Abstract
Objective: A fully closed-loop insulin-only system was developed to provide glucose control in patients with type 1 diabetes without requiring announcement of meals or activity. Our goal was to assess initial safety and efficacy of this system.
Research Design and Methods: The multiple model probabilistic controller (MMPPC) anticipates meals when the patient is awake. The controller used the subject's basal rates and total daily insulin dose for initialization. The system was tested at two sites on 10 patients in a 30-h inpatient study, followed by 15 subjects at three sites in a 54-h supervised hotel study, where the controller was challenged by exercise and unannounced meals. The system was implemented on the UVA DiAs system using a Roche Spirit Combo Insulin Pump and a Dexcom G4 Continuous Glucose Monitor.
Results: The mean overall (24-h basis) and nighttime (11 PM–7 AM) continuous glucose monitoring (CGM) values were 142 and 125 mg/dL during the inpatient study. The hotel study used a different daytime tuning and manual announcement, instead of automatic detection, of sleep and wake periods. This resulted in mean overall (24-h basis) and nighttime CGM values of 152 and 139 mg/dL for the hotel study and there was also a reduction in hypoglycemia events from 1.6 to 0.91 events/patient/day.
Conclusions: The MMPPC system achieved a mean glucose that would be particularly helpful for people with an elevated A1c as a result of frequent missed meal boluses. Current full closed loop has a higher risk for hypoglycemia when compared with algorithms using meal announcement.
Keywords: : Artificial pancreas, Closed-loop systems, Postprandial blood glucose, Continuous glucose monitoring.
Introduction
The development of artificial pancreas systems—closed-loop systems that modulate insulin delivery based upon continuous glucose values—has progressed rapidly in recent years. There have been a number of algorithms developed by academic and commercial groups that can generally be separated into three basic types: (i) proportional-integral-derivative (PID)1 control, (ii) model predictive control (MPC),2 and (iii) fuzzy logic control.3 There has been a healthy debate about the advantages and disadvantages of PID4 and MPC,5 and a recent clinical study has compared the two in a randomized clinical trial.6 Closed-loop systems include insulin-only approaches as well as bihormonal systems that use both insulin and glucagon. Both types seek to reduce the burden of care for people with type 1 diabetes (T1D) and improve average glucose levels while reducing hypoglycemia.
In clinical studies with supervised premeal dosing, also known as announced meals, postprandial hyperglycemia remains a challenge. This becomes increasingly difficult with free-living conditions when missed boluses are frequent.7–9 We developed a fully closed-loop insulin-only system (multiple model probabilistic predictive control or multiple model probabilistic controller [MMPPC]) that does not require meal announcement. The controller anticipates meals when the patient is awake and assumes no current or future meals during sleep. Hence, a major consideration in the design of the proposed MMPPC strategy was to achieve reasonable glucose control with unannounced meals and not to detect meals while the patient is sleeping. Our system initially interpreted the signal from a Zephyr BioHarness 3.0 accelerometer to provide automated sleep/wake information.
Previous work has assessed full closed-loop glucose control using bihormonal control with delivery of both glucagon and insulin and no premeal announcements.10,11 Van Bon et al.10 studied 11 patients over 2 days in their home environment comparing 2 days of open-loop control with 2 days of full closed-loop control. With closed-loop control, there was comparable time in euglycemia compared with open-loop control on day 1 of closed-loop therapy. With adjustments to the algorithm, there was a significantly lower median glucose level on day 2 of closed-loop control at the expense of more time in hypoglycemia, with 0.77 hypo alerts per day and 78% time spent between 70 and 180 mg/dL. This same group developed an integrated bihormonal control system with the pumps and algorithm on the same device and studied 10 patients for 3 days in their home environment using full closed-loop control without meal announcement.11 They had more hyperglycemia following breakfast, but significantly improved glucose control overnight, and overall, there were an average of 0.4 treatments for hypoglycemia each day and 84.7% time was spent between 70 and 180 mg/dL compared with 68.5% of the time in open loop.
The primary objectives of this work were to determine the feasibility, safety, and efficacy of an insulin-only controller in patients with T1D without meal announcements, exercise announcements, or glucagon. We describe the results of an inpatient study and the follow-up hotel study. The inpatient study illustrated the challenges of using a developmental accelerometer for sleep/wake detection and the controller did not utilize the accelerometer data for exercise detection. The hotel study was more cautious and used manual sleep/wake announcement instead.
Research Design and Methods
Closed-loop system
The MMPPC algorithm, part of the class of MPC algorithms, has been previously described11 and tested in preliminary feasibility trials using a laptop-based system.2 There are several novel features of MMPPC when compared with a standard MPC.
First, standard MPC algorithms use a prediction of the mean glucose into the future. The MMPPC algorithm, in addition to predicting the mean glucose for 5 h into the future, also predicts the uncertainty of future glucose measurements. This controller uses the uncertainty predictions to directly control a risk of a hypoglycemic event in the future rather than targeting a set point or zone of glucose values and subsequently indirectly penalizing insulin delivery to account for glucose uncertainty.
Second, standard MPC algorithms may use current meals or exercise to modify glucose predictions, but future meals, sleep, and exercise are not anticipated. Our algorithm uses data from the National Health and Nutrition Examination Survey12 and the American Time use Survey13 to provide population-level assumptions about meal and sleep behavior. This information has enabled us to anticipate future meals and better detect unannounced meals. For example, if a subject has a meal at 10 AM, then it is unlikely that the next meal will occur before 11:30 AM or after 1 PM. It also allows us to adapt to new information. In our example, our initial estimate was that a meal after 1 PM was unlikely. However, if 3 h have passed since 10 AM without a meal being detected, the probability adapts to a 100% chance of the patient eating after 1 PM.
The Zephyr Bioharness accelerometer was used to determine if the patient was asleep, awake, and/or exercising. Assuming that the accelerometer was firmly fixed to the patient's torso, the controller assumed that if the torso was more than 60° from vertical, the patient was asleep. The proprietary Zephyr activity measure was used to detect exercise using a simple threshold. The sleep–wake information was a way to blend control between daytime and nighttime modes. Specifically, if the patient had been awake for roughly 15 h, then the controller would be more cautious because the patient is likely to sleep soon and unlikely to have a large meal. The daytime mode featured meal anticipation as well as the potential for large automated boluses. This mode does not provide a basal rate, but instead relies on superboluses, that is, the algorithm computes a meal-related bolus that assumes 50% of the basal rate over the prediction horizon. The controller reevaluates the data every 5 min, allowing for the potential of a long series of meal boluses as the controller becomes more certain of a meal and its size. The nighttime mode seeks to be more cautious and explicitly infuses only 1/12th of the desired bolus at every sample time.11,14
The Artificial Pancreas algorithm was implemented on the UVA DiAs system15 and used a Roche Spirit Combo Insulin Pump with a Dexcom G4 Continuous Glucose Monitor and a Zephyr BioHarness 3.0 accelerometer. A 20-min gap in sensor readings or a loss of Bluetooth connection to the pump automatically took the system out of closed-loop mode.
Protocol
The protocol was approved by the Food and Drug Administration (IDE G150058) and the Stanford, Barbara Davis Center, Rensselaer Polytechnic Institute, and Mt. Sinai Institutional Review Boards. Patients were between 18 and 48 years of age with diabetes duration of more than 1 year and insulin pump use for more than 3 months. Patients were required to attend a preadmission visit. During this visit, subjects consented to study, completed enrollment procedures, had hemoglobin A1c measured, and were trained as per manufacturer instructions to insert and wear an unblinded Dexcom G4 CGM. The unblinded Dexcom was worn for 1 to 3 days before the inpatient studies and for at least 5 days before the start of the hotel studies.
For the inpatient studies, subjects arrived fasting before 8 AM on the day of admission. Blood samples for YSI or StatStrip readings were obtained every hour. If there were sensor alarms for glucose <70 or >300 mg/dL, a reference glucose reading was obtained. If a reference glucose was <70 mg/dL, reference readings were obtained every 15 min until the reference glucose reading was >70 mg/dL. Closed-loop control was generally initiated by 10 AM before breakfast. Subjects were remotely monitored using the DiAs web-monitoring system16 for the next 32 h.
For the hotel studies, subjects wore an unblinded Dexcom G4 CGM for 5–7 days before their arrival to the hotel to collect a minimum of 72 h of baseline data. Subjects arrived at the hotel in the morning following breakfast at home. They were trained on the system and closed-loop control was initiated before lunch. Subjects were supervised for the next 3 days and 2 nights. Subjects were allowed to travel within 20 min walking distance of the hotel, but were not allowed to drive. Subjects slept in the hotel every night and research staff were present in the hotel and obtained 3 AM glucose readings each night. Subjects made their own food choices, including alcohol for adults. Accompanying research staff were not involved in the patients' diabetes management decisions such as carbohydrate counting for meals, but assisted in technical issues with using the system. Research staff only intervened if sensor glucose values were below 70 mg/dL and there was no response by the subject within 15 min or if there was no response for sensor glucose values >300 mg/dL for 30 min. The hotel study used manual sleep/wake announcements in addition to detecting sleep with Zephyr Bioharness data. Zephyr activity level was collected, but not used by the algorithm in determining insulin doses. When study staff determined that an insulin infusion set or sensor needed to be changed, closed loop was manually suspended until the sensor or pump was again functional. Unblinded CGM data obtained before the hotel admission were used as baseline data in the analysis of open-loop glucose control.
In both settings, patients were strongly encouraged, although not required to exercise once each day. Examples of patient exercise include walks of about 1 h, trampoline/volleyball for 1.5 h, 40 min of treadmill running, or a half-hour of ultimate Frisbee. Exercise was necessarily lighter in the inpatient setting, compared with the hotel setting, due to hourly reference glucose testing. For the purposes of analysis, we use the staff-recorded activity logs and did not use Zephyr data.
Results
Demographics
The 10 patients in the inpatient study had an average age of 30 ± 9 years (mean ± SD); four patients were male. All patients identified their race as white. The average duration of T1D was 11 ± 7 years. The average total daily dose was 44 ± 12 U. Normalizing by body weight, the average total daily dose was 0.65 ± 0.23 U/kg. The mean A1c was 7.0% ± 1.2%. The average weight was 70 ± 12 kg.
The 15 patients in the hotel study had an average age of 30 ± 13 years; two patients were male. All patients identified ethnicity as Caucasian. The average duration of T1D was 18 years ±13 years. The average total daily dose was 48 ± 26 U. Normalizing by body weight, the average total daily dose was 0.71 ± 0.38 U/kg. The mean A1c was 7.0% ± 1.1%. The average weight was 68 ± 12 kg. The expanded demographics data are shown in the Supplementary Data (Supplementary Data are available online at www.liebertpub.com/dia).
System performance
From initial activation to study completion, the system remained in closed-loop control during 95% of the inpatient studies and 96% of the hotel studies. The Zephyr Bioharness used to measure activity was operational for 86% of the time for the inpatient study. During the inpatient study, subjects consumed an average of 239 g of carbohydrates per day over an average of 5.7 meals/snacks per day. During the hotel study, subjects consumed an average of 210 g of carbohydrates per day over an average of 4.0 meals/snacks per day.
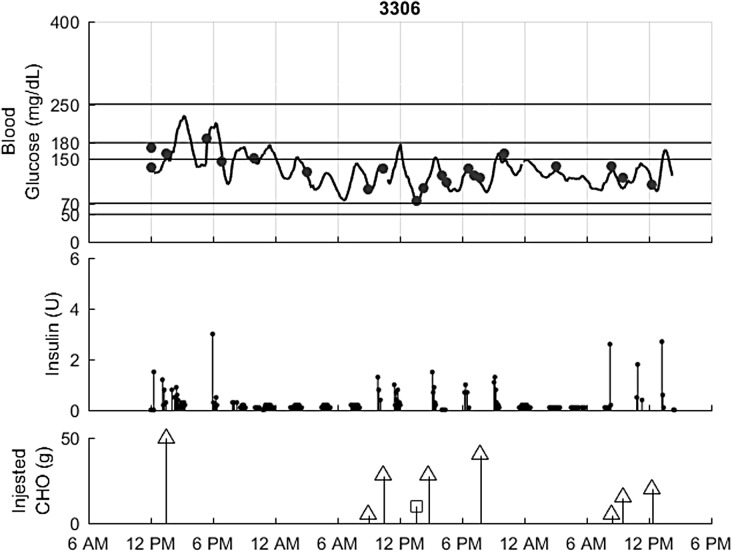
The closed-loop results for a representative patient in the hotel study are shown in Figure 1. Individual plots for all of the inpatient and hotel subjects are included in the Supplementary Data.
FIG. 1.
Hotel closed-loop performance for an example subject. Top: Glucose, CGM (black), Reference values (circles). Second: Insulin delivered. Third: Carbs, intervention (squares) and meals (triangles).
Table 1 summarizes the closed-loop results. Since we have more day periods than night periods, and some gaps in our closed loop data, we adjusted the data to allow comparison with other reported 24-h data. Specifically, our adjustment averages over the date, then over the time-specific blocks. The Supplementary Data include the results both per patient and over the study period. The hotel open-loop baseline data were derived from the sensor and insulin doses over at least 3 days before the admission to the hotel study.
Table 1.
Summary of CGM Glucose Results During Day, Night (11 PM–7 AM), and Overall with Average Daily Insulin Delivered
| Time | Mean (mg/dL) | CGM <50 (%) | 50–70 (%) | 70–180 (%) | 180–250 (%) | CGM >250 (%) | CGM >300 (%) | Daily insulin (U) | |
|---|---|---|---|---|---|---|---|---|---|
| Inpatient | Day | 152 | 0.05 | 2.5 | 70 | 20 | 6.6 | 2.8 | 37 |
| Night | 125 | 0.0 | 1.7 | 92 | 4.3 | 2.5 | 0 | 9 | |
| 24 h | 142 | 0.03 | 2.2 | 78 | 15 | 5.2 | 1.9 | 47 | |
| Hotel | Day | 158 | 0.2 | 1.9 | 68 | 22 | 7.5 | 1.9 | 39 |
| Night | 139 | 0.0 | 0.17 | 85 | 12 | 2.9 | 1.0 | 11 | |
| 24 h | 152 | 0.13 | 1.3 | 73 | 19 | 6.0 | 1.6 | 51 | |
| Hotel open loop | Day | 154 | 0.4 | 3.7 | 66 | 20 | 9.3 | 2.6 | NA |
| Night | 174 | 0.63 | 3.5 | 54 | 26 | 15 | 5.5 | NA | |
| 24 h | 160 | 0.50 | 3.6 | 62 | 22 | 11 | 3.6 | 48 |
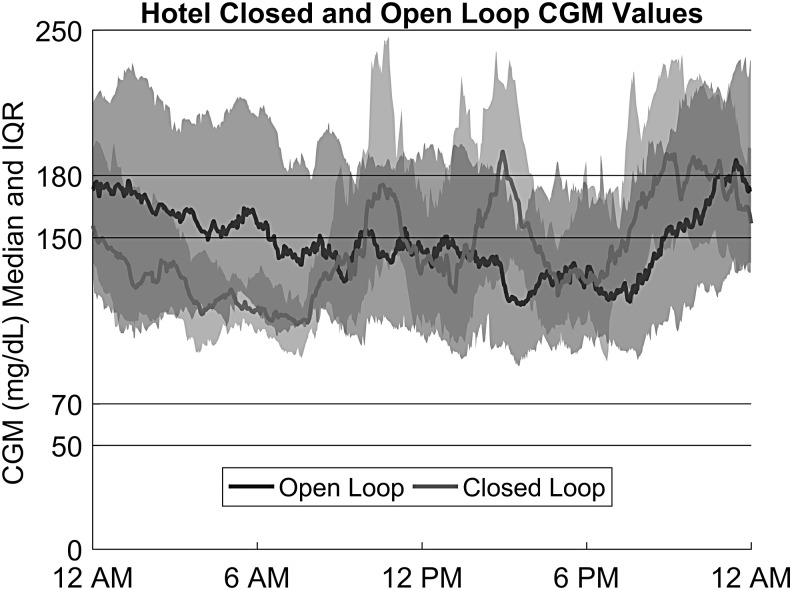
Figure 2 summarizes the results by showing the behavior of the CGM values on a 24-h basis for the hotel closed-loop and hotel baseline data.
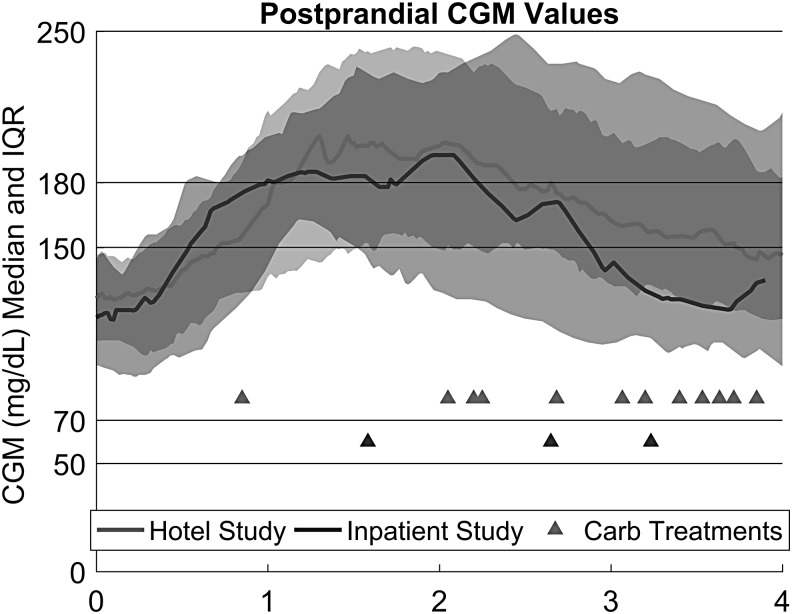
FIG. 2.
Postprandial control in the inpatient and hotel phase of the trial. Each curve draws from meals that are more than 25 g CHO and patients do not have another meal greater than 25 g CHO within the next 4 h. Twenty-one of 45 inpatient meals and 63 of 100 hotel meals met this criterion.
Meal glucose control with this fully closed-loop controller is shown in Figure 3. All of the meals in Figure 3 were unannounced, larger than 25 g CHO, and had no voluntary meals greater than 25 g CHO in the next 4 h. This means that a given meal response may have been altered by hypoglycemic interventions and/or exercise. Hypoglycemic interventions are shown as triangles in Figure 3. In the 4 h following a meal, there was a carbohydrate intervention 18% of the time. We do not have the frequency of carbohydrate interventions in the open-loop phase of the study, but this is probably a higher incidence than would be expected. We therefore examined the amount of insulin delivered for meals. For these meals, the ingested carbohydrates averaged 59 g. The controller delivered 9.4 units of insulin in the 1 h before to 2 h after the meal. Using their open-loop settings, subjects would have received 8.1 units for meal boluses (their carbohydrate-to-insulin ratio for that time of the day) and their basal insulin for 1 h before and 2 h after the meal.
FIG. 3.
Median sensor glucose and interquartile ranges (dotted lines) for 15 hotel subjects during closed-loop and open-loop phases.
Hypoglycemia
We defined a hypoglycemia event as YSI or meter glucose <70 mg/dL with at least 30 min of glucose values >70 mg/dL separating events. There were 12.5 patient days in the inpatient study with 21 hypoglycemic events and 29 hypoglycemia treatments. Events are described as a string of treatments each within 20 min of another. Of the inpatient events, 38% occurred more than 4 h after a meal and 5 occurred overnight; 33% occurred after a meal or snack; and 29% occurred during or immediately after exercise. There were 31.7 hotel patient days and there were 29 hypoglycemic events and 37 treatments: 14% can be attributed to missed meals, 10% to hardware issues, 33% to poor postprandial control, and 37% were associated with exercise. In addition, patients received carbohydrates on 18 separate occasions without glucose >70 mg/dL. These treatments had a median and interquartile range of 77 (74,104) mg/dL. These occurred with system-generated alerts for predicted hypoglycemia, at which time it was recommended the subject take 16 g of CHO, or with exercise when subjects were feeling symptomatic or were concerned about the negative rate of change of their glucose values.
CGM
The CGM values were compared with reference glucose values obtained during the inpatient study. Based on 663 paired sensor and reference readings, the mean absolute relative difference was 14% and the mean absolute difference was 18.2 mg/dL. The reference glucose demonstrated an overall bias of 0.1 mg/dL below sensor values.
Discussion
In this study, we report the performance of the MMPPC algorithm in patients with T1D in both inpatient and hotel study environments. This control algorithm does not require announcement of any meals or exercise. Furthermore, there were no restrictions or guidelines in meal choices for the duration of the study in an attempt to simulate outpatient living conditions.
For patients living with T1D, there is a significant self-management burden implicit in the treatment that includes, but is not limited to, meal carbohydrate estimation and insulin dosing calculations, as well as the uncertainty of glycemic response to increased physical activity. Thus far, the majority of closed-loop controllers require users to announce meals and activity. There is great heterogeneity in the level of self-management that patients employ in management of T1D. It has been described that there are populations (particularly adolescents) that miss meal boluses.7 Treatment of T1D should be tailored to the individual patient. The natural progression, as closed-loop control becomes a reality, is that algorithms will also be tailored to patients. The MMPPC algorithm would therefore have particular value in the subset of patients who frequently miss meal boluses. We expect that as the consequences of missed meal boluses are reduced by artificial pancreas systems, this subset may grow.
Despite the complete absence of meal announcement during the hotel trials, the daytime mean closed-loop sensor glucose during the study period (158 mg/dL) was similar to the pretrial open-loop period (154 mg/dL). However, during the night, subjects in closed loop had a significantly lower mean sensor glucose of 139 mg/dL by comparison with 154 mg/dL in open-loop control (P = 0.064% by the two-sample Kolmogorov–Smirnov test). The overnight control was responsible for the overall lower 24-h mean sensor glucose.
Fully closed-loop systems must not only detect meals quickly to initiate insulin delivery to cover a meal excursion but must also be careful not to overdeliver insulin in response to erratic glucose excursions. This means that these systems have an increased risk of hypoglycemia relative to hybrid glucose control when tuned to similar mean glucose levels. Although our system has this implicit risk, it demonstrated comparable time spent below 70 mg/dL.17,18 The time spent <70 mg/dL was partly mitigated by subjects being immediately treated for any meter BG <70 mg/dL, and if the subject had symptoms of hypoglycemia even if their glucose was >70 mg/dL.
Our system did not use the acquired accelerometer data to adjust insulin delivery. This caused the system to deliver insulin for carbohydrates that were consumed before and during exercise (exercise carbohydrates). In addition, the lack of a pre-exercise announcement meant that the controller could not act to prevent high levels of insulin on board during exercise. This controller was particularly susceptible to overdelivering insulin for the amount of carbohydrates consumed following small amounts of fast-acting carbohydrates. The initial high rate of change in the glucose level prompted an overestimation of the meal size and overdelivery of insulin.
Another component of the MMPPC controller was the integration of the accelerometer to determine when the subject was asleep or awake. Effective and accurate use of the accelerometer used in these studies required that it be firmly fixed to the torso of the patients. Unfortunately, the chest straps used reliably in a previous study19 were too uncomfortable for patients and therefore three-dimensional printed belt clips were manufactured by us and used by the subjects. These clips were less reliable. Challenges encountered included device connectivity, rotation of the clip leading to discomfort overnight, and possible misidentification of sleep/wake status. The inpatient beds were particularly prone to this issue since they were used in a semireclined position during the daytime period. Consequently, as the trial continued, we used the accelerometer less and less, instead directly informing the system when the patient would be asleep or awake. Because of these issues, the accelerometer was not used in the hotel study to announce sleep or wake states.
There was a 50% reduction in the rate of hypoglycemic events per patient per day between the inpatient and hotel phases. We attribute this both to not using the accelerometer to detect wake and sleep and to raising the daytime hypoglycemia threshold during the day. Removing the accelerometer before the subject went to sleep eliminated all overnight hypoglycemia, but did require additional user input to announce sleep and wake status. Since the remaining hypoglycemic events were split evenly between overly aggressive control and not anticipating exercise, future generations of this system will investigate exercise announcement options. We expect these to include activity sensors on smart watches and profile-based sleep/wake announcements.
Conclusion
These results show promise for full closed-loop control. Further adjustments are needed to reduce hypoglycemic risk and develop more wearable accelerometer options. In the future, we plan to conduct outpatient trials, and to correct the issues with using accelerometers to detect when the patient is awake or asleep, and make further adjustments to the algorithm to decrease the number of hypoglycemic events associated with exercise and with small meals associated with rapidly absorbed carbohydrates.
Supplementary Material
Acknowledgments
This research has been supported by an NIH grant 1R01DK102188-02. The authors gratefully acknowledge contributions from other members of the MMPPC study group (Michelle Clay, Liana Hsu, and Ryan Kingman of Stanford University, and Lindsey Schulhoff of the University of Colorado at Denver) and Patrick Keith-Hynes and Benton Mize at the University of Virginia for DiAs development and benchtop testing. F.M.C. acts as guarantor for this work. Results were presented at the Advanced Technology and Therapeutics for Diabetes conference in Milan, Italy, in 2016.
Author Contributions
All authors contributed to the review, editing, and discussion of the article and its ideas. Drs. Ly, Buckingham, Maahs, Levy, Lam, and Forlenza, as well as research coordinators Clinton, Messer, Westfall, Levister, and Xie, ran the studies that collected the data. Engineers Baysal, Howsmon, Patek, and Bequette developed the device that was tested in the studies.
Author Disclosure Statement
D.M.M. is on the Scientific Advisory board for Insulet, consulting for Abbott, and has benefited from research support to his institution from Medtronic and G.P.F. is a paid consultant for Abbott Diabetes Care. C.J.L. is a paid consultant for NovoNordisk and has had research support from NovoNordisk, Lexicon, and Roche. B.W.B. has consulted for BD. B.A.B. is on Medical Advisory Boards for NovoNordisk, Sanofi, and Convatec and has received research support from Medtronic, Roche, Tandem, and Dexcom.
References
- 1.Dauber A, Corcia L, Safer J, et al. : Closed-loop insulin therapy improves glycemic control in children aged <7 years. Diabetes Care 2013;36:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron F, Niemeyer G, Wilson DM, et al. : Inpatient trial of an artificial pancreas based on multiple model probabilistic predictive control with repeated large unannounced meals. Diabetes Technol Ther 2014;16:728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimri R, Muller I, Atlas E, et al. : Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes 2014;15:91–99 [DOI] [PubMed] [Google Scholar]
- 4.Steil GM: Algorithms for a closed-loop artificial pancreas: the case for proportional-integral-derivative control. J Diabetes Sci Technol 2013;7:1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bequette BW: Algorithms for a closed-loop artificial pancreas: the case for model predictive control. J Diabetes Sci Technol 2013;7:1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinsker JE, Lee JB, Dassau E, et al. : Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care 2016;39:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdick J, Chase H, Slover R, et al. : Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics 2004;113:e221–e224 [DOI] [PubMed] [Google Scholar]
- 8.O'Connell MA, Donath S, Cameron FJ: Poor adherence to integral daily tasks limits the efficacy of CSII in youth. Pediatr Diabetes 2011;12:556–559 [DOI] [PubMed] [Google Scholar]
- 9.Patton SR, Clements MA, Fridlington A, et al. : Frequency of mealtime insulin bolus as a proxy measure of adherence for children and youths with type 1. Diabetes Technol Ther 2013;15:124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Bon AC, Luijf YM, Koebrugge R, et al. : Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther 2014;16:131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron F, Niemeyer G, Bequette BW: Extended multiple model prediction with application to blood glucose regulation. J Process Control 2012;22:1422–1432 [Google Scholar]
- 12.CDC, NCHS: National Health and Nutrition Examination Survey Data. Hyattsville, MD: Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), 2004 [Google Scholar]
- 13.Statistics B of L. American Time Use Survey [Internet]. 2013. www.bls.gov/tus/ (accessed July20, 2017)
- 14.Cameron F, Bequette B, Buckingham B, et al. : A closed-loop artificial pancreas based on risk management. J Diabetes Sci Technol 2011;5:368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith-hynes P, Guerlain S, Mize B, et al. : DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 2013;7:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Place J, Sc M, Robert A, et al. : DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol 2013;7:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ly TT, Buckingham BA, Desalvo DJ, et al. : Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care 2016;39:106–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thabit H, Tauschmann M, Allen J, et al. : Home use of an artificial beta cell in type 1 diabetes. New Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenerson M, Cameron F, Wilson D, et al. : The impact of accelerometer and heart rate data on hypoglycemia mitigation in type 1 diabetes. J Diabetes Sci Technol 2014;8:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.