Abstract
Cells in multicellular organisms communicate extensively with neighboring cells and distant organs using a variety of secreted proteins and small molecules. Cells also reside in a structural extracellular matrix (ECM), and changes in its composition, mechanical properties, and post-translational modifications provide additional layers of communication. This Forum addresses emerging mechanisms by which redox signaling controls and is controlled by changes in the ECM, focusing on the roles of matricellular proteins. These proteins engage specific cell surface signaling receptors, integrins, and proteoglycans to regulate the biosynthesis and catabolism of redox signaling molecules and the activation of their signal transducers. These signaling pathways, in turn, regulate the composition of ECM and its function. Covalent post-translational modifications of ECM by redox molecules further regulate its structure and function. Recent studies of acute injuries and chronic disease have identified important pathophysiological roles for this cross-talk and new therapeutic opportunities. Antioxid. Redox Signal. 27, 771–773.
Keywords: : matricellular proteins, nitric oxide, reactive oxygen species, hydrogen sulfide, hypoxia, extracellular matrix
Reactive nitrogen species, reactive oxygen species (ROS), carbon monoxide, and hydrogen sulfide metabolites play important autocrine and paracrine roles in cell signaling. A variety of cues from the cellular microenvironment regulate the biosynthesis and catabolism of these mediators by mitochondria, nitric oxide synthases (NOSs), heme oxygenases, oxidases, peroxidases, superoxide dismutases, cystathionine β-synthase, and cystathionine γ-lyase. Activation of biosynthetic and catabolic pathways for these redox signaling molecules is controlled, in part, by signal transduction pathways that are activated by binding of secreted factors to cell surface receptors and by mechanotransduction induced by changes in fluid shear flow or rigidity of the extracellular matrix (ECM) that anchors most cells in multicellular organisms. Recent studies have expanded our understanding of the mechanisms by which the ECM regulates redox signaling and mechanisms by which dysregulation of this signaling contributes to the pathogenesis of major acute and chronic diseases. This Forum reviews some significant recent advances in this field.
Matricellular proteins are a dynamically regulated subset of ECM proteins that have emerged as important regulators of cellular redox signaling during development and in responses to injury and stress. Matricellular proteins differ from structural ECM proteins in that they typically appear only transiently in the ECM (6). Although several matricellular proteins interact with classical ECM protein receptors such as integrins, they also engage specific signaling receptors through which they control intracellular signaling. For example, CD47 is a major signaling receptor for the matricellular protein thrombospondin-1 that regulates NO and H2S signaling (9). In the vasculature, NO produced by endothelial NOS (NOS3) controls the activation of soluble guanylate cyclase in vascular smooth muscle cells and thereby regulates regional blood flow and central blood pressure. Hypoxia and fluid shear stress activate NOS3 to increase blood flow, and adrenergic tone opposes this vasodilator activity of NO to maintain physiological blood pressure. Studies using mice lacking thrombospondin-1 or CD47 demonstrated a physiological role for CD47 signaling to limit both NO biosynthesis and cGMP-mediated signaling pathways controlled by NO in the vasculature (9). Thrombospondin-1 accumulates in the vasculature in response to ischemic injuries and several chronic diseases of aging, thereby limiting NO signaling and tissue perfusion. Two articles in this Forum review physiological and pathophysiological functions of this pathway and the potential to therapeutically suppress CD47 signaling to restore NO signaling in acute ischemic injuries, cardiovascular disease, and aging (5, 9).
Studies of other matricellular proteins are also discussed that demonstrate regulation of inducible NOS (NOS2) as well as NOS3-mediated NO synthesis by matricellular proteins, and the ability of matricellular proteins to control ROS and H2S signaling as well as NO signaling (9). CD47 signaling limits the autocrine induction of H2S biosynthesis and signaling that supports T cell activation, and thrombospondin-1 signaling through CD47 inhibits T cell activation. Consistent with this inhibitory pathway, therapeutic inhibitors of CD47 signaling enhance adaptive antitumor immunity by increasing CD8+ T cell killing of tumor cells.
Integrins are a major class of receptors that anchor cells in the ECM by binding to specific ECM ligands. Integrins also mediate bidirectional signaling across the plasma membrane. Huang et al. review the role of redox signaling as a target of outside-in signaling through integrins that induces ROS production through mitochondrial- and NADPH oxidase-dependent mechanisms (2). The role of the ECM protein fibulin-5 as a negative modulator of ROS production is also discussed, including its potential as a therapeutic target to control aberrant ROS production in tumors.
Proteoglycans are proteins that are covalently modified with polymeric carbohydrate chains known as glycosaminoglycans. Extracellular proteoglycans and the nonproteoglycan glycosaminoglycan hyaluronan serve as structural components of the ECM by interacting with glycosaminoglycan binding sites on other ECM components. A subset of transmembrane signaling receptors on the plasma membrane bear covalent glycosaminoglycan modifications (e.g., syndecans, CD44, and CD47), associate laterally with other proteoglycans in the plasma membrane, or interact with pericellular proteoglycans. Nastase et al. review mechanisms by which these proteoglycans function as coreceptors for growth factors, cytokines, and matricellular proteins that contain glycosaminoglycan binding sites (7). Recently recognized roles of these proteoglycans are discussed in the physiological and pathophysiological regulation of cellular redox signaling. In addition to revealing novel signaling mechanisms, these studies shed light on the pathogenesis of diseases involving dysregulation of cellular redox signaling and identify potential therapeutic targets. For example, calcium signaling induced by amyloid-β binding to cell surface heparan sulfate proteoglycans mediates the hyperproduction of ROS by vascular cells in Alzheimer's disease. Despite considerable progress, the authors emphasize that the mechanisms by which many proteoglycans regulate redox signaling remain poorly defined and require further investigation.
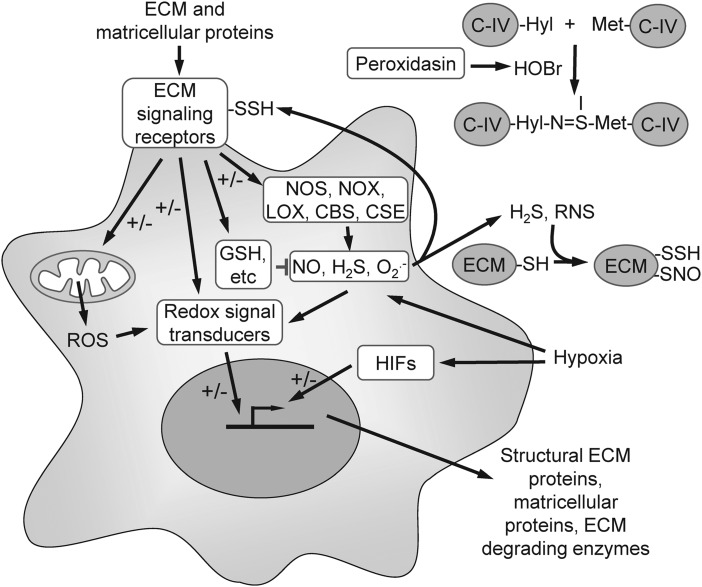
Redox signaling can be mediated by noncovalent binding to a sensor protein such as soluble guanylate cyclase or by covalent modifications of sensor proteins, including thiol oxidation, sulfhydration, and nitrosation. However, the functions of these covalent modifications extend beyond signal transduction. The recent discovery of sulfilimine cross-linking in basement membranes revealed an unexpected structural role of covalent protein modification by HOBr in the ECM (1). Colon et al. review the role of the heme peroxidase peroxidasin in formation of sulfilimine cross-links, which utilizes bromide as a substrate to generate HOBr. The HOBr reacts with Met and hydroxy-Lys residues in type IV collagen to form sulfilimine cross-links (Fig. 1). Type IV collagen is a major structural component of the basement membrane that underlies all epithelial and endothelial cells, and this sulfilimine cross-link is required for its stability. The identification of this novel pathway also explains why bromine is an essential element and why peroxidasin is essential for proper basement membrane formation during development.
FIG. 1.
Bidirectional cross-talk between ECM and redox signaling. A subset of structural proteins in the ECM and dynamically regulated matricellular proteins engage specific cell surface receptors that regulate the biosynthesis and catabolism of redox signaling molecules produced by nitric oxide synthases (NOSs), NADPH oxidases (NOXs), lipoxygenases (LOX), and the H2S biosynthetic enzymes cystathionine β-synthase and cystathionine γ-lyase. The matricellular protein thrombospondin-1 also regulates signal transduction downstream of NO. Redox signal transducers, in turn, regulate matricellular and ECM protein expression, degradation, and post-translational modifications, including the peroxidasin-mediated sulfilimine cross-linking of type IV collagen (C-IV) in basement membranes. Hypoxia regulates ECM protein expression by altering cellular redox chemistry that regulates multiple targets, including the hypoxia-induced transcription factors. ECM, extracellular matrix.
In addition to post-translational modification of ECM proteins, redox signaling is an important regulator of ECM gene expression (Fig. 1). Pathological changes in redox signaling, therefore, can lead to alterations in the composition of ECM that contribute to disease pathogenesis. Kunkemoeller et al. review the impact of this dysregulation on diabetic wound healing (3). Dysregulation of mitochondrial ROS production caused by hyperglycemia leads to overexpression of matrix metalloproteinases that degrade ECM and growth factors required for its maintenance and repair. Advanced glycation end products further enhance the generation of ROS in diabetic tissues. Kunkemoeller et al. also review emerging therapeutic strategies employing antioxidants to correct these defects in ECM homeostasis.
Hypoxia contributes to the pathogenesis of diseases associated with acute and chronic ischemia and to tumor growth and metastasis. Hypoxia alters cellular redox signaling directly and through transcriptional regulation by hypoxia-induced factors (HIFs). Labrousse-Arias et al. focus on the roles of the matricellular proteins thrombospondin-1, osteopontin, periostin, tenascins, CCN family proteins, and fibulin-5 in hypoxia associated with malignant and nonmalignant diseases (4). The regulation of these matricellular proteins by hypoxia is tissue- and cell type-dependent and involves both HIF1α- and HIF2α-dependent transcription regulation and modulation of the concentrations of NO, ROS, and H2S metabolites (Fig. 1).
Recent insights into pathological dysregulation of the cross-talk between ECM and redox signaling are providing a better mechanistic understanding of some genetic disorders involving matrix genes such as Marfan syndrome, where genetic or pharmacological inactivation of Nos2 was recently shown to prevent aortic aneurysms in fibrillin-1 mutant mice (8). Ongoing studies are also exploring therapeutic applications of drugs targeting specific matricellular protein receptors to correct the dysregulation of redox signaling in several diseases. Antibody or antisense blockade of thrombospondin-1 signaling through CD47 protects mice from a variety of ischemic injuries by increasing NO/cGMP signaling and preserves resistance to these injuries in aging mice (9). This strategy could overcome the resistance to NO-donating drugs associated with diseases of aging. Experimental therapeutics targeting CD47 are currently in several human clinical trials for cancer (9), but their potential effects on redox signaling in tumor cells or the tumor microenvironment will need to be assessed to maximize their antitumor efficacy and understand their potential side effects. Articles in this Forum provide guidance for future basic and applied research that could lead to novel therapeutic strategies for correcting the pathological dysregulation of redox signaling based on drugs that target specific ECM proteins or their receptors.
Abbreviations Used
- ECM
extracellular matrix
- NOSs
nitric oxide synthases
- ROS
reactive oxygen species
Acknowledgment
This work was supported by the Intramural Research Program of the NIH/NCI.
References
- 1.Colon S, Page-McCaw P, and Bhave G. Role of hypohalous acids in basement membrane homeostasis. Antioxid Redox Signal 27: 839–854, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H, Du W, and Brekken RA. Extracellular matrix induction of intracellular reactive oxygen species. Antioxid Redox Signal 27: 774–784, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Kunkemoeller B. and Kyriakides TR. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid Redox Signal 27: 823–838, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labrousse-Arias D, Martínez-Ruiz A, and Calzada MJ. Hypoxia and redox signaling on extracellular matrix remodeling: from the mechanisms to the pathological implications. Antioxid Redox Signal 27: 802–822, 2017 [DOI] [PubMed] [Google Scholar]
- 5.LeBlanc AJ. and Kelm NQ. Thrombospondin-1, free radicals, and the coronary microcirculation: the aging conundrum. Antioxid Redox Signal 27: 785–801, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy-Ullrich JE. and Sage EH. Revisiting the matricellular concept. Matrix Biol 37: 1–14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nastase M-V, Janicova A, Wygrecka M, and Schaefer L. Signaling at the crossroads: Matrix-derived proteoglycan and reactive oxygen species signaling. Antioxid Redox Signal 27: 855–873, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Oller J, Mendez-Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, Briones AM, Alberca R, Lozano-Vidal N, Hurle MA, Milewicz D, Evangelista A, Salaices M, Nistal JF, Jimenez-Borreguero LJ, De Backer J, Campanero MR, and Redondo JM. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med 23: 200–212, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Roberts DD, Kaur S, and Isenberg JS. Regulation of cellular redox signaling by matricellular proteins in vascular biology, immunology, and cancer. Antioxid Redox Signal 27: 874–911, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]