Abstract
Significance: Basement membranes (BMs) are sheet-like structures of specialized extracellular matrix that underlie nearly all tissue cell layers including epithelial, endothelial, and muscle cells. BMs not only provide structural support but are also critical for the development, maintenance, and repair of organs. Animal heme peroxidases generate highly reactive hypohalous acids extracellularly and, therefore, target BMs for oxidative modification. Given the importance of BMs in tissue structure and function, hypohalous acid-mediated oxidative modifications of BM proteins represent a key mechanism in normal development and pathogenesis of disease.
Recent Advances: Peroxidasin (PXDN), a BM-associated animal heme peroxidase, generates hypobromous acid (HOBr) to form sulfilimine cross-links within the collagen IV network of BM. These cross-links stabilize BM and are critical for animal tissue development. These findings highlight a paradoxical anabolic role for HOBr, which typically damages protein structure leading to dysfunction.
Critical Issues: The molecular mechanism whereby PXDN uses HOBr as a reactive intermediate to cross-link collagen IV, yet avoid collateral damage to nearby BM proteins, remains unclear.
Future Directions: The exact identification and functional impact of specific hypohalous acid-mediated modifications of BM proteins need to be addressed to connect these modifications to tissue development and pathogenesis of disease. As seen with the sulfilimine cross-link of collagen IV, hypohalous acid oxidative events may be beneficial in select situations rather than uniformly deleterious. Antioxid. Redox Signal. 27, 839–854.
Keywords: : hypohalous acid, basement membrane, peroxidasin, hypobromous acid, peroxidase
Introduction
The extracellular matrix (ECM) is a noncellular, organized network of proteins that provides a structural scaffold for cells. But more than a simple scaffold, the ECM provides chemical and mechanical cues. Cells sense these cues via cell surface receptors, such as integrins (53). This forms a bidirectional relationship wherein ECM modifies cell behavior and cells modify their surrounding ECM (11, 53). These cell–ECM interactions are critical for tissue development, maintenance, and response to injury (19, 111). The exact composition of the ECM is tissue specific and changes during and after development (29, 111).
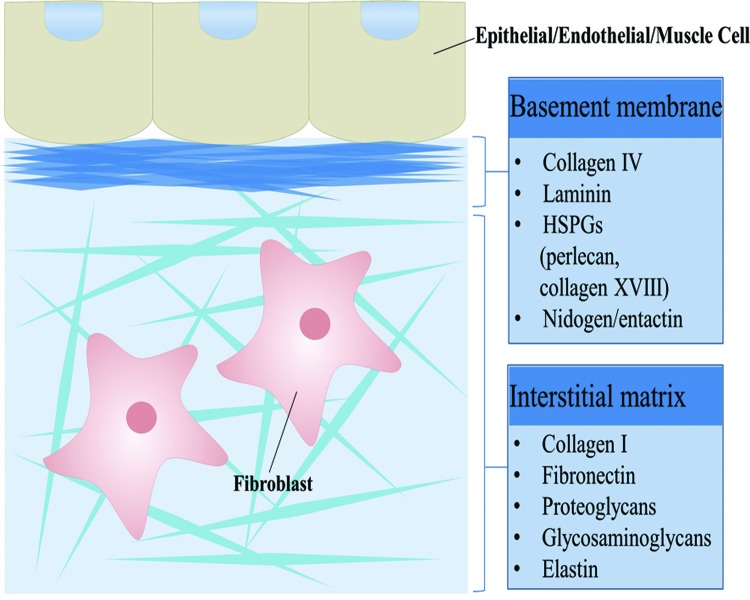
ECM can be classified into two subtypes, interstitial matrix and the basement membrane (BM) (11, 29). The BM and interstitial matrix form separate but adjacent structures in vivo (65). Interstitial matrix surrounds mesenchymal cells and provides structural support and protection against compression. The BM, in contrast, is a specialized sheet-like form of ECM that underlies epithelial, endothelial, and muscle cells separating them from underlying stroma (Fig. 1). BMs are intimately tied to the primary cells of nearly all tissues governing their polarity, migration, proliferation, and differentiation (160). Therefore, BMs are critical for the development and maintenance of tissues playing a critical role in normal physiology and pathogenesis of disease. Given the critical importance of BM to multicellular tissues, it is no surprise that BMs are the primordial ECM with its core protein constituents conserved to the earliest animals (52, 147). These core proteins are collagen IV, laminins, proteoglycans, and nidogens (160).
FIG. 1.
Major types of ECM. Schematic representation of the two main types of ECM, the interstitial matrix and the BM. The interstitial matrix surrounds cells and is composed of mostly collagen I, fibronectin, proteoglycans, glycosaminoglycans, and elastin. The BM underlies most epithelial, endothelial, and muscle cells. It is composed mostly of collagen IV, laminin, perlecan, collagen XVIII, and nidogen. Both types of ECM provide cells with a scaffold upon which to adhere or embed, while helping to regulate cellular processes such as growth, migration, and differentiation. BM, basement membrane; ECM, extracellular matrix. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Recent work in BM structure and function revealed a novel mechanism of BM assembly. Peroxidasin (PXDN), a BM-associated animal heme peroxidase, generates hypobromous acid (HOBr) to cross-link collagen IV, thus mechanically reinforcing BMs and promoting tissue integrity (10, 72). This work highlights the role of hypohalous acids, such as HOBr, in the structure and function of BMs. Although PXDN is directly associated with BM, other animal heme peroxidases, namely myeloperoxidase (MPO) and eosinophil peroxidase (EPO), are secreted by activated leukocytes into the extracellular space and often directly bind to BM (6, 57, 105). MPO produces hypochlorous acid (HOCl) and EPO generates HOBr, which may, therefore, preferentially target BM. In the context of chronic inflammation with tissue infiltration of activated leukocytes, HOBr and HOCl modify and damage BM proteins (17). Since BM proteins are typically long-lived, these modifications can promote maladaptive cell behavior over prolonged time frames to drive chronic disease. Thus, hypohalous acids can alter BM structure and function in a detrimental way, but also promote BM assembly and integrity. The focus of this review will be to examine these contrasting roles in detail beginning with an overview of BM biology and then considering hypohalous acid-driven BM damage and the role of PXDN and HOBr in BM assembly.
Overview of BM Biology and Its Components
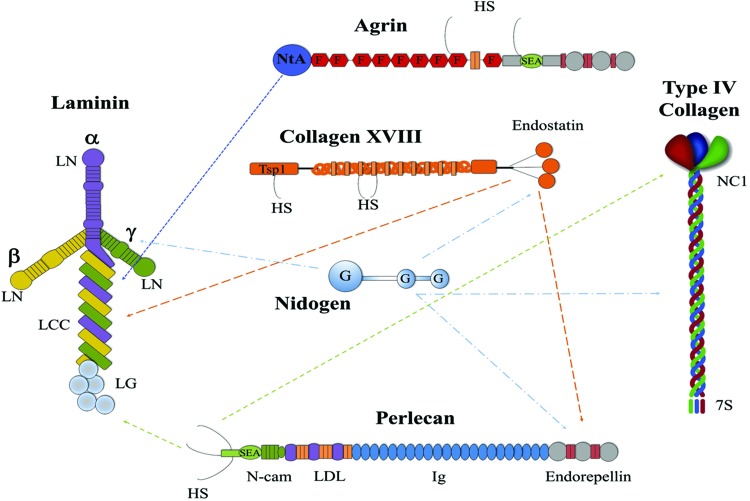
The BM is a specialized sheet-like layer of insoluble material that provides a scaffold for cellular attachment, migration, and differentiation. It is the most primitive form of ECM that dates back to the appearance of multicellular organisms. The core structural components of most BMs are collagen IV, laminins, proteoglycans (perlecan, agrin, collagen XVIII, and collagen XV), and nidogens (Fig. 2). Laminins and collagen IV form two distinct polymeric networks, with proteoglycans and nidogens often acting as bridging molecules between these networks and cell surface receptors (52, 76, 160).
FIG. 2.
The major components of basement membranes. Schematic representation of the domain structure for the main components of BM: laminin, type IV collagen, nidogen, agrin, collagen XVIII, and perlecan. Arrows indicate known binding interactions between BM components. HS, heparin sulfate; NtA, N-terminal agrin domain; F, folstatin-like repeats; SEA, sea urchin sperm protein, enterokinase, and agrin domain; LN domain, laminin N-terminal domain; LCC, laminin coiled-coil domain; LG, laminin globular domain; Tsp1, trombospondin-1-like; G, globular-like domains; N-CAM, neural cell adhesion molecule; LDL, low-density lipoprotein receptor; Ig, immunoglobulin repeats; NC1, noncollagenous domain; 7S, N-terminal “7S” domain. A portion of this figure was adapted from Miner (67) with permission from Elsevier. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Collagen IV
Collagens are one of the most abundant proteins in animals making up ∼30% of total protein. They are characterized by the presence of a collagenous domain, a triple helical structure with a stereotyped amino acid repeat structure in which glycine residues occur in every third residue. Lysine and prolines and their hydroxylated variants commonly occupy the other two positions (107, 122). Type IV collagen is the predominant collagen found in BMs. Most vertebrate genomes encode for six alpha chains, α1 (IV)–α6 (IV), which form heterotrimeric triple helical molecules. Each alpha chain consists of three distinct domains, an N-terminal “7S” domain, a long central triple helical collagenous domain, and a C-terminal noncollagenous (NC1) domain. Although many combinations of the six alpha chains may assemble into triple helical “protomers,” only three protomers have been found in mammalian systems, namely α1α2α1, α3α4α5, and α5α5α6. These protomers self-oligomerize to form three sheet-like networks: the 112 network composed purely of α1α2α1 protomers, the 345 network made up of α3α4α5 protomers, and the 1256 network composed of a variable combination of α1α2α1 and α5α5α6 protomers (62). The 112 network is found broadly in nearly all tissues in mammals and its homologue is the only network found in invertebrates (62, 160). Conversely, the 345 and 1256 networks have restricted tissue localization. For instance, the 345 network is restricted to the glomerular BM (GBM) of kidney, the lens epithelial BM, cochlear BMs, and in small quantities in lung alveolar and testicular BMs (1, 37, 59, 60, 114).
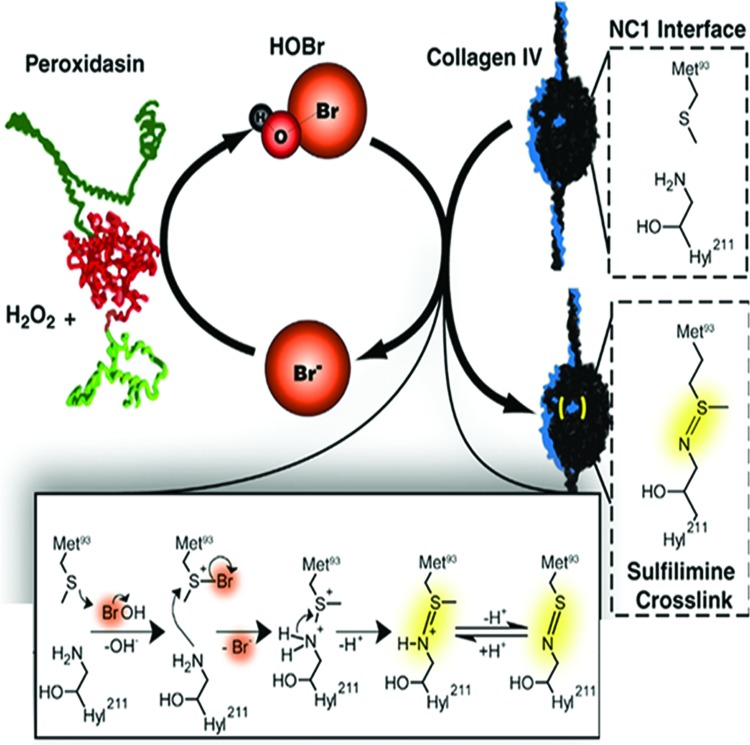
The oligomerization process of collagen IV involves three distinct associations. N-terminal 7S domains from four protomers associate to form the 7S tetramer (109). Lateral associations between central collagenous domains occur with variable stoichiometry (162). Finally, two trimeric protomers join via their NC1 domains to form an NC1 hexamer (12). Intermolecular covalent bonds between protomers, often termed cross-links, uniquely reinforce the collagen IV network. The 7S tetramer is reinforced by disulfide bonds and lysine to lysine cross-links (2, 109). Disulfide bonds also intermittently cross-link the central collagenous domains of protomers to one another (36). Finally, a unique sulfilimine (S = N) bond between a methionine sulfur and hydroxylysine nitrogen bridges the NC1 trimer–trimer interface within the NC1 hexamer (136).
Collagen IV mechanically stabilizes BMs and, therefore, tissues. Mice lacking the collagen 112 network demonstrate normal formation of BM, but eventual embryonic lethality because of widespread BM rupture and mechanical instability (99). Similarly, in Drosophila egg development, collagen IV deficiency prevents normal egg elongation, which involves the tightening of a “molecular corset” created by collagen IV and its interactions with cell surface receptors (38). Finally, in human Alport syndrome, loss of the collagen IV alpha 345 network leads to breaks in the renal GBM with compromise of renal filtration and progressive renal failure (50). Much of the mechanical strength of the collagen IV network may be related to its extensive covalent cross-links reinforcing the oligomeric network. Indeed, recent work has found that loss of the NC1 bridging sulfilimine cross-links reduces BM stiffness (9).
Laminin
Laminin is a (∼400–800 kDa) heterotrimer comprising one each of five α, four β, and three γ subunits, joined through a long coiled-coil domain. Despite the multitude of possible heterotrimers, 16 isoforms have been identified (160). These isoforms are typically named based on their chain composition. For example, the laminin isoform consisting of α1, β1, and γ1 chains is termed laminin-111 (3). All chains generally share common structural features, such as a large globular N-terminal domain (LN domain), a rod-like stretch of laminin-type epidermal growth factor-like repeats (LE) domain that contains one or two globular domains (one in β or γ chains; two in α chains). The β and γ chains end with a laminin coiled-coil domain, which is involved in trimerization, and the α chains contain five C-terminal laminin globular (LG) domains (LG1–5) (161).
Heterotrimeric laminins assemble into polymeric networks via their LN domains. Initial formation of dimers is facilitated by cell surface receptors with subsequent self-polymerization in a calcium-dependent manner (161). Laminins have tissue-restricted expression and, therefore, have tissue-specific functions in development and physiology. The function of specific laminin isoforms is beyond the scope of this review (77, 161). In general, although collagen IV mechanically reinforces BM, laminins spatially define the BM during development and are, therefore, critical for its formation. For instance, knockout of the laminin γ1 chain, which eliminates nearly all laminin networks, results in early embryonic lethality as BMs completely fail to form, including Reichert's membrane and embryonic BM (125).
Proteoglycans
Proteoglycans are glycoproteins studded with glycosaminoglycans (GAGs), polymers of disaccharides, such as heparan sulfate (HS). There are ∼43 proteoglycan genes classified into four major proteoglycan classes based on cellular and subcellular location, overall gene/protein homology, and the presence of specific protein modules within their respective protein cores. These classes encompass nearly all the known proteoglycans of the mammalian genome (56). The addition of GAGs typically endows proteoglycans a negative charge and enables them to sequester both water and divalent cations. This allows them to take on space-filling and lubrication functions within the ECM (81). GAGs also bind growth factors within the ECM, providing for regulation of cell–matrix interactions (115).
The heparan sulfate proteoglycans (HSPGs) perlecan, agrin, and collagen XVIII and the chondroitin sulfate proteoglycan collagen XV are the major proteoglycans found in BMs (56). Perlecan is characterized by a ∼500 kDa protein core subdivided into five domains with homology to SEA (sea urchin sperm protein, enterokinase, and agrin), N-CAM (neural cell adhesion molecule), IgG (immunoglobulin G), LDL (low-density lipoprotein) receptor, and laminin. Perlecan carries three HS chains at its N-terminus (26, 56). It is expressed by both vascular and avascular tissues, with localization at the apical cell surface and BM (35). Perlecan interacts with numerous ligands and cell surface receptors giving it a role in the regulation of many cellular processes such as adhesion, growth, and survival. It is particularly known to have dual functions in regulating angiogenesis. Cleavage of the HS chains by heparanase and its core protein by proteases releases various proangiogenic factors, whereas the C-terminal domain V, known as endorepellin, inhibits angiogenesis (24, 106).
Agrin has a modular structural organization homologous to that of perlecan with the potential to generate several splice isoforms (73). The N-terminal region can be spliced to generate two isoforms. The N-terminal-agrin (NtA) domain isoform has a high affinity for the coiled-coil domain of laminin γ1. This affinity allows it to function as a link between the cell surface and BM. The NtA domain is followed by nine follistatin-like (FS) repeats with two laminin (LE) domains inserted between the last two repeats. After the FS repeats, there are two Ser/Thr (S/T)-rich domains separated by an SEA module. The structural organization of the C-terminus is similar to endorepellin in perlecan. The central region of the agrin protein core contains attachment sites for HS chains. Multiple cell surface receptors including receptor tyrosine kinases bind the LG-rich C-terminus of agrin (73). Most work on agrin has focused on its role in the development of the neuromuscular junction (8). Mutations in the agrin gene are known to cause congenital myasthenic syndrome (51, 85).
Collagens XV and XVIII are proteoglycans, but are also members of the multiplexin collagen family (39). Multiplexins are proteins with multiple collagen-like triple-helical domains and interruptions (91). Collagen XVIII is composed of 10 collagenous repeats alternating with 11 noncollagenous repeats and can be expressed as 1 of 3 N-terminal isoforms (113). The “short form” has a trombospondin-1-like (Tsp1) sequence at the N-terminus, and the longest isoform contains a domain of unknown function and a frizzled domain (FZC18). Through alternative splicing, the FZC18 domain is not present in the intermediate sized variant (117). The C-terminal 20 kDa endostatin fragment found in all isoforms has potent antiangiogenic properties when proteolytically cleaved (140). Collagen XVIII often localizes to the BM–interstitial matrix interface, which suggests a role in anchoring the BM to the interstitial matrix (117). In humans, mutations in collagen XVIII are linked to Knobloch syndrome, an autosomal recessive disorder characterized by the occurrence of high myopia, vitreoretinal degeneration with retinal detachment, macular abnormalities, and occipital encephalocele (118).
Type XV collagen is a trimeric chondroitin sulfate proteoglycan with a 250 kDa core protein linked by interchain disulfide bonds (66). Collagen XV is similar to collagen XVIII in its primary structure, but with more extensive interruptions of its collagenous regions. Similar to endostatin, collagen XV may proteolytically release a 22 kDa C-terminal fragment, restin, which also displays antiangiogenic activity (102).
Nidogens
The nidogen family, also known as entactins, consists of two sulfated monomeric glycoproteins, nidogens 1 and 2, one or both of which are found in all BMs (47). At ∼150 kDa, they are the smallest BM glycoproteins (40). They are composed of 10% carbohydrate, and the core protein is made up of three globular-like domains (G domains), an N-terminal G1, G2, and C-terminal G3. These G domains are separated by two rod-like segments. Nidogens are known as ECM linker molecules because of their wide range of binding partners, including type IV collagen, perlecan, laminin, and fibronectin. Although animal studies indicate that nidogens are not required for the formation of BM, these bridging interactions may play a role in BM stabilization particularly under stress (47).
Hypohalous Acid-Mediated Oxidative Damage to BMs
Unlike the intracellular compartment, the extracellular space is relatively devoid of antioxidant enzymes. The primary extracellular defense against oxidant injury lies in the chelation of transition metals to prevent the generation of highly reactive species such as the hydroxyl radical. Most of this chelation is via binding proteins such as transferrin and ceruloplasmin, although some may also involve binding to negatively charged GAGs of the ECM (17, 41). The fate of oxidatively damaged ECM proteins starkly contrasts with intracellular or even cell surface proteins. Although disulfide reductase activities may be present to reduce inappropriately oxidized cysteines, few other reducing mechanisms exist extracellularly (17, 41, 159). The cellular compartment possesses robust mechanisms, such as the ubiquitin-proteosome and lysosomal pathways, to degrade oxidatively damaged and misfolded proteins. Conversely, damaged ECM proteins have unclear mechanisms of damage detection and turnover. Given their inherent insolubility, ECM proteins are presumably proteolyzed with cellular uptake of solubilized fragments. A large cadre of metalloproteases, such as matrix metalloproteases (MMPs), have been described, which degrade ECM proteins, but how they are activated and targeted after oxidative damage to ECM proteins is unclear (11).
The turnover of ECM proteins is probably slower than cellular proteins. Based on limited data, BM proteins turnover slowly at rates that vary with location. Furthermore, BM components have different turnover rates with collagen IV being quite long lived (Table 1). Taken together, oxidative damage to BM may only be slowly addressed via degradation, synthesis, and assembly with a significant potential for maladaptive cellular responses related to “outside-in” signaling between long-lived, damaged BM proteins and cell surface receptors, such as integrins (54).
Table 1.
Normal Basement Membrane Turnover Is Slow and Varies by Location and Basement Membrane Protein
| Species and tissue | Technique | Estimated half-life | Reference |
|---|---|---|---|
| Hair follicle | Silver labeling | ||
| Rat | >2 years | (142) | |
| Human | >10 years | (141) | |
| Colon | Silver labeling | ||
| Rat | 2 weeks | (142) | |
| Human | 1–4 months | (141) | |
| Jejunum—mouse | Laminin IF | (132) | |
| Epithelial | 2–4 weeks | ||
| Smooth muscle | >4 weeks | ||
| Endothelial | 1–4 weeks | ||
| Glomerulus | Silver labeling | ||
| Rat | 1–3 months | (142) | |
| Human | 1–5 years | (141) | |
| Glomerulus—rat | Radioactive AA labeling | (100) | |
| Overall | 1–2 months | ||
| Collagen | >3 months |
AA, amino acid; IF, immunofluorescence.
Animal heme peroxidases are the primary source of hypohalous acids. These enzymes utilize hydrogen peroxide and a halide (I−, Br−, or Cl−) or pseudohalide (SCN−) anion as substrates to generate the corresponding hypohalous acids, namely hypoiodous acid (HOI), HOBr, HOCl, and hypothiocyanous acid (HOSCN). The halide substrates for these peroxidases exhibit relatively narrow plasma and extracellular concentrations. The normal plasma iodide level is 0.5–1 μM, SCN− is 25–50 μM, Br− is 50–100 μM, and Cl− is 95–105 mM (48, 79, 133). I− and Br− levels may rise in rare intoxications, whereas Cl− levels can vary mildly with tonicity and acid-base disorders (14, 116, 135). SCN− levels can increase about threefold in smokers (79, 133). In contrast to halides, hydrogen peroxide concentrations can vary significantly in normal physiology and disease, thereby regulating peroxidase generation of hypohalous acids.
A clear source of extracellular hydrogen peroxide is the membrane proteins known as NADPH oxidases (NOX), which consist of NOX1–5 and DUOX (dual oxidase) 1 and 2. These proteins reduce extracellular O2 to either superoxide (NOX1–3, NOX5) or directly to hydrogen peroxide (NOX4, DUOX1, and DUOX2), while oxidizing NADPH intracellularly (64). Superoxide spontaneously or via superoxide dismutase forms hydrogen peroxide (32). Whether hydrogen peroxide generated intracellularly diffuses extracellularly to be available for animal heme peroxidases is unclear. Hydrogen peroxide diffuses easily across cell membranes. However, depending on the intracellular milieu of thiols, intracellularly generated hydrogen peroxide may quantitatively react with thiols or escape these reactions to end up in the extracellular space (148).
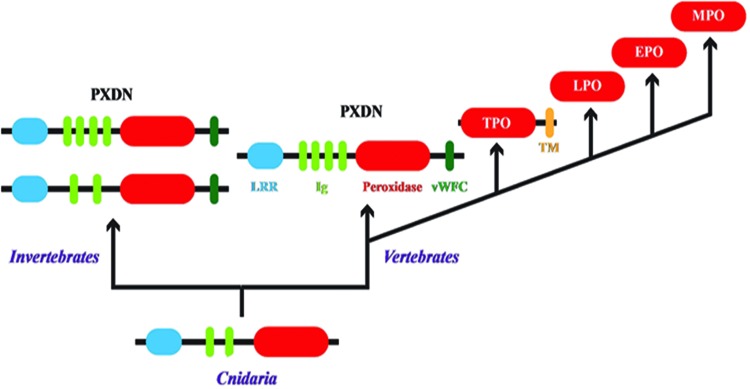
The animal heme peroxidase family consists of PXDN, thyroid peroxidase (TPO), lactoperoxidase (LPO), EPO, and MPO. Several peroxidases have been purified from various tissues, including salivary, gastric, intestinal, uterine, and placental peroxidases. Although salivary peroxidase is likely encoded by the LPO gene, the molecular identity of the other tissue peroxidases has not been clearly defined (23, 49, 55, 58, 63). As discussed later in this review, PXDN is the ancestral member of this family conserved to the basal phylum Cnidaria, which reflects its function in the BM assembly, a critical aspect of tissue genesis in animals (25, 27, 127). TPO arises in early chordates with thyroid hormone signaling possibly related to endothermy (69). EPO and MPO appear in vertebrates with the development of specialized leukocytes, namely eosinophils and neutrophils, whereas LPO may be ancestral within this group as it is primarily expressed by glandular epithelia (Fig. 3) (163). The human genome location reflects the evolutionary ontogeny of the animal heme peroxidases with PXDN and TPO located adjacent to one another on chromosome 2 and LPO, EPO, and MPO residing as a cluster on chromosome 17.
FIG. 3.
Evolution of animal peroxidases. The ancestral animal heme peroxidase found in Cnidaria is PXDN. The addition of two Ig domains and the vWFC domain occurs in lower invertebrates. PXDN likely gave rise to TPO through gene duplication, deletion of noncatalytic domains, and fusion with a TM domain. LPO, EPO, and MPO were likely the result of successive gene duplications after the loss of the TM domain found in TPO. This figure was adapted with permission from work originally published by Ero-Tolliver et al. (25) ©2015 by American Society for Biochemistry and Molecular Biology. EPO, eosinophil peroxidase; PXDN, peroxidasin; LPO, lactoperoxidase; MPO, myeloperoxidase; TPO, thyroid peroxidase; TM, transmembrane; vWFC, von Willebrand factor type C. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
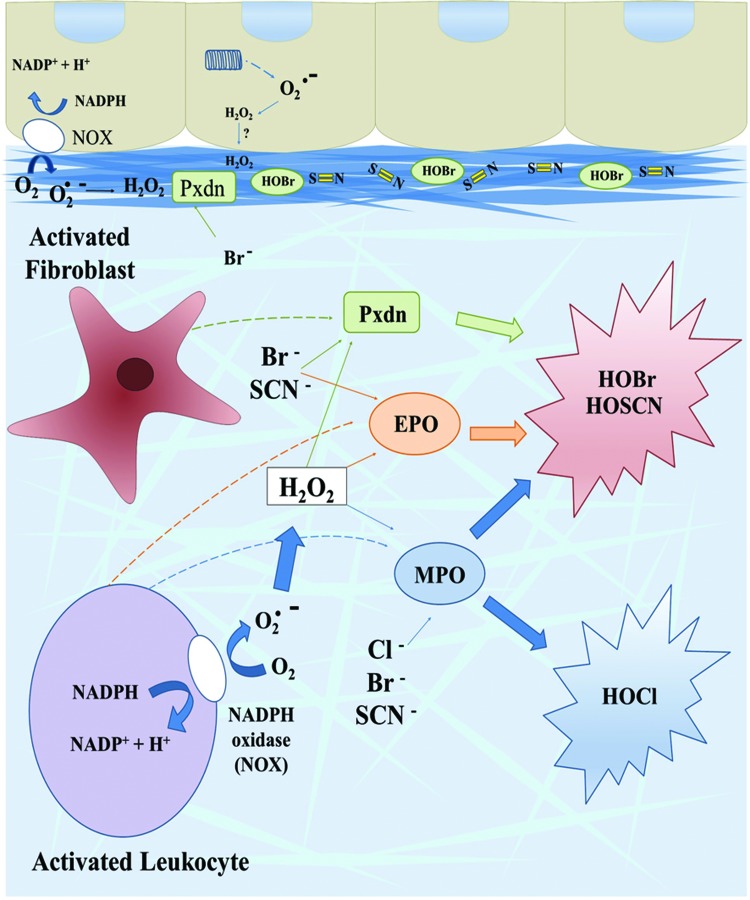
We review basic aspects of peroxidase enzymology and function herein, but more detailed information is available in excellent reviews (22, 87). The ease of oxidation of halides has a general rank order of I− ≅ SCN− > Br− > Cl− so that all animal heme peroxidases oxidize I− and SCN−. PXDN and EPO also oxidize Br−, whereas only MPO oxidizes Cl− to any appreciable degree (10, 22, 87, 126). Based on normal halide plasma concentrations and oxidation profile, LPO primarily generates HOSCN, EPO and PXDN produce HOBr and HOSCN, and MPO creates HOCl and HOSCN (Fig. 4) (10, 22, 87, 126). In mammals, TPO is compartmentalized within the thyroid gland with iodide concentrations in the low millimolar range and thus solely oxidizes iodide to modify thyroglobulin in the biosynthetic pathway for thyroxine (112).
FIG. 4.
Oxidation reactions of animal heme peroxidases in tissues. PXDN found in basement membranes generates HOBr to form sulfilimine (S = N) cross-links in collagen IV. Activated fibroblasts may also secrete PXDN into interstitial matrix, whereas MPO and EPO are released from activated, infiltrating leukocytes. NADPH oxidases generate superoxide (O2. −), which dismutates into hydrogen peroxide, a substrate along with halide (Br− and Cl−) or pseudohalide (SCN−) anions for peroxidase generation of hypohalous acids. Whether intracellular hydrogen peroxide may act as a substrate extracellularly is unclear. PXDN, EPO, and MPO generate HOSCN and HOBr, whereas only MPO produces HOCl in appreciable quantities. HOBr, hypobromous acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
LPO, EPO, and MPO are thought to function in pathogen defense. LPO is found in many exocrine secretions most prominently in saliva and milk and is thought to defend against microorganisms (4). MPO is a major component of the antimicrobial toolbox of neutrophils, which act as effector cells in both innate and adaptive immunity (83). Upon activation of neutrophils, MPO is delivered to the phagolysosome-containing engulfed pathogens. Coincident to MPO delivery to the phagolysosome, a membrane NOX is assembled and generates superoxide, which dismutates to H2O2 to provide substrate for MPO-driven HOCl generation (82). MPO may also be secreted extracellularly in the normal degranulation process, packaged within neutrophil extracellular traps, or released after neutrophil cell death (88). Monocytes and macrophages also express MPO, but at significantly lower concentrations (74). EPO is part of the antimicrobial armamentarium of eosinophils, which are tasked to destroy helminths. Given the large size of their target, eosinophils do not phagocytize pathogens but rather secrete EPO-containing granules and target a NOX to the plasma membrane to generate superoxide (143).
Hypohalous acids are powerful oxidants capable of broadly modifying proteins, lipids, and nucleic acids, and consuming antioxidants such as glutathione. Indeed, these reactions underlie their use to destroy pathogens. For the purpose of this review, we provide a broad overview of hypohalous acid reactions with biomolecules, but refer the reader to more detailed reviews for further information (22, 92, 93, 103). HOSCN is thought to be a “milder” oxidant primarily targeting thiols on proteins and low molecular weight substances (124). Although chemically circumspect, HOSCN may more potently disrupt cellular function because it specifically targets functionally important thiols. For example, HOSCN induces apoptosis in a murine macrophage cell line at lower concentrations than HOBr and HOCl (71).
HOBr and HOCl are much more chemically promiscuous. In addition to targeting thiols, they also potently target amines to form haloamines on protein side chains, free amino acids, nucleic acids, and amine-containing phospholipids or haloamides on GAG side chains. These haloamines and haloamides essentially retain the oxidative capacity of the parent hypohalous acid and, therefore, can generate downstream oxidative modifications. HOBr and HOCl also efficiently react with aromatic rings in amino acids such as tyrosine and tryptophan and nucleic acids. Their reaction with tyrosine to generate the respective 3-halotyrosine and 3,5-dihalotyrosine is quantitatively minor, but notable as specific biomarkers of oxidative damage because of HOBr and HOCl (22, 92, 93, 149, 152).
Except in the case of phagolysosomal MPO, animal heme peroxidases are secreted into the extracellular space where they generate hypohalous acids. Given their high reactivity, in particular HOBr and HOCl, one would predict that extracellular targets, including ECM and BM, would be preferentially modified. Indeed, in a study examining oxidative modifications in human atherosclerotic plaques, the vast majority of these modifications were found in the ECM fraction (151). Hypohalous acids freely permeate cell membranes, but their conjugate anions are relatively impermeable. The pKa of the hypohalous acid and the environmental pH will determine the rate at which a hypohalous acid will enter cells and modify intracellular biomolecules. The pKa of HOSCN is lowest at 5.3, HOCl is next at 7.59, whereas HOBr has the highest pKa at 8.7 (80, 101, 130). Thus, at physiological pH, OSCN− and HOBr will predominate along with a mixture of HOCl and OCl−.
Animal heme peroxidases and hypohalous-acid mediated oxidant injury have garnered significant attention in inflammatory diseases. In these disorders, neutrophils, monocytes-macrophages, or eosinophils infiltrate tissues often after injury but in the absence of infective pathogens. With inappropriate activation, these leukocytes release peroxidases, cytokines, and growth factors to drive injury and maladaptive repair such as fibrosis. The role of MPO and EPO and hypohalous acid-mediated injury has, therefore, been a focus of investigation in the pathogenesis of inflammatory renal, bowel, and lung disease and atherosclerosis (22, 28, 45, 89). Although many targets for hypohalous acid modification have been studied in the literature, in this review, we focus our attention on modification of BMs and their constituents.
In vitro studies using vascular ECM, a combination of BM and interstitial matrix, have demonstrated that HOBr and HOCl fragment and release ECM proteins and proteoglycan carbohydrates (104, 150). The fate of the remaining insoluble ECM protein was less clear. Studies using other oxidants such as hydroxyl radical and peroxynitrous acid have shown that BM proteins are not only degraded but are also cross-linked and aggregated (18, 108). Although hypohalous acids aggregate and cross-link model and interstitial matrix proteins, the extent to which HOBr and HOCl cross-link BM proteins remains unclear (42, 43, 139).
Although HOBr and HOCl directly solubilize BM proteins and carbohydrates, other more subtle actions have also been identified. In earlier work using subendothelial ECM, which closely resembles BM, the cell or integrin binding region of fibronectin was most sensitive to HOCl-mediated oxidative damage, whereas laminin and the collagen binding region of fibronectin were less sensitive as assessed by antibody binding. The work found little evidence for ECM protein fragmentation or cross-linking, which may reflect lower HOCl exposure than other studies (137). Integrins often bind the amino acid sequence RGD on fibronectin and the arginine would be particularly sensitive to oxidative modification by HOCl (128). Similarly, HOBr and HOCl selectively damage the core protein of perlecan, leaving its GAGs relatively unscathed (105). In studies using renal GBM exposed to activated neutrophils, MPO-generated HOCl did not directly degrade BM proteins, but increased their susceptibility to the proteolytic action of neutrophil or endogenous BM MMPs (138).
HOCl also has complex effects on proteases and their endogenous inhibitors themselves. HOCl modulates the activity of MMPs often with activation at low concentrations and inhibition at higher concentrations (75, 96, 146). Recent work on MMP7 suggests low concentrations of HOCl oxidize a cysteine thiol within the critical cysteine switch region, leading to protease activation (31). Conversely, higher concentrations of HOCl oxidize a tryptophan residue leading to MMP7 inactivation (30). HOCl also inactivates protease inhibitors, such as alpha-1 proteinase inhibitor and tissue inhibitor of metalloproteases (TIMPs) (144, 145). Similarly, HOBr and HOCl modify and inhibit trypsin inhibitor (42, 43). At a molecular level, HOCl oxidizes the N-terminal cysteine of TIMP1 leading to its inactivation (144). Taken together, these data suggest that HOBr and HOCl directly damage BM constituents, leading to protein solubilization and susceptibility to proteolysis and also shift the balance of proteases and their inhibitors toward proteolysis of BM constituents. The loss or alteration of BM proteins by hypohalous acids reduces cell adhesion to BM proteins, which may alter cell behavior and thereby promote inflammatory disease pathogenesis (105, 137).
Localization of animal heme peroxidases to BMs may further target hypohalous acid damage to BM proteins. As discussed later, PXDN is a BM resident protein with the potential to locally generate HOBr and damage nearby BM constituents (10, 84). MPO has been found to tightly bind to subendothelial and GBM and undergo transcytosis across endothelial cells to reach the BM (6, 57). Furthermore, MPO directly binds to perlecan via an electrostatic interaction between cationic MPO and the anionic HS side chains of perlecan (105).
Direct demonstrations of the functional consequences of hypohalous acid-mediated oxidative modifications of BM proteins are limited. In a classic study, Johnson et al. demonstrated that infusion of MPO and H2O2 into the renal artery led to histological damage and proteinuria, a functional marker of renal glomerular damage. MPO directly bound to the GBM and coinfusion of radiolabeled iodide demonstrated iodination and thus oxidative modification of the GBM. This study demonstrated that MPO-generated HOCl has the potential to directly damage the GBM, leading to renal injury (57).
A correlative approach to define the role of hypohalous acid modification of BM proteins in disease pathogenesis is to determine whether these oxidative modifications are found in greater abundance from diseased tissues. Although tissue bromo- and chlorotyrosine content has been found to be increased in many disease states (15, 44–46, 119, 120, 131, 134, 153), demonstration of these modifications specifically on BM proteins is scant. Examination of oxidative modifications of ECM (i.e., mixed BM and interstitial matrix) from atherosclerotic plaques demonstrated increased levels of the oxidized amino acids, dihydroxyphenylalanine, dityrosine, and 2-hydroxyphenylalanine. Although these modifications are not specific to HOCl, reaction of vascular ECM with HOCl produced a similar oxidative modification pattern suggestive of a pathogenic relationship (151). Recently, Brown et al. demonstrated that tryptophan 28 of the α2 NC1 domain and tryptophan 192 of the α1 NC1 domain of collagen IV are oxidized and chlorinated to a greater extent in diabetic animals. These residues were similarly modified when purified collagen IV was treated with HOCl. Furthermore, HOCl treatment of collagen IV promoted unfolding and reduced binding to α1β1 integrin, suggesting a functional impact of HOCl oxidation (13).
Anabolic Role of HOBr in BM Assembly
Although most work has focused on how hypohalous acids damage BM, recent work has uncovered surprising mechanisms, whereby HOBr is utilized as an anabolic agent to facilitate collagen IV and BM assembly. As noted earlier, a sulfilimine (S = N) bond bridges the NC1 trimer–trimer interface within the NC1 hexamer to reinforce assembled collagen IV networks. The sulfilimine bond connects the sulfur of methionine 93 on one NC1 trimer subunit and the nitrogen of hydroxylysine (or lysine) 211 on an opposing NC1 trimer subunit (136). PXDN, an ECM-localized animal heme peroxidase, catalyzes the formation of these sulfilimine cross-links (10). As alluded to earlier, the sulfilimine bond of collagen IV and its catalytic enzyme, PXDN, are conserved back to Cnidaria, a basal phylum of the animal kingdom, suggesting that the formation of this cross-link was an early step in the development of multicellular eumetazoans (27, 127). In the absence of PXDN, animals form easily fractured BMs, suggesting a role in the structural integrity of collagen IV, which imparts BMs their mechanical strength.
In Drosophila, PXDN loss of function leads to larval lethality caused by gut perforation. Examination of the fly midgut BM with GFP-labeled collagen IV demonstrates torn and missing collagen IV networks (10). Using electron microscopy, loss of PXDN produces thickened and more diffuse-appearing BMs (72). In Drosophila egg development, loss of PXDN produces a milder phenocopy of the loss of collagen IV, with less elongation related to the “corset” action of collagen IV (38, 72). In C. elegans, lack of PXDN (pxn-2) results in ultrastructural defects in BM reminiscent of collagen IV mutants with early lethality because of failure of elongation, a process associated with the onset of smooth muscle activity. Pharmacological muscle paralysis ameliorates the phenotype, suggesting an inability to defend against mechanical strain associated with muscle contraction (34).
These data suggested that abrogation of PXDN function in mammals would lead to phenotypes reminiscent of, yet milder than collagen IV loss of function, which results in embryonic lethality (99). Surprisingly, PXDN knockout mice and humans lacking PXDN are completely viable and only exhibit an ocular developmental defect known as anterior segment dysgenesis (9, 61, 154). The reason for the discrepancy between invertebrate and mammalian phenotypes is unclear. Mice do not possess other genes similar to PXDN, whereas the human genome encodes a gene known as PXDN-like. The resultant PXDN-like protein, however, lacks critical residues in its peroxidase domain for catalytic activity (98). Collagen IV isolated from PXDN knockout mice possesses about one-third of normal sulfilimine cross-link content, confirming the importance of PXDN in forming these cross-links (9). This reduction in cross-links is similar to that observed in Drosophila PXDN loss of function (10, 72). Invertebrates may be much more dependent on sulfilimine cross-links for development either because of more rapid remodeling and growth or because the remainder of the collagen IV network may be less cross-linked. For instance, mammalian collagen IV is cross-linked via disulfide and lysine-lysine cross-links in the 7S and collagenous regions, but invertebrate collagen IV may not be as densely cross-linked (62, 86).
PXDN generates HOBr and HOSCN (10, 68, 126), but whether it produces HOCl is unclear. Endpoint assays based on taurine haloamine oxidation of 3,3′,5,5′-tetramethylbenzidine demonstrated low levels of HOCl generation (10, 68). But, detailed spectral studies found that Cl− cannot reduce compound I of PXDN, which is generated after hydrogen peroxide-mediated oxidation of the heme moiety. These presteady state measurements imply that PXDN is incapable of producing HOCl (94). Although these discrepant results may simply reflect assay differences, the weight of evidence suggests that PXDN does not generate HOCl.
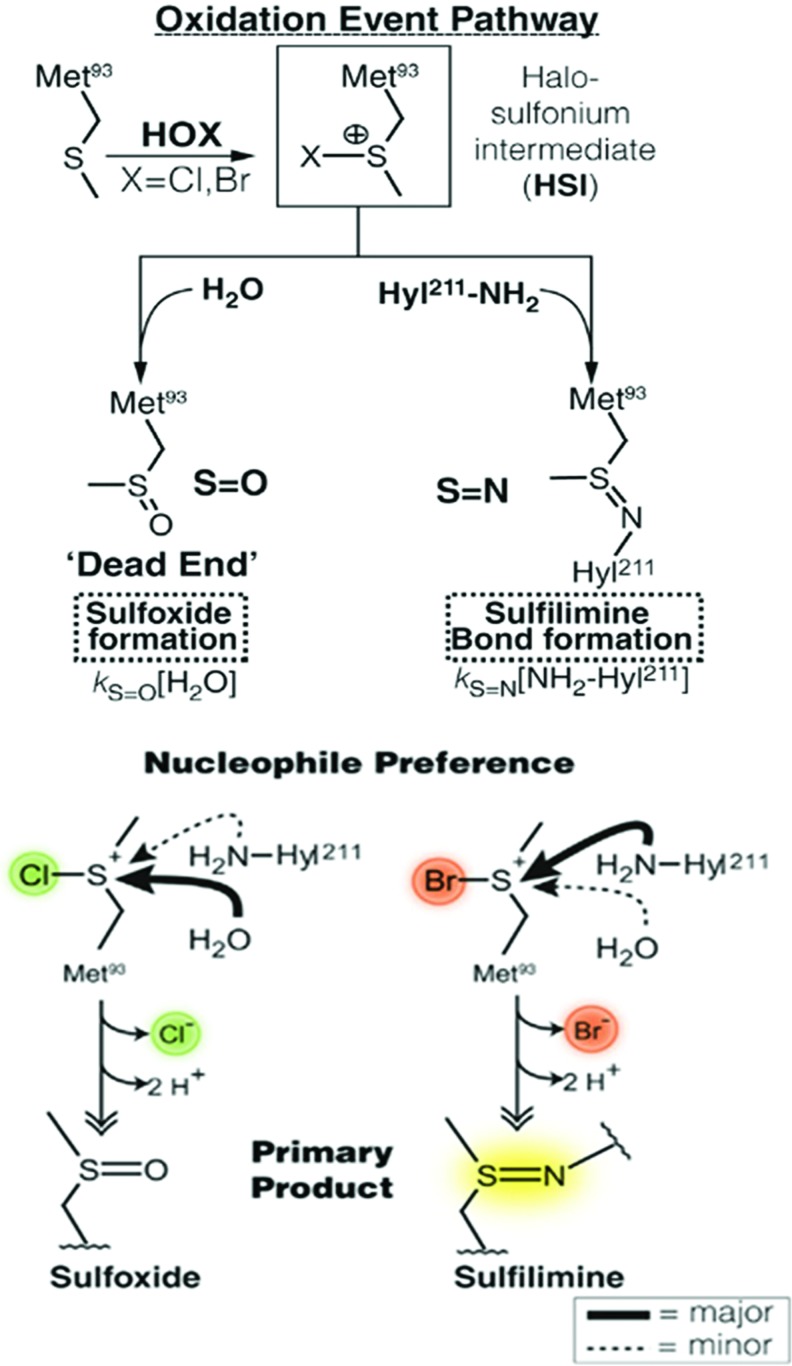
PXDN primarily uses HOBr to form sulfilimine cross-links in collagen IV. When directly applied to solubilized collagen IV in vitro, HOBr vastly outperforms HOCl in the generation of sulfilimine cross-links (10, 72). Thus, as discussed earlier, not only does PXDN probably fail to generate HOCl, but also HOCl poorly cross-links collagen IV. Cells grown in media devoid of Br− fail to cross-link collagen IV, whereas addition of Br− reinstates these cross-links (72). Therefore, PXDN generates HOBr, which acts as a reactive intermediate to catalyze sulfilimine bond formation in collagen IV. The most likely reaction pathway involves the formation of a bromosulfonium ion at methionine 93, which then undergoes nucleophilic attack by the hydroxylysine 211 nitrogen to create the sulfilimine bond and release Br− (Fig. 5). The analogous chlorosulfonium intermediate formed by HOCl undergoes nucleophilic attack by solvent water more readily than the juxtaposed hydroxylysine nitrogen, which results in the formation of methionine sulfoxide, a “dead-end” product (Fig. 6) (72, 110). Notably, addition of SCN− to cultured cells inhibits sulfilimine bond formation in collagen IV (72). Based on kinetic data, SCN− outcompetes Br− for compound I of PXDN, which leads to preferential formation of HOSCN (94). HOSCN poorly reacts with methionine and, therefore, cannot generate sulfilimine cross-links or the dead-end sulfoxide (7, 124).
FIG. 5.
Model for PXDN and HOBr-mediated sulfilimine bond formation in collagen IV. Schematic representation of oxidation of bromide to form HOBr that acts as a reactive intermediate to form sulfilimine cross-links in collagen IV via a bromosulfonium intermediate at methionine 93 of the collagen IV NC1 hexamer. This figure was adapted from work originally published by McCall et al. (72) with permission from Elsevier. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 6.
Preference of HOBr over HOCl for sulfilimine bond formation in collagen IV. A model of the oxidative formation of a sulfilimine bond versus the “dead-end” product methionine sulfoxide. The chlorosulfonium intermediate at methionine 93 preferentially undergoes nucleophilic attack by solvent water, whereas the bromosulfonium intermediate tends to react with the juxtaposed lysine nitrogen. kS = O and kS = N denote rate constants in the formation of sulfoxides and sulfilimines, respectively. This figure was adapted from work originally published by McCall et al. (72) with permission from Elsevier. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Since HOBr is the critical intermediate for sulfilimine bond formation in collagen IV, the trace element bromine may be essential for the maintenance of BM and tissue integrity in animals. Indeed, Drosophila grown without dietary bromine died prematurely because of gut pathology with BM defects very similar to those found in PXDN mutants (72). The phenotypic similarity between bromine and PXDN deficiency suggested that bromine is an essential trace element primarily because it is a required substrate for PXDN to cross-link collagen IV. The significance of bromine to human health is less clear. First, bromine is ubiquitously found in the diet so that only select populations have been found to be bromine deficient, namely dialysis patients and patients on chronic total parenteral nutrition (21, 78, 90). Second, as noted earlier, mice and humans deficient in PXDN only exhibit an eye phenotype with normal BM ultrastructure in kidney, suggesting that bromine deficiency would produce these same limited phenotypes. In contrast, residual sulfilimine cross-links, about one-third normal, are present within collagen IV isolated from PXDN null mice (9). Bromine deficiency may completely abrogate sulfilimine cross-links and thereby lead to greater BM abnormalities than loss of PXDN.
The source of hydrogen peroxide substrate for PXDN to generate HOBr and form cross-links in collagen IV is unclear. TPO, MPO, and EPO clearly have NOX/DUOX isoforms coregulated and spatially localized to provide hydrogen peroxide substrate (82, 112, 143). Since PXDN function is required in nearly all tissues, a single NOX/DUOX isoform is unlikely to couple with PXDN (123). Rather, PXDN may associate with different NOX/DUOX family members in different tissues. Consistent with this notion, PXDN consumed hydrogen peroxide to oxidize an exogenous substrate with coexpression of nearly all NOX proteins (16). As alluded to earlier, diffusion of intracellularly generated hydrogen peroxide may also be a source of hydrogen peroxide substrate for PXDN.
The use of HOBr as a reactive intermediate to form sulfilimine cross-links and assemble BM raised a critical question of how a destructive, highly reactive oxidant could execute this anabolic function without collateral damage to nearby biomolecules. Although the vertebrate animal heme peroxidases are simple enzymes with a single catalytic domain, PXDN is a multidomain protein with N-terminal leucine-rich repeat (LRR) domains, followed by immunoglobulin (Ig) domains, a heme peroxidase catalytic region, and a C-terminal von Willebrand factor type C (vWFC) domain (10, 84). The LRR, Ig, and catalytic domains of PXDN are conserved down to Cnidaria, suggesting that this multidomain structure is critical for function (25, 27, 127). Thus, TPO, LPO, EPO, and MPO evolutionarily arose with the loss of PXDN's noncatalytic domains (Fig. 3). Based on these data, a model is envisioned where the noncatalytic domains of PXDN somehow “target” HOBr to collagen IV and thereby avoid collateral damage to nearby molecules.
Deletion studies of PXDN suggested that the catalytic and Ig domains are required to efficiently cross-link collagen IV. The catalytic domain alone of PXDN and EPO produces HOBr and inefficiently cross-links insoluble collagen IV within BM (25). Although unmeasured, one wonders whether these catalytic enzymes lacking noncatalytic domains also oxidatively damaged nearby BM protein targets with spatially indiscriminate HOBr generation. How does the Ig domain of PXDN promote cross-link formation in collagen IV? The simplest hypothesis is that the Ig domains interact directly with collagen IV to bring the PXDN catalytic domain within spatial proximity of methionine 93 of the collagen IV NC1 domain. Unfortunately, no direct, stable interaction between PXDN and collagen IV has been demonstrated (25). Several possibilities may explain this inability to demonstrate a collagen IV–PXDN interaction. The interaction may be low affinity with transient association of PXDN and collagen IV driven by the high local concentration of collagen IV in the insoluble BM, which has not been recapitulated in vitro. Alternatively, PXDN may associate with a bridging molecule such as perlecan or nidogen to allow PXDN to reside in close quarters with its collagen IV substrate. The last possible hypothesis is that PXDN generates HOBr within the general vicinity of the collagen IV NC1 domain with some low level of “acceptable” collateral oxidative damage.
PXDN exhibits a relatively low catalytic efficiency (kcat/KM) of bromide oxidation to HOBr compared with EPO and MPO (94). The catalytic efficiency is increased with a truncated PXDN construct containing only the Ig and heme peroxidase domains (94, 126), suggesting that the LRR and/or the vWFC domains may “auto-inhibit” catalytic activity. Indeed, proprotein convertase processing of PXDN with resulting loss of the vWFC domain enhances PXDN-mediated HOBr generation (20). Whether proteolytic processing or conformational changes involving the LRR domain also activate PXDN is unknown. Taken together, these data suggest that PXDN is a modest generator of HOBr with tight internal controls of activity and spatial localization dictated by its noncatalytic domains to minimize collateral oxidative damage. Conversely, the vertebrate animal heme peroxidases, particularly EPO and MPO, have unencumbered catalytic domains that generate robust bursts of hypohalous acids to destroy pathogens as part of the innate immune system.
The discovery of PXDN arose from a search for the catalytic activity responsible for the formation of sulfilimine cross-links in collagen IV. However, PXDN may generate HOBr to subserve other roles in normal physiology or disease. Invertebrates lack leukocytes and their heme peroxidases (e.g., EPO and MPO) and, therefore, could use PXDN in a dual role to generate HOBr to cross-link collagen IV and defend against foreign pathogens. Host responses to pathogens may increase H2O2 generation and upregulate PXDN so that it generates lethal quantities of HOBr to destroy pathogens rather than cross-link collagen IV. Indeed, mosquito PXDN is upregulated in the gut after bacterial infection and knockdown slows pathogen clearance and decreases survival (33). Similarly, in human plasma, the presence of circulating PXDN capable of producing microbicidal levels of hypohalous acids has been reported (67).
Dissociation of PXDN-generated HOBr from collagen IV sulfilimine bond formation may occur in mammalian disease. A series of studies on PXDN, referred to as vascular peroxidase 1 given its high expression in cardiac and vascular tissue (16), has examined its role in vascular disease. These studies demonstrate that PXDN generates hypohalous acids, which oxidize lipoproteins and endothelial targets to promote endothelial dysfunction and atherosclerosis (5, 70, 95, 121, 129, 155–158). Similarly, Péterfi et al. have demonstrated that transforming growth factor beta upregulates PXDN expression in fibroblasts in vitro and in vivo within interstitial regions in the unilateral ureteral obstruction model of renal fibrosis (97). As discussed earlier, collagen IV is scarcely found in interstitial matrix. Thus, the pathological expression of PXDN within interstitial matrix could allow it to generate HOBr to drive oxidant injury rather than cross-link collagen IV. Taken together, derangements in PXDN expression, post-translational processing, and/or localization have the potential to decouple PXDN-mediated HOBr generation from collagen IV, leading to oxidative tissue injury rather than assembly and repair.
Conclusions
BMs are specialized forms of ECM that underlie and, therefore, intimately associate with epithelial, endothelial, and muscle cells to determine their function. Thus, BM homeostasis is critical for tissue genesis and integrity. Hypohalous acids generated by PXDN located within the BM itself or MPO and EPO from infiltrating leukocytes can target BM proteins for oxidative modification. Although the vast majority of these modifications are functionally detrimental or neutral, the formation of sulfilimine cross-links in collagen IV by HOBr suggests that some modifications may support BM integrity and function. Future work will need to define specific sites of modification on BM proteins and determine their functional significance to address their role in normal BM function and dysfunction in disease.
Abbreviations Used
- BMs
basement membranes
- DUOX
dual oxidase
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EPO
eosinophil peroxidase
- FS
follistatin-like
- FZC18
frizzled domain
- G domains
globular-like domains
- GAGs
glycosaminoglycans
- GBM
glomerular basement membrane
- H2O2
hydrogen peroxide
- HOBr
hypobromous acid
- HOCl
hypochlorous acid
- HOSCN
hypothiocyanous acid
- HOX
hypohalous acid
- HS
heparan sulfate
- HSI
halosulfonium intermediate
- HSPGs
heparan sulfate proteoglycans
- Ig
immunoglobulin
- kDa
kilodalton
- LDL
low-density lipoprotein
- LE
laminin-type epidermal growth factor-like repeats
- LG
laminin globular
- LN domain
laminin N-terminal domain
- LPO
lactoperoxidase
- LRR
leucine-rich repeat
- MMPs
matrix metalloproteases
- MPO
myeloperoxidase
- N-CAM
neural cell adhesion molecule
- NC1
noncollagenous domain
- NOX
NADPH oxidase
- NtA
N-terminal-agrin
- PXDN
peroxidasin
- S
N-sulfilimine bond
- S/T
Ser/Thr
- SEA
sea urchin sperm protein, enterokinase, and agrin
- TIMPs
tissue inhibitor of metalloproteases
- TPO
thyroid peroxidase
- Tsp1
trombospondin-1-like
- vWFC
von Willebrand factor type C
Acknowledgments
This work was supported by NIH grant K08 DK 097306, a Burroughs-Wellcome Fund Career Award for Medical Scientists (13030995), and developmental funds from the Vanderbilt University Medical Center Division of Nephrology to G.B.
References
- 1.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, and St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol 20: 1471–1479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anazco C, Lopez-Jimenez AJ, Rafi M, Vega-Montoto L, Zhang MZ, Hudson BG, and Vanacore RM. Lysyl Oxidase-like-2 Cross-links Collagen IV of Glomerular Basement Membrane. J Biol Chem 291: 25999–26012, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, and Yurchenco PD. A simplified laminin nomenclature. Matrix Biol 24: 326–332, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bafort F, Parisi O, Perraudin JP, and Jijakli MH. Mode of action of lactoperoxidase as related to its antimicrobial activity: a review. Enzyme Res 2014: 517164, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai YP, Hu CP, Yuan Q, Peng J, Shi RZ, Yang TL, Cao ZH, Li YJ, Cheng G, and Zhang GG. Role of VPO1, a newly identified heme-containing peroxidase, in ox-LDL induced endothelial cell apoptosis. Free Radic Biol Med 51: 1492–1500, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, and Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest 108: 1759–1770, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beal JL, Foster SB, and Ashby MT. Hypochlorous acid reacts with the N-terminal methionines of proteins to give dehydromethionine, a potential biomarker for neutrophil-induced oxidative stress. Biochemistry 48: 11142–11148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezakova G. and Ruegg MA. New insights into the roles of agrin. Nat Rev Mol Cell Biol 4: 295–308, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bhave G, Colon S, and Ferrell N. The Sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. Am J Physiol Renal Physiol: ajprenal. 00096.2017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-00 IA, Rafi M, Kang J-S, Pedchenko V, Fessler LI, Fessler JH, and Hudson BG. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8: 784–790, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnans C, Chou J, and Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786–801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, and Hudson BG. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem 275: 30716–30724, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Brown KL, Darris C, Rose KL, Sanchez OA, Madu H, Avance J, Brooks N, Zhang MZ, Fogo A, Harris R, Hudson BG, and Voziyan P. Hypohalous acids contribute to renal extracellular matrix damage in experimental diabetes. Diabetes 64: 2242–2253, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows B, Niden AH, and Barclay WR. Goiter and myxedema due to iodide administration. Ann Intern Med 52: 858–870, 1960 [DOI] [PubMed] [Google Scholar]
- 15.Buss IH, Senthilmohan R, Darlow BA, Mogridge N, Kettle AJ, and Winterbourn CC. 3-Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: association with adverse respiratory outcome. Pediatr Res 53: 455–462, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Cheng G, Salerno JC, Cao Z, Pagano PJ, and Lambeth JD. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic Biol Med 45: 1682–1694, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang CY, Degendorfer G, and Davies MJ. Oxidation and modification of extracellular matrix and its role in disease. Free Radic Res 48: 970–989, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Chuang CY, Degendorfer G, Hammer A, Whitelock JM, Malle E, and Davies MJ. Oxidation modifies the structure and function of the extracellular matrix generated by human coronary artery endothelial cells. Biochem J 459: 313–322, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Clause KC. and Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol 24: 830–833, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colon S. and Bhave G. Proprotein Convertase Processing Enhances Peroxidasin Activity to Reinforce Collagen IV. J Biol Chem 291: 24009–24016, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlstrom KA, Ament ME, Medhin MG, and Meurling S. Serum trace elements in children receiving long-term parenteral nutrition. J Pediatr 109: 625–630, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Davies MJ, Hawkins CL, Pattison DI, and Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 1199–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 23.De SK. and Banerjee RK. Purification, characterization and origin of rat gastric peroxidase. Eur J Biochem 160: 319–325, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Douglass S, Goyal A, and Iozzo RV. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect Tissue Res 56: 381–391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ero-Tolliver IA, Hudson BG, and Bhave G. The Ancient Immunoglobulin Domains of Peroxidasin Are Required to Form Sulfilimine Cross-links in Collagen IV. J Biol Chem 290: 21741–21748, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farach-Carson MC, Warren CR, Harrington DA, and Carson DD. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol 34: 64–79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, Bhave G, Yin VP, Stothers CL, Rose KL, McDonald WH, Clark TA, Borza DB, Steele RE, Ivy MT, Aspirnauts , Hudson JK, and Hudson BG. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc Natl Acad Sci U S A 111: 331–336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, Foster PS, and Hogan SP. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol 172: 5664–5675, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Frantz C, Stewart KM, and Weaver VM. The extracellular matrix at a glance. J Cell Sci 123: 4195–4200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu X, Kassim SY, Parks WC, and Heinecke JW. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J Biol Chem 278: 28403–28409, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Fu X, Kassim SY, Parks WC, and Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem 276: 41279–41287, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Fukai T. and Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garver LS, Xi Z, and Dimopoulos G. Immunoglobulin superfamily members play an important role in the mosquito immune system. Dev Comp Immunol 32: 519–531, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotenstein JR, Swale RE, Fukuda T, Wu Z, Giurumescu CA, Goncharov A, Jin Y, and Chisholm AD. The C. elegans peroxidasin PXN-2 is essential for embryonic morphogenesis and inhibits adult axon regeneration. Development 137: 3603–3613, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gubbiotti MA, Neill T, and Iozzo RV. A current view of perlecan in physiology and pathology: a mosaic of functions. Matrix Biol 57–58: 285–298, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, and Hudson BG. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J Biol Chem 273: 8767–8775, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Gunwar S, Bejarano PA, Kalluri R, Langeveld JP, Wisdom BJ, Jr., Noelken ME, and Hudson BG. Alveolar basement membrane: molecular properties of the noncollagenous domain (hexamer) of collagen IV and its reactivity with Goodpasture autoantibodies. Am J Respir Cell Mol Biol 5: 107–112, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Haigo SL. and Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331: 1071–1074, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halfter W, Dong SC, Schurer B, and Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem 273: 25404–25412, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Halfter W, Oertle P, Monnier CA, Camenzind L, Reyes-Lua M, Hu H, Candiello J, Labilloy A, Balasubramani M, Henrich PB, and Plodinec M. New concepts in basement membrane biology. FEBS J 282: 4466–4479, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Halliwell B. and Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, United Kingdom: Oxford University Press, 2015 [Google Scholar]
- 42.Hawkins CL. and Davies MJ. Inactivation of protease inhibitors and lysozyme by hypochlorous acid: role of side-chain oxidation and protein unfolding in loss of biological function. Chem Res Toxicol 18: 1600–1610, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Hawkins CL. and Davies MJ. The role of aromatic amino acid oxidation, protein unfolding, and aggregation in the hypobromous acid-induced inactivation of trypsin inhibitor and lysozyme. Chem Res Toxicol 18: 1669–1677, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Hazen SL. and Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 99: 2075–2081, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinecke JW. The role of myeloperoxidase in HDL oxidation and atherogenesis. Curr Atheroscler Rep 9: 249–251, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Himmelfarb J, McMenamin ME, Loseto G, and Heinecke JW. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic Biol Med 31: 1163–1169, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Ho MSP, Böse K, Mokkapati S, Nischt R, and Smyth N. Nidogens—Extracellular matrix linker molecules. Microsc Res Tech 71: 387–395, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Holzbecher J. and Ryan DE. The rapid determination of total bromine and iodine in biological fluids by neutron activation. Clin Biochem 13: 277–278, 1980 [DOI] [PubMed] [Google Scholar]
- 49.Hosoya T, Sasaki K, and Wagai N. Spectroscopic and kinetic properties of the estrogen-induced peroxidase in rat uterine fluid. J Biochem 89: 1453–1463, 1981 [DOI] [PubMed] [Google Scholar]
- 50.Hudson BG, Tryggvason K, Sundaramoorthy M, and Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Huze C, Bauche S, Richard P, Chevessier F, Goillot E, Gaudon K, Ben Ammar A, Chaboud A, Grosjean I, Lecuyer HA, Bernard V, Rouche A, Alexandri N, Kuntzer T, Fardeau M, Fournier E, Brancaccio A, Ruegg MA, Koenig J, Eymard B, Schaeffer L, and Hantai D. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet 85: 155–167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol 196: 671–679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hynes RO. Extracellular matrix: not just pretty fibrils. Science (New York, NY) 326: 1216–1219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Ihalin R, Loimaranta V, and Tenovuo J. Origin, structure, and biological activities of peroxidases in human saliva. Arch Biochem Biophys 445: 261–268, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Iozzo RV. and Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11–55, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson RJ, Couser WG, Chi EY, Adler S, and Klebanoff SJ. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest 79: 1379–1387, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph P, Srinivasan NS, and Kulkarni AP. Placental peroxidase—further purification of the enzyme and oxidation of thiobenzamide. Placenta 14: 309–319, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Kahsai TZ, Enders GC, Gunwar S, Brunmark C, Wieslander J, Kalluri R, Zhou J, Noelken ME, and Hudson BG. Seminiferous tubule basement membrane. Composition and organization of type IV collagen chains, and the linkage of alpha3(IV) and alpha5(IV) chains. J Biol Chem 272: 17023–17032, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Kalluri R, Gattone VH, 2nd, and Hudson BG. Identification and localization of type IV collagen chains in the inner ear cochlea. Connect Tissue Res 37: 143–150, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Khan K, Rudkin A, Parry DA, Burdon KP, McKibbin M, Logan CV, Abdelhamed ZI, Muecke JS, Fernandez-Fuentes N, Laurie KJ, Shires M, Fogarty R, Carr IM, Poulter JA, Morgan JE, Mohamed MD, Jafri H, Raashid Y, Meng N, Piseth H, Toomes C, Casson RJ, Taylor GR, Hammerton M, Sheridan E, Johnson CA, Inglehearn CF, Craig JE, and Ali M. Homozygous mutations in PXDN cause congenital cataract, corneal opacity, and developmental glaucoma. Am J Hum Genet 89: 464–473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khoshnoodi J, Pedchenko V, and Hudson BG. Mammalian collagen IV. Microsc Res Tech 71: 357–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimura S. and Jellinck PH. Studies on mammalian intestinal peroxidase. Biochem J 205: 271–279, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lambeth JD. and Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol 9: 119–145, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Laurila P. and Leivo I. Basement membrane and interstitial matrix components form separate matrices in heterokaryons of PYS-2 cells and fibroblasts. J Cell Sci 104 (Pt 1): 59–68, 1993 [DOI] [PubMed] [Google Scholar]
- 66.Li D, Clark CC, and Myers JC. Basement membrane zone type XV collagen is a disulfide-bonded chondroitin sulfate proteoglycan in human tissues and cultured cells. J Biol Chem 275: 22339–22347, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Li H, Cao Z, Moore DR, Jackson PL, Barnes S, Lambeth JD, Thannickal VJ, and Cheng G. Microbicidal activity of vascular peroxidase 1 in human plasma via generation of hypochlorous acid. Infect Immun 80: 2528–2537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H, Cao Z, Zhang G, Thannickal VJ, and Cheng G. Vascular peroxidase 1 catalyzes the formation of hypohalous acids: characterization of its substrate specificity and enzymatic properties. Free Radic Biol Med 53: 1954–1959, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Little AG. and Seebacher F. The evolution of endothermy is explained by thyroid hormone-mediated responses to cold in early vertebrates. J Exp Biol 217: 1642–1648, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Liu Y, Xu Q, Peng H, Tang Y, Yang T, Yu Z, Cheng G, Zhang G, and Shi R. Critical role of vascular peroxidase 1 in regulating endothelial nitric oxide synthase. Redox Biol 12: 226–232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lloyd MM, van Reyk DM, Davies MJ, and Hawkins CL. Hypothiocyanous acid is a more potent inducer of apoptosis and protein thiol depletion in murine macrophage cells than hypochlorous acid or hypobromous acid. Biochem J 414: 271–280, 2008 [DOI] [PubMed] [Google Scholar]
- 72.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, and Hudson BG. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157: 1380–1392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCarthy KJ. The Basement Membrane Proteoglycans Perlecan and Agrin: something Old, Something New. Curr Top Membr 76: 255–303, 2015 [DOI] [PubMed] [Google Scholar]
- 74.McMillen TS, Heinecke JW, and LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation 111: 2798–2804, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Michaelis J, Vissers MC, and Winterbourn CC. Different effects of hypochlorous acid on human neutrophil metalloproteinases: activation of collagenase and inactivation of collagenase and gelatinase. Arch Biochem Biophys 292: 555–562, 1992 [DOI] [PubMed] [Google Scholar]
- 76.Miner JH. The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miner JH. Laminins and their roles in mammals. Microsc Res Tech 71: 349–356, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Miura Y, Nakai K, Suwabe A, and Sera K. Trace elements in renal disease and hemodialysis. Nucl Instrum Meth B 189: 443–449, 2002 [Google Scholar]
- 79.Morgan PE, Pattison DI, Talib J, Summers FA, Harmer JA, Celermajer DS, Hawkins CL, and Davies MJ. High plasma thiocyanate levels in smokers are a key determinant of thiol oxidation induced by myeloperoxidase. Free Radic Biol Med 51: 1815–1822, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Morris JC. Acid ionization constant of HOCl from 5 to 35 degrees. J Phys Chem 70: 3798–3805, 1966 [Google Scholar]
- 81.Naba A. and Hynes RO. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4: a004903, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nauseef WM. Myeloperoxidase in human neutrophil host defence. Cell Microbiol 16: 1146–1155, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nauseef WM. and Borregaard N. Neutrophils at work. Nat Immunol 15: 602–611, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, and Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J 13: 3438–3447, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nicole S, Chaouch A, Torbergsen T, Bauche S, de Bruyckere E, Fontenille MJ, Horn MA, van Ghelue M, Loseth S, Issop Y, Cox D, Muller JS, Evangelista T, Stalberg E, Ioos C, Barois A, Brochier G, Sternberg D, Fournier E, Hantai D, Abicht A, Dusl M, Laval SH, Griffin H, Eymard B, and Lochmuller H. Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain 137: 2429–2443, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Noelken ME, Wisdom BJ, Jr, Dean DC, Hung CH, and Hudson BG. Intestinal basement membrane of Ascaris suum. Molecular organization and properties of the collagen molecules. J Biol Chem 261: 4706–4714, 1986 [PubMed] [Google Scholar]
- 87.Obinger C. Chemistry and biology of human peroxidases. Arch Biochem Biophys 445: 197–198, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Odobasic D, Kitching AR, and Holdsworth SR. Neutrophil-mediated regulation of innate and adaptive immunity: the role of myeloperoxidase. J Immunol Res 2016: 2349817, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Odobasic D, Kitching AR, Semple TJ, and Holdsworth SR. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol 18: 760–770, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Oe PL, Vis RD, Meijer JH, Vanlangevelde F, Allon W, Vandermeer C, and Verheul H. Adding of bromine to dialysate to improve quality of sleep of patients on hemodialysis. Artif Organs 5: 200–200, 1981 [Google Scholar]
- 91.Oh SP, Kamagata Y, Muragaki Y, Timmons S, Ooshima A, and Olsen BR. Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc Natl Acad Sci U S A 91: 4229–4233, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pattison DI. and Davies MJ. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem 13: 3271–3290, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Pattison DI, Davies MJ, and Hawkins CL. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic Res 46: 975–995, 2012 [DOI] [PubMed] [Google Scholar]
- 94.Paumann-Page M, Katz RS, Bellei M, Schwartz I, Edenhofer E, Sevcnikar B, Soudi M, Hofbauer S, Battistuzzi G, Furtmuller PG, and Obinger C. Pre-steady-state kinetics reveal the substrate specificity and mechanism of halide oxidation of truncated human peroxidasin 1. J Biol Chem 292: 4583–4592, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng H, Chen L, Huang X, Yang T, Yu Z, Cheng G, Zhang G, and Shi R. Vascular peroxidase 1 up regulation by angiotensin II attenuates nitric oxide production through increasing asymmetrical dimethylarginine in HUVECs. J Am Soc Hypertens 10: 741–751 e743, 2016 [DOI] [PubMed] [Google Scholar]
- 96.Peppin GJ. and Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci U S A 83: 4322–4326, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Péterfi Z, Donkó Á, Orient A, Sum A, Prókai Á, Molnár B, Veréb Z, Rajnavölgyi É, Kovács KJ, Müller V, Szabó AJ, and Geiszt M. Peroxidasin is secreted and incorporated into the extracellular matrix of myofibroblasts and fibrotic kidney. Am J Pathol 175: 725–735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peterfi Z, Toth ZE, Kovacs HA, Lazar E, Sum A, Donko A, Sirokmany G, Shah AM, and Geiszt M. Peroxidasin-like protein: a novel peroxidase homologue in the human heart. Cardiovasc Res 101: 393–399, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, and Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131: 1619–1628, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Price RG. and Spiro RG. Studies on the metabolism of the renal glomerular basement membrane. Turnover measurements in the rat with the use of radiolabeled amino acids. J Biol Chem 252: 8597–8602, 1977 [PubMed] [Google Scholar]
- 101.Prutz WA, Kissner R, Koppenol WH, and Ruegger H. On the irreversible destruction of reduced nicotinamide nucleotides by hypohalous acids. Arch Biochem Biophys 380: 181–191, 2000 [DOI] [PubMed] [Google Scholar]
- 102.Ramchandran R, Dhanabal M, Volk R, Waterman MJ, Segal M, Lu H, Knebelmann B, and Sukhatme VP. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem Biophys Res Commun 255: 735–739, 1999 [DOI] [PubMed] [Google Scholar]
- 103.Rayner BS, Love DT, and Hawkins CL. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radic Biol Med 71: 240–255, 2014 [DOI] [PubMed] [Google Scholar]
- 104.Rees MD, McNiven TN, and Davies MJ. Degradation of extracellular matrix and its components by hypobromous acid. Biochem J 401: 587–596, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rees MD, Whitelock JM, Malle E, Chuang CY, Iozzo RV, Nilasaroya A, and Davies MJ. Myeloperoxidase-derived oxidants selectively disrupt the protein core of the heparan sulfate proteoglycan perlecan. Matrix Biol 29: 63–73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reiland J, Sanderson RD, Waguespack M, Barker SA, Long R, Carson DD, and Marchetti D. Heparanase degrades syndecan-1 and perlecan heparan sulfate: functional implications for tumor cell invasion. J Biol Chem 279: 8047–8055, 2004 [DOI] [PubMed] [Google Scholar]
- 107.Ricard-Blum S. The Collagen Family. Cold Spring Harb Perspect Biol 3: a004978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Riedle B. and Kerjaschki D. Reactive oxygen species cause direct damage of Engelbreth-Holm-Swarm matrix. Am J Pathol 151: 215–231, 1997 [PMC free article] [PubMed] [Google Scholar]
- 109.Risteli J, Bachinger HP, Engel J, Furthmayr H, and Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem 108: 239–250, 1980 [DOI] [PubMed] [Google Scholar]
- 110.Ronsein GE, Winterbourn CC, Di Mascio P, and Kettle AJ. Cross-linking methionine and amine residues with reactive halogen species. Free Radic Biol Med 70: 278–287, 2014 [DOI] [PubMed] [Google Scholar]
- 111.Rozario T. and DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 341: 126–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ruf J. and Carayon P. Structural and functional aspects of thyroid peroxidase. Arch Biochem Biophys 445: 269–277, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, and Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol 153: 611–626, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saito K, Yonezawa T, Minaguchi J, Kurosaki M, Suetsugu S, Nakajima A, Nomoto H, Morizane Y, Sado Y, Sugimoto M, Kusachi S, and Ninomiya Y. Distribution of alpha(IV) collagen chains in the ocular anterior segments of adult mice. Connect Tissue Res 52: 147–156, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Sarrazin S, Lamanna WC, and Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3: a004952, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seifter JL. Integration of acid-base and electrolyte disorders. N Engl J Med 371: 1821–1831, 2014 [DOI] [PubMed] [Google Scholar]
- 117.Seppinen L. and Pihlajaniemi T. The multiple functions of collagen XVIII in development and disease. Matrix Biol 30: 83–92, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Sertie AL, Sossi V, Camargo AA, Zatz M, Brahe C, and Passos-Bueno MR. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Hum Mol Genet 9: 2051–2058, 2000 [DOI] [PubMed] [Google Scholar]
- 119.Shao B, Oda MN, Oram JF, and Heinecke JW. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem Res Toxicol 23: 447–454, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shao B, Tang C, Heinecke JW, and Oram JF. Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res 51: 1849–1858, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shi R, Hu C, Yuan Q, Yang T, Peng J, Li Y, Bai Y, Cao Z, Cheng G, and Zhang G. Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc Res 91: 27–36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shoulders MD. and Raines RT. Collagen structure and stability. Annu Rev Biochem 78: 929–958, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sirokmany G, Donko A, and Geiszt M. Nox/Duox family of NADPH oxidases: lessons from knockout mouse models. Trends Pharmacol Sci 37: 318–327, 2016 [DOI] [PubMed] [Google Scholar]
- 124.Skaff O, Pattison DI, and Davies MJ. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: absolute rate constants and assessment of biological relevance. Biochem J 422: 111–117, 2009 [DOI] [PubMed] [Google Scholar]
- 125.Smyth N, Vatansever HS, Meyer M, Frie C, Paulsson M, and Edgar D. The targeted deletion of the LAMC1 gene. Ann N Y Acad Sci 857: 283–286, 1998 [DOI] [PubMed] [Google Scholar]
- 126.Soudi M, Paumann-Page M, Delporte C, Pirker KF, Bellei M, Edenhofer E, Stadlmayr G, Battistuzzi G, Boudjeltia KZ, Furtmuller PG, Van Antwerpen P, and Obinger C. Multidomain human peroxidasin 1 is a highly glycosylated and stable homotrimeric high spin ferric peroxidase. J Biol Chem 290: 10876–10890, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soudi M, Zamocky M, Jakopitsch C, Furtmuller PG, and Obinger C. Molecular evolution, structure, and function of peroxidasins. Chem Biodivers 9: 1776–1793, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takagi J. Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins. Biochem Soc Trans 32: 403–406, 2004 [DOI] [PubMed] [Google Scholar]
- 129.Tang Y, Xu Q, Peng H, Liu Z, Yang T, Yu Z, Cheng G, Li X, Zhang G, and Shi R. The role of vascular peroxidase 1 in ox-LDL-induced vascular smooth muscle cell calcification. Atherosclerosis 243: 357–363, 2015 [DOI] [PubMed] [Google Scholar]
- 130.Thomas EL. Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibria between oxidized forms of thiocyanate. Biochemistry 20: 3273–3280, 1981 [DOI] [PubMed] [Google Scholar]
- 131.Thomson E, Brennan S, Senthilmohan R, Gangell CL, Chapman AL, Sly PD, Kettle AJ, Australian Respiratory Early Surveillance Team for Cystic F, Balding E, Berry LJ, Carlin JB, Carzino R, de Klerk N, Douglas T, Foo C, Garratt LW, Hall GL, Harrison J, Kicic A, Laing IA, Logie KM, Massie J, Mott LS, Murray C, Parsons F, Pillarisetti N, Poreddy SR, Ranganathan SC, Robertson CF, Robins-Browne R, Robinson PJ, Skoric B, Stick SM, Sutanto EN, and Williamson E. Identifying peroxidases and their oxidants in the early pathology of cystic fibrosis. Free Radic Biol Med 49: 1354–1360, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Trier JS, Allan CH, Abrahamson DR, and Hagen SJ. Epithelial basement membrane of mouse jejunum. Evidence for laminin turnover along the entire crypt-villus axis. J Clin Invest 86: 87–95, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsuge K, Kataoka M, and Seto Y. Cyanide and thiocyanate levels in blood and saliva of healthy adult volunteers. J Health Sci 46: 343–350, 2000 [Google Scholar]
- 134.van Dalen CJ, Aldridge RE, Chan T, Senthilmohan R, Hancox RJ, Cowan JO, Taylor DR, Town GI, and Kettle AJ. Bromotyrosines in sputum proteins and treatment effects of terbutaline and budesonide in asthma. Ann Allergy Asthma Immunol 103: 348–353, 2009 [DOI] [PubMed] [Google Scholar]
- 135.van Leeuwen FX. and Sangster B. The toxicology of bromide ion. Crit Rev Toxicol 18: 189–213, 1987 [DOI] [PubMed] [Google Scholar]
- 136.Vanacore R, Ham AJL, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, and Hudson BG. A sulfilimine bond identified in collagen IV. Science 325: 1230–1234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vissers MC. and Thomas C. Hypochlorous acid disrupts the adhesive properties of subendothelial matrix. Free Radic Biol Med 23: 401–411, 1997 [DOI] [PubMed] [Google Scholar]
- 138.Vissers MC. and Winterbourn CC. The effect of oxidants on neutrophil-mediated degradation of glomerular basement membrane collagen. Biochim Biophys Acta 889: 277–286, 1986 [DOI] [PubMed] [Google Scholar]
- 139.Vissers MC. and Winterbourn CC. Oxidative damage to fibronectin. I. The effects of the neutrophil myeloperoxidase system and HOCl. Arch Biochem Biophys 285: 53–59, 1991 [DOI] [PubMed] [Google Scholar]
- 140.Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, and Azar DT. Endostatin's emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta 1850: 2422–2438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Walker F. Basement‐membrane turnover in man. J Pathol 107: 123–125, 1972 [DOI] [PubMed] [Google Scholar]
- 142.Walker F. Basement‐membrane turnover in the rat. J Pathol 107: 119–121, 1972 [DOI] [PubMed] [Google Scholar]
- 143.Wang J. and Slungaard A. Role of eosinophil peroxidase in host defense and disease pathology. Arch Biochem Biophys 445: 256–260, 2006 [DOI] [PubMed] [Google Scholar]
- 144.Wang Y, Rosen H, Madtes DK, Shao B, Martin TR, Heinecke JW, and Fu X. Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: an oxidative mechanism for regulating proteolysis during inflammation. J Biol Chem 282: 31826–31834, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weiss SJ, Curnutte JT, and Regiani S. Neutrophil-mediated solubilization of the subendothelial matrix: oxidative and nonoxidative mechanisms of proteolysis used by normal and chronic granulomatous disease phagocytes. J Immunol 136: 636–641, 1986 [PubMed] [Google Scholar]
- 146.Weiss SJ, Peppin G, Ortiz X, Ragsdale C, and Test ST. Oxidative autoactivation of latent collagenase by human neutrophils. Science 227: 747–749, 1985 [DOI] [PubMed] [Google Scholar]
- 147.Whittaker CA, Bergeron K-F, Whittle J, Brandhorst BP, Burke RD, and Hynes RO. The echinoderm adhesome. Dev Biol 300: 252–266, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008 [DOI] [PubMed] [Google Scholar]
- 149.Winterbourn CC. and Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med 29: 403–409, 2000 [DOI] [PubMed] [Google Scholar]
- 150.Woods AA. and Davies MJ. Fragmentation of extracellular matrix by hypochlorous acid. Biochem J 376: 219–227, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Woods AA, Linton SM, and Davies MJ. Detection of HOCl-mediated protein oxidation products in the extracellular matrix of human atherosclerotic plaques. Biochem J 370: 729–735, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu W, Chen Y, d'Avignon A, and Hazen SL. 3-Bromotyrosine and 3,5-dibromotyrosine are major products of protein oxidation by eosinophil peroxidase: potential markers for eosinophil-dependent tissue injury in vivo. Biochemistry 38: 3538–3548, 1999 [DOI] [PubMed] [Google Scholar]
- 153.Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, and Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest 105: 1455–1463, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan X, Sabrautzki S, Horsch M, Fuchs H, Gailus-Durner V, Beckers J, Hrabe de Angelis M, and Graw J. Peroxidasin is essential for eye development in the mouse. Hum Mole Genet 23: 5597–5614, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang L, Bai Y, Li N, Hu C, Peng J, Cheng G, Zhang G, and Shi R. Vascular VPO1 expression is related to the endothelial dysfunction in spontaneously hypertensive rats. Biochem Biophys Res Commun 439: 511–516, 2013 [DOI] [PubMed] [Google Scholar]
- 156.Yang W, Liu Z, Xu Q, Peng H, Chen L, Huang X, Yang T, Yu Z, Cheng G, Zhang G, and Shi R. Involvement of vascular peroxidase 1 in angiotensin II-induced hypertrophy of H9c2 cells. J Am Soc Hypertens 2016. [Epub ahead of print]; DOI: 10.1016/j.jash.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 157.Yang Y, Cao Z, Tian L, Garvey WT, and Cheng G. VPO1 mediates ApoE oxidation and impairs the clearance of plasma lipids. PLoS One 8: e57571, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang Y, Shi R, Cao Z, Zhang G, and Cheng G. VPO1 mediates oxidation of LDL and formation of foam cells. Oncotarget 7: 35500–35511, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yi MC. and Khosla C. Thiol-disulfide exchange reactions in the mammalian extracellular environment. Annu Rev Chem Biomol Eng 7: 197–222, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3: a004911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yurchenco PD. Integrating activities of laminins that drive basement membrane assembly and function. Curr Top Membr 76: 1–30, 2015 [DOI] [PubMed] [Google Scholar]
- 162.Yurchenco PD. and Ruben GC. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol 105: 2559–2568, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zamocky M, Jakopitsch C, Furtmuller PG, Dunand C, and Obinger C. The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins 72: 589–605, 2008 [DOI] [PubMed] [Google Scholar]