Abstract
Objective: This study's objective was to determine whether two distinct carbohydrate (CHO)-modified diets and a standard portion-controlled (PC) diet differentially impacted children's eating behaviors and whether eating behavior scores predicted lower BMI among children with obesity.
Methods: Children (n = 102) aged 7–12 years with obesity were randomly assigned to a 12-month intervention of a low-carbohydrate (LC), reduced glycemic load (RGL), or standard PC diet. The Three-Factor Eating Questionnaire (TFEQ) was completed at baseline, 3, 6, and 12 months by parents to characterize their child's hunger (H), disinhibition (D), and cognitive restraint (CR). Baseline and follow-up TFEQ scores by diet were evaluated relative to BMI status over time.
Results: All diet groups showed increased CR and decreased H and D from baseline to 3 months, with differences from baseline remaining at 12 months for CR and H. Lower BMI status during study follow-up was associated with different TFEQ scores by diet group (LC and RGL: higher CR; PC: lower H), adjusting for sex, age, and race. Higher CR at follow-up was predicted by race and higher baseline CR; only lower H at baseline predicted lower H at follow-up.
Conclusion: Eating behaviors improved significantly with all diets during the initial 3 months; higher CR and lower H were sustained at treatment's end. BMI outcomes were associated with different eating behaviors in CHO-modified diet groups compared with PC diets. Targeting diets of children with obesity with specific baseline characteristics may lead to improved outcomes.
Keywords: : dietary intake, eating behaviors, pediatric obesity, weight management
Introduction
Obesity among American youth continues to be a major public health concern, with an estimated prevalence of 17.0% among 2- to 19-year-olds from 2011 through 2014.1 Pediatric obesity has been shown to be managed most effectively when interventions with multiple components, such as dietary changes, physical activity, supportive parental involvement, and behavioral strategies, are included.2 While a calorie-restricted low-fat diet has been a widely promoted dietary strategy, our recent findings suggest that a variety of dietary approaches [e.g., low-carbohydrate (LC), reduced glycemic load (RGL), and nutrient-balanced portion-controlled (PC) diets] can be similarly effective in helping youth with overweight and obesity improve their weight status.3 However, it is not known how these different dietary approaches impact children's eating behaviors within the context of a multicomponent pediatric weight management intervention. Understanding if initial eating behaviors or change in these eating behaviors during treatment predicts child weight status changes or differs based on the treatment approach has implications for matching children to treatment approach and/or tailoring approaches for maximum efficacy. Indeed, given that a variety of dietary approaches appear to have similar average efficacy, more investigation is needed to identify individual-level factors that result in a specific dietary approach yielding the best outcomes.
One set of potential factors are the psychological dimensions of eating behaviors, which include (1) cognitive restraint: the ability to restrict eating voluntarily; (2) disinhibition: the inability to stop eating to prevent overconsumption; and (3) hunger: subjective feelings of hunger and food cravings.4 Psychological attributes related to dysfunctional eating behaviors (e.g., loss of control,5 disinhibition,6 and response to external cues7) were found to be predictors of subsequent increase in BMI or correlates of greater BMI z-scores during childhood and adolescence.
Furthermore, we hypothesize that different dietary interventions may change eating behaviors and cognitions about eating, as well as the type and/or amount of macronutrients consumed.
For example, an LC diet [LC: 0–60 g carbohydrate (CHO)/day] affects hunger by promoting a state of ketosis, leading to an appetite suppressant effect that has been associated with improved weight status.8 Another dietary intervention with desirable effects on hunger is an RGL diet. This dietary approach limits the intake of high glycemic-index foods (e.g., white bread, concentrated sugars) that are associated with a rapid rise and fall of blood glucose levels that can promote hunger and lead to greater caloric intake.9
Clinical trials of these CHO-modified dietary interventions involving youth with obesity have varied in the type and amount of CHO modification, dietary fat, caloric restriction, and intervention duration, yet all of these studies report an improvement in weight status at the completion of the intervention.3,9–13 However, only our trial of the LC, RGL, and PC diets3 collected data to evaluate the psychological dimensions on eating behaviors among children with obesity, using the Three-Factor Eating Questionnaire (TFEQ), a validated instrument that assesses cognitive restraint (CR), disinhibition, and hunger.4
In this article, we analyzed the TFEQ data on psychological dimensions of eating behaviors among children with obesity who participated in our 12-month randomized controlled trial.3 The aim of the present study was to evaluate whether baseline or changes in psychological dimensions of eating behaviors could account for variability in weight status outcomes. The study hypotheses are (1) CHO-modified diets will differentially affect psychological attributes of eating behaviors (i.e., decreased hunger and disinhibition with no change in cognitive restraint) compared with a standard PC diet and (2) baseline or favorable changes in eating behaviors will be associated with improvement in weight status at completion of the trial.
Methods
As described in detail previously,3 children were recruited from referrals to a pediatric weight management program at Cincinnati Children's Hospital Medical Center (CCHMC) who lacked health insurance coverage for the CCHMC program. Study announcements were also sent to community pediatricians and CCHMC employees to broaden the potential subject pool. The inclusion criteria were age 7–12 years, a fasting blood glucose level ≤100 mg/dl, and BMI z-score of 1.60–2.65. Exclusion criteria included medications known to affect appetite (e.g., stimulants and atypical antipsychotics), those with developmental or physical disabilities, and medical conditions such as diabetes, cardiac disease, or significant mental illness. Written informed consent was obtained from each child's parent/guardian and assent was obtained from each child age 11 and older. The study was approved by CCHMC's Institutional Review Board.
Subjects were randomly assigned to one of three diet groups: LC (n = 35); RGL (n = 36); or PC (n = 31). As previously described,3 subjects in the LC diet group were instructed to limit CHO intake to no more than 60 g/day. Subjects in the RGL diet group were instructed to limit their intake of high-glycemic index (GI) foods and drinks, using a stoplight approach that classified these items according to their GI. Subjects in the PC diet group were given age-appropriate, calorie-restricted meal plans (55%–60% CHO; 10%–15% protein, and 30% fat), resulting in a 500 kcal/day deficit relative to expected energy requirements. The subjects in all three diet groups were advised to take a daily vitamin/mineral supplement and to consume adequate fluids, with a goal of 48 ounces/day, preferably water.
As previously described,3 the 12-month study was designed to begin with a 3-month intervention, and then a 9-month follow-up period, with contact limited to the 6- and 12-month reassessment visits. The intervention was the diet assignment, with all groups receiving group exercise/education sessions and individual counseling for each subject by registered dietitians. Assessments were conducted at baseline, 3, 6, and 12 months. Assessment visits included measurement of height and weight using standardized protocols. The TFEQ was completed by the parent/guardian regarding the following eating behavior attributes of their children: (1) cognitive restraint: the ability to restrict eating voluntarily; (2) disinhibition: the inability to stop eating to prevent overconsumption; and (3) hunger: subjective feelings of hunger and food cravings. In the absence of standardized measures to evaluate eating behaviors based on self-report among young children, parental reports have been used to assess different aspects of their child's eating behaviors, including interest in eating and appetite.14 The TFEQ includes 36 true/false statements and 15 questions with ratings of 1–4.4 Examples of statements/questions and their associated factor [i.e., cognitive restraint (CR), disinhibition (D), and hunger (H)] include the following:
• I usually eat too much at social occasions like parties and picnics. (D)
• I am usually so hungry that I eat more than three times a day. (H)
• I deliberately take small helpings as a means of controlling my weight. (CR)
• How likely are you to consciously eat slowly to cut down on how much you eat? (CR)
• How frequently do you skip dessert because you are no longer hungry? (H)
• Do you go on eating binges even though you are not hungry? (D)
Statistical Analyses
Statistical analyses were conducted using SAS v.9.3 (SAS Institute, Cary, NC). Baseline variables are described using median (interquartile range, IQR) or number (percent) and compared across diets using nonparametric Wilcoxon or Fisher's exact tests for continuous and categorical data, respectively. Longitudinal mixed models were used to evaluate trajectories of TFEQ scores from baseline by diet, accounting for multiple measurements per person; Bonferroni-corrected p < 0.002 was considered significant (n = 21 comparisons). Categorical weight status criteria were based on BMI percent of 95th percentile as follows: Obesity-Class 1 (OB1): 100%–120% of 95th percentile for BMI and BMI <35 kg/m2; Severe Obesity-Class 2 (OB2): 121%–140% of 95th percentile or BMI = 35–39 kg/m2, whichever is lower; and Severe Obesity-Class 3 (OB3): >140% of 95th percentile or BMI >40 kg/m2, whichever is lower.15,16 To assess the relationship between TFEQ scores and BMI percent of the 95th percentile, accounting for multiple measurements per person, longitudinal mixed models were fitted, adjusting for sex, race, visit, and diet group assignment. To test for potential differences of TFEQ scores by visit, appropriate interaction terms were tested and retained if Type 3 estimates for fixed effects were significant. Diet group-specific models were also constructed to test for potential differing relationships of TFEQ scores with outcomes by diet group. For all model-based analyses, p < 0.05 was considered significant.
Results
Baseline Characteristics and TFEQ Scores During 12 Months of Follow-Up
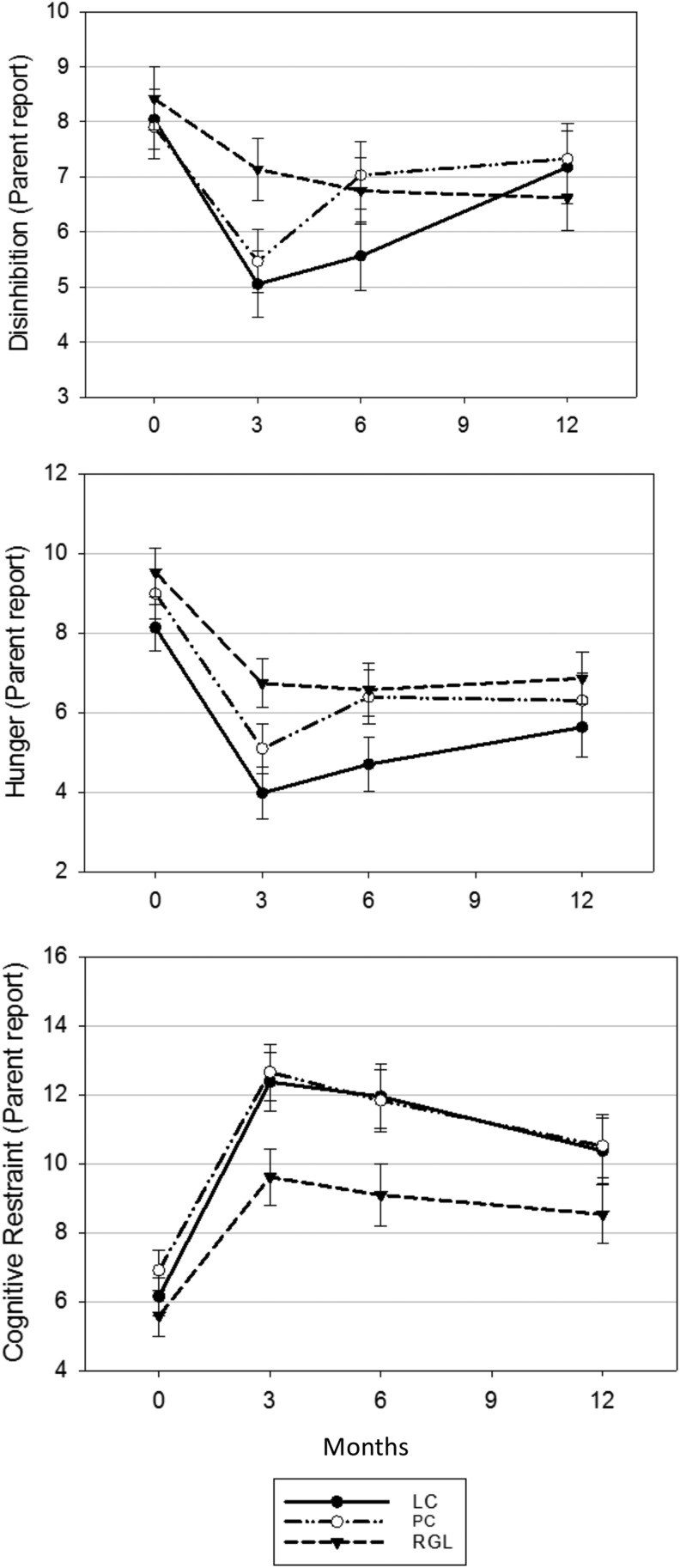
As previously reported3 and expected from randomization, the diet groups did not differ by demographic, BMI status, or parent-reported child TFEQ score variables at baseline (Table 1). All three diet groups demonstrated significant increases in child cognitive restraint and decreases in child hunger and disinhibition within the first 3 months of intervention (Fig. 1); changes in hunger and cognitive restraint scores were attenuated, but still differed from baseline at the 12-month follow-up visit in all three diet groups, while disinhibition remained significantly lower at 12 months only for the RGL group. TFEQ scores in the three diet groups did not differ from each other at any visit (all pairwise p > 0.002, Fig. 1). As previously reported for BMI z-score,3 the 3-month change in BMI percent of 95th percentile decreased more for the LC group than the other diets, but 6-month and 12-month child weight status outcomes did not differ by diet assignment (data not shown).
Table 1.
Clinical and Three-Factor Eating Questionnaire Characteristics at Baseline
| LC | PC | RGL | p | |
|---|---|---|---|---|
| N | 35 | 31 | 36 | |
| Age, years | 10.4 (9.4–12.1) | 10.5 (9.2–11.3) | 10.5 (9.0–11.8) | 0.83 |
| Sex (% male) | 16 (46) | 19 (53) | 8 (26) | 0.07 |
| Race (% white) | 26 (74) | 22 (71) | 31 (86) | 0.25 |
| BMI, kg/m2 | 29.7 (26.7–32.6) | 28.9 (25.8–31.4) | 30.1 (25.3–32.1) | 0.78 |
| BMI percent of 95th percentile | 128 (121–143) | 127 (113–136) | 128 (117–138) | 0.77 |
| Weight status category (%) | ||||
| Obesity—Class 1 | 8 (23) | 11 (35) | 10 (28) | |
| Severe obesity—Class 2 | 17 (49) | 16 (52) | 19 (53) | 0.58 |
| Severe obesity—Class 3 | 10 (29) | 4 (13) | 7 (19) | |
| TFEQ scoresa | ||||
| Restraint | 5.5 (4.5–7) | 7 (4–9.5) | 6 (3–8) | 0.42 |
| Hunger | 8.5 (5–11) | 9.5 (7.5–12) | 10 (7–12) | 0.28 |
| Disinhibition | 8 (6–10) | 7 (5.5–11) | 8 (6–11) | 0.71 |
N (%) or median (IQR) presented; p-values across groups from Fisher's exact or Wilcoxon rank-sum tests, respectively.
Maximum TFEQ scores are 21, 14, and 16 for cognitive restraint, hunger, and disinhibition, respectively.
IQR, interquartile range; LC, low-carbohydrate; PC, portion-controlled; RGL, reduced glycemic load; TFEQ, Three-Factor Eating Questionnaire.
Figure 1.
TFEQ scores for disinhibition, hunger, and cognitive restraint by diet group over time. TFEQ, Three-Factor Eating Questionnaire.
Relationships of TFEQ Scores with BMI Percent of 95th Percentile
In longitudinal models, including all diet groups and adjusting for sex (p = 0.05, boys higher), race (p = 0.05, nonwhite higher), and diet group assignment (p = 0.90), disinhibition score was not significantly associated with BMI percent of 95th percentile. However, a lower hunger score was associated with lower weight status across all visits (p = 0.002), and cognitive restraint scores differed in their relationship by study visit (p interaction = 0.005), with higher cognitive restraint associated with lower weight status particularly at the 12-month visit (Table 2).
Table 2.
Longitudinal Mixed Models of Three-Factor Eating Questionnaire Scores in Relation to BMI Percent of 95th Percentile
| Model Components | Overall | LC | RGL | PC |
|---|---|---|---|---|
| Disinhibition score | Ns | ns | ns | ns |
| Hunger score | 0.31 ± 0.10* | ns | ns | 0.62 ± 0.17* |
| Restraint score | −0.51 ± 0.14* | −0.72 ± 0.23* | −0.46 ± 0.09* | ns |
| Restraint by visit interactiona | p = 0.006 | p = 0.0003 | ns | ns |
| Restraint: baseline | 0.59 ± 0.23* | 1.0 ± 0.41** | ||
| Restraint: 3 months | 0.53 ± 0.16* | 0.88 ± 0.24* | ||
| Restraint: 6 months | 0.29 ± 0.13** | 0.43 ± 0.24 | ||
| Restraint: 12 months | 0 (Ref.) | 0 (Ref.) |
All models adjust for visit, age, sex, and race. The overall model also adjusts for diet group assignment.
Interpretation note regarding interaction: to obtain overall estimates of the effect of restraint score by visit, the restraint offset at that visit should be added to the restraint main effect; for example, estimate for restraint at baseline = −0.51 + 0.59 = 0.08 BMI percent of 95th percentile. p-Values in this section indicate whether each visit's offset differs significantly from the restraint estimate at 12 months (reference visit).
p < 0.01; **p < 0.05.
To determine whether these overall relationships differed by diet group, we next constructed diet-specific mixed models, adjusting for sex and race. In the LC model, sex (p = 0.03, boys higher) and cognitive restraint were associated with BMI percent of 95th percentile, again interacting by visit (p interaction = 0.0001); as with the overall model, higher cognitive restraint was associated with lower weight status particularly at the 12-month visit (Table 2). In the RGL model, sex and race were not significant, but higher cognitive restraint was associated with lower weight status across all visits (p < 0.0001) with no evidence of interaction with visit. In the portion control model, sex and race were not significant, but lower hunger scores were associated with lower weight status across all visits (p = 0.0006).
Factors Associated with Higher Cognitive Restraint and Lower Hunger TFEQ Scores
Given that cognitive restraint (LC and RGL group) and hunger (PC group) emerged as independent predictors of the child's BMI status, we conducted a post hoc analysis to determine which baseline factors were related to variability in these TFEQ factors at the 3-, 6-, and 12-month visits. Variables of interest included sex, race, baseline age, baseline obesity category, and baseline TFEQ scores to predict later TFEQ scores. Examining cognitive restraint in the LC and RGL groups and adjusting for visit-level differences, restraint was higher among those assigned to the LC diet (1.87 ± 0.77, p = 0.02), among white vs. nonwhite participants (4.14 ± 1.21, p = 0.001), and those with higher baseline cognitive restraint scores (0.33 ± 0.13, p = 0.02). Examining hunger in the PC group, adjusting for visit-level differences, only a lower baseline hunger score was associated with lower hunger scores at later visits (0.62 ± 0.12, p < 0.0001).
Discussion
The current analysis of psychological dimensions of eating behaviors among children in response to different dietary interventions provides novel findings regarding trajectory of change in these behaviors related to changes in children's BMI status up to 1 year after treatment initiation. In contrast to the original hypothesis, eating behavior changes, measured by the Three-Factor Eating Questionnaire (TFEQ), did not differ across diets. The LC diet, the RGL diet, and the PC diet demonstrated significant increases in children's cognitive restraint, decreases in hunger and disinhibition scores during the 3-month intervention, and all except disinhibition scores in the RGL group were sustained throughout the 12-month follow-up. This stands in contrast to literature that suggests hunger scores are more attenuated in those adhering to modified CHO diets due to the decreased excursion of blood glucose and insulin.17 In a randomized crossover trial, adult subjects reported greater satiation and fewer food cravings on a low-GL diet compared with a high-GL diet.18 The results of the present study suggest that children experience changes in psychological dimensions of eating behaviors similarly across distinct diets in ways that adults do not.
Strict adherence to an LC diet (≤60 g CHO/day) leads to a state of ketosis, which has been an explanation for the reported reduction in appetite and subsequent decrease in caloric intake that results in improved weight status.18 However, subjects assigned to the LC group did not strictly adhere to the LC diet at any time point as evidenced by 3-day food records [CHO g/day, mean ± standard deviation (SD): 3 months: 85 ± 12; 6 months: 108 ± 12; and 12 months: 123 ± 11].3 Furthermore, only 16% of Keto-Diastix results were positive for urinary ketones at any time point. Yet, the LC group reported a significant reduction in CHO intake at all time points when compared with baseline (242 ± 62 g/day; p < 0.003). This result suggests that a less restrictive LC diet still may lead to reduction in hunger measured by the TFEQ, but in the absence of ketosis, the mechanism is not known.
Although there were similar changes in eating behaviors across diets, the relationship between these eating behavior changes and BMI change during 12 months of follow-up differed by diet. In both the LC and RGL groups, higher cognitive restraint scores were associated with lower BMI status during the study, while in the PC group, only lower hunger scores were associated with lower BMI status.
By contrast, greater 6-month decreases in disinhibition scores were associated with greater improvement in 12-month BMI status, but only in the RGL group. This lowering of disinhibition may be explained by the RGL diet's effect on satiety. An RGL diet is designed to limit the intake of high-GI foods (e.g., white bread, concentrated sugars), which are associated with a rapid postprandial rise and fall of blood glucose levels that can promote hunger and lead to overconsumption.9 Instead, consuming more low-GI foods, such as those high in fiber (e.g., fruit, nonstarchy vegetables, 100% whole grains), and meat, fish, and poultry without breading, will slow the rate of digestion and thus sustain satiety longer.19 In addition, dietary adherence of subjects assigned to the RGL diet was consistently high (>75% at all time points).3 Therefore, the association between the decrease in disinhibition and improved weight status may reflect the RGL diet's effect on satiety combined with subjects' consistent adherence to the assigned diet.
In contrast to the findings relating cognitive restraint to BMI status in CHO-modified diets, lower hunger emerged as an important independent factor in relation to BMI status in the standard PC diet.
These findings suggest that changes in different aspects of children's eating behaviors may be more critical for improvement of BMI in the context of CHO-modified diets than PC diets. In a feeding study of healthy-weight adult women, those with high TFEQ disinhibition scores (i.e., tendency to overeat) had greater energy intake following a high-CHO meal than those with low scores, while energy intake following a high-fat meal did not differ based on participants' disinhibition scores.20 These results are generally consistent with our findings of CHO-specific effects of changes in psychological attributes, such as disinhibition, on subsequent BMI status, although we detected no differences in child BMI status outcomes based on baseline TFEQ scores regardless of dietary approach. Perhaps fluctuations in blood glucose associated with intake of high glycemic foods differentially affect the appetite of those with varying disinhibition scores. On the other hand, participants' TFEQ restraint scores in the aforementioned feeding study20 did not predict energy intake. Therefore, those results are not congruent with our current findings that link increased restraint scores with decreased BMI status among individuals assigned to a low-CHO diet.
Our post hoc analysis pursues the question of whether we could identify baseline factors that could help predict which participants would have higher cognitive restraint or lower hunger TFEQ scores as these were related to BMI status in the LC and RGL, or in the PC, diet groups, respectively. The findings from this analysis suggest that the race of the child (i.e., white participants having higher restraint scores in the LC and RGL groups) and higher baseline TFEQ scores could help predict higher TFEQ scores at later visits.
The results of this study may extend our potential to improve long-term treatment outcomes by tailoring strategies to the type of dietary intervention and modification of these eating behaviors. However, there is insufficient evidence at this time to make recommendations on how to favorably change cognitive restraint, disinhibition, or emotional eating within the course of treatment. To help address this knowledge gap, these eating behaviors need to be measured more routinely during treatment. In addition, studies need to be designed that can test interventions aimed to modify these eating behaviors. This could include more content focus on emotional eating in the behavioral component of treatment through basic feelings identification, monitoring links between feelings and eating behavior, and having alternative noneating responses to strong feelings, etc. Research studies are also needed to test the interaction between different treatment components aimed to modify these eating behaviors and different dietary approaches.
Strengths/Limitations
The strengths of this study include the use of a randomized clinical trial design over a 12-month period with quality control procedures to ensure treatment fidelity within and across the dietary approaches. The high retention rate (≥77% across the three diet groups) and percentage of subjects with complete data at all time points (78%) increased the validity of the reported findings. However, there are several limitations in this study. When the study was conducted, a validated self-assessment tool to measure the psychological attributes of eating behaviors among children was not available. Therefore, the TFEQ, which is validated for adults,4 was selected and completed by the child's parent or guardian, who may or may not be privy to the child's experiences of cognitive restraint, disinhibition, or hunger. However, it has been noted in the literature that there is value in a parent/guardian assessment of their child's eating behaviors.14 Analysis of a subset of participants 11 or 12 years of age who self-reported the TFEQ in addition to their parent's report revealed Spearman correlations that ranged from 0.40 to 0.65 for all three scales and across all study visits (all p < 0.02). These moderate and consistent correlations suggest that parents and children are reasonably consistent regarding the TFEQ scales, although the parent proxy is not perfect. Self-reporting by children may be influenced by social desirability as well as an inability to recognize or understand the reasons for their behavior, whereas caregivers may have more awareness and insight into their child's eating behaviors.
Braet et al. compared child and parent ratings of eating behaviors of overweight and obese children, ages 7–15 years, who had been referred to an outpatient weight management program.21 Significant positive correlations of child and parent scores were found for all subscales of the Dutch Eating Behavior Questionnaire (i.e., emotional eating, external eating, and restrained eating). Although both parent and child perspectives lend valuable information when available, the researchers concluded that parent reporting alone is an adequate measure of the child's eating behaviors. Therefore, in our study, caregivers able to observe the child's eating behaviors over time were regarded as appropriate surrogates for reporting this information.
Conclusions
This study found that eating behaviors improved significantly with all diets, and increased cognitive restraint and decreased hunger were sustained at 12 months; disinhibition also remained significantly lower at 12 months for the RGL group. Different aspects of the children's eating behaviors were associated with BMI outcomes by diet group, with higher cognitive restraint more important in CHO-modified diets and lower hunger more important in standard PC diets. The results of this study may extend our potential to improve long-term treatment outcomes for children with obesity by tailoring intervention strategies to the type of dietary approach and the child's eating behaviors.
Targeting diets to youth with obesity who have specific characteristics such as sex, race, and/or lower baseline hunger or higher baseline cognitive restraint scores may lead to improved results. However, more research is needed to determine whether tailoring diets based on either baseline characteristics, as reported with this study, or metabolic outcomes, as previously reported,3 are effective strategies to optimize outcomes in the context of a monitored dietary intervention for pediatric weight management.
Acknowledgments
This study was supported by the Thrasher Research Fund and an Institutional Clinical and Translational Science Award (NIH/National Center for Research Resources grant 5UL1RR026314-02).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ogden CL, Carroll MD, Lawman HG, et al. . Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016;315:2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock EP, O'Conner EA, Williams SB, et al. . Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics 2010;125:e396–e418 [DOI] [PubMed] [Google Scholar]
- 3.Kirk S, Brehm B, Saelens BE, et al. . Role of carbohydrate modification in weight management among obese children: A randomized clinical trial. J Pediatr 2012;161:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 5.Tanofsky-Kraff M, Yanovski SZ, Schvey NA, et al. . A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord 2009;42:26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant AR, Tremblay A, Perusse L, et al. . The three-factor eating questionnaire and BMI in adolescents: Results from the Quebec Family study. Br J Nutr 2010;104:1074–1079 [DOI] [PubMed] [Google Scholar]
- 7.Ho M, Gow M, Halim J, et al. . Effect of a prescriptive dietary intervention on psychological dimensions of eating behavior in obese adolescents. Int J Behav Nutr Phys Act 2013;10:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bravata DM, Sanders L, Huang J, et al. . Efficacy and safety of low-carbohydrate diets: A systematic review. JAMA 2003;289:1837–1850 [DOI] [PubMed] [Google Scholar]
- 9.Ebbeling CB, Ludwig DS. Treating obesity in youth: Should dietary glycemic load be a consideration? Adv Pediatr 2001;48:179–212 [PubMed] [Google Scholar]
- 10.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factors in overweight adolescents. J Pediatr 2003;142:253–258 [DOI] [PubMed] [Google Scholar]
- 11.Demol S. Yackobovitch-Gavan M, Shalitin S, et al. . Low-carbohydrate (low & high fat) versus high-carbohydrate low-fat diets in the treatment of obesity in adolescents. Acta Paediatr 2009;98:346–351 [DOI] [PubMed] [Google Scholar]
- 12.Diaz RG, Esparza-romero J, Moya-Camarena SY, et al. . Lifestyle intervention in primary care settings improves obesity parameters among Mexican youth. J Am Diet Assoc 2010;110:285–290 [DOI] [PubMed] [Google Scholar]
- 13.Spieth LE, Harnish JD, Lenders CM, et al. . A low-glycemic load diet in the treatment of pediatric obesity. Arch Pediatr Adolesc Med 2000;154:947–951 [DOI] [PubMed] [Google Scholar]
- 14.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behavior Questionnaire. J Child Psychol Psychiatry 2001;42:963–970 [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM.1, Wei R, Ogden CL, et al. . Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr 2009;90:1314–1320 [DOI] [PubMed] [Google Scholar]
- 16.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr 2014;168:561–566 [DOI] [PubMed] [Google Scholar]
- 17.Kong APS, Chan RSM, Nelson AS, Chan JCN. Role of low-glycemic index diet in management of childhood obesity. Obes Rev 2011;12:492–498 [DOI] [PubMed] [Google Scholar]
- 18.Chang KT, Lampe JW, Schwarz Y, et al. . Low glycemic load experimental diet more satiating than high glycemic load diet. Nutr Cancer 2012;64:666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig DS. Dietary glycemic index and obesity. J Nutr 2000;130:280S–283S [DOI] [PubMed] [Google Scholar]
- 20.Chambers L, Yeomans MR. Individual differences in satiety response to carbohydrate and fat. Predictions from the Three Factor Eating Questionnaire (TFEQ). Appetite 2011;56:316–323 [DOI] [PubMed] [Google Scholar]
- 21.Braet C, Soetens B, Moens E, et al. . Are two informants better than one? Parent-child agreement on the eating styles of children who are overweight. Eur Eat Disord Rev 2007;15:410–417 [DOI] [PubMed] [Google Scholar]