, from its beginnings as a fibrin clot through remodeling into granulation tissue and a permanent scar [reviewed in Refs. (4, 64, 139, 170)]. Wound ECM not only provides structure and support to the tissue, but also serves as a reservoir for growth factors and mediates cell–cell, cell–matrix, and matrix–protein interactions. Through these interactions, ECM influences cell behavior and function (including adhesion, proliferation, migration, differentiation, and gene expression), and thus its own remodeling and maturation. The importance of ECM is reflected in the diabetic wound, where its irregularity has numerous effects on wound fibroblasts, keratinocytes, and endothelial cells, as already described. In diabetes, the structure and function of ECM is marred by fibroblast dysfunction, changes in protein deposition, degradation, and remodeling, and post-translational modification by advanced glycation end products (Fig. 4).
FIG. 4.
Changes in ECM in diabetes. The structure and function of the ECM are altered in diabetes via changes in fibroblast function, post-translational modification by glucose (glycation), and an imbalance of ECM deposition and remodeling. These changes influence matrix composition and assembly, cell–matrix interactions, and development of granulation tissue, and ultimately contribute to delayed wound healing in diabetes. Changes described in this figure have been found in human diabetic tissues and wounds. TIMP, tissue inhibitors of metalloproteinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Fibroblast function in diabetes
Fibroblasts are key to ECM production and remodeling in wound healing, but several of their essential functions are compromised in diabetes. For example, fibroblasts isolated from diabetic wounds proliferate more slowly than fibroblasts isolated from uninjured skin and nondiabetic chronic wounds (79). Diabetes also induces fibroblast apoptosis; there are increased TUNEL and caspase-3 positive fibroblasts in diabetic gingival wounds than in normal controls (49). Similarly, hyperglycemia has been shown to inhibit proliferation and induce apoptosis in dermal fibroblasts in vitro (78, 189). Migration is similarly reduced; fibroblasts derived from the db/db mouse exhibit decreased invasion in Boyden chamber assays, and high glucose impairs migration of normal fibroblasts by suppressing c-Jun n-terminal kinase (JNK) phosphorylation in vitro (97, 189). Moreover, fibroblasts isolated from diabetic wounds respond abnormally to TGF-β1, a growth factor that induces collagen and ECM synthesis, and retain collagen intracellularly (110). All together, these defects in fibroblast function result in poor ECM deposition, which is discussed in detail below.
ECM deposition in diabetes
The deposition of collagen, the most abundant ECM protein in normal tissue and the healing wound, is significantly altered in diabetes (Fig. 3). At baseline, skin biopsies from diabetes patients exhibit lower expression of collagens I and III, as detected by Western blot and immunohistochemistry (22, 192). The ratio of collagen I to collagen III, which is correlated with ECM tensile strength, is also reduced (22). Moreover, the ECM in diabetic skin is more disorganized, with increased spacing between collagen fibrils (123, 192). Diabetes may also impact collagen fibril diameter, but it remains unclear whether fibril thickness is increased or decreased (123, 192). Similar decreases in collagen I and collagen III levels have been reported in diabetic rodent models, and histological analysis reveals degeneration of collagen fibers and disorganized epithelial structure as shown in human skin (39, 92, 93, 146). Real-time PCR analysis has shown increased Col1a2, Col3a1, and Col5a1 gene expression in patients with low skin collagen levels, which suggests post-transcriptional regulation of collagen in diabetes, but most studies report only protein or hydroxyproline content (22, 123, 192). In addition, gene expression levels of collagens I, III, IV, V, VI, XIV, and XVII are decreased in diabetic rats, which is consistent with the collagen protein levels in other diabetic rodent models (146, 163).
The collagen levels in diabetic wounds are also significantly decreased, as determined from hydroxyproline assay and Masson's trichrome staining of diabetic human and mouse wounds (25, 74, 80, 120). Specifically, collagen I and III levels are decreased compared with those in nondiabetic wounds, although Col1a1 and Col3a1 gene expression was elevated in diabetic wounds in one study (34, 163). Notably, diabetic wounds exhibit increased expression of miR-29a, a key negative regulator of collagen I and collagen III expression, which is a potential mechanism of post-transcriptional regulation of collagen in diabetes (34, 114). Despite the major deficits in collagen deposition in diabetic wounds, few studies have addressed mechanisms that mediate changes in transcription or post-transcriptional regulation of collagen in this environment, such as decreased TGF-β1 signaling and microRNA regulation (6, 24, 55, 75, 110).
FN is another major component of granulation tissue and an essential antecedent of collagen I deposition. FN is elevated in the dermis of chronic diabetic ulcers analyzed by immunohistochemistry (and persists 12–18 months postwounding), but has also been reported to be highly fragmented in wounds and diseased gums in diabetes patients (106, 165). FN is overrepresented in ECM produced by diabetic ulcer-derived fibroblasts, but the same fibroblasts exhibit dampened FN expression in response to TGF-β1 stimulation (110). One study reported a threefold decrease in FN RNA expression in diabetic ulcers, but this was in comparison with normal uninjured skin, which complicates interpretation (61). Although further study is required to fully understand FN expression in diabetic wounds, multiple studies have shown that treatment with exogenous FN improves healing rate and hydroxyproline content in diabetic wounds (73, 138).
ECM remodeling in diabetes
MMPs, which cleave collagen, FN, and other components of the ECM, are highly active in diabetes (175). Skin biopsy samples from diabetes patients exhibit increased expression of active MMP-1, MMP-2, and MMP-9, as determined by ELISA and gelatin zymography (95, 192). Similarly, MMP-2 and MMP-3 are elevated in the skin in rat models of diabetes (92, 93). Wound tissue homogenates from diabetes patients have significantly elevated levels of MMP-2, MMP-3, MMP-8, and MMP-9 compared with nondiabetic controls, and analysis of diabetic wound exudate also demonstrates elevated MMP-2 and MMP-9 (102, 177). Moreover, fibroblasts derived from diabetic wounds secrete more MMP-2 and MMP-3 in culture (178). Comparably, MMP-2, MMP-3, and MMP-13 expression is increased in wounds of the diabetic Zucker rat, and MMP-9 activity is increased in granulation tissue of diabetic mice (C57BL/6-db) (146, 163). One study has indicated that MMP-2 and MMP-9 are actually decreased in diabetic mouse wounds, but these conclusions were based on RNA expression data rather than protein quantification (177). Diabetic wounds also typically contain high levels of bacterial proteases, which can activate human proteases, including MMP-2 (116).
Elevated MMP activity in diabetes is compounded by decreased expression of TIMPs, which bind to and inhibit activated MMPs. TIMP-1 and TIMP-2 levels are decreased at baseline in skin biopsy samples from diabetes patients and rodent models (92, 93, 192). TIMP-2 is also reduced in diabetic wound homogenates (102). An increase in MMP/TIMP ratio disrupts the normal balance of ECM synthesis and degradation, which impacts ECM composition and fragmentation. Specifically, high MMP-9/TIMP ratio is predictive of poor healing in diabetes patients (99). Because of this, there is significant interest in targeting MMP activity to treat diabetic wounds; one recent study demonstrated that MMP-9 knockout or inhibition improved healing in diabetic mice (66).
ECM glycation in diabetes
In diabetes, ECM structure and function is also changed by glycation, a nonenzymatic reaction between glucose and proteins (39, 123, 124). Glycation leads to the formation of intermolecular crosslinks, which significantly alter the biomechanical properties of ECM (69). For example, in vitro glycation of skin biopsy samples increases direction-dependent stiffness of the tissue (143). Similarly, glycated collagen matrices are less flexible and more rigid than nonglycated collagen matrices, which impairs their contraction by myofibroblasts, an essential aspect of scar formation (100, 134). Glycation of collagen side chains alters the overall charge of the molecule, which interferes with its interaction with other matrix components and disrupts normal matrix assembly (67, 69). Glycated collagen is also more resistant to MMP-mediated degradation, which disrupts matrix remodeling (45, 69).
ECM glycation also alters cell–matrix interactions and cell behavior (14, 134). For example, contact with glycosylated matrix induced cell cycle arrest and apoptosis in cultured human dermal fibroblasts, an effect mediated by activation of the receptor for advanced glycation end products (RAGE) (39, 124). This increase in apoptosis is consistent with the increased TUNEL staining observed in histological sections of human diabetic wounds (123, 124). Furthermore, fibroblasts cultured on glycated collagen exhibit decreased migration because of poor integrin binding and reduced expression of collagen, FN, elastin, and MMP-1 (100, 123, 170). Keratinocytes and endothelial cells similarly exhibit reduced migration and adhesion on glycated ECM (134). Multiple studies have attempted to improve wound healing in diabetes by inhibiting ECM glycation; however, treatment with aminoguanidine, which decreases the formation of advanced glycation end products (AGE), has yielded mixed results, although differences may be related to the diabetes models used (21, 191).
Redox Signaling in Wound Healing
ROS, including superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radical, and other reactive oxygen derivatives, are produced in the cell as an unavoidable byproduct of oxidative phosphorylation. ROS can damage cells by oxidizing lipids and proteins, so levels are tightly controlled by the presence of ROS scavenging enzymes and small molecule antioxidants. Despite the potential harm posed by ROS, signaling through these molecules is essential for many cellular processes (154).
Redox signaling regulates several wound healing processes (Fig. 1) [reviewed in Refs. (53, 149, 154, 156)]. H2O2, a reactive species produced by dismutation of O2−, acts as the principal secondary messenger in wound healing and is present at low concentrations (100–250 μM) in normal wounds (53, 149). The critical role of ROS in healing has been shown in systems with NADPH oxidase (Nox) deficiency or antioxidant overexpression; wounds with low levels of ROS because of these defects exhibit impaired angiogenesis, abnormal remodeling, and delayed closure in patients and mouse models (62, 98, 147).
Redox regulation of wound healing begins in the inflammation phase, when ROS levels peak (130). Platelet aggregation in response to collagen induces production of O2− and H2O2, which facilitate further aggregation and platelet recruitment (46, 135, 156). O2− and H2O2 also modulate platelet aggregation in response to various stimuli in vitro, including collagen, adenosine diphosphate (ADP), and arachidonic acid (10, 173). ROS generated during hemostasis may also contribute to inflammatory cell recruitment to the wound by stimulating chemotaxis and adhesion molecule expression (105, 154). High levels of O2− and H2O2 are generated by neutrophils and macrophages via Nox, which is rapidly expressed after wounding, and subsequent dismutation (53, 149, 154, 156). This “oxidative burst” serves as the primary mechanism of bacterial killing and prevention of wound infection, and is accompanied by a temporary downregulation of some ROS scavenging enzymes (148, 156). ROS also stimulate release of cytokines and growth factors, including macrophage colony-stimulating factor, platelet-derived growth factor (PDGF), and TNF-α.
Redox signaling is also critical for the proliferation phase. ROS promote fibroblast proliferation and migration, and mediate TGF-β1 signaling, which results in migration, collagen and FN production, and basic fibroblast growth factor (bFGF) expression (7, 176, 193). ROS also facilitate angiogenesis; H2O2 stimulates VEGF expression by macrophages, keratinocytes, and fibroblasts independent of hypoxia inducible factor, and is required for signaling downstream of VEGF receptor binding (35, 40, 147, 155, 156). Furthermore, exogenous H2O2 induces endothelial cell migration, and low levels of exogenous H2O2 increase angiogenesis in mouse wounds (56, 105). H2O2 also stimulates keratinocyte proliferation and migration, facilitating re-epithelialization (104).
ROS generated in wounds are tightly regulated by ROS scavenging enzymes, such as superoxide dismutases (Cu/ZnSOD, MnSOD, and SOD3), peroxidases (catalase [CAT], phospholipid hydroperoxide glutathione peroxidase), and peroxiredoxins, as well as small molecule antioxidants, such as vitamin E and glutathione (149, 166). Many of the enzymes are upregulated in healing wounds, whereas levels of the small molecule antioxidants drop as they are depleted by ROS (149). Disrupting the redox balance provided by these antioxidant enzymes is sufficient to make wounds chronic, as does overwhelming the antioxidant mechanisms by adding exogenous H2O2 (50, 105, 147). A balance of ROS generation and scavenging is required for efficient and timely wound healing.
Redox Signaling in Diabetes
ROS levels are elevated in various tissues in diabetes patients through a combination of mechanisms that increase ROS production and reduce antioxidant defenses (Fig. 5) (68, 112). Thus, diabetic wounds are characterized by high levels of ROS, particularly O2− and H2O2 (50, 126, 179, 189). Several pathological mechanisms contribute to the accumulation of ROS in diabetic wounds, all of them secondary to hyperglycemia (33, 57, 68, 133). When considered with the central role of redox signaling in wound repair, the redox imbalance in diabetic wounds described hereunder has major implications for the pathogenesis of delayed healing in diabetes.
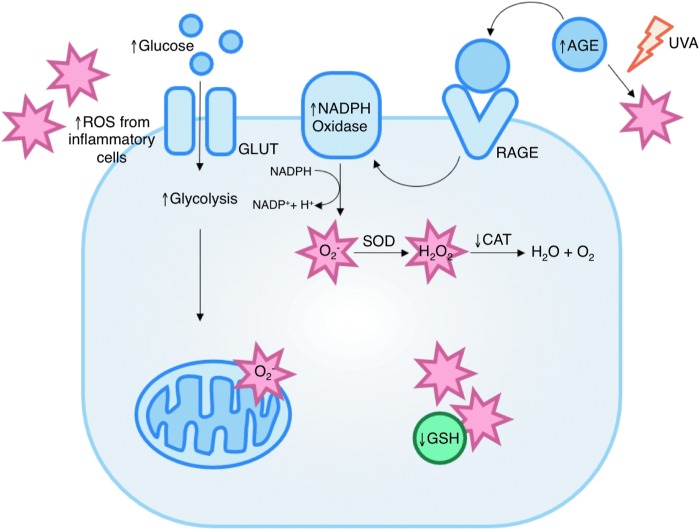
FIG. 5.
Sources of oxidative stress in diabetic wounds. Several mechanisms contribute to increased ROS levels in diabetes (indicated by stars). These include increased mitochondrial superoxide production, formation of advanced glycation end-products, increased activity of ROS-generating enzymes such as NADPH oxidase, and decreased expression of antioxidant enzymes and small molecules. AGE, advanced glycation end products; CAT, catalase; GLUT, glucose transporter; GSH, glutathione; RAGE, receptor for advanced glycation end products; SOD, superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mitochondrial superoxide production
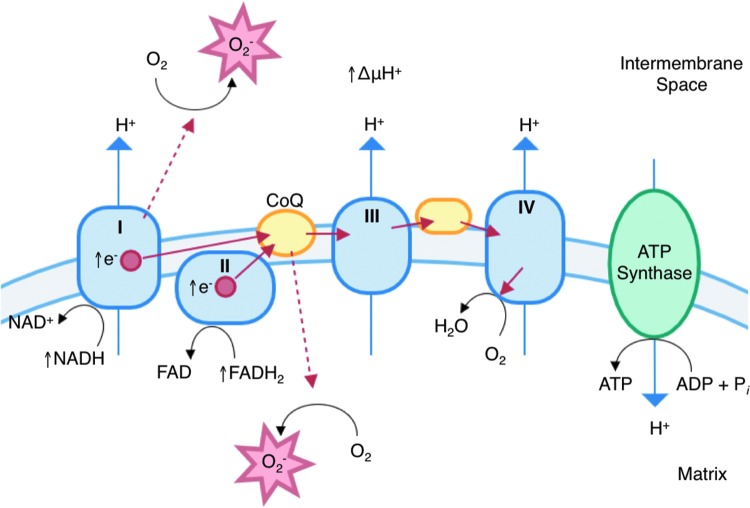
High glucose significantly increases O2− levels in cells and skin in vitro through the mitochondrial electron transport chain (42, 109, 133, 157). Superoxide is an unavoidable byproduct of oxidative phosphorylation, but under normal conditions, <10% of all oxygen consumed in aerobic metabolism is reduced to O2− (68, 133). Hyperglycemia increases O2− production by increasing the amount of pyruvate oxidation in the TCA cycle and consequently the availability of electron donors NADH and FADH2. Increased electron flux then increases the proton gradient across the inner mitochondrial membrane, which at a critical threshold disrupts electron transport through complex III (68). Then, electron transport is largely mediated by coenzyme Q, which transfers only one electron to oxygen, producing excess O2− and H2O2 (Fig. 6) (68, 122).
FIG. 6.
Excess mitochondrial superoxide production in diabetes. Hyperglycemia induces excess superoxide production by increasing the number of electron donors available to the electron transport chain. This increases the proton gradient past a critical level, and allows electron leakage (indicated by dashed lines) at complex I and CoQ. CoQ, coenzyme Q. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Excessive superoxide production in the mitochondria further impacts ROS levels by altering the flux through several intracellular pathways (33, 67, 68, 122). ROS leads to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) inhibition by poly(ADP-ribose) modification, which increases levels of glycolysis intermediates upstream of GAPDH. This provides increased substrate levels for the polyol, protein kinase C (PKC), and hexosamine pathways (68, 122, 129). Activation and interaction of these pathways ultimately alter gene expression, deplete antioxidant resources, and favor the production of advanced glycation end products (60, 68, 122, 133).
Advanced glycation end products
AGE are formed through nonenzymatic reactions between glucose or other reducing sugars and proteins. The carbonyl group of the sugar reacts with the free amino group of an amino acid, such as lysine or arginine, to form a Schiff base (69). Then, rearrangement leads to the formation of a stable Amadori product, which may be further rearranged, condensed, oxidized, or dehydrated to form new AGE or crosslinks and adducts with additional proteins (68, 69). AGE accumulate more rapidly in high glucose cell culture as well as diabetic patients and animal models (67, 127, 159, 188).
AGE increase intracellular ROS levels by several mechanisms, even in normal glucose conditions (38, 42, 115, 159). AGE binding with the RAGE produces O2− and H2O2 through activation of Nox and by increasing the expression of Nox subunits, including Nox4 and p22phox (38, 107, 159, 163, 188). This ROS production has been shown to exacerbate excessive mitochondrial superoxide production in diabetes; cytosolic H2O2 produced after RAGE binding decreases the activity of complex I, resulting in increased superoxide leakage in diabetic conditions (42). ROS produced by the mitochondria, in turn, increase RAGE expression, perpetuating further ROS generation (18). There is also evidence that AGE induce ROS production through α1β1 integrin binding and Nox activation independent of RAGE and under UVA radiation, which is particularly relevant in the skin (107, 131). AGE-induced ROS production in endothelial cells has been inhibited by treatment with an anti-RAGE antibody, but further research must be done to address RAGE-independent mechanisms of ROS generation by AGE (18).
Increased ROS-generating enzymes
ROS production by several ROS-generating enzymes is elevated in diabetic wounds. As already discussed, expression and activity of Nox, the major source of ROS in many cell types, are increased in response to RAGE binding (132). Nox activity is also increased downstream of hyperglycemia-induced PKC activation in smooth muscle and endothelial cells (82). PKC phosphorylates the p47phox subunit of Nox, which induces its translocation to the cell membrane and assembly of the functional Nox complex (133). Similarly, hyperglycemia-induced angiotensin II type 1 receptor (AT1) activation increases expression of p47phox and enhances ROS production by Nox (136). AT1 is expressed by several cell types in the wound, including myofibroblasts and keratinocytes (169). In addition, expression of Rac2, an activator of Nox, is elevated in the Zucker rat model, but the mechanism of its upregulation has not been determined (163).
Expression and activity of H2O2-producing enzymes xanthine oxidase (XO) and p66Shc are significantly increased in diabetic mouse wounds, and healing is improved when either protein is knocked down (58, 180). XO and p66Shc are also elevated in fibroblasts cultured in high glucose, but the mechanisms mediating their increased expression remain unknown (58, 180). Deeper understanding of the molecular mechanisms underlying increased activity of ROS-generating enzymes in diabetic wounds is needed.
Decreased ROS-scavenging mechanisms
Increased production of ROS in diabetes is coupled with a reduction in antioxidant defenses, which intensifies the redox imbalance (67, 164, 179). Levels of glutathione, a free radical scavenger, are significantly reduced in wound tissue from diabetic patients and mouse models (13, 121). Nitric oxide (NO), which can neutralize O2−, is also reduced in diabetic wounds, but its role in the redox balance of diabetic wounds remains unclear (109, 112).
The expression of antioxidant enzymes is also reduced in diabetes. CAT levels are low in diabetic mice at baseline, and lymphocytes isolated from diabetes patients exhibit decreased CAT activity (13, 141). Analysis of blood collected from diabetes patients showed reduced SOD, CAT, and glutathione peroxidase activity, and an overall decrease in antioxidant status (172). There is conflicting evidence regarding MnSOD expression and activity in diabetes; studies in mice have demonstrated decreased expression and activity in diabetes, whereas analysis of human samples has indicated increased MnSOD activity in diabetic wounds (13, 113, 172). Further characterization of chronic wounds in diabetes must be performed to fully understand the antioxidant activity.
A significant factor that influences antioxidant enzyme levels in diabetes is impaired signaling through the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidant gene expression (23, 164). The expression and nuclear translocation of Nrf2 are decreased in diabetic dermal fibroblasts, which leads to decreased expression of CAT, NADPH dehydrogenase quinone 1 (NOQ1), glutathione reductase, and glutathione s-transferase in response to oxidative stress (23). In fibroblasts cultured in high glucose, Nrf2 is retained in the cytoplasm by its regulator Keap1, and transcription of MnSOD and NOQ1 is reduced (164). The activity of other transcription factors is similarly altered in hyperglycemia, including AP-1 and NF-κB, which also regulate transcription of antioxidant enzymes (118, 145, 196). The role of these transcription factors in the diabetic environment should be further explored.
Redox Modulation of ECM
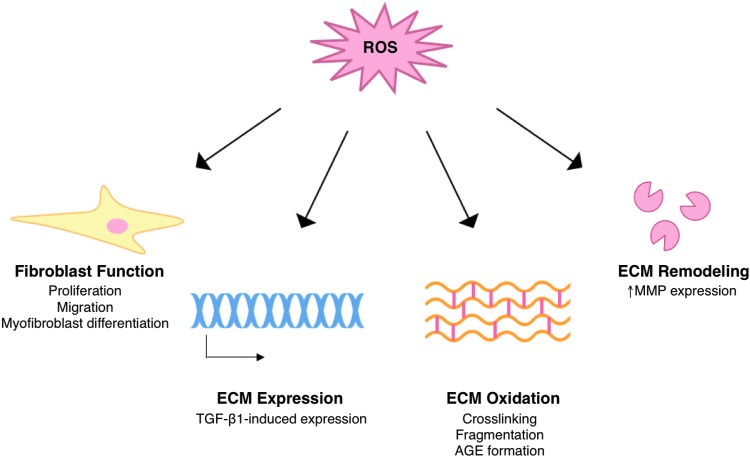
Oxidative stress in diabetic wounds has major implications for the ECM, and thus the progression of wound healing. Excessive ROS can alter ECM structure and composition through modulation of wound fibroblast function, direct oxidative damage, and changes in gene expression and matrix remodeling (Fig. 7).
FIG. 7.
Redox modulation of ECM. Redox signaling regulates ECM structure during normal wound healing, and excess ROS can cause pathological changes in ECM structure and function. TGF-β1, transforming growth factor-β1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Fibroblast function
Redox signaling is an essential mediator of fibroblast functions critical to wound healing, including proliferation, migration, ECM production, and contraction. Treatment with low levels of ROS (i.e., 100 μM H2O2) stimulates fibroblast proliferation through activation of JNK and p38 MAPK; a similar effect is observed with partial inhibition of Cu/ZnSOD, indicating that intracellular ROS also influence cell proliferation (89). ROS production is also required for TGF-β1-induced ECM expression and fibroblast migration in response to bFGF stimulation (36, 158, 193). In fact, siRNA knockdown of the Nox2 subunit of NADPH inhibits proliferation, migration, and expression of collagen I, FN, bFGF, and PAI-1 in human dermal fibroblasts (193). In addition, Nox4 expression is required for the differentiation of fibroblasts to myofibroblasts, a transition that facilitates ECM expression and contraction (36, 83, 193).
Conversely, high levels of ROS have negative effects on fibroblast function. Fibroblasts treated with 500 μM H2O2 proliferate at a lower rate than untreated fibroblasts, as do fibroblasts lacking SOD3, which exhibit higher levels of intracellular ROS than control cells (63, 89). Oxidative stress can induce apoptosis in fibroblasts and particularly affects those stimulated to proliferate in wound healing assays (41, 152, 168). Increases in intracellular ROS can also lead to cellular senescence in fibroblasts, which prevents fibrosis in normal healing but may be detrimental in diabetes (85, 90). Furthermore, fibroblast migration in wound healing assays is inhibited by increased mitochondrial production of O2−, and is correspondingly increased with antioxidant treatment (84, 111). High ROS can also interfere with fibroblast contractile function, evidenced by reduced collagen gel contraction in cells treated with curcumin (151). Although these ROS-mediated changes in fibroblast function have been identified, the underlying mechanisms remain poorly understood. Further research could inform the development of cell-based therapeutics to improve wound healing in high-ROS environments.
ECM production
Although low levels of ROS facilitate ECM synthesis, a redox imbalance as is observed in diabetes can interfere with normal ECM production. Treatment with high concentrations of H2O2 (>150 mM) reduces the amount of connective tissue, and particularly collagen levels, in mouse wounds and retards wound closure (105). Similarly, treatment of fibroblasts with H2O2 or the SOD inhibitor diethyldithiocarbamic acid decreases both fibrillar and nonfibrillar collagen synthesis in vitro (162). Moreover, high levels of ROS can interfere with rather than support TGF-β1 signaling by reducing expression of the type II TGF-β receptor and Smad3 transcription factor in dermal fibroblasts (77). ROS exposure also increases expression of cysteine-rich protein 61 (CCN1), a negative regulator of collagen I production, in dermal fibroblasts and human skin (137).
Direct oxidative damage
The ECM is particularly susceptible to oxidative damage, even in normal conditions, because of low levels of antioxidant enzymes in the intercellular space (142). ROS-mediated protein damage is a result of oxidation of amino acid side chains, and most amino acids are easily modified by radical ROS, particularly cysteine and methionine (142). Oxidation of these amino acids can result in the formation of disulfide bridges and protein adducts, which interfere with protein structure and function (142). ROS can also significantly damage proteoglycans present in the ECM (86).
Oxidation of collagen disrupts its triple helical structure and induces inappropriate inter- and intramolecular crosslinking (54, 142). Such crosslinking can cause the formation of collagen aggregates and increase resistance to degradation by MMPs (69, 142). Oxidation can also cause protein cleavage at proline residues, leading to fragmentation of collagen, FN, and glycosaminoglycans (43, 44, 51, 138). Most studies of ECM oxidation focus solely on individual components, so the effects on overall ECM structure are not well understood.
ROS also mediate AGE accumulation in the ECM (20, 86, 127, 142). Elevated ROS in diabetes favor the formation of AGE by inhibiting GAPDH activity, and causing the accumulation of glyceradehyde-3-phosphate (G3P), a glycolysis intermediate upstream of GAPDH. G3P can be nonenzymatically converted to methylglyoxal, a highly reactive and abundant intracellular AGE precursor. ROS also contribute to the formation of AGE through both glycation and oxidation reactions; these glycoxidation products include pentosidine and carboxymethyllysine, which are among the best studied AGE. These species can further glycate collagen, elastin, and FN in the ECM (69). Like direct oxidative damage to the ECM, glycation alters mechanical proteins and cellular interactions, as already discussed (see ECM glycation in diabetes section).
MMP expression
Redox signaling also regulates the expression of MMPs, and thus influences ECM remodeling. ROS generation is required for the expression of MMP-1, MMP-2, and MMP-3 in human dermal fibroblasts exposed to UV light, but does not influence the expression of TIMPs (32, 76). Direct treatment with H2O2 also induces expression of MMP-1 in human fibroblasts in vitro; a similar effect is achieved by inhibition of CAT or glutathione peroxidase, enzymes that detoxify H2O2 (31). Notably, no change in MMP-1 was observed when Cu/ZnSOD was inhibited, indicating that H2O2 exerts a specific effect on the signaling pathway (31). Similarly, H2O2 treatment increases MMP-8 expression in granulation tissue in diabetic wounds, although the mechanism of this effect is unknown (105).
Redox-Based Wound Therapy
Treatment of diabetic wounds is largely limited to standard wound care practices, including surgical debridement, antibiotic treatment, moisture dressing, and pressure off-loading, as well as close management of blood glucose levels (171). Recent advances have focused on specific defects in the diabetic wound environment, including topical application of growth factors, introduction of bone marrow-derived endothelial and epithelial cells, and collagen-based tissue-engineered grafts (171). Notably, research has also focused on modulating the redox environment of the diabetic wound; such approaches will be reviewed hereunder.
The efficacy of altering ROS levels to improve healing in diabetes has been well established in a variety of preclinical studies. Many of these target ROS-generating mechanisms. For example, decreasing the activity of XO by topical application of an siRNA targeting its precursor, xanthine dehydrogenase (XDH), significantly improves healing in db/db diabetic mice (180). Wounds treated with siXDH exhibited a dramatic reduction in ROS levels and healed 7 days sooner than those treated with scramble siRNA control (180). Similarly, genetic deletion of the H2O2-generating enzyme p66Shc in diabetic mice decreased concentration of nitrotyrosine (a marker of oxidative stress) and improved healing rate, with increased granulation tissue thickness and collagen deposition as well as reduced apoptosis in the wound bed (58). Notably, topical treatment with galectin-1, which increases ROS generation through Nox, improved healing in a diabetic mouse model (101). However, diabetic wounds were not the focus of the study, and, therefore, were not extensively characterized.
Increasing antioxidant capacity has also proven to be an effective strategy; in vivo transfer of MnSOD improved healing rate by nearly 15% in streptozotocin (STZ)-induced diabetic mice (109). These mice exhibited increased MnSOD activity and decreased levels of O2− in addition to the rapid reduction of wound area (109). This study also demonstrated that increased NO availability further improved healing, but this is difficult to interpret in the context of redox signaling because NO has both oxidant and antioxidant properties [reviewed in Refs. (108, 184, 186)]. Analogously, restoration of signaling through Nrf2 accelerated healing in db/db diabetic mice (164). Nrf2 signaling was improved through topical application of siRNA for Keap1, the regulatory protein that sequesters Nrf2 in the cytoplasm. This treatment improved expression of Nrf2 target antioxidant genes, including NQO1, HO-1, glutathione reductase, and glutathione s-transferase, in the wound and improved healing time by 9 days (164). Even supplemental growth factor treatment may influence healing through ROS; topical PDGF was recently shown to increase levels of small-molecule antioxidants in the diabetic wounds, although the mechanisms must be studied more in depth (70).
Nonspecific methods of reducing ROS have also been explored recently. Topical treatment with vitamin C, a dietary antioxidant, improved wound closure at days 7 and 14 postwounding in STZ diabetic rats (96). The wounds exhibited increased collagen deposition, based on Masson's trichrome staining and hydroxyproline assays, as well as reduced apoptosis (96). Topical application of 0.3% bilirubin ointment, which scavenges ROS at low concentrations, has also been shown to improve closure rate and collagen deposition in diabetic wounds (140). Bilirubin-treated wounds also had lower MMP-9 expression and increased TGF-β1 expression relative to controls (140). Similar effects were observed with oral administration of antioxidant; 12 weeks of treatment with the mitochondria-targeted antioxidant SkQ1 improved granulation tissue deposition—the collagen was more mature and organized and blood vessel density significantly increased (48). SkQ1-treated mice also had a greater number of α-smooth muscle actin-positive fibroblasts (48). Comparable improvements in healing were observed with SkQ1 treatment in aged mice (47). Systemic treatment with antioxidant (N-acetyl cysteine) also improved healing in an incisional wound model (5).
Antioxidant-based therapy is just beginning to be tested in the clinic. For example, oral administration of the polyphenol antioxidant resveratrol (RSV) was used in diabetes patients with newly diagnosed ulcers in addition to standard wound management techniques (17, 187). Patients treated with RSV showed significant improvement in ulcer size relative to the control group after 60 days, and there is evidence that RSV may decrease MMP expression and increase fibroblast proliferation in vitro (16, 17). However, the study was small (only 24 patients) and ECM-related parameters were not measured, so few conclusions about the efficacy of the treatment or its mechanism of action can be drawn. When combined with the successful preclinical models already described, this promising clinical data demonstrate the value of redox-based therapeutics for wound healing in diabetes. There must be further development of current antioxidant treatment strategies and evaluation of new targets to address imbalances in redox signaling in diabetes.
Conclusions
It has been recently demonstrated that ROS are critical to wound healing, and redox imbalance significantly influences ECM production and remodeling. Oxidative stress is also a critical cause of diabetic complications, including impaired wound healing. Comparison of the literature on these topics reveals several overlapping pathological mechanisms, including fibroblast dysfunction, reduced collagen deposition, oxidative damage, and dysregulated remodeling by MMPs. Given this intersection, attention must be paid to the role of ROS in diabetic wound healing. Further study of the sources and consequences of oxidative stress in the diabetic wound, with particular focus on the ECM, may allow for the development of ROS-based therapies for chronic diabetic ulcers. Based on the success of preclinical studies on antioxidant treatment, this may represent a novel and effective strategy to improve healing and prevent limb loss in diabetes patients.
Abbreviations Used
- AGE
advanced glycation end products
- AP-1
activator protein 1
- bFGF
basic fibroblast growth factor
- CAT
catalase
- CCN1
cysteine-rich protein 61
- ECM
extracellular matrix
- FN
fibronectin
- G3P
glyceradehyde-3-phosphate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
glutathione
- IL-1β
interleukin-1
- IL-6
interleukin-6
- JNK
c-Jun n-terminal kinase
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- NOQ1
NADPH dehydrogenase quinone 1
- Nox
NADPH oxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- PDGF
platelet-derived growth factor
- PKC
protein kinase C
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- RSV
resveratrol
- SOD
superoxide dismutase
- STZ
streptozotocin
- TGF-β1
transforming growth factor-β1
- TIMP
tissue inhibitor of metalloproteinase
- TNF
tumor necrosis factor
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- VEGF
vascular endothelial growth factor
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
Acknowledgments
This work was supported by NIH grants HL107205, GM 072194, and the Gruber Science Fellowship (to B.K.). We thank Dr. Amelia Luciano and Nicole Calabro for their careful review of the article.
References
- 1.National Diabetes Statistics, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control [Google Scholar]
- 2.Global report on diabetes. Geneva, Switzerland: World Health Organization; 2014 [Google Scholar]
- 3.Acosta JB, del Barco DG, Vera DC, Savigne W, Lopez-Saura P, Guillen Nieto G, and Schultz GS. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J 5: 530–539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agren MS. and Werthen M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Low Extrem Wounds 6: 82–97, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Aktunc E, Ozacmak VH, Ozacmak HS, Barut F, Buyukates M, Kandemir O, and Demircan N. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol 35: 902–909, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Al-Mulla F, Leibovich SJ, Francis IM, and Bitar MS. Impaired TGF-beta signaling and a defect in resolution of inflammation contribute to delayed wound healing in a female rat model of type 2 diabetes. Mol Biosyst 7: 3006–3020, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Alexandrova AY, Kopnin PB, Vasiliev JM, and Kopnin BP. ROS up-regulation mediates Ras-induced changes of cell morphology and motility. Exp Cell Res 312: 2066–2073, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Alexiadou K. and Doupis J. Management of diabetic foot ulcers. Diabetes Ther 3: 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida ME, Monteiro KS, Kato EE, Sampaio SC, Braga TT, Camara NO, Lamers ML, and Santos MF. Hyperglycemia reduces integrin subunits alpha v and alpha 5 on the surface of dermal fibroblasts contributing to deficient migration. Mol Cell Biochem 421: 19–28, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Ambrosio G, Golino P, Pascucci I, Rosolowsky M, Campbell WB, DeClerck F, Tritto I, and Chiariello M. Modulation of platelet function by reactive oxygen metabolites. Am J Physiol 267: H308–H318, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Andreassen TT. and Oxlund H. The influence of experimental diabetes and insulin treatments on the biochemical properties of rat skin incisional wounds. Acta Chir Scand 153: 405–409, 1987 [PubMed] [Google Scholar]
- 12.Arden GB. and Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev 7: 291–304, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Arya AK, Pokharia D, and Tripathi K. Relationship between oxidative stress and apoptotic markers in lymphocytes of diabetic patients with chronic non healing wound. Diabetes Res Clin Pract 94: 377–384, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Avery NC. and Bailey AJ. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol (Paris) 54: 387–395, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Baltzis D, Eleftheriadou I, and Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 31: 817–836, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Bashmakov YK, Assaad-Khalil S, and Petyaev IM. Resveratrol may be beneficial in treatment of diabetic foot syndrome. Med Hypotheses 77: 364–367, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Bashmakov YK, Assaad-Khalil SH, Abou Seif M, Udumyan R, Megallaa M, Rohoma KH, Zeitoun M, and Petyaev IM. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol 2014: 816307, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, and De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol 25: 1401–1407, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Baum CL. and Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg 31: 674–686; discussion 686, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 40: 405–412, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Berdal M. and Jenssen T. Effects of AGE inhibition with aminoguanidine in a diabetic db/db mouse wound model. J Diabetes Mellitus 4: 107–114, 2014 [Google Scholar]
- 22.Bermudez DM, Herdrich BJ, Xu J, Lind R, Beason DP, Mitchell ME, Soslowsky LJ, and Liechty KW. Impaired biomechanical properties of diabetic skin implications in pathogenesis of diabetic wound complications. Am J Pathol 178: 2215–2223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitar MS. and Al-Mulla F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am J Physiol Endocrinol Metab 301: E1119–E1129, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Bitar MS. and Labbad ZN. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 61: 113–119, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Black E, Vibe-Petersen J, Jorgensen LN, Madsen SM, Agren MS, Holstein PE, Perrild H, and Gottrup F. Decrease of collagen deposition in wound repair in type 1 diabetes independent of glycemic control. Arch Surg 138: 34–40, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Blakytny R. and Jude EB. Altered molecular mechanisms of diabetic foot ulcers. Int J Low Extrem Wounds 8: 95–104, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Boulton AJ, Kirsner RS, and Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 351: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, and Apelqvist J. The global burden of diabetic foot disease. Lancet 366: 1719–1724, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Brandner JM, Zacheja S, Houdek P, Moll I, and Lobmann R. Expression of matrix metalloproteinases, cytokines, and connexins in diabetic and nondiabetic human keratinocytes before and after transplantation into an ex vivo wound-healing model. Diabetes Care 31: 114–120, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Brem H. and Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117: 1219–1222, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenneisen P, Briviba K, Wlaschek M, Wenk J, and Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic Biol Med 22: 515–524, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Brenneisen P, Wenk J, Klotz LO, Wlaschek M, Briviba K, Krieg T, Sies H, and Scharffetter-Kochanek K. Central role of ferrous/ferric iron in the ultraviolet B irradiation-mediated signaling pathway leading to increased interstitial collagenase (matrix-degrading metalloprotease (MMP)-1) and stromelysin-1 (MMP-3) mRNA levels in cultured human dermal fibroblasts. J Biol Chem 273: 5279–5287, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Caskey RC, Zgheib C, Morris M, Allukian M, Dorsett-Martin W, Xu J, Wu W, and Liechty KW. Dysregulation of collagen production in diabetes following recurrent skin injury: contribution to the development of a chronic wound. Wound Repair Regen 22: 515–520, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Cavalla F, Osorio C, Paredes R, Valenzuela MA, Garcia-Sesnich J, Sorsa T, Tervahartiala T, and Hernandez M. Matrix metalloproteinases regulate extracellular levels of SDF-1/CXCL12, IL-6 and VEGF in hydrogen peroxide-stimulated human periodontal ligament fibroblasts. Cytokine 73: 114–121, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Chan EC, Peshavariya HM, Liu GS, Jiang F, Lim SY, and Dusting GJ. Nox4 modulates collagen production stimulated by transforming growth factor beta1 in vivo and in vitro. Biochem Biophys Res Commun 430: 918–925, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Chao CY. and Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev 25: 604–614, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Chen SC, Guh JY, Hwang CC, Chiou SJ, Lin TD, Ko YM, Huang JS, Yang YL, and Chuang LY. Advanced glycation end-products activate extracellular signal-regulated kinase via the oxidative stress-EGF receptor pathway in renal fibroblasts. J Cell Biochem 109: 38–48, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Chen XF, Lin WD, Lu SL, Xie T, Ge K, Shi YQ, Zou JJ, Liu ZM, and Liao WQ. Mechanistic study of endogenous skin lesions in diabetic rats. Exp Dermatol 19: 1088–1095, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Cho M, Hunt TK, and Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol 280: H2357–H2363, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Chowdhury AR, Ghosh I, and Datta K. Excessive reactive oxygen species induces apoptosis in fibroblasts: role of mitochondrially accumulated hyaluronic acid binding protein 1 (HABP1/p32/gC1qR). Exp Cell Res 314: 651–667, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, and Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20: 742–752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David-Raoudi M, Tranchepain F, Deschrevel B, Vincent JC, Bogdanowicz P, Boumediene K, and Pujol JP. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen 16: 274–287, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta 1703: 93–109, 2005 [DOI] [PubMed] [Google Scholar]
- 45.DeGroot J, Verzijl N, Budde M, Bijlsma JWJ, Lafeber FPJG, Tekoppele JM, and TeKoppele JM. Accumulation of advanced glycation end products decreases collagen turnover by bovine chondrocytes. Exp Cell Res 266: 303–310, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Del Principe D, Menichelli A, De Matteis W, Di Giulio S, Giordani M, Savini I, and Agro AF. Hydrogen peroxide is an intermediate in the platelet activation cascade triggered by collagen, but not by thrombin. Thromb Res 62: 365–375, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Demyanenko IA, Popova EN, Zakharova VV, Ilyinskaya OP, Vasilieva TV, Romashchenko VP, Fedorov AV, Manskikh VN, Skulachev MV, Zinovkin RA, Pletjushkina OY, Skulachev VP, and Chernyak BV. Mitochondria-targeted antioxidant SkQ1 improves impaired dermal wound healing in old mice. Aging 7: 475–485, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demyanenko IA, Zakharova VV, Ilyinskaya OP, Vasilieva TV, Fedorov AV, Manskikh VN, Zinovkin RA, Pletjushkina OY, Chernyak BV, Skulachev VP, and Popova EN. Mitochondria-targeted antioxidant SkQ1 improves dermal wound healing in genetically diabetic mice. Oxid Med Cell Longev 2017: 10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desta T, Li J, Chino T, and Graves DT. Altered fibroblast proliferation and apoptosis in diabetic gingival wounds. J Dent Res 89: 609–614, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, Lyubovitsky J, Lonardi S, Nothnagel EA, Schiller N, and Martins-Green M. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014: 562625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dijkgraaf LC, Zardeneta G, Cordewener FW, Liem RSB, Schmitz JP, de Bont LGM, and Milam SB. Crosslinking of fibrinogen and fibronectin by free radicals: a possible initial step in adhesion formation in osteoarthritis of the temporomandibular joint. J Oral Maxillofac Surg 61: 101–111, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, and Veves A. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 61: 2937–2947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, and Georgopoulos NT. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J 14: 89–96, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eble JA. and de Rezende FF. Redox-relevant aspects of the extracellular matrix and its cellular contacts via integrins. Antioxid Redox Signal 20: 1977–1993, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El Gazaerly H, Elbardisey DM, Eltokhy HM, and Teaama D. Effect of transforming growth factor Beta 1 on wound healing in induced diabetic rats. Int J Health Sci (Qassim) 7: 160–172, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eligini S, Arenaz I, Barbieri SS, Faleri ML, Crisci M, Tremoli E, and Colli S. Cyclooxygenase-2 mediates hydrogen peroxide-induced wound repair in human endothelial cells. Free Radic Biol Med 46: 1428–1436, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Erciyas F, Taneli F, Arslan B, and Uslu Y. Glycemic control, oxidative stress, and lipid profile in children with type 1 diabetes mellitus. Arch Med Res 35: 134–140, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Fadini GP, Albiero M, Menegazzo L, Boscaro E, Pagnin E, Iori E, Cosma C, Lapolla A, Pengo V, Stendardo M, Agostini C, Pelicci PG, Giorgio M, and Avogaro A. The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing. Diabetes 59: 2306–2314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Feng B, Ruiz MA, and Chakrabarti S. Oxidative-stress-induced epigenetic changes in chronic diabetic complications. Can J Physiol Pharmacol 91: 213–220, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Fu X, Yang Y, Sun T, Wang Y, and Sheng Z. Comparative study of fibronectin gene expression in tissues from hypertrophic scars and diabetic foot ulcers. Chin Med Sci J 17: 90–94, 2002 [PubMed] [Google Scholar]
- 62.Fu XJ, Peng YB, Hu YP, Shi YZ, Yao M, and Zhang X. NADPH oxidase 1 and its derived reactive oxygen species mediated tissue injury and repair. Oxid Med Cell Longev 2014: 282854, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujiwara T, Duscher D, Rustad KC, Kosaraju R, Rodrigues M, Whittam AJ, Januszyk M, Maan ZN, and Gurtner GC. Extracellular superoxide dismutase deficiency impairs wound healing in advanced age by reducing neovascularization and fibroblast function. Exp Dermatol 25: 206–211, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gailit J. and Clark RA. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol 6: 717–725, 1994 [DOI] [PubMed] [Google Scholar]
- 65.Galkowska H, Wojewodzka U, and Olszewski WL. Low recruitment of immune cells with increased expression of endothelial adhesion molecules in margins of the chronic diabetic foot ulcers. Wound Repair Regen 13: 248–254, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Gao M, Nguyen TT, Suckow MA, Wolter WR, Gooyit M, Mobashery S, and Chang M. Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc Natl Acad Sci U S A 112: 15226–15231, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gary Sibbald R and Woo KY. The biology of chronic foot ulcers in persons with diabetes. Diabetes Metab Res Rev 24 Suppl 1: S25–S30, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Giacco F. and Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gkogkolou P. and Bohm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol 4: 259–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goksen S, Balabanli B, and Cevher SC. Application of platelet derived growth factor-BB and diabetic wound healing: the relationship with oxidative events. Free Radic Res 51: 498–505, 2017 [DOI] [PubMed] [Google Scholar]
- 71.Guo S. and Dipietro LA. Factors affecting wound healing. J Dent Res 89: 219–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gurtner GC, Werner S, Barrandon Y, and Longaker MT. Wound repair and regeneration. Nature 453: 314–321, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Hamed S, Ullmann Y, Egozi D, Daod E, Hellou E, Ashkar M, Gilhar A, and Teot L. Fibronectin potentiates topical erythropoietin-induced wound repair in diabetic mice. J Invest Dermatol 131: 1365–1374, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Hamed S, Ullmann Y, Masoud M, Hellou E, Khamaysi Z, and Teot L. Topical erythropoietin promotes wound repair in diabetic rats. J Invest Dermatol 130: 287–294, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Hansen SL, Young DM, and Boudreau NJ. HoxD3 expression and collagen synthesis in diabetic fibroblasts. Wound Repair Regen 11: 474–480, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Hantke B, Lahmann C, Venzke K, Fischer T, Kocourek A, Windsor LJ, Bergemann J, Stab F, and Tschesche H. Influence of flavonoids and vitamins on the MMP- and TIMP-expression of human dermal fibroblasts after UVA irradiation. Photochem Photobiol Sci 1: 826–833, 2002 [DOI] [PubMed] [Google Scholar]
- 77.He T, Quan T, Shao Y, Voorhees JJ, and Fisher GJ. Oxidative exposure impairs TGF-beta pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age (Dordr) 36: 9623, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hehenberger K, Heilborn JD, Brismar K, and Hansson A. Inhibited proliferation of fibroblasts derived from chronic diabetic wounds and normal dermal fibroblasts treated with high glucose is associated with increased formation of l-lactate. Wound Repair Regen 6: 135–141, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Hehenberger K, Kratz G, Hansson A, and Brismar K. Fibroblasts derived from human chronic diabetic wounds have a decreased proliferation rate, which is recovered by the addition of heparin. J Dermatol Sci 16: 144–151, 1998 [DOI] [PubMed] [Google Scholar]
- 80.Hennessey PJ, Ford EG, Black CT, and Andrassy RJ. Wound collagenase activity correlates directly with collagen glycosylation in diabetic rats. J Pediatr Surg 25: 75–78, 1990 [DOI] [PubMed] [Google Scholar]
- 81.Hu SC. and Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci 84: 121–127, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, and Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GRS, and Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem 288: 770–777, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janda J, Nfonsam V, Calienes F, Sligh JE, and Jandova J. Modulation of ROS levels in fibroblasts by altering mitochondria regulates the process of wound healing. Arch Dermatol Res 308: 239–248, 2016 [DOI] [PubMed] [Google Scholar]
- 85.Jun JI. and Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kennett EC, Chuang CY, Degendorfer G, Whitelock JM, and Davies MJ. Mechanisms and consequences of oxidative damage to extracellular matrix. Biochem Soc Trans 39: 1279–1287, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Kern PA, Ranganathan S, Li C, Wood L, and Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280: E745–E751, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, and Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5: e9539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim BY, Han MJ, and Chung AS. Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic Biol Med 30: 686–698, 2001 [DOI] [PubMed] [Google Scholar]
- 90.Kim YJ, Cha HJ, Nam KH, Yoon Y, Lee H, and An S. Centella asiatica extracts modulate hydrogen peroxide-induced senescence in human dermal fibroblasts. Exp Dermatol 20: 998–1003, 2011 [DOI] [PubMed] [Google Scholar]
- 91.King AJF. The use of animal models in diabetes research. Br J Pharmacol 166: 877–894, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knas M, Niczyporuk M, Zalewska A, and Car H. The unwounded skin remodeling in animal models of diabetes types 1 and 2. Physiol Res 62: 519–526, 2013 [DOI] [PubMed] [Google Scholar]
- 93.Knas M, Wolosik K, Zalewska A, Mikucka-Niczyporuk A, Kasacka I, and Niczyporuk M. The skin remodeling in type 1 diabetes and insulin resistance animal models. Physiol Res 64: 875–881, 2015 [DOI] [PubMed] [Google Scholar]
- 94.Lamers ML, Almeida ME, Vicente-Manzanares M, Horwitz AF, and Santos MF. High glucose-mediated oxidative stress impairs cell migration. PLoS One 6: e22865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lateef H, Stevens MJ, and Varani J. All-trans-retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture. Am J Pathol 165: 167–174, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee YH, Chang JJ, Chien CT, Yang MC, and Chien HF. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Exp Diabetes Res 2012: 504693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lerman OZ, Galiano RD, Armour M, Levine JP, and Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 162: 303–312, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levigne D, Modarressi A, Krause KH, and Pittet-Cuenod B. NADPH oxidase 4 deficiency leads to impaired wound repair and reduced dityrosine-crosslinking, but does not affect myofibroblast formation. Free Radic Biol Med 96: 374–384, 2016 [DOI] [PubMed] [Google Scholar]
- 99.Li Z, Guo S, Yao F, Zhang Y, and Li T. Increased ratio of serum matrix metalloproteinase-9 against TIMP-1 predicts poor wound healing in diabetic foot ulcers. J Diabetes Complications 27: 380–382, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Liao H, Zakhaleva J, and Chen W. Cells and tissue interactions with glycated collagen and their relevance to delayed diabetic wound healing. Biomaterials 30: 1689–1696, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin YT, Chen JS, Wu MH, Hsieh IS, Liang CH, Hsu CL, Hong TM, and Chen YL. Galectin-1 accelerates wound healing by regulating the neuropilin-1/Smad3/NOX4 pathway and ROS production in myofibroblasts. J Invest Dermatol 135: 258–268, 2015 [DOI] [PubMed] [Google Scholar]
- 102.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, and Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 45: 1011–1016, 2002 [DOI] [PubMed] [Google Scholar]
- 103.Lobmann R, Schultz G, and Lehnert H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care 28: 461–471, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Loo AE. and Halliwell B. Effects of hydrogen peroxide in a keratinocyte-fibroblast co-culture model of wound healing. Biochem Biophys Res Commun 423: 253–258, 2012 [DOI] [PubMed] [Google Scholar]
- 105.Loo AE, Wong YT, Ho R, Wasser M, Du T, Ng WT, and Halliwell B. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLoS One 7: e49215, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, and Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 111: 850–857, 1998 [DOI] [PubMed] [Google Scholar]
- 107.Loughlin DT. and Artlett CM. Precursor of advanced glycation end products mediates ER-stress-induced caspase-3 activation of human dermal fibroblasts through NAD(P)H oxidase 4. PLoS One 5: e11093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo JD. and Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin 26: 259–264, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Luo JD, Wang YY, Fu WL, Wu J, and Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 110: 2484–2493, 2004 [DOI] [PubMed] [Google Scholar]
- 110.Maione AG, Smith A, Kashpur O, Yanez V, Knight E, Mooney DJ, Veves A, Tomic-Canic M, and Garlick JA. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen 24: 630–643, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mao G, Goswami M, Kalen AL, Goswami PC, and Sarsour EH. N-acetyl-L-cysteine increases MnSOD activity and enhances the recruitment of quiescent human fibroblasts to the proliferation cycle during wound healing. Mol Biol Rep 43: 31–39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maritim AC, Sanders RA, Watkins JB, 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17: 24–38, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Marrotte EJ, Chen DD, Hakim JS, and Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest 120: 4207–4219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, and Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62: 1733–1743, 2010 [DOI] [PubMed] [Google Scholar]
- 115.McCarthy AD, Etcheverry SB, Bruzzone L, Lettieri G, Barrio DA, and Cortizo AM. Non-enzymatic glycosylation of a type I collagen matrix: effects on osteoblastic development and oxidative stress. BMC Cell Biol 2: 16, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McCarty SM, Cochrane CA, Clegg PD, and Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen 20: 125–136, 2012 [DOI] [PubMed] [Google Scholar]
- 117.Minossi JG, de Oliveira Lima F, Caramori CA, Hasimoto CN, Ortolan EV, Rodrigues PA, and Spadella CT. Alloxan diabetes alters the tensile strength, morphological and morphometric parameters of abdominal wall healing in rats. Acta Cir Bras 29: 118–124, 2014 [DOI] [PubMed] [Google Scholar]
- 118.Morgan MJ. and Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21: 103–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moura LI, Dias AM, Carvalho E, and de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater 9: 7093–7114, 2013 [DOI] [PubMed] [Google Scholar]
- 120.Moura LI, Dias AM, Suesca E, Casadiegos S, Leal EC, Fontanilla MR, Carvalho L, de Sousa HC, and Carvalho E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim Biophys Acta 1842: 32–43, 2014 [DOI] [PubMed] [Google Scholar]
- 121.Mudge BP, Harris C, Gilmont RR, Adamson BS, and Rees RS. Role of glutathione redox dysfunction in diabetic wounds. Wound Repair Regen 10: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- 122.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, and Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 123.Niu Y, Cao X, Song F, Xie T, Ji X, Miao M, Dong J, Tian M, Lin Y, and Lu S. Reduced dermis thickness and AGE accumulation in diabetic abdominal skin. Int J Low Extrem Wounds 11: 224–230, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Niu Y, Xie T, Ge K, Lin Y, and Lu S. Effects of extracellular matrix glycosylation on proliferation and apoptosis of human dermal fibroblasts via the receptor for advanced glycosylated end products. Am J Dermatopathol 30: 344–351, 2008 [DOI] [PubMed] [Google Scholar]
- 125.Nomura S, Shouzu A, Omoto S, Nishikawa M, and Fukuhara S. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol 121: 437–443, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nouvong A, Ambrus AM, Zhang ER, Hultman L, and Coller HA. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol Genomics 48: 889–896, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nowotny K, Jung T, Hohn A, Weber D, and Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5: 194–222, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Loughlin A, McIntosh C, Dinneen SF, and O'Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds 9: 90–102, 2010 [DOI] [PubMed] [Google Scholar]
- 129.Obrosova IG, Pacher P, Szabo C, Zsengeller Z, Hirooka H, Stevens MJ, and Yorek MA. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes 54: 234–242, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ojha N, Roy S, He G, Biswas S, Velayutham M, Khanna S, Kuppusamy P, Zweier JL, and Sen CK. Assessment of wound-site redox environment and the significance of Rac2 in cutaneous healing. Free Radic Biol Med 44: 682–691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okano Y, Masaki H, and Sakurai H. Pentosidine in advanced glycation end-products (AGEs) during UVA irradiation generates active oxygen species and impairs human dermal fibroblasts. J Dermatol Sci 27 Suppl 1: S11–S18, 2001 [DOI] [PubMed] [Google Scholar]
- 132.Panday A, Sahoo MK, Osorio D, and Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol 12: 5–23, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Panigrahy SK, Bhatt R, and Kumar A. Reactive oxygen species: sources, consequences and targeted therapy in type 2 diabetes. J Drug Target 25: 93–101, 2017 [DOI] [PubMed] [Google Scholar]
- 134.Peppa M, Stavroulakis P, and Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen 17: 461–472, 2009 [DOI] [PubMed] [Google Scholar]
- 135.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, and Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 91: 484–490, 1998 [PubMed] [Google Scholar]
- 136.Privratsky JR, Wold LE, Sowers JR, Quinn MT, and Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension 42: 206–212, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Qin Z, Robichaud P, He T, Fisher GJ, Voorhees JJ, and Quan T. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts. PLoS One 9: e115402, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qiu ZY, Kwon AH, and Kamiyama Y. Effects of plasma fibronectin on the healing of full-thickness skin wounds in streptozotocin-induced diabetic rats. J Surg Res 138: 64–70, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J 8: 823–831, 1994 [DOI] [PubMed] [Google Scholar]
- 140.Ram M, Singh V, Kumawat S, Kant V, Tandan SK, and Kumar D. Bilirubin modulated cytokines, growth factors and angiogenesis to improve cutaneous wound healing process in diabetic rats. Int Immunopharmacol 30: 137–149, 2016 [DOI] [PubMed] [Google Scholar]
- 141.Rasik AM. and Shukla A. Antioxidant status in delayed healing type of wounds. Int J Exp Pathol 81: 257–263, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rees MD, Kennett EC, Whitelock JM, and Davies MJ. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med 44: 1973–2001, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Reihsner R. and Menzel EJ. Two-dimensional stress-relaxation behavior of human skin as influenced by non-enzymatic glycation and the inhibitory agent aminoguanidine. J Biomech 31: 985–993, 1998 [DOI] [PubMed] [Google Scholar]
- 144.Ribu L, Birkeland K, Hanestad BR, Moum T, and Rustoen T. A longitudinal study of patients with diabetes and foot ulcers and their health-related quality of life: wound healing and quality-of-life changes. J Diabetes Complications 22: 400–407, 2008 [DOI] [PubMed] [Google Scholar]
- 145.Rochette L, Zeller M, Cottin Y, and Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta 1840: 2709–2729, 2014 [DOI] [PubMed] [Google Scholar]
- 146.Rodgers KE, Ellefson DD, Espinoza T, Hsu YH, diZerega GS, and Mehrian-Shai R. Expression of intracellular filament, collagen, and collagenase genes in diabetic and normal skin after injury. Wound Repair Regen 14: 298–305, 2006 [DOI] [PubMed] [Google Scholar]
- 147.Roy S, Khanna S, Nallu K, Hunt TK, and Sen CK. Dermal wound healing is subject to redox control. Mol Ther 13: 211–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roy S, Khanna S, Rink C, Biswas S, and Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics 34: 162–184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schafer M. and Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 58: 165–171, 2008 [DOI] [PubMed] [Google Scholar]
- 150.Schaffer MR, Tantry U, Efron PA, Ahrendt GM, Thornton FJ, and Barbul A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: a possible pathophysiologic correlation. Surgery 121: 513–519, 1997 [DOI] [PubMed] [Google Scholar]
- 151.Scharstuhl A, Mutsaers HA, Pennings SW, Szarek WA, Russel FG, and Wagener FA. Curcumin-induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase: implications for scar formation. J Cell Mol Med 13: 712–725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schreml S, Landthaler M, Schaferling M, and Babilas P. A new star on the H(2)O(2)rizon of wound healing? Exp Dermatol 20: 229–231, 2011 [DOI] [PubMed] [Google Scholar]
- 153.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, and Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol 163: 257–268, 2010 [DOI] [PubMed] [Google Scholar]
- 154.Sen CK. The general case for redox repair of wound repair. Wound Repair Regen 11: 431–438, 2003 [DOI] [PubMed] [Google Scholar]
- 155.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, and Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem 277: 33284–33290, 2002 [DOI] [PubMed] [Google Scholar]
- 156.Sen CK. and Roy S. Redox signals in wound healing. Biochim Biophys Acta 1780: 1348–1361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shemirani F. and Yazdanparast R. The interplay between hyperglycemia-induced oxidative stress markers and the level of soluble receptor for advanced glycation end products (sRAGE) in K562 cells. Mol Cell Endocrinol 393: 179–186, 2014 [DOI] [PubMed] [Google Scholar]
- 158.Shi HX, Cheng Y, Ye JJ, Cai PT, Zhang JJ, Li R, Yang Y, Wang ZG, Zhang HY, Lin C, Lu XH, Jiang LP, Hu AP, Zhu XB, Zeng QQ, Fu XB, Li XK, and Xiao J. bFGF promotes the migration of human dermal fibroblasts under diabetic conditions through reactive oxygen species production via the PI3K/Akt-Rac1-JNK pathways. Int J Biol Sci 11: 845–859, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi L, Chen H, Yu X, and Wu X. Advanced glycation end products delay corneal epithelial wound healing through reactive oxygen species generation. Mol Cell Biochem 383: 253–259, 2013 [DOI] [PubMed] [Google Scholar]
- 160.Singer AJ. and Clark RA. Cutaneous wound healing. N Engl J Med 341: 738–746, 1999 [DOI] [PubMed] [Google Scholar]
- 161.Singh DK, Winocour P, and Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7: 176–184, 2011 [DOI] [PubMed] [Google Scholar]
- 162.Siwik DA, Pagano PJ, and Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280: C53–C60, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Slavkovsky R, Kohlerova R, Tkacova V, Jiroutova A, Tahmazoglu B, Velebny V, Rezacova M, Sobotka L, and Kanta J. Zucker diabetic fatty rat: a new model of impaired cutaneous wound repair with type II diabetes mellitus and obesity. Wound Repair Regen 19: 515–525, 2011 [DOI] [PubMed] [Google Scholar]
- 164.Soares MA, Cohen OD, Low YC, Sartor RA, Ellison T, Anil U, Anzai L, Chang JB, Saadeh PB, Rabbani PS, and Ceradini DJ. Restoration of Nrf2 signaling normalizes the regenerative niche. Diabetes 65: 633–646, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stanley CM, Wang Y, Pal S, Klebe RJ, Harkless LB, Xu XP, Chen ZH, and Steffensen B. Fibronectin fragmentation is a feature of periodontal disease sites and diabetic foot and leg wounds and modifies cell behavior. J Periodontol 79: 861–875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Steiling H, Munz B, Werner S, and Brauchle M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp Cell Res 247: 484–494, 1999 [DOI] [PubMed] [Google Scholar]
- 167.Stojadinovic O, Pastar I, Gordon KA, and Tomic-Canic M. Physiology and pathophysiology of wound healing in diabetes. In: The Diabetic Foot: Medical and Surgical Management, edited by Veves A, Giurini JM, and LoGerfo FW. Totowa, NJ: Humana Press, 2012, pp. 127–149 [Google Scholar]
- 168.Takahashi A, Aoshiba K, and Nagai A. Apoptosis of wound fibroblasts induced by oxidative stress. Exp Lung Res 28: 275–284, 2002 [DOI] [PubMed] [Google Scholar]
- 169.Takeda H, Katagata Y, Hozumi Y, and Kondo S. Effects of angiotensin II skin wound healing receptor signaling during. Am J Pathol 165: 1653–1662, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tracy LE, Minasian RA, and Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care (New Rochelle) 5: 119–136, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tsourdi E, Barthel A, Rietzsch H, Reichel A, and Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int 2013: 385641, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vairamon SJ, Babu M, and Viswanathan V. Oxidative stress markers regulating the healing of foot ulcers in patients with type 2 diabetes. Wounds 21: 273–279, 2009 [PubMed] [Google Scholar]
- 173.Vavaev AV, Buryachkovskaya LI, Uchitel IA, Tishchenko EG, and Maksimenko AV. Effect of hydrogen peroxide and catalase derivatives on functional activity of platelets. Bull Exp Biol Med 152: 307–312, 2012 [DOI] [PubMed] [Google Scholar]
- 174.Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev 17: 246–249, 2001 [DOI] [PubMed] [Google Scholar]
- 175.Visse R. and Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003 [DOI] [PubMed] [Google Scholar]
- 176.Wagner S, Hussain MZ, Hunt TK, Bacic B, and Becker HD. Stimulation of fibroblast proliferation by lactate-mediated oxidants. Wound Repair Regen 12: 368–373, 2004 [DOI] [PubMed] [Google Scholar]
- 177.Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, and Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol 119: 91–98, 2002 [DOI] [PubMed] [Google Scholar]
- 178.Wall SJ, Sampson MJ, Levell N, and Murphy G. Elevated matrix metalloproteinase-2 and -3 production from human diabetic dermal fibroblasts. Br J Dermatol 149: 13–16, 2003 [DOI] [PubMed] [Google Scholar]
- 179.Wang X, Sng MK, Foo S, Chong HC, Lee WL, Tang MB, Ng KW, Luo B, Choong C, Wong MT, Tong BM, Chiba S, Loo SC, Zhu P, and Tan NS. Early controlled release of peroxisome proliferator-activated receptor beta/delta agonist GW501516 improves diabetic wound healing through redox modulation of wound microenvironment. J Control Release 197: 138–147, 2015 [DOI] [PubMed] [Google Scholar]
- 180.Weinstein AL, Lalezarzadeh FD, Soares MA, Saadeh PB, and Ceradini DJ. Normalizing dysfunctional purine metabolism accelerates diabetic wound healing. Wound Repair Regen 23: 14–21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wellen KE. and Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, and Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 115: 245–253, 2000 [DOI] [PubMed] [Google Scholar]
- 183.Wicks K, Torbica T, and Mace KA. Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Semin Immunol 26: 341–353, 2014 [DOI] [PubMed] [Google Scholar]
- 184.Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, and Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal 3: 203–213, 2001 [DOI] [PubMed] [Google Scholar]
- 185.Witte MB. and Barbul A. General principles of wound healing. Surg Clin North Am 77: 509–528, 1997 [DOI] [PubMed] [Google Scholar]
- 186.Witte MB. and Barbul A. Role of nitric oxide in wound repair. Am J Surg 183: 406–412, 2002 [DOI] [PubMed] [Google Scholar]
- 187.Wu H, Li GN, Xie J, Li R, Chen QH, Chen JZ, Wei ZH, Kang LN, and Xu B. Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-beta/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc Disord 16: 5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu B, Chiu J, Feng B, Chen S, and Chakrabarti S. PARP activation and the alteration of vasoactive factors and extracellular matrix protein in retina and kidney in diabetes. Diabetes Metab Res Rev 24: 404–412, 2008 [DOI] [PubMed] [Google Scholar]
- 189.Xuan YH, Bin Huang B, Tian HS, Chi LS, Duan YM, Wang X, Zhu ZX, Cai WH, Zhu YT, Wei TM, Ye HB, Cong WT, and Jin LT. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLos One 9: e108182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.This reference has been deleted
- 191.Yavuz D, Tugtepe H, Cetinel S, Uyar S, Kaya H, Haklar G, Civelek S, Deyneli O, San T, Burcak G, and Akalin S. Collagen ultrastructure and TGF-beta1 expression preserved with aminoguanidine during wound healing in diabetic rats. Endocr Res 31: 229–243, 2005 [DOI] [PubMed] [Google Scholar]
- 192.Zeng W, Tahrani A, Shakher J, Varani J, Hughes S, Dubb K, and Stevens MJ. Effects of a synthetic retinoid on skin structure, matrix metalloproteinases, and procollagen in healthy and high-risk subjects with diabetes. J Diabetes Complications 25: 398–404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang GY, Wu LC, Dai T, Chen SY, Wang AY, Lin K, Lin DM, Yang JQ, Cheng B, Zhang L, Gao WY, and Li ZJ. NADPH oxidase-2 is a key regulator of human dermal fibroblasts: a potential therapeutic strategy for the treatment of skin fibrosis. Exp Dermatol 23: 639–644, 2014 [DOI] [PubMed] [Google Scholar]
- 194.Zhang P, Lu J, Jing Y, Tang S, Zhu D, and Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis dagger. Ann Med 49: 106–116, 2017 [DOI] [PubMed] [Google Scholar]
- 195.Zhao R, Liang H, Clarke E, Jackson C, and Xue M. Inflammation in chronic wounds. Int J Mol Sci 17: 2085, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou LZ, Johnson AP, and Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med 31: 1405–1416, 2001 [DOI] [PubMed] [Google Scholar]